Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory syndrome designated by synovial joint inflammation leading to cartilage degradation and bone damage as well as progressive disability. Synovial inflammation is promoted through the infiltration of mononuclear immune cells, dominated by CD4+ T cells, macrophages and dendritic cells (DCs), together with fibroblast‐like synoviocytes (FLS), into the synovial compartment. Berberine is a bioactive isoquinoline alkaloid compound showing various pharmacological properties that are mainly attributed to immunomodulatory and anti‐inflammatory effects. Several lines of experimental study have recently investigated the therapeutic potential of berberine and its underlying mechanisms in treating RA condition. The present review aimed to clarify determinant cellular and molecular targets of berberine in RA and found that berberine through modulating several signalling pathways involved in the joint inflammation, including PI3K/Akt, Wnt1/β‐catenin, AMPK/lipogenesis and LPA/LPA1/ERK/p38 MAPK can inhibit inflammatory proliferation of FLS cells, suppress DC activation and modulate Th17/Treg balance and thus prevent cartilage and bone destruction. Importantly, these molecular targets may explore new therapeutic targets for RA treatment.
Keywords: berberine, immunomodulatory, rheumatoid arthritis, synovial joint inflammation
1. INTRODUCTION
Rheumatoid arthritis (RA) is a destructive, chronic, immune‐mediated inflammatory syndrome designated by synovial joint inflammation leading to cartilage degradation and bone damage as well as progressive disability. 1 , 2 Synovial inflammation reflecting joint swelling is promoted through the infiltration of mononuclear immune cells, dominated by CD4+ T cells, macrophages and dendritic cells (DCs) into the synovial compartment. 1 , 2 The inflammatory milieu triggers a strong tissue response, mainly activation of fibroblast‐like synoviocytes (FLS) together with increased synovial osteoclastogenesis and chondrocyte catabolism, causing articular destruction. Cartilage and bone damage stems from synovial invasion into neighbour articular structures. The dominant synovial cells responsible for cartilage damage are the activated FLS with the invasive phenotype that generate tremendous amounts of proteases, prominently matrix metalloproteinases (MMPs) contributing to local matrix destruction, 3 , 4 and whereby readily emigrate from joint to joint to propagate disease. 5 Pro‐inflammatory and tissue‐damaging cellular responses in synovitis are integrated by cytokine networks. Cytokines bind cognate receptors to provoke various intracellular signalling pathways, the intermediaries between extracellular events and activation of an array of genes that result or aggravate inflammation and damage. Among inflammatory cytokines, tumour necrosis factor (TNF)‐α and interleukin (IL)‐6 have been known to be essential in disease pathogenesis, through activating immune cells, stimulating MMP generation and provoking pain. However, others such as IL‐1 and IL‐17 seem to be involved with less extent. 6 Synovial cells generate cytokines that promote and exacerbate the inflammatory response by activating endothelial cells and attracting immune cells to infiltrate into the synovial environment. These cells, in turn, produce additional cytokines that can activate adjacent FLS, T cells and dendritic cells in the joint compartment. Activated fibroblasts and the infiltrated activated immune cells eventually promote bone erosion through inducing generation and maturation of osteoclasts via the production of TNF‐α, IL‑6 and IL‑1, together with receptor activator of nuclear factor κ B ligand (RANKL) that interact with RANK receptor on preosteoclasts. 6 , 7 , 8 Osteoclasts are bone‐resorbing cells that degrade the mineralized bone matrix by secreting proteases, such as cathepsin K. Besides, cytokines can trigger cartilage damage by affecting chondrocyte catabolism 9 (Figure 1).
Figure 1.
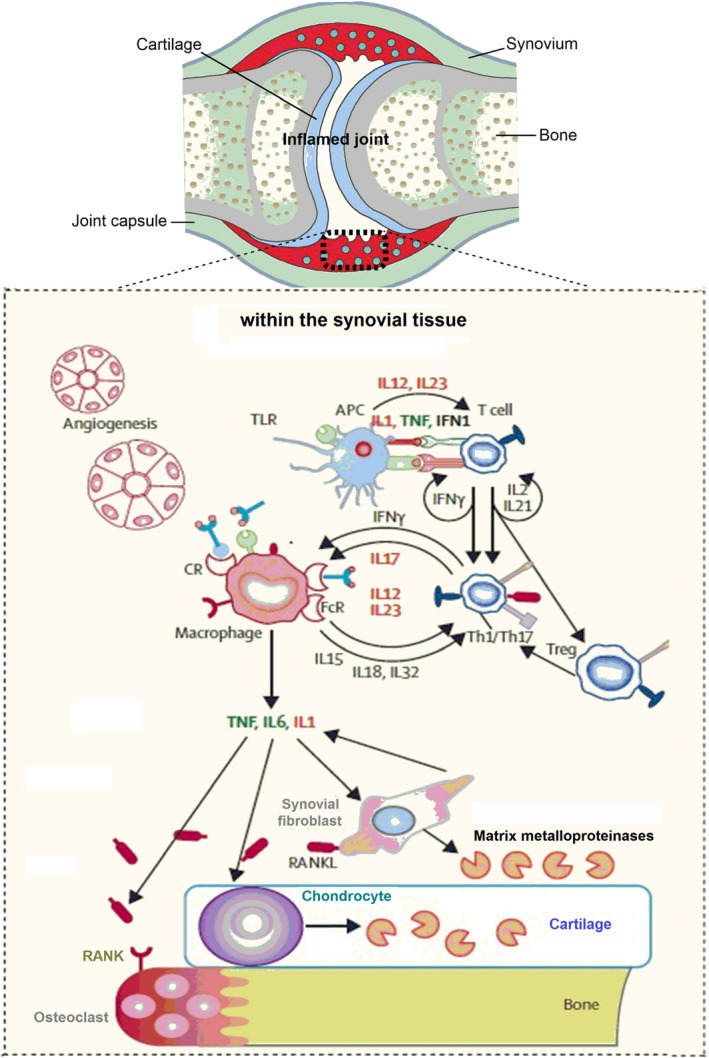
Schematic view of cellular pathways in the inflamed joint in rheumatoid arthritis
In recent years, monotherapy or combination therapy with immunosuppressive drugs including synthetic disease‐modifying anti‐rheumatic drugs (DMARDs) such as methotrexate, sulfasalazine, hydroxychloroquine and leflunomide; biological DMARDs such as infliximab, adalimumab, etanercept, rituximab, abatacept, rituximab, tocilizumab and tofacitinib; non‐steroidal anti‐inflammatory drugs (NSAIDs); and glucocorticoids have become the therapeutic anchor for RA management. 1 , 2 Despite their partial clinical success, these therapies manifest some important limitations and adverse effects in long‐term use such as infections, liver injury, gastrointestinal damage and heart failure. 10 , 11 NSAIDs, while relieving pain and stiffness and enhancing physical ability, do not affect mechanisms underlying joint damage and are hence not disease‐modifying. On the other hand, synthetic DMARDs and glucocorticoids possess disease‐modifying properties, but their serious side effects impede long‐term use. 1 , 2 Moreover, biological DMARDs are significantly more expensive than other treatments. 12 As estimated by a comprehensive meta‐analysis, the annual direct medical costs in the USA, for biological users, are approximately three times greater than those using any treatment regimen according. 13 Therefore, there is an urgent need to address adjuvant immunomodulatory treatments for the management of RA. The better complement for the currently prescribed medicine, eliciting minimal side effects, can be the purified compounds from natural plant sources.
