Abstract
Objective
To examine the associations between neuraxial anaesthesia or general anaesthesia and clinical outcomes, length of hospital stay, and readmission in adults undergoing lower limb revascularisation surgery.
Design
Comparative effectiveness study using linked, validated, population based databases.
Setting
Ontario, Canada, 1 April 2002 to 31 March 2015.
Participants
20 988 patients Ontario residents aged 18 years or older who underwent their first lower limb revascularisation surgery in hospitals performing 50 or more of these surgeries annually.
Main outcome measures
Primary outcome was 30 day all cause mortality. Secondary outcomes were in-hospital cardiopulmonary and renal complications, length of hospital stay, and 30 day readmissions. Multivariable, mixed effects regression models, adjusting for patient, procedural, and hospital characteristics, were used to estimate associations between anaesthetic technique and outcomes. Robustness of analyses were evaluated by conducting instrumental variable, propensity score matched, and survival sensitivity analyses.
Results
Of 20 988 patients who underwent lower limb revascularisation surgery, 6453 (30.7%) received neuraxial anaesthesia and 14 535 (69.3%) received general anaesthesia. The percentage of neuraxial anaesthesia use ranged from 0.6% to 90.6% across included hospitals. Furthermore, use of neuraxial anaesthesia declined by 17% over the study period. Death within 30 days occurred in 204 (3.2%) patients who received neuraxial anaesthesia and 646 (4.4%) patients who received general anaesthesia. After multivariable, multilevel adjustment, use of neuraxial anaesthesia compared with use of general anaesthesia was associated with decreased 30 day mortality (absolute risk reduction 0.72%, 95% confidence interval 0.65% to 0.79%; odds ratio 0.68, 95% confidence interval 0.57 to 0.83; number needed to treat to prevent one death=139). A similar direction and magnitude of association was found in instrumental variable, propensity score matched, and survival analyses. Use of neuraxial anaesthesia compared with use of general anaesthesia was also associated with decreased in-hospital cardiopulmonary and renal complications (odds ratio 0.73, 0.63 to 0.85) and a reduced length of hospital stay (−0.5 days, −0.3 to−0.6 days).
Conclusions
Use of neuraxial anaesthesia compared with general anaesthesia for lower limb revascularisation surgery was associated with decreased 30 day mortality and hospital length of stay. These findings might have been related to reduced cardiopulmonary and renal complications after neuraxial anaesthesia and support the increased use of neuraxial anaesthesia in patients undergoing these surgeries until the results of a large, confirmatory randomised trial become available.
Introduction
Although endovascular treatments are increasingly being used for peripheral artery disease, they are less durable, are not suitable for many patients’ arterial disease, and there is equipoise about whether to offer endovascular or surgical revascularisation to candidates suitable for both.1 2 3 4 Therefore, lower limb revascularisation surgeries (endarterectomy, patch angioplasty, thromboembolectomy, and arterial bypass) remain commonly performed, despite being costly and high risk procedures.5 6 7 8 9 In the United States, 15 000-20 000 lower limb bypass surgeries are performed annually.5 6 People who undergo these procedures are typically older and have multiple comorbidities.10 11 These patients have a high risk of postoperative morbidity and mortality and often require long hospital stays.1 9 12 The cost of each lower limb revascularisation procedure can exceed $120 000 (£91 552; €101 406.5 6 Interventions to improve clinical outcomes and decrease resource use are required for patients undergoing these surgeries.
Neuraxial (epidural or spinal) anaesthesia is commonly used worldwide and could represent one approach to improving outcomes in patients after lower limb revascularisation. Neuraxial anaesthesia has been shown to improve coagulation, increase peripheral blood flow, avoid airway instrumentation and mechanical ventilation, and blunt surgical stress responses.13 14 15 16 17 Systematic reviews of randomised controlled trials have found that, compared with general anaesthesia, neuraxial anaesthesia might reduce the risk of pneumonia, venous thromboembolism, and mortality after heterogeneous types of surgery.18 19 However, a systematic review limited to four small randomised controlled trials enrolling patients who underwent lower limb revascularisation surgery reported that only the risk of pneumonia was lower after neuraxial anaesthesia.20 Observational studies comparing neuraxial anaesthesia with general anaesthesia for lower limb revascularisation surgery report inconsistent findings and are limited owing to confounding by indication.21 22 23 This confounding occurs because anaesthetic techniques might be chosen based on patient, provider, and hospital characteristics, and these characteristics might be associated with outcomes.24
To better understand the relations between type of anaesthesia for lower limb revascularisation surgery and clinical outcomes, we conducted a population based comparative effectiveness study using recommended methods to account for confounding by indication.24 We hypothesised that use of neuraxial anaesthesia instead of general anaesthesia for adults undergoing lower limb revascularisation surgery would be associated with improved clinical outcomes.
Methods
Design, setting, and protocol
We conducted a population based comparative effectiveness study in Ontario, Canada. Except when indicated, all study methods were preplanned and prespecified in a protocol registered at the Center for Open Science (https://osf.io/sy4xu/) before the study was initiated. Because our study relied on routinely collected, anonymised data, it was exempt from research ethics review. Reporting followed recommended guidelines.25 26
Data sources
Ontario residents with a valid healthcare card receive universal access to publicly funded healthcare in Ontario. Ontario healthcare data are recorded in administrative datasets by ICES, an independent health research institute.27 28 In this study we used several ICES datasets: the Discharge Abstract Database, which captures hospital admissions and discharges, diagnoses, and procedures; the Ontario Health Insurance Plan Database, which captures physician claims; the National Ambulatory Care Reporting System, which captures emergency and outpatient care; the Continuing Care Reporting System, which captures long term and respite care; the Ontario Drug Benefits Database, which captures prescription drug claims for residents aged 65 years and older; and the Registered Persons Database, which captures deaths.
Participants
We identified Ontario residents aged 18 years and older who underwent lower limb revascularisation surgery, including an infrainguinal arterial bypass or repair (ie, endarterectomy, patch angioplasty, or thromboembolectomy) between 1 April 2002 and 31 March 2015 using Canadian Classification of Intervention (CCI) codes (starting with 1KG76 and 1KG80, except 1KG80GQRN (endovascular stent repair)). These codes have been validated and used in previous studies conducted at ICES.29 30 31 To ensure patient level data, we only included data on the first surgery for patients who underwent multiple lower limb revascularisation surgeries during the study period. We excluded patients who underwent a lower limb revascularisation surgery that required suprainguinal inflow (eg, aortobifemoral or axillobifemoral bypass). We also excluded those receiving surgery at hospitals performing fewer than 50 lower limb revascularisation surgeries annually, as vascular surgery is provided in regional centres in Ontario and therefore procedures at low volume hospitals represent atypical care.
Exposure variables
The type of anaesthesia was captured from the Discharge Abstract Database; reabstraction (ie, duplicate data extraction from the same chart by independent reviewers) shows 94% agreement.32 Contraindications to use of neuraxial anaesthesia include patient refusal, infection at the intended injection site, use of anticoagulants or certain antiplatelet agents, severe uncorrected hypotension, increased intracranial pressure, an anticipated long duration of surgery, and an inability to lie still (eg, secondary to cognitive impairment or agitation).33 Patients who received an epidural or spinal anaesthetic without general anaesthesia were coded as having received neuraxial anaesthesia; patients who received general anaesthesia or neuraxial anaesthesia plus general anaesthesia were coded as having received general anaesthesia. As a sensitivity analysis, we also coded the types of anaesthesia as neuraxial, general, or neuraxial plus general. We did not stratify by type of neuraxial anaesthesia as epidural anaesthesia accounted for only 3.8% of all neuraxial anaesthetics.
