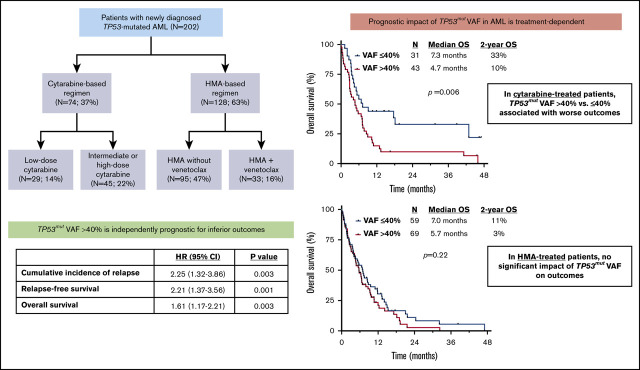
Key Points
TP53mut VAF >40% was independently associated with higher rates of relapse and worse relapse-free survival and OS.
Treatment with a cytarabine-based regimen preferentially benefited patients with 1 TP53 mutation with VAF ≤40%.
Abstract
TP53 mutations are associated with poor outcomes in acute myeloid leukemia (AML). The prognostic impact of mutant TP53 (TP53mut) variant allelic frequency (VAF) is not well established, nor is how this information might guide optimal frontline therapy. We retrospectively analyzed 202 patients with newly diagnosed TP53-mutated AML who underwent first-line therapy with either a cytarabine- or hypomethylating agent (HMA)–based regimen. By multivariate analysis, TP53mut VAF >40% was independently associated with a significantly higher cumulative incidence of relapse (P = .003) and worse relapse-free survival (P = .001) and overall survival (OS; P = .003). The impact of TP53mut VAF on clinical outcomes was driven by patients treated with a cytarabine-based regimen (median OS, 4.7 vs 7.3 months for VAF >40% vs ≤40%; P = .006), whereas VAF did not significantly affect OS in patients treated with HMA. The addition of venetoclax to HMA did not significantly affect OS compared with HMA without venetoclax, both in the entire TP53-mutated population and in patients stratified by TP53mut VAF. Among patients with TP53mut VAF ≤40%, OS was superior in those treated with higher-dose cytarabine, whereas OS was similarly poor for patients with TP53mut VAF >40% regardless of therapy. The best long-term outcomes were observed in those with 1 TP53 mutation with VAF ≤40% who received a frontline cytarabine-based regimen (2-year OS, 38% vs 6% for all others; P < .001). In summary, TP53mut VAF provides important prognostic information that may be considered when selecting frontline therapy for patients with newly diagnosed TP53-mutated AML.
Visual Abstract
Introduction
Acute myeloid leukemia (AML) is a heterogeneous myeloid neoplasm with a widely variable prognosis that is strongly influenced by karyotypic and molecular alterations.1 Several of these recurrent cytomolecular abnormalities have been incorporated into consensus risk stratification guidelines that may inform decisions for optimal postremission strategies, including consideration of allogeneic hematopoietic stem cell transplantation (HSCT) in first remission.2 Mutations of the TP53 gene have been described in up to 20% of patients with newly diagnosed AML; they are enriched in patients with prior exposure to cytotoxic chemotherapy or radiation therapy and have a strong association with the presence of a complex karyotype.3-5 Compared with TP53 wild-type (WT) disease, TP53-mutated AML has consistently been shown to be associated with a lower likelihood of response to conventional chemotherapy and dismal outcomes, with a median overall survival (OS) of 4 to 6 months and a 2-year OS rate <10%.3-7 HSCT in first remission is often recommended for patients who respond to frontline therapy, although outcomes remain poor even when HSCT is performed, because the rate of post-HSCT relapse is high.8
Similar to TP53, FLT3–internal tandem duplication mutations are associated with poor prognosis in AML.1 A higher FLT3–internal tandem duplication allelic ratio has been shown to confer worse outcomes in patients with FLT3-mutated AML, and this information is now routinely used for risk stratification and postremission decision making.9-11 However, it is largely unknown whether assessment of mutant TP53 (TP53mut) variant allelic frequency (VAF) likewise provides additional prognostic information in patients with TP53-mutated AML. In TP53-mutated myelodysplastic syndrome (MDS), a higher TP53mut VAF has been reported to be associated with worse survival, particularly in the presence of a complex karyotype.12 In contrast, previous retrospective studies in AML have yielded conflicting data as to whether higher TP53mut VAF is predictive for clinical outcomes.6,13,14 Notably, these reports were not designed to assess whether the impact of TP53mut VAF on outcomes might be treatment dependent. To better understand whether assessment of TP53mut VAF might have prognostic or therapeutic utility, we performed a retrospective study of patients with TP53-mutated AML receiving frontline therapy and evaluated the impact of TP53mut VAF on clinical outcomes and its interaction with type of therapy received. The goal of this analysis was to establish whether determination of TP53mut VAF might rationally inform the selection of optimal frontline therapy for patients with TP53-mutated AML.
Methods
Study design and participants
This was a retrospective study evaluating the prognostic impact of TP53 mutation characteristics in adults with newly diagnosed AML receiving frontline therapy. Patients with previously treated secondary AML (eg, AML arising from previously treated MDS) were excluded because of the distinctly poor outcomes of these patients, as previously described.15 Treatments were divided into 4 categories for analysis: (1) intermediate- (IDAC) or high-dose cytarabine (HDAC) based, (2) low-dose cytarabine (LDAC) based, (3) hypomethylating agent (HMA) with or without a second investigational agent (excluding venetoclax), and (4) HMA plus venetoclax. Groups 1 and 2 (the cytarabine group) and groups 3 and 4 (the HMA group) were combined for some analyses. This study was conducted at a single academic center (The University of Texas MD Anderson Cancer Center), approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center, and conducted in accordance with the Declaration of Helsinki.
TP53 mutation assessment
Mutation analysis was performed on bone marrow specimens using a 28-, 53-, or 81-gene targeted next-generation sequencing (NGS) panel as previously described.16,17 Genomic DNA was extracted from bone marrow aspirates. A minimum sequencing coverage of 250X (bidirectional true paired-end sequencing) and minimum input of 250 ng of DNA were required. The analytical sensitivity was established at 5% mutant reads against a background of WT reads. Established bioinformatic pipelines were used to identify somatic variants. In cases of multiple TP53 mutations, the highest TP53mut VAF was used for analysis.
