Key Points
Question
What are the risks of primary hepatic malignancies or death by age 75 years in hemochromatosis HFE p.C282Y homozygotes identified in genotyping in a community sample?
Findings
In this cohort study that included 451 186 individuals, the risk of primary hepatic malignancy among 1294 male homozygous participants compared with men without pathogenic variants was 7.2% vs 0.6%, and the risk of all-cause death was 19.5% vs 15.1%; both differences were statistically significant. There were no statistically significant associations for women.
Meaning
Among men, HFE p.C282Y homozygosity was significantly associated with increased risk for incident primary hepatic malignancy and excess mortality.
Abstract
Importance
Hereditary hemochromatosis is predominantly caused by the HFE p.C282Y homozygous pathogenic variant. Liver carcinoma and mortality risks are increased in individuals with clinically diagnosed hereditary hemochromatosis, but risks are unclear in mostly undiagnosed p.C282Y homozygotes identified in community genotyping.
Objective
To estimate the incidence of primary hepatic carcinoma and death by HFE variant status.
Design, Setting, and Participants
Cohort study of 451 186 UK Biobank participants of European ancestry (aged 40-70 years), followed up from baseline assessment (2006-2010) until January 2018.
Exposures
Men and women with HFE p.C282Y and p.H63D genotypes compared with those with neither HFE variants.
Main Outcomes and Measures
Two linked co–primary outcomes (incident primary liver carcinoma and death from any cause) were ascertained from follow-up via hospital inpatient records, national cancer registry, and death certificate records, and from primary care data among a subset of participants for whom data were available. Associations between genotype and outcomes were tested using Cox regression adjusted for age, assessment center, genotyping array, and population genetics substructure. Kaplan-Meier lifetable probabilities of incident diagnoses were estimated from age 40 to 75 years by HFE genotype and sex.
Results
A total of 451 186 participants (mean [SD] age, 56.8 [8.0] years; 54.3% women) were followed up for a median (interquartile range) of 8.9 (8.3-9.5) years. Among the 1294 male p.C282Y homozygotes, there were 21 incident hepatic malignancies, 10 of which were in participants without a diagnosis of hemochromatosis at baseline. p.C282Y homozygous men had a higher risk of hepatic malignancies (hazard ratio [HR], 10.5 [95% CI, 6.6-16.7]; P < .001) and all-cause mortality (n = 88; HR, 1.2 [95% CI, 1.0-1.5]; P = .046) compared with men with neither HFE variant. In lifetables projections for male p.C282Y homozygotes to age 75 years, the risk of primary hepatic malignancy was 7.2% (95% CI, 3.9%-13.1%), compared with 0.6% (95% CI, 0.4%-0.7%) for men with neither variant, and the risk of death was 19.5% (95% CI, 15.8%-24.0%), compared with 15.1% (95% CI, 14.7%-15.5%) among men with neither variant. Among female p.C282Y homozygotes (n = 1596), there were 3 incident hepatic malignancies and 60 deaths, but the associations between homozygosity and hepatic malignancy (HR, 2.1 [95% CI, 0.7-6.5]; P = .22) and death (HR, 1.2 [95% CI, 0.9-1.5]; P = .20) were not statistically significant.
Conclusions and Relevance
Among men with HFE p.C282Y homozygosity, there was a significantly increased risk of incident primary hepatic malignancy and death compared with men without p.C282Y or p.H63D variants; there was not a significant association for women. Further research is needed to understand the effects of early diagnosis and treatment.
This cohort study uses UK Biobank data to estimate the risk for primary hepatic carcinoma and cancer-related death by HFE variant status among community-dwelling individuals of European ancestry in England, Scotland, and Wales.
Introduction
In European ancestry groups, the iron overload condition hereditary hemochromatosis is predominantly caused by HFE gene variants (OMIM #613609)1; a meta-analysis from 20102 suggested that 81% of patients with hemochromatosis have the p.C282Y variant and 5% have the p.C282Y/p.H63D compound heterozygote genotype. The p.C282Y variant is carried by 10% to 15% of the population of northern European descent,3 with 0.64% of people of European ancestry in the UK being p.C282Y homozygous.4 In the Hemochromatosis and Iron Overload Screening (HEIRS) study5 of primary care patients from 5 North American clinical centers, p.C282Y homozygosity prevalence was 0.44% in non-Hispanic White patients and 0.11% in Native American patients, and less common in other racial groups. Hereditary hemochromatosis is the most common autosomal recessive disorder among individuals of northern European ancestry, with a prevalence of 1 in 300 to 1 in 500 individuals.6 Hemochromatosis-associated iron overload can lead to liver cirrhosis and liver cancers, mainly hepatocellular carcinomas,7 which are a major cause of premature death in untreated hereditary hemochromatosis.8 Hepatic morbidity is more common in men who are p.C282Y homozygous,4 with women partly protected by menstrual iron losses.9 However, hereditary hemochromatosis is easily managed with phlebotomy10; if started before irreversible end organ damage, treatment can regress liver fibrosis and reduce risks of liver cancer.11,12
Elevated liver enzymes (especially alanine aminotransferase and aspartate aminotransferase) are common in community-screened p.C282Y homozygotes.13 However, progression rates to liver disease and carcinoma are unclear. Because iron accumulates with age, clinical expression is best estimated as a lifetime incidence. Grosse et al14 estimated the lifetime incidence of severe liver disease (cirrhosis or hepatocellular carcinoma) was 9% (95% CI, 2.6%-15.3%) in 152 untreated male p.C282Y homozygotes in 4 cohort studies.
Given the limited information on key outcomes, follow-up data from a large genotyped community sample were used to estimate the incidence of primary hepatic carcinomas and deaths by HFE variant status in participants of European descent.
Methods
This analysis used UK Biobank data,15 which received ethical approval from the North West Multi-centre Research Ethics Committee. Participants gave written informed consent for data collection, genotyping, and linkage to electronic medical records through electronic signature at baseline assessment. The current cohort analysis was approved under Biobank application 14631.
Study Population
A total of 502 492 community volunteers were recruited who were aged 40 to 70 years at baseline and living near 22 assessment centers in England, Scotland, and Wales.15 Baseline assessments (from 2006 to 2010) included information about demographics, lifestyle, and disease history. Participants were notified of relevant health related findings at the baseline assessment, but individuals are not notified of subsequent findings, including genotyping.
