Abstract
Extramedullary plasmacytoma of the head and neck is a rare indolent neoplasm. Radiotherapy is often the preferred treatment option with excellent local control and survival. The risk of local recurrence or transformation to multiple myeloma is 10-30%. In our case-cohort, thorough, sensitive initial evaluation for disseminated clonal disease and the incorporation of surgery led to excellent results with no recurrences or systemic progression.
Key words: Extramedullary plasmacytoma, surgery, radiotherapy, head and neck
Introduction
Extramedullary Plasmacytoma (EMP) is a rare disease that presents 3% of plasma cell neoplasms.1,2 More than 80% of EMP arise in the head and neck region, especially the nasal cavity, paranasal sinuses, and nasopharynx.1–3 The median age at presentation is between 50–60 years with a male predominance 3:1.4,5 The course is indolent with 10–20 % local recurrence rates and 10–30 % progressing to multiple myeloma (MM).1–3,6 Because of rarity, most treatment data are from case cohorts and retrospective analysis of datasets.
EMP is highly radiosensitive, and radiation doses above 40-45 Gy are associated with improved survival as compared to lower doses.2,4,6–9 Some centers prefer radiation therapy because of the mutilating procedure of curative surgery. While some studies suggest the benefit of radiation, others show better survival for patients after radical surgery without additional radiation.5,10 At our center, surgery has been used more frequently in recent years, reserving radiation only for patients with EMP locations not manageable with surgery. Often the diagnosis of EMP of the head and neck region was established only after surgery for an unknown mass lesson. We retrospectively analyzed recent patients with EMP of the head and neck region to determine the effectiveness of surgery.
Materials and Methods
The institutional database was used for the identification of patients with a diagnosis of EMP in the years 2009–2019. Medical records were reviewed to select patients meeting inclusion criteria. Patients were eligible if they had a solitary lesion of the head and neck with histologic confirmation of plasmacytoma, with clonality by immunohistochemical staining for kappa and lambda, and a negative bone marrow biopsy. Patients with multiple soft tissue lesions, >10% plasma cells in the bone marrow or osteolytic lesions were excluded from further analysis. Patients with local bone destruction as a result of direct EMP extension were included.
Patients
From January 2009 till January 2019 we treated eight patients, seven male, and one female. All patients were Caucasian. Three had EMP in the nose, 4 in the pharynx and 1 in a lymph node. The median age of patients was 60 years (23-83). All patients were evaluated for disseminated disease or MM. The initial evaluation included bone marrow cytology and histology with immunohisto - chemical staining, serum protein electrophoresis, Free Light Chain evaluation (FLC), and standard skeletal X-rays. Seven patients had a PET-CT scan. Flow cytometry for determination of plasma cell immunophenotype and infiltration percentage was done in three patients.
Results
Seven patients were treated with radical surgery. Four patients had surgery performed for a biopsy confirmed EMP. Three patients had surgery performed for an unknown lesion that was histologically confirmed as EMP after surgery. All lesions were removed with negative margins at the final histopathology report. One patient (number 6) had extirpation of the lymph node in the left neck and received 50Gy adjuvant radiation to left neck regions.
Only one patient (number 5) had a monoclonal protein IgG/lambda of 5.2 g/L before treatment with no signs of disseminated disease or MM by bone marrow cytology, immunohistology, and a PET-CT scan. Due to the localization in the oropharynx, the patient received radiation (50Gy). Three months after radiation, the monoclonal protein disappeared and was not detected during the 61 months of follow up. All other patients had no signs of clonality or MM by bone marrow immunohistology, serum electrophoresis, FLC and PET-CT.
During follow-up, serum protein electrophoresis and FLC were measured at 6-month intervals for the first year and annually thereafter. At the physician’s discretion measurements were done at shorter intervals. Patient 5 had a follow up CT scan at 2- and 4-months after radiation. At 2-months a residual mass was present on CT scan and a biopsy was undertaken, that revealed no signs of residual EMP. At 4- months no lesion on the CT scan was left. During a median follow up of 39 months (14–101 months) there was no local recurrence or progression to MM. Patient characteristics are shown in Table 1 and Figure 1.