Outcome variables
The primary outcome was 30 day all cause mortality (captured from the Discharge Abstract Database and Registered Persons Database). Secondary outcomes were in-hospital cardiopulmonary and renal complications (including major adverse cardiac event (acute coronary syndrome, heart failure, ventricular arrhythmia, or cardiac arrest), pneumonia, venous thromboembolism, or acute kidney injury), captured from type 2 (arising in hospital) diagnostic codes (see supplementary eTable 1), postoperative length of hospital stay (measured from the Discharge Abstract Database as number of days from surgery to hospital discharge), and 30 day readmissions (identified as a new admission in the Discharge Abstract Database within 30 days of the index discharge date from hospital).
Covariates
We captured known, measured covariates that could influence choice of anaesthetic technique and outcomes (ie, produce confounding by indication).21 22 23 34 35 Patient characteristics were identified from the Discharge Abstract Database and Canadian Census. Validated case ascertainment algorithms and ICD codes (international classification of diseases, 10th revision) from Discharge Abstract Database records were used to identify high priority diagnoses (coronary artery disease, diabetes, chronic obstructive pulmonary disease, heart failure, and hypertension) and Elixhauser comorbidities in the three years before surgery, respectively.36 37 38 39 40 As smoking status is not available in administrative data, we captured physician billing codes for smoking cessation consultations. Preoperative residence in a long term care facility was identified from the Continuing Care Reporting System. Hospital admissions and emergency department visits in the year before surgery were identified from the Discharge Abstract Database and National Ambulatory Care Reporting System, respectively. We calculated Hospital-patient One-year Mortality Risk (HOMR) scores (range from −12 to 76, with higher scores denoting greater risk of death)41 and the hospital volume of lower limb revascularisation surgery before each index surgery. The Johns Hopkins Adjusted Clinical Group system was used to identify healthcare resource utilisation bands and frailty.42 43 44 45 The surgical procedure (using the full 10 digit CCI code), a unique identifier for each hospital, and year of surgery were recorded from the Discharge Abstract Database. Urgency for surgery was categorised as elective (elective admission for surgery), urgent (non-elective admission and surgery ≥72 hours after admission), emergent (non-elective admission and surgery 24-72 hours after admission), and critical (surgery <24 hours after admission) instead of just elective or emergent, to more accurately reflect variations in urgency faced by vascular surgery patients. For people aged 65 years and older, a count of all unique outpatient drugs and a set of specific outpatient drugs (opioids, anticoagulants, antiplatelets, antipsychotics, benzodiazepines, β blockers, dementia drugs, insulin, steroids, and oral diabetes drugs) received in the six months before surgery were identified.46 We also collected data on the annual prevalence of antiplatelet and anticoagulant use by these patients.
Statistical analyses
Based on reported differences in mortality between patients who received neuraxial versus general anaesthesia after lower limb revascularisation surgery (2.2% v 1.1%),23 we estimated that we would require 2817 participants or more in each study arm to detect a difference in 30 day mortality at a 5% significance level with 90% power. A conservative estimate of 10 events for each covariate for our prespecified regression model required about 650 deaths.47
An investigator who was aware of treatment group status performed all the statistical analyses. We compared covariate balance using standardised differences, with values greater than 0.1 indicating substantive differences.48 Outcomes were analysed on an unadjusted and adjusted basis. Assumptions for regression models were tested and met. SAS (SAS Institute) version 9.4 was used for all analyses.
Preplanned analyses
Our primary approach to adjustment used mixed effects regression models that included a random intercept for each hospital. Binary outcomes were modelled using logit-links and binary distributions. We analysed in-hospital cardiopulmonary and renal complications as a composite outcome to improve power. As length of hospital stay typically has a right skewed distribution, this outcome was modelled using a log-link and gamma distribution.49 Absolute differences in adjusted outcomes were calculated using predicted estimates, generated across 1000 bootstrap samples using 1:1 resampling with replacement.50 The centile method was used to define effect sizes and 95% confidence intervals.
Adjusted models included anaesthetic type, age, sex, rural versus urban residence, neighbourhood income fifth, surgical urgency, the specific surgical procedure, a multiplicative interaction between the surgical procedure and urgency, comorbidities (coronary artery disease, chronic obstructive pulmonary disease, diabetes, heart failure, hypertension, and each Elixahauser comorbidity not captured by these validated case ascertainment algorithms),36 37 38 39 40 preadmission residence in a long term care facility, frailty,42 43 44 hospital admission and emergency department visits in the year before surgery, resource utilisation band (Adjusted Clinical Group),51 hospital lower limb revascularisation volume, HOMR score, smoking cessation consultations, and year of surgery. Supplementary eTable 2 provides the form of all covariates.
We evaluated the robustness of our primary outcome analysis by performing prespecified sensitivity analyses. A Durbin-Wu-Hausman test of endogeneity was used to evaluate if our primary adjusted analysis was subject to unmeasured confounding.52 53 54 To account for unmeasured confounding, we conducted a two stage residual inclusion instrumental variable analysis, which is appropriate for binary and non-normally distributed continuous outcomes.52 53 55 Instrumental variable analyses leverage a source of naturally occurring variation to block unmeasured confounders through pseudo-randomisation.52 53 55 A commonly used instrumental variable is the hospital preference instrument; this approach assumes that local practice patterns (ie, proportion of neuraxial anaesthesia use for all lower limb revascularisation surgeries at each hospital in the year before each patients’ surgery) influences receipt of the intervention (ie, neuraxial anaesthesia)55 56 without influencing outcome except through the intervention. This analysis makes three key assumptions (the first two of which are verifiable): patients who receive care at a hospital that provides a high proportion of neuraxial anaesthesia are more likely to receive such anaesthesia (ie, the instrumental variable is highly correlated with use of neuraxial anaesthesia); attending a hospital with higher use of neuraxial anaesthesia does not influence outcome, except through use of neuraxial anaesthesia (ie, the instrumental variable is not correlated with 30 day mortality except through use of neuraxial anaesthesia), and attending a hospital with high use of neuraxial anaesthesia is not influenced by patients’ unmeasured characteristics.55 57 Next, we performed a propensity score matched analysis, when we matched exactly on hospital and then within 0.2 standard deviations of the logit of the propensity score using a greedy matching algorithm.58 The propensity score was derived from a logistic regression model using anaesthetic type as the dependent variable and all measured covariate data as predictors. We then ran our primary analysis using a Cox proportional hazards model with a random effect for each hospital. The analysis was rerun limited to patients aged 65 years and older so we could adjust for outpatient prescription drug use (anticoagulants, antiplatelet agents, opioids, antipsychotics, benzodiazepines, insulin, oral diabetic agents, oral corticosteroids, and total number of prescription drugs). Finally, we conducted a formal quantitative bias analysis, using the E-value method as previously described, to estimate the effect size of a missing confounder that would be required to move our primary effect estimate to the null value.59
We evaluated for possible effect measure modification by introducing multiplicative interaction terms between anaesthetic type and covariates into our primary regression model. These covariates included chronic obstructive pulmonary disease, surgical urgency, frailty, previous diagnosis of peripheral artery disease, and the specific surgical procedure.
Data were missing for 0.09% of participants for the covariate rural versus urban residence and 0.29% of participants for the covariate neighbourhood income fifth. We imputed these covariates with the central measure of tendency (for neighbourhood income fifth) or most common value (for rural versus urban residence).