Genotyping
Genotyping data were available from Affymetrix microarrays (800 000 markers directly genotyped); however, because HFE p.C282Y (dbSNP rs1800562) was not directly genotyped, standard imputation methods were applied16 (see eMethods in the Supplement for details). HFE p.H63D (dbSNP rs1799945) was directly genotyped in the microarray data.
Baseline Variables
Participants self-reported alcohol use frequency, smoking status, and physician-diagnosed disease at baseline (including hepatitis, cirrhosis, and diabetes). Body mass index and waist circumference measures were from baseline assessment. Baseline liver enzyme concentrations were measured using International Federation of Clinical Chemistry standards (on Beckman Coulter AU5800 series analyzer17), with concentrations dichotomized (normal vs above reference ranges) in analyses for alanine aminotransferase (ALT; >50 U/L18) and aspartate aminotransferase (AST; >45 U/L19). Hemoglobin concentration and hematocrit percentage were from baseline, with polycythemia defined as elevated hemoglobin (>16.5 g/dL for men and >16 g/dL for women) and hematocrit (>49% for men and >48% for women).20 No data were available on ferritin concentrations or transferrin saturation.
Outcomes
Prevalent diagnoses were derived from baseline questionnaires plus International Classification of Diseases, 10th Revision (ICD-10) coded hospital inpatient data (National Health Service Hospital Episode Statistics) from 1996 to the time of the baseline assessment. Incident disease diagnoses were from the baseline assessment (2006-2010), with follow-up from hospital inpatient (to March 2017), national cancer registry (to March 2016), and death registration (to January 2018) data. Given the literature, the 2 linked co–primary outcomes of interest were any incident primary liver cancer (ICD-10 code C22*) and all-cause death, with excess mortality related to hemochromatosis mainly linked to liver complications.21 Secondary outcomes included any noncancer liver disease (K70-K77*), alcoholic liver disease (K70*), fibrosis and cirrhosis (K74*), chronic hepatitis (K73*), hepatitis B (B16*, B17.0, B18.0, B18.1, B19.1), hepatitis C (B17.1, B18.2, B19.2), hepatocellular carcinoma (C22.0), and intrahepatic bile duct carcinoma/cholangiocarcinoma (C22.1). These diagnoses were not mutually exclusive, and individuals could be counted as having more than 1 category of disease. Because unmanaged hemochromatosis tends to progress from mild liver fibrosis through liver cirrhosis to malignancy and then to higher death rates, these outcomes cannot be separated.
Primary Care Data
Primary care records were available for a participant subset, providing additional incident disease diagnoses after baseline until 2016 or 2017 (depending on the computer system supplier of the general practice). Liver outcomes listed above were ascertained in primary care “Read codes” (a hierarchical coding system including ICD diagnoses22).
Statistical Analysis
Baseline characteristics of participants with and without primary care follow-up data were compared (final follow-up in January 2018) using logistic regression for categorical variables and t tests for continuous variables. Cox proportional hazards regression models tested sex-specific genotype associations with incident liver outcomes and mortality. Incident liver outcomes excluded participants with each baseline prevalent diagnosis for each outcome only (eg, participants with noncancer liver disease at baseline were excluded from incident noncancer liver disease analyses, but other participants were included irrespective of other studied baseline diagnoses). Models were adjusted for age, assessment center, genotyping array, and 10 genetic principal components generated in participants of European descent, accounting for population genetics substructure. Proportional hazards assumptions for the main outcomes were tested with Schoenfeld residuals, which did not indicate violation of the assumption. Kaplan-Meier curves provided probabilities of incident liver outcomes by age, within genotypes, and by sex. Lifetable probabilities of incident outcomes were estimated from age 40 to 75 years in 5-year bands, by HFE genotypes and sex, applying observed incidence rates in each age group to a notional cohort, estimating cumulative incident case numbers from age 40 to 75 years.
Given the literature, the 2 linked co–primary outcomes of interest were incident primary liver carcinoma and death from any cause in male p.C282Y homozygotes vs men with neither p.C282Y or p.H63D HFE variants. The 2 co–primary outcomes were not adjusted for multiple comparisons because they are linked and not independent. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. In the main text, results are presented for the higher-risk p.C282Y homozygotes and p.C282Y/p.H63D genotypes vs neither HFE variants (see eTables and eFigures in the Supplement for full results). All P values were 2-sided, with statistical significance set at P <.05. All analyses were performed using Stata, version 15.1 (StataCorp).
Sensitivity Analysis
Potential confounding factors23 (sex, high alcohol intake, smoking, body mass index, waist circumference, and diabetes) were tested for interactions with genotypes in sensitivity analyses. An additional sensitivity analysis excluded participants diagnosed with hemochromatosis at baseline to provide separate estimates for community-identified undiagnosed participants.
Missing Data
For the 451 186 included participants of European descent with HFE p.C282Y genotypes, all of the main variables used in analyses had complete data (genotype, age, sex, assessment center, genotyping array, and the first 10 genetic principal components). Data were complete for incident liver outcomes and death based on ascertainment through clinical data sources (hospital admissions, cancer registry, and death records). Less than 0.3% of participants has missing data for covariates used in sensitivity analyses, including self-reported baseline disease diagnoses, alcohol intake, smoking status, or waist circumference. Less than 5% of participants had missing data for biological measurements from blood assays (ALT, AST, hemoglobin, or hematocrit), but these variables were used for descriptive purposes only and not in the main analyses. Given the low level of missing data, we excluded participants with specific missing data from each analysis as needed.
Results
Characteristics of Participants
Analyses included 451 186 participants of European descent aged 40 to 70 years at baseline with available HFE p.C282Y (rs1800562) genotype data (mean [SD] age, 56.8 [8.0] years; 244 834 [54.3%] women), including 209 811 (46.5%) with additional primary care data available. Characteristics of participants with and without primary care data were similar, although large sample sizes resulted in statistically significant, but clinically unimportant, differences (eg, mean age of 56.7 years for participants with primary care data vs 56.8 for participants without; P < .001; see eTable 1 in the Supplement for details). Overall, 2890 (0.6%) participants were p.C282Y homozygous (including 1382 in the subset of participants with primary care data available) (Table 1; see eTable 2 in the Supplement for details of all HFE genotypes). To check imputation quality, imputed p.C282Y genotypes (rs1800562) were compared with exome sequencing genotypes available for 49 772 participants. There was high sequencing vs imputation correlation (r2 = 0.998), and only 1 of 231 (0.4%) imputed p.C282Y homozygotes was incorrectly classified (sequenced genotype = p.C282Y heterozygote).
