Keywords: COVID-19, neonatal outcomes, pathophysiology, placenta, pregnancy
Abstract
There are many unknowns for pregnant women during the coronavirus disease 2019 (COVID-19) pandemic. Clinical experience of pregnancies complicated with infection by other coronaviruses e.g., Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome, has led to pregnant woman being considered potentially vulnerable to severe SARS-CoV-2 infection. Physiological changes during pregnancy have a significant impact on the immune system, respiratory system, cardiovascular function, and coagulation. These may have positive or negative effects on COVID-19 disease progression. The impact of SARS-CoV-2 in pregnancy remains to be determined, and a concerted, global effort is required to determine the effects on implantation, fetal growth and development, labor, and neonatal health. Asymptomatic infection presents a further challenge regarding service provision, prevention, and management. Besides the direct impacts of the disease, a plethora of indirect consequences of the pandemic adversely affect maternal health, including reduced access to reproductive health services, increased mental health strain, and increased socioeconomic deprivation. In this review, we explore the current knowledge of COVID-19 in pregnancy and highlight areas for further research to minimize its impact for women and their children.
-
1.
The risk of severe coronavirus disease 2019 (COVID-19) during pregnancy may be higher than in the general population.
-
2.
The risk factors for severe COVID-19 are similar in pregnancy to the general population.
-
3.
Vertical transmission is plausible, but mechanisms are uncertain. Severe neonatal disease appears to be rare.
-
4.
Antenatal corticosteroid use for threatened preterm birth is likely to be safe for the mother, and corticosteroid use for severe maternal disease may be beneficial.
-
5.
Clinicians should have a low threshold for thromboprophylaxis in mothers with COVID-19, and for investigation of possible thromboembolic events.
-
6.
Mothers with COVID-19 should be encouraged to breastfeed if they are able, but should wear personal protective equipment to do so.
-
7.
Asymptomatic COVID-19 in pregnancy appears to be common, but is of uncertain clinical significance.
-
8.
Clinicians should be mindful of the wider implications of the pandemic and ensure that screening takes place for mental health distress and intimate partner violence whenever possible.
I. BACKGROUND
In December 2019, a cluster of four cases of pneumonia of unknown etiology in Wuhan, China, were reported to the World Health Organization (WHO) (70). Since then, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly across the world. On March 12, 2020 the WHO defined the outbreak as a pandemic (141). Many countries responded by restricting freedom of movement and limiting nonemergency health care to focus resources on COVID-19 care provision (139). As pregnant women are at greater risk of complications and severe disease from infection with other coronaviruses, including Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS), they were identified as a vulnerable group and were advised to take additional precautions as the COVID-19 pandemic unfolded (22, 33, 143). To reduce transmission risks for both pregnant women and health care workers, the International Federation of Gynecology and Obstetrics (FIGO) recommended the suspension of much routine antenatal care and replacement with video or telephone consultations whenever possible (12, 103, 109).
In this review, we evaluate the evidence of the effects of SARS-CoV-2 infection throughout pregnancy. We will examine the physiological adaptations to pregnancy and the implications for COVID-19, as well as COVID-19’s impact on pregnancy outcomes, and consider areas of uncertainty where more research is needed. We conducted a literature search to identify all articles relating to COVID-19 in pregnancy until August 17, 2020. Search terms included combinations of coronavirus, 2019-nCoV, COVID-19, SARS-CoV-2, and pregnancy and were input into Medline, Embase, Cochrane, Web of Science, and Cinahl. All case series and cohort studies describing maternal outcomes are summarized in Table 1, listed in order of study size. Single case studies and non-English language articles are not included.
Table 1.
List of studies on pregnant women with COVID-19
| Authors | Title | Location | Article Type | Number of Women | Gestation | Maternal Course | Neonatal Course | Neonatal Infection |
|---|---|---|---|---|---|---|---|---|
| Ellington et al. (38) | Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020 |
USA | Retrospective cohort | 8,207 pregnant vs 83,205 non-pregnant women | Not given | Pregnant women more likely to require admission to ICU and mechanical ventilation | Not reported | Not reported |
| Tekbali et al. (122) | Pregnant versus non-pregnant SARS-CoV-2 and COVID-19 Hospital 1 Admissions: The first 4 wk in New York |
New York | Retrospective cohort | Review of all (female) admissions to the hospital, 3,064 pregnant, 18,916 nonpregnant | Not given | Pregnant women less likely to require hospital admission than non-pregnant | Not reported | Not reported |
| Takemoto et al. (119) | The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting |
Brazil | Retrospective cohort | 978 (NB only testing women with “Severe” symptoms | Not given | 124 deaths (12.7% mortality rate) | Not reported | Not reported |
| Knight et al. (64) | Characteristics and outcomes of pregnant women hospitalized with confirmed SARS-CoV-2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS) |
UK | Prospective cohort | 427 | Majority 3rd trimester (Median = 34w, IQR29–38) | 40 women (9%) required critical care admission. Three deaths | 52% gave birth- 48% still pregnant. 74% term delivery. All PTB iatrogenic. Five neonatal deaths- 3 definitely not COVID, 2 uncertain. 64 admissions to NICU | 12 positive, 6 of whom within 12 h of birth |
| Blitz et al. (10) | Intensive care unit admissions for pregnant and non-pregnant women with COVID-19 |
New York | Retrospective cohort | 82 | Not given | No sig diff between ICU admission rates of pregnant and non-pregnant women | COVID +ve baby symptomatic | Not reported |
| Lokken et al. (77) | Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State |
USA | Retrospective cohort | 46 | 3 1st trimester, 20 2nd trimester, 23 3rd trimester | 7 hospitalized, 1 ICU | 1 PTB at 33 wk- iatrogenic due to maternal condition. One stillbirth etiology unknown | Not tested |
| Oncel et al. (94) | A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society |
Turkey | Retrospective cohort | 125 | All 3rd trimester | 8 ICU admissions, 6 deaths | 4 positive and required minor respiratory support | 4 positive |
| Breslin et al. (15) | Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals |
New York | Retrospective cohort | 43 | All 3rd trimester | 4 severe and 2 critical cases | No adverse outcomes | All negative |
| Ferrazzi et al. (40) | Vaginal delivery in SARS‐CoV‐2 infected pregnant women in Northern Italy: a retrospective analysis |
Italy | Retrospective cohort | 42 | All 3rd trimester | 4 admitted to critical care, 7 required O2. All made good recovery | 5 spontaneous PTB, no other complications listed | 1 tested positive (after 3 days) |
| Gabriel et al. (79) | Multi-center Spanish study found no incidences of viral transmission in infants born to mothers with COVID-19 |
Spain | Retrospective cohort | 42 | All 3rd trimester | 3 required ICU care and 1 died due to massive VTE | 9 preterm (6 late preterm), 9 required NICU, all discharged well | 3 tested positive in first 24 h of life |
| Liu et al. (74) | Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children |
China | Case-control | 41 pregnant women vs 14 non-pregnant women | 2nd and 3rd trimester | No severe disease | No adverse outcomes | Not tested |
| Li et al. (69) | Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study |
China | Case series | 34 | All 3rd trimester | 5 women had preterm CS due to maternal pneumonia, all made good recovery | No adverse outcomes | All negative |
| Wu et al. (145) | Radiological findings and clinical characteristics of pregnant women with COVID‐19 pneumonia |
China | Case series | 23 | Twenty 3rd trimester, three 1st trimester | No severe disease | 1 neonatal jaundice, 20 well babies | 4 tested negative, others all clinically negative |
| Baergen and Heller (6) | Placental pathology in Covid-19 positive mothers: preliminary findings |
New York | Prospective cohort | 20 | All 3rd trimester | 20 placentas examined, 10 cases showed poor perfusion or thrombus but significance is unclear |
||
| Chen et al. (24) | Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients |
China | Case series | 17 | All 3rd trimester | No severe disease. Indication for Cesarean not given | No adverse outcomes | All negative |
| Khan et al. (61) | Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women |
China | Case series | 17 | All 3rd trimester | No severe disease | 3 PTB by elective CS- indication not given. All well | All negative, although 2 neonates were suspected to have COVID-19 |
| Zhang et al. (152) | Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province |
China | Case-control | 16 cases vs 45 controls | All 3rd trimester | From the women with COVID, none developed severe pneumonia | No sig difference in neonatal outcomes between the two groups. | Not tested |
| Liu et al. (73) | Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis |
China | Case series | 15 | Three in 2nd trimester, 9 in 3rd trimester | No severe disease | No adverse outcomes | Not tested |
| Yang et al. (147) | Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019 |
China | Case-control | 13 cases vs 42 pregnant controls | All 3rd trimester | No severe disease | No adverse outcomes | All negative |
| Liu et al. (76) | Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy |
China | Case series | 13 | Two 2nd trimester, eleven 3rd trimester | 1 severe case requiring ICU + mechanical ventilation, 3 discharged still pregnant- others all underwent C-section | 1 stillbirth— cause not given | Not tested |
| Penfield et al. (97) | Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples |
USA | Prospective cohort | 11 | All 3rd trimester | 3 “critical”, 2 “severe” | No adverse outcomes | All negative |
| Liao et al. (72) | Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID‐19 pandemic |
China | Case-control | 10 cases and 53 controls | All 3rd trimester | No severe disease | No adverse outcomes | All negative |
| Chen et al. (23) | Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records |
China | Case series | 9 | All 3rd trimester | No severe disease | No adverse outcomes | None |
| Khalil et al. (60) | SARS-CoV-2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg |
London | Retrospective cohort | 9 positive | All 3rd trimester | Only 1 had symptoms (mild) | No adverse outcomes | Not reported |
| Zhu et al. (155) | Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia |
China | Case series | 9 | All 3rd trimester | No severe disease | 1 neonatal death- cause not given | Not tested |
| Govind et al. (42) | Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital |
London | Retrospective cohort | 9 | All 3rd trimester | 7 mild symptoms, 2 more unwell | COVID +ve neonate treated for viral pneumonia | 1 tested positive |
| Wu et al. (144) | Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women |
China | Case series | 8 | All 3rd trimester | No severe disease | ||
| Yu et al. (149) | Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-center, descriptive study. |
China | Case series | 7 | All 3rd trimester | No severe disease | No adverse outcomes | 1 neonate positive 36 h after delivery |
| Juusela et al. (58) | Two cases of coronavirus 2019-related cardiomyopathy in pregnancy |
USA | Case series | 7 | All 3rd trimester | 2 out of 7 patients with severe disease developed cardiomyopathy, however sample size too small to be generalizable | Not reported | Not reported |
| Hu et al. (52a) | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia |
China | Retrospective cohort | 7 | All 3rd trimester | No severe disease | No adverse outcomes | 1 tested positive at 36 h |
| Chen et al. (26) | Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia | China | Case series | 5 | All 3rd trimester | No severe disease | No adverse outcomes | All negative |
| Perrone et al. (98) | Report of a series of healthy term newborns from convalescent mothers with COVID-19 |
Italy | Retrospective cohort | 4 | All 3rd trimester | Not reported | No adverse outcomes | Not reported |
| Chen et al. (25) | Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases |
China | Case series | 3 | All 3rd trimester | No severe disease. Placental tissue all negative for COVID-19 | No adverse outcomes | None |
| Tanacan et al. (120) | The rate of SARS-CoV-2 positivity in asymptomatic pregnant women admitted to hospital for delivery: experience of a pandemic center in Turkey |
Turkey | Retrospective cohort | 3 women (out of 206 tested | All 3rd trimester | Only one symptomatic, no severe disease | Not reported | Not reported |
CS, cesarean section; ICU, intensive care unit; IQR, interquartile range; NICU, neonatal intensive care unit; PTB, preterm birth; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2.
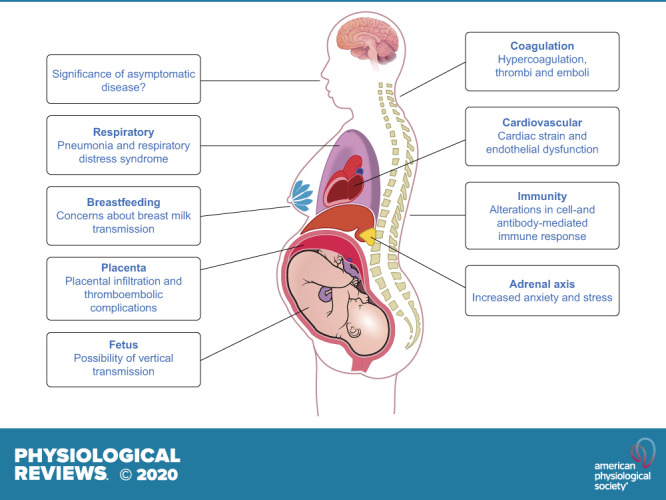
II. PHYSIOLOGICAL ADAPTATIONS TO PREGNANCY AND THE IMPLICATIONS FOR COVID-19
A. Immunological Response
COVID-19 is a capsulated single-stranded RNA virus (21). The immunological response to COVID-19, like other viruses, relies on a working immune system (21). COVID-19 infection can result in mild disease, in which the virus is cleared effectively by the immune system or severe disease with high mortality rates (21). The position for pregnant women on this spectrum is unclear. The immune system adapts during pregnancy to allow for the growth of a semiallogenic fetus (57), resulting in an altered immune response to infections during pregnancy (55, 115). To understand the COVID-19 phenotype during pregnancy, it is important to understand the pathophysiology and molecular mechanisms of COVID-19 and examine these in the context of the modulated maternal immune response.
SARS-CoV-2, which is transmitted by respiratory droplets, direct contact with fomites, close person-to-person contact and possibly by aerosols generated (27, 28, 39, 45, 111, 142), enters the body via the nasal passage and infects pulmonary cells via the SARS-CoV receptor angiotensin-converting enzyme 2 (ACE2) and uses transmembrane serine protease 2 (TMPRSS2) for S protein priming (21, 51, 121). Cells in which ACE-2 and TMPRSS2 are colocalized are likely to be most susceptible to entry by SARS-CoV-2 (104). Infection with SARS-CoV-2 is followed by viral replication and release of the virus, causing pyroptosis [inflammation-mediated programmed cell death occurring in response to a pathological stimulus (9)] of the host cell (134). This releases damage-associated molecular patterns (DAMPs), including ATP and nucleic acids, that trigger an inflammatory response from neighboring cells (121). This proinflammatory response includes production of IL-6, C-X-C motif chemokine 10 (CXCL10), and type 1 interferons, which act as chemoattractants for monocytes, macrophages, and T cells to the infection site (92, 121). This positive feedback loop may lead to excessive inflammation and damage the integrity of the lung (121), resulting in infection with other (host) microbes (21, 121). The inflammation caused by SARS-CoV-2 may also result in a “cytokine storm” that can lead to multisystem organ failure (121). This excessive inflammation is thought to be the cause of severe COVID-19 and is associated with high morbidity and mortality (21, 121). Among patients with mild symptoms, it is likely that the immune system reacts appropriately to viral infection. The inflammation caused by viral entry attracts T-helper 1 cluster differentiation 4 (Th1 CD4+) T cells that can clear infected cells before further spread and replications of the virus occurs (50, 121). The virus is blocked further by neutralizing antibodies, and macrophages clear the neutralized viruses and apoptotic cells by phagocytosis (121).
The modulations of the maternal immune system in pregnancy may affect the response to infections, and specifically to viruses (115). The altered inflammatory response to viruses during pregnancy is thought to be mediated, at least in part, by
-
1.
A shift in CD4+ T cell population toward the Th2 phenotype over Th1 during pregnancy (a response that promotes humoral responses over cellular immune responses) (100). For the immune response to viral infections, a decrease in Th1 reactivity can result in an altered clearance of infected cells. However, an overt Th1 and Th2 response to SARS-CoV-2 has been implicated in the pathogenesis of severe COVID-19 (154).
-
2.
A decrease in circulating natural killer (NK) cells during pregnancy (130). NK cells play an important role in the innate immune system’s viral clearance, and a decrease in these cell populations may alter the ability to clear viruses (14). However, it is unclear whether this decrease in circulating NK cells has clinical implications for COVID-19.
-
3.
A decrease in circulating plasmacytoid dendritic cells (pDCs) (84, 128). These cells are key for type 1 interferon production against viruses (106). Moreover, pDCs from pregnant women have also been shown to have an attenuated inflammatory response to the H1N1/09 virus (128). This is thought to be one of the reasons why pregnant women were more severely affected by the H1N1 pandemic in 2009 (54, 84, 106, 128).
-
4.
An increase in circulating progesterone levels (114). Progesterone is a steroid hormone that has immunomodulatory properties (37). Progesterone also has the ability to enhance lung repair of damage induced by influenza virus (47), making high levels during pregnancy potentially beneficial for the recovery after viral lung infections. However, in a mouse model of influenza A infection, treatment with progesterone or the progestin, levonorgestrel, also resulted in a decrease in virus-specific antibody levels, as well as a decrease in virus-specific CD8+ T cells in mice (48). When these mice were rechallenged with influenza A, this resulted in more severe disease (48). Further studies are needed to understand the role of pregnancy-related changes in progesterone and other hormones, including estrogen and androgens, which may contribute to immunoregulation (63) in the response to COVID-19 infection.
-
5.
Alterations in the innate immune system, including the pattern recognition receptors Toll-like receptors (TLRs) during pregnancy (5, 148). COVID-19 infection causes pyroptosis of host cells and release of DAMPs, which can be TLR ligands and further enhance inflammation. The role that the innate immune system and TLRs play in the COVID-19 immune response still needs to be investigated to understand how pregnancy affects this particular aspect of the viral response.