Assessment of TP53 loss by conventional cytogenetics
To evaluate the presence of TP53 locus deletion, all patients had conventional karyotyping performed. Loss of TP53 was determined as previously described12 and included any of the following cytogenetic abnormalities: monosomy 17; isochromosome i(17)(q10); del(17)(pvar[variable]) with pvar centromeric to p13.1; unbalanced translocations, including der(var)t(var;17)(var;qvar),−17; der(var)t(var;17)(var;pvar),−17 with pvar centromeric to p13.1; der(17)t(17;var)(pvar;var)der(17)t(var;17)(var;pvar) with pvar centromeric to p13.1; der(var)t(var;17)(var;qvar) with dicentric der; der(var)t(var;17)(var;pvar) with pvar centromeric to p13.1 and dicentric der; balanced translocation and 17p13 breakpoint: t(17;var)(p13;var) or t(var;17)(var;p13) in the presence of TP53 deletion by fluorescence in situ hybridization (FISH); additive material: add(17)(pvar) in the presence of TP53 deletion by FISH; dicentric chromosome dic(var;17)(var;pvar); and ring chromosome r(17)(pvarqvar) with the presence of TP53 deletion by FISH.
Response and outcome definitions
Responses were defined according to European LeukemiaNet consensus guidelines.2 For purposes of response-based analyses, patients who achieved either complete remission (CR) or CR with incomplete hematological recovery (CRi) were considered responders, and all others were considered nonresponders. Cumulative incidence of relapse (CIR) was calculated from the time of CR/CRi until relapse, censored for death in morphological remission or if the patient was alive at last follow-up. Relapse-free survival (RFS) was calculated from the time of CR/CRi until relapse or death resulting from any cause, censored if the patient was alive at last follow-up. OS was calculated from the time of treatment initiation until death resulting from any cause, censored if the patient was alive at last follow-up. Survival estimates were not censored at the time of HSCT.
Statistical methods
Patient characteristics were summarized using median (range) for continuous variables and frequencies (percentages) for categorical variables. To compare 2 groups, Fisher’s exact test was performed for categorical variables, and the Wilcoxon rank-sum test was performed for continuous variables. A univariate recursive partitioning analysis was implemented to identify an optimal cutoff for TP53mut VAF on OS, where the minimum number of patients in any subgroup was set to 20. Univariate Cox proportional hazards models were used to evaluate the risk factors associated with survival outcomes. A multivariate proportional hazards model was obtained by first including the factors with P < .20 on univariate analysis and then finalizing via backward elimination until all remaining factors had P < .05. Statistical analyses were conducted in R (version 3.5.1).
Results
Patient characteristics and study cohort
Between October 2012 and April 2019, we identified 202 patients with TP53-mutated AML who received frontline therapy at our institution. Baseline characteristics of the study population are listed in Table 1. The median age was 70 years (range, 20-90 years). One hundred and sixty-six patients (83%) had complex cytogenetics, and 17 (9%) had a normal karyotype. Ninety-three patients (47%) had evidence of TP53 loss by conventional karyotype. The median TP53mut VAF was 43% (range, 1% to 100%). Forty-eight patients (24%) had >1 TP53 mutation detected (2 mutations, n = 46; 3 mutations, n = 2). First-line therapies received were IDAC/HDAC in 45 patients (22%), LDAC in 29 patients (14%), HMA without venetoclax in 95 patients (47%), and HMA with venetoclax in 33 patients (16%).
Table 1.
Patient characteristics (N = 202)
| Characteristic | n/N (%) |
|---|---|
| Age, y | |
| Median | 70 |
| Range | 20-90 |
| WBC, × 109/L | |
| Median | 3 |
| Range | 1-77 |
| Platelets, × 109/L | |
| Median | 32 |
| Range | 2-321 |
| BM blasts, % | |
| Median | 32 |
| Range | 3-97 |
| t-AML | 52 (26) |
| s-AML | 30 (15) |
| Cytogenetics | |
| Complex | 166 (83) |
| Diploid | 17 (9) |
| Nondiploid, noncomplex | 17 (9) |
| Insufficient metaphases/not done | 2 (1) |
| No. of TP53 mutations | |
| 1 | 154 (76) |
| >1 | 48 (24) |
| TP53mut VAF, % | |
| Median | 43 |
| Range | 1-100 |
| TP53mut VAF, % | |
| ≤40 | 90 (45) |
| >40 | 112 (56) |
| TP53 loss | 93/200 (47) |
| Regimen | |
| Cytarabine based | 74 (37) |
| HMA based | 128 (63) |
| Regimen | |
| IDAC/HDAC | 45 (22) |
| LDAC | 29 (14) |
| HMA (no venetoclax) | 95 (47) |
| HMA plus venetoclax | 33 (16) |
BM, bone marrow; s-AML, secondary AML; t-AML, therapy-related AML; WBC, white blood cell count.
Overall response rates for the entire cohort are shown in supplemental Table 1. The overall CR rate was 35%, and the composite CR/CRi rate was 47%. Twenty patients (10%) underwent HSCT in first remission. Among patients who achieved CR/CRi, the rate of HSCT was 21%, and among patients ≤70 years of age who achieved CR/CRi, it was 43%. The median duration of follow-up of the entire cohort was 38.4 months. The median duration of response was 7.0 months, and the 1- and 2-year CIR rates were 65% and 75%, respectively. The median RFS was 5.3 months, with 1- and 2-year RFS rates of 27% and 16%, respectively. The median OS was 5.8 months, with 1- and 2-year OS rates of 26% and 12%, respectively.
Overall, 88 patients (44%) received at least 1 subsequent salvage regimen. First salvage therapy was received in an investigational clinical trial for 45 patients, whereas 43 patients received a standard-of-care regimen. Among the 42 patients treated with a frontline cytarabine-based regimen, 24 received an HMA-based regimen as first salvage, 12 received further cytarabine-based chemotherapy, and 6 received another investigational therapy. Among the 46 patients treated with a frontline HMA-based regimen, 19 received a cytarabine-based regimen as first salvage, 14 received further HMA-based therapy, and 13 received another investigational therapy.