Table 1. Baseline Characteristics of Participants in a Study of the Association of Hemochromatosis HFE p.C282Y Homozygosity With Hepatic Malignancy (N = 451 186)a.
| Characteristics | No. (%) | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| C282Y +/+b | C282Y+/H63D+ | No variants | C282Y +/+b | C282Y+/H63D+ | No variants | |
| Total participants | 1294 | 4955 | 122 860 | 1596 | 5746 | 145 719 |
| Participants with primary care records | 612 | 2297 | 56 425 | 770 | 2754 | 67 916 |
| Age at baseline, mean (SD), y | 56.9 (8.2) | 57.0 (8.1) | 57.0 (8.1) | 56.9 (8.0) | 56.5 (7.9) | 56.6 (7.9) |
| Obesity (BMI >30) | 295 (22.8) | 1223 (24.7) | 31 767 (25.9) | 354 (22.2) | 1353 (23.6) | 33 997 (23.3) |
| Waist circumference, mean (SD), cm | 96.9 (11.1) | 97.0 (11.3) | 97.0 (11.3) | 84.6 (12.5) | 84.9 (12.6) | 84.6 (12.5) |
| Current smoker | 189 (14.6) | 612 (12.4) | 14 953 (12.2) | 138 (8.7) | 509 (8.9) | 12 837 (8.8) |
| Consuming alcohol daily | 324 (25.1) | 1231 (24.9) | 32 471 (26.5) | 256 (16.0) | 932 (16.2) | 24 603 (16.9) |
| Polycythemia (from baseline blood counts)c | 35 (2.8) | 191 (4.0) | 2386 (2.0) | 1 (0.1) | 13 (0.2) | 131 (0.1) |
| Liver enzymes | ||||||
| Alanine aminotransferase >50 U/L [95% CI] | 181 (14.7) [12.8-16.9] | 338 (7.1) [6.4-7.9] | 7197 (6.1) [6.0-6.3] | 62 (4.1) [3.1-5.2] | 139 (2.5) [2.1-3.0] | 3041 (2.2) [2.1-2.3] |
| Aspartate aminotransferase >45 U/L [95% CI] | 129 (10.6) [8.9-12.4] | 226 (4.8) [4.2-5.4] | 4814 (4.1) [4.0-4.2] | 54 (3.6) [2.7-4.6] | 135 (2.5) [2.1-2.9] | 2792 (2.0) [1.9-2.1] |
| Prevalent disease | ||||||
| Diabetes (any type) | 113 (8.7) | 311 (6.3) | 7876 (6.4) | 59 (3.7) | 193 (3.4) | 4478 (3.1) |
| Hepatitis (any) | 5 (0.4) | 24 (0.5) | 675 (0.6) | 5 (0.3) | 30 (0.5) | 648 (0.4) |
| Cirrhosis | 6 (0.5) | 2 (<0.1) | 85 (0.1) | 1 (0.1) | 3 (0.1) | 93 (0.1) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
p.C282Y heterozygous, p.H63D heterozygous, and p.H63D homozygous individuals are not shown (see eTable 2 in the Supplement for details).
HFE p.C282Y homozygosity.
Polycythemia defined as elevated hemoglobin (>16.5 g/dL for men; >16 g/dL for women) and hematocrit (>49% for men; >48% for women).20
At baseline, 156 of 1294 male (12.1%) and 54 of 1596 female (3.4%) p.C282Y homozygotes were diagnosed with hemochromatosis (Table 2); the median (interquartile range) age of diagnosis was 55.2 (48.8-59.5) years for men and 58.1 (52.2-61.8) years for women. Obesity was slightly less common in male p.C282Y homozygotes (22.8%) than in men with neither p.C282Y or p.H63D variants (25.9%) (P = .01). Mean waist circumference was 97.1 cm among men and 84.6 cm among women, with no differences between p.C282Y homozygotes and those with no pathogenic variants (P = .59 for men and P = .97 for women). Male p.C282Y homozygotes were more likely than men with neither variant to have diabetes (8.7% vs 6.4%; P < .001) and be smokers (14.6% vs 12.2%; P < .01). Overall, 26.3% of men and 16.8% of women consumed alcohol daily, with male p.C282Y heterozygotes (25.7%) and men with p.C282Y and p.H63D variants (24.9%) slightly less likely to drink daily (P = .02 and P = .01) than men with neither variant (26.5%). Polycythemia was more common in p.C282Y homozygote men than men with neither variant (2.8% vs 2.0%). Hepatitis diagnoses were not more common in male or female p.C282Y homozygotes than in those with neither variant. Cirrhosis was more common in male p.C282Y homozygotes than in men with neither variant (0.5% vs 0.1%), with no significant difference for women. Liver enzyme concentrations above clinical cut points were more common in p.C282Y homozygote men than in men with neither variant (ALT: 14.7% [95% CI, 12.8%-16.9%] vs 6.1% [95% CI, 6.0%-6.3%]; AST: 10.6% [95% CI, 8.9%-12.4%] vs 4.1% [95% CI, 4.0%-4.2%]), with smaller differences among women (Table 1; see eTable 2 in the Supplement for details of all HFE genotypes).
Table 2. Incident Disease and Death During Follow-up in a Study of the Association of Hemochromatosis HFE p.C282Y Homozygosity With Hepatic Malignancya.