Discussion
Extramedullary plasmacytoma is a rare disease with most treatment data available from retrospective patient databases. Based on available data, current NCCN clinical practice guidelines recommend radiotherapy for the treatment of solitary plasmacytoma including EMP.11 Studies by Li, Bachar and Wen showed a survival benefit for radiation compared to surgery alone.1,2,6 In the study by Li the 5-year local progression free survival for patients with EMP was 100% vs 100% vs 61.0% (p=0.029) for patients receiving radiation, surgery combined with radiation or surgery alone respectively.6 In addition, the risk of progression to MM was reduced by the addition of local radiation. The 5-year MM free survival was 100% vs 100% vs 83.3% (p=0.14) for radiation, surgery combined with radiation or surgery alone respectively.6 In the study by Bachar the local recurrence rate for patient with EMP in the head and neck area was 12.5 % for patients treated with either surgery or radiation, but the risk of regional recurrence (5.1 % vs 25 % for radiation and surgery respectively) and progression to myeloma (18 % vs 50 % for radiation and surgery respectively) was reduced with radiotherapy treatment.1 Similar results were published by Wen with a decreased 5-year local progression free survival 88.7 % vs 73.1 % (p=0.069) and 5-year MM free survival 92.9 % vs. 70.3 % (p=0.027) for patients with EMP of the head and neck region receiving radiation or surgery respectively.2
In the largest study of solitary plasmacytoma with 5,056 patients, including 1528 EMP patients, combined surgery and radiation was associated with improved mortality compared to either monotherapy, but because of the design of the study, the authors cautioned against combining surgery and radiotherapy in all patients, due to the possible difference in patient and disease characteristic between the groups.4 The retrospective study from the Surveillance, Epidemiology and End Results (SEER) database, with records of 1691 patients with solitary plasmacytoma, including 540 patients with EMP, calculated the 5-year relative survival.5 Although the combination of surgery and radiation was beneficial in patients with skeletal solitary plasmacytoma, in patients with EMP of the upper and lower respiratory tract and gastrointestinal tract, surgery alone was superior to radiation therapy and the combination of surgery and radiotherapy.5 The 5- year relative survival for upper respiratory tract (sinonasal, mouth and pharynx) EMP was 96.7 % for surgery, 73.8 % for radiation and 93 % (p<0.05) for the combination treatment.5 The study did not report data on local progression or progression to MM. Because of the survival benefit of patients with EMP treated with surgery only, adjuvant radiation following radical surgery is questionably. In the study by Strojan, the authors were reluctant to adjuvant radiation in patients with a complete resection with negative surgical margins.12
In the studies by Li, Bachar and Wen there was an increased risk of progression to MM in patients receiving surgery as compared to patients treated with radiation, but the risk of local progression was statistically significant only in the study by Li.1,2,6 The higher risk of local progression in surgery is likely due to difficulties in achieving an adequate negative surgical margin in patients with a large EMP in sensitive areas. Radiation and surgery are local means of treatment and the difference in progression rates to MM are difficult to explain. Due to the nature of data sources and lack of randomization, differences in patient groups are possible. In the studies there is paucity of data on the plasma cell clonality in the bone marrow, monoclonal protein concentration and isotype, FLC ratio and imaging studies used. Monoclonal Gammopathy of Undetermined Significance (MGUS) precedes MM and the risk of progression is increased in patients with higher monoclonal protein and skewed FLC ratio.13,14 In patients with EMP the presence of monoclonal protein is a risk factor for dissemination and progression to MM.2,8 In our cohort, only one patient had a monoclonal protein that disappeared following local treatment and was probably secreted by the local tumor. Further sensitive initial test in our patients showed no clonal plasma cells in the bone marrow by immunohistology or a skewed FLC ratio. All but one of the patients had imaging with PET-CT and no disseminated disease or signs of MM were present. A study in patients with osseous plasmacytoma showed a higher risk of progression to MM in patients with a skewed FLC ration and additional changes present by PET-CT.15 The published risk of 5-year progression of EMP to MM is 10–30 %.1–3,6Absence of clonality or dissemination by sensitive tests can potentially distinguish a patient population with low risk of progression to MM irrespective of the EMP treatment modality. We observed no local progression or progression to MM during the median follow up of 39 months (14–101 months).
Figure 1.
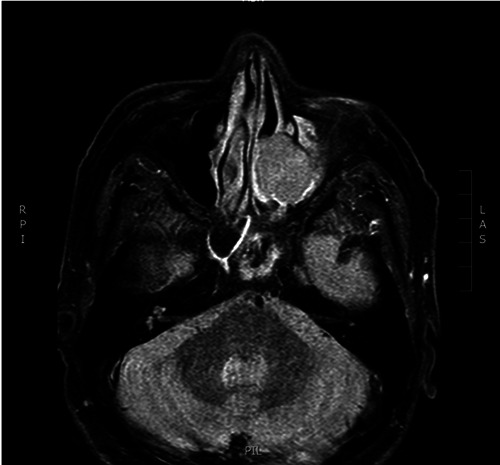
MRI STIR sequence of patient number 8 showing nasal extramedullary plasmacytoma.