Factors associated with TP53mut VAF
Patients with diploid cytogenetics had a significantly lower TP53mut VAF than those with nondiploid cytogenetics (median VAF, 4.4% vs 44.8%, respectively; P < .001). The number of TP53 mutations was inversely associated with TP53mut VAF (median VAF, 45.8% and 38.6% for patients with 1 and >1 mutation, respectively; P = .01). Age, diagnosis of therapy-related or secondary AML, TP53 loss by cytogenetics, and type of frontline therapy were not associated with TP53mut VAF.
Factors associated with response to therapy
To assess predictors of response, patients were divided into groups according to frontline treatment received. The impact of TP53mut VAF on response rates was treatment dependent (supplemental Table 2). In the HMA group (n = 128), no baseline variables were significantly associated with likelihood of response (supplemental Table 3). In contrast, in the cytarabine group, factors associated with lower response rates by univariate analysis included older age, nondiploid cytogenetics, >1 TP53 mutation, and higher TP53mut VAF (supplemental Table 4). By multivariate analysis, independent predictors for response in the cytarabine-treated group included older age (odds ratio, 0.92; 95% confidence interval [CI], 0.87-0.98; P = .004), >1 TP53 mutation (odds ratio, 0.10; 95% CI, 0.01-0.79; P = .03), and TP53mut VAF >40% (odds ratio, 0.24; 95% CI, 0.09-0.065; P = .005).
Among cytarabine-treated patients, response rate was 48% for patients with 1 TP53 mutation vs 8% for those with >1 mutation (P = .01); response rate was 61% for patients with TP53mut VAF ≤40% vs 28% if VAF >40% (P = .01). Among patients who received an IDAC/HDAC regimen, response rate was 79% for patients with TP53mut VAF ≤40% vs 35% for those with VAF >40% (P = .007); a similar pattern was observed in patients who received an LDAC regimen, with responses of 47% and 8%, respectively (P = .03). Among patients with TP53mut VAF >40%, response rates were significantly higher in patients who received HMA-based therapy compared with those who received cytarabine-based therapy (54% and 28%, respectively; P = .008), whereas response rates were not significantly different among patients with VAF ≤40% (44% and 61%, respectively; P = .12).
Predictors for relapse and survival outcomes in the entire cohort
By univariate analyses, TP53mut VAF was associated with OS in patients who received cytarabine (P = .001) but not in those who received HMA-based therapy (P = .20). Recursive partitioning analysis identified a VAF of 40% as the optimal cutoff within the cytarabine-treated population, and this stratification (ie, TP53mut VAF >40% vs ≤40%) was used for subsequent analyses and Kaplan-Meier estimates of relapse and survival outcomes.
Univariate analyses for predictors of CIR, RFS, and OS are summarized in supplemental Table 5. TP53mut VAF >40% vs ≤40% was associated with higher CIR (hazard ratio [HR], 1.95; 95% CI, 1.16-3.28; P = .01) and shorter RFS (HR, 1.97; 95% CI, 1.24-3.14; P = .004) and OS (HR, 1.51; 95% CI, 1.11-2.05; P = .01). In the whole cohort stratified by TP53mut VAF >40% vs ≤40%, the 1-year CIR rates were 85% and 48%, the 1-year RFS rates were 10% and 44%, and the 1-year OS rates were 19% and 35%, respectively (Figure 1A-C). Table 2 summarizes the multivariate analyses for CIR, RFS, and OS, including HSCT as a time-dependent variable. TP53mut VAF retained its independent prognostic significance for all outcomes (CIR: HR, 2.25; 95% CI, 1.32-3.86; P = .003; RFS: HR, 2.21; 95% CI, 1.37-3.56; P = .001; OS: HR, 1.61; 95% CI, 1.17-2.21; P = .003). Number of TP53 mutations and TP53 loss by cytogenetics were independently prognostic for OS (P < .001 and P = .04, respectively) but not for CIR or RFS.
Figure 1.
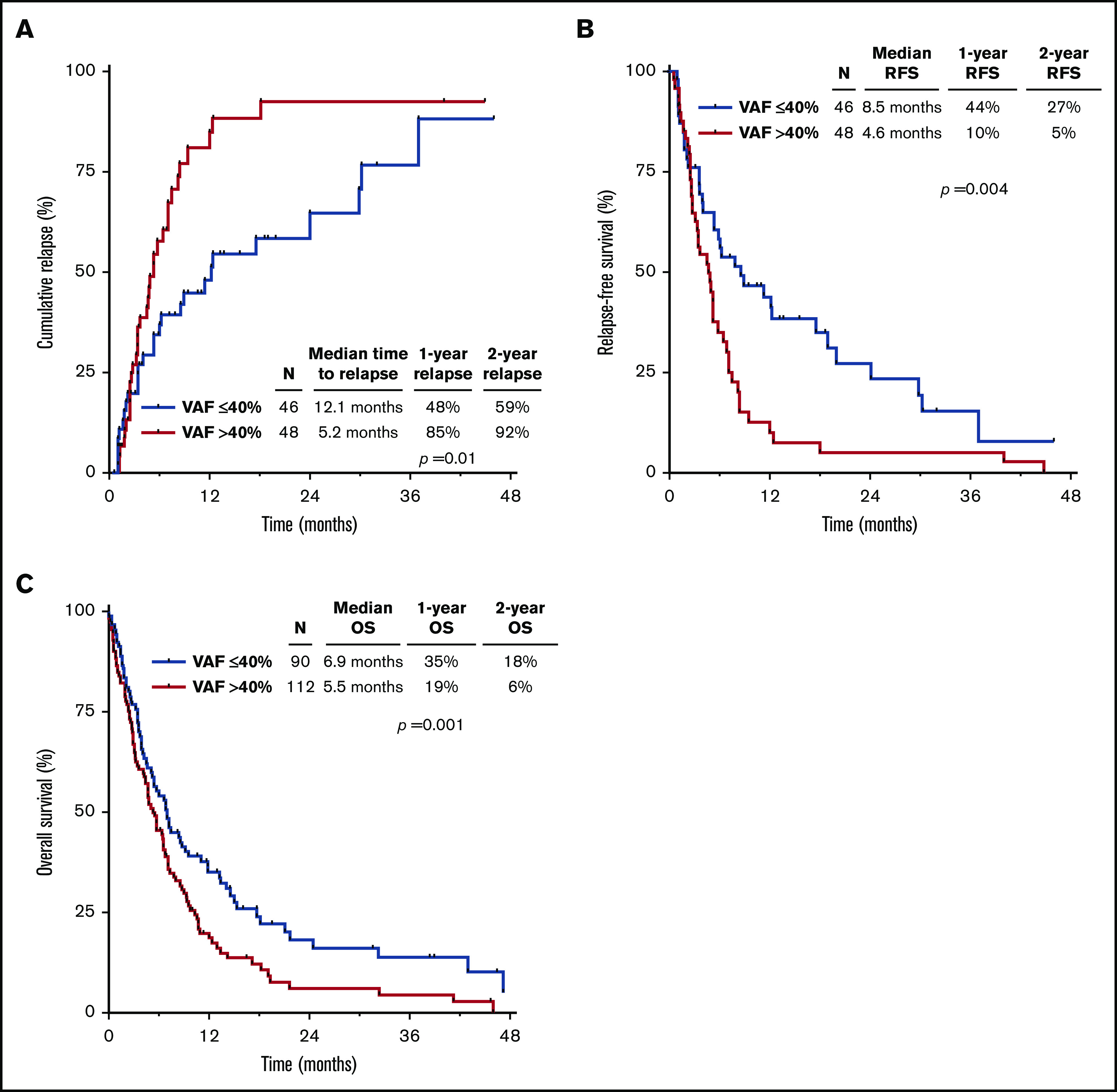
Outcomes by TP53mutVAF. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified by TP53mut VAF.
