Shekhar and Hoeprich et al. show that Twinfilin bypasses the normal requirement for actin filament aging (Pi release) and drives barbed end depolymerization of newly formed filaments. This occurs even under assembly-promoting conditions, where actin monomer levels are far above the critical concentration.
Abstract
Cellular actin networks grow by ATP-actin addition at filament barbed ends and have long been presumed to depolymerize at their pointed ends, primarily after filaments undergo “aging” (ATP hydrolysis and Pi release). The cytosol contains high levels of actin monomers, which favors assembly over disassembly, and barbed ends are enriched in ADP-Pi actin. For these reasons, the potential for a barbed end depolymerization mechanism in cells has received little attention. Here, using microfluidics-assisted TIRF microscopy, we show that mouse twinfilin, a member of the ADF-homology family, induces depolymerization of ADP-Pi barbed ends even under assembly-promoting conditions. Indeed, we observe in single reactions containing micromolar concentrations of actin monomers the simultaneous rapid elongation of formin-bound barbed ends and twinfilin-induced depolymerization of free barbed ends. The data show that twinfilin catalyzes dissociation of subunits from ADP-Pi barbed ends and thereby bypasses filament aging prerequisites to disassemble newly polymerized actin filaments.
Introduction
Rapid remodeling and turnover of the actin cytoskeleton is essential for many cellular processes, including cell migration, endocytosis, cytokinesis, and wound healing (Pollard and Borisy, 2003). Actin networks are assembled by nucleation and elongation factors, which seed the polymerization of new filaments and facilitate addition of ATP-actin subunits at filament barbed ends (Pollard and Borisy, 2003; Shekhar et al., 2016). Newly added subunits immediately hydrolyze ATP, producing ADP-Pi F-actin, and then release Pi at a much slower rate (0.005 s−1), producing ADP F-actin (Carlier, 1987; Carlier and Pantaloni, 1986). ADP-Pi and ADP F-actin have distinct conformations, dynamics, and binding partners (Chou and Pollard, 2019; Merino et al., 2018). Notably, cofilin and its cofactors selectively target ADP (“aged”) F-actin and catalyze severing and pointed end depolymerization (Carlier et al., 1997; Maciver et al., 1991). Thus, the nucleotide state of F-actin can serve as an internal “timer” for turnover of actin networks, directing disassembly to older regions of F-actin. However, some cellular actin networks, such as those at the leading edge, and sites of endocytosis turn over at a rate much faster than the actin aging process (Kaksonen et al., 2003; Lacy et al., 2019; Watanabe and Mitchison, 2002). These observations suggest that our understanding of actin disassembly mechanisms remains incomplete and that there may be cellular factors that target ADP-Pi F-actin to drive barbed end depolymerization and/or accelerate filament aging.
For two reasons, barbed end depolymerization as a potential mechanism for promoting actin disassembly in vivo has received little attention. First, barbed ends are enriched in ADP-Pi actin, which is more stable than ADP actin and does not attract cofilin. Second, the cytosol contains micromolar levels of assembly-competent ATP-actin monomers, well above the critical concentration for assembly at barbed ends (Carlier and Shekhar, 2017; Skruber et al., 2018). Therefore, under cellular conditions, free barbed ends should grow rather than depolymerize, unless there are specialized barbed end–associated factors that can promote the net depolymerization of ADP-Pi and/or ADP barbed ends even in the presence of ATP-actin monomers. Until now, such factors have not been reported.
Two evolutionarily conserved members of the ADF-homology (ADF-H) family, cofilin and twinfilin, promote actin depolymerization in vitro. Cofilin binds cooperatively to the sides of ADP-actin filaments and induces severing to promote disassembly (reviewed in Hild et al., 2014). In the absence of actin monomers, cofilin alone also modestly increases the rate of depolymerization at pointed ends, to ∼2–3 subunits s−1 (Shekhar and Carlier, 2017; Wioland et al., 2017) and, together with cyclase-associated protein (Srv2/CAP), increases the rate of pointed end depolymerization by >300-fold, to >50 subunits s−1 (Kotila et al., 2019; Shekhar et al., 2019). In contrast to cofilin, twinfilin binds poorly to the sides of actin filaments but readily associates with barbed ends (Helfer et al., 2006; Hilton et al., 2018; Johnston et al., 2015; Paavilainen et al., 2007). Indeed, twinfilin localizes to and functions in vivo at sites of dynamic actin assembly enriched in barbed ends, including the tips of filopodia and stereocilia, lamellipodial networks, and yeast cortical actin patches (Hakala et al., 2019 Preprint; Johnston et al., 2015; Rzadzinska et al., 2009). A number of different in vitro activities have been reported for twinfilin, including filament capping, depolymerization, and monomer sequestration (Goode et al., 1998; Helfer et al., 2006; Hilton et al., 2018; Johnston et al., 2015; Paavilainen et al., 2007; Vartiainen et al., 2002). Clarification of twinfilin’s effects on actin filament dynamics represents a crucial step in understanding its biological functions. Here, we attempted to resolve this open question using newly developed microfluidics-assisted total internal reflection fluorescence (mf-TIRF) microscopy.
Results and discussion
Two previous studies from our laboratory, both using conventional total internal reflection fluorescence (TIRF) microscopy, reported that budding yeast and mouse twinfilins accelerate barbed end depolymerization in the absence of actin monomers (Hilton et al., 2018; Johnston et al., 2015). However, a recent study using mf-TIRF microscopy reported that twinfilin slows rather than accelerates barbed end depolymerization (Hakala et al., 2019 Preprint). This discrepancy inspired us to carefully reexamine twinfilin’s effects at barbed ends, comparing them side by side in conventional and mf-TIRF assays. Further, we compared twinfilin’s effects on actin filaments in two different nucleotide states (ADP and ADP-Pi) given that in our previous studies we had not aged the filaments to the same extent as in a previous study (Hakala et al., 2019 Preprint).
