Abstract
Background
Malaria is a major public-health problem, with over 40% of the world’s population (more than 3.3 billion people) at risk from the disease. Malaysia has committed to eliminate indigenous human malaria transmission by 2020. The objective of this descriptive study is to understand the epidemiology of malaria in Malaysia from 2000 through 2018 and to highlight the threat posed by zoonotic malaria to the National Malaria Elimination Strategic Plan.
Methods
Malaria is a notifiable infection in Malaysia. The data used in this study were extracted from the Disease Control Division, Ministry of Health Malaysia, contributed by the hospitals and health clinics throughout Malaysia. The population data used in this study was extracted from the Department of Statistics Malaysia. Data analyses were performed using Microsoft Excel. Data used for mapping are available at EPSG:4326 WGS84 CRS (Coordinate Reference System). Shapefile was obtained from igismap. Mapping and plotting of the map were performed using QGIS.
Results
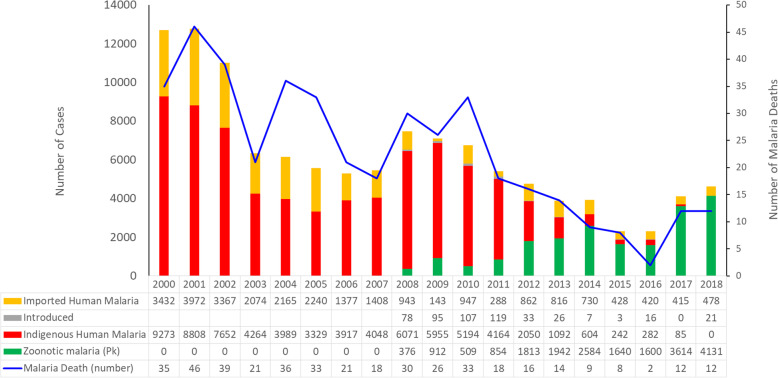
Between 2000 and 2007, human malaria contributed 100% of reported malaria and 18–46 deaths per year in Malaysia. Between 2008 and 2017, indigenous malaria cases decreased from 6071 to 85 (98.6% reduction), while during the same period, zoonotic Plasmodium knowlesi cases increased from 376 to 3614 cases (an 861% increase). The year 2018 marked the first year that Malaysia did not report any indigenous cases of malaria caused by human malaria parasites. However, there was an increasing trend of P. knowlesi cases, with a total of 4131 cases reported in that year. Although the increased incidence of P. knowlesi cases can be attributed to various factors including improved diagnostic capacity, reduction in human malaria cases, and increase in awareness of P. knowlesi, more than 50% of P. knowlesi cases were associated with agriculture and plantation activities, with a large remainder proportion linked to forest-related activities.
Conclusions
Malaysia has entered the elimination phase of malaria control. Zoonotic malaria, however, is increasing exponentially and becoming a significant public health problem. Improved inter-sectoral collaboration is required in order to develop a more integrated effort to control zoonotic malaria. Local political commitment and the provision of technical support from the World Health Organization will help to create focused and concerted efforts towards ensuring the success of the National Malaria Elimination Strategic Plan.
Keywords: Malaria, Malaysia, Epidemiology, Zoonotic malaria, Elimination
Introduction
Malaria is a major public health challenge, with over 40% of the world’s population (3.3 billion people) at risk from the disease. According to the latest World Malaria Report (2019), there were an estimated 228 million cases of malaria worldwide in 2018, with the majority of cases occurring in Africa.
Malaysia has come a long way in the prevention and control of malaria since the introduction of the Malaria Eradication Program in 1961 in Sabah and Sarawak, and in 1967 in Peninsular Malaysia [1]. In 2011, Malaysia recorded less than 1 case per 1000 people and thereby entered into the elimination phase of malaria control [2]. The National Malaria Elimination Strategic Plan was introduced in the same year with the target of achieving “malaria-free” status by 2020. Malaria-free status here refers to human indigenous malaria species such as P. falciparum, P. malariae, P. ovale, and P. vivax. In 2019, the WHO Western Pacific Region, which includes Malaysia, reported the second lowest malaria incidence among all WHO Regions [3]. Under the Regional Action Framework for Malaria Control and Elimination in the Western Pacific (2016–2020), three countries/provinces including Malaysia were targeted to achieve malaria elimination by 2020 [4].
Plasmodium knowlesi is a zoonotic malaria parasite transmitted between non-human primate hosts by Anopheles mosquitoes and causes spill-over infections in humans where the parasite, vector, host, and human converge. The first naturally acquired knowlesi malaria case was reported in 1965 in a man who returned to the USA after visiting peninsular Malaysia [5], followed by a second naturally occurring transmission reported in 1971 [6]. Plasmodium knowlesi is the fifth species that can cause malaria in humans and gained attention as a possible emerging public health threat in 2004 when a large focus of naturally acquired P. knowlesi infections was confirmed in blood samples from 2002 to 2004 in Sarawak, Malaysian Borneo [7]. Human P. knowlesi infections occurred in Sarawak from as early as 1996 [8], and also from 2000 to 2002 [7] and 2001 to2006 [9]. P. knowlesi cases were also described in Pahang in 2004 and 2005, and in Sabah from 2003 to 2005 [9].