These modulations in the maternal immune system have consequences for the clinical trajectory of COVID-19 and for the treatment and prevention of COVID-19 in pregnancy. However, it remains to be determined whether these adaptations result in a higher susceptibility and/or morbidity or are, in fact, protective against COVID-19. It is unclear whether disease severity has consequences for COVID-19 immunity in the nonpregnant population. In future studies, it will be important to investigate the inflammatory response, viral load, antibody production, and level of immunity acquired in pregnant women across different gestations. This will be important when considering the efficacy and immunological responses to potential COVID-19 vaccination, to ensure that it is administered at the right time (i.e., at a specific timepoint during pregnancy or postpartum) and is safe and effective for women throughout the reproductive lifespan.
B. Respiratory Response
In addition to the systemic immunological changes of pregnancy that have the potential to have an impact on lung function, anatomical changes also are present in the respiratory system. Physiological alterations to the chest shape and elevation of the diaphragm due to diaphragmatic splinting by the gravid uterus cause changes to the respiratory function. Although there is a 30–40% increase in tidal volume, the reduction in chest volume leads to a decrease in functional residual capacity, end-expiratory volumes, and residual volumes from early in pregnancy. The reduction in total lung capacity and inability to clear secretions can make pregnant women more susceptible to severe respiratory infections (41).
C. Coagulation Response
In the general population, COVID-19 is associated with high rates of thromboembolic complications, with a study including 184 critically unwell patients (24% female) reporting that 31% had thrombotic events (64). This is due to activation of coagulation pathways and potential progression to disseminated vascular coagulopathy (DIC) and fibrinolysis with resultant dynamic hypercoagulation occurring alongside thrombocytopenia (56).
Pregnancy is a hypercoagulable state with increased thrombin production and an increase in intravascular inflammation (34). During pregnancy, there are higher levels of circulating coagulation and fibrinolytic factors, such as plasmin, and these may be implicated in the pathogenesis of SARS-CoV-2 infection (56). Pregnant women are at increased risk of thromboembolic events with associated mortality (31, 81). Therefore, pregnant women with COVID-19 may have additive or synergistic risk factors for thrombosis. This hypothesis is supported by a case report describing mortality in a woman at 29 wk gestation with COVID-19 due to a large pulmonary embolism and basilar artery embolism (2). Current guidelines recommend that all pregnant women with confirmed COVID-19 should have thromboprophylaxis until 10 days postnatal and that their clinicians have a low threshold for investigation of possible thromboembolism (124).
D. Endothelial Cell Function
Mortality in COVID-19 is predominantly due to acute respiratory distress syndrome (ARDS) (146). Emerging evidence suggests that pulmonary endothelial cell dysfunction has an important role in the onset and progression of ARDS (123, 129). In health, endothelial cells are surrounded by mural cells (pericytes) and limit inflammation by restricting immune cell entry and prevent coagulation via expression of anticoagulant factors. In ARDS, this endothelial barrier is damaged, leading to tissue edema, excessive inflammation, and hypercoagulability. Risk factors for COVID-19 (increasing age, obesity, diabetes mellitus, and cardiovascular disease) are all associated with endothelial cell dysfunction (71).
Maternal vascular adaptation to pregnancy is critical for optimal pregnancy outcomes. At implantation, the specialized uterine spiral arterioles are remodeled to form sinuses that become placental villi. Systemic vascular physiology also undergoes significant adaptations to pregnancy (18). Maternal blood volume increases, heart rate and stroke volume increase cardiac output by 30–50%, and vascular resistance decreases. The impact of this increased vasodilation on pulmonary endothelial cell function (immune cell adhesions and activation of coagulation) has yet to be determined.
Pregnancies affected by preeclampsia are characterized by hypertension and proteinuria. Preeclampsia is associated with significant maternal (stroke, cardiac arrest, renal failure, liver failure) and fetal (intrauterine growth restriction (IUGR), preterm birth, stillbirth) complications (85). Women with preeclampsia have an insufficient decrease in vascular resistance in middle to late gestation and associated endothelial cell dysfunction (19). Given the potential importance of endothelial cell function in the development and progression of COVID-19, these women may be at particular risk, if infected (33), and an early systematic review found higher rates of preeclampsia in pregnant women hospitalized with COVID-19 (33).
E. Placental Responses and Mechanisms of Vertical Transmission
The role of the placenta in SARS-CoV-2 infection is currently poorly understood. Although it appears vertical transmission can occur, the mechanisms underlying this type of transmission are uncertain.
1. Physiology of the placenta and viral interaction
The placenta is usually an effective barrier that prevents maternal infection spreading to the fetus (vertical transmission). It is well recognized that certain pathogens can overcome this barrier, with sometimes devastating effects on the developing pregnancy (30). Cytomegalovirus (CMV), herpes simplex virus (HSV), varicella zoster virus, and Zika virus (ZIKV) can all cause congenital syndromes, with variable rates of transmission and severity of effects that depend, in part, on the stage of pregnancy that infection occurs. Of note, many of these infections may have only minor effects on the mother, and there is little recognized correlation between maternal symptomology and severity of fetal effects. Experience of viral infections in pregnancy has led to three other key observations regarding congenital infection, in general. First, the presence of the virus on the placental surface does not necessarily indicate placental infection—vertical transmission of viruses depends on some kind of breach of the placental barrier. Second, viral infection of placental cells does not necessarily mean that there is transmission to the fetus. Third, even when fetal infection occurs, responses are heterogeneous; thus, fetal infection does not always mean fetal damage.
The human placenta is hemochorial, meaning that maternal blood is in direct contact with the placental chorionic villi. The placenta is formed predominantly of specialized, fetally derived, cells called trophoblasts, of which there are three main types. Terminally differentiated multinuclear syncytiotrophoblast cells line the villus tree and are in direct contact with maternal blood. Progenitor villous cytotrophoblast cells underlie the syncytiotrophoblast. Invasive extravillous trophoblast cells anchor the chorionic villi to the uterus and modify its vasculature. A number of potential mechanisms may be involved in vertical transmission of viruses, including direct damage to the villous tree, with breaks in the protective syncytiotrophoblast layer; spread from virally infected maternal endothelium to extravillous trophoblast; traffic of infected maternal immune cells across the syncytiotrophoblast or paracellular or transcellular transport (e.g., immunoglobulin-mediated transcytosis) into fetal capillaries; and/or ascending infection from the vagina (30).
2. SARS-CoV-2 and the placenta
There have been a series of case reports examining the placentas of women with COVID-19. SARS-CoV-2 expression has been detected in samples taken from midtrimester placentae, but it remains unclear whether the presence of virus was due to primary infection or facilitated by placental damage from other pathologies. SARS-CoV-2 was found on RT-PCR of swabs and biopsies following a spontaneous fetal loss at 19 wk gestation (136). SARS-CoV-2 was also highly expressed in placental and umbilical cord biopsies following a termination of pregnancy at 22-wk gestation (52). The pregnancy was terminated as a result of placental abruption and severe maternal preeclampsia with thrombocytopenia and coagulopathy. In this case, electron microscopy (52) revealed virus-like particles in the cytosol of placental cells; however, no viral expression was detected in fetal tissues tested. In both case reports (7, 52), macrophage infiltrates and fibrin deposits were seen on placental histology, which the authors attributed to being most likely associated with viral infection. However, such intervillositis can also be idiopathic, autoimmune, or associated with other infections, and so could be unrelated to the presence of SARS-CoV-2. A further case study found both placental swabs and amniotic fluid were positive for SARS-CoV-2 PCR. On microscopic examination, the placenta also had evidence of perivillous fibrin deposition with infarction and intervillositis. In this case, the neonate tested positive on nasal and rectal swabs, and required NICU for respiratory support (132).
Two other publications report placental histological findings in women with SARS-CoV-2 infection (6). In a study of the placentae from 20 women found to be positive for SARS-CoV-2 on routine testing at the time of birth (32 to 40 wk gestation), 10 placentae showed signs of possible fetal vascular malperfusion or fetal vascular thrombosis (6). However, there was no control group for comparison, making interpretation of findings difficult. Findings were mainly low grade and could be related to other etiologies. Another study examined the placentas from 16 women with SARS-CoV-2 infection (112). The placentas were from babies born between 16 to 40 wk gestation, with 11 of the maternal SARS-CoV-2 infections diagnosed around the time of birth, and five diagnosed earlier in pregnancy. Twelve of the 15 third-trimester placentas were reported as showing signs of maternal vascular malperfusion (villous infarctions, villous agglutination, or decidual arteriopathy), a statistically significant higher proportion when compared with pathology reports from historical controls (identified by natural language processing). However, pathologists performing the examination were not blind to the SARS-CoV-2 status of the mother. As the pathological examination was indicated by maternal SARS-CoV-2 infection in the majority of cases, and histological signs of placental vascular malperfusion are somewhat subjective, these findings need to be interpreted with caution. Further research is required, including standardized examination of placental samples from women with SARS-CoV-2 and matched negative controls, by pathologists unaware of SARS-CoV-2 status, to verify these preliminary reports of potential vascular and thrombotic effects in the placenta associated with maternal COVID-19. In addition, these findings should be correlated to clinical status of the fetus, ideally with longer-term follow-up (66).