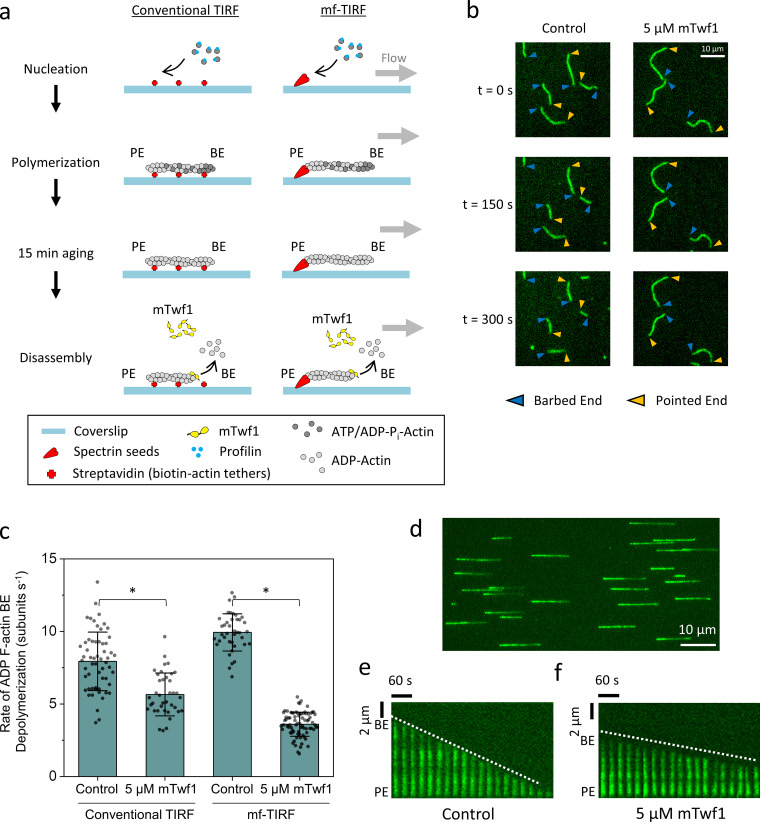
We first examined the effects of mouse twinfilin 1 (mTwf1) on aged (ADP) F-actin barbed ends in a conventional TIRF experiment (Fig. 1 a, left) using fluorescently labeled filaments anchored to streptavidin-functionalized glass coverslips. After polymerization, filaments were incubated in TIRF buffer for 15 min to allow complete conversion to ADP F-actin. These filaments were then exposed to TIRF buffer with or without 5 µM mTwf1 (in the absence of G-actin). In the presence of 5 µM mTwf1, barbed ends depolymerized at a rate of 5.6 ± 1.5 subunits s−1 (± SD; Fig. 1, b and c), slightly slower than in control reactions lacking mTwf1 (7.9 ± 2.0 subunits s−1). These effects are consistent with mTwf1 binding to barbed ends and slowing the dissociation of ADP-actin subunits.
Figure 1.
mTwf1 slows the depolymerization of aged (ADP) actin filament barbed ends. (a) Schematics of conventional TIRF and mf-TIRF experiments to monitor barbed end depolymerization of ADP-actin filaments. In conventional TIRF (left), filaments are polymerized using 2 µM G-actin (15% Alexa-488 labeled and 0.5% biotinylated) and 3 µM profilin. In mf-TIRF assays (right), filaments with free barbed ends are polymerized by exposing coverslip-anchored spectrin-actin seeds to 2 µM G-actin (15% Alexa-488 labeled) and 5 µM profilin. In both setups, filaments are aged to produce ADP F-actin by incubation for 15 min in TIRF buffer or mf-TIRF buffer containing 0.1 µM G-actin (critical concentration at the barbed end, to prevent change in filament length). Then 5 µM mTwf1 or control buffer is flowed in, and barbed end depolymerization is monitored over time. Note that G-actin and profilin are no longer present when depolymerization is monitored. BE, barbed end; PE, pointed end. (b) Representative time-lapse images from conventional TIRF assays showing the effects of 5 µM mTwf1 on barbed end depolymerization. Blue arrowheads, barbed ends. Yellow arrowheads, pointed ends. (c) Comparison of single filament rates (mean ± SD) for barbed end depolymerization in the presence and absence of 5 µM mTwf1 and in conventional TIRF and mf-TIRF assays. *, Statistical comparison by two-sample t test between indicated conditions (P < 0.05). Number of filament ends analyzed for each condition (left to right): 58, 38, 40, and 78. Conventional TIRF experiments were performed two times and mf-TIRF experiments three times and yielded similar results. Data shown are from one experiment each. (d) Representative field of view from mf-TIRF assay, showing filaments aligned under flow in the microfluidic chamber with anchored pointed ends (left) and free barbed ends (right). (e) Representative kymograph of an Alexa-488–labeled actin filament (green) depolymerizing in control buffer from mf-TIRF assay. (f) Same as e but in the presence of 5 µM mTwf1. White dotted lines indicate the slope of the depolymerizing barbed ends.
We next performed mf-TIRF assays in which the filaments were only anchored at their pointed ends (Fig. 1 a, right). These assays alleviate concerns that anchoring filaments along their lengths might affect depolymerization rates. They also provide a more accurate measurement of change in filament length over time and more precise control when flowing in ingredients (Jégou et al., 2011; Shekhar, 2017). After polymerization, filaments were aged for 15 min as above. In the presence of 5 µM mTwf1, ADP barbed ends depolymerized at 3.6 ± 0.8 subunits s−1 (Fig. 1, c–f), whereas in control reactions lacking mTwf1, they depolymerized at 9.9 ± 1.3 subunits s−1. Thus, mTwf1 exhibited the same or perhaps even stronger inhibition of depolymerization at ADP barbed ends in mf-TIRF compared with that in conventional TIRF assays. These results demonstrate that the above-mentioned discrepancy in twinfilin effects at barbed ends does not stem from differences in TIRF methods.
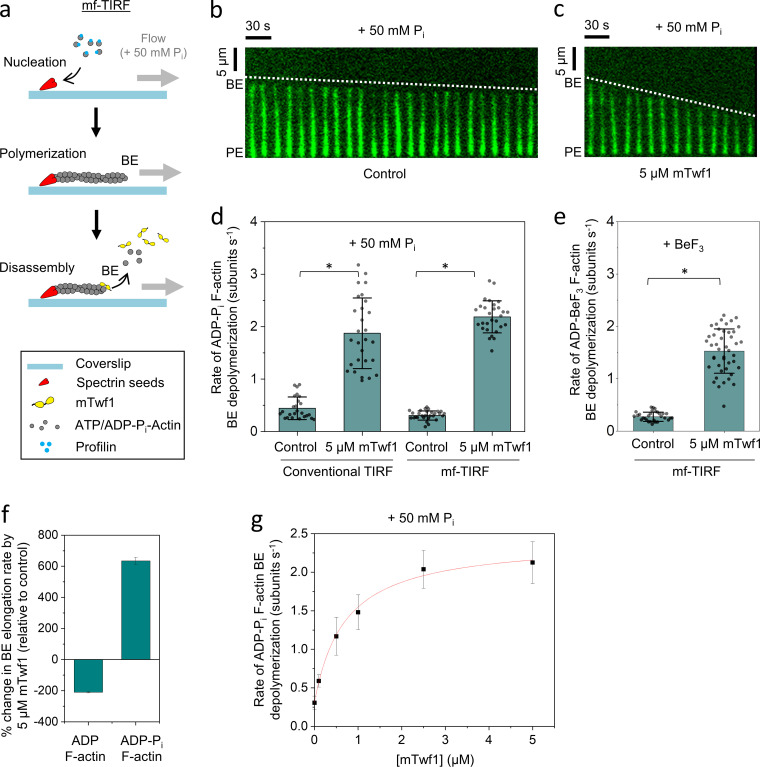
The recent study from Hakala et al., (2019 Preprint) investigated the effects of twinfilin on extensively aged ADP-actin filaments, and we noticed that in our earlier studies we had not aged filaments for as long, suggesting they may have consisted of a mixture of ADP-Pi and ADP subunits. This prompted us to directly investigate how the nucleotide state of the filaments influences twinfilin’s effects at the barbed end. To keep filaments in the ADP-Pi state, they were maintained in buffer supplemented with 50 mM Pi (Fig. 2 a; Carlier and Pantaloni, 1988). In the presence of 5 µM mTwf1, ADP-Pi barbed ends depolymerized at 2.2 ± 0.3 subunits s−1, approximately sevenfold faster than ADP-Pi barbed ends in control reactions lacking mTwf1 (0.3 ± 0.1 subunits s−1; Fig. 2, b–d). Similar effects were observed in conventional TIRF assays (Fig. 2 d). Further, mTwf1 accelerated the rate of barbed end depolymerization for ADP-actin filaments incubated with BeF3 (without 50 mM Pi), which essentially mimics the ADP-Pi state (Combeau and Carlier, 1988, 1989; Fig. 2 e). Thus, the ability of mTwf1 to increase the rate of barbed end depolymerization is not the result of high free Pi levels in solution, but rather the nucleotide state (ADP-Pi) of the filaments.