Most human P. knowlesi cases are chronic and symptomatic, but some can be severe and lead to death [10]. Clinical studies in Sarawak indicated that more than 10% of patients with P. knowlesi malaria developed severe disease as classified by the WHO with an approximately 1% case fatality rate (CFR) [11]. Plasmodium knowlesi has the shortest asexual replication cycle of all human-infecting malaria parasites leading to rapidly increasing parasitemia levels [11]. It is highly sensitive to artemisinin, and variably and moderately sensitive to chloroquine and mefloquine [12].
Here, we analyze Malaysia’s successful anti-malaria campaign from an epidemiological perspective, with the aim that the results can be reference for other countries experiencing malaria elimination plan. The objective of this study is to understand the epidemiology of malaria in Malaysia from 2000 through 2018 and to highlight the threat that zoonotic malaria poses to the National Malaria Elimination Strategic Plan.
Methods
Here, we present a descriptive study on the epidemiology of malaria in Malaysia from 2000 through 2018. The study site is whole Malaysia. The case definitions for classification of malaria are given in Table 1 [13]. Human malaria was defined as malaria caused by P. falciparum, P. malariae, P. ovale, and P. vivax. Only these four species of Plasmodium were only considered in indigenous malaria. Imported versus indigenous malaria cases were classified according to the criteria set by the WHO, which were consideration of travel history and incubation period, and the evidences of epidemiology link in traveled destination and the absence of epidemiology link locally during case investigation. The data used in this study were provided by the Disease Control Division, Ministry of Health Malaysia, and were initially contributed by hospitals and health clinics throughout Malaysia. Malaria is a mandatory notifiable disease under the Malaysian Infectious Disease Prevention and Control Act 1988. Passive surveillance is performed through routine notification of district health offices by local health facilities, while active surveillance among high-risk groups such as land scheme settlers and migrant workers is carried out in endemic areas. State health departments compile data for the whole state before sending it to the national level central health department. The population data used in this study was extracted from the Department of Statistics Malaysia. Data analyses were performed using Microsoft Excel. Data used for mapping are available at EPSG:4326 WGS84 CRS (Coordinate Reference System). Shapefile was obtained from igismap [14]. Mapping and plotting of the map were performed using QGIS [15].
Table 1.
Case definition for the classification of malaria
| Malaria case | Confirmed malaria case (or infection) in which the parasite has been detected in a diagnostic test, i.e., microscopy, a rapid test, or a molecular diagnostic test |
| Malaria case, introduced | A case contracted locally, with strong epidemiological evidence linking it directly to a known imported case (first-generation local transmission) |
| Malaria case, imported | Malaria case or infection in which the infection was acquired outside the area in which it is diagnosed |
| Malaria case, indigenous | A case contracted locally with no evidence of importation and no direct link to transmission from an imported case |
| Malaria elimination | Interruption of local transmission (reduction to zero incidence of indigenous case) of a specified (human) malaria parasite in a defined geographical area as a result of deliberate activities. Continued measures to prevent re-establishment of transmission are required |
Results
The highest numbers of indigenous malaria cases (9273) were recorded in 2000. Cases decreased gradually to 3329 in 2005 (Fig. 1). There followed a gradual increase over the next 3 years to a peak of 6071 cases in 2008, followed by a further decrease to 2050 cases in 2012, and by 2018, no cases were detected. Converse to this trend, Plasmodium knowlesi malaria was first identified in 2008 (Figs. 1 and 2) and by 2018 accounted for a total of 4131 cases of malaria. Between 2000 and 2007, human malaria contributed to 100% of reported malaria cases in Malaysia including 18–46 deaths per year. Between 2008 and 2017, however, indigenous malaria cases decreased from 6071 to 85 (a 98.6% reduction), while P. knowlesi cases increased from 376 to 3614 cases.
Fig. 1.
Annual reported malaria cases and deaths in Malaysia, 2000–2018
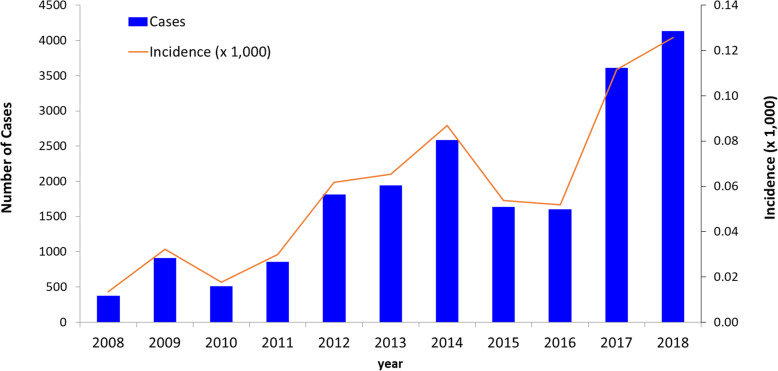
Fig. 2.