2. BERBERINE; A POTENTIAL ADJUVANT FOR RA MANAGEMENT
Natural product derivatives found in plant extracts interact with biological systems in interesting ways. The majority of today's therapeutically important drugs are based on the structure of natural products. When a natural product shows an inhibitory effect on the severity and progression of a disease, evaluating its molecular targets can assist to improve understanding of key underlying molecular mechanisms of the disease pathogenesis and thus explore potential therapeutic targets for developing new drugs and reliable assessment tools. Berberine is one such natural products that have been frequently investigated for pharmacological effects in the various disease condition, such as cancer, diabetes, atherosclerosis and cardiovascular diseases and thereby are found to have several biological activities including antioxidant, anti‐tumorigenic, anti‐hyperlipidemic, anti‐inflammatory and immunosuppressive effects. 14 , 15 , 16 , 17 , 18 , 19
There is growing evidence that berberine can ameliorate adjuvant‐induced arthritis (AIA) and/or collagen‐induced arthritis (CIA) in experimental animals witnessed through the immunomodulatory effects and the suppression of numbers of inflammatory signalling cascades involved in the joint inflammation and bone destruction. In the present review, details regarding the current evidence on the therapeutic impacts of berberine on RA pathogenesis, together with the mechanisms of action, are covered. Importantly, the findings can reveal new potential therapeutic targets for RA management.
2.1. In vivo effects of berberine on clinical symptoms in experimental models of RA
Several lines of in vivo studies show that berberine can exert anti‐inflammatory and/or immunosuppressive effects and thus alleviate disease progression and severity in animals with AIA and CIA. Among the established experimental models of human RA, AIA and CIA models are the most commonly used standard ones, reflecting a number of clinical characteristics of RA in humans, including inflammation with swelling of the joints, proliferation of synovial tissue and destruction of cartilage and bone. Overall, joint lesions of AIA are most severe and consistent, while structural and immunological changes of CIA best resemble RA. 20 , 21 Berberine has been found to reduce the intensity and incidence of CIA and AIA in rodents. Histologic analysis of joints from rodents with CIA or AIA indicated severe proliferation and hyperplasia of the synovium, with significant infiltration of inflammatory cells, pannus formation, narrowing of joint space, cartilage damage and bone erosion. Of note, berberine treatment inhibited these pathologic events and improved the joint rigidity in both CIA 22 , 23 , 24 and AIA 25 , 26 , 27 , 28 , 29 models.
Of note, berberine treatment caused a significant reduction in the level of anti‐CII (type II collagen) IgG in CIA rats. 22 , 23 Moreover, berberine decreased the production of inflammatory cytokines IFN γ, IL‐17 and IL‐2 by collagen‐stimulated splenocytes. 22 These findings exhibit that berberine can exert anti‐arthritic effects through suppressing both the humoral and cell‐mediated immune responses. 22 The progression of joint destruction, bone loss and uncontrolled proliferation of synoviocytes in RA are mainly mediated by pro‐inflammatory mediators circulating in the bloodstream and synovial fluid. 30 In CIA rats, the levels of pro‐inflammatory mediators RANKL, TNF‐α, IL17, IL‐6 and IL‐1β were indicated to be increased in the blood and synovium, and berberine treatment significantly diminished the levels. 24 Similarly, berberine treatment decreased plasma levels of these proinflammatory mediators in AIA rats, which was accompanied with considerable suppression of pathological inflammatory signs and events in the joint. 25 , 27 , 28 Further studies on AIA rats showed that berberine reduced bone loss and increased calcium retainability by reducing the proteolytic activity of osteoclasts through reducing RANKL release in the joint region, 28 together with a reduction in the just‐mentioned pro‐inflammatory cytokines. 25 , 27 , 28
The suppressive effect of berberine on bone erosion is further supported by studies that show berberine can directly attenuate RANKL‐mediated osteoclastogenic differentiation by inhibiting the nuclear factor κB (NF‐κB) and Akt activation. 31 , 32 RANKL induces the differentiation of osteoclast precursor cells, stimulates bone resorption by osteoclasts, and supports the survival of mature osteoclasts through activating the NF‐κB pathway and phosphatidylinositide (PI) 3‐kinase/Akt signalling. 33 Particularly, berberine suppresses RANKL‐induced activation of NF‐κB through inhibiting phosphorylation of IκBα kinase β, phosphorylation and degradation of IκBα, and NF‐κB p65 nuclear translocation. 32 PI3K/Akt signalling mediates osteoclastogenesis via up‐regulation of nuclear factor of activated T cells 1 (Nfatc1) that results in increased levels of various bone resorptive enzymes including tartarate acid phosphatase (TRAP), cathepsin K and MMP9 mediated through RANKL. 34 , 35 , 36 Of note, berberine can inhibit RANKL‐induced Akt phosphorylation 32 and, thereby, lead to suppression of PI3K dependent nuclear factor of activated T cells 1 (Nfatc1) induction and inhibition of mentioned resorptive enzymes. 31 The synovium infiltration by inflammatory cells and proliferation of fibroblast‐like synoviocytes (FLS) results in the formation of an invasive pannus, an inflammatory fibrovascular tissue that invades the joint and destroys the adjacent cartilage and bone. 37 In this event, angiogenesis has a critical role, whereby newly formed vessels can maintain the chronic inflammatory state by transporting inflammatory cells to sites of synovitis, and supply nutrients and oxygen to the pannus. 38 Importantly, berberine could significantly decrease microvessel density and pannus formation in synovial tissues and thereby prevent cartilage destruction and bone erosion in both CIA and AIA models. 22 , 23 , 25
Since more than 95% of the functional pathways in the human and rodent catalogs are identical, 39 , 40 developing the experimental arthritis model in rodents can provide convincing conclusions. Therefore, the just‐mentioned berberine's effects on joint inflammation as well as the cartilage and bone damage in rodent models of RA can reveal effective molecular targets behind this scenario, which may be accounted as therapeutic targets for RA management. In the following sections, cellular and molecular evidence behind berberine's effects on the joint inflammation are discussed.
2.2. Inhibitory effects of berberine on the inflammatory proliferation of FLS cells
FLS cells resembling tumour‐like proliferation are the prominent infiltrated cells circulating hyperplastic synovium in the joint space that majorly contribute to both initiation and progression of RA, including pannus formation and secretion of proinflammatory that induce inflammation, neovascularization and cartilage degradation. 37 , 41 There are numbers of signalling pathways detected in recent years to induce the cellular survival/proliferation of FLS cells, 42 and berberine has been identified to affect such pathways (discussed below) and thereby suppress proinflammatory proliferation of these cells in RA.