Figure 2.
mTwf1 accelerates the depolymerization of newly polymerized (ADP + Pi) actin filament barbed ends. (a) Schematic showing the experimental strategy for measuring barbed end depolymerization of ADP-Pi actin filaments in mf-TIRF assays. Actin filaments with free barbed ends were polymerized using coverslip-anchored spectrin-actin seeds by the addition of 2 µM G-actin (15% Alexa-488 labeled) and 5 µM profilin in mf-TIRF buffer containing 50 mM Pi. Next, mf-TIRF buffer (supplemented with 50 mM Pi) with or without 5 µM mTwf1 was introduced into the chamber, and depolymerization at barbed ends was monitored. Note that G-actin and profilin were no longer present when depolymerization was monitored. BE, barbed end; PE, pointed end. (b) Representative kymograph of Alexa-488–labeled actin filament (green) depolymerizing in mf-TIRF buffer with 50 mM Pi (Control). (c) Same as b but in the presence of 5 µM mTwf1. White dotted lines indicate the slope of depolymerizing barbed ends. (d) Rates (mean ± SD) of barbed end depolymerization with and without 5 µM mTwf1 in conventional TIRF versus mf-TIRF assays. *, Statistical comparison by two-sample t test between indicated conditions (P < 0.05). Number of filament ends analyzed for each condition (left to right): 25, 28, 29, and 29. Note that the scale in this panel is different than that in Fig. 1 c. (e) Rates (mean ± SD) of barbed end depolymerization in the presence of BeF3 with and without 5 µM mTwf1. *, Statistical comparison by two-sample t test between indicated conditions (P < 0.05). Number of filament ends analyzed for each condition (left to right): 77 and 109. (f) Percent change in the rate of barbed end depolymerization induced by 5 µM mTwf1 for ADP- (left) or ADP-Pi–actin (right) filaments (mean ± SEM for two replicates). (g) Rates (mean ± SD) of barbed end depolymerization for ADP-Pi filaments as a function of mTwf1 concentration in mf-TIRF. Number of filament ends analyzed for each concentration (left to right): 29, 45, 41, 36, 42, and 46. The line is a fit to a hyperbolic binding curve (see Materials and methods). Conventional TIRF experiments were performed two times and mf-TIRF experiments three times and yielded similar results. Data shown are from one experiment each.
Taken together, these observations reveal that mTwf1 has strikingly opposite effects at barbed ends depending on their nucleotide state, accelerating depolymerization at ADP-Pi ends and slowing depolymerization at ADP ends (Fig. 2 f). Interestingly, in the absence of additional cellular factors, free ADP barbed ends depolymerize almost 33-fold faster than free ADP-Pi barbed ends (0.3 subunits s−1 vs. 9.9 subunits s−1). In contrast, the depolymerization rates for ADP-Pi and ADP barbed ends are quite similar in the presence of mTwf1 (2.2 subunits s−1 and 3.6 subunits s−1, respectively). Therefore, in the presence of mTwf1, new and old regions of F-actin will depolymerize at approximately the same rate. This is an effect that may be used to control the disassembly of cellular actin structures where twinfilin is known to reside (e.g., filopodia tips, stereocilia tips, and lamellipodia; Hakala et al., 2019 Preprint; Peng et al., 2009; Rzadzinska et al., 2009).
Our data also show that mTwf1’s effects on ADP-Pi barbed end depolymerization are saturable (Fig. 2 g). The concentration dependence is consistent with a simple mechanism in which mTwf1 molecules bind independently to a site at the filament barbed end, and the maximum disassembly rate is reached when that site is fully occupied. The estimated cytosolic concentrations of twinfilin in mammalian cells and Saccharomyces cerevisiae are 0.5 and 1 µM, respectively (Johnston et al., 2015, 2018). Our in vitro experiments predict that these concentrations of twinfilin could strongly accelerate depolymerization.
We next considered whether mTwf1’s depolymerization effects at ADP-Pi barbed ends can occur under actin assembly-promoting conditions (i.e., ATP-actin monomer levels above the barbed end critical concentration). In cells, the cytosol contains micromolar levels of assembly-competent actin monomers (free and profilin bound), well above the ∼0.1-µM critical concentration for assembly. This favors growth over disassembly at free barbed ends, as well as at barbed ends bound to polymerization factors such as formins or Ena/VASP (Bear and Gertler, 2009; Goode and Eck, 2007). A study (Hakala et al., 2019 Preprint) recently called into question twinfilin’s ability to drive barbed end depolymerization in the presence of G-actin and instead proposed that twinfilin sequesters actin monomers and thereby lowers the concentration of assembly-competent G-actin to indirectly cause net depolymerization under actin assembly–promoting conditions.