Table 2.
Multivariate analysis for CIR, RFS, and OS
| Characteristic | CIR | RFS | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Platelets, × 109/L | — | — | — | — | 0.73 (0.62-0.87) | <.001 |
| No. of TP53 mutations >1 vs 1 | — | — | — | — | 1.98 (1.36-2.87) | <.001 |
| TP53mut VAF >40% vs ≤40% | 2.25 (1.32-3.86) | .003 | 2.21 (1.37-3.56) | .001 | 1.61 (1.17-2.21) | .003 |
| TP53 loss | — | — | — | — | 1.38 (1.01-1.89) | .04 |
| Transplantation (time dependent) | 0.18 (0.08-0.44) | <.001 | 0.30 (0.15-0.57) | <.001 | 0.27 (0.15-0.49) | <.001 |
Dash (—) indicates lack of statistical significance in the multivariate model. Variables considered in the multivariate analysis included: age (years), white blood cells (× 109/L), platelets (× 109/L), bone marrow blast percentage, therapy-related AML vs non–therapy-related AML, secondary AML vs nonsecondary AML, cytogenetics (diploid vs nondiploid), n of TP53 mutations (>1 vs 1), TP53mut VAF, TP53 loss by cytogenetics, and transplantation (time dependent).
Impact of therapy on the prognostic impact of TP53mut VAF
The worse outcomes for patients with TP53mut VAF >40% were driven primarily by those who received cytarabine-based therapy. Among patients treated with a cytarabine-based regimen, those with TP53mut VAF >40% had significantly worse outcomes than those with VAF ≤40% (median OS, 4.7 vs 7.3 months, respectively; P = .006), whereas there was no significant difference in outcomes according to VAF among HMA-treated patients (median OS, 5.7 vs 7.0 months, respectively; P = .22; Figure 2A-B). Similarly, among patients who received a more intensive IDAC/HDAC-based regimen, TP53mut VAF was strongly correlated with OS. Patients with VAF ≤40% who received IDAC/HDAC had a median OS of 18.1 months and 2-year OS rate of 41%; in contrast, those with VAF >40% who received IDAC/HDAC had a median OS of only 5.0 months and 2-year OS rate of 14% (P = .02; supplemental Figure 1A). Among patients who received LDAC, a similar but nonsignificant trend was also observed when patients were stratified by TP53mut VAF (VAF ≤40%: median OS, 5.4 months; 2-year OS rate, 26% vs VAF >40%: median OS, 3.8 months; 2-year OS rate, 0%; P = .06; supplemental Figure 1B).
Figure 2.
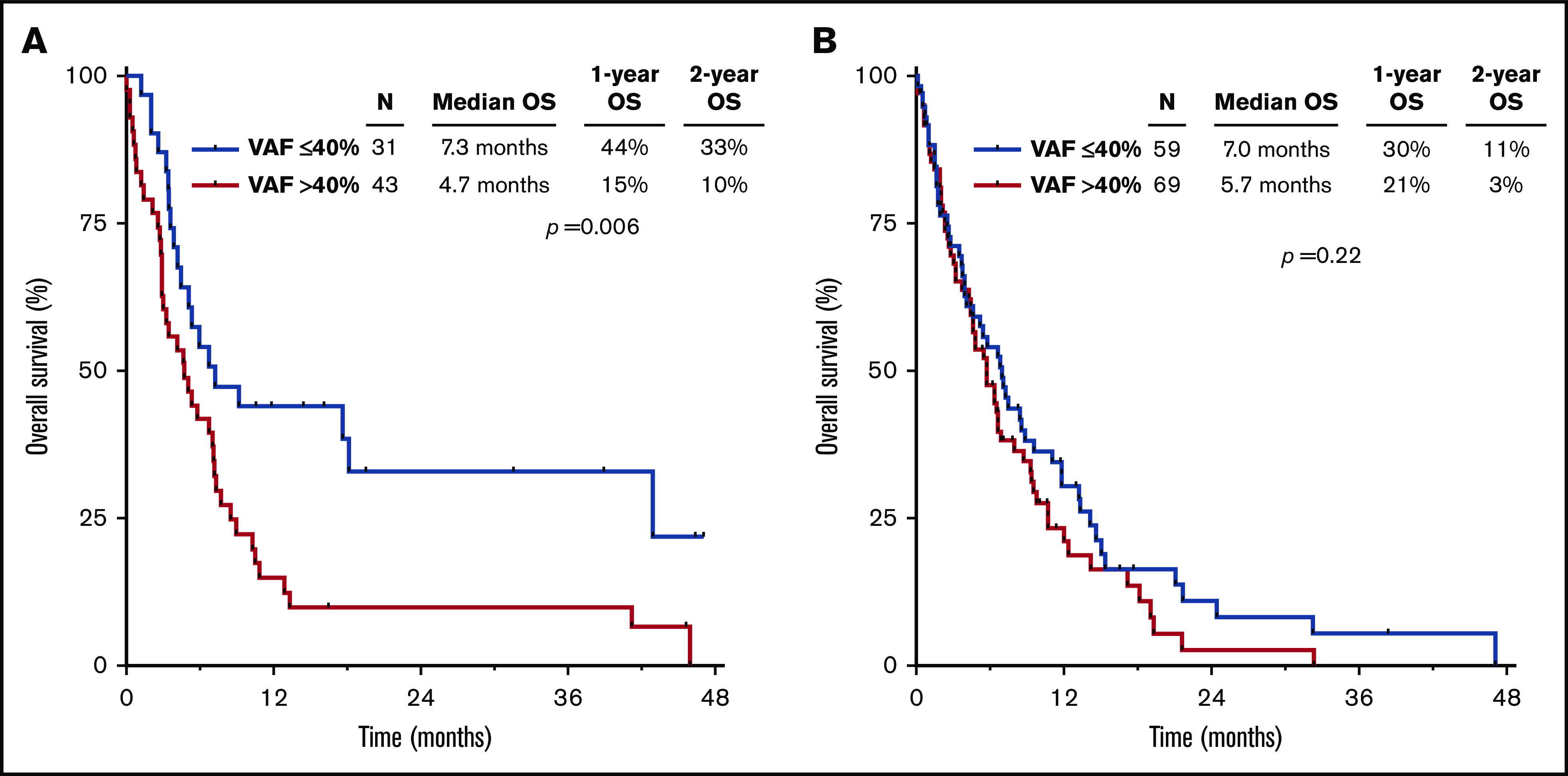
OS by therapy received, stratified by TP53mutVAF. OS for patients receiving a cytarabine-based regimen (A) or an HMA-based regimen (B), stratified by TP53mut VAF.