2.2.1. Berberine reverse defective cell cycle arrest and apoptosis in FLS cells
Defective cell cycle arrest and apoptosis in rheumatoid arthritis FLS (RAFLS) cells have been known to be an effectual mechanism underlying uncontrolled cell proliferation and synovial hyperplasia. In vitro studies on RA‐FLS cells isolated from RA patients indicate that berberine dose‐dependently inhibits cell proliferation through inducing apoptosis and cell cycle arrest. 43 Molecular studies reveal that berberine arrests cell cycle at the G0/G1 phase through stimulating cyclin‐dependent kinase (CDK) inhibitors Kip1/p27 and Cip1/p21, which suppress the progression of cells through the G0/G1 to S phase, and reducing protein levels of Cdks and cyclins, including CDK2, CDK4 and CDK6, and cyclins D1, D2 and E, which mediate cell cycle progression. 43 Likewise, berberine‐induced apoptosis in RAFLS cells is identified to be mediated through elevating the expression of pro‐apoptotic protein Bax and reducing the expression of anti‐apoptotic proteins Bcl‐2 and Bcl‐xl, disruption of mitochondrial membrane potential, and activation of caspase‐3, caspase‐9 and poly (ADP‐ribose) polymerase. 43
2.2.2. Berberine reverse defective autophagy in FLS cells
Pro‐inflammatory cytokines circulating in the synovial compartment have been found to hamper apoptosis and induce libertine proliferation of RAFLS cells through promoting autophagic responses in RA. 44 , 45 , 46 , 47 , 48 , 49 Autophagy is a cellular homeostatic process providing energy to support cellular survival during stressful states; however, its aberrant activity has been found to provoke pathogenesis of autoimmune diseases such as RA. 50 , 51 It has been recently shown that IL‐21/IL‐21 receptor (IL‐21R) interaction can induce autophagic influx and consequent uncontrolled proliferation in adjuvant‐induced arthritic FLS (AAFLS) cells through activating the PI3K/Akt proinflammatory signalling pathway that influences the expression of both apoptosis‐ and autophagy‐related genes. 31 , 52 Upon IL‐21 stimulation, autophagy is induced in AAFLS cells through promoting PI3K/Akt pathway that results in up‐regulation of autophagy‐related 5 (Atg5), Beclin‐1 and LC3‐phosphatidylethanolamine conjugate 3‐II (LC3‐II) via the utilization of p62 and suppression of C/EBP homologous protein (CHOP) transcription factor. 52
Interestingly, berberine is found to decrease the gene and protein levels of IL‐21R complex in AAFLS cells and whereby suppress IL‐21/IL‐21R dependent‐autophagy mediated through PI3K/Akt signalling via inhibiting autophagic mediators, p62 sequestration and promotion of CHOP in a dose‐dependent manner. 52 Besides, this autophagic pathway is also known to evade apoptosis through induction of B‐cell lymphoma 2 (Bcl‐2) anti‐apoptotic transcription factor and diminish the expression of Bcl‐2 associated X protein (BAX) pro‐apoptotic protein in AAFLS cells, which is markedly attenuated by berberine treatment. 31 , 52 In conclusion, these findings indicate that each member of IL21/IL21R‐autophagy mediators‐PI3K/Akt axis can serve an efficient therapeutic target for treating RA.
2.2.3. Berberine inhibits Wnt1/β‐catenin signalling involved in the proliferation of FLS cells
The Wnt signalling pathways involve important signalling transducers, which are recruited for both paracrine and autocrine routes of cellular communications through which regulate vital aspects of cell proliferation, differentiation, migration and organogenesis. 53 Wnt signalling pathway has been known to prominently participate in bone deformities in RA. Importance of Wnt signalling in RA pathogenesis was initially evidenced by observing the high expression of Wnt protein and its receptor, frizzled (Fz) complex, in the synovial joint region of patients with RA. 54 Further studies in recent years have shown that Wnt signalling through β‐catenin activation contributes to pleomorphic changes in osteocytes/chondrocytes leading to bone erosion and cartilage degradation in RA. 55 , 56 , 57 Wnt signalling promotes this effect through the interaction of circulating soluble Wnt proteins with Fz receptor complex majorly expressed on the surface of RAFLS cells. Among Wnt protein family, Wnt1 is mostly produced in FLS cells, which induces their tumour‐like proliferation, MMP secretion and generation of pro‐inflammatory cytokines. 58 Wnt1/β‐catenin signalling is triggered when Wnt1 protein binds to the cell surface FZD4 receptor, resulting in activation of downstream intracellular mediators including LDL receptor‐related protein 5 (LRP5) and Dishevelled segment polarity protein 1 (Dvl‐1) that leads to sequestration of a β‐catenin transcription factor in RAFLS cells. 53 , 59 The excessive activation of β‐catenin inside the cells promotes uncontrolled secretion of inflammatory cytokines, aberrant cell proliferation, bone erosion, cartilage degradation and pannus formation. Such cellular events are mainly mediated through excessive release of inflammatory cytokines and RANKL that elevate the proteolytic activity of osteoclasts at the joint. 59 The Wnt1/β‐catenin signalling is known to be naturally suppressed by both LRP inhibitor (Dickkopf homolog 1 [DKK1]) and Dvl‐1 inhibitor (CYLD; a cell cycle regulator) that lead to the dormancy of β‐catenin inside the FLS cells, while are repressed in FLS cells during RA condition. 60 , 61 , 62
Recent research reveals that berberine suppresses the Wnt1/β‐catenin signalling in AAFLS cells by decreasing the levels of FZD4, LRP5 and Dvl‐1 via inducing CYLD, which result in the decreased levels of β‐catenin. Moreover, berberine can reduce the β‐catenin levels through the up‐regulation of miR‐23a that is found to be an LRP5 inhibitor. These effects further decrease the expression levels of various pro‐inflammatory cytokines (TNF‐α, IL‐1β, IL‐6 and IL‐23) and the release of RANKL, whereby reverse the excessive levels of inflammation, cartilage degradation and bone erosion, as well as pannus formation and immune cells infiltration at the joint space in AIA rats. 28 To sum up, the aforementioned findings revealing the crucial impact of the Wnt1/β‐catenin signalling pathway in RA progression and the inhibitory effect of berberine on this pathway accompanied by amelioration of RA clinical complications suggest the potential of Wnt1/β‐catenin signalling mediators as the effective therapeutic targets for treating RA.
2.2.4. Berberine inhibits the lipid‐mediated signals involved in the proliferation of FLS cells
Hyperlipidemia, a leading cause of inflammatory atherosclerosis, has been found to exacerbate arthritis development in RA patients, 63 , 64 , 65 while lipid‐reducing drugs, such as statins, are known to considerably decrease the risk of RA. 66 Lipid metabolism alterations through affecting the signal pathways that regulate cell growth, energy homeostasis 67 and inflammation 68 have been indicated to involve in the pathogenesis of various diseases. 69 , 70 There is growing evidence showing a positive and impressive association of abnormal lipid metabolism with the development and pathogenesis of RA. 63 , 64 , 71 , 72 , 73 In recent years, the cellular signalling pathways such as AMPK/lipogenesis and LPA/MAPK have been recognized to mediate the inflammatory effect of lipids on FLS proliferation in RA condition, and berberine has been found to regulate such pathways and thereby decrease complications of the disease, as discussed in following subsections.
AMPK/lipogenesis signalling pathway
Highly proliferative cells, such as RAFLSs, possess an excessive lipogenesis that provides the energy and lipids for supporting cellular growth and survival. 74 Pro‐inflammatory cytokines, such as TNF‐α is involved in the pathogenesis of RA‐related atherosclerosis. 75 , 76 Of note, TNFα‐stimulated RAFLS cells are known to acquire inflammatory phenotype with high intracellular levels of pro‐inflammatory cytokines including IL‐1β, IL‐6, IL‐8, IL‐25, and IL‐33 as well as the increase lipogenic activity and the elevated expression of key lipid metabolism regulators such as sterol regulatory element‐binding protein 1 (SREBP‐1). 27 Interestingly, berberine suppressed inflammatory growth of TNF‐stimulated RAFLS cells by reducing the increased intracellular levels of pro‐inflammatory cytokines as wells as decreasing the intracellular level of palmitic acid, the key intermediate metabolite of lipogenesis, via down‐regulating the increased expression of SREBP‐1. 27 The anti‐lipogenic activity of berberine is found to attribute to a direct inhibitory impact on the mitochondrial respiratory chain, which led to Adenosine 5'‐monophosphate (AMP)‐activated protein kinase (AMPK) activation. AMPK is an energy sensor and regulates energy homeostasis of cells by detecting changes in the AMP/ATP ratio. 26 , 27 In this way, berberine through direct targeting respiration chain complex I deprives ATP levels and consequently promotes AMPK activity that suppresses downstream acetyl‐CoA carboxylase and thus inhibits the fatty acid synthesis and SREBP‐1 expression, resulting in lipogenesis reduction in RAFLS cells. 26 , 27 Importantly, such an effect of berberine in AAFLS cells has been found to associate with reduced bone erosion and RA complications in AIA rats. 27 In summary, above‐mention findings indicate that increased lipogenesis in FLS cells plays important role in the pathogenesis of RA, and berberine trough enhancing AMPK signalling pathway can effectively suppress lipogenic activity, which leads to decrease inflammatory growth of FLS cells, resulting in ameliorating effects on the progression of bone damage in RA. Therefore, AMPK/lipogenesis signalling cascade can be the candidate as a valuable therapeutic target for RA treatment.