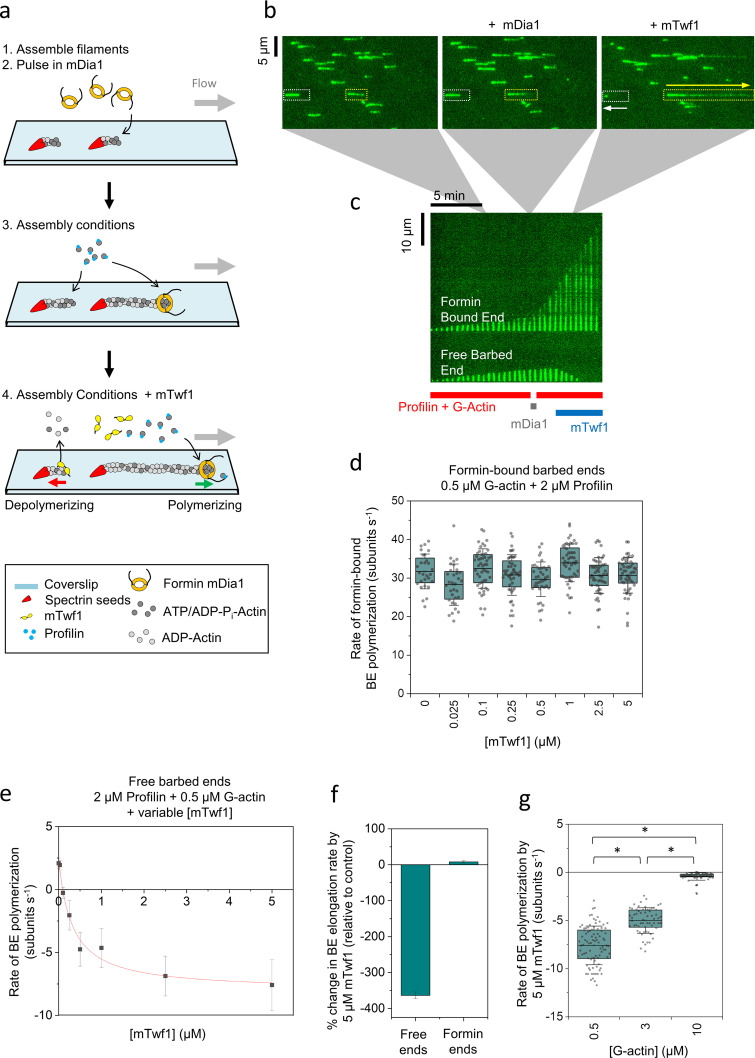
To clarify how twinfilin promotes barbed end depolymerization, we prepared a mixed population of filaments, some with free and some with formin (mDia1)-capped barbed ends (Fig. 3, a–c). Approximately 20% of barbed ends were capped by mDia1, as judged by their faster growth rates upon exposure to G-actin and profilin (Video 1 and Video 2). When this mixed filament population was then exposed to mTwf1 (25 nM to 5 µM) in the presence of G-actin and profilin, the formin-bound barbed ends continued to grow rapidly, at rates similar to those observed before the addition of mTwf1 (Fig. 3 d). Thus, mTwf1 does not significantly reduce the concentration of assembly-competent actin monomers under these conditions. In contrast, mTwf1 changed the dynamics of free barbed ends, slowing net polymerization at lower concentrations of mTwf1 and inducing net depolymerization at higher concentrations of mTwf1 (Fig. 3 e). We note that the rate of barbed end depolymerization observed here is higher than in Fig. 1 c. This is likely due to these reactions containing free profilin, which was included to enable formins to polymerize the barbed ends. Profilin alone increases the rate of depolymerization at barbed ends (Pernier et al., 2016). Thus, the higher rates of depolymerization we observed in these experiments could be due to the combined effects of mTwf1 and profilin on barbed end depolymerization. Importantly, our observation that free barbed ends rapidly shortened while formin-bound barbed ends continued to rapidly grow, all within the same reactions (Fig. 3, c and f; and Video 1), demonstrates that mTwf1 promotes net depolymerization of barbed ends under assembly-promoting conditions (0.5 µM G-actin) and that these effects are not due to actin monomer sequestration by mTwf1. At higher concentrations of G-actin (3 µM and 10 µM), mTwf1 continued to suppress growth and promote net depolymerization of filaments but at slower rates (Fig. 3 g). Overall, these observations show that under assembly-promoting conditions, mTwf1 attenuates growth and promotes barbed end depolymerization.
Figure 3.
mTwf1 induces depolymerization of newly polymerized barbed ends even under assembly-promoting conditions. (a) Schematic showing the experimental strategy for investigating the effects of mTwf1 on barbed ends in assembly-promoting conditions. Filaments with free barbed ends were polymerized by exposing coverslip-anchored spectrin-actin seeds to a flow containing 0.5 µM G-actin (15% Alexa-488 labeled) and 2 µM profilin. 20 nM mDia1 was then introduced for 20 s, which resulted in ∼20% of barbed ends being capped by mDia1. The filaments were then briefly exposed to 0.5 µM G-actin (15% Alexa-488 labeled) and 2 µM profilin in mf-TIRF buffer (<1 min) to identify the fast-growing mDia1-capped ends. Next, mf-TIRF buffer containing 0.5 µM G-actin (15% Alexa-488 labeled), 2 µM profilin, and 0–5 µM mTwf1 was introduced into the chamber, and barbed end dynamics were monitored. (b) Representative field of view showing filaments growing at their barbed ends before mDia1 flow in (left), then the same filaments growing at their free barbed ends (white dotted rectangles) or growing more rapidly at their mDia1-capped barbed ends (yellow dotted rectangles) in the presence of actin and profilin (middle), and finally the same barbed ends shortening or elongating, respectively, after flowing in 5 µM mTwf1 with G-actin and profilin (right). White arrow denotes a depolymerizing free barbed end, and the yellow arrow denotes a rapidly elongating formin-bound barbed end. (c) Kymographs of the same two filaments highlighted in b, grown in the presence of 0.5 µM G-actin and 2 µM profilin (red bar) before and after pulsing in 20 nM mDia1 (gray bar). Upon subsequent flow in of 5 µM mTwf1 with 0.5 µM G-actin and 2 µM profilin (blue bar), the free barbed end starts to depolymerize (bottom), while the mDia1-capped barbed end continues to rapidly elongate (top). (d) Rates (mean ± SD) of mDia1-capped barbed end elongation in the presence of 0.5 µM G-actin (15% Alexa-488 labeled), 2 µM profilin, and different concentrations of mTwf1. BE, barbed end. (e) Rates (mean ± SD) of free barbed end elongation in the presence of 0.5 µM G-actin, 2 µM profilin, and different concentrations of mTwf1. The red line is a fit to a hyperbolic binding curve (see Materials and methods). (f) Percent change in elongation rate by 5 µM mTwf1 (compared with control reactions) for free barbed ends versus mDia1-capped barbed ends (mean ± SEM for three replicates). Reactions contained 0.5 µM G-actin and 2 µM profilin, with or without 5 µM mTwf1. (g) Effects of 5 µM mTwf1 on rate (mean ± SD) of depolymerization of free barbed ends in the presence of different concentrations of profilin-bound actin monomers: 0.5 µM G-actin with 2 µM profilin, 3 µM G-actin with 4 µM profilin, and 10 µM G-actin with 15 µM profilin. *, Statistical comparison by two-sample t test between indicated conditions (P < 0.05). Number of filament ends analyzed for each condition (left to right): 90, 61, and 78. All experiments were performed at least three times and yielded similar results. Data shown are from one experiment each.
Video 1.
mTwf1 promotes the depolymerization of free barbed ends even under assembly-promoting conditions. Alexa-488–labeled actin filaments (green) anchored at their pointed ends (magenta arrowheads) and growing at their free barbed ends (yellow arrowheads) were transiently exposed to 20 nM of the formin mDia1 (FH1-FH2-C). Filaments were then exposed to a continuous flow of 0.5 µM G-actin and 2 µM profilin to identify fast-growing mDia1-capped barbed ends (red arrowheads) and slow-growing free barbed ends (yellow arrowheads). Finally, filaments were exposed to a continuous flow of 0.5 µM G-actin, 2 µM profilin, and 5 µM mTwf1. Under these assembly-promoting conditions, the mDia1-capped barbed ends continued to rapidly elongate, while free barbed ends depolymerized. Playback speed is 15 frames per second.
Video 2.
Control reaction under assembly-promoting conditions showing that in the absence of mTwf1, free and formin-capped barbed ends both continue to polymerize, as expected. Alexa-488–labeled actin filaments (green) anchored at their pointed ends (magenta arrowheads) and growing at their free barbed ends (yellow arrowheads) were transiently exposed to 20 nM of the formin mDia1 (FH1-FH2-C). Filaments were then exposed to a continuous flow of 0.5 µM G-actin and 2 µM profilin. Under these assembly-promoting conditions, free barbed ends (yellow arrowheads) and mDia1-capped barbed ends (red arrowheads) elongated. Playback speed is 25 frames per second.