Among patients with TP53mut VAF ≤40%, those treated with a cytarabine-based regimen had superior OS compared with those who received an HMA-based regimen (1-year OS rates of 44% and 31%, respectively; P = .04), with the best outcomes in patients who received IDAC/HDAC-based therapy (Figure 3A). However, among those with TP53mut VAF >40%, outcomes were similar among the different treatment groups, and survival was universally poor, with 1-year OS rate <25% in all groups (Figure 3B). Patients with VAF >40% who received an LDAC-based regimen had particularly poor outcomes, with a median OS of 3.8 months, and none of these 17 patients remained alive after 1 year.
Figure 3.
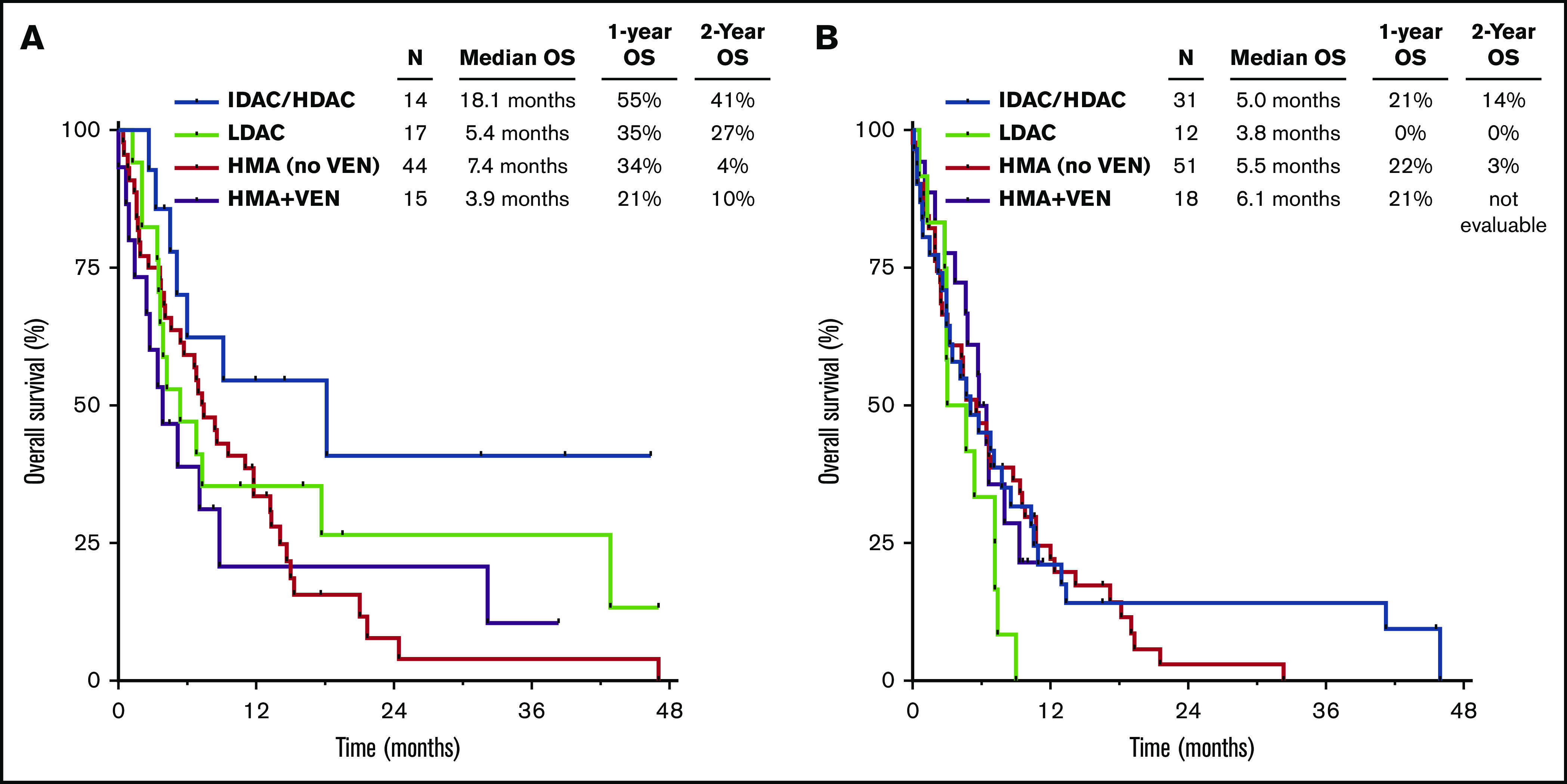
OS by therapy received. OS by regimen in patients with TP53mut VAF ≤40% (A) and >40% (B). VEN, venetoclax.