Post hoc analyses
We conducted several post hoc analyses requested by peer reviewers. Firstly, we re-estimated our primary outcome association after also including those who received surgery in hospitals performing fewer than 50 lower limb revascularisation surgeries annually. Secondly, we re-ran our sensitivity analysis limited to patients aged 65 years and older to additionally adjust for outpatient β blocker and dementia drug use. Thirdly, we included cerebrovascular events (transient ischaemic attack or ischaemic or haemorrhagic stroke, captured from type 2 diagnostic codes) (see supplementary eTable 2) as a secondary outcome and estimated fully adjusted associations between anaesthetic type and each of the components of our composite cardiopulmonary and renal complications outcome.
Patient and public involvement
This was a secondary analysis of healthcare data making it difficult to involve patients or members of the public in this research as it required specialist research training. No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on interpretation or writing up of the results.
Results
Among 26 871 lower limb revascularisation surgeries performed in the Ontario hospitals in this study, 20 988 patients were included (fig 1). Of these patients, 6453 (30.7%) received neuraxial anaesthesia and 14 535 (69.3%) general anaesthesia. The percentage of neuraxial anaesthesia use ranged from 0.6% to 90.6% across the hospitals. Use of neuraxial anaesthesia declined by 17% over the study period (fig 2).
Fig 1.
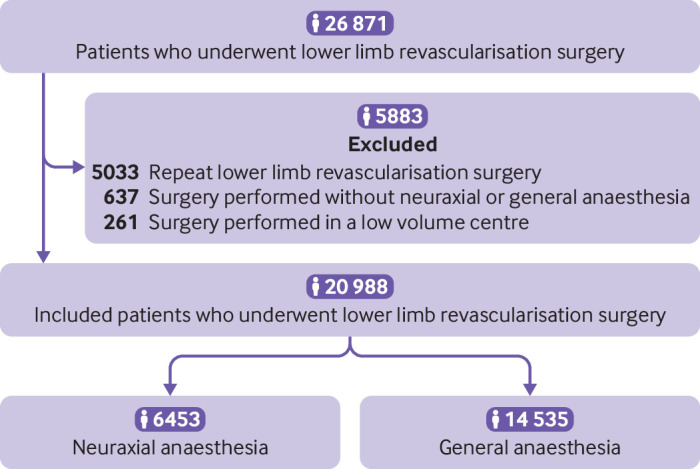
Flow of patients through the study
Fig 2.
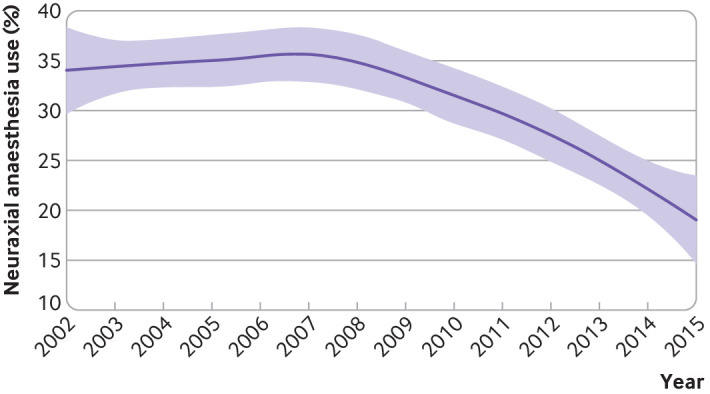
Loess smoothed plot of percentage of neuraxial anaesthesia use across the study period. Shaded areas around the plotted line represent 95% confidence intervals
Table 1 shows the characteristics of the study patients. The participants had multiple associated comorbidities and most received an infrainguinal arterial bypass (78.6%) either electively (60.1%) or urgently (27.5%). Patients who received neuraxial anaesthesia were older; more often had chronic obstructive pulmonary disease, diabetes, and a previous diagnosis of peripheral artery disease; and more often received an arterial bypass and underwent surgery on an elective basis. Patients who received general anaesthesia more often had cardiac valvular disease, underwent surgery on a critical basis, and received an infrainguinal arterial repair (endarterectomy, patch angioplasty, or thromboembolectomy).
Table 1.
Characteristics of 20 988 participants stratified by anaesthetic technique used for lower limb revascularisation surgery. Values are numbers (percentages) unless stated otherwise
| Characteristics | Neuraxial anaesthesia (n=6453) | General anaesthesia (n=14 535) | Absolute standardised difference* |
|---|---|---|---|
| Personal characteristics | |||
| Mean (SD) age (years) | 70.6 (10.7) | 67.6 (12.7) | 0.25 |
| Women | 2132 (33.0) | 4763 (32.8) | 0 |
| Rural residence | 1071 (16.6) | 2639 (16.3) | 0 |
| Long term care facility resident | 159 (2.5) | 233 (1.6) | 0.06 |
| Income fifth: | |||
| 1 (lowest) | 1569 (24.3) | 3554 (24.5) | 0 |
| 2 | 1419 (22.0) | 3167 (21.8) | 0 |
| 3 | 1231 (19.1) | 2758 (19.0) | 0 |
| 4 | 1173 (18.2) | 2618 (18.0) | 0.01 |
| 5 (highest) | 1061 (16.4) | 2438 (16.8) | 0.01 |
| Smoking cessation consultation in year before surgery | 168 (2.6) | 375 (2.6) | 0 |
| Mean (SD) HOMR score† | 31.7 (6.0) | 31.0 (6.5) | 0.11 |
| Comorbidities recorded in 3 years before surgery | |||
| Alcohol misuse | 176 (2.7) | 417 (2.9) | 0.01 |
| Atrial fibrillation | 569 (8.8) | 1228 (8.5) | 0.01 |
| Blood loss anaemia | 755 (11.7) | 1647 (11.3) | 0.01 |
| Cancer | 382 (5.9) | 865 (6.0) | 0 |
| Cardiac valvular disease | 219 (3.4) | 908 (6.3) | 0.14 |
| Cerebrovascular disease | 432 (6.7) | 881 (6.1) | 0.02 |
| Chronic kidney disease | 587 (9.1) | 997 (6.9) | 0.08 |
| Chronic obstructive pulmonary disease | 2639 (40.9) | 5260 (36.2) | 0.10 |
| Coagulopathy | 190 (2.9) | 656 (4.5) | 0.08 |
| Deficiency anaemia | 65 (1.0) | 121 (0.8) | 0.02 |
| Dementia | 126 (2.0) | 219 (1.5) | 0.04 |
| Depression | 182 (2.8) | 427 (2.9) | 0.01 |
| Diabetes | 3162 (49.0) | 6301 (43.4) | 0.11 |
| Dialysis | 322 (5.0) | 558 (3.8) | 0.06 |
| Drug misuse | 30 (0.5) | 63 (0.4) | 0.01 |
| Frailty | 1383 (21.4) | 2710 (18.6) | 0.07 |
| Heart failure | 1588 (24.6) | 3047 (21.0) | 0.09 |
| Hemiparesis or hemiplegia | 59 (0.9) | 106 (0.7) | 0.02 |
| Hypertension | 5199 (81.0) | 11 306 (77.8) | 0.08 |
| Liver disease | 78 (1.2) | 164 (1.1) | 0.01 |
| Metastatic cancer | 45 (0.7) | 146 (1.0) | 0.03 |
| Myocardial infarction | 871 (13.5) | 1919 (13.2) | 0 |
| Obesity | 134 (2.1) | 350 (2.4) | 0.02 |
| Peptic ulcer disease | 134 (2.1) | 243 (1.7) | 0.03 |
| Previous diagnosis of peripheral artery disease | 2579 (40.0) | 4786 (32.9) | 0.15 |
| Psychosis | 23 (0.4) | 75 (0.5) | 0.01 |
| Pulmonary circulatory disease | 96 (1.5) | 295 (2.0) | 0.04 |
| Rheumatic disease | 69 (1.1) | 153 (1.1) | 0 |
| Venous thromboembolism | 78 (1.2) | 185 (1.3) | 0.01 |
| Weight loss | 113 (1.8) | 219 (1.5) | 0.02 |
| Healthcare resource use | |||
| Emergency department visit in past year | 4072 (63.1) | 9001 (61.9) | 0.02 |
| Hospital admission in past year | 2594 (40.2) | 5919 (40.7) | 0.01 |
| Resource utilisation band: | |||
| 1 – healthy user | ‡ | ‡ | N/A |
| 2 – low morbidity | ‡ | ‡ | N/A |
| 3 – moderate | 827 (12.8) | 1658 (11.4) | 0.04 |
| 4 – high | 1509 (23.4) | 3453 (23.8) | 0.01 |
| 5 – very high | 4113 (63.7) | 9373 (64.5) | 0.02 |
| Lower limb revascularisation surgery | |||
| Mean (SD) hospital volume of lower limb revascularisation yearly | 1450 (784) | 1480 (842) | 0.04 |
| Urgency: | |||
| Elective | 4112 (63.7) | 8492 (58.4) | 0.11 |
| Urgent | 1847 (28.6) | 3922 (27.0) | 0.04 |