Combining the new observations here with previous work, we present a working model for twinfilin’s overall regulatory effects at barbed ends (Fig. 4). Twinfilin first binds, alone or together with its interaction partner capping protein (Johnston et al., 2018; Palmgren et al., 2001), to newly polymerized (ADP-Pi) barbed ends. When capping protein alone or twinfilin and capping protein together are bound to a barbed end, filament growth is paused. Twinfilin catalyzes capping protein dissociation from barbed ends (Hakala et al., 2019 Preprint), leaving twinfilin alone at the barbed end to processively depolymerize ADP-Pi actin filaments (accelerating subunit dissociation while blocking the addition of new subunits).
Figure 4.
Working model for twinfilin activities at actin filament barbed ends. Actin filament barbed ends grow by the addition of ATP-actin monomers, which rapidly hydrolyze their bound ATP, producing ADP-Pi–actin subunits. Subsequently, barbed ends are bound by twinfilin alone, capping protein (CP) alone, or twinfilin-capping protein complexes, in each case inhibiting barbed end growth. Twinfilin catalyzes the dissociation of capping protein from barbed ends (Hakala et al., 2019 Preprint), leaving twinfilin alone at the barbed end. Twinfilin then induces the depolymerization of ADP-Pi barbed ends, even in the presence of G-actin at concentrations far above the critical concentration for assembly. We postulate that twinfilin achieves these effects using a processive barbed end tracking mechanism, which was directly observed for yeast twinfilin (Johnston et al., 2015). Once twinfilin reaches regions of ADP F-actin, its interactions with the barbed end slow the rate of depolymerization (compared with that of free ADP barbed ends).
To date, no other cellular factors have been shown to promote the net depolymerization of ADP-Pi barbed ends in the presence of excess G-actin (as found in the cytosol). Cofilin is capable of increasing the rate of dissociation of actin subunits from ADP-Pi (and ADP) barbed ends, but not in presence of actin monomers (Shekhar and Carlier, 2017; Wioland et al., 2017). We speculate that the unique ability of twinfilin to drive barbed end depolymerization under assembly-promoting conditions may arise from an ability to suppress ATP-actin subunit exchange at barbed ends while permitting the exchange of ADP-Pi-actin and ADP-actin subunits. Further, these novel capabilities of twinfilin may stem from its specific molecular architecture, consisting of two connected ADF-H domains and a short C-terminal tail, with each domain binding to actin and contributing to its in vitro and in vivo functions (Johnston et al., 2015; Palmgren et al., 2001).
Finally, our observations reinforce the view that different ADF-H domain proteins have evolved to perform distinct roles in promoting cellular actin turnover (Hild et al., 2014). Under assembly-promoting conditions, twinfilin promotes capping protein dissociation from barbed ends and drives depolymerization of ADP-Pi F-actin, while cofilin works with AIP1 and Srv2/CAP to induce severing and accelerated pointed end depolymerization of ADP F-actin (Fig. 4; Andrianantoandro and Pollard, 2006; Blanchoin and Pollard, 1999; Brieher et al., 2006; Carlier et al., 1997; Jansen et al., 2014; Kotila et al., 2019; Michelot et al., 2007; Nadkarni and Brieher, 2014; Shekhar and Carlier, 2017; Shekhar et al., 2019; Wioland et al., 2017). The nucleotide-dependent effects of twinfilin at the barbed end allow it to bypass filament aging requirements (Pi release) and promote disassembly of recently polymerized F-actin. These activities may play an important role in the regulation of cellular actin structures that undergo rapid turnover (Carlier, 1987; Carlier and Pantaloni, 1986) and/or whose lengths are tightly controlled by growth and disassembly at barbed ends (e.g., filopodia and stereocilia tips), where twinfilin has been localized (Hakala et al., 2019 Preprint; Peng et al., 2009; Rzadzinska et al., 2009).
Materials and methods
Purification and labeling of actin
Rabbit skeletal muscle actin was purified from acetone powder (Spudich and Watt, 1971) generated from frozen ground hind leg muscle tissue of young rabbits (Pel-Freez). Lyophilized acetone powder stored at −80°C was mechanically sheared in a coffee grinder, resuspended in G-buffer (5 mM Tris-HCl pH 7.5, 0.5 mM DTT, 0.2 mM ATP, and 0.1 mM CaCl2), and then cleared by centrifugation for 20 min at 50,000 ×g. Supernatant was collected and further filtered with Whatman paper. Actin was polymerized by the addition of 2 mM MgCl2 and 50 mM NaCl to the filtrate and overnight incubation at 4°C with slow stirring. Next morning, NaCl powder was added to a final concentration of 0.6 M, and stirring was continued for another 30 min at 4°C. F-actin was pelleted by centrifugation for 150 min at 120,000 ×g, and the pellet was solubilized by dounce homogenization and dialyzed against G-buffer for 48 h at 4°C. Monomeric actin was then precleared at 435,000 ×g and loaded onto an S200 (16/60) gel-filtration column (GE Healthcare) equilibrated in G-buffer. Fractions containing actin were stored at 4°C.
To biotinylate actin, the F-actin pellet above was dounced and dialyzed against G-buffer lacking DTT. Monomeric actin was then polymerized by the addition of an equal volume of 2× labeling buffer (50 mM imidazole pH 7.5, 200 mM KCl, 0.3 mM ATP, and 4 mM MgCl2). After 5 min, the actin was mixed with a fivefold molar excess of NHS-XX-Biotin (Merck KGaA) and incubated in the dark for 15 h at 4°C. The F-actin was pelleted as above, and the pellet was rinsed with G-buffer, then homogenized with a dounce, and dialyzed against G-buffer for 48 h at 4°C. Biotinylated monomeric actin was purified further on an S200 (16/60) gel-filtration column as above. Aliquots of biotin-actin were snap frozen in liquid N2 and stored at −80°C.
To fluorescently label actin, G-actin was polymerized by dialyzing overnight against modified F-buffer (20 mM Pipes pH 6.9, 0.2 mM CaCl2, 0.2 mM ATP, and 100 mM KCl; Shekhar, 2017). F-actin was incubated for 2 h at room temperature with a fivefold molar excess of Alexa-488 NHS ester dye (Life Technologies). The F-actin was then pelleted by centrifugation at 450,000 ×g for 40 min at room temperature, and the pellet was resuspended in G-buffer, homogenized with a dounce, and incubated on ice for 2 h to depolymerize the filaments. The monomeric actin was then repolymerized on ice for 1 h by the addition of 100 mM KCl and 1 mM MgCl2, and the F-actin was pelleted by centrifugation for 40 min at 450,000 ×g at 4°C. The pellet was homogenized with a dounce and dialyzed overnight at 4°C against 1 liter of G-buffer. The solution was precleared by centrifugation at 450,000 ×g for 40 min at 4°C. The supernatant was collected, and the concentration and the labeling efficiency of actin were determined by measuring the absorbance at 280 nm and 495 nm, respectively. Molar extinction coefficients used were as follows: ε280 actin = 45,840 M−1 cm−1, ε495 Alexa-488 = 71,000 M−1 cm−1, and ε280 AF488 = 7,810 M−1 cm−1.