Incidence of knowlesi malaria in Malaysia, 2008–2018
Imported cases peaked in 2001 with a nadir in 2009 (Fig. 1). Although there are variable rates of imported cases throughout the study period, a decreasing trend has been observed. The imported cases were mostly from Papua New Guinea and Indonesia, with few cases from Nigeria and Pakistan.
Introduced cases were first identified in 2008 when they accounted for 78 cases (Fig. 1). Their number peaked in 2011 with 119 cases and then decreased to only 33 cases in 2012. In 2017, no introduced cases were detected; however, in 2018, 21 cases were detected. Overall, in 2018, there were a total of 4630 malaria cases reported in Malaysia, of which 4131 cases were P. knowlesi and 499 human malaria. The Plasmodium species responsible for human malaria were in the following order: P. vivax (284), P. falciparum (182), P. ovale (14), P. malariae (13), and mixed infection [6].
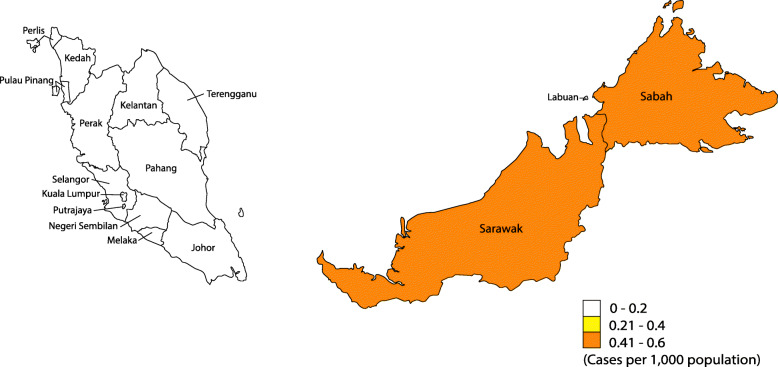
As illustrated in Fig. 3, zoonotic malaria cases, only refers to P. knowlesi cases in this manuscript, in Malaysia are not restricted to Sabah and Sarawak, two states located on the island of Borneo, where a significant proportion of the population is at risk of the disease, but also occurs in states with high forest coverage in Peninsular Malaysia, such as Kelantan, Perak, and Pahang [16].
Fig. 3.
Distribution of Plasmodium knowlesi malaria cases by state in Malaysia, 2018
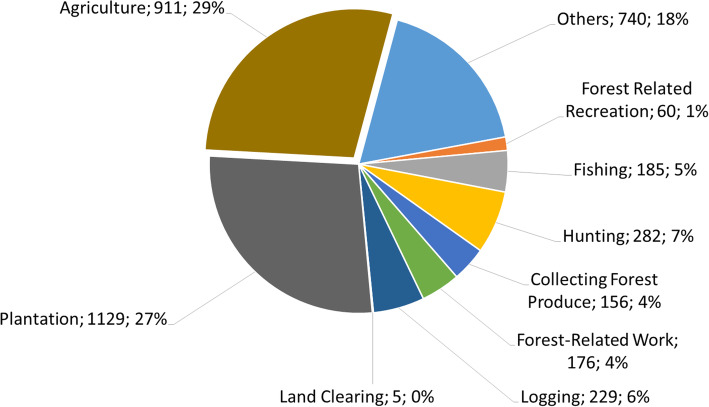
Figure 4 illustrates the distribution of zoonotic malaria according to risk activities in Malaysia in 2018. More than 50% of cases were in individuals involved with agriculture and plantation activities, with a large remainder proportion occurring in people engaged in forest-related activities.
Fig. 4.
Distribution of Plasmodium knowlesi malaria by risk activities, Malaysia, 2018. Others are people in the dependent group such as housewife, student, and elderly
Figure 2 illustrates the annual reported incidence trend for knowlesi malaria in Malaysia from 2008 to 2018. With current emphasis given by the Ministry of Health Malaysia on mandating hospital referral and admission policies for all malaria cases in both public and private sectors, mandatory referral for PCR testing of all microscopy-positive blood to be conducted by designated Public Health Laboratory, and surveillance linked to both systems, it is likely that the significant majority of symptomatic malaria cases were identified. Overall, P. knowlesi cases and incidence rates are increasing, with the highest reported case number of 4131 and the highest incidence rate of 0.13 case per 1000 population occurring in 2018.