| Characteristics | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Males | Females | ||||||
| C282Y +/+ | C282Y+/H63+ | No variants | C282Y +/+ | C282Y+/H63+ | No variants | ||
| Total participantsb | (n = 1294) | (n = 4955) | (n = 122 860) | (n = 1596) | (n = 5746) | (n = 145 719) | |
| Hemochromatosis diagnosis at baseline | 156 (12.1) | 29 (0.6) | 30 (<0.1) | 54 (3.4) | 12 (0.2) | 8 (<0.1) | |
| Hemochromatosis diagnosis at the end of follow-up (baseline or incident) | 327 (25.3) | 89 (1.8) | 74 (0.1) | 200 (12.5) | 35 (0.6) | 25 (<0.1) | |
| Follow-up time, median (IQR), y | 8.9 (8.3-9.5) | 8.9 (8.3-9.5) | 8.9 (8.3-9.5) | 9.0 (8.4-9.5) | 8.9 (8.3-9.5) | 8.9 (8.3-9.5) | |
| Incident diagnoses | |||||||
| Noncancer liver disease | 48 (3.7) | 80 (1.6) | 1598 (1.3) | 23 (1.5) | 68 (1.2) | 1433 (1.0) | |
| Fibrosis and cirrhosis | 17 (1.3) | 16 (0.3) | 264 (0.2) | 4 (0.3) | 9 (0.2) | 229 (0.2) | |
| Alcoholic liver disease | 8 (0.6) | 18 (0.4) | 309 (0.3) | 4 (0.3) | 2 (<0.1) | 77 (0.1) | |
| Chronic hepatitis | 1 (0.1) | 2 (<0.1) | 23 (<0.1) | 1 (0.1) | 1 (<0.1) | 26 (<0.1) | |
| Hepatitis B | 0 | 3 (0.1) | 44 (<0.1) | 1 (0.1) | 1 (<0.1) | 11 (<0.1) | |
| Hepatitis C | 0 | 2 (<0.1) | 53 (<0.1) | 1 (0.1) | 0 | 14 (<0.1) | |
| Liver carcinomas (primary) | 20 (1.6) | 10 (0.2) | 174 (0.1) | 3 (0.2) | 4 (0.1) | 129 (0.1) | |
| Hepatocellular carcinoma | 14 (1.1) | 7 (0.1) | 81 (0.1) | 1 (0.1) | 0 | 30 (<0.1) | |
| Intrahepatic bile duct carcinoma | 5 (0.4) | 3 (0.1) | 63 (0.1) | 0 | 3 (0.1) | 69 (0.1) | |
| Death | |||||||
| All-cause | 88 (6.8) | 260 (5.3) | 6560 (5.3) | 60 (3.8) | 154 (2.7) | 4362 (3.0) | |
| Noncancer liver disease | 1 (0.1) | 5 (0.1) | 141 (0.1) | 0 | 2 (<0.1) | 43 (<0.1) | |
| Liver carcinomas | 14 (1.1) | 6 (0.1) | 102 (0.1) | 3 (0.2) | 3 (0.1) | 78 (0.1) | |
| Participants with primary care recordsc | (n = 612) | (n = 2297) | (n = 56 425) | (n = 770) | (n = 2754) | (n = 67 916) | |
| Incident diagnosis | |||||||
| Noncancer liver disease | 42 (7.1) | 64 (2.8) | 1448 (2.6) | 22 (2.9) | 70 (2.6) | 1305 (1.9) | |
| Fibrosis and cirrhosis | 14 (2.3) | 10 (0.4) | 148 (0.3) | 4 (0.5) | 6 (0.2) | 151 (0.2) | |
| Alcoholic liver disease | 9 (1.5) | 12 (0.5) | 272 (0.5) | 4 (0.5) | 4 (0.2) | 72 (0.1) | |
| Liver carcinomas (primary) | 12 (2.0) | 3 (0.1) | 75 (0.1) | 1 (0.1) | 1 (<0.1) | 62 (0.1) | |
| Hepatocellular carcinoma | 6 (1.0) | 3 (0.1) | 31 (0.1) | 0 | 0 | 16 (<0.1) | |
| Intrahepatic bile duct carcinoma | 4 (0.7) | 0 | 31 (0.1) | 0 | 1 (<0.1) | 31 (0.1) | |
Excluding individuals with each prevalent diagnosis at baseline only (eg, the incident liver cancer analysis included participants with baseline noncancer liver disease). p.C282Y heterozygous, p.H63D heterozygous and p.H63D homozygous individuals are not shown (see eTable 3 in the Supplement for details).
Total sample (n = 451 186) with follow-up data from Hospital Episode Statistics, the Cancer Registry, and death records.
Subset of participants (n = 209 811) with additional follow-up data available from primary care records.
Hazard Ratios for Incident Liver Outcomes
By the end of follow-up (median [interquartile range], 8.9 [8.3-9.5] years), 25.3% of p.C282Y homozygote men and 12.5% of female homozygotes had hemochromatosis diagnoses (Table 2; eTable 3 in the Supplement).
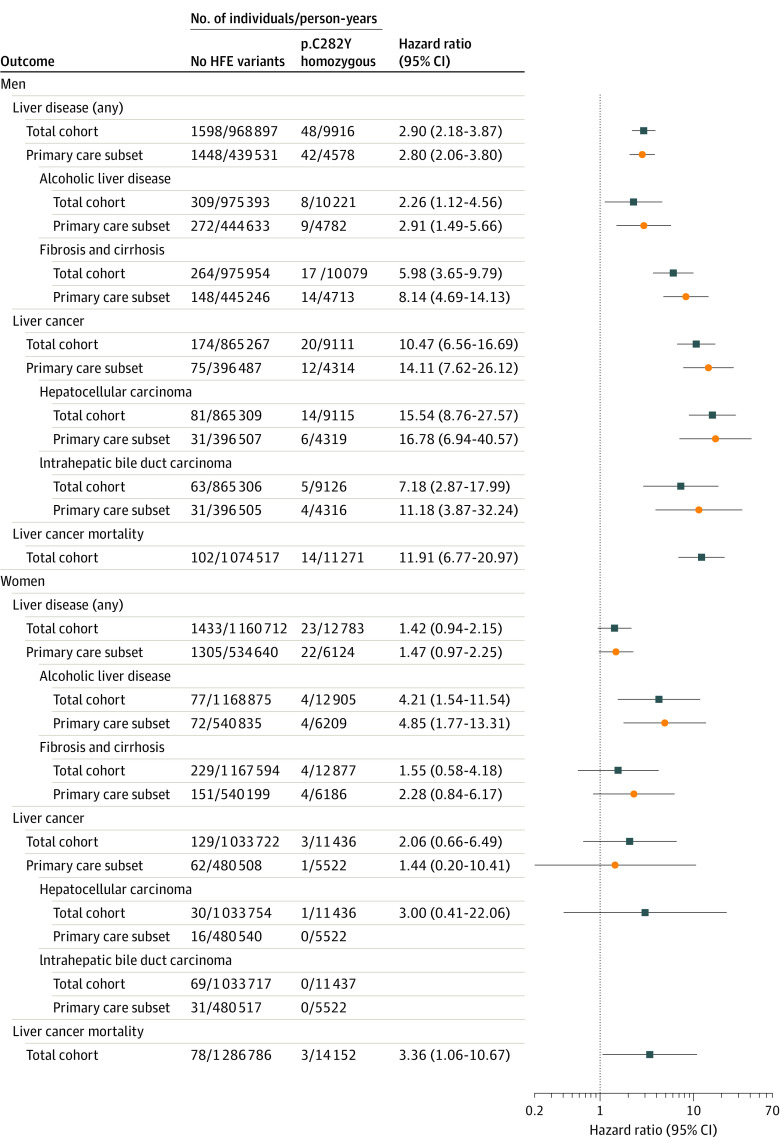
During follow-up, 48 of 1294 male homozygotes (3.7%) were diagnosed with incident noncancer liver diseases vs 1598 of 122 860 men with neither variant (1.3%) (difference, 2.41% [95% CI, 1.4%-3.6%]; hazard ratio [HR] for male p.C282Y homozygotes, 2.90 [95% CI, 2.18-3.87]; P < .001), excluding baseline noncancer liver disease, irrespective of hemochromatosis diagnoses (Table 2; Figure 1; eTables 3 and 4 in the Supplement). Incident primary hepatic malignancies were diagnosed in 1.55% (20 of 1294) of p.C282Y homozygous men vs 0.14% (174 of 122 860) of individuals with neither HFE variant (difference, 1.41% [95% CI, 0.73%-2.10%]; HR, 10.47 [95% CI, 6.56-16.69]; P < .001), excluding participants with liver cancers at baseline. Seven of the 20 male p.C282Y homozygotes with hepatic malignancies had (noncancer) liver disease at baseline, but none had hepatitis diagnoses at baseline or during follow-up (including chronic hepatitis, hepatitis B, or hepatitis C). Eleven of the 20 male p.C282Y homozygotes with hepatic malignancies had hemochromatosis diagnoses at baseline (9 were undiagnosed). Of the hepatic malignancies, the majority were hepatocellular (n = 14 in p.C282Y homozygote men; HR, 15.54 [95% CI, 8.76-27.57]; P < .001 vs individuals with neither variant), and there were 5 homozygote men with intrahepatic bile duct carcinoma/cholangiocarcinoma (HR, 7.18 [95% CI, 2.87%-17.99%]; P < .001) (Figure 1; eTable 4 in the Supplement).