LPA/MAPK signalling pathway
Lysophosphatidic acid (LPA) is an inflammatory phospholipid driving a potent lipid‐signalling medium with growth‐factor‐like activities and has been detected to be elevated in RA. 71 The levels of plasma and synovial LPA in RA patients are found to be significantly higher than those in healthy subjects. 77 LPA is suggested to be an independent risk factor of synovial hyperplasia in RA in addition to the high burden of inflammation. 77 During inflammation, LPA is majorly generated in activated platelets through the degradation of lysophosphatidylcholine by secretory lysophospholipase D termed as autotaxin (ATX) that is detected to be highly elevated in the activated arthritic synovial fluid from RA patients and AIA rats, 77 , 78 and berberine has been indicated to decrease the expression of ATX in RAFLS cells and thus decrease LPA levels in RA condition. 77
LPA can induce the inflammatory proliferation and growth of various cell types, and the LPA signalling has been confirmed to be involved in the inflammatory response of RAFLS cells. 77 , 79 In particular, LPA mediates its effects through binding to G‐protein‐coupled LPA receptors that activate downstream mitogen‐activated protein kinases (MAPK) signalling pathways. 77 , 79 Principally, the MAPK cascades have been known to participate in the proliferation, apoptosis, stress responses, inflammation and joint destruction of RA. 80 In RAFLS cells, two main MAPK pathways including the ERK1/2 and the p38 MAPK are found to be promoted by the binding of LPR to the plasma membrane G‐protein‐coupled receptor, followed by activation of the small G‐protein Ras. 77 , 79 In brief, activated Ras recruits and activates the protein kinase Raf that activates downstream ERK/p38 MAPKs. 77 , 79 Among LPA receptors, LPA receptor 1 (LPR1) is essential in RAFLS cells, presents in large amounts in the synovial fluids of RA patients 79 ; berberine is found to block LPR1 and, thereby, suppress the proliferation and redundant generation of inflammatory cytokines IL‐6 and TNF‐α in RAFLS cells through inhibiting activation of Ras/Raf/ERK/p38 axis. 77
To sum up, LPA possesses mitogenic and proinflammatory effects in RAFLS cells, and berberine can inhibit the proliferation and inflammatory phenotype of RAFLS cells through both inhibiting ATX‐mediated LPA activation and blocking LPA/LPA1/ERK/p38 MAPK signalling, which suggests a potential therapeutic target for developing anti‐RA drugs.
2.3. Inhibitory effects of berberine on dendritic cells
The inflammatory process of RA is promoted by maturation and activation of dendritic cells (DCs) that present arthritis‐associated antigens to T cells, resulting in the increased production of inflammatory cytokines, the inflammatory proliferation of synovitis, and joint destruction. 81 , 82 , 83 Timely elimination of mature DCs is important to prevent aberrant activation of the inflammatory immune responses. Apoptosis deficiency in DCs leads to the accumulation and prolonged activity of DCs that, in turn, result in long‐last activation of lymphocytes and progression of autoimmunity response. 84 Bone marrow (BM)‐derived myeloid DCs (MDCs) and plasmacytoid DCs (PDCs) are the main DC subsets contributing to RA progression. MDCs express a great level of IL‐12 whereby promoting Th1 responses and representing pro‐inflammatory activities. PDCs are known to activate and generate type I IFN through viral infection and whereby derive protective antiviral inflammation. Besides, long‐last induction of PDCs and the production of type I IFN in the absence of infection can lead to autoimmune diseases such as RA. 82 Of note, immunosuppressive drugs inhibiting T‐cell activation, including rapamycin, cyclosporine A and dexamethasone, have been shown to effectively inhibit DC functions or to promote DC apoptosis. 82 , 85 , 86 , 87 Therefore, DCs have been suggested to provide a novel and efficient target for immunosuppressive therapy in RA. Despite the improving effect of immunosuppressive agents on RA treatment, their clinical utilization is restricted because of potential toxic effects, indicating the necessity of the alternative therapeutic drugs.
Berberine has been shown to exert anti‐apoptotic effects on DCs in in vitro and in vivo models of RA. 22 Berberine could time‐ and dose‐dependently induce apoptosis in murine BM‐derived MDCs. 22 Freshly isolated BM cells are found to be insensitive to berberine, and the susceptibility to berberine‐promoted apoptosis is increased during DC differentiation, in which mature IL‐12‐producing DCs show higher sensitivity to berberine than immature DCs. Thus, berberine can selectively trigger apoptosis in mature DCs and whereby restrict DC maturation and shorten their lifespan. 22 Although the exact intracellular mechanisms underlying selective pro‐apoptotic effect in DCs remain unknown, it has been shown that the production of reactive oxygen species (ROS) and mitochondrial depolarization, as well as caspase 3 activation, are involved in berberine‐mediated apoptosis induction. 22 Interestingly, berberine at the same concentrations, which strongly induces apoptosis in BMDCs, shows no significant pro‐apoptotic effect in murine peritoneal macrophages, Jurkat cells, or RAW 264.7 cells, indicating the specific pro‐apoptotic effect of berberine to DCs. 22 Berberine is also found to induce apoptosis in splenic DCs that exhibit higher sensitivity to berberine‐promoted apoptosis than splenic T and B cells. 22 Moreover, both MDCs and PDCsn subsets, in both BM‐derived DCs and splenic DCs, indicate similar sensitivity to berberine‐promoted apoptosis, showing that the pro‐apoptotic effect of berberine is DC subset independent. 22 In accordance with the aforementioned in vitro findings, berberine can markedly reduce the ratio of mature to immature DCs in spleens, confirming its selective pro‐apoptotic effect in mature DCs in vivo. 22 In this regards, berberine treatment can cause a considerable loss of DCs and an elevation in the apoptosis of DCs within spleens and lymph nodes in CIA mice, which is accompanied by the antiarthritic and immunosuppressive effects in these mice. 22 Since mature DCs play the crucial roles in pathogenic inflammation and immune responses in RA, berberine‐induced apoptosis in mature DCs provides a major mechanism of immunomodulation that can account, at least in part, for the immunosuppressive and antiarthritic impacts observed in animal models of RA.
