Abstract
Background
The majority of antimicrobial use occurs in the ambulatory setting. Antimicrobial stewardship programs (ASPs) are effective in improving appropriate prescribing and are now required by accreditation bodies.
Methods
This was a cross-sectional, multicenter survey describing the current state of ambulatory ASPs in a national cohort of Vizient member hospitals with ambulatory healthcare settings and serves as a benchmark for stewardship strategies related to program effectiveness.
Results
One hundred twenty-nine survey responses from a variety of institution types across 44 states were received. Survey respondents reported a fully functioning ASP in 7% (9 of 129) of ambulatory practices compared with 88% (114 of 129) of inpatient institutions. Effectiveness in at least 1 antibiotic use-related outcome (ie, utilization, resistance, Clostridioides difficile infection, or cost) in the past 2 years was reported in 18% (18 of 100) of ambulatory and 84% (103 of 123) of inpatient ASPs. Characteristics of ambulatory ASPs demonstrating effectiveness were institution guidelines (89%, 16 of 18), rapid diagnostic testing for respiratory viruses or group A Streptococcus (89% 16 of 18), outpatient antibiograms (78% 14 of 18), and dedicated pharmacist support (72%, 13 of 18). Ambulatory ASP effectiveness was shown to increase as programs met more of the Centers for Disease Control and Prevention (CDC) Core Elements of Outpatient Antimicrobial Stewardship (P < .001).
Conclusions
Antimicrobial stewardship programs are needed in the ambulatory setting, but they are not common. Currently, few ambulatory ASPs in this survey self-identify as fully functioning. The CDC Core Elements of antimicrobial stewardship should remain foundational for ASP development and expansion.
Keywords: antimicrobial stewardship, antimicrobial use, ambulatory practice
A national cohort of ambulatory stewardship program characteristics and activities compared with inpatient stewardship programs. Ambulatory ASP effectiveness was shown to increase as programs met more of the Centers of Disease Control and Prevention Core Elements of Outpatient Antimicrobial Stewardship.
Antimicrobial resistance is an ongoing public health concern driven by antibiotic overuse across the care continuum [1]. It has been estimated that 80%–90% of all antibiotic use in humans occurs in the ambulatory setting [2, 3]. In 2016, there were 270.2 million outpatient antibiotic prescriptions written in the United States, surmounting to 5 prescriptions per 6 people, as reported by the Centers for Disease Control and Prevention (CDC) [4]. Furthermore, data suggest that 30%–52% of outpatient antibiotic prescriptions are unnecessary [5, 6]. This excessive use of antibiotics has been described as a primary cause of resistance and leads to adverse events such as secondary Clostridioides difficile infections [7–10].
With such data indicating the possible overuse and misuse of antibiotics, it is essential to evaluate and implement effective stewardship practices. Although antimicrobial stewardship programs (ASPs) vary in characteristics, they have been shown to be beneficial in the inpatient setting, and, although less common, ASPs in ambulatory settings have also been shown to improve antibiotic therapy selection and minimize inappropriate use of antibiotics [11–14]. As a result, The Joint Commission has published new standards for ASPs in the ambulatory setting effective January 2020 [15]. The CDC Core Elements of Antimicrobial Stewardship along with the Quality Innovation Network-Quality Improvement Organization (QIN-QIO) Field Guide help to provide guidance for stewardship programs in the ambulatory setting [16, 17]. Despite these resources, compared with inpatient ASPs, there are no recommendations regarding priority interventions for ambulatory ASP program success. Ambulatory antimicrobial stewardship remains generally uncharted, and detailed information on the current state of practice across the country is lacking.
Due to the high rates of unnecessary and inappropriate antibiotic use along with new regulatory requirements, it is prudent to assess ASP strategies currently established in the ambulatory setting. This study reports survey findings from a national cohort describing the current state of ambulatory ASPs and provides a benchmark of strategies leading to reported effectiveness in ambulatory ASPs.