| Emergent | 109 (1.7) | 326 (2.2) | 0.04 |
| Critical | 385 (6.0) | 1795 (12.4) | 0.22 |
| Procedure: | |||
| Infrainguinal bypass | 5583 (86.5) | 10 917 (75.1) | 0.29 |
| Using autogenous graft material | 3393 (52.6) | 6368 (43.8) | 0.18 |
| Using synthetic graft material | 2044 (31.7) | 4171 (28.7) | 0.07 |
| Using composite graft material | 146 (2.3) | 378 (2.6) | 0.02 |
| Repair of arteries below inguinal ligament | 653 (10.1) | 3158 (21.7) | 0.32 |
| Using autograft material | 159 (2.5) | 665 (4.6) | 0.11 |
| Using synthetic material | 288 (4.5) | 871 (6.0) | 0.07 |
| Without using above materials | 206 (3.2) | 1622 (11.2) | 0.31 |
HOMR=Hospital-patient One-year Mortality Risk; NA=not applicable.
Difference in prevalence of binary covariates, or average of continuous covariates, between treatment groups without influence of sample size; values greater than 0.1 indicate substantive differences.
Range from −12 to 76, with higher scores denoting greater risk of death.
Privacy legislation does not allow reporting of cell sizes of 6 or less, or cells that allow calculation of a cell of 6 or less.
For the 13 176 included patients aged 65 years and older with outpatient prescription drug data, use of anticoagulants remained relatively stable over time, whereas yearly antiplatelet use increased by nearly 10% over the study period (see supplementary eFigure 1).
Primary outcome
Death within 30 days occurred in 204 (3.2%) patients who received neuraxial anaesthesia and 646 (4.4%) who received general anaesthesia (unadjusted odds ratio 0.70, 95% confidence interval 0.61 to 0.83; P<0.001). After multivariable, multilevel adjustment, the risk of 30 day mortality remained lower in patients who received neuraxial anaesthesia (0.68, 0.57 to 0.83; P<0.001) (supplementary eTable 3 provides results of the fully adjusted mixed effects model). This difference corresponds to a bootstrap estimated adjusted absolute risk reduction of 0.72% (95% confidence interval 0.65% to 0.79%) or number needed to treat of 139 (95% confidence interval 127 to 154) to prevent one death at 30 days with use of neuraxial anaesthesia instead of general anaesthesia.
Primary outcome sensitivity and subgroup analyses
Sensitivity analyses
In our quantitative bias analysis using the E-value we estimated that our calculated adjusted odds ratio for 30 day mortality could only be attenuated from 0.68 to 1.0 (the null value) by an unmeasured confounder strongly associated (by an odds ratio of 2.3, above and beyond the measured confounders already accounted for in our analyses) with both use of neuraxial anaesthesia and mortality. Furthermore, the Durbin-Wu-Hausman test suggested that unmeasured confounding was present in the primary outcome adjusted analysis (P<0.001). The hospital preference instrumental variable appeared valid (ie, a strong association was found with use of neuraxial anaesthesia (F statistic=3791), but no correlation with 30 day mortality (ρ=0.02).
Table 2 shows the results of sensitivity analyses. The association between use of neuraxial anaesthesia and decreased 30 day mortality was consistent in instrumental variable, propensity score matched, and survival analyses, and after adjusting for different outpatient prescription drug use (see supplementary eTable 4 for characteristics of the propensity score matched cohorts, eTable 5 for characteristics of the patients with outpatient prescription drug data, and eTable 6 for results of the fully adjusted mixed effects model that included these patients). The association was also consistent in a post hoc analysis that included all patients who underwent lower limb revascularisation surgery in Ontario during the study, including those who received surgery in hospitals performing fewer than 50 lower limb revascularisation surgeries annually.
Table 2.
Results of prespecified and post hoc sensitivity analyses examining association between anaesthetic technique and 30 day mortality in patients who underwent lower limb revascularisation surgery
| Analysis | Adjusted odds ratio (95% CI)* | Adjusted absolute difference (95% CI) (%) | P value |
|---|---|---|---|
| Prespecified | |||
| Multivariable, mixed effects models (primary approach to adjustment) | 0.68 (0.57 to 0.83) | 0.72 (0.65 to 0.79) | <0.001 |
| Instrumental variable analysis | 0.82 (0.73 to 0.93) | 0.61 (0.68 to 0.75) | NA† |
| Propensity score matched analysis | 0.72 (0.58 to 0.89) | 1.1 (0.40 to 1.8) | <0.001 |
| Survival analysis | 0.72 (0.60 to 0.82) | NA‡ | <0.001 |
| Multivariable, mixed effects model using only patients with outpatient prescription drug data to adjust for different preoperative drug use§ | 0.74 (0.51 to 0.90) | 0.99 (0.85 to 1.11) | 0.003 |
| Post hoc | |||
| Multivariable, mixed effects model using only patients with outpatient prescription drug data and also adjusting for preoperative β blocker and dementia drug use¶ | 0.74 (0.60 to 0.90) | 0.93 (0.80 to 1.1) | 0.003 |
| Multivariable, mixed effects model also included patients who had lower limb revascularisation surgery in a low volume centre** | 0.74 (0.61 to 0.90) | 0.38 (0.30 to 0.46) | 0.002 |
NA=not applicable.
Survival analysis measure of association is a hazard ratio instead of odds ratio.
P values are not generated for effect measures derived from bootstrap analyses.
Absolute differences cannot be calculated with Cox proportional hazards models as they are rank based instead of probability based.
Model also adjusted for differences in outpatient opioid, anticoagulant, antiplatelet, antipsychotic, benzodiazepine, insulin, steroid, and oral diabetes drug use.
Model also adjusted for differences in outpatient opioid, anticoagulant, antiplatelet, antipsychotic, benzodiazepine, β blocker, dementia drug, insulin, steroid, and oral diabetes drug use.