2.4. Modulatory effect of berberine on inflammatory M1 macrophages
Clinical studies indicate that macrophages are crucial in inducing the progression of inflammation and exacerbation of joint destruction in RA. 88 , 89 The number of infiltrating macrophages in the synovial intimal lining layer associates with the degree of disease activity and joint erosion in the inflamed synovial tissue. 90 , 91 , 92 , 93 Macrophages differentiate into two different polarization states serving opposite function; M1 macrophages can engage in inflammatory reactions by secreting pro‐inflammatory cytokines, such as IL‐1β, IL‐6 and TNF‐α, and inducible nitric oxide synthase (iNOS), and M2 macrophages alleviate inflammation and induce tissue repair by secreting anti‐inflammatory cytokines, such as IL‐10, transforming growth factor (TGF)‐β1 and by inducing arginase 1(Arg1). 94 Importantly, the ratio of M1/M2 macrophages has been found to be remarkably elevated in the synovial fluid of RA patients. 95 , 96
It has been also shown that peritoneal macrophages in AIA rats are mainly of the M1 polarization status, and berberine treatment can restore the balance of the peritoneal M1/M2 by reducing the levels of M1 cytokines (TNF‐α, IL‐1β and IL‐6), increasing the levels of M2 cytokines (IL‐10 and TGF‐β1), increasing the expression of Arg1 (M2 marker) and decreasing the expression of iNOS (M1 marker). 29 Mechanisms underlying the modulatory effect of berberine on macrophages are based on the theory that the inflammatory response promoted by tissue injury is closely related to metabolic regulation. AMPK is activated when the ratio of ATP/AMP is low in cells. 97 In M1 macrophages, the level of reactive oxygen species (ROS) is increased and the expression of iNOS is up‐regulated because of the elevated glycolysis flux needed to maintain the ATP level for the biosynthesis of inflammatory cytokines and the potential of the mitochondrial membrane as well as to prolong the lifespan of macrophages. 98 AMPK has been found to play an important role in the inhibition of inflammation by inducing polarization of macrophages to the M2 phenotype. 97 AMPK in macrophages negatively regulates NF‐κB activity, which is a central transcription factor for regulating the expression of pro‐inflammatory cytokines. 99
Mechanistic studies on AMPK/NF‐κB pathway in peritoneal macrophages isolated from berberine‐treated AIA rats showed that berberine induces M1 macrophage polarization to the anti‐inflammatory M2 functional phenotype and inhibits the production of inflammatory cytokines by activating AMPK and remarkably attenuating the NF‐кB activation. 29 In sum, berberine was found to exert the anti‐arthritic effect on AIA rats by modulating the polarization of macrophages through the AMPK/NF‐кB pathway. 29 Therefore, it can be concluded that drugs targeting the regulation of the macrophage polarization may become a valuable prospective therapeutic approach for RA.
2.5. Modulatory effects of berberine on Th17/Treg cells responses
Th17/Treg cells imbalance has been known to have a key role in the development and progression of RA; Th17 cells govern the disease development in which function and frequency of Th17 cells are increased in both synovial fluid and circulating blood of RA patients, whereas Treg cells, which are functionally defective in patients with RA, can inhibit pro‐inflammatory responses and hamper the destructing effects of Th17 cells by decreasing its accumulation at the inflammation niches. 100 , 101 The function of Th17 cells stems from the secretion of inflammatory cytokines, such as IL‐17. 102 , 103 In this regard, an increased level of IL‐17 in the RA synovial fluid can highlight the importance of IL‐17 in the development of RA. 104 Likewise, IL‐17/Th17 deficiency 105 or treatment with IL‐17R antagonist/IL‐17 neutralizing antibody 106 have been shown to ameliorate arthritis development in mice coincided with reduced joint damage. 107 Th17 cells in the RA synovium undergo uncontrolled differentiation 108 , 109 and express high levels of CD196, a Th17‐specific marker and RAR‐related orphan receptor gamma T (RORγt) transcription factor that is essential for Th17 differentiation and survival. 110
There have been several in vitro and in vivo studies that indicate the modulatory effects of berberine on function and proliferation of Th17 and Treg cells in RA. Berberine is shown to significantly reduce the blood levels of Th17 population and the serum levels of IL‐17 in CIA rats. Of note, this effect is accompanied by the decreased expression of IL‐17 in synovium and Th17 transcription factor RORγt in the spleen. 24 An in vitro study on naïve T cells isolated from the spleen of AIA rats reveals that berberine treatment can significantly decrease differentiation and survival of Th17 cells, in a concentration‐dependent manner, through down‐regulating surface marker CD196 and RORγt transcription factor. In contrast, berberine‐treated naïve CD4+ T cells differentiated into CD4+ CD25+ Foxp3+ Treg cells through activating AhR/CYP1A1/Foxp3 axis. 52 The differentiation and survival of Treg cells rely on the induction of Foxp3, which is induced through aryl hydrocarbon receptor (AhR) transcription factor and elevation in levels of cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1), a downstream element of AhR. 111 In mechanism, berberine activates AhR transcription factor by which up‐regulates CYP1A1 levels and subsequently increases Foxp3 expression. 52 These findings can be further supported by reports that show berberine treatment can modulate Th17/Treg responses in other autoimmune conditions, such as experimental colitis 112 , 113 , 114 and type 1 diabetes 115 as well as experimental autoimmune encephalomyelitis 116 and myocarditis. 117 In conclusion, berberine treatment can inhibit differentiation of Th17 cells via suppressing its proliferation and induce differentiation of CD4+ CD25+ Foxp3+ Treg cells through activation of AhR transcription factor, therefore modulates Th17/Treg imbalance in RA.
2.6. The gut‐mediated immunomodulatory effects of berberine on CIA
Berberine has shown to exert higher ameliorating effects on CIA progression by oral administration than intravenous injection. 24 However, because of poor intestinal absorption, the oral bioavailability of berberine is very low. Once berberine was orally administered in rats at a dose of 200 mg/kg, the peak blood levels were less than 0.06 µM, 22 , 118 which was far from its minimal effective concentration in vitro (<50 µM). Therefore, berberine absorption after oral treatment is not adequate to directly alleviate arthritis, and the systemic ameliorating mode of berberine might in part be under gut‐dependent mechanisms. 24 The gastrointestinal tract is known to be an endocrine organ secreting various immunosuppressive neuropeptides, such as cortistatin (CST), that play key roles in modulating the balance of proinflammatory and anti‐inflammatory responses as well as the balance of Th17 and Treg cells in autoimmune diseases. 119 , 120 , 121 There is evidence that shows unabsorbed berberine might accumulate in the gastrointestinal tract and provoke the production of CST in enteric neurons and endocrine cells and thus direct an anti‐arthritic impact in AIA rats. 24 The other possible mechanism by which berberine modulates pro‐inflammatory responses in RA condition is gut microbiota‐dependent. 122 RA patients possess a distinct composition of the gut microbiota 123 , 124 that is known to be one of the environmental triggers of RA progression. 125 , 126 , 127 In vivo study on CIA rats with the alterations in the gut microbiota similar to RA patients revealed that oral administration of berberine could ameliorate CIA in rats in a gut microbiota‐dependent manner through increasing the abundance of butyrate‐producing bacteria, inducing the expression and activity of butyryl‐CoA: acetate‐CoA transferase (BUT) and increasing the intestinal butyrate level. 122 Of note, berberine can adjust the host intestinal environment to a condition that is more conducive to the growth of butyrate‐producing bacterial by limiting the production of nitrate and stabilizing the local physiologic hypoxia in the intestine, 128 , 129 and this adjustment can be reversed by suppressing BUT activity. 122 In conclusion, berberine may also ameliorate RA severity and progression through affecting gut‐joint axis via activating CST and BUT function, and thus the novel therapeutic agents that target CST and BUT might be promising for the management of RA.