Purification of twinfilin
mTwf1 was expressed as a GST fusion protein in Escherichia coli strain BL21 (pRARE). Cells were grown to log phase at 37°C, and then expression was induced for 16 h at 18°C by the addition of 0.4 mM IPTG. Cells were harvested by centrifugation, and the pellets were stored at −−80°C. Frozen cell pellets were resuspended in 10 ml of PBS supplemented freshly with 0.5 mM DTT, 1 mM PMSF, and protease inhibitors (0.5 µM each of pepstatin A, antipain, leupeptin, aprotinin, and chymostatin). Lysozyme (0.5 mg/ml) was added, and the cells were lysed by sonication using a tip sonicator while keeping the tubes on ice. The lysate was clarified by centrifugation at 12,500 ×g for 20 min, and then the supernatant was incubated at 4°C (rotating) for 2–3 h with 0.5 ml glutathione–agarose beads (Sigma-Aldrich). Beads were washed three times in cold PBS supplemented with 1 M NaCl and then washed twice in cold PBS.
mTwf1 was released from the beads by incubation with PreScission Protease (GE Healthcare) overnight at 4°C (rotating), thus leaving GST on the beads. The released mTwf1 was concentrated to 0.3 ml, then loaded on a Superose12 size-exclusion column (GE Healthcare) equilibrated in 20 mM Hepes pH 7.5, 1 mM EDTA, 50 mM KCl, and 0.5 mM DTT. Peak fractions were pooled, concentrated, aliquoted, snap frozen in liquid N2, and stored at −80°C.
Purification of profilin
Human profilin-1 was expressed in E. coli BL21 DE3 by growing cells to log phase at 37°C in Terrific Broth (TB) medium and inducing expression with 1 mM IPTG at 37°C for 3 h. Cells were harvested by centrifugation, and pellets were stored at −80°C. Cell pellets were resuspended in lysis buffer (50 mM Tris-HCl pH 8.0, 1 mM EDTA, 0.2% Triton X-100, and lysozyme + protease inhibitors as above), kept on ice for 30 min, and then further lysed by sonication. Lysates were cleared for 25 min at 272,000 ×g at 4°C, and the supernatant was collected and fractionated on a HiTrap Q column (buffer: 20 mM Tris-HCl, pH 8.0). Peak fractions were concentrated and then purified further on a Superdex 75 column equilibrated in 20 mM Tris-HCl pH 8.0, and 50 mM NaCl. Peak fractions were pooled, aliquoted, snap frozen in liquid N2, and stored at −80°C.
Conventional TIRF microscopy
Glass coverslips (60 × 24 mm; Thermo Fisher Scientific) were first cleaned by sonication in detergent for 60 min, followed by successive sonications in 1 M KOH and 1 M HCl for 20 min each and in ethanol for 60 min. Coverslips were then washed extensively with H2O and dried in an N2 stream. The cleaned coverslips were coated with 2 mg/ml methoxy–poly(ethylene glycol [PEG])–silane MW 2,000 and 2 µg/ml biotin-PEG-silane MW 3,400 (Laysan Bio) in 80% ethanol pH 2.0, and incubated overnight at 70°C. Flow cells were assembled by rinsing PEG-coated coverslips with water, drying with N2, and adhering to μ-Slide VI0.1 (0.1 × 17 × 1 mm) flow chambers (Ibidi) with double-sided tape (2.5 cm × 2 mm × 120 µm) and 5-min epoxy resin (Devcon). Before each reaction, the flow cell was sequentially incubated for 1 min each with 4 µg/ml streptavidin in HEK buffer (20 mM Hepes pH 7.4, 1 mM EDTA, 50 mM KCl, and 5% glycerol) and 1% BSA in HEK buffer. The flow cell was then equilibrated with TIRF buffer (10 mM imidazole pH 7.4, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, 10 mM DTT, 15 mM glucose, 20 µg/ml catalase, 100 µg/ml glucose oxidase, and 0.5% methylcellulose [4,000 cP]). Actin filaments were grown using 2 µM G-actin (15% Alexa-488 labeled and 0.5% biotinylated G-actin) and 3 µM profilin until they reached lengths of ∼10–20 µm. After this assembly phase, the filaments were aged (to produce ADP actin) by incubating them for 15 min in TIRF buffer in the presence of 0.1 µM G-actin (to prevent their depolymerization during aging). We then flowed in mTwf1 diluted in TIRF buffer or buffer alone and monitored change in filament length over time. For experiments with ADP-Pi–actin filaments, the filaments were polymerized using 2 µM ATP–G-actin and 3 µM profilin in modified TIRF buffer containing 50 mM PO4 (the 50 mM KCl was replaced with 34.8 mM K2HPO4 and 15.2 mM KH2PO4). Effects of mTwf1 on ADP-Pi barbed ends were monitored in the continuous presence of modified TIRF buffer.
mf-TIRF microscopy
Actin filaments were first assembled in mf-TIRF flow cells (Jégou et al., 2011; Shekhar, 2017; Shekhar and Carlier, 2016, 2017). Coverslips were first cleaned by sonication in detergent for 60 min, followed by successive sonications in 1 M KOH and 1 M HCl for 20 min each and in ethanol for 60 min. Coverslips were then washed extensively with H2O and dried in an N2 stream. The cleaned coverslips were coated with an 80% ethanol solution adjusted to pH 2.0 with HCl containing 2 mg/ml methoxy-PEG–silane MW 2,000 and incubated overnight at 70°C. A 40-µm-high Polydimethylsiloxane (PDMS) mold with three inlets and one outlet was mechanically clamped onto a PEG-silane–coated coverslip. The chamber was then connected to a Maesflo microfluidic flow-control system (Fluigent), rinsed with mf-TIRF buffer (10 mM imidazole pH 7.4, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, 10 mM DTT, 15 mM glucose, 20 µg/ml catalase, and 100 µg/ml glucose oxidase) and incubated with 1% BSA and 10 µg/ml streptavidin in mf-TIRF buffer for 5 min. Spectrin-actin seeds were nonspecifically anchored on the glass coverslip. Actin filaments with free barbed ends were formed by exposing spectrin-actin seeds to a flow containing 2 µM G-actin (15% Alexa-488 labeled) and 5 µM profilin in mf-TIRF buffer at room temperature.
After filaments were polymerized in mf-TIRF flow cells, barbed-end depolymerization was monitored at room temperature as follows. For experiments on ADP-actin filaments, newly polymerized filaments were aged for 15 min under continuous flow in mf-TIRF buffer containing 0.1 µM G-actin (unlabeled) before monitoring depolymerization. For experiments on ADP-Pi actin filaments, the polymerized filaments were maintained in the presence of modified mf-TIRF buffer containing 50 mM PO4 (where 50 mM KCl was replaced by 34.8 mM K2HPO4 and 15.2 mM KH2PO4). For experiments on ADP-BeF3 actin filaments, the polymerized filaments were maintained in the presence of modified mf-TIRF buffer containing 5 mM NaF and 100 µM BeSO4 (Combeau and Carlier, 1988, 1989).