3. Vertical transmission of SARS-CoV-2
Viral infection of placental cells does not necessarily mean fetal infection or fetal harm. So far, 15 reports include neonatal test results for SARS-CoV-2 (15, 24, 26, 40, 61, 64, 69, 72, 79, 145, 147, 149, 150) with positive cases occurring only in the minority (40, 64, 79, 149, 150). Significant neonatal respiratory diseases appear to be rare, even in the presence of SARS-CoV-2 positivity. It is unclear from reports of PCR-based SARS-CoV-2 testing whether infection occurs in utero or during the labor or birth; or whether transmission occurs from the infected mother or asymptomatic hospital staff in the immediate newborn period. However, the availability of antibody tests has provided new evidence that vertical transmission can occur. Some babies born to mothers with COVID-19 have increased concentrations of both immunoglobulin (Ig)M and IgG for SARS-CoV-2 (35, 151). Although IgG can be passively transferred from mother to baby in utero, IgM has a larger molecular weight and cannot cross the placenta. Circulating SARS-CoV-2 IgM in the neonate, thus, indicates vertical transmission of virus, although all the infants in reports so far have been asymptomatic and tested negative for SARS-CoV-2 viral RNA at birth (35, 151).
Mechanisms of viral invasion of the placenta have yet to be clearly established. In the lungs, SARS-CoV-2 uses the ACE2 receptor to enter cells, and serine protease TMPRSS2 is implicated in cleaving the spike glycoprotein to allow fusion. Three studies have included analysis of single-cell RNA sequencing data to determine whether ACE2 +/− TMPRSS2 are expressed on placental cells. Li et al. (68) performed secondary analysis of publicly available single-cell transcriptome profiles from decidua and placenta samples at 6–12 wk gestation (131), with additional data from a previous study of two-term placentae samples (96). They reported ACE2 gene expression in first-trimester decidual stromal and perivascular cells, and in the villous cytotrophoblast and syncytiotrophoblast in both first trimester and term samples. However, another study using the same first trimester data set (131) found generally only very low level expression of ACE2 in placental and decidual cell populations (153). Pique-Regi et al. (102) also used the same resource for first-trimester analysis (131), along with newly generated data from a single second-trimester placenta, and their own existing data from third-trimester placenta and fetal membranes. They also included data from single-nuclear RNA sequencing, to overcome the potential bias from low fractions of syncytiotrophoblast cells in single-cell transcriptomic studies, which can result from lack of dissociation of large multinucleated cells. They specifically looked at colocalization of ACE2 and TMPRSS2, which they found to be negligible in both the placenta throughout gestation and fetal membranes in the third trimester. A further study studied human embryos to investigate possible transmission routes in the first trimester, and they found expression of ACE2 and coexpression of TMPRSS2 in the trophoblast, the blastocyst, and the hypoblast, which suggests that fetal infection via this pathway is possible (133).
Given the lack of ACE2 and TMPRSS2 coexpression in the placenta, it, therefore, seems likely that SARS-CoV-2 enters the placental tissues via an alternative mechanism (66, 102). A number of other proteases have also been implicated. DPP4 and CD147 are both highly expressed in the placenta throughout gestation and may have e role in cell entry (66). Furin, trypsin, and cathepsins B and L have all been demonstrated to have the ability to cleave the spike glycoprotein binding at the S1/S2 site (51, 53, 102). Additionally plasmin can cleave this site, and has been identified as a possible therapeutic target with tranexamic acid, to prevent cell entry (91). SARS-Cov-2 viral RNA has been detected in the amniotic fluid in case reports of serious maternal disease, although neonatal positivity at birth was variable (52, 132, 150).
III. COVID-19 AND ITS IMPACT ON PREGNANCY
A. Pregnancy Response to Other Viral Exposure
A number of viruses have established effects on the mother and the fetus during pregnancy and may provide information on the potential impact and mechanism of COVID-19 in pregnancy.
1. Viruses and the mother
A systematic literature review examining the effects of influenza A (H1N1), found H1N1 caused significantly more severe disease in pregnant women compared with age-matched non-pregnant controls. The review encompassed 3,110 women and reported an 8% maternal mortality rate and a 30% preterm birth rate (86). Pregnancy was a risk factor for hospitalization, intensive care unit (ICU) admission and death.
Other coronaviruses—SARS and MERS—had significant adverse maternal outcomes with a 25.8% and 28.6% maternal mortality rate, respectively, and were associated with preterm birth, fetal growth restriction, and perinatal death. However sample sizes were small, with only 12 recorded cases of MERS in pregnancy and 26 cases of SARS (33).
2. Viruses and the fetus
Viral illness during pregnancy increases the risk of a range of adverse outcomes for the child (86). A study using birth registry data in the United States found that early pregnancy coinciding with high population levels of seasonal influenza, was significantly associated with preterm birth, neonatal and infant mortality (36). A 13-yr cohort study in Nova Scotia found that women hospitalized with influenza during pregnancy were significantly more likely to have a baby who was small for gestational age (OR 1.66, 95% CI: 1.11–2.49) (82). In a UK cohort, H1N1 infection during pregnancy was associated with increase in preterm birth (OR 4.0, 95% CI:2.7–5.9) and perinatal mortality (OR 5.7, 95% CI:2.2–15.1) (101). Influenza infection has also been associated with congenital abnormalities, such as cleft palate and neuronal tube and congenital heart defects (86).
Viruses can have long-lasting detrimental impacts on the fetus. High levels of maternal inflammation in response to viral infection can impact several aspects of fetal brain development, leading to wide-ranging neurological sequelae (75). Influenza during pregnancy is associated with higher rates of bipolar disorder (20, 78) and schizophrenia (83) in offspring. In CMV, 20–25% of infected fetuses develop postnatal neurological sequelae (90), and vertically acquired Zika virus infection causes a broad range of neurological abnormalities, as well as increasing rates of fetal growth restriction and perinatal death (29). Data from a large Swedish cohort study found maternal exposure to any viral infection during pregnancy increased offspring diagnosis of autism (HR 1.79; 95% confidence interval (CI), 1.34–2.40) and depression (HR, 1.24; 95% CI, 1.08–1.42) (3).
B. How SARS-CoV-2 Impacts Pregnancy
1. SARS-CoV-2 and early pregnancy
There is little evidence about the possible impact of COVID in early pregnancy (up to 12 wk gestation). Seasonal influenza has been associated with higher rates of miscarriage (36) and population level monitoring and upscale of community testing will be needed to ascertain whether this is also the case with COVID-19.
2. SARS-CoV-2 and late pregnancy
Extrapolating from effects of other viruses in late pregnancy (greater than 24 wk gestation) (36, 86), we can expect that COVID-19 infection could cause increased rates of adverse pregnancy outcomes such as fetal growth restriction, preterm birth and perinatal mortality. It will be important to evaluate population level data on these outcomes, as they become available, to identify trends related to the COVID-19 pandemic.
Since January 2020, a number of case series and cohort studies have described the presentation and clinical course of COVID-19 in pregnancy (Table 1). To date, the majority of studies have been reassuring and the risk of severe COVID-19 in pregnancy appears to be no greater than for the general population. Thirty-one relevant studies were identified reporting on outcomes of pregnant women with confirmed SARS-CoV-2 infection, and their babies, encompassing 12,260 women. (6, 10, 15, 23–26, 38, 40, 42, 58, 60, 61, 64, 69, 72–74, 76, 77, 79, 97, 122, 132, 144, 145, 147, 149, 152, 155) The majority of women were in the third trimester and had mild to moderate symptoms only. A minority of women required critical care admission. So far 146 deaths have been reported, the vast majority of these (124) are from Brazil (38, 64, 119). There were a number of preterm births reported; however, all of these were iatrogenic due to worsening maternal COVID-19 or were due to obstetric complications unrelated to COVID-19 disease (70, 73, 106).
The largest cohort study to date from the United States (Colombia and New York) includes population-level data from 91,412 women aged 15–44, 8,207 of whom were pregnant (38). Pregnancy was associated with significantly increased chance of hospitalization (RR, 5.4; 95% CI, 5.1–5.6), ICU admission (RR, 1.5; 95% CI, 1.2–1.8) and need for mechanical ventilation (RR, 1.7; 95% CI, 1.2–2.4). There was no significant reduction in mortality (RR, 0.9; 95% CI, 0.5–1.5). An earlier smaller study using the same population, but only including data from the first 4 wk of the pandemic in New York, reported that pregnant women were less likely to require admission than nonpregnant women with COVID-19, although in this study, they did not age-match the control group (122). It is difficult to extrapolate the results of these studies more widely, as criteria for hospital/ICU admission and ventilation are not provided, and these results may reflect the health care setting rather than the clinical status of the women, particularly given that there was no increased mortality. A cohort study from the UK, including 427 women, found risk factors identified for hospitalization with COVID-19 disease are similar to those in the general population, including having comorbidities such as asthma, hypertension, or diabetes (combined OR, 1.52; 95% CI, 1.12–2.06); being overweight (OR, 1.91; 95% CI, 1.37–2.68) or obese (OR, 2.20; 95% CI, 1.56–3.10), and being a member of a black or ethnic minority ethnic group (OR, 4.49; 95% CI, 3.37–6.00) (64). Remaining cohorts are limited by small numbers and lack of a population denominator. The majority, however, report no severe disease and no mortality. A further limitation to the data is most of the studies tested only those women with COVID-19 symptoms. One study did test all women on admission to the labor ward and found only 9/129 women who tested positive were symptomatic (11.1%) (60). Therefore, the reported cases are likely to represent only the tip of the iceberg. This means that first, the likelihood of an adverse event will be significantly overestimated, but also that subacute or long-term complications will be difficult to accurately assess or predict.