Defined as a centre performing fewer than 50 lower limb revascularisation surgeries annually.
Subgroup analyses
No significant heterogeneity was observed for associations between use of neuraxial anaesthesia and 30 day mortality within subgroups of patients with a history of chronic obstructive pulmonary disease (P=0.43), frailty (P=0.68), or peripheral artery disease (P=0.39); those with differing surgical urgency (P=0.14); or among patients who underwent different infrainguinal arterial procedures (P=0.45). When we re-ran our primary analysis with a three level categorical variable as our main exposure, isolated neuraxial anaesthesia (odds ratio 0.71, 95% confidence interval 0.58 to 0.88; P=0.001) but not neuraxial anaesthesia plus general anaesthesia (0.84, 0.64 to 1.10; P=0.21) was significantly associated with an adjusted decrease in 30 day mortality compared with general anaesthesia alone.
Secondary outcomes
Table 3 shows the results of secondary outcome analyses (see supplementary eTable 7 for the incidence of each secondary outcome and unadjusted absolute differences in these outcomes between groups). Compared with patients who received general anaesthesia, those who received neuraxial anaesthesia had a lower unadjusted odds of a major adverse cardiac event, heart failure, pneumonia, venous thromboembolism, acute kidney injury, and cerebrovascular event. After multivariable, multilevel adjustment, use of neuraxial anaesthesia instead of general anaesthesia was associated with a decreased odds of in-hospital cardiopulmonary and renal complications (0.73, 0.63 to 0.85; P<0.001) and a reduced length of hospital stay (−0.5 days, −0.3 to−0.6 days; P<0.001). In a post hoc analysis, after multivariable, multilevel adjustment, neuraxial anaesthesia was also associated with a decreased odds of a major adverse cardiac event (0.72, 0.60 to 0.87; P<0.001).
Table 3.
Results of analyses examining associations between anaesthetic technique and secondary outcomes among the 20 988 study patients who underwent lower limb revascularisation surgery
| Secondary outcomes | Unadjusted odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value |
|---|---|---|---|---|
| In-hospital cardiac, pulmonary, or renal complications* | 0.74 (0.66 to 0.83) | <0.001 | 0.73 (0.63 to 0.85) | <0.001 |
| Major adverse cardiac event† | 0.80 (0.69 to 0.92) | <0.001 | 0.72 (0.60 to 0.87) | <0.001 |
| Heart failure | 0.90 (0.71 to 1.13) | 0.39 | ‡ | ‡ |
| Pneumonia | 0.74 (0.57 to 0.96) | 0.02 | 0.81 (0.60 to 1.11) | 0.19 |
| Venous thromboembolism | 0.42 (0.24 to 0.73) | 0.001 | ‡ | ‡ |
| Deep vein thrombosis | 0.52 (0.29 to 0.95) | 0.04 | ‡ | ‡ |
| Pulmonary embolism | 0.17 (0.04 to 0.70) | 0.004 | ‡ | ‡ |
| Acute kidney injury | 0.48 (0.34 to 0.67) | <0.001 | ‡ | ‡ |
| Cerebrovascular event§ | 0.38 (0.16 to 0.90) | 0.03 | ‡ | ‡ |
| Hospital readmissions | 1.00 (0.91 to 1.10) | 0.98 | 0.59 (0.32 to 1.10) | 0.11 |
| Length of hospital stay (days) | Absolute difference −0.2 (−0.1 to −0.2) | <0.001 | Absolute difference −0.5 (−0.3 to −0.6) | <0.001 |
It was decided a priori to analyse the adjusted odds of in-hospital cardiopulmonary and renal complications as a composite outcome to improve power.
Includes acute coronary syndrome, heart failure, ventricular arrhythmia, or cardiac arrest.
Number of events was too low to allow for fully adjusted regression modelling.
Post hoc analysis; includes transient ischaemic attack and ischaemic or haemorrhagic stroke.
Discussion
In this population based comparative effectiveness study of 20 988 adults who underwent lower limb revascularisation surgery, including infrainguinal arterial bypass or repair, use of neuraxial anaesthesia instead of general anaesthesia was associated with decreased 30 day mortality. This finding persisted in adjusted analyses (controlling for potential confounding factors and clustering of patient data within hospitals) and instrumental variable, propensity score matched, survival, and other sensitivity analyses. Our secondary outcome analyses suggested that decreased mortality after neuraxial anaesthesia might have been related to reduced cardiopulmonary and renal complications among patients who received neuraxial anaesthesia. Neuraxial anaesthesia was also associated with a decreased adjusted length of hospital stay. Despite this, we observed wide variation in use of this type of anaesthesia across hospitals in Ontario, Canada, and an overall noticeable decline in use over the study period.
Our findings are biologically plausible and consistent with results of randomised controlled trials conducted among other populations. Adults undergoing lower limb revascularisation surgery are at high risk of postoperative complications, typically because they are older, are current or former cigarette smokers, and have multiple comorbidities, including diabetes and coronary artery, pulmonary, and chronic kidney disease.9 60 61 As neuraxial anaesthesia has been associated with a reduced stress response to vascular surgery and improved postoperative coagulation compared with general anaesthesia, it theoretically might be safer than general anaesthesia for these patients.13 14 15 16 17 In potential support of this, we observed statistically significant reductions in cardiac, renal, and thromboembolic complications in adults receiving neuraxial anaesthesia compared with general anaesthesia for lower limb revascularisation surgery, a finding consistent with systematic reviews of randomised controlled trials of patients having various types of surgery.18 19 Furthermore, when these patients received neuraxial anaesthesia and general anaesthesia together, the mortality benefit of neuraxial anaesthesia was no longer present, suggesting that provision of mechanical ventilation, general anaesthesia, or both may negate some of the benefits of neuraxial anaesthesia.
Strengths and weaknesses of this study
Our findings should be considered in the context of the study’s strengths and limitations. We used health administrative data that lack information on patient level physiological measures such as blood pressure that might mediate the relation between anaesthetic type and outcome. Additionally, our data were not collected for research purposes and are therefore at risk of misclassification bias. However, our exposure (anaesthetic type) has been validated,32 and mortality was ascertained from the reference standard for mortality data in Ontario. Finally, our data on cardiopulmonary and renal complications were not prospectively collected, and typically complications captured from administrative data have high specificity but variable sensitivity.62 Therefore our methods might underestimate the risks of complications, although this would not be expected to differ between treatment groups.
Although we adjusted for the specific type of surgery using the entire 10 digit CCI code describing details of the type of operation performed, we were unable to identify certain potentially important surgical details (eg, arterial bypass inflow and outflow sources or exact anticipated operative durations). This limitation could bias the results of our primary outcome (and some of our sensitivity analyses) away from the null if patients with more complex types of lower limb revascularisation surgeries were more likely to receive general anaesthesia. However, we believe that it would be unlikely for our findings to be entirely explained by differences in surgical complexity between treatment groups or other residual or unmeasured potentially confounding factors, for several reasons. In our quantitative bias analysis we estimated that the calculated adjusted odds ratio for 30 day mortality could only be attenuated to the null by an unmeasured confounder (eg, surgical complexity) strongly associated with both use of neuraxial anaesthesia and mortality.59 Furthermore, when we used a prespecified instrumental variable analysis to block the effects of both measured and unmeasured confounders, our effect size remained statistically significant and only slightly attenuated towards the null when compared with methods used to account for measured confounding alone. Therefore, for patients who would be eligible for either neuraxial anaesthesia or general anaesthesia, there might be stronger than associative evidence of benefit for use of neuraxial anaesthesia.