Image acquisition and analysis
Single-wavelength time-lapse TIRF imaging was performed on a Nikon-Ti2000 inverted microscope equipped with a 150-mW Argon laser (Melles Griot), a 60× TIRF-objective with a numerical aperture of 1.49 (Nikon Instruments Inc.), and an electron-multiplying charge-coupled device (EMCCD) camera (Andor Ixon). One pixel was equivalent to 143 × 143 nm. Focus was maintained by the Perfect Focus system (Nikon Instruments Inc.). Images were acquired every 5 s or 10 s and exposed for 100 ms using imaging software Elements (Nikon Instruments Inc.).
Images were analyzed in Fiji (Schindelin et al., 2012). Drift correction was performed using coverslip-anchored streptavidin-functionalized TransFluoSpheres (Thermo Fisher Scientific) and processing of the images with the MultiStackReg plugin. Background subtraction was conducted using the rolling ball background subtraction algorithm (ball radius, 5 pixels). For conventional TIRF assays, depolymerization rates were determined by tracking the initial and final positions of the actin filament barbed ends using the MTrackJ plugin. For mf-TIRF assays, the kymograph plugin was used to draw kymographs of individual filaments. The kymograph slope was used to calculate barbed end depolymerization rate of each individual filament (assuming one actin subunit contributes 2.7 nm to filament length). Data analysis and curve fitting were performed in Microcal Origin. Depolymerization rate (D) data (Fig. 2 g) and polymerization rate (P) data (Fig. 3 e) were fit to the hyperbolic binding curve:
where C is the mTwf1 concentration, D0 is the rate of depolymerization in the absence of mTwf1, Dmax is the rate of depolymerization at saturating mTwf1 concentration, and K is the mTwf1 concentration at half-saturation. Fitting the data in Fig. 2 g to this equation gives Dmax = 2.4 ± 0.1 subunits s−1, D0 = 0.32 ± 0.02 subunits s−1, and K = 0.7 ± 0.1 µM, and fitting the data in Fig. 3 e gives Dmax = 8.0 ± 0.9 subunits s−1, D0 = 2.3 ± 0.2 subunits s−1, and K = 0.3 ± 0.1 µM.
Percent changes in elongation rate in the presence of mTwf1 (Fig. 2 f and Fig. 3 f) were determined using the following equation:
where RT and Rc are the rates of barbed end elongation in the presence of twinfilin (RT) or control buffer (Rc).
Statistical analysis
As indicated in the legend for each figure, all experiments were repeated multiple times and yielded similar results. Data shown are from one experiment each. Means and errors (SD or SEM) were calculated using Microcal Origin. Data distributions were assumed to be normal, but this was not formally tested. Statistical comparison between indicated conditions was conducted using the two-sample t test (Fig. 1 c; Fig. 2, d and e; and Fig. 3 g). Differences were considered significant if the P value was <0.05 (*).
Data availability
Data supporting the findings of this manuscript are available from the corresponding author upon reasonable request.
Online supplemental material
Video 1 and Video 2 show that twinfilin promotes depolymerization of actin filament–free barbed ends under assembly-promoting conditions while having no effect on the rate of elongation of formin-capped barbed ends in the same reactions.
Supplementary Material
Acknowledgments
We thank Marie-France Carlier and Tom Pollard for advice on how to keep actin filaments in the ADP-Pi state and how to use BeF3 to mimic the ADP-Pi state.
This research was supported by the Brandeis University National Science Foundation Materials Research Science and Engineering Center, grant 142038, and by grants from the National Institutes of Health to B.L. Goode (R35 GM134895) and to B.L. Goode and J. Gelles (R01 GM098143).
The authors declare no competing financial interests.
Author contributions: S. Shekhar, G.J. Hoeprich, J. Gelles, and B.L. Goode designed the experiments and wrote the manuscript. S. Shekhar and G.J. Hoeprich performed experiments and analyzed the data.
References
- Andrianantoandro E., and Pollard T.D.. 2006. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell. 24:13–23. 10.1016/j.molcel.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Bear J.E., and Gertler F.B.. 2009. Ena/VASP: towards resolving a pointed controversy at the barbed end. J. Cell Sci. 122:1947–1953. 10.1242/jcs.038125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L., and Pollard T.D.. 1999. Mechanism of interaction of Acanthamoeba actophorin (ADF/Cofilin) with actin filaments. J. Biol. Chem. 274:15538–15546. 10.1074/jbc.274.22.15538 [DOI] [PubMed] [Google Scholar]
- Brieher W.M., Kueh H.Y., Ballif B.A., and Mitchison T.J.. 2006. Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J. Cell Biol. 175:315–324. 10.1083/jcb.200603149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.F. 1987. Measurement of Pi dissociation from actin filaments following ATP hydrolysis using a linked enzyme assay. Biochem. Biophys. Res. Commun. 143:1069–1075. 10.1016/0006-291X(87)90361-5 [DOI] [PubMed] [Google Scholar]
- Carlier M.F., and Pantaloni D.. 1986. Direct evidence for ADP-inorganic phosphate-F-actin as the major intermediate in ATP-actin polymerization. Rate of dissociation of inorganic phosphate from actin filaments. Biochemistry. 25:7789–7792. 10.1021/bi00372a001 [DOI] [PubMed] [Google Scholar]
- Carlier M.F., and Pantaloni D.. 1988. Binding of phosphate to F-ADP-actin and role of F-ADP-Pi-actin in ATP-actin polymerization. J. Biol. Chem. 263:817–825. [PubMed] [Google Scholar]
- Carlier M.F., and Shekhar S.. 2017. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat. Rev. Mol. Cell Biol. 18:389–401. 10.1038/nrm.2016.172 [DOI] [PubMed] [Google Scholar]
- Carlier M.F., Laurent V., Santolini J., Melki R., Didry D., Xia G.X., Hong Y., Chua N.H., and Pantaloni D.. 1997. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 136:1307–1322. 10.1083/jcb.136.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S.Z., and Pollard T.D.. 2019. Mechanism of actin polymerization revealed by cryo-EM structures of actin filaments with three different bound nucleotides. Proc. Natl. Acad. Sci. USA. 116:4265–4274. 10.1073/pnas.1807028115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combeau C., and Carlier M.F.. 1988. Probing the mechanism of ATP hydrolysis on F-actin using vanadate and the structural analogs of phosphate BeF-3 and A1F-4. J. Biol. Chem. 263:17429–17436. [PubMed] [Google Scholar]
- Combeau C., and Carlier M.F.. 1989. Characterization of the aluminum and beryllium fluoride species bound to F-actin and microtubules at the site of the gamma-phosphate of the nucleotide. J. Biol. Chem. 264:19017–19021. [PubMed] [Google Scholar]
- Goode B.L., and Eck M.J.. 2007. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76:593–627. 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- Goode B.L., Drubin D.G., and Lappalainen P.. 1998. Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomer-sequestering protein. J. Cell Biol. 142:723–733. 10.1083/jcb.142.3.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakala M., Wioland H., Tolonen M., Jegou A., Romet-Lemonne G., and Lappalainen P.. 2019. Twinfilin uncaps filament barbed ends to promote turnover of lamellipodial actin networks. bioRxiv. doi:10.1101/864769 (Preprint posted December 4, 2019) [DOI] [PubMed] [Google Scholar]