Although response rates were numerically higher in the HMA plus venetoclax group compared with those who received HMA without venetoclax (61% and 45%, respectively), the addition of venetoclax to HMA did not seem to significantly affect OS compared with HMA without venetoclax, both in the entire TP53-mutated population and when patients were stratified by TP53mut VAF (supplemental Figure 2A-B). Among those with TP53mut VAF ≤40%, the median OS durations for patients who received HMA with or without venetoclax were 3.9 and 7.4 months, respectively (P = .65). Among those with VAF >40%, the median OS durations were 6.1 and 5.5 months, respectively (P = .97).
Integration of TP53mut VAF, number of TP53 mutations, and therapy
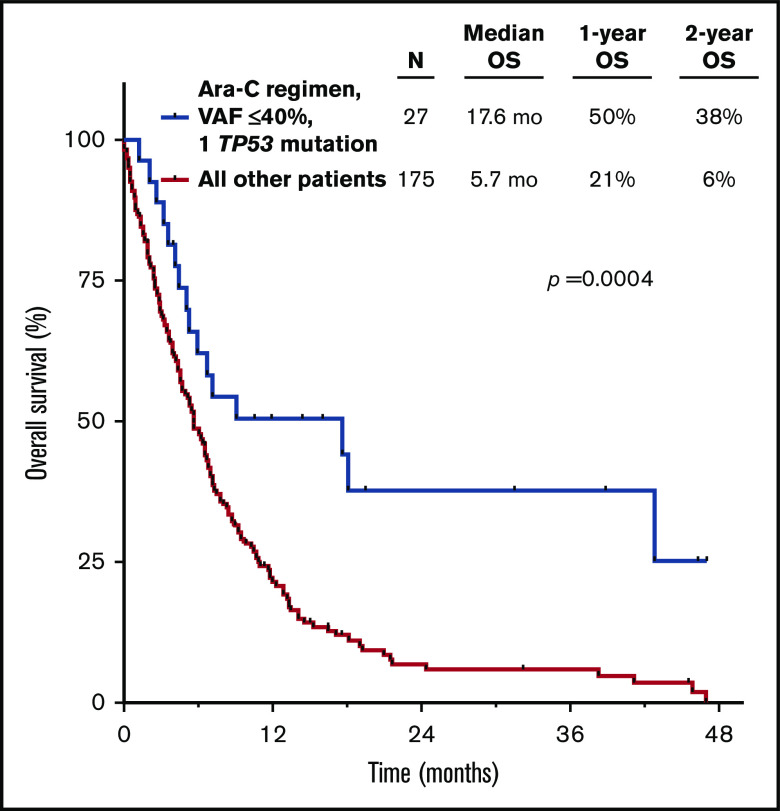
Given the strong independent prognostic significance of both TP53mut VAF and number of TP53 mutations by multivariate analysis, as well as the potential contribution of type of therapy received, we sought to integrate all these parameters to identify patients with TP53-mutated AML who may have relatively favorable outcomes. When patients were stratified by TP53mut VAF, number of TP53 mutations, and treatment with a cytarabine- or HMA-based regimen, the best outcomes were observed in those with 1 TP53 mutation with VAF ≤40% who received frontline cytarabine-based therapy (median OS, 17.6 months; 2-year OS rate, 38% vs median OS, 5.7 months; 2-year OS rate, 6% for other patients; P = .0004; Figure 4). Outcomes were poor for all patients who had at least 1 poor-risk TP53-related feature (ie, VAF >40% and/or >1 mutation) and/or who received an HMA-based regimen, with a median OS <12 months and 2-year OS rate <25% for all individual subgroups (supplemental Figure 3). To contextualize the outcomes of patients with 1 TP53 mutation with VAF ≤40% treated with a frontline cytarabine-based regimen, we compared these patients with a similar historical cohort of patients with newly diagnosed TP53 WT AML (median age, 67 years) who were treated at our institution with an LDAC- (n = 224) or IDAC/HDAC-based regimen (n = 61). Interestingly, OS was similar between patients with 1 TP53 mutation with VAF ≤40% and those with WT TP53 (median OS, 17.6 vs 14.3 months; 2-year OS rate, 38% vs 36%, respectively; P = .86; supplemental Figure 4).
Figure 4.
OS for patients with 1 TP53 mutation with VAF ≤40% who received a cytarabine (Ara-C)-based regimen.
Impact of TP53mut VAF on outcomes with HSCT
Among the 20 patients undergoing transplantation, the median time from start of therapy to HSCT was 3.6 months (range, 2.1-6.1 months). The rate of HSCT in first remission was similar between patients with TP53mut VAF ≤40% and >40% (10 patients in each group; 11% and 9%, respectively). Using a landmark analysis in which patients who died or were lost to follow-up before 3.6 months (ie, median time to HSCT) were excluded, HSCT in first remission was associated with a significant improvement in OS compared with no HSCT (median OS, 17.6 months; 2-year OS rate, 50% vs median OS, 9.1 months; 2-year OS rate, 12%, respectively; P = .006; supplemental Figure 5A). By multivariate analysis including HSCT as a time-dependent variable, HSCT in first remission was independently associated with improved CIR, RFS, and OS (P < .001 for all; Table 2). Among patients with TP53mut VAF ≤40%, the median OS was 32.2 months for patients who underwent transplantation vs 9.5 months for patients who did not (P = .01; supplemental Figure 5B). Among patients with VAF >40%, the difference in OS between those undergoing or not undergoing transplantation was less pronounced (median OS, 9.8 and 8.0 months, respectively; P = .09; supplemental Figure 5C). Patients undergoing transplantation with TP53mut VAF ≤40% had a trend toward better OS compared with patients undergoing transplantation with VAF >40% (P = .07).
Discussion
In this study, we have shown that clinical outcomes of TP53-mutated AML are driven by TP53mut VAF and that this association is treatment dependent. Among patients treated with a cytarabine-based regimen, TP53mut VAF >40% was associated with lower rate of response and worse OS; in contrast, TP53mut VAF did not significantly affect outcomes of patients treated with an HMA-based regimen. Although outcomes were similarly poor for patients with TP53mut VAF >40% regardless of therapy received, treatment with a cytarabine-based regimen was associated with improved OS in patients with TP53mut VAF ≤40%. Together, these findings suggest that TP53mut VAF has both prognostic importance and might also rationally influence selection of frontline therapy.