Strengths and weaknesses in comparison to other studies
Our findings seem to be consistent with a recent cohort study of 16 052 patients who underwent lower limb arterial bypass.23 In that study, use of neuraxial anaesthesia was associated with a shorter adjusted length of hospital stay (−1.66 days, 95% confidence interval −0.02 to−3.29 days) compared with use of general anaesthesia. Also, although underpowered for mortality, the reported absolute risk difference for mortality in that study (1.1%) was similar to the value reported in our study (0.72%). Importantly, our study deals with limitations of previously reported observational studies comparing neuraxial anaesthesia with general anaesthesia after lower limb revascularisation surgery, including that of the recent cohort study.23 We adjusted for a more complete set of potential confounding factors, conducted sensitivity analyses designed to account for unmeasured confounding, and evaluated a cohort in which the distribution of anaesthetic types was more balanced—that is, only 3.5% of participants in the published cohort study received neuraxial anaesthesia compared with 30.7% of participants in our study.23
Meaning of the study, unanswered questions, and future research
Our findings have potentially important implications for practice and future research. Despite evidence of benefit with no signal towards harm, use of neuraxial anaesthesia in patients undergoing lower limb revascularisation surgery was recently reported to decrease by 40% between 2011 and 2016 in the US.23 We also observed a decline in use of neuraxial anaesthesia by 17% for these types of surgeries in Ontario between 2002 and 2015. Utilisation of neuraxial anaesthesia for lower limb revascularisation surgeries was also highly variable between the Ontario hospitals included in our study (ranging from 0.6% to 90.6%).
Variation in use of neuraxial anaesthesia for other types of surgical procedures has also been reported. For example, one study reported wide variation between hospitals in the United Kingdom in use of neuraxial, general, and local anaesthesia for patients undergoing endovascular abdominal aortic aneurysm repair.63 Although the frequency of neuraxial anaesthesia use for total hip and knee arthroplasty has remained relatively unchanged or might have even declined slightly with time in the US, Canada, and the UK,64 65 66 its use seems to be increasing for hip fracture surgery in Canada.67
We are unable to fully explain the observed decline in use of neuraxial anaesthesia for lower limb revascularisation surgery in our study. Although the increasing use of antiplatelet agents observed during the study period could partly explain the decline, more than 80% of the study patients with outpatient prescription drug data were not taking prescription antiplatelet or anticoagulant drugs preoperatively. Furthermore, no financial incentives are provided for use of neuraxial anaesthesia or general anaesthesia in Ontario. Reported reasons for variation in use of neuraxial anaesthesia for other operations have included patient factors; the specific hospital or treating anaesthetist; whether the treating anaesthetist is board certified; patient’s knowledge, attitudes, perceptions, and concerns about anaesthesia; and surgeon and anaesthetist preferences.34 68 69 70 71 72 73 Future research is required to evaluate contributors to declining use of neuraxial anaesthesia for lower limb revascularisation surgery.
With a number needed to treat for mortality estimated at 139, our data suggest that increased use of neuraxial anaesthesia could result in more than 100 lives saved among the 20 000 patients who underwent lower limb revascularisation surgeries performed annually in the US. These estimates must be interpreted with caution given the observational nature of our data, and they highlight the need for a confirmatory randomised controlled trial. However, we believe that confirmatory data from a randomised controlled trial are unlikely to be available soon. Based on our data, powering a randomised controlled trial to show a difference in mortality or end organ complications would require more than 2500 participants in each study arm and would be the largest trial to date of neuraxial anaesthesia in any setting, requiring a large amount of funding and years of multicentre, possibly international recruitment. Use of a validated continuous outcome, such as the Comprehensive Complications Index or World Health Organization Disability Assessment Schedule could substantially reduce the required sample size.74 75 Until a confirmatory randomised controlled trial is completed, our findings, combined with the low risk of complications attributable to neuraxial anaesthesia (estimated incidence of permanent injury is 3 to 6 per 100 000 patients treated with this anaesthetic technique76), support increased use of neuraxial anaesthesia in patients undergoing lower limb revascularisation surgery without contraindications.
Conclusions
In this population based comparative effectiveness study, we observed wide variation in use of neuraxial anaesthesia for lower limb revascularisation surgery across Ontario hospitals and an overall noticeable decline in its use for this type of surgery over the study period. Despite this, use of neuraxial anaesthesia compared with general anaesthesia for lower limb revascularisation surgery was associated with decreased adjusted mortality and length of hospital stay. These findings might have been related to reduced cardiopulmonary and renal complications after neuraxial anaesthesia. Until results of a large, confirmatory randomised controlled trial become available, this study supports increased use of neuraxial anaesthesia in patients undergoing lower limb revascularisation surgery.
What is already known on this topic
Leg revascularisation surgeries are frequently performed, yet are costly and high risk procedures
Commonly used neuraxial (epidural or spinal) anaesthesia improves coagulation, increases peripheral blood flow, avoids airway instrumentation and mechanical ventilation, and blunts surgical stress responses
Small randomised trials and larger cohort studies limited by confounding by indication have reported inconsistent improvements in clinical outcomes between neuraxial anaesthesia and general anaesthesia
What this study adds
Use of neuraxial anaesthesia for lower limb revascularisation surgery varied widely across hospitals in Ontario, Canada, and use noticeably declined for this type of surgery over the study period
After accounting for confounding by indication, use of neuraxial anaesthesia for lower limb revascularisation surgery was associated with decreased 30 day mortality and length of hospital stay compared with use of general anaesthesia
These findings might have been related to reduced cardiopulmonary and renal complications after neuraxial anaesthesia and support its increased use in patients undergoing lower limb revascularisation surgery until results of a large, randomised trial are available
Acknowledgments
This study was registered with the Center for Open Science (https://osf.io/sy4xu/).
Web extra.
Extra material supplied by authors
Supplementary information: additional tables e1-e7 and fig e1
Contributors: DJR conceived the study. DJR, SN, DK, TB, HTS, MML, AJF, CJLM, and DIM designed the study. DIM acquired and analysed the data, which was interpreted by DJR, SN, DK, TB, HTS, MML, AJF, CJLM, and DIM. DJR drafted the manuscript. SN, DK, TB, HTS, MML, AJF, CJLM, and DIM revised the article critically for important intellectual content. DJR, SN, DK, TB, HTS, MML, AJF, CJLM, and DIM read the final version of the manuscript and gave approval for it to be published. DIM had access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. DJR is the guarantor.