Discussion
A Malaria Eradication Program was launched in Malaysia in 1961 when there were recorded 243,870 cases per year throughout the country. However, it was not until the 1980s, following a renewed emphasis on malaria control by the Health Ministry, that a significant reduction in malaria cases was achieved. This resulted in a 6-fold reduction of reported malaria cases (44,226 cases) in 1980. Under Malaria Eradication Program and Malaria Control Program in Malaysia, vector control was performed by ensuring that drains and open pools of water (including seepage rainwater) be regularly and routinely sprayed with oil, large unused pools drained, residual spraying of homes with insecticide, and the use of mosquito net. Following further strenuous efforts over the subsequent 30 years, a further 7-fold reduction of malaria cases was achieved, and Malaysia entered the elimination phase in 2011. Malaria elimination is defined by the WHO as the interruption of local transmission (reduction to zero incidence of indigenous case) of a specified (human) malaria parasite in a defined geographical area as a result of deliberate activities. Malaysia achieved zero case of indigenous malaria in 2018, 2 years ahead of the scheduled target of 2020. Therefore, 2019 and 2020 are crucial for achieving malaria elimination status.
From 2008, PCR-based malaria diagnosis for P. knowlesi was initiated by Public Health Laboratory. Subsequently, significant increases in the incidence of this zoonotic parasite were observed to date. This could be due to various factors including improved diagnostic capacity, the reduction in human malaria cases, and a change in land use patterns creating increased opportunities for spillover of infections to humans through closer associations with natural reservoir hosts or access to infected vectors [17]. The successful control of human malaria in Malaysia may increase the susceptibility of populations to P. knowlesi due to the removal of cross-protective immunity. However, further research is needed to explain this phenomenon as a possibility.
The paradox for Malaysia is that despite the excellent efforts to control human malaria, and the elimination of these parasites from the country, malaria cases are rising due to increasing P. knowlesi transmission to humans. It is possible that Malaysia will achieve “elimination” of malaria despite higher malaria case numbers in the last 2 years than in 2014. Plasmodium knowlesi is classified as a zoonotic parasite as there is no evidence to date that human-to-human transmission can occur under natural conditions. Should such evidence arise, then P. knowlesi may be re-classified as human malaria, and Malaysia’s “elimination” status would need to be revised accordingly. Malaysia also faces the threat of imported malaria and the reintroduction of malaria due to the number of undocumented migrant workers traveling from endemic regions [18]. The number of imported malaria cases is further confounded by Malaysians returning from work in the agricultural and logging sectors of endemic countries [4].
Accurate identification of P. knowlesi is critical to the design and implementation of effective malaria interventions. Due to morphological similarities at the trophozoite stages, P. knowlesi is often mistaken for P. malariae by microscopy. It may also be mistaken for P. falciparum or P. malariae due to similar morphological features at some stages [19]. Thus, in the past, P. knowlesi may have been underdiagnosed, and its true incidence was likely considerably underestimated. For this reason, the definitive diagnosis of P. knowlesi is now carried out by PCR in Malaysia.
There are reports suggesting a lack of reliability among commonly used rapid diagnostic tests for the detection of P. knowlesi infections. Pan-plasmodium Rapid Diagnostic Tests (RDTs) can be used for screening (they will give a positive result for malaria parasites) but not for species confirmation of P. knowlesi. Detection of P. knowlesi using RDTs has demonstrated low sensitivity [20]. PCR, on the other hand, can detect and diagnose P. knowlesi with high sensitivity and specificity; however, it is expensive and requires specialized equipment and highly trained personnel [21].
Loop-mediated isothermal amplification is, potentially, a reliable and convenient substitute for the molecular diagnosis of Plasmodium infection particularly in high malaria transmission regions as it displays high sensitivity and specificity as well as its low cost and high simplicity, making it particularly useful as a point-of-care diagnosis [22]. Other methods such as recombinase polymerase amplification (RPA) also have the potential to be developed as point-of-care molecular diagnostic tools for P. knowlesi infections [23]. Poor diagnostics contribute to inadequate surveillance for P. knowlesi, and calls have been made to include specific P. knowlesi reporting to the WHO to encourage improved regional surveillance [24].