Our observation that TP53mut VAF is strongly predictive for response rates and survival outcomes with cytarabine-based therapy but not with HMA-based therapy suggests biological differences in how TP53-mutated leukemic cells respond to these different types of therapy. It is well established that TP53 mutations are associated with resistance to conventional cytotoxic chemotherapeutics in AML and in other cancers,3,18 and therefore, an association of higher TP53 mutation burden with worse outcomes with standard chemotherapy (eg, cytarabine) might have been reasonably expected. In contrast, it has been proposed that TP53 mutations might actually epigenetically prime cells to respond to HMAs.19 This was suggested by a study in which a prolonged, 10-day schedule of decitabine resulted in marrow remission (ie, bone marrow blasts <5%) in 100% of patients with TP53-mutated AML or MDS vs only 41% of patients with TP53 WT disease.19 Although these high rates of remission with a 10-day schedule of decitabine were not replicated in a subsequent randomized study,16 these observations nevertheless suggest an important distinction between response of TP53-mutated myeloid neoplasms to conventional cytotoxic chemotherapy and HMAs. Although we did not observe higher rates of response with HMAs among patients with higher TP53mut VAF, the lack of association is nevertheless notable, particularly in contrast to the strong negative impact of higher TP53mut VAF in patients treated with a cytarabine-based regimen. This discrepancy highlights important mechanistic differences between these 2 types of therapy that should be further explored in future studies in AML and other myeloid neoplasms.
Despite the generally poor prognosis of TP53-mutated AML, we were able to identify some patients with relatively favorable outcomes. By multivariate analysis, both TP53mut VAF ≤40% and presence of only 1 TP53 mutation were associated with superior OS. Patients with both of these more favorable TP53-related characteristics (26% of whom underwent HSCT in first remission) had a median OS of 17.6 months and 2-year OS rate of 38% when treated with a cytarabine-based regimen, in contrast to a 1-year OS <25% for all other groups. Acknowledging the limitations of such a retrospective comparison and that there may be some bias toward treatment of younger and more fit patients with an intensive cytarabine-based regimen rather than HMA, this finding is suggestive that cytarabine-based regimens (consisting of either IDAC/HDAC or LDAC) might be preferentially considered over HMA-based therapy for patients with only 1 TP53 mutation with VAF ≤40%. Notably, patients with both of these more favorable TP53 characteristics represented 32% of our entire cohort, suggesting that this consideration is relevant to a substantial proportion of patients with TP53-mutated AML. It is important to note, however, that although these patients had relatively favorable outcomes within this cohort of TP53-mutated AML, their outcomes still remain suboptimal.
In contrast, the universally poor outcomes in patients with TP53mut VAF >40% highlight the great unmet need for these patients. In older patients with newly diagnosed AML who are unfit for intensive chemotherapy, the addition of the oral Bcl-2 inhibitor venetoclax to HMAs has resulted in a promising CR/CRi rate of 67% and a median OS of 17.5 months, representing a new standard of care for these patients.20 However, in the context of mutated TP53, response rates have been reported to be lower (47%) and OS shorter (7.2 months). Interestingly, we found no significant difference in OS between patients who received an HMA with or without venetoclax (irrespective of TP53mut VAF), suggesting a lack of clinical benefit in this context. This is consistent with other reports that TP53 mutations may confer resistance to venetoclax-based therapy.21-23 Therefore, novel therapies are needed for these patients. For example, APR-246 is a small molecule that has been reported to restore the transcriptional activity of unfolded WT or mutant p53, leading to induction of apoptosis in cells with mutant p53.24 In early results from an ongoing phase 1b/2 study in patients with high-risk TP53-mutated MDS or oligoblastic AML (20% to 30% blasts), the combination of APR-246 and azacitidine resulted in an overall response rate of 88% and a CR rate of 59%, with 11% of patients achieving measurable residual disease negativity by NGS of TP53.25 A randomized phase 3 study of APR-246 plus azacitidine vs azacitidine alone in TP53-mutated MDS is going (registered at www.clinicaltrials.gov as #NCT03745716), and several studies with APR-246 combinations in AML are currently enrolling. Promising early results with the anti-CD47 monoclonal antibody magrolimab in combination with azacitidine have also been reported in patients with newly diagnosed TP53-mutated AML, with CR/CRi observed in 7 (78%) of 9 patients and median duration of response not yet reached with a median follow-up of 6.9 months.26 Longer follow-up will be needed to assess whether these novel agents will improve the poor outcomes of patients with TP53-mutated AML, particularly those with TP53mut VAF >40%.
Current consensus guidelines define TP53 mutations as high-risk genetic lesions and recommend allogeneic HSCT in first remission for fit patients with TP53-mutated AML.2 However, some experts have questioned the routine use of HSCT for patients with TP53-mutated AML in light of their poor post-HSCT outcomes, which are driven primarily by a high rate of relapse.27-29 In the present study, we observed a highly significant reduction of CIR and prolonged RFS and OS with HSCT. Although the outcomes of patients with TP53-mutated AML remain suboptimal compared with their TP53 WT counterparts, our findings are consistent with the recommendation for strong consideration of HSCT in first remission, particularly in patients with TP53mut VAF ≤40%, in whom the median OS was 32 months and the 2-year OS rate was 67%. Even in patients with TP53mut VAF >40%, the 2-year OS rate was 30% in patients who underwent HSCT (compared with only 3% in patients not undergoing transplantation), suggesting that HSCT is a reasonable consolidation strategy in this population. An important caveat to these observations it that the number of patients undergoing transplantation in our cohort was small (20 patients total; 10% of the entire cohort), in part because of the older age of this population (median age, 70 years) and the low response rate to frontline therapy. Therefore, although our results support consideration of HSCT in patients with a TP53 mutation, definitive conclusions about the role of HSCT in this poor-risk AML subgroup is limited and should be confirmed in larger studies.