- Helfer E., Nevalainen E.M., Naumanen P., Romero S., Didry D., Pantaloni D., Lappalainen P., and Carlier M.F.. 2006. Mammalian twinfilin sequesters ADP-G-actin and caps filament barbed ends: implications in motility. EMBO J. 25:1184–1195. 10.1038/sj.emboj.7601019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild G., Kalmár L., Kardos R., Nyitrai M., and Bugyi B.. 2014. The other side of the coin: functional and structural versatility of ADF/cofilins. Eur. J. Cell Biol. 93:238–251. 10.1016/j.ejcb.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Hilton D.M., Aguilar R.M., Johnston A.B., and Goode B.L.. 2018. Species-Specific Functions of Twinfilin in Actin Filament Depolymerization. J. Mol. Biol. 430:3323–3336. 10.1016/j.jmb.2018.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S., Collins A., Golden L., Sokolova O., and Goode B.L.. 2014. Structure and mechanism of mouse cyclase-associated protein (CAP1) in regulating actin dynamics. J. Biol. Chem. 289:30732–30742. 10.1074/jbc.M114.601765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégou A., Niedermayer T., Orbán J., Didry D., Lipowsky R., Carlier M.F., and Romet-Lemonne G.. 2011. Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol. 9:e1001161 10.1371/journal.pbio.1001161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.B., Collins A., and Goode B.L.. 2015. High-speed depolymerization at actin filament ends jointly catalysed by Twinfilin and Srv2/CAP. Nat. Cell Biol. 17:1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.B., Hilton D.M., McConnell P., Johnson B., Harris M.T., Simone A., Amarasinghe G.K., Cooper J.A., and Goode B.L.. 2018. A novel mode of capping protein-regulation by twinfilin. eLife. 7:e41313 10.7554/eLife.41313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., and Drubin D.G.. 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 115:475–487. 10.1016/S0092-8674(03)00883-3 [DOI] [PubMed] [Google Scholar]
- Kotila T., Wioland H., Enkavi G., Kogan K., Vattulainen I., Jégou A., Romet-Lemonne G., and Lappalainen P.. 2019. Mechanism of synergistic actin filament pointed end depolymerization by cyclase-associated protein and cofilin. Nat. Commun. 10:5320 10.1038/s41467-019-13213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy M.M., Baddeley D., and Berro J.. 2019. Single-molecule turnover dynamics of actin and membrane coat proteins in clathrin-mediated endocytosis. eLife. 8:e52355 10.7554/eLife.52355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver S.K., Zot H.G., and Pollard T.D.. 1991. Characterization of actin filament severing by actophorin from Acanthamoeba castellanii. J. Cell Biol. 115:1611–1620. 10.1083/jcb.115.6.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino F., Pospich S., Funk J., Wagner T., Küllmer F., Arndt H.D., Bieling P., and Raunser S.. 2018. Structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution revealed by cryo-EM. Nat. Struct. Mol. Biol. 25:528–537. 10.1038/s41594-018-0074-0 [DOI] [PubMed] [Google Scholar]
- Michelot A., Berro J., Guérin C., Boujemaa-Paterski R., Staiger C.J., Martiel J.L., and Blanchoin L.. 2007. Actin-filament stochastic dynamics mediated by ADF/cofilin. Curr. Biol. 17:825–833. 10.1016/j.cub.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Nadkarni A.V., and Brieher W.M.. 2014. Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends. Curr. Biol. 24:2749–2757. 10.1016/j.cub.2014.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavilainen V.O., Hellman M., Helfer E., Bovellan M., Annila A., Carlier M.F., Permi P., and Lappalainen P.. 2007. Structural basis and evolutionary origin of actin filament capping by twinfilin. Proc. Natl. Acad. Sci. USA. 104:3113–3118. 10.1073/pnas.0608725104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren S., Ojala P.J., Wear M.A., Cooper J.A., and Lappalainen P.. 2001. Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. J. Cell Biol. 155:251–260. 10.1083/jcb.200106157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A.W., Belyantseva I.A., Hsu P.D., Friedman T.B., and Heller S.. 2009. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J. Neurosci. 29:15083–15088. 10.1523/JNEUROSCI.2782-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernier J., Shekhar S., Jegou A., Guichard B., and Carlier M.F.. 2016. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev. Cell. 36:201–214. 10.1016/j.devcel.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D., and Borisy G.G.. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Rzadzinska A.K., Nevalainen E.M., Prosser H.M., Lappalainen P., and Steel K.P.. 2009. MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One. 4:e7097 10.1371/journal.pone.0007097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. . 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9:676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S. 2017. Microfluidics-Assisted TIRF Imaging to Study Single Actin Filament Dynamics. Curr. Protoc. Cell Biol. 77:12.13.1–12.13.24. [DOI] [PMC free article] [PubMed]
- Shekhar S., and Carlier M.F.. 2016. Single-filament kinetic studies provide novel insights into regulation of actin-based motility. Mol. Biol. Cell. 27:1–6. 10.1091/mbc.E15-06-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S., and Carlier M.F.. 2017. Enhanced Depolymerization of Actin Filaments by ADF/Cofilin and Monomer Funneling by Capping Protein Cooperate to Accelerate Barbed-End Growth. Curr. Biol. 27:1990–1998.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S., Pernier J., and Carlier M.F.. 2016. Regulators of actin filament barbed ends at a glance. J. Cell Sci. 129:1085–1091. 10.1242/jcs.179994 [DOI] [PubMed] [Google Scholar]
- Shekhar S., Chung J., Kondev J., Gelles J., and Goode B.L.. 2019. Synergy between Cyclase-associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat. Commun. 10:5319 10.1038/s41467-019-13268-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skruber K., Read T.A., and Vitriol E.A.. 2018. Reconsidering an active role for G-actin in cytoskeletal regulation. J. Cell Sci. 131:jcs203760 10.1242/jcs.203760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J.A., and Watt S.. 1971. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246:4866–4871. [PubMed] [Google Scholar]
- Vartiainen M.K., Mustonen T., Mattila P.K., Ojala P.J., Thesleff I., Partanen J., and Lappalainen P.. 2002. The three mouse actin-depolymerizing factor/cofilins evolved to fulfill cell-type-specific requirements for actin dynamics. Mol. Biol. Cell. 13:183–194. 10.1091/mbc.01-07-0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., and Mitchison T.J.. 2002. Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science. 295:1083–1086. 10.1126/science.1067470 [DOI] [PubMed] [Google Scholar]
- Wioland H., Guichard B., Senju Y., Myram S., Lappalainen P., Jégou A., and Romet-Lemonne G.. 2017. ADF/Cofilin Accelerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Curr. Biol. 27:1956–1967.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this manuscript are available from the corresponding author upon reasonable request.