METHODS
Survey
This was a cross-sectional survey of the Vizient network and associated provider care practices, characterizing the current state of ambulatory ASPs nationally. In addition, survey respondents were asked to compare their ambulatory ASP to their inpatient ASP where applicable. Vizient, Inc. is the largest member-driven, healthcare performance improvement company in the United States that encompasses more than 3200 acute care hospitals, and service to 95% of US academic medical centers, and more than 20% of the nation’s ambulatory market. The survey was sent to pharmacy directors, antimicrobial stewardship listserv, and ambulatory/outpatient pharmacy listserv, and pharmacy contacts in the community-based network were invited to participate regardless of presence of any ASP. Incomplete or duplicate surveys were excluded.
The Institutional Review Board-approved electronic survey comprised 51 questions and was distributed to participants via email. Questions were designed to capture participant demographics, volume metrics, presence of stewardship program, compliance with CDC Core Elements of Outpatient Antimicrobial Stewardship, and components of ambulatory stewardship (Qualtrics, Provo, UT) (see Supplementary Appendix). Participants answering on behalf of health systems were directed to respond to questions based on the majority of their practice sites, when applicable. Survey responses were collected from September 19 to October 18, 2019 with 2 reminders sent via email.
Definitions
Presence of ASPs were classified by respondents in 4 categories that include fully functional, in development, no program but considering a small project, or no program with no plans to develop one. Fully function was defined as stewardship activities at multiple ambulatory locations and/or for multiple diagnoses, whereas programs in development were those providing stewardship activities to 1 ambulatory location and/or 1 diagnosis. Stewardship interventions (eg, written justification in medical record, prescriber-level audit feedback, communication training) were all defined and grouped (ie, commitment, action and policy, tracking and reporting, education) according to the 2016 CDC Core Elements of Outpatient Antibiotic Stewardship [16]. Antimicrobial stewardship program effectiveness was defined as self-reported achievement in at least one of the following areas within the past 2 years: (1) cost savings or cost avoidance related to antimicrobials; (2) decreased antimicrobial utilization; (3) decreased C difficile infection; (4) decreased rate of drug-resistant organisms [18]. For additional survey definitions, see Supplementary Appendix.
Analysis
Data were analyzed using statistical software, Stata (version 13.1; StataCorp LLC, College Station, TX). Participant survey responses were summarized using descriptive statistics. Inferential group comparisons were analyzed using χ 2 or Fisher’s exact test for nominal data, as appropriate, and the Mann-Whitney U test for continuous non-parametric data. Kruskal-Wallis testing was used for ranked data. Statistical significance was defined as P < .05.
RESULTS
Demographics
Surveys were sent to 1662 individuals, and 172 (10.3%) responses were received. Forty-three responses were excluded (7 redundant and 36 incomplete) leaving 129 included surveys. Respondents represented a variety of institution types and were geographically distributed across 44 states (Table 1). A majority of respondents answered on behalf of a health system and identified an affiliation with an academic medical center.
Table 1.
Characteristics of Survey Participantsa
| Characteristics | N = 129 |
|---|---|
| Location | |
| South | 39 (30.2) |
| Midwest | 37 (28.6) |
| Northeast | 27 (20.9) |
| West | 26 (20.5) |
| Answering on behalf of a health system | 68 (52.7) |
| Institution typesb | |
| Academic medical center | 76 (58.9) |
| Ambulatory care/outpatient clinic | 61 (47.3) |
| Medium sized community hospital (100–300 beds) | 36 (27.9) |
| Large community hospital (>300 beds) | 34 (26.4) |
| Children’s hospital | 28 (21.7) |
| Critical access hospital | 22 (17.1) |
| Behavioral health facility | 20 (15.5) |
| Small sized community hospital (<100 beds) | 17 (13.2) |
| Long-term acute care facility | 16 (12.4) |
| Freestanding oncology hospital | 6 (4.7) |
aAll data reported as n (%).
bMultiple selections possible when answering for health system.