Figure 1. Hazard Ratios for Incident Liver Outcomes Comparing p.C282Y Homozygous Genotype With Individuals With No HFE Variants.
Hazard ratios adjusted for age, assessment center, genotyping array, and population genetics substructure using principal components. Individuals with each prevalent diagnosis at baseline only were excluded (eg, the incident liver cancer analysis included participants with baseline noncancer liver disease). The participants in the total cohort had incident diagnoses taken from Hospital Episode Statistics, the Cancer Registry, and death records (n = 451 186) (see eTables 4, 5, and 7 in the Supplement for details of all HFE genotypes). Participants in the primary care subset had primary care data available from general practices (n = 209 811).
In the subsample of 209 811 participants with additional primary care data (46.5%), there was 1 additional hepatic malignancy recorded in a male homozygote (ie, a total of 21 male p.C282Y homozygotes with incident hepatic malignancy ascertained) (male homozygote [vs men with neither variant] HR, 14.11 [95% CI, 7.62-26.12]; P < .001) (Figure 1; eTable 5 in the Supplement). Ten of the 21 male p.C282Y homozygotes with incident hepatic malignancies were undiagnosed with hemochromatosis at baseline.
The incidence of hepatic malignancies (n = 3; HR, 2.06 [95% CI, 0.66-6.49]; P = .22) in female p.C282Y homozygotes and women with neither variant was not significantly different, but female p.C282Y homozygotes did have a significantly increased risk of alcoholic liver disease diagnoses (HR, 4.21 [95% CI, 1.54-11.54]; P = .01). Male p.C282Y heterozygotes also had a significantly increased risk of alcoholic liver disease (HR, 1.48 [95% CI, 1.18-1.87]; P = .001). There was no significant increase in risk of incident liver outcomes in either male or female p.C282Y/p.H63D compound heterozygotes (eTable 4 in the Supplement).
Lifetable Risks of Incident Clinical Liver Diagnoses During Follow-up
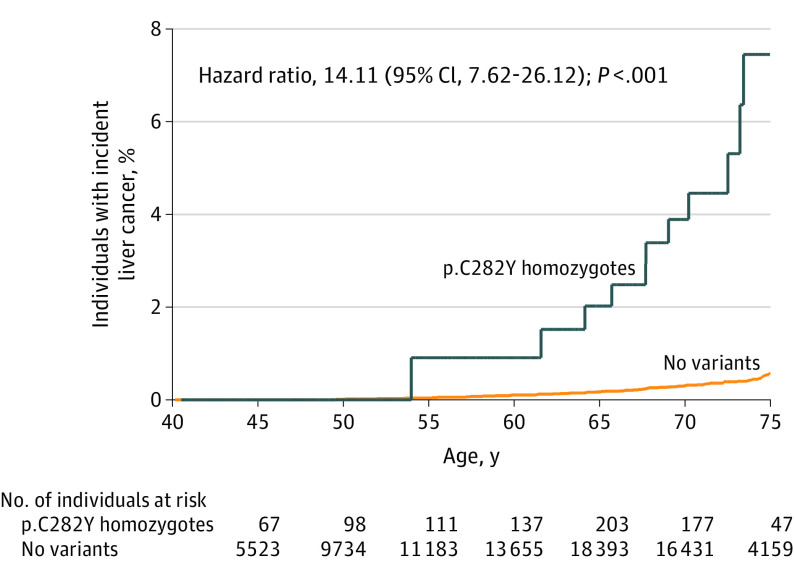
Kaplan-Meier curves for diagnosis of liver carcinoma in male p.C282Y homozygotes, from age 40 to 75 years, can be seen in Figure 2 (see eFigures 1 to 4 in the Supplement for other outcomes and groups).
Figure 2. Kaplan-Meier Curve for Incidence of Diagnosed Liver Cancer in Male HFE p.C282Y Homozygotes Compared With Those With No Variants in Subset of Participants With Primary Care Data .
Incident diagnosis from Hospital Episode Statistics, the Cancer Registry, death records, and primary care data (n = 209 811). Participants with prevalent liver cancer diagnosis at baseline were excluded, but those with noncancer liver disease at baseline were included. Median (interquartile range) follow-up time was 7.2 (6.5-7.8) years for p.C282Y homozygotes and 7.1 (6.5-7.7) years for those with no variants.