3. Low- and middle-income settings
Two Brazilian studies represent the only data from low- and middle-income settings (LMICs). The first reports on all female patients admitted to hospital with COVID-18 and found that 18.9% were pregnant, which is much higher than the pregnancy rate in the general population (93). The second study includes 978 pregnant women reported with COVID-19 infection and with a mortality rate of 12.7%. This is likely to be an overestimate, as only women with “severe” symptoms were tested. However, the authors raised concern that only 22.6% of the women who died were admitted to the ICU, and only 64% of these women were ventilated, raising the possibility that much of the excess mortality is due to inability to access critical care. Although there is limited data on COVID-19 in pregnancy in LMICs, COVID-19 cases in these countries are increasing and a WHO estimate suggests up to 190,000 people in Sub-Saharan Africa could die (140). There may be additional complications in this setting relating to higher rates of both malnutrition and TB among women of child-bearing age, and it is unknown whether this will impact on COVID-19 infection (137). In resource-constrained settings, pregnant women are particularly vulnerable to the adverse impact that COVID-19 has on health services at all levels (17).
4. Corticosteroid use
Antenatal corticosteroids given to women at threat of preterm birth have been shown to confer significant morbidity and mortality benefit for neonates (59). Early observational data showed that corticosteroid use in nonpregnant patients with COVID-19 was associated with increased mortality and poorer disease outcomes when given in severe infection, although findings were not adjusted for disease severity or comorbidity (44, 113). In contrast, the first results of the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated benefits for 454 nonpregnant participants randomized to treatment with dexamethasone: mortality was significantly reduced in those requiring mechanical ventilation (RR, 0.65; 95% CI, 0.48–0.88) and in those required supplementary oxygen (RR, 0.80; 95% CI, 0.67–0.96) (105). Because dexamethasone crosses the placenta, the corticosteroid arm of the RECOVERY trial was modified to treatment with prednisolone or hydrocortisone, although no pregnant women were included in this first report. These findings suggest that in the context of iatrogenic preterm delivery in SARS-CoV-2-positive pregnant patients, due to the maternal condition (40, 61, 64), corticosteroid administration should continue as per current guidelines. Additionally, it is reasonable to consider corticosteroid administration during pregnancy or the postpartum period, where maternal illness severity warrants this (110).
C. SARS-CoV-2 and Postpartum
1. Neonatal outcomes
In the majority of studies reporting on neonatal outcomes, no serious adverse outcomes have been observed in neonates born to SARS-CoV-2-positive mothers (15, 23–26, 40, 60, 61, 69, 72–74, 97, 98, 145, 147, 149, 152). In studies comparing pregnant women who were unwell with confirmed COVID-19 disease and pregnant women who were unwell but SARS-CoV-2-negative, there were no significant differences in rates of adverse neonatal outcomes (147, 152). Thirteen studies tested neonates for SARS-CoV-2 and only three studies identified positive cases. Even when neonates tested positive for SARS-CoV-2, they were largely asymptomatic or had mild self-limiting symptoms (40, 42, 149). Three studies reported neonatal deaths. In two of these, the cause was not identified.
A number of studies have reported high rates of preterm birth, although none had a denominator population for comparison. Where cause of preterm birth was given, all were iatrogenic because of deteriorating maternal condition (40, 61, 64, 77, 79). Conversely, observational data from Ireland and Denmark have seen dramatic decreases in population level rates of preterm birth during the COVID-19 pandemic, the cause of which is unclear (49, 99). Whether COVID-19 infection is an independent risk factor for preterm birth has not yet been established, and this is an important area for future research.
2. Breastfeeding
It is uncertain whether SARS-CoV-2 is transmitted through breast milk. There has been one case study in which breast milk tested positive for SARS-CoV-2 on four different occasions (43). In another study, samples of breast milk of nine SARS-CoV-2-positive mothers were tested, and none were positive (23). Current guidance is for mothers to continue breastfeeding even if they have tested positive during birth and the postpartum period. Basic hygiene advice and handwashing should be followed, and women with confirmed COVID-19 should wear a medical mask while feeding, if available (88, 138). Given that neonatal infection is generally mild and often asymptomatic, the benefits of breastfeeding may outweigh the potential transmission risk.
3. Asymptomatic disease
Most studies of COVID-19 in pregnancy identify cases by testing women with symptoms. Data from centers in London and New York where all pregnant women admitted to give birth had routine nasal/throat swab PCR testing, found that of the women who tested positive, 88% were asymptomatic (60, 118). This suggests that the cases reported in the literature are likely to represent only a small proportion of overall cases. The clinical significance of having asymptomatic infection during pregnancy at any gestation is unknown.
IV. UNINTENDED CONSEQUENCES OF COVID-19 FOR MATERNAL HEALTH
The unintended consequences of the COVID-19 pandemic pose a threat to the health of pregnant women. As observed with the Ebola outbreak, women and girls are likely to bear a heavier burden of the downstream health, social, and economic consequences of this pandemic (46, 125, 134). In many LMICs, pregnancy-care provision is already stretched and under-resourced, and the indirect mortality impact of the crisis may exceed the direct mortality from COVID-19 itself (116).
Concerns about overwhelming resource-constrained healthcare systems has led to a reduction or suspension of many reproductive health care services (126). Substantial alterations have been made to the running of routine sexual and reproductive health services, which may increase gender inequalities in health, economic well-being, and social status (46, 108). This reduction in clinical services, alongside supply chain and manufacturing problems, may make contraception access difficult (107, 108, 126, 127). These factors will also impede access to and provision of safe abortions (8, 127).
Regarding antenatal care, staff and equipment have been diverted to the provision of acute medical care, meaning clinic capacity is limited, reducing the ability to screen for conditions such as gestational diabetes (107). In many settings, policies on social distancing have necessitated the restructuring of health care services to reduce face-to-face contact between doctors and patients, making diagnosis and support of mental health conditions more challenging (16). During this pandemic, many women are more vulnerable to intimate partner violence (13, 95) and associated adverse pregnancy outcomes (4), and they are less able to seek support (4, 134). Increased stress and anxiety during pregnancy upregulate inflammatory pathways and correlate strongly with neuropsychiatric disease for offspring (1). The pandemic is already widening social inequalities, and the likely impending economic crisis will only exacerbate this, increasing inequality and pushing more women into poverty (80, 117). Socioeconomic deprivation is a clear driver of maternal morbidity and mortality (11, 32, 81, 89), and a positive gradient has been observed between deprivation indicators and a range of adverse maternal child health outcomes (32). We can expect the downstream effects of COVID-19 to be apparent for a number of years.
V. CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE RESEARCH
From the current evidence base, it is difficult to draw absolute conclusions on whether pregnant women are at increased risk of severe consequences of COVID-19. Most women will experience mild or asymptomatic disease with no lasting consequences; however, some centers have seen increased rates of ICU admission, and the need for mechanical ventilation in pregnant women. The lack of granular population level data makes identification of risk factors, and the definitive comparison of pregnant and nonpregnant cohorts impossible. The lack of universal COVID-19 testing means that it is likely that the majority of cases go undetected. Vertical transmission is probable, but it appears rare, and in the majority of neonates, it has minimal impact. However, once more, this is impossible to fully assess until neonatal testing is routine. There are a number of unknowns, in particular, whether COVID-19 is an independent risk factor for preterm birth, whether infection during pregnancy is likely to lead to long-term adverse effects in offspring, and whether this effect is dependent on gestational age at infection. To answer these questions, the establishment of both data repositories and biobanks of women with confirmed or suspected COVID-19 is crucial. Data should be shared and made available wherever possible, and a concerted effort must be made to ensure a wide range of population groups are included, particularly those at increased risk. Granular epidemiological information is critical to identify differential population responses and subgroup analysis, for example, about impact of socioeconomic and ethnicity. The COVIPREG database has been set up to rapidly gather and synthesize data on COVID-19 infection in pregnancy worldwide, which can be used to inform evidence-based decision making from health care practitioners and policy makers (67).