Characteristics of ambulatory and inpatient ASPs are described in Table 2. Ambulatory ASPs were reported as fully functional in 7% (9 of 129) of surveys compared with 88% (114 of 129) in the inpatient setting. Most respondents, 78% (100 of 129), expressed an interest in or current development of an ambulatory ASP, and 53% (68 of 129) of programs reported that ambulatory antimicrobial stewardship was a priority for their institution. The established duration of ASPs differed between ambulatory and inpatient programs with more ambulatory ASPs developed within the last year at 22% (29 of 129), whereas more inpatient ASPs were established for greater than 7 years at 44% (57 of 129). The minority of respondents noted somewhat (16%) or strongly (4%) agreed that financial resources were adequate for ambulatory ASP compared with inpatient ASP where 44% somewhat and 17% strongly agreed.
Table 2.
Comparison of Ambulatory and Inpatient Stewardship Programsa
| Characteristics | Ambulatory N = 129 | Inpatient N = 129 |
|---|---|---|
| Description of Program | ||
| Fully functional | 9 (7) | 114 (88) |
| In development | 47 (36) | 12 (9) |
| No program, considering small project | 53 (41) | 1 (1) |
| No program, no current plans to develop one | 20 (16) | 2 (2) |
| Dedicated Support | ||
| Pharmacist | 53 (41) | 115 (89) |
| Informatics technology | 47 (36) | 82 (64) |
| Physician | 45 (35) | 109 (85) |
| Infection preventionist | 44 (34) | 88 (68) |
| Administrative | 36 (28) | 50 (39) |
| Microbiologist | 22 (17) | 93 (72) |
| Core Element: Commitment | 74 (57) | - |
| Commitment posters | 44 (34) | 35 (27) |
| Ambulatory stewardship is an institutional priority | 68 (53) | - |
| Core Element: Action and Policy | 77 (60) | 112 (87) |
| Institution-specific treatment guidelines | 58 (45) | 112 (87) |
| Cascade reporting | 40 (31) | 69 (54) |
| Set at least 1 annual goal | 27 (21) | 105 (81) |
| Electronic decision support | 18 (14) | 81 (63) |
| Provider incentives | 14 (11) | 22 (17) |
| Healthcare Effectiveness Data and Information Set | 13 (10) | 16 (12) |
| Required antimicrobial indications | 13 (10) | 82 (64) |
| Written accountable justification | 8 (6) | 32 (25) |
| Core Element: Tracking and Reporting | 72 (56) | 116 (90) |
| Antibiogram | 59 (46) | 106 (82) |
| Per-visit antibiotic prescription rates per diagnosis | 19 (15) | 6 (5) |
| Prescriber-level audit and feedback | 18 (14) | 72 (56) |
| Provider-level prescribing data | 14 (11) | 22 (17) |
| Special project outcomes | 14 (11) | 69 (53) |
| Prescribing rates | 14 (11) | 22 (17) |
| Progress on annual goals | 10 (8) | 75 (58) |
| Clostridioides difficile infection | 5 (4) | 101 (78) |
| Drug-resistant organisms | 5 (4) | 90 (70) |
| Antibiotic days of therapy | 3 (2) | 96 (74) |
| Pediatric-specific data | 2 (2) | 25 (19) |
| Purchasing costs | 2 (2) | 77 (60) |
| Antibiotic length of therapy | 2 (2) | 17 (13) |
| Daily defined doses | 0 | 17 (13) |
| Core Element: Education | 43 (33) | 67 (52) |
| Patient education materials | 37 (29) | 56 (43) |
| Communication training | 21 (16) | 42 (33) |
| Symptomatic prescribing pad | 13 (10) | 3 (2) |
aAll data reported as n (%).