In lifetable estimates based on observed 5-year age group–specific incidence rates from age 40 to 75 years (including primary care data), 22.7% (95% CI, 17.1%-29.9%) of p.C282Y homozygote men were projected to develop (noncancer) liver disease by age 75 years compared with 10.2% (95% CI, 9.5%-10.9%) of men with neither HFE variant (difference, 12.5% [95% CI, 7.6%-19.0%]) (eTable 6 in the Supplement). Similarly, 7.2% (95% CI, 3.9%-13.1%) of male p.C282Y homozygotes were projected to develop primary hepatic carcinomas by age 75 years compared with 0.6% (95% CI, 0.4%-0.7%) of men without HFE variants (difference, 6.6% [95% CI, 3.5%-12.3%]) (Table 3).
Table 3. Lifetable Estimates for Incident Liver Cancer and Mortality by Sex and HFE Genotypes .
| Outcome | No variants | C282Y homozygotes | Difference, % | ||||
|---|---|---|---|---|---|---|---|
| Cases | Survival function | Incident diagnosis (95% CI), %a | Cases | Survival function | Incident diagnosis (95% CI), %a | ||
| Liver cancerb | |||||||
| Male age group, y | |||||||
| 40-45 | 0 | 1.0000 | 0 | 1 | |||
| 46-50 | 1 | 0.9999 | 0.0 (0.0-0.1) | 0 | 1 | ||
| 51-55 | 3 | 0.9996 | 0.0 (0.0-0.1) | 1 | 0.9909 | 0.9 (0.1-6.3) | 0.9 |
| 56-60 | 8 | 0.9990 | 0.1 (0.1-0.2) | 0 | 0.9909 | 0.9 (0.1-6.3) | 0.8 |
| 61-65 | 11 | 0.9983 | 0.2 (0.1-0.3) | 2 | 0.9799 | 2.0 (0.6-6.3) | 1.8 |
| 66-70 | 25 | 0.9969 | 0.3 (0.2-0.4) | 4 | 0.9617 | 3.8 (1.8-8.1) | 3.5 |
| 71-75 | 21 | 0.9944 | 0.6 (0.4-0.7) | 4 | 0.9279 | 7.2 (3.9-13.1) | 6.6 |
| Female age group, y | |||||||
| 40-45 | 0 | 1.0000 | 0 | 1 | |||
| 46-50 | 3 | 0.9997 | 0.0 (0.0-0.1) | 0 | 1 | ||
| 51-55 | 1 | 0.9997 | 0.0 (0.0-0.1) | 0 | 1 | ||
| 56-60 | 2 | 0.9995 | 0.0 (0.0-0.1) | 0 | 1 | ||
| 61-65 | 16 | 0.9988 | 0.1 (0.1-0.2) | 0 | 1 | ||
| 66-70 | 19 | 0.9979 | 0.2 (0.2-0.3) | 1 | 0.9962 | 0.4 (0.0-2.7) | 0.2 |
| 71-75 | 17 | 0.9964 | 0.4 (0.3-0.5) | 0 | 0.9962 | 0.4 (0.0-2.7) | 0.0 |
| Mortalityc | |||||||
| Male age group, y | |||||||
| 40-45 | 18 | 0.997 | 0 | 1.000 | |||
| 46-50 | 131 | 0.990 | 1.0 (0.8-1.3) | 3 | 0.985 | 1.5 (0.5-4.6) | 0.5 |
| 51-55 | 286 | 0.980 | 2.0 (1.8-2.3) | 3 | 0.975 | 2.5 (1.1-5.6) | 0.5 |
| 56-60 | 504 | 0.965 | 3.5 (3.2-3.9) | 4 | 0.963 | 3.7 (2.0-6.9) | 0.2 |
| 61-65 | 980 | 0.943 | 5.7 (5.4-6.1) | 17 | 0.927 | 7.3 (5.0-10.7) | 1.6 |
| 66-70 | 1814 | 0.909 | 9.1 (8.8-9.5) | 27 | 0.880 | 12.0 (9.2-15.6) | 2.9 |
| 71-75 | 2086 | 0.849 | 15.1 (14.7-15.5) | 27 | 0.805 | 19.5 (15.8-24.0) | 4.4 |
| Female age group, y | |||||||
| 40-45 | 22 | 0.997 | 0.3 (0.2-0.5) | 0 | 1.000 | ||
| 46-50 | 90 | 0.993 | 0.7 (0.5-0.9) | 2 | 0.993 | 0.7 (0.2-2.8) | 0.0 |
| 51-55 | 240 | 0.986 | 1.4 (1.2-1.6) | 2 | 0.988 | 1.2 (0.5-3.3) | −0.1 |
| 56-60 | 380 | 0.977 | 2.3 (2.1-2.5) | 7 | 0.973 | 2.7 (1.5-4.9) | 0.4 |
| 61-65 | 711 | 0.964 | 3.6 (3.4-3.8) | 8 | 0.959 | 4.1 (2.6-6.5) | 0.6 |
| 66-70 | 1176 | 0.945 | 5.5 (5.3-5.8) | 15 | 0.936 | 6.4 (4.5-8.9) | 0.8 |
| 71-75 | 1271 | 0.910 | 9.0 (8.7-9.3) | 24 | 0.883 | 11.7 (9.1-15.0) | 2.7 |
Lifetable projections based on observed 5-year age group–specific incidence rates from ages 40 and 75 years (eg, in male p.C282Y homozygotes, 7.2% were projected to develop liver cancer by age 75 years compared with 0.6% of men without HFE variants; difference, 6.6%).
Liver cancer estimates are based on the subset of participants with primary care data (n = 209 811). Individuals with prevalent diagnosis at baseline were excluded.
All-cause mortality estimates based on total cohort (n = 451 186).
All-Cause and Liver-Related Mortality
During follow-up, 6.8% (88 of 1294) of p.C282Y homozygous men died, compared with 5.3% (6560 of 122 860) of men with neither HFE variant (difference, 1.5% [95% CI, 0.1%-2.8%]; HR, 1.24 [95% CI, 1.004-1.53]; P = .046) (Table 2; eTables 3 and 7 in the Supplement). Based on death certificates, 14 male p.C282Y homozygotes were recorded as having liver carcinomas vs 102 men with neither variant (HR, 11.91 [95% CI, 6.77-20.97]; P < .001). There were 60 deaths among female p.C282Y homozygotes (n = 1596), but there was no significant association for all-cause mortality (HR, 1.18 [95% CI, 0.91-1.52]; P = .20). However, significantly more female homozygotes had liver carcinomas specifically noted on their death certificates (n = 3) than women with neither variant (HR, 3.36 [95% CI, 1.06-10.67]; P = .04) (Figure 1; eTable 7 in the Supplement). Both male and female p.C282Y heterozygotes showed no excess mortality.