3. CONCLUSION
Accumulation of scientific evidence from many in vitro and in vivo experimental study exhibits that berberine may be beneficial for ameliorating RA complications. Berberine can suppress synovial joint inflammation together with cartilage and bone damage through inhibiting inflammatory proliferation of FLS cells, suppressing DC activation, modulating Th17/Treg balance, as well as inducing the gut‐mediated immunosuppression and adjusting the gut microbiota. Multiple signalling pathways including cell cycle arresting signalling, apoptosis‐mediating pathways, PI3K/Akt, Wnt1/β‐catenin, AMPK/lipogenesis and LPA/LPA1/ERK/p38 MAPK, CST and BUT are the common molecular targets of berberine in RA disorder, which can be considered as the efficient therapeutic target for managing RA. To our knowledge, all reported ameliorating effects of berberine on RA complications are based on preclinical and cell culture investigations. Hence, further investigations are needed to determine the clinical efficiency of berberine in RA patients.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest and financial support for the present review article.
AUTHOR CONTRIBUTIONS
Peng Shen: Writing‐original draft (lead). Yang Jiao: Conceptualization (equal); project administration (lead); validation (equal). Li Miao: Writing‐review and editing (equal). Ji‐hua Chen: Data curation (lead); investigation (equal). Amir Abaas Momtazi‐Borojeni: Conceptualization (lead); supervision (supporting); validation (equal).
CODE AVAILABILITY
Not applicable.
ACKNOWLEDGEMENT
This work was financially supported by the Open Project of State Key Laboratory of Military Stomatology (No. 2018KA02).
Shen P, Jiao Y, Miao L, Chen J‐H, Momtazi‐Borojeni AA. Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J Cell Mol Med. 2020;24:12234–12245. 10.1111/jcmm.15803
Web of Science ResearcherID: AAC‐6972‐2019
Scopus ID: 37039724400
Contributor Information
Yang Jiao, Email: jiaoyang1981731@163.com.
Amir Abbas Momtazi‐Borojeni, Email: amirabbas.momtazi64@gmail.com, Email: abbasmomtazi@yahoo.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023‐2038. [DOI] [PubMed] [Google Scholar]
- 3. Bartok B, Firestein GS. Fibroblast‐like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bottini N, Firestein GS. Duality of fibroblast‐like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lefèvre S, Knedla A, Tennie C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redlich K, Hayer S, Ricci R, et al. Osteoclasts are essential for TNF‐α–mediated joint destruction. J Clin Investig. 2002;110:1419‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. The American journal of pathology. 2001;159:1689‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martel‐Pelletier J, Welsch DJ, Pelletier J‐P. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805‐829. [DOI] [PubMed] [Google Scholar]
- 10. Laev SS, Salakhutdinov NF. Anti‐arthritic agents: progress and potential. Bioorg Med Chem. 2015;23:3059‐3080. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11:276. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh P‐H, Wu O, Geue C, McIntosh E, McInnes IB, Siebert S. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis. 2020;79:771‐777. [DOI] [PubMed] [Google Scholar]
- 13. Hresko A, Lin TC, Solomon DH. Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta‐analysis. Arthritis Care Res (Hoboken). 2018;70:1431‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fatahian A, Mohammadian Haftcheshmeh S, Azhdari S, Kaboli Farshchi H, NIkdar B, Momtazi‐Borojeni A. Promising Anti‐atherosclerotic Effect of Berberine: Evidence from In Vitro, In Vivo, and Clinical Studies Reviews of Physiology, Biochemistry and Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2020:1–28. [DOI] [PubMed] [Google Scholar]
- 15. Kumar A, Ekavali , Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015;761:288‐297. [DOI] [PubMed] [Google Scholar]
- 16. Cicero AF, Baggioni A. Berberine and its role in chronic disease. Anti‐inflammatory Nutraceuticals and Chronic Diseases. Cham, Switzerland: Springer; 2016:27‐45. [DOI] [PubMed] [Google Scholar]
- 17. Zhang M, Feng L, Li J, Chen L. Therapeutic potential and mechanisms of berberine in cardiovascular disease. Curr Pharmacol Rep. 2016;2:281‐292. [Google Scholar]
- 18. Ayati SH, Fazeli B, Momtazi‐Borojeni AA, Cicero AF, Pirro M, Sahebkar A. Regulatory effects of berberine on microRNome in cancer and other conditions. Crit Rev Oncol/Hematol. 2017;116:147‐158. [DOI] [PubMed] [Google Scholar]
- 19. Mortazavi H, Nikfar B, Esmaeili S‐A, et al. Potential cytotoxic and anti‐metastatic effects of berberine on gynaecological cancers with drug‐associated resistance. Eur J Med Chem. 2019;187:111951. [DOI] [PubMed] [Google Scholar]
- 20. Benson RA, McInnes IB, Garside P, Brewer JM. Model answers: Rational application of murine models in arthritis research. Eur J Immunol. 2018;48:32‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bevaart L, Vervoordeldonk MJ, Tak PP. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62:2192‐2205. [DOI] [PubMed] [Google Scholar]
- 22. Hu Z, Jiao Q, Ding J, et al. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Rheum. 2011;63:949‐959. [DOI] [PubMed] [Google Scholar]
- 23. Wang Z, Chen Z, Yang S, et al. Berberine ameliorates collagen‐induced arthritis in rats associated with anti‐inflammatory and anti‐angiogenic effects. Inflammation. 2014;37:1789‐1798. [DOI] [PubMed] [Google Scholar]
- 24. Yue M, Xia Y, Shi C, et al. Berberine ameliorates collagen‐induced arthritis in rats by suppressing Th17 cell responses via inducing cortistatin in the gut. FEBS J. 2017;284:2786‐2801. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, He X, Zhang C‐F, Guo C‐R, Wang C‐Z, Yuan C‐S. Anti‐arthritic effect of berberine on adjuvant‐induced rheumatoid arthritis in rats. Biomed Pharmacother. 2017;89:887‐893. [DOI] [PubMed] [Google Scholar]
- 26. Fan X‐X, Xu M‐Z, Leung EL‐H, Jun C, Yuan Z, Liu L. ROS‐Responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nano‐Micro Letters. 2020;12:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan X‐X, Leung EL‐H, Xie Y, et al. Suppression of lipogenesis via reactive oxygen species–AMPK signaling for treating malignant and proliferative diseases. Antioxid Redox Signal. 2018;28:339‐357. [DOI] [PubMed] [Google Scholar]
- 28. Sujitha S, Dinesh P, Rasool M. Berberine encapsulated PEG‐coated liposomes attenuate Wnt1/β‐catenin signaling in rheumatoid arthritis via miR‐23a activation. Eur J Pharm Biopharm. 2020;149:170‐191. [DOI] [PubMed] [Google Scholar]
- 29. Zhou J, Yu Y, Yang X, et al. Berberine attenuates arthritis in adjuvant‐induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF‐кB pathway. Eur J Pharmacol. 2019;852:179‐188. [DOI] [PubMed] [Google Scholar]
- 30. Moreland LW, Curtis JR. Systemic nonarticular manifestations of rheumatoid arthritis: focus on inflammatory mechanisms. Semin Arthritis Rheum. 2009;39:132‐143. [DOI] [PubMed] [Google Scholar]
- 31. Dinesh P, Rasool M. Berberine inhibits IL‐21/IL‐21R mediated inflammatory proliferation of fibroblast‐like synoviocytes through the attenuation of PI3K/Akt signaling pathway and ameliorates IL‐21 mediated osteoclastogenesis. Cytokine. 2018;106:54‐66. [DOI] [PubMed] [Google Scholar]
- 32. Hu J‐P, Nishishita K, Sakai E, et al. Berberine inhibits RANKL‐induced osteoclast formation and survival through suppressing the NF‐κB and Akt pathways. Eur J Pharmacol. 2008;580:70‐79. [DOI] [PubMed] [Google Scholar]
- 33. Lee ZH, Kim H‐H. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Comm. 2003;305:211‐214. [DOI] [PubMed] [Google Scholar]
- 34. Moon JB, Kim JH, Kim K, et al. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J Immunol. 2012;188:163‐169. [DOI] [PubMed] [Google Scholar]