Table 1.
Patient characteristics.
| Patient | Gender | Age (years) | Location | Size (mm) | Therapy | M-protein | FLC ratio | PET-CT | R/P to MM | Follow up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | Oropharynx | 30 | Surgery | neg | neg | neg | No | 16 |
| 2 | M | 49 | Nasal | 8 | Surgery | neg | neg | neg | No | 101 |
| 3 | M | 57 | Nasal | 40 | Surgery | neg | neg | neg | No | 14 |
| 4 | M | 63 | Hypopharynx | 15 | Surgery | neg | neg | ND | No | 51 |
| 5 | M | 57 | Oropharynx | 33 | Radiotherapy | IgG/lambda 5.2 g/L | neg | neg | No | 61 |
| 6 | M | 23 | Lymph node | 25 | Surgery/ Radiotherapy | neg | neg | neg | No | 26 |
| 7 | F | 64 | Epipharynx | 20 | Surgery | neg | neg | neg | No | 93 |
| 8 | M | 83 | Nasal | 15 | Surgery | neg | neg | neg | No | 4 |
M-protein=monoclonal protein; FLC=free light chains; MM=multiple myeloma, R/P to MM=recurrence or progression to multiple myeloma.
Size of the tumor is an important prognostic factor. In a multicenter study of 258 patients, including 52 EMP patients, the 10-year OS was better for tumors measuring <4cm (72%) than for those ≥4cm (61%, P<0.0002).16 In the study by Wen a tumor size <4cm was associated with lower risk of MM free survival (88.1% vs 56.1%, P=0.013), 5-year progression free survival (75% vs 44.5 %, P=0.022) and overall survival (89.0% vs 46.2%, P=0.044) compared to a tumor size ≥4 cm.2 Both studies showed no increase in local recurrence in larger tumor size. Other studies used a cutoff value of 5 cm to distinguish patients according to risk of progression to MM and overall survival.6,17 The median tumor size in our group of patients was 3cm (0.3cm–4cm). The small tumor size, in addition to lack of disseminated or clonal disease, explains the favorable outcome of patients treated at our institution. The median age of patients in our study was 60 years, which is consistent with data from larger patient cohorts.4,5 In those cohorts, older age was associated with worse overall survival and MM free survival.2,4,5 Different age cutoffs were used in studies, some using a cutoff of 50 years2 while others used a higher cutoff value of 60-65 years.4,5,17 Because the age of our patients is like that in published studies, we believe that our patients do not represent a favorable age cohort.
Recent advances in reconstructive oral and maxillofacial surgery have reduced the mutilation of radical surgery for EMP. Similar to surgery, advances in the field of radiotherapy have led to significant reduction of acute and chronic side effects that were historically prevalent, such as mucositis, dermatitis, xerostomia and even lymphocyte decline.18
The role of adjuvant chemotherapy or systemic therapy in patients with EMP is not known. Although British guidelines recommend the use of adjuvant chemotherapy in patients with EMP with a size >5 cm and in those with high grade tumors, there is limited data suggesting such an approach and there is no data on the use of novel agents that are currently used in MM.19,20 In recent years there is increased evidence that treatment of smoldering myeloma with novel agents delays progression and increases overall survival.21 In the study by Elsayad including 84 patients with solitary plasmacytoma (including 30 patients with EMP) the addition of systemic therapy improved MM free survival and PFS.9 Only 4 patients with EMP received systemic therapy thereby rendering a conclusion in EMP patients impossible. The study by Mignot included 46 patients with solitary plasmacytoma (including 6 EMP) treated by either radiotherapy alone or concomitant radiotherapy and systemic therapy with 4 cycles of lenalidomide and dexamethasone.22MM free survival and PFS were significantly higher in patients receiving concomitant systemic therapy. Only two patients with EMP received systemic therapy at the discretion of the treating physician. The addition of systemic therapy decreases the progression to MM in patients with solitary bone plasmacytoma, the data in patients with EMP is still insufficient.
The findings of this study have some limitations. Foremost, this study is retrospective and includes a small number of patients and there is an inherent risk of selection bias. Because EMP is a rare disease, prospective single center trials are not possible. This limitation is also an advantage. Being a single center study, we had full access to patient records. All patients were initially thoroughly evaluated with sensitive laboratory and imaging investigations and underwent surgery by the oncological team at the maxillofacial department.