In lifetable estimates from age 40 to 75 years in a cohort of male p.C282Y homozygotes, 19.5% (95% CI, 15.8%-24.0%) were projected to die (of any cause) by age 75 years compared with 15.1% (95% CI, 14.7%-15.5%) among individuals with neither variant (difference, 4.4% [95% CI, 1.2%-8.5%]). Among female p.C282Y homozygotes, 11.7% (95% CI, 9.1%-15.0%) were projected to die by age 75 years compared with 9.0% (95% CI, 8.7%-9.3%) among women with neither variant (difference, 2.7%) (Table 3).
Sensitivity Analysis
Statistical interactions were tested between p.C282Y homozygotes vs individuals with neither HFE variant and potential confounding clinical factors (obesity [body mass index >30], waist circumference, prevalent diabetes, current smoking, and daily alcohol consumption) for incident liver carcinoma, but there were no significant statistical interactions in men or women (P > .05), implying that risks are additive rather than multiplicative. There was a statistically significant interaction between p.C282Y homozygote genotype (vs individuals with neither HFE variant) and male sex for risk of liver cancer (HR, 5.37 [95% CI, 1.56-18.45]; P = .01), but no significant interaction for all-cause mortality (P = .70). The association between male p.C282Y homozygosity and incident liver cancer remained significant after excluding 354 participants with hemochromatosis diagnosed at baseline from the total sample (n = 450 832) (HR, 5.42 [95% CI, 2.77-10.63]; eTable 8 in the Supplement).
Discussion
In the UK Biobank community cohort, male HFE p.C282Y homozygotes had significantly increased risks of incident hepatic carcinomas (including hepatocellular and intrahepatic bile duct carcinomas) and mortality (all-cause and from hepatic malignancy) compared with individuals without the 2 studied HFE variants. In female HFE p.C282Y homozygotes, there were no significant increases in the risk of incident primary hepatic malignancy or all-cause mortality, but there was a significant increase in risk of death with hepatic malignancy noted on death certificates.
Direct comparisons with large prospective community studies are difficult to make. Findings from this analysis were consistent with the HEIRS study, which examined cross-sectional associations in 299 p.C282Y homozygotes and found 3-fold increases in risk of any liver disease in male p.C282Y homozygotes.5 The results for hepatocellular carcinoma in male homozygotes (whole sample HR, 15.54 [95% CI, 8.76-27.57]; P < .001) were within the CIs of the meta-analysis of 202 mainly cross-sectional hemochromatosis studies, which reported that p.C282Y homozygotes (men and women together) had an odds ratio of 11.0 (99% CI, 3.7-34.0) for hepatocellular carcinoma.24 The lifetable estimate (7.2% [95% CI, 3.9%-13.1%]) of male p.C282Y homozygotes developing liver cancer by age 75 years was difficult to compare, given the paucity of similar data, although it was comparable in magnitude to the study by Grosse et al,14 which estimated that the lifetime incidence of severe liver disease (cirrhosis or hepatocellular carcinoma combined) was 9% (95% CI, 2.6%-15.3%) in untreated male HFE p.C282Y homozygotes.
A potential problem in assessing the increased incidence of liver malignancies in p.C282Y homozygotes is whether this group may have had high rates of viral hepatitis. A 2019 study25 reported that chronic hepatitis C viral infection prevalence in referred HFE p.C282Y homozygous adults in North America was similar to that of controls without the variant. In the current analysis, none of the male p.C282Y homozygotes with incident liver cancers had diagnoses of hepatitis.
Mortality data for large numbers of mostly undiagnosed (and therefore untreated) community p.C282Y male homozygotes are scarce; for example, the Atherosclerosis Risk in Communities study analysis reported no excess mortality, but was based on combining data on 21 male and 24 female p.C282Y homozygotes, and CIs (rate ratio, 0.97 [95% CI, 0.39-2.00])26 overlap estimates from the current analysis, which shows moderately increased mortality in 1294 male p.C282Y homozygotes. Reports of p.C282Y homozygote prevalence in older adults were also based on small samples. For example, Willis et al27 reported 4 p.C282Y homozygotes among 600 (1:150) English men aged 70 to 97 years, while, in a study by Van Aken et al,28 2 homozygotes were found among 1265 (1:633) Dutch adults older than 85 years. CIs around such estimates would be very wide and would not exclude limited excess mortality.
The p.C282Y allele frequency in the current study (7.3%) was similar to other UK studies reported in dbSNP,29 including the Avon Longitudinal Study of Parents and Children (7.9%) and the TwinsUK study (6.9%), and homozygosity prevalence (0.64%) was similar to the 0.68% prevalence reported from a sample of 10 000 Welsh blood donors.30 The age-specific and sex-specific rates of hemochromatosis diagnosis were also comparable to the eMERGE study that included 7 US hospitals (n = 98 homozygotes).31 By the end follow-up in the current study (mean age, 65.3 years), 25.3% of male p.C282Y homozygotes and 12.5% of female homozygotes were diagnosed with hemochromatosis compared with approximately 22% of male p.C282Y homozygotes and 14% of female homozygotes by age 70 years in the eMERGE study.31 The prevalence of elevated liver enzymes was also similar to UK general population studies. For example, in the Health Survey for England of 5049 adults with blood samples, 2.7% of men and 1.3% of women aged 55 to 64 years had an ALT above a cut point of greater than 60 U/L32; using the same age group and cut point in the current study, 2.7% of men and 1.4% of women had increased liver enzyme levels. Therefore, overall there was no evidence of significant bias in the analyses presented.
Limitations
This study has several limitations. First, participants were somewhat healthier than the general population33 at baseline, but estimates were from incident disease during follow-up and should be less influenced by baseline characteristics. Second, genotype status for p.C282Y was based on imputation, but on comparison with sequencing data in a subset, only 1 p.C282Y homozygote was misclassified as a p.C282Y heterozygote, thus slightly “diluting” the excess risks with the homozygote genotype. Third, given the evidence for excess liver cancers and the controversy regarding a possible mortality excess in male p.C282Y homozygotes, these were the 2 primary outcomes of the analyses, and other estimates were included for completeness and should be regarded as exploratory. Fourth, primary care data were only available for a subset of participants, so estimates of cumulative penetrance to liver outcomes based on hospital data only were likely to be underestimated. Fifth, the European ancestry sample studied may be relatively ancestrally homogeneous, and the results of this study may not generalize to more diverse populations. More research is needed on longer-term liver outcomes, especially in women with HFE pathogenic variants.