- 35. Blüml S, Friedrich M, Lohmeyer T, et al. Loss of phosphatase and tensin homolog (PTEN) in myeloid cells controls inflammatory bone destruction by regulating the osteoclastogenic potential of myeloid cells. Ann Rheum Dis. 2015;74:227‐233. [DOI] [PubMed] [Google Scholar]
- 36. Yuan F‐L, Xu R‐S, Jiang D‐L, et al. Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF‐κB and PI3K/Akt signaling pathways. Bone. 2015;75:128‐137. [DOI] [PubMed] [Google Scholar]
- 37. Bustamante MF, Garcia‐Carbonell R, Whisenant KD, Guma M. Fibroblast‐like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marrelli A, Cipriani P, Liakouli V, et al. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011;10:595‐598. [DOI] [PubMed] [Google Scholar]
- 39. Nilsson S, Helou K, Walentinsson A, Szpirer C, Nerman O, Ståhl F. Rat–mouse and rat–human comparative maps based on gene homology and high‐resolution zoo‐FISH. Genomics. 2001;74:287‐298. [DOI] [PubMed] [Google Scholar]
- 40. Gibbs RA, Weinstock GM, Metzker ML, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493‐520. [DOI] [PubMed] [Google Scholar]
- 41. Tu J, Hong W, Zhang P, Wang X, Körner H, Wei W. Ontology and function of fibroblast‐like and macrophage‐like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol. 2018;9:1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falconer J, Murphy AN, Young SP, et al. Synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2018;70:984‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang X‐H, Jiang S‐M, Sun Q‐W. Effects of berberine on human rheumatoid arthritis fibroblast‐like synoviocytes. Exp Biol Med. 2011;236:859‐866. [DOI] [PubMed] [Google Scholar]
- 44. Kim EK, Kwon J‐E, Lee S‐Y, et al. IL‐17‐mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2018;8:e2565‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang L, Feng X, Gao W. Proliferation of rheumatoid arthritis fibroblast‐like synoviocytes is enhanced by IL‐17‐mediated autophagy through STAT3 activation. Connect Tissue Res. 2019;60:358‐366. [DOI] [PubMed] [Google Scholar]
- 46. Connor AM, Mahomed N, Gandhi R, Keystone EC, Berger SA. TNFα modulates protein degradation pathways in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2012;14:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin N‐Y, Beyer C, Gießl A, et al. Autophagy regulates TNFα‐mediated joint destruction in experimental arthritis. Ann Rheum Dis. 2013;72:761‐768. [DOI] [PubMed] [Google Scholar]
- 48. Meng Q, Du X, Wang H, Gu H, Zhan J, Zhou Z. Astragalus polysaccharides inhibits cell growth and pro‐inflammatory response in IL‐1β‐stimulated fibroblast‐like synoviocytes by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Apoptosis. 2017;22:1138‐1146. [DOI] [PubMed] [Google Scholar]
- 49. Yang R, Zhang Y, Wang L, et al. Increased autophagy in fibroblast‐like synoviocytes leads to immune enhancement potential in rheumatoid arthritis. Oncotarget. 2017;8:15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vomero M, Barbati C, Colasanti T, et al. Autophagy and rheumatoid arthritis: current knowledges and future perspectives. Front Immunol. 2018;9:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Y‐M, Chang C‐Y, Chen H‐H, et al. Association between autophagy and inflammation in patients with rheumatoid arthritis receiving biologic therapy. Arthritis Res Ther. 2018;20:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dinesh P, Rasool M. Berberine mitigates IL‐21/IL‐21R mediated autophagic influx in fibroblast‐like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis. 2019;24:644‐661. [DOI] [PubMed] [Google Scholar]
- 53. Miao C‐G, Yang Y‐Y, He X, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25:2069‐2078. [DOI] [PubMed] [Google Scholar]
- 54. Sen M, Reifert J, Lauterbach K, et al. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002;46:2867‐2877. [DOI] [PubMed] [Google Scholar]
- 55. Daoussis D, Andonopoulos AP, Liossis S‐NC. Wnt pathway and IL‐17: novel regulators of joint remodeling in rheumatic diseases. Looking beyond the RANK‐RANKL‐OPG axis. Semin Arthritis Rheum. 2010;39:369‐383. [DOI] [PubMed] [Google Scholar]
- 56. Miao C, Chang J, Dou J, Xiong Y, Zhou G. DNA hypermethylation of SFRP2 influences the pathology of rheumatoid arthritis through the canonical Wnt signaling in model rats. Autoimmunity. 2018;51:319‐332. [DOI] [PubMed] [Google Scholar]
- 57. Zhou Y, Lin J, Shao J, et al. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone. 2019;127:324‐333. [DOI] [PubMed] [Google Scholar]
- 58. de Sousa RF, da Mota LMH, Lima RAC, et al. The Wnt signaling pathway and rheumatoid arthritis. Autoimmun Rev. 2010;9:207‐210. [DOI] [PubMed] [Google Scholar]
- 59. Liu XZ, Fan J, Qi KE, et al. Dishevelled2 promotes apoptosis and inhibits inflammatory cytokine secretion in rheumatoid arthritis fibroblast‐like synoviocytes through crosstalk with the NF‐κB pathway. Oncotarget. 2017;8:12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sun J, Yan P, Chen Y, et al. MicroRNA‐26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK‐3β/β‐catenin pathway. Diagnostic Pathol. 2015;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu X, Zhang Y, Ju W, Li C, Mu Y. MiR‐21 relieves rheumatoid arthritis in rats via targeting Wnt signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:96‐103. [DOI] [PubMed] [Google Scholar]
- 62. Liu L, Zuo Y, Xu Y, Zhang Z, Li Y, Pang J. MiR‐613 inhibits proliferation and invasion and induces apoptosis of rheumatoid arthritis synovial fibroblasts by direct down‐regulation of DKK1. Cell Mol Biol Lett. 2019;24:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kerekes G, Nurmohamed MT, González‐Gay MA, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10:691. [DOI] [PubMed] [Google Scholar]
- 64. Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513. [DOI] [PubMed] [Google Scholar]
- 65. Curtis JR, John A, Baser O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti–tumor necrosis factor therapy. Arthritis Care Res (Hoboken). 2012;64:1282‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tascilar K, Dell'Aniello S, Hudson M, Suissa S. Statins and risk of rheumatoid arthritis: a nested case‐control study. Arthritis Rheumatol. 2016;68:2603‐2611. [DOI] [PubMed] [Google Scholar]
- 67. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610‐2623. [DOI] [PubMed] [Google Scholar]
- 68. Spiegel S, Milstien S. The outs and the ins of sphingosine‐1‐phosphate in immunity. Nat Rev Immunol. 2011;11:403‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qu Q, Zeng F, Liu X, Wang Q, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kosinska MK, Liebisch G, Lochnit G, et al. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013;65:2323‐2333. [DOI] [PubMed] [Google Scholar]
- 72. Maradit‐Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population‐based study. Arthritis Rheum. 2005;52:722‐732. [DOI] [PubMed] [Google Scholar]
- 73. Kaplan MJ. Cardiovascular complications of rheumatoid arthritis: assessment, prevention, and treatment. Rheumatic Disease Clinics. 2010;36:405‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016;531:651‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Végh E, Kerekes G, Pusztai A, et al. Effects of 1‐year anti‐TNF‐α therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol Int. 2020;40:427‐436. [DOI] [PubMed] [Google Scholar]
- 76. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology. 2014;53:2143‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang H, Tu S, Yang S, et al. Berberine Modulates LPA Function to Inhibit the Proliferation and Inflammation of FLS‐RA via p38/ERK MAPK Pathway Mediated by LPA1. Evid‐Based Complement Alternat Med. 2019;2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nikitopoulou I, Oikonomou N, Karouzakis E, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J Exp Med. 2012;209:925‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao C, Fernandes MJ, Prestwich GD, et al. Regulation of lysophosphatidic acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? Mol Pharmacol. 2008;73:587‐600. [DOI] [PubMed] [Google Scholar]