Conclusions
Extramedullary plasmacytoma of the head and neck is a rare disease with treatment based on data from case cohorts and retrospective database analysis. According to our data, the use of sensitive tests to exclude clonality of plasma cells in the bone marrow or disseminated disease can distinguish a favorable patient group with excellent long-term prognosis and low risk of progression to MM. In patients with EMP of the head and neck, surgery results in high local control rates and the benefit of adjuvant radiation is limited. Concomitant systemic therapy cannot be recommended as standard clinical practice.
References
- 1.Bachar G, Goldstein D, Brown D, et al. Solitary extramedullary plasmacytoma of the head and neck-Long-term outcome analysis of 68 cases. Head Neck 2008;30:1012-9. [DOI] [PubMed] [Google Scholar]
- 2.Wen G, Wang W, Zhang Y, et al. Management of extramedullary plasmacytoma: Role of radiotherapy and prognostic factor analysis in 55 patients. Chin J Cancer Res 2017;29:438-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma. Cancer 1999;85:2305-14. [PubMed] [Google Scholar]
- 4.Goyal G, Bartley AC, Funni S, et al. Treatment approaches and outcomes in plasmacytomas: analysis using a national dataset. Leukemia 2018;32:1414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thumallapally N, Meshref A, Mousa M, Terjanian T. Solitary plasmacytoma: population-based analysis of survival trends and effect of various treatment modalities in the USA. BMC Cancer 2017;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li QW, Niu SQ, Wang HY, et al. Radiotherapy alone is associated with improved outcomes over surgery in the management of solitary plasmacytoma. Asian Pacific J Cancer Prev 2015;16: 3741-5. [DOI] [PubMed] [Google Scholar]
- 7.Tsang RW, Campbell BA, Goda JS, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys 2018;101:794-808. [DOI] [PubMed] [Google Scholar]
- 8.Tournier-Rangeard L, Lapeyre M, Graff- Caillaud P, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: A dose greater than 45 Gy to the target volume improves the local control. Int J Radiat Oncol 2006;64:1013-7. [DOI] [PubMed] [Google Scholar]
- 9.Elsayad K, Oertel M, König L, et al. Maximizing the clinical benefit of radiotherapy in solitary plasmacytoma: an international multicenter analysis. Cancers (Basel) 2020;12:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerry D, Lentsch EJ. Epidemiologic evidence of superior outcomes for extramedullary plasmacytoma of the head and neck. Otolaryngol Neck Surg 2013;148:974-81. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, Version 3.2017, NCCN Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:230-69. [DOI] [PubMed] [Google Scholar]
- 12.Strojan P, Soba E, Lamovec J, Munda A. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol Biol Phys 2002;53:692-701. [DOI] [PubMed] [Google Scholar]
- 13.Lindqvist EK, Lund SH, Costello R, et al. Risk of progression in monoclonal gammopathy of undetermined significance (MGUS): results from a population-based screening study. Blood 2016;128:5650. [Google Scholar]
- 14.Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med 2018;378:241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouquet G, Guidez S, Herbaux C, et al. Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma. Clin Cancer Res 2014;20:3254-60. [DOI] [PubMed] [Google Scholar]
- 16.Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: A multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol 2006;64: 210-7. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesulu B, Mallick S, Giridhar P, et al. Pattern of care and impact of prognostic factors on the outcome of head and neck extramedullary plasmacytoma: a systematic review and individual patient data analysis of 315 cases. Eur Arch Oto-Rhino-Laryngology 2018;275:595-606. [DOI] [PubMed] [Google Scholar]
- 18.Dovšak T, Ihan A, Didanovič V, et al. Influence of surgical treatment and radiotherapy of the advanced intraoral cancers on complete blood count, body mass index, liver enzymes and leukocyte CD64 expression. Radiol Oncol 2009;43:282-92. [Google Scholar]
- 19.Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A Review for solitary plasmacytoma of bone and extramedullary plasmacytoma. Sci World J 2012;2012:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol 2004;124: 717-26. [DOI] [PubMed] [Google Scholar]
- 21.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med 2013;369:438-47. [DOI] [PubMed] [Google Scholar]
- 22.Mignot F, Schernberg A, Arsène-Henry A, et al. Solitary plasmacytoma treated by lenalidomide-dexamethasone in combination with radiation therapy: clinical outcomes. Int J Radiat Oncol Biol Phys 2020;106:589-96. [DOI] [PubMed] [Google Scholar]