Antimicrobial Stewardship Program Characteristics and Core Elements
Commitment to ambulatory stewardship was present in 57% of programs as exhibited by the identification of ambulatory stewardship as an institutional priority. Institutional priority included programs with an approved statement of commitment from executive leadership, ambulatory stewardship-related duties in position descriptions, or the appointment of a single leader to direct stewardship activities. The most common stewardship personnel in both settings were pharmacists at 41% (53 of 129) in ambulatory programs and 89% (115 of 129) in inpatient programs. This was closely followed by informatics support at 37% (48 of 129) and physician support at 35% (45 of 129) in ambulatory programs. For technology support, electronic health record (EHR) systems were used in 98% (127 of 129) of all ASPs, and the majority (82%) reported capabilities for sharing information between inpatient and ambulatory sites. Technology add-ons were only present in 13% (17 of 129) of ambulatory programs. The primary add-on technology for both ambulatory and inpatient ASPs was the use of Epic ASP Module at 5% (6 of 129) and 30% (39 of 129), respectively.
Broadly assessing ASP actions, inpatient programs consistently used more components than ambulatory programs (Table 2). The use of institution-specific treatment guidelines was the most common stewardship action in both ambulatory and inpatient programs (45% vs 87%, respectively). Ambulatory treatment guidelines targeted urinary tract infections at 21% (27 of 129), pharyngitis at 19% (25 of 129), sinusitis at 19% (25 of 129), and otitis at 16% (20 of 129). Second to treatment guidelines, cascade antimicrobial susceptibility testing reporting and the use of annual goals were reported in 31% and 21% of ambulatory ASPs, respectively. Microbiological testing support was most commonly seen with the use of rapid diagnostic testing (RDT) for respiratory viruses (59%), group A Streptococcus (62%), and blood cultures (40%) in ambulatory programs. Overall, stewardship actions less commonly reported included the use of EHR clinical decision support, provider incentives, antimicrobial indications, and written accountable justifications.
Antimicrobial tracking and reporting was less frequently used in the ambulatory setting with the exception of antibiograms, which were reported in 46% (59 of 129) of programs (Table 2). Additional reporting included per-visit antibiotic prescription rates for a particular diagnosis (ie, bronchitis, upper respiratory tract infection, and sinusitis) at 15% (19 of 129). Comparatively in the inpatient setting, almost all programs (90%) reported a component of tracking and reporting.
Education was a component of 33% of ambulatory ASPs and was primarily in the form of patient education materials (29%). Other described methods of education included communication training (16%) and the use of symptomatic prescribing pads (10%).
Antimicrobial Stewardship Program Outcomes
Only ASPs who reported measuring antimicrobial utilization, cost, resistance, and/or C difficile infections in the past 2 years were included in the effectiveness assessment (ambulatory programs = 100, inpatient programs = 123). Overall, self-reported effectiveness was less common in the ambulatory setting at 18% (18 of 100) compared with 84% (103 of 124) of inpatient ASPs (Table 3). For both ambulatory and inpatient ASPs, effectiveness was most commonly reported as demonstrating decreased antibiotic utilization at 94% (17 of 18) and 87% (90 of 103), respectively. Decreases in antimicrobial resistance was the lowest reported measure of effectiveness for both ambulatory and inpatient ASPs (2 of 18 [11%] versus 38 of 103 [37%], respectively).
Table 3.
Self-Reported Program Effectivenessa
| Type of Effectiveness Reported | Ambulatory | Inpatient |
|---|---|---|
| Effectiveness reported | 18/100 (18)b | 103/123 (84)b |
| Decreased antimicrobial utilization | 17/18 (94) | 90/103 (87) |
| Antimicrobial cost savings | 4/18 (22) | 87/103 (84) |
| Decreased Clostridioides difficile infection | 3/18 (17) | 67/103 (65) |
| Decreased antimicrobial resistance | 2/18 (11) | 38/103 (37) |
aAll data reported as n (%).
bDenominator includes programs who answered as tracking effectiveness.