Conclusions
Among men with HFE p.C282Y homozygosity, there was a significantly increased risk of incident primary hepatic malignancy and death compared with those without p.C282Y or p.H63D pathogenic variants; there was not a significant association for women. Further research is needed to understand the effects of early diagnosis and treatment in preventing these outcomes.
eMethods
eTables
eFigures
References
- 1.Kawabata H. The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis. Int J Hematol. 2018;107(1):31-43. doi: 10.1007/s12185-017-2365-3 [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol. 2010;53(1):3-22. doi: 10.1016/j.jhep.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Bomford A. Genetics of haemochromatosis. Lancet. 2002;360(9346):1673-1681. doi: 10.1016/S0140-6736(02)11607-2 [DOI] [PubMed] [Google Scholar]
- 4.Pilling LC, Tamosauskaite J, Jones G, et al. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ. 2019;364:k5222. doi: 10.1136/bmj.k5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams PC, Reboussin DM, Barton JC, et al. ; Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators . Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352(17):1769-1778. doi: 10.1056/NEJMoa041534 [DOI] [PubMed] [Google Scholar]
- 6.Porter JL, Rawla P. Hemochromatosis. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 7.Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221-230. doi: 10.1056/NEJMoa073286 [DOI] [PubMed] [Google Scholar]
- 8.Niederau C, Strohmeyer G, Stremmel W. Epidemiology, clinical spectrum and prognosis of hemochromatosis. Adv Exp Med Biol. 1994;356:293-302. doi: 10.1007/978-1-4615-2554-7_31 [DOI] [PubMed] [Google Scholar]
- 9.Harrison-Findik DD. Gender-related variations in iron metabolism and liver diseases. World J Hepatol. 2010;2(8):302-310. doi: 10.4254/wjh.v2.i8.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowdley KV, Brown KE, Ahn J, Sundaram V. ACG clinical guideline: hereditary hemochromatosis. Am J Gastroenterol. 2019;114(8):1202-1218. doi: 10.14309/ajg.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 11.Bardou-Jacquet E, Morandeau E, Anderson GJ, et al. Regression of fibrosis stage with treatment reduces long-term risk of liver cancer in patients with hemochromatosis caused by mutation in HFE. Clin Gastroenterol Hepatol. 2020;18(8):1851-1857. doi: 10.1016/j.cgh.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 12.Powell LW, Dixon JL, Ramm GA, et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med. 2006;166(3):294-301. doi: 10.1001/archinte.166.3.294 [DOI] [PubMed] [Google Scholar]
- 13.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359(9302):211-218. doi: 10.1016/S0140-6736(02)07447-0 [DOI] [PubMed] [Google Scholar]
- 14.Grosse SD, Gurrin LC, Bertalli NA, Allen KJ. Clinical penetrance in hereditary hemochromatosis: estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet Med. 2018;20(4):383-389. doi: 10.1038/gim.2017.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Companion document for serum biomarker data. UK Biobank. Published November 3, 2019. Accessed May 20, 2019. http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1227
- 18.Bhavnani M, Lloyd D, Bhattacharyya A, Marples J, Elton P, Worwood M. Screening for genetic haemochromatosis in blood samples with raised alanine aminotransferase. Gut. 2000;46(5):707-710. doi: 10.1136/gut.46.5.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin LK, Tong KS. Elevated ALT and AST in an asymptomatic person: what the primary care doctor should do? Malays Fam Physician. 2009;4(2-3):98-99. [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Harris N., et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues International Agency for Research on Cancer; 2017. [Google Scholar]
- 21.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313(20):1256-1262. doi: 10.1056/NEJM198511143132004 [DOI] [PubMed] [Google Scholar]
- 22.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827-836. doi: 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimpin L, Cortez-Pinto H, Negro F, et al. ; EASL HEPAHEALTH Steering Committee . Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718-735. doi: 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 24.Ellervik C, Birgens H, Tybjaerg-Hansen A, Nordestgaard BG. Hemochromatosis genotypes and risk of 31 disease endpoints: meta-analyses including 66,000 cases and 226,000 controls. Hepatology. 2007;46(4):1071-1080. doi: 10.1002/hep.21885 [DOI] [PubMed] [Google Scholar]
- 25.Barton JC, Barton JC, Adams PC. Prevalence and characteristics of anti-HCV positivity and chronic hepatitis C virus infection in HFE p.C282Y homozygotes. Ann Hepatol. 2019;18(2):354-359. doi: 10.1016/j.aohep.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Pankow JS, Boerwinkle E, Adams PC, et al. HFE C282Y homozygotes have reduced low-density lipoprotein cholesterol: the Atherosclerosis Risk in Communities (ARIC) Study. Transl Res. 2008;152(1):3-10. doi: 10.1016/j.trsl.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis G, Wimperis JZ, Smith KC, Fellows IW, Jennings BA. Haemochromatosis gene C282Y homozygotes in an elderly male population. Lancet. 1999;354(9174):221-222. doi: 10.1016/S0140-6736(99)02195-9 [DOI] [PubMed] [Google Scholar]
- 28.Van Aken MO, De Craen AJM, Gussekloo J, et al. No increase in mortality and morbidity among carriers of the C282Y mutation of the hereditary haemochromatosis gene in the oldest old: the Leiden 85-plus study. Eur J Clin Invest. 2002;32(10):750-754. doi: 10.1046/j.1365-2362.2002.01062.x [DOI] [PubMed] [Google Scholar]
- 29.dbSNP: short genetic variations. National Library of Medicine. Accessed February 6, 2020. https://www.ncbi.nlm.nih.gov/snp/rs1800562
- 30.Jackson HA, Carter K, Darke C, et al. HFE mutations, iron deficiency and overload in 10,500 blood donors. Br J Haematol. 2001;114(2):474-484. doi: 10.1046/j.1365-2141.2001.02949.x [DOI] [PubMed] [Google Scholar]
- 31.Gallego CJ, Burt A, Sundaresan AS, et al. Penetrance of hemochromatosis in HFE genotypes resulting in p.Cys282Tyr and p.[Cys282Tyr];[His63Asp] in the eMERGE Network. Am J Hum Genet. 2015;97(4):512-520. doi: 10.1016/j.ajhg.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng Fat L, Mindell J, Roderick P. Health Survey for England 2016: Kidney and Liver Disease Health and Social Care Information Centre; 2017. Accessed February 1, 2020. http://healthsurvey.hscic.gov.uk/media/63736/HSE2016-Adult-kid-liv.pdf
- 33.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTables
eFigures