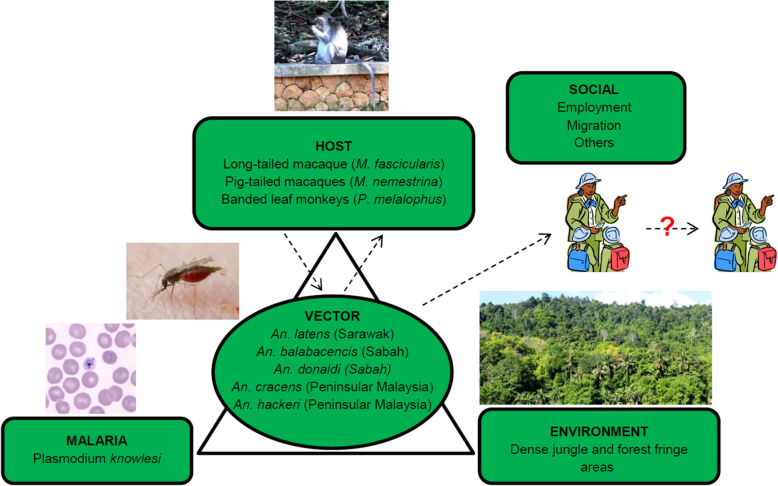
Figure 5 describes the epidemiologic triad of zoonotic malaria infections. Plasmodium knowlesi cases are proportionally higher in states with higher coverage of intact forest as opposed to forest loss areas [16]. Vector mosquitoes, members of the An. leucosphyrus group, are found throughout forested regions of Malaysia and are particularly associated with dense jungle and forest fringes. Anopheles leucosphyrus mosquitoes are exophagic, typically feeding and resting outdoors after dusk. In Sarawak, the forest-breeding An. latens was found to be the primary vector of P. knowlesi [25]. Anopheles latens has also been found to harbor other simian malaria parasites including Plasmodium inui, Plasmodium coatneyi, and Plasmodium fieldi. This species mainly breeds in forests in locations comprised of shaded temporary pools and natural containers on the ground [26].
Fig. 5.
Epidemiologic triad and factors for zoonotic malaria infections
Anopheles balabacensis has been implicated as a vector of P. knowlesi in Sabah. This species prefers to breed in ground pools formed in, for example, fruit orchards and rubber and palm oil plantations [27]. In Peninsular Malaysia, the major P. knowlesi vector is thought to be Anopheles cracens [26]. However, knowledge regarding the distribution of these vectors in the country is sparse [28].
Risk-assessment maps for P. knowlesi have been produced based upon the ranges of the host species, vectors, land use patterns, and confirmed infection of humans, macaques, and mosquitos [29]. Plasmodium knowlesi transmission to humans peaks in June [30], and this is associated with peak average monthly rainfall and humidity with a lag time of 3 months [31].
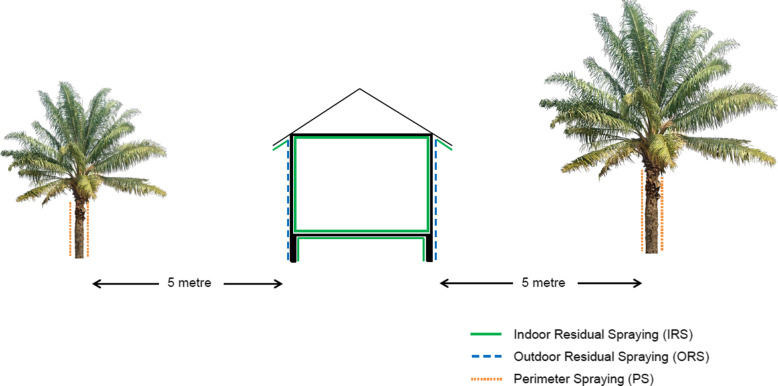
Vector control methods for knowlesi malaria include indoor residual spraying (IRS), and experimental methods of outdoor residual spraying (ORS), and perimeter spraying (Fig. 6). Outdoor residual spraying is done to the outside walls of a house. Perimeter spraying on the other hand is conducted up to the height of trunks for all trees in the range of less than 5 m from a house (where adult vectors are predominantly found resting). Based on a study conducted by Malaysia’s Institute of Medical Research (IMR) in Tenom, Sabah, a polymer-enhanced suspension concentrate (SC-PE) synthetic pyrethroid dose of 25 mg/m2 is effective to maintain 80% mortality of An. balabacensis for at least 6 months when applied to timber, bamboo, and concrete [32].
Fig. 6.
Schematic diagram of insecticidal sprays used for vector control
Control and prevention of zoonotic malaria remains a major challenge especially in rural areas, where macaques are common [33]. Macaque populations tend to congregate near village areas (average 6 km away) as well as within evergreen and deciduous forest cover, and are associated with lower elevations and higher temperatures [34]. Reducing macaque population density would be an effective means of controlling P. knowlesi transmission, but implementing such a measure is impractical and difficult as well as unethical.
The highest incidences of zoonotic malaria occur in the states of Sabah, Sarawak, and the Gua Musang district of Kelantan state [35]. In an attempt to identify the environmental risk factors for P. knowlesi in Sabah, it was found that agricultural expansion and forest fragmentation positively affect P. knowlesi exposure, supporting linkage between land use change and P. knowlesi transmission [36]. Factors that bring human populations into closer and more frequent contact with macaque monkeys exacerbate zoonotic transmission of pathogens, including malaria, from non-human primates to man. Land-use change also affects macaque habitat use and ranging behavior, with repercussions for changes in human disease risk [37]. Indonesia and Malaysia have increased oil palm cultivation from 2.6 million ha in 1990 to over 15 million ha by 2014, leading to large scale deforestation [38].