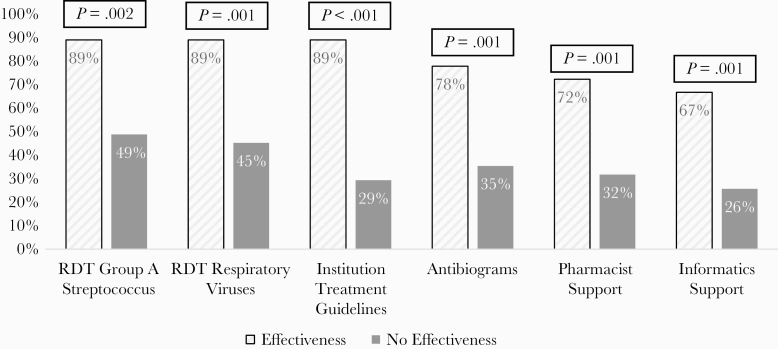
The ASP activities most commonly present in ambulatory programs that reported effectiveness included institutional guidelines, RDT for respiratory viruses, and RDT of group A Streptococcus (Figure 1). The 2 most common personnel support types in effective ambulatory ASPs were pharmacist at 72% (13 of 18) and informatics at 67% (12 of 18).
Figure 1.
Characteristics and activities of ambulatory antimicrobial stewardship programs based on program effectiveness. Percentages calculated as presence of characteristic with programs demonstrating effectiveness (n = 18) or within programs not demonstrating effectiveness (n = 82). Excludes those programs not tracking effectiveness (n = 29). RDT, rapid diagnostic testing.
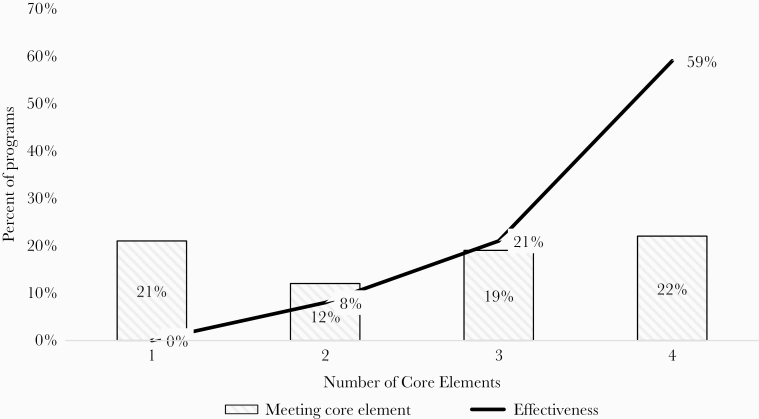
The relationship between the number of CDC Core Elements of Outpatient Antimicrobial Stewardship implemented and measured effectiveness is shown in Figure 2. For ASPs meeting only one Core Element, zero programs reported effectiveness compared with 59% when all 4 Core Elements were met (P < .001).
Figure 2.
Centers for Disease Control and Prevention (CDC) Core Elements for ambulatory stewardship and reported effectiveness. Relationship between ambulatory antimicrobial stewardship programs meeting core elements and reporting effectiveness.
Discussion
This national survey found that a minority of institutions reported a fully functional ambulatory ASP with only 13 institutions reporting they met all 4 CDC core elements of outpatient stewardship. A vast majority (78%) expressed an interest in or current development of an ambulatory ASP, whereas only 20% reported having adequate financial resources. Furthermore, results show inpatient stewardship programs to be more prevalent and consistently use more stewardship activities compared with ambulatory ASPs. This finding aligns with the higher rates of effectiveness seen in inpatient ASPs when compared with ambulatory ASPs. More importantly, the longer duration of ASPs and higher frequency of tracking and reporting in the inpatient setting likely had a significant contribution on this difference. The intent of this research is to serve as a benchmark to those ambulatory ASPs in development and provide a framework for stewardship activities that are commonly seen in effective programs.