Despite concerns about increased vulnerability of pregnant women to COVID-19, in more than 300 clinical trials investigating potential therapeutic options, pregnant women are almost universally excluded (135). Very few clinical trials include pregnant women, even those investigating treatments with a well-established safety record in pregnancy, such as hydroxychloroquine. Researchers should be urged to consider inclusion of pregnant women and other underrepresented groups to create a balanced and informed evidence base with data from a representative population.
GRANTS
We acknowledge the Medical Research Council Centre for Reproductive Health (MRC CRH) Grant MR/N022556/1 and the support of Tommy’s and the British Heart Foundation (RE/18/5/34216). S. J. Stock and J. A. Maybin are supported by Wellcome Trust Clinical Career Development Fellowships 209560/Z/17/Z and 209589/Z/17/Z, respectively.
DISCLOSURES
H. O. D. Critchley has clinical research support for laboratory consumables and staff from Bayer AG and provides consultancy advice (but with no personal remuneration) for Bayer AG, PregLem SA, Gedeon Richter, Vifor Pharma UK, AbbVie, and Myovant Sciences. H. O. D. Critchley receives royalties from UpToDate for an article on abnormal uterine bleeding. No conflicts of interest, financial or otherwise, are declared by any of the other authors.
ACKNOWLEDGMENTS
We thank C. Wastnedge for assistance in creating the graphics.
E. A. N. Wastnedge and R. M. Reynolds are joint first authors.
Correspondence: H. O. D. Critchley (e-mail: hilary.critchley@ed.ac.uk).
REFERENCES
- 1.Abdoli A, Falahi S, Kenarkoohi A, Shams M, Mir H, Jahromi MAM. The COVID-19 pandemic, psychological stress during pregnancy, and risk of neurodevelopmental disorders in offspring: a neglected consequence. J Psychosom Obstet Gynaecol 41: 247–248, 2020. doi: 10.1080/0167482X.2020.1761321. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed I, Azhar A, Eltaweel N, Tan BK. First COVID-19 maternal mortality in the UK associated with thrombotic complications. Br J Haematol 190: e37–e38, 2020. doi: 10.1111/bjh.16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Haddad BJS, Jacobsson B, Chabra S, Modzelewska D, Olson EM, Bernier R, Enquobahrie DA, Hagberg H, Östling S, Rajagopal L, Adams Waldorf KM, Sengpiel V. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 76: 594–602, 2019. doi: 10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhusen JL, Ray E, Sharps P, Bullock L. Intimate partner violence during pregnancy: maternal and neonatal outcomes. J Womens Health (Larchmt) 24: 100–106, 2015. doi: 10.1089/jwh.2014.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amirchaghmaghi E, Taghavi SA, Shapouri F, Saeidi S, Rezaei A, Aflatoonian R. The role of Toll-like receptors in pregnancy. Int J Fertil Steril 7: 147–154, 2013. [PMC free article] [PubMed] [Google Scholar]
- 6.Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 23: 177–180, 2020. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 323: 2198–2200, 2020. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayefsky MJ, Bartz D, Watson KL. Abortion during the Covid-19 pandemic - ensuring access to an essential health service. N Engl J Med 382: e47, 2020. doi: 10.1056/NEJMp2008006. [DOI] [PubMed] [Google Scholar]
- 9.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blitz MJ, Grünebaum A, Tekbali A, Bornstein E, Rochelson B, Nimaroff M, Chervenak FA. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol 223: 290–291, 2020. doi: 10.1016/j.ajog.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med 39: 263–272, 2010. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Bourne T, Kyriacou C, Coomarasamy A, Al-Memar M, Leonardi M, Kirk E, Landolfo C, Blanchette-Porter M, Small R, Condous G, Timmerman D. ISUOG Consensus Statement on rationalization of early-pregnancy care and provision of ultrasonography in context of SARS-CoV-2. Ultrasound Obstet Gynecol 55: 871–878, 2020. doi: 10.1002/uog.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs 29: 2047–2049, 2020. doi: 10.1111/jocn.15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandstadter JD, Yang Y. Natural killer cell responses to viral infection. J Innate Immun 3: 274–279, 2011. doi: 10.1159/000324176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, Ring L, Landau R, Purisch S, Friedman AM, Fuchs K, Sutton D, Andrikopoulou M, Rupley D, Sheen J-J, Aubey J, Zork N, Moroz L, Mourad M, Wapner R, Simpson LL, D’Alton ME, Goffman D. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2: 100118, 2020. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395: 912–920, 2020. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buekens P, Alger J, Bréart G, Cafferata ML, Harville E, Tomasso G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob Health 8: e877–e878, 2020. doi: 10.1016/S2214-109X(20)30206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 96: 1509–1565, 2016. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366: l2381, 2019. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 20.Canetta SE, Bao Y, Co MDT, Ennis FA, Cruz J, Terajima M, Shen L, Kellendonk C, Schaefer CA, Brown AS. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry 171: 557–563, 2014. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) (Online). https://www.ncbi.nlm.nih.gov/pubmed/32150360 [27 May 2020]. [PubMed]
- 22.Centers for Disease Control and Prevention Pregnancy, Breastfeeding, or Caring for Newborns (Online). https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Fpregnant-women-faq.html [14 May 2020].
- 23.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395: 809–815, 2020. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth 67: 655–663, 2020. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, Nie X, Huang BX. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases]. Zhonghua Bing Li Xue Za Zhi 49: 418–423, 2020. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 92: 1556–1561, 2020. doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, Sridhar S, Chan JFW, Ho PL, Yuen KY. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol 41: 493–498, 2020. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Sutjipto S, Lee PH, Son TT, Young BE, Milton DK, Gray GC, Schuster S, Barkham T, De PP, Vasoo S, Chan M, Ang BSP, Tan BH, Leo YS, Ng O-T, Wong MSY, Marimuthu K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun 11: 2800, 2020. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chibueze EC, Tirado V, Lopes KD, Balogun OO, Takemoto Y, Swa T, Dagvadorj A, Nagata C, Morisaki N, Menendez C, Ota E, Mori R, Oladapo OT. Zika virus infection in pregnancy: a systematic review of disease course and complications. Reprod Health 14: 28, 2017. doi: 10.1186/s12978-017-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol 14: 707–715, 2016. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 31.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol 130: 366–373, 2017. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daoud N, O’Campo P, Minh A, Urquia ML, Dzakpasu S, Heaman M, Kaczorowski J, Levitt C, Smylie J, Chalmers B. Patterns of social inequalities across pregnancy and birth outcomes: a comparison of individual and neighborhood socioeconomic measures. BMC Pregnancy Childbirth 14: 393, 2015. doi: 10.1186/s12884-014-0393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V, D’Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2: 100107, 2020. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Renzo GC, Giardina I. Coronavirus disease 2019 in pregnancy: consider thromboembolic disorders and thromboprophylaxis. Am J Obstet Gynecol 223: 135, 2020. doi: 10.1016/j.ajog.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 323: 1846–1848, 2020. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorélien A. The effects of in utero exposure to influenza on birth and infant outcomes in the US. Popul Dev Rev 45: 489–523, 2019. doi: 10.1111/padr.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Druckmann R, Druckmann M-A. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol 97: 389–396, 2005. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, Nahabedian J, Anderson K, Gilboa SM. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 69: 769–775, 2020. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M, Momeniha F, Mokamel A, Hassanvand MS, MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ 725: 138401, 2020. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrazzi E, Frigerio L, Savasi V, Vergani P, Prefumo F, Barresi S, Bianchi S, Ciriello E, Facchinetti F, Gervasi MT, Iurlaro E, Kustermann A, Mangili G, Mosca F, Patanè L, Spazzini D, Spinillo A, Trojano G, Vignali M, Villa A, Zuccotti GV, Parazzini F, Cetin I. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG 127: 1116–1121, 2020. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med 33, Suppl: S390–S397, 2005. doi: 10.1097/01.CCM.0000182483.24836.66. [DOI] [PubMed] [Google Scholar]
- 42.Govind A, Essien S, Karthikeyan A, Fakokunde A, Janga D, Yoong W, Nakhosteen A. Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol 251: 272–274, 2020. doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groß R, Conzelmann C, Müller JA, Stenger S, Steinhart K, Kirchhoff F, Münch J. Detection of SARS-CoV-2 in human breastmilk. Lancet 395: 1757–1758, 2020. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen K-Y, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Z-D, Wang Z-Y, Zhang S-F, Li X, Li L, Li C, Cui Y, Fu R-B, Dong Y-Z, Chi X-Y, Zhang M-Y, Liu K, Cao C, Liu B, Zhang K, Gao Y-W, Lu B, Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 26: 1583–1591, 2020. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall KS, Samari G, Garbers S, Casey SE, Diallo DD, Orcutt M, Moresky RT, Martinez ME, McGovern T. Centring sexual and reproductive health and justice in the global COVID-19 response. Lancet 395: 1175–1177, 2020. doi: 10.1016/S0140-6736(20)30801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol 10: 1097–1107, 2017. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 48.Hall OJ, Nachbagauer R, Vermillion MS, Fink AL, Phuong V, Krammer F, Klein SL. Progesterone-based contraceptives reduce adaptive immune responses and protection against sequential influenza a virus infections. J Virol 91: e02160-16, 2017. doi: 10.1128/JVI.02160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedermann G, Hedley PL, Baekvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, Breindahl M, Melbye M, Hougaard D, Christiansen M, Lausten-Thomsen U. Changes in premature birth rates during the Danish nationwide COVID-19 lockdown: a nationwide register-based prevalence proportion study (Preprint). MedRxiv 20109793, 2020. doi: 10.1101/2020.05.22.20109793. [DOI]