Funding: DJR receives salary support from the The Ottawa Hospital Department of Surgery. DIM receives salary support from The Ottawa Hospital Department of Anesthesiology and Pain Medicine; DIM is supported by the Canadian Anesthesiologists’ Society Career Scientist Award. HTS is supported by an Embedded Clinician Researcher Award from the Canadian Institutes of Health Research. This study was supported by the International Credential Evaluation Service (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long term Care (MOHLTC). No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
The lead author (DJR) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Because participants cannot be identified from our anonymised and deidentified study data source, we are unable to directly disseminate results of the study to its participants. However, to promote use of the results of the study both locally and nationally, we purposefully engaged several key local policymakers and national stakeholders at the outset or design phase of the project who have the authority to implement or assist in implementing practice change. These included heads of the Divisions of Vascular and Endovascular Surgery (SKN) and the Department of Anesthesiology and Pain Medicine (CJLM), the vice president of Innovation and Quality at The Ottawa Hospital and vice chair of Quality and Clinical Services at the University of Ottawa (AJF), and stakeholder members of the Canadian Society for Vascular Surgery (SKN, TB, DK) and Canadian Anesthesiologists’ Society (MML, CJLM, DIM). To disseminate results of the study, we presented our findings at local (University of Ottawa Department of Surgery Division of Vascular and Endovascular Surgery 2019 Vascular Research Day and the University of Ottawa Department of Surgery Collins Surgical Day 2019), national (Canadian Society for Vascular Surgery 41st Annual Meeting on Vascular Surgery), and international (Society for Vascular Surgery 2020 Vascular Annual Meeting) vascular and endovascular surgery academic meetings. In addition to disseminating the study findings through social media communications, we will partner with media officers at The Ottawa Hospital and ICES to communicate findings to local, national, and international communities by way of media releases. ICES disseminates results of studies conducted using ICES data to patients, researchers, healthcare workers, policymakers, and other stakeholders on its website, in newsletters, and on social media websites. We also plan to share our summarised findings and the entire published study with vascular and endovascular surgery and anaesthesia and pain medicine professional societies in Canada, the United Kingdom, and the United States.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Adam DJ, Beard JD, Cleveland T, et al. BASIL trial participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925-34. 10.1016/S0140-6736(05)67704-5 [DOI] [PubMed] [Google Scholar]
- 2. Bradbury AW, Adam DJ, Bell J, et al. BASIL trial Participants Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51(Suppl):5S-17S. 10.1016/j.jvs.2010.01.073 [DOI] [PubMed] [Google Scholar]
- 3. Menard MT, Farber A, Assmann SF, et al. Design and Rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc 2016;5:e003219. 10.1161/JAHA.116.003219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Popplewell MA, Davies H, Jarrett H, et al. BASIL-2 Trial Investigators Bypass versus angio plasty in severe ischaemia of the leg - 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016;17:11. 10.1186/s13063-015-1114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mustapha JA, Katzen BT, Neville RF, et al. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J Am Heart Assoc 2018;7:e009724. 10.1161/JAHA.118.009724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nejim BJ, Wang S, Arhuidese I, et al. Regional variation in the cost of infrainguinal lower extremity bypass surgery in the United States. J Vasc Surg 2018;67:1170-1180.e4. 10.1016/j.jvs.2017.08.055 [DOI] [PubMed] [Google Scholar]
- 7. Goodney PP, Travis LL, Brooke BS, et al. Relationship between regional spending on vascular care and amputation rate. JAMA Surg 2014;149:34-42. 10.1001/jamasurg.2013.4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antoniou GA, Georgiadis GS, Antoniou SA, Makar RR, Smout JD, Torella F. Bypass surgery for chronic lower limb ischaemia. Cochrane Database Syst Rev 2017;4:CD002000. 10.1002/14651858.CD002000.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaMuraglia GM, Conrad MF, Chung T, Hutter M, Watkins MT, Cambria RP. Significant perioperative morbidity accompanies contemporary infrainguinal bypass surgery: an NSQIP report. J Vasc Surg 2009;50:299-304, 304.e1-4. 10.1016/j.jvs.2009.01.043 [DOI] [PubMed] [Google Scholar]
- 10. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017;14:156-70. 10.1038/nrcardio.2016.179 [DOI] [PubMed] [Google Scholar]
- 11. Veronese N, Cereda E, Stubbs B, et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev 2017;35:63-73. 10.1016/j.arr.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siracuse JJ, Menard MT, Eslami MH, et al. Vascular Quality Initiative Comparison of open and endovascular treatment of patients with critical limb ischemia in the Vascular Quality Initiative. J Vasc Surg 2016;63:958-65.e1. 10.1016/j.jvs.2015.09.063 [DOI] [PubMed] [Google Scholar]
- 13. Rosenfeld BA, Beattie C, Christopherson R, et al. Perioperative Ischemia Randomized Anesthesia Trial Study Group The effects of different anesthetic regimens on fibrinolysis and the development of postoperative arterial thrombosis. Anesthesiology 1993;79:435-43. 10.1097/00000542-199309000-00005 [DOI] [PubMed] [Google Scholar]
- 14. Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg 1991;73:696-704. 10.1213/00000539-199112000-00005 [DOI] [PubMed] [Google Scholar]
- 15. Breslow MJ, Parker SD, Frank SM, et al. The PIRAT Study Group Determinants of catecholamine and cortisol responses to lower extremity revascularization. Anesthesiology 1993;79:1202-9. 10.1097/00000542-199312000-00010 [DOI] [PubMed] [Google Scholar]
- 16. Parker SD, Breslow MJ, Frank SM, et al. Perioperative Ischemia Randomized Anesthesia Trial Study Group Catecholamine and cortisol responses to lower extremity revascularization: correlation with outcome variables. Crit Care Med 1995;23:1954-61. 10.1097/00003246-199512000-00003 [DOI] [PubMed] [Google Scholar]
- 17. Christopherson R, Glavan NJ, Norris EJ, et al. Perioperative Ischemia Randomized Anesthesia Trial (PIRAT) Study Group Control of blood pressure and heart rate in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. J Clin Anesth 1996;8:578-84. 10.1016/S0952-8180(96)00139-0 [DOI] [PubMed] [Google Scholar]
- 18. Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000;321:1493. 10.1136/bmj.321.7275.1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014;(1):CD010108. 10.1002/14651858.CD010108.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbosa FT, Jucá MJ, Castro AA, Cavalcante JC. Neuraxial anaesthesia for lower-limb revascularization. Cochrane Database Syst Rev 2013;(7):CD007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghanami RJ, Hurie J, Andrews JS, et al. Anesthesia-based evaluation of outcomes of lower-extremity vascular bypass procedures. Ann Vasc Surg 2013;27:199-207. 10.1016/j.avsg.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh N, Sidawy AN, Dezee K, et al. The effects of the type of anesthesia on outcomes of lower extremity infrainguinal bypass. J Vasc Surg 2006;44:964-8, discussion 968-70. 10.1016/j.jvs.2006.06.035 [DOI] [PubMed] [Google Scholar]
- 23. Sgroi MD, McFarland G, Mell MW. Utilization of regional versus general anesthesia and its impact on lower extremity bypass outcomes. J Vasc Surg 2019;69:1874-9. 10.1016/j.jvs.2018.08.190 [DOI] [PubMed] [Google Scholar]
- 24. Sox HC, Goodman SN. The methods of comparative effectiveness research. Annu Rev Public Health 2012;33:425-45. 10.1146/annurev-publhealth-031811-124610 [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benchimol EI, Smeeth L, Guttmann A, et al. RECORD Working Committee The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vision and mandate. http://www.cihi.ca/CIHI-ext-portal/internet/EN/SubTheme/about+cihi/vision+and+mandate/cihi010703.
- 28. ICES About ICES. https://www.ices.on.ca/About-ICES.