The reporting of stewardship characteristics commonly seen in effective programs may help to guide institutions who are currently in development of ambulatory ASPs. Rapid diagnostics for group A Streptococcus and respiratory viruses, antibiograms, and institutional-specific guidelines were common in programs who reported effectiveness, suggesting possible target areas for ASPs in development. Use of RDT to improve diagnosis of group A Streptococcus or respiratory virus infections during an ambulatory office visit can assist with antibiotic selection and make a large impact given the high rates of inappropriate or unnecessary antibiotic prescriptions for these disease states [5, 6, 19–21]. Adopting these tests requires economic commitment, electronic medical record integration, personnel training, collaboration with microbiology, and prescriber support, which may pose road blocks to quick implementation [22]. However, for institutions who currently use these tests in their inpatient setting, expansion to ambulatory sites may be a logical first step to ambulatory ASP development. Effective ambulatory ASPs in this survey often incorporated institutional guidelines for urinary tract infections and otitis, providing additional conditions to target. Previous literature has also suggested that pursuing ASP activities for these disease states may improve antimicrobial therapy [23, 24]. More research is needed to guide effective ambulatory antimicrobial stewardship interventions. In addition, research into the sustainability of these intervention is desperately needed given the dearth of resources in this area.
Although we identify high-priority areas for ambulatory ASPs, it is important to note that successful ASPs are multifaceted, as seen with our survey respondents reporting greater rates of effectiveness as more CDC Core Elements of Outpatient Antimicrobial Stewardship are met [16, 25, 26]. Likewise, March-López et al [25] report that a multilayered outpatient study using interventions in line with the CDC Core Elements resulted in a 17% reduction in overall antibiotic utilization. A key component of this success was dependent on the personnel support provided to the ASP. In our survey, in ambulatory programs that demonstrated effectiveness, 72% had pharmacist support and 67% had informatics support. This integral role of pharmacist support and technology add-ons on ASP effectiveness is essential given the high (98%) prevalence of EHRs and has also been described in the acute-care setting [18, 27]. More importantly, physician support was more common in the inpatient setting as were overall rates of effectiveness. Although studies have evaluated physician and pharmacist full-time equivalent (FTE) support and effectiveness in inpatient ASPs, information is lacking in the ambulatory setting [18]. Further evaluation of FTE support in the ambulatory ASPs is warranted.
This study was not without limitations. First, the use of Vizient-affiliated institutions and low response rates may have resulted in selection bias and overestimated the actual current state of ambulatory ASP. However, the survey respondents comprised of a variety of institutional types and were well represented geographically from 44 states. Second, the use of self-reported effectiveness and lack of standardization for degree of effectiveness seen by ASPs may have led to an over- or underreporting of outcomes. In addition, although these definitions have been previously used in studies to demonstrate inpatient antimicrobial stewardship effectiveness, it is unknown whether all components are applicable to outpatient ASP effectiveness [18]. The survey did not capture other additional infection control or hospital-related interventions that may impact rates of C difficile infection and multidrug-resistant infections. Finally, a large percentage of participants answered on behalf of a health system and were directed to answer questions based on the practice at the majority of their affiliated sites. This may make it difficult to apply the results of our study to all institution types. In light of new regulatory requirements and need for ambulatory antimicrobial stewardship, future assessments to evaluate this evolving field will be informative. Assessing more discrete markers of program effectiveness in future surveys may be beneficial because programs that are currently in development will have more time to drive change in antimicrobial utilization.
Conclusions
In summary, this study serves as a benchmark to ambulatory antimicrobial stewardship that has been largely undescribed, demonstrates the importance of a multifaceted program on success, and provides stewardship areas of focus for institutions with ambulatory ASPs currently in development.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. G. L. B. is an employee of Vizient, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 27 June 2020.