- 50.Heller KN, Gurer C, Münz C. Virus-specific CD4+ T cells: ready for direct attack. J Exp Med 203: 805–808, 2006. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, Yasumoto Y, Vogels CB, Casanovas-Massana A, Vijayakumar P, Geng B, Odio CD, Fournier J, Brito AF, Fauver JR, Liu F, Alpert T, Tal R, Szigeti-Buck K, Perincheri S, Larsen C, Gariepy AM, Aguilar G, Fardelmann KL, Harigopal M, Taylor HS, Pettker CM, Wyllie AL, Cruz CD, Ring AM, Grubaugh ND, Ko AI, Horvath TL, Iwasaki A, Reddy UM, Lipkind HS. SARS-CoV-2 infection of the placenta. J Clin Invest 130: 4947–4953, 2020. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Hu X, Gao J, Luo X, Feng L, Liu W, Chen J, Benachi A, De Luca D, Chen L. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers With coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol 136: 65–67, 2020. doi: 10.1097/AOG.0000000000003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaimes JA, Millet JK, Whittaker GR. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience 23: 101212, 2020. doi: 10.1016/j.isci.2020.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ; Novel Influenza A (H1N1) Pregnancy Working Group . H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 374: 451–458, 2009. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 55.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 12: 1638–1643, 2006. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji H-L, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100: 1065–1075, 2020. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John E, Jorge M, Jonathan W, Vicki L, Smith R. Mechanisms of maternal immune tolerance during pregnancy In: Recent Advances in Research on the Human Placenta, edited by Zhang J. London: InTech, 2012. [Google Scholar]
- 58.Juusela A, Nazir M, Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM 2: 100113, 2020. doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update 22: 240–259, 2016. doi: 10.1093/humupd/dmv047. [DOI] [PubMed] [Google Scholar]
- 60.Khalil A, Hill R, Ladhani S, Pattisson K, O’Brien P. SARS-CoV-2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol 0: 2020. doi: 10.1016/j.ajog.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan S, Jun L, Nawsherwan, Siddique R, Li Y, Han G, Xue M, Nabi G, Liu J. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect 26: 788–790, 2020. doi: 10.1016/j.cmi.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 16: 626–638, 2016. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 64.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O’Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 369: m2107, 2020. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kreis N-N, Ritter A, Louwen F, Yuan J. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells 9: 1777, 2020. doi: 10.3390/cells9081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau LS, Samari G, Moresky RT, Casey SE, Kachur SP, Roberts LF, Zard M. COVID-19 in humanitarian settings and lessons learned from past epidemics. Nat Med 26: 647–648, 2020. doi: 10.1038/s41591-020-0851-2. [DOI] [PubMed] [Google Scholar]
- 68.Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 15: e0230295, 2020. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, Yue L, Li Q, Sun G, Chen L, Yang L. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin Infect Dis ciaa352, 2020. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382: 1199–1207, 2020. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Sun X, Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab 30: 414–433, 2019. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet 150: 53–57, 2020. doi: 10.1002/ijgo.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, Zheng C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol 215: 127–132, 2020. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 74.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 80: e7–e13, 2020. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H, Wang L-L, Zhao S-J, Kwak-Kim J, Mor G, Liao A-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol 139: 103122, 2020. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect S0163-4453(20)30109-2, 2020. doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lokken EM, Walker CL, Delaney S, Kachikis A, Kretzer NM, Erickson A, Resnick R, Vanderhoeven J, Hwang JK, Barnhart N, Rah J, McCartney SA, Ma KK, Huebner EM, Thomas C, Sheng JS, Paek BW, Retzlaff K, Kline CR, Munson J, Blain M, LaCourse SM, Deutsch G, Adams Waldorf KM. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol S0002-9378(20)30558-5, 2020. doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Machón RA, Mednick SA, Huttunen MO. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 54: 322–328, 1997. doi: 10.1001/archpsyc.1997.01830160040006. [DOI] [PubMed] [Google Scholar]
- 79.Marín Gabriel MA, Cuadrado I, Álvarez Fernández B, González Carrasco E, Alonso Díaz C, Llana Martín I, Sánchez L, Olivas C, Heras S, Criado E; Neo-COVID-19 Research Group . Multicentre Spanish study found no incidences of viral transmission in infants born to mothers with COVID-19. Acta Paediatr apa.15474, 2020. doi: 10.1111/apa.15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marmot M, Allen J, Boyce T, Goldblatt P, Morrison J.. Marmot Review 10 Years On (Online). http://www.instituteofhealthequity.org/resources-reports/marmot-review-10-years-on [19 May 2020].
- 81.MBRRACE-UK Lessons learning to inform maternity care from the UK and Ireland Confidential ENquiries into Maternal Deaths and Morbidity 2015–17 (Online). https://www.npeu.ox.ac.uk/mbrrace-uk/reports [18 May 2020].
- 82.McNeil SA, Dodds LA, Fell DB, Allen VM, Halperin BA, Steinhoff MC, MacDonald NE. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol 204, Suppl 1: S54–S57, 2011. doi: 10.1016/j.ajog.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 83.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 45: 189–192, 1988. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 84.Yang M, Yang L, Wang X, Wang Y, Wei Y, Zhao Y. Decline of plasmacytoid dendritic cells and their subsets in normal pregnancy are related with hormones [Online]. [27 May 2020]. J Reprod Med 60: 423–429, 2015. https://pubmed.ncbi.nlm.nih.gov/26592069/ [PubMed] [Google Scholar]
- 85.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet 387: 999–1011, 2016. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 86.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 205: 10–18, 2011. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 88.Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol 55: 586–592, 2020. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- 89.Nair M, Kurinczuk JJ, Brocklehurst P, Sellers S, Lewis G, Knight M. Factors associated with maternal death from direct pregnancy complications: a UK national case-control study. BJOG 122: 653–662, 2015. doi: 10.1111/1471-0528.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navti O, Hughes BL, Tang JW, Konje J. Comprehensive review and update of cytomegalovirus infection in pregnancy. Obstet Gynaecol 18: 301–307, 2016. doi: 10.1111/tog.12309. [DOI] [Google Scholar]
- 91.Ness T. Tranexamic Acid (TXA) and corona virus 2019 (COVID19) in inpatients (Online). https://clinicaltrials.gov/ct2/show/NCT04338126 [3 August 2020].
- 92.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 53: 66–70, 2020. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niquini RP, Lana RM, Pacheco AG, Cruz OG, Coelho FC, Carvalho LM, Villela DAM, Gomes MFDC, Bastos LS. SRAG por COVID-19 no Brasil: descrição e comparação de características demográficas e comorbidades com SRAG por influenza e com a população geral. Cad Saude Publica 36: e00149420, 2020. doi: 10.1590/0102-311x00149420. [DOI] [PubMed] [Google Scholar]
- 94.Oncel MY, Akın IM, Kanburoglu MK, Tayman C, Coskun S, Narter F, Er I, Oncan TG, Memisoglu A, Cetinkaya M, Oguz D, Erdeve O, Koc E; Neo-Covid Study Group . A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur J Pediatr 2020. doi: 10.1007/s00431-020-03767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pal A, Rao R. Intimate Partner Violence During Pregnancy In: Labour Room Emergencies, edited by Sharma A. Singapore: Springer Nature, 2020, p. 515–520. doi: 10.1007/978-981-10-4953-8_52. [DOI] [Google Scholar]
- 96.Pavličev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, Kallapur SG, Muglia L, Jones H. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res 27: 349–361, 2017. doi: 10.1101/gr.207597.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, Meyer JA, Roman AS. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM 2: 100133, 2020. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perrone S, Deolmi M, Giordano M, D’Alvano T, Gambini L, Corradi M, Frusca T, Ghi T, Esposito S. Report of a series of healthy term newborns from convalescent mothers with COVID-19. Acta Biomed 91: 251–255, 2020. doi: 10.23750/ABM.V91I2.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, O’Connell NH, Dunne CP. Reduction in preterm births during the COVID-19 lockdown in Ireland: a natural experiment allowing analysis of data from the prior two decades (Preprint). MedRxiv 20121442, 2020. doi: 10.1101/2020.06.03.20121442. [DOI] [PMC free article] [PubMed]
- 100.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res 15: 141–150, 1996. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 101.Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M; UKOSS . Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 342, jun14 2: d3214, 2011. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, Leng Y, Hsu CD, Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 9: e58716, 2020. doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poon LC, Yang H, Kapur A, Melamed N, Dao B, Divakar H, McIntyre HD, Kihara AB, Ayres-de-Campos D, Ferrazzi EM, Di Renzo GC, Hod M. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynaecol Obstet 149: 273–286, 2020. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens 9: 231, 2020. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N Engl J Med NEJMoa2021436, 2020. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity 50: 37–50, 2019. doi: 10.1016/j.immuni.2018.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low- and middle-income countries. Int Perspect Sex Reprod Health 46: 73–76, 2020. doi: 10.1363/46e9020. [DOI] [PubMed] [Google Scholar]
- 108.Roberton T, Carter ED, Chou VB, Stegmuller AR, Jackson BD, Tam Y, Sawadogo-Lewis T, Walker N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health 8: e901–e908, 2020. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Royal College for Obstetrics and Gynaecology Guidance for antenatal and postnatal services in the evolving coronavirus (COVID-19) pandemic (Online). https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-10-guidance-for-antenatal-and-postnatal.pdf.