Men working in agricultural areas have the highest risk of P. knowlesi infection [39, 40], with occupations in industries such as forestry, agriculture, and hunting identified as risk factors [39, 41, 42]. In Sabah, P. knowlesi infections were recorded affecting a range of ethnic groups, with Rungus, Dusun, and Murut accounting for 32%, 28%, and 15% of infections, respectively [43]. This may be related to their residence in forest fringe areas and the fact that their livelihoods are primarily based on forest-related activities.
The prevention and control of knowlesi malaria in rural settings relies primarily on vector control, most importantly the use of personal anti-mosquito measures when working in forest-fringe areas. This involves the use of insect repellents, but these are prohibitively expensive for subsistence farmers, hunters, logging camp workers, and other rural people whose daily activities take them to the forest and forest fringes. Other, more novel interventions could be considered, for example, the use of insecticide impregnated hammocks which have been successful for controlling forest malaria in Vietnam [44]. However, although some people stay overnight in the forest in Malaysian Borneo, hammocks are not traditionally used here, and so such a control measure would be difficult to implement successfully.
There is an urgent need to foster inter-sectoral collaboration for the control of knowlesi malaria. Additional efforts by the Ministry of Health may include the strengthening of health education and health promotion by local health authorities as well as the strengthening of the currently existing community workforce, Sukarelawan Penjagaan Kesihatan Am (SPKA) also known as General Healthcare Volunteer, and increasing rural community resilience. The Wildlife Department could undertake the role of screening macaques for malaria parasites, controlling their numbers, and may also assist the Ministry of Health in the provision of health education for hunters. The Forestry Department could monitor forest activities in view of infection prevention such as keeping full record of people going in and out of forest and advocating use of mosquito bite prevention methods. The Malaysian Armed Forces could conduct screening of staff as well as the supervision and monitoring of the uptake of chemoprophylaxis. Due to the location of camps and activities involving forest areas, this group is deemed to be of high risk for P. knowlesi malaria infection [45]. For the plantation sector, key players can be involved by appointment of a “Malaria Ambassador” who will collaborate with the State Health Department in malaria screening and also ensure provision of insecticide-treated bed nets to workers to prevent outdoor biting as many people prefer to sleep outdoor during hot weather.
Additional research on mapping of vector breeding site, determining prevalence of malaria parasite infection, identifying and tracking areas of macaque population, and stricter policy on planning of land opening will aid in eliminating zoonotic malaria.
Conclusion
The changing epidemiology of malaria in Malaysia from indigenous malaria to zoonotic malaria has had a severe negative impact on the gains made by more than half a century’s effort by the Malaysian government in the battle against the disease. More research is required in order to effectively combat and eliminate P. knowlesi in humans. Improved inter-sectoral collaboration is required in order to develop a more effective, integrated effort that involves all stakeholders. Local political commitment and provision of technical support from the World Health Organization can help to create focused and concerted efforts towards ensuring the success of the National Malaria Elimination Strategic Plan.
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for giving permission to publish this article.
Abbreviations
- CI
Confidence interval
- PCR
Polymerase chain reaction
- CFR
Case fatality rate
- SPKA
Sukarelawan Penjagaan Kesihatan Am/General Healthcare Volunteer
- IRS
Indoor residual spraying
- ORS
Outdoor residual spraying
- IMR
Institute of Medical Research
- SC-PE
Suspension Concentrate Synthetic Pyrethroid
Authors’ contributions
AZC contributed to the concept/design, data analysis/interpretation, critical revision of the article, and approval of the article. MM contributed to data collection, statistics, data analysis, and approval of the article. JJ contributed to design, data analysis/interpretation, data collection, and approval of the article. MSJ contributed to critical review of the article and approval of the article. RC and KA contributed to the concept/design, data interpretation, drafting the article, and approval of the article. The authors read and approved the final manuscript.
Funding
The study was supported by Launching Grant for Centre of Excellence from Universiti Malaysia Sabah (Grant Number: AM 18006).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from JJ, Disease Control Division, Ministry of Health, Putrajaya, Selangor, Malaysia, on reasonable request.