- 29. Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet 2008;372:562-9. 10.1016/S0140-6736(08)61121-6 [DOI] [PubMed] [Google Scholar]
- 30. Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. A population-based study of anesthesia consultation before major noncardiac surgery. Arch Intern Med 2009;169:595-602. 10.1001/archinternmed.2009.3 [DOI] [PubMed] [Google Scholar]
- 31. Wijeysundera DN, Beattie WS, Karkouti K, Neuman MD, Austin PC, Laupacis A. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: population based cohort study. BMJ 2011;342:d3695. 10.1136/bmj.d3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards J, Brown A, Homan C. The data quality study of the Canadian Discharge Abstract Database. Proc Stat Canada Symp 2001. [Google Scholar]
- 33. Miller’s Anesthesia. 9th ed Elsevier, 2019. [Google Scholar]
- 34. McIsaac DI, Wijeysundera DN, Bryson GL, Huang A, McCartney CJL, van Walraven C. Hospital-, anesthesiologist-, and patient-level variation in primary anesthesia type for hip fracture surgery: a population-based cross-sectional analysis. Anesthesiology 2018;129:1121-31. 10.1097/ALN.0000000000002453 [DOI] [PubMed] [Google Scholar]
- 35. Bennett KM, Kent KC, Schumacher J, Greenberg CC, Scarborough JE. Targeting the most important complications in vascular surgery. J Vasc Surg 2017;65:793-803. 10.1016/j.jvs.2016.08.107 [DOI] [PubMed] [Google Scholar]
- 36. Tu K, Mitiku T, Guo H, Lee DS, Tu JV. Myocardial infarction and the validation of physician billing and hospitalization data using electronic medical records. Chronic Dis Can 2010;30:141-6. 10.24095/hpcdp.30.4.06 [DOI] [PubMed] [Google Scholar]
- 37. Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18-26. [PMC free article] [PubMed] [Google Scholar]
- 38. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013;33:160-6. 10.24095/hpcdp.33.3.06 [DOI] [PubMed] [Google Scholar]
- 39. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512-6. 10.2337/diacare.25.3.512 [DOI] [PubMed] [Google Scholar]
- 40. Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009;6:388-94. 10.1080/15412550903140865 [DOI] [PubMed] [Google Scholar]
- 41. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ 2015;187:725-33. 10.1503/cmaj.150209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen G, Lix L, Tu K, et al. Hypertension Outcome and Surveillance Team Influence of Using Different Databases and ‘Look Back’ Intervals to Define Comorbidity Profiles for Patients with Newly Diagnosed Hypertension: Implications for Health Services Researchers. PLoS One 2016;11:e0162074. 10.1371/journal.pone.0162074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sternberg SA, Bentur N, Abrams C, et al. Identifying frail older people using predictive modeling. Am J Manag Care 2012;18:e392-7. [PubMed] [Google Scholar]
- 44. McIsaac DI, Bryson GL, van Walraven C. Association of Frailty and 1-Year Postoperative Mortality Following Major Elective Noncardiac Surgery: A Population-Based Cohort Study. JAMA Surg 2016;151:538-45. 10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 45.The Johns Hopkins ACG System. Excerpt from Version 11.0 Technical Reference Guide. https://www.hopkinsacg.org/document/acg-system-version-11-technical-reference-guide/.
- 46. McIsaac DI, Wong CA, Bryson GL, van Walraven C. Association of polypharmacy with survival, complications, and healthcare resource use after elective noncardiac surgery: a population-based cohort study. Anesthesiology 2018;128:1140-50. 10.1097/ALN.0000000000002124 [DOI] [PubMed] [Google Scholar]
- 47. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 48. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228-34 10.1080/03610910902859574 https://www.tandfonline.com/doi/abs/10.1080/03610910902859574. [DOI] [Google Scholar]
- 49. Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Stat Med 2003;22:2799-815. 10.1002/sim.1442 [DOI] [PubMed] [Google Scholar]
- 50. Austin PC, Laupacis A. A tutorial on methods to estimating clinically and policy-meaningful measures of treatment effects in prospective observational studies: a review. Int J Biostat 2011;7:6. 10.2202/1557-4679.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The ACG® system. http://www.hopkinsacg.org/applications/-impact.
- 52. Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ 2008;27:531-43. 10.1016/j.jhealeco.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Terza JV. Two-stage residual inclusion estimation in health services research and health economics. Health Serv Res 2018;53:1890-9. 10.1111/1475-6773.12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koladjo BF, Escolano S, Tubert-Bitter P. Instrumental variable analysis in the context of dichotomous outcome and exposure with a numerical experiment in pharmacoepidemiology. BMC Med Res Methodol 2018;18:61. 10.1186/s12874-018-0513-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lousdal ML. An introduction to instrumental variable assumptions, validation and estimation. Emerg Themes Epidemiol 2018;15:1. 10.1186/s12982-018-0069-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brookhart MA, Schneeweiss S. Preference-based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat 2007;3:14. 10.2202/1557-4679.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444-55 10.1080/01621459.1996.10476902. [DOI] [Google Scholar]
- 58. Austin PC, Stuart EA. Estimating the effect of treatment on binary outcomes using full matching on the propensity score. Stat Methods Med Res 2017;26:2505-25. 10.1177/0962280215601134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268-74. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 60. Barshes NR, Menard MT, Nguyen LL, Bafford R, Ozaki CK, Belkin M. Infrainguinal bypass is associated with lower perioperative mortality than major amputation in high-risk surgical candidates. J Vasc Surg 2011;53:1251-1259.e1. 10.1016/j.jvs.2010.11.099 [DOI] [PubMed] [Google Scholar]
- 61. Tsay C, Luo J, Zhang Y, Attaran R, Dardik A, Ochoa Chaar CI. Perioperative outcomes of lower extremity revascularization for rest pain and tissue loss. Ann Vasc Surg 2020;66:493-501. 10.1016/j.avsg.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 62. Romano PS, Mull HJ, Rivard PE, et al. Validity of selected AHRQ patient safety indicators based on VA National Surgical Quality Improvement Program data. Health Serv Res 2009;44:182-204. 10.1111/j.1475-6773.2008.00905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dovell G, Rogers CA, Armstrong R, Harris RA, Hinchliffe RJ, Mouton R. The effect of mode of anaesthesia on outcomes after elective endovascular repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2020;59:729-38. 10.1016/j.ejvs.2020.01.031 [DOI] [PubMed] [Google Scholar]
- 64. Cozowicz C, Poeran J, Memtsoudis SG. Epidemiology, trends, and disparities in regional anaesthesia for orthopaedic surgery. Br J Anaesth 2015;115(Suppl 2):ii57-67. 10.1093/bja/aev381 [DOI] [PubMed] [Google Scholar]
- 65. Cozowicz C, Poeran J, Zubizarreta N, Mazumdar M, Memtsoudis SG. Trends in the use of regional anesthesia: neuraxial and peripheral nerve blocks. Reg Anesth Pain Med 2016;41:43-9. 10.1097/AAP.0000000000000342 [DOI] [PubMed] [Google Scholar]
- 66. Memtsoudis SG, Fiasconaro M, Soffin EM, et al. Enhanced recovery after surgery components and perioperative outcomes: a nationwide observational study. Br J Anaesth 2020;124:638-47. 10.1016/j.bja.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 67. McIsaac DI, Wijeysundera DN, Huang A, Bryson GL, van Walraven C. Association of hospital-level neuraxial anesthesia use for hip fracture surgery with outcomes: a population-based cohort study. Anesthesiology 2018;128:480-91. 10.1097/ALN.0000000000001899 [DOI] [PubMed] [Google Scholar]
- 68. Yılmaz İnal F, Yılmaz Y, Daşkaya H, et al. Evaluation of the attitudes of surgeons about regional anesthesia: a survey study. Local Reg Anesth 2019;12:89-95. 10.2147/LRA.S211469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fleischut PM, Eskreis-Winkler JM, Gaber-Baylis LK, et al. Variability in anesthetic care for total knee arthroplasty: an analysis from the anesthesia quality institute. Am J Med Qual 2015