- 110.Saad AF, Chappell L, Saade GR, Pacheco LD. Corticosteroids in the management of pregnant patients with coronavirus disease (COVID-19). Obstet Gynecol 136: 823–826, 2020. doi: 10.1097/AOG.0000000000004103. [DOI] [PubMed] [Google Scholar]
- 111.Santarpia JL, Rivera DN, Herrera V, Morwitzer MJ, Creager H, Santarpia GW, Crown KK, Brett-Major DM, Schnaubelt E, Jana Broadhurst MJ, Lawler JV, Reid SP, Lowe JJ. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center (Preprint). MedRxiv 20039446, 2020. doi: 10.1101/2020.03.23.20039446. [DOI]
- 112.Shanes ED. Placental pathology in COVID-19 (Preprint). MedRxiv 20093229, 2020. doi: 10.1101/2020.05.08.20093229. [DOI]
- 113.Shang J, Du R, Lu Q, Wu J, Xu S, Ke Z, Cai Z, Gu Y, Huang Q, Zhan Y, Yang J, Liu Y, Hu Y, Zhang H, Huang H, Xie Z, Li X, Hu W, Gong J, Ke W, Shao Z, Liu Z, Xie J. The treatment and outcomes of patients with COVID-19 in Hubei, China: a multi-centered, retrospective, observational study. Lancet 2020. doi: 10.2139/ssrn.3546060. [DOI] [Google Scholar]
- 114.Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. Progesterone and maintenance of pregnancy: is progesterone nature’s immunosuppressant? Ann N Y Acad Sci 286, 1 Biochemical A: 384–397, 1977. doi: 10.1111/j.1749-6632.1977.tb29431.x. [DOI] [PubMed] [Google Scholar]
- 115.Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol 73: 199–213, 2015. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sochas L, Channon AA, Nam S. Counting indirect crisis-related deaths in the context of a low-resilience health system: the case of maternal and neonatal health during the Ebola epidemic in Sierra Leone. Health Policy Plan 32, Suppl 3: iii32–iii39, 2017. doi: 10.1093/heapol/czx108. [DOI] [PubMed] [Google Scholar]
- 117.Staton B. Effects of pandemic will widen inequality, report finds (Online). https://www.ft.com/content/5e6330de-1e95-4343-8424-184d19dc34b9 [19 May 2020].
- 118.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 382: 2163–2164, 2020. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takemoto MLS, Menezes MO, Andreucci CB, Nakamura-Pereira M, Amorim MMR, Katz L, Knobel R. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int J Gynaecol Obstet ijgo.13300, 2020. doi: 10.1002/ijgo.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanacan A, Erol SA, Turgay B, Anuk AT, Secen EI, Yegin GF, Ozyer S, Kirca F, Dinc B, Unlu S, Yapar Eyi EG, Keskin HL, Sahin D, Surel AA, Tekin OM. The rate of SARS-CoV-2 positivity in asymptomatic pregnant women admitted to hospital for delivery: Experience of a pandemic center in Turkey. Eur J Obstet Gynecol Reprod Biol 253: 31–34, 2020. doi: 10.1016/j.ejogrb.2020.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tekbali A, Grünebaum A, Saraya A, McCullough L, Bornstein E, Chervenak FA. Pregnant vs nonpregnant severe acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 hospital admissions: the first 4 weeks in New York. Am J Obstet Gynecol 223: 126–127, 2020. doi: 10.1016/j.ajog.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 20: 389–391, 2020. doi: 10.1038/s41577-020-0343-0. A correction for this article is available at https://doi.org/10.1038/s41577-020-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.The Royal College of Obstetricians and Gynaecologists Information for healthcare professionals coronavirus (COVID-19) infection and abortion care (Online). https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-abortion/ 2020.
- 125.UNFPA COVID-19: A Gender Lens | UNFPA - United Nations Population Fund (Online). https://www.unfpa.org/resources/covid-19-gender-lens [17 May 2020].
- 126.UNFPA Impact of the COVID-19 Pandemic on Family Planning and Ending Gender-based Violence, Female Genital Mutilation and Child Marriage | UNFPA - United Nations Population Fund (Online). https://www.unfpa.org/resources/impact-covid-19-pandemic-family-planning-and-ending-gender-based-violence-female-genital [15 May 2020].
- 127.UNFPA UNFPA Supplies COVID-19 update - 16 April 2020 | UNFPA - United Nations Population Fund (Online). https://www.unfpa.org/resources/unfpa-supplies-covid-19-update-16-april-2020 [15 May 2020].
- 128.Vanders RL, Gibson PG, Murphy VE, Wark PAB. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection [Online]. [27 May 2020]. J Infect Dis 208: 1062–1070, 2013. doi: 10.1093/infdis/jit296. https://pubmed.ncbi.nlm.nih.gov/23861550/ [DOI] [PubMed] [Google Scholar]
- 129.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update 9: 347–357, 2003. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 131.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polański K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563: 347–353, 2018. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 11: 3572, 2020. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weatherbee BAT, Glover DM, Zernicka-Goetz M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol 10: 200162, 2020. doi: 10.1098/rsob.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wenham C, Smith J, Morgan R; Gender and COVID-19 Working Group . COVID-19: the gendered impacts of the outbreak. Lancet 395: 846–848, 2020. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Whitehead CL, Walker SP. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet 395: e92, 2020. doi: 10.1016/S0140-6736(20)31029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324: 259–269, 2020. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.WHO HIV/AIDS (Online). https://www.who.int/news-room/fact-sheets/detail/hiv-aids [16 May 2020].
- 138.WHO Breastfeeding and COVID-19 for healthcare workers (Online). www.who.int/publications-detail/clinical-management-of-severe-acute-.
- 139.WHO WHO Director-General’s opening remarks at the media briefing on COVID-19 (Online). https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---13-april-2020 [14 May 2020].
- 140.New WHO estimates: Up to 190 000 people could die of COVID-19 in Africa if not controlled WHO Regional Office for Africa (Online). https://www.afro.who.int/news/new-who-estimates-190-000-people-could-die-covid-19-africa-if-not-controlled [8 November 2020].
- 141.WHO WHO/Europe. Coronavirus disease (COVID-19) outbreak - WHO announces COVID-19 outbreak a pandemic (Online). https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic [8 November 2020].
- 142.Wong SCY, Kwong RTS, Wu TC, Chan JWM, Chu MY, Lee SY, Wong HY, Lung DC. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 105: 119–127, 2020. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PWY, Ho LC, To WWK, Lai ST, Yan WW, Tan PYH. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 191: 292–297, 2004. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu C, Yang W, Wu X, Zhang T, Zhao Y, Ren W, Xia J. Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women. Virol Sin 35: 305–310, 2020. doi: 10.1007/s12250-020-00227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet 150: 58–63, 2020. doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 147.Yang H, Sun G, Tang F, Peng M, Gao Y, Peng J, Xie H, Zhao Y, Jin Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect 81: e40–e44, 2020. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Young BC, Stanic AK, Panda B, Rueda BR, Panda A. Longitudinal expression of Toll-like receptors on dendritic cells in uncomplicated pregnancy and postpartum. Am J Obstet Gynecol 210: 445.e1–445.e6, 2014. doi: 10.1016/j.ajog.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, Liu Y, Xiao J, Liu H, Deng D, Chen S, Zeng W, Feng L, Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 20: 559–564, 2020. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn pd.5713, 2020. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 323: 1848–1849, 2020. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, Tian JH, Dong L, Hu RH. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province]. Zhonghua Fu Chan Ke Za Zhi 55: 166–171, 2020. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 153.Zheng Q-L, Duan T, Jin L-P. Single-cell RNA expression profiling of ACE2 and AXL in the human maternal–Fetal interface. Reprod Dev Med 4: 7, 2020. doi: 10.4103/2096-2924.278679. [DOI] [Google Scholar]
- 154.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 7: 998–1002, 2020. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 9: 51–60, 2020. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]