Ethics approval and consent to participate
The study was registered, and ethical clearance obtained from the National Medical Research Register NMRR-20-785-54761. All data obtained in this study was saved in a password-protected computer that is only accessible to the researchers. The confidentiality of each patient is maintained as no identifiable individual information was recorded.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ho K. Current status of malaria and anti-malaria programme in Malaysia. In: First Asia and Pacific Congress on Malaria, Honolulu, Hawaii, 22-27 April, 1985. 1985. pp. 63–74. [Google Scholar]
- 2.Ministry of Health Malaysia and the World Health Organization and the University of California, San Francisco . Eliminating malaria: case-study 8 | Progress towards elimination in Malaysia. Geneva: World Health Organization; 2015. [Google Scholar]
- 3.World Health Organization . World malaria report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 4.World Health Organization . The E-2020 initiative of 21 malaria-eliminating countries: 2019 progress report. Geneva: World Health Organization; 2019. [Google Scholar]
- 5.Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. doi: 10.1126/science.149.3686.865. [DOI] [PubMed] [Google Scholar]
- 6.Fong YL, Cadigan FC, Coatney GR. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans R Soc Trop Med Hyg. 1971;65:839–840. doi: 10.1016/0035-9203(71)90103-9. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Cox-Singh J, Brooke G, Matusop A, Singh B. Plasmodium knowlesi from archival blood films: further evidence that human infections are widely distributed and not newly emergent in Malaysian Borneo. Int J Parasitol. 2009;39:1125–1128. doi: 10.1016/j.ijpara.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PC, Singh B. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–860. doi: 10.1086/605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–184. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatih FA, Staines HM, Siner A, Ahmed MA, Woon LC, Pasini EM, Kocken CH, Singh B, Cox-Singh J, Krishna S. Susceptibility of human Plasmodium knowlesi infections to anti-malarials. Malar J. 2013;12:425. doi: 10.1186/1475-2875-12-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . WHO malaria terminology. Geneva: World Health Organization; 2016. [Google Scholar]
- 14.Acharya J. Download Malaysia Shapefile area map free- country boundary, state, polygon [Internet]. 2020 [cited 2020 Apr 14]. Available from: https://www.igismap.com/download-malaysia-shapefile-area-map-free-country-boundary-state-polygon/.
- 15.QGIS Development Team. QGIS Geographic Information System [Internet]. Open Source Geospatial Foundation Project; 2020. [cited 2020 Apr 14]. Available from: http://qgis.osgeo.org.
- 16.Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 17.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeffree SM, Ahmed K, Safian N, Hassan R, Mihat O, Lukman KA, Shamsudin SB, Kamaludin F. Falciparum malaria outbreak in Sabah linked to an immigrant rubber tapper. Am J Trop Med Hyg. 2018;98:45–50. doi: 10.4269/ajtmh.17-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KS, Cox-Singh J, Singh B. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar J 2009;8:1-0. [DOI] [PMC free article] [PubMed]
- 20.Foster D, Cox-Singh J, Mohamad DS, Krishna S, Chin PP, Singh B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar J. 2014;13:60. doi: 10.1186/1475-2875-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeremiah SS, Janagond AB, Parija SC. Challenges in diagnosis of Plasmodium knowlesi infections. Trop Parasitol. 2014;4:25. doi: 10.4103/2229-5070.129156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau YL, Lai MY, Fong MY, Jelip J, Mahmud R. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am J Trop Med Hyg. 2016;94:336–339. doi: 10.4269/ajtmh.15-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai MY, Ooi CH, Lau YL. Rapid detection of Plasmodium knowlesi by isothermal recombinase polymerase amplification assay. Am J Trop Med Hyg. 2017;97:1597–1599. doi: 10.4269/ajtmh.17-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber BE, Rajahram GS, Grigg MJ, William T, Anstey NM. World Malaria Report: time to acknowledge Plasmodium knowlesi malaria. Malar J. 2017;16:1–3. doi: 10.1186/s12936-017-1787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans R Soc Trop Med Hyg. 2006;100:1087–1088. doi: 10.1016/j.trstmh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Sallum MA, Peyton EL, Wilkerson RC. Six new species of the Anopheles leucosphyrus group, reinterpretation of An. elegans and vector implications. Med Vet Entomol. 2005;19:158–199. doi: 10.1111/j.0269-283X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad R, Lim LH, Omar MH, Abd Rahman AA, Majid MA, Nor ZM, Xin YK, Jelip J, Husin T. Characterization of the larval breeding sites of Anopheles balabacensis (Baisas), in Kudat, Sabah, Malaysia. Southeast Asian J Trop Med Pub Health. 2018;49:566–579. [Google Scholar]
- 28.Vythilingam I, Wong ML, Wan-Yussof WS. Current status of Plasmodium knowlesi vectors: a public health concern? Parasitol. 2018;145:32–40. doi: 10.1017/S0031182016000901. [DOI] [PubMed] [Google Scholar]