- 2. Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report. Available at: https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report. Accessed 27 June 2020.
- 3. Public Health Agency of Sweden, National Veterinary Institute. Consumption of antibiotics and occurance of antibiotic resistance in Sweeden. Available at: https://www.folkhalsomyndigheten.se/pagefiles/20281/Swedres-Svarm-2014–14027.pdf. Accessed 27 June 2020.
- 4. Centers for Disease Control and Prevention. Antibiotic use in the United States, 2018 update: progress and opportunitites. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 5. Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ 2019; 364:k5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. . Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Office-related antibiotic prescribing for persons aged </= 14 years--United States, 1993–1994 to 2007–2008. MMWR Morb Mortal Wkly Rep 2011; 60:1153–6. [PubMed] [Google Scholar]
- 8. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47:735–43. [DOI] [PubMed] [Google Scholar]
- 11. Meeker D, Knight TK, Friedberg MW, et al. . Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drekonja DM, Filice GA, Greer N, et al. . Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36:142–52. [DOI] [PubMed] [Google Scholar]
- 13. Meeker D, Linder JA, Fox CR, et al. . Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patros C, Sabol M, Paniagua A, Lans D. Implementation and evaluation of an algorithm-based order set for the outpatient treatment of urinary tract infections in the spinal cord injury population in a VA Medical Center. J Spinal Cord Med 2018; 41:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Joint Commission. R3 report issue 23: antimicrobial stewardship in ambulatory health care. Available at: https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_23_antimicrobial_stewardship_amb_6_14_19_final2.pdf. Accessed 27 June 2020.
- 16. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Telligen, the Quality Innovation Network National Coordinating Center. A field guide to antibiotic stewardship in outpatient settings. Available at: https://qioprogram.org/sites/default/files/editors/141/C310_Field_Guide_20180730_FNL.pdf. Accessed 27 June 2020.
- 18. Doernberg SB, Abbo LM, Burdette SD, et al. . Essential resources and strategies for antibiotic stewardship programs in the acute care setting. Clin Infect Dis 2018; 67:1168–74. [DOI] [PubMed] [Google Scholar]
- 19. Havers FP, Hicks LA, Chung JR, et al. . Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open 2018; 1:e180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olesen SW, Barnett ML, MacFadden DR, et al. . Trends in outpatient antibiotic use and prescribing practice among US older adults, 2011-15: observational study. BMJ 2018; 362:k3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dodd M, Adolphe A, Parada A, et al. . Clinical impact of a rapid streptococcal antigen test on antibiotic use in adult patients. Diagn Microbiol Infect Dis 2018; 91:339–44. [DOI] [PubMed] [Google Scholar]
- 22. Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol 2017; 55:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wattengel BA, DiTursi S, Schroeck J, et al. . Outpatient antimicrobial stewardship: targets for urinary tract infections. Am J Infect Control 2020; doi: 10.1016/j.ajic.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 24. White AT, Clark CM, Sellick JA, Mergenhagen KA. Antibiotic stewardship targets in the outpatient setting. Am J Infect Control 2019; 47:858–63. [DOI] [PubMed] [Google Scholar]
- 25. March-López P, Madridejos R, Tomas R, et al. . Impact of a multifaceted antimicrobial stewardship intervention in a primary health care area: a quasi-experimental study. Front Pharmacol 2020; 11:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hürlimann D, Limacher A, Schabel M, et al. ; Swiss Sentinel Working Group. Improvement of antibiotic prescription in outpatient care: a cluster-randomized intervention study using a sentinel surveillance network of physicians. J Antimicrob Chemother 2015; 70:602–8. [DOI] [PubMed] [Google Scholar]
- 27. Blanchette L, Gauthier T, Heil E, et al. . The essential role of pharmacists in antibiotic stewardship in outpatient care: an official position statement of the Society of Infectious Diseases Pharmacists. J Am Pharm Assoc 2018; 58:481–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.