- 80. Arthur JSC, Ley SC. Mitogen‐activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679‐692. [DOI] [PubMed] [Google Scholar]
- 81. Santiago‐Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory‐type responses. J Immunol. 2001;167:1758‐1768. [DOI] [PubMed] [Google Scholar]
- 82. Cavanagh LL, Boyce A, Smith L, et al. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res Ther. 2005;7:R230‐R240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lutzky V, Hannawi S, Thomas R. Cells of the synovium in rheumatoid arthritis. Dendritic cells. Arthritis Res Therapy. 2007;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tarbell KV, Rahman MJ. Dendritic Cells in Autoimmune Disease. The Autoimmune Diseases. : Elsevier; 2020:213‐227. [Google Scholar]
- 85. Penna G, Adorini L. 1α, 25‐dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405‐2411. [DOI] [PubMed] [Google Scholar]
- 86. Woltman AM, Massacrier C, de Fijter JW, Caux C, van Kooten C. Corticosteroids prevent generation of CD34+‐derived dermal dendritic cells but do not inhibit Langerhans cell development. J Immunol. 2002;168:6181‐6188. [DOI] [PubMed] [Google Scholar]
- 87. Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down‐regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818‐2824. [DOI] [PubMed] [Google Scholar]
- 88. Gent YYJ, ter Wee MM, Voskuyl AE, et al. Subclinical synovitis detected by macrophage PET, but not MRI, is related to short‐term flare of clinical disease activity in early RA patients: an exploratory study. Arthritis Res Ther. 2015;17:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Rycke L, Baeten D, Foell D, et al. Differential expression and response to anti‐TNFα treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol. 2005;206:17‐27. [DOI] [PubMed] [Google Scholar]
- 90. Soler Palacios B, Estrada‐Capetillo L, Izquierdo E, et al. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A‐dependent pro‐inflammatory profile. J Pathol. 2015;235:515‐526. [DOI] [PubMed] [Google Scholar]
- 91. Vieira‐Sousa E, Gerlag DM, Tak PP Suppl 1: synovial tissue response to treatment in rheumatoid arthritis. Open Rheumatol J. 2011;5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56:3869‐3871. [DOI] [PubMed] [Google Scholar]
- 93. Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang Y, Han C‐C, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol. 2017;50:345‐352. [DOI] [PubMed] [Google Scholar]
- 95. Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol. 2018;8:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115‐124. [DOI] [PubMed] [Google Scholar]
- 97. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′‐monophosphate‐activated protein kinase promotes macrophage polarization to an anti‐inflammatory functional phenotype. J Immunol. 2008;181:8633‐8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo‐starvation. Nature. 2013;493:346‐355. [DOI] [PubMed] [Google Scholar]
- 99. Huang B‐P, Lin C‐H, Chen H‐M, Lin J‐T, Cheng Y‐F, Kao S‐H. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF‐κB signaling in murine macrophages. DNA Cell Biol. 2015;34:133‐141. [DOI] [PubMed] [Google Scholar]
- 100. Alunno A, Manetti M, Caterbi S, et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators Inflamm. 2015;2015:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Frontiers Oncol. 2014;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yao Z, Painter SL, Fanslow WC, et al. Human IL‐17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483‐5486. [PubMed] [Google Scholar]
- 103. van den Berg WB, Miossec P. IL‐17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549‐553. [DOI] [PubMed] [Google Scholar]
- 104. Chabaud M, Durand JM, Buchs N, et al. Human interleukin‐17: AT cell–derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963‐970. [DOI] [PubMed] [Google Scholar]
- 105. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen‐induced arthritis in IL‐17‐deficient mice. J Immunol. 2003;171:6173‐6177. [DOI] [PubMed] [Google Scholar]
- 106. Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin‐17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802‐805. [DOI] [PubMed] [Google Scholar]
- 107. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668‐677. [DOI] [PubMed] [Google Scholar]
- 108. Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876‐2885. [DOI] [PubMed] [Google Scholar]
- 109. Chen W, Wang J, Xu Z, et al. Apremilast ameliorates experimental arthritis via suppression of Th1 and Th17 cells and enhancement of CD4+ Foxp3+ regulatory T cells differentiation. Front Immunol. 2018;9:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kaneko S, Kondo Y, Yokosawa M, et al. The RORγt‐CCR6‐CCL20 axis augments Th17 cells invasion into the synovia of rheumatoid arthritis patients. Mod Rheumatol. 2018;28:814‐825. [DOI] [PubMed] [Google Scholar]
- 111. Tong B, Yuan X, Dou Y, et al. Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. Int J Biochem Cell Biol. 2016;75:63‐73. [DOI] [PubMed] [Google Scholar]
- 112. Li Y‐H, Xiao H‐T, Hu D‐D, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium‐induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227‐239. [DOI] [PubMed] [Google Scholar]
- 113. Li C, Xi Y, Li S, et al. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol. 2015;67:444‐454. [DOI] [PubMed] [Google Scholar]
- 114. Cui H, Cai Y, Wang LI, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Frontiers Pharmacol. 2018;9:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420‐28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qin X, Guo BT, Wan B, et al. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185:1855‐1863. [DOI] [PubMed] [Google Scholar]
- 117. Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016;79:222‐230. [DOI] [PubMed] [Google Scholar]
- 118. Liu Y‐T, Hao H‐P, Xie H‐G, et al. Extensive intestinal first‐pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos. 2010;38:1779‐1784. [DOI] [PubMed] [Google Scholar]
- 119. Gonzalez‐Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti‐inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52‐63. [DOI] [PubMed] [Google Scholar]
- 120. Broglio F, Papotti M, Muccioli G, Ghigo E. Brain–gut communication: cortistatin, somatostatin and ghrelin. Trends Endocrinol Metab. 2007;18:246‐251. [DOI] [PubMed] [Google Scholar]
- 121. Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015;213:442‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yue M, Tao Y, Fang Y, et al. The gut microbiota modulator berberine ameliorates collagen‐induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. 2019;33:12311‐12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Teng F, Klinger CN, Felix KM, et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer's patch T follicular helper cells. Immunity. 2016;44:875‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Darrah E, Andrade F. Rheumatoid arthritis and citrullination. Curr Opin Rheumatol. 2018;30:72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chatzidionisyou A, Catrina AI. The lung in rheumatoid arthritis, cause or consequence? Curr Opin Rheumatol. 2016;28:76‐82. [DOI] [PubMed] [Google Scholar]
- 127. Gilbert JA, Quinn RA, Debelius J, et al. Microbiome‐wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94‐103. [DOI] [PubMed] [Google Scholar]
- 128. Byndloss MX, Olsan EE, Rivera‐Chávez F, et al. Microbiota‐activated PPAR‐γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kelly C, Zheng L, Campbell E, et al. Crosstalk between microbiota‐derived short‐chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