- 29.Moyes CL, Henry AJ, Golding N, Huang Z, Singh B, Baird JK, Newton PN, Huffman M, Duda KA, Drakeley CJ, Elyazar IR. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Negl Trop Dis. 2014;8:e2780. doi: 10.1371/journal.pntd.0002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, Grigg MJ, Yeo TW, Anstey NM, Barber BE. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl Trop Dis. 2013;7:e2026. doi: 10.1371/journal.pntd.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper DJ, Rajahram GS, William T, Jelip J, Mohammad R, Benedict J, Alaza DA, Malacova E, Yeo TW, Grigg MJ, Anstey NM. Plasmodium knowlesi malaria in Sabah, Malaysia, 2015–2017: ongoing increase in incidence despite near-elimination of the human-only Plasmodium species. Clin Infect Dis. 2020;70:361–367. doi: 10.1093/cid/ciz604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohani A, Fakhriy HA, Suzilah I, Zurainee MN, Najdah WW, Ariffin MM, Shakirudin NM, Afiq MM, Jenarun J, Tanrang Y, Lee HL. Indoor and outdoor residual spraying of a novel formulation of deltamethrin K-Othrine®(Polyzone) for the control of simian malaria in Sabah, Malaysia. PLoS One. 2020;15:e0230860. doi: 10.1371/journal.pone.0230860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KS, Divis PC, Zakaria SK, Matusop A, Julin RA, Conway DJ, Cox-Singh J, Singh B. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011;7:e1002015. doi: 10.1371/journal.ppat.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyes CL, Shearer FM, Huang Z, Wiebe A, Gibson HS, Nijman V, Mohd-Azlan J, Brodie JF, Malaivijitnond S, Linkie M, Samejima H. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasit Vectors. 2016;9:1–2. doi: 10.1186/s13071-016-1527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shearer FM, Huang Z, Weiss DJ, Wiebe A, Gibson HS, Battle KE, Pigott DM, Brady OJ, Putaporntip C, Jongwutiwes S, Lau YL. Estimating geographical variation in the risk of zoonotic Plasmodium knowlesi infection in countries eliminating malaria. PLoS Neg Trop Dis. 2016;10:e0004915. doi: 10.1371/journal.pntd.0004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fornace KM, Brock PM, Abidin TR, Grignard L, Herman LS, Chua TH, Daim S, William T, Patterson CL, Hall T, Grigg MJ. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across northern Sabah, Malaysia: a population-based cross-sectional survey. Lancet Planet Health. 2019;3:e179–e186. doi: 10.1016/S2542-5196(19)30045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stark DJ, Fornace KM, Brock PM, Abidin TR, Gilhooly L, Jalius C, Goossens B, Drakeley CJ, Salgado-Lynn M. Long-tailed macaque response to deforestation in a Plasmodium knowlesi-endemic area. EcoHealth. 2019;16:638–646. doi: 10.1007/s10393-019-01403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cramb R, McCarthy JF, McCarthy JF. Characterising oil palm production in Indonesia and Malaysia. In: Cramb R, editor. The oil palm complex. Singapore: NUS Press; 2016. pp. 27–77. [Google Scholar]
- 39.Grigg MJ, Cox J, William T, Jelip J, Fornace KM, Brock PM, von Seidlein L, Barber BE, Anstey NM, Yeo TW, Drakeley CJ. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: a case-control study. Lancet Planet Health. 2017;1:e97–104. doi: 10.1016/S2542-5196(17)30031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herdiana H, Cotter C, Coutrier FN, Zarlinda I, Zelman BW, Tirta YK, Greenhouse B, Gosling RD, Baker P, Whittaker M, Hsiang MS. Malaria risk factor assessment using active and passive surveillance data from Aceh Besar, Indonesia, a low endemic, malaria elimination setting with Plasmodium knowlesi, Plasmodium vivax, and Plasmodium falciparum. Malar J. 2016;15:468. doi: 10.1186/s12936-016-1523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fornace KM, Herman LS, Abidin TR, Chua TH, Daim S, Lorenzo PJ, Grignard L, Nuin NA, Ying LT, Grigg MJ, William T. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, the Philippines. PLoS Negl Trop Dis. 2018;12:e0006432. doi: 10.1371/journal.pntd.0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigg MJ, William T, Drakeley CJ, Jelip J, Von Seidlein L, Barber BE, Fornace KM, Anstey NM, Yeo TW, Cox J. Factors that are associated with the risk of acquiring Plasmodium knowlesi malaria in Sabah, Malaysia: a case-control study protocol. BMJ Open. 2014;4. [DOI] [PMC free article] [PubMed]
- 43.Naing DK, Anderios F, Lin Z. Geographic and ethnic distribution of P. knowlesi infection in Sabah, Malaysia. Int J Collab Res Intern Med Public Health. 2011;3:391–400. [Google Scholar]
- 44.Thang ND, Erhart A, Speybroeck N, Xa NX, Thanh NN, Van Ky P, Hung LX, Coosemans M, D’Alessandro U. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS One. 2009;4:e7369. doi: 10.1371/journal.pone.0007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roe K, Thangarajan A, Lilley K, Dowd S, Shanks D. Plasmodium knowlesi infection in an Australian soldier following jungle warfare training in Malaysia. J Military Veterans Health. 2020;28:53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from JJ, Disease Control Division, Ministry of Health, Putrajaya, Selangor, Malaysia, on reasonable request.