There are several limitations to our analysis. Because TP53 mutations are relatively rare in newly diagnosed AML, we pooled patients treated with IDAC/HDAC and LDAC for some analyses. Although we did observe that both LDAC- and IDAC/HDAC-treated patients had lower response rates and worse survival when TP53mut VAF was >40%, it is important to acknowledge that pooled analyses of cytarabine-treated patients (ie, including both LDAC and IDAC/HDAC regimens) represent a heterogeneous group, limiting the generalizability of some of these analyses. Another limitation is the use of absolute TP53mut VAF. We evaluated outcomes based on absolute VAF, because this is most consistent with what has been studied in other recent reports of TP53mut VAF.12,14 However, it is possible that more complex analyses using copy number–adjusted VAF could yield different results. Furthermore, TP53 mutations can vary in their impact on p53 activity, which may in turn affect how they influence disease biology and clinical outcomes.30 Prospective studies accounting for the functional impact of individual TP53 mutations (eg, gain vs loss of function) may therefore provide a more nuanced view of the impact of TP53 mutations in AML. Use of single-cell sequencing to exclude the presence of TP53 mutations in nonmalignant cells (particularly in the case of patients with normal karyotype AML and low VAF TP53 mutations) would also be informative, as would future studies using high-throughput NGS assays capable of achieving higher levels of sensitivity. Such studies might also reveal any discrepancies between our data and those from studies in MDS. Subclonal TP53 mutations in MDS may be related to clonal hematopoiesis of indeterminate potential and/or may clonally evolve over time.31,32 TP53 mutations may therefore have differential prognostic impacts in MDS (in which treatment is often delayed from the time of diagnosis) and AML (in which therapy is almost always started immediately).
In conclusion, we have shown that TP53mut VAF is highly prognostic for response, relapse, and survival in patients with TP53-mutated AML, particularly for those treated with conventional cytotoxic chemotherapy. Given the treatment-dependent impact of TP53mut VAF on clinical outcomes, we propose that VAF should be assessed when considering frontline therapy options. Although there may be benefit in the use of a cytarabine-based regimen for select patients with only 1 TP53 mutation with VAF ≤40%, outcomes for patients with TP53-mutated AML remain suboptimal, even for those who undergo HSCT in first remission. Further development of effective novel therapies is therefore needed for this poor-risk subgroup of patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by an MD Anderson Cancer Center Support grant (CA016672 from the National Institutes of Health, National Cancer Institute) and a Specialized Programs of Research Excellence grant. N.J.S. is supported by the K12 Paul Calabresi Clinical Oncology Scholar Award and the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Footnotes
For original data, please contact the corresponding author, Tapan M. Kadia (tkadia@mdanderson.org).
Authorship
Contribution: N.J.S. and T.M.K. designed the study, collected and analyzed the data, and wrote the manuscript; G.M.-B. collected and analyzed the data; H.H. and J.N. performed statistical analyses; M.J.F. created tables and figures; R.K.-S. and K.P.P. performed sequencing and pathological analyses; C.D.D., F.R., G.G.-M., K.T., M.K., N.D., G.C.I., M.A., and H.K. treated patients; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tapan M. Kadia, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: tkadia@mdanderson.org.
References
- 1.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016;122(22):3484-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23(1):203-206. [DOI] [PubMed] [Google Scholar]
- 5.Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114-2121. [DOI] [PubMed] [Google Scholar]
- 6.Goel S, Hall J, Pradhan K, et al. High prevalence and allele burden-independent prognostic importance of p53 mutations in an inner-city MDS/AML cohort. Leukemia. 2016;30(8):1793-1795. [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia. 2018;32(12):2546-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciurea SO, Chilkulwar A, Saliba RM, et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood. 2018;131(26):2989-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlenk RF, Kayser S, Bullinger L, et al. ; German-Austrian AML Study Group . Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441-3449. [DOI] [PubMed] [Google Scholar]
- 10.Pratcorona M, Brunet S, Nomdedéu J, et al. ; Grupo Cooperativo Para el Estudio y Tratamiento de las Leucemias Agudas Mieloblásticas . Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734-2738. [DOI] [PubMed] [Google Scholar]
- 11.Ho AD, Schetelig J, Bochtler T, et al. ; Study Alliance Leukemia . Allogeneic stem cell transplantation improves survival in patients with acute myeloid leukemia characterized by a high allelic ratio of mutant FLT3-ITD. Biol Blood Marrow Transplant. 2016;22(3):462-469. [DOI] [PubMed] [Google Scholar]
- 12.Montalban-Bravo G, Kanagal-Shamanna R, Benton CB, et al. Genomic context and TP53 allele frequency define clinical outcomes in TP53-mutated myelodysplastic syndromes. Blood Adv. 2020;4(3):482-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki K, Kanagal-Shamanna R, Montalban-Bravo G, et al. Impact of the variant allele frequency of ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 on the outcomes of patients with newly diagnosed acute myeloid leukemia. Cancer. 2020;126(4):765-774. [DOI] [PubMed] [Google Scholar]
- 14.Prochazka KT, Pregartner G, Rücker FG, et al. Clinical implications of subclonal TP53 mutations in acute myeloid leukemia. Haematologica. 2019;104(3):516-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Short NJ, Kantarjian HM, Loghavi S, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol. 2019;6(1):e29-e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99(3):465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8(5):8921-8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chyla B, Daver N, Doyle K, et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am J Hematol. 2018;93(8):E202-E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoukier M, Konopleva M, Dinardo CD, et al. Activity of venetoclax-based therapy in TP53-mutated acute myeloid leukemia [abstract] J Clin Oncol. 2019;37(15 suppl). Abstract 7034 [Google Scholar]
- 24.Lehmann S, Bykov VJ, Ali D, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30(29):3633-3639. [DOI] [PubMed] [Google Scholar]
- 25.Sallman DA, DeZern AE, Garcia-Manero G, et al. Phase 2 results of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML) [abstract]. Blood. 2019;134(suppl 1). Abstract 676.
- 26.Sallman DA, Asch AS, Al Malki MM, et al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results [abstract]. Blood. 2019;134(suppl 1). Abstract 569.
- 27.Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Della Porta MG, Gallì A, Bacigalupo A, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2016;34(30):3627-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin T, Wu S, Zhao H, et al. Molecular predictors of post-transplant survival in acute myeloid leukemia. Blood Cancer J. 2017;7(12):641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta S, Pregartner G, Rücker FG, et al. Functional classification of TP53 mutations in acute myeloid leukemia. Cancers (Basel). 2020;12(3):E637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Wang Q, Yu H, et al. Mutant p53 drives clonal hematopoiesis through modulating epigenetic pathway [published correction appears in Nat Commun. 2020;11(1):3856]. Nat Commun. 2019;10(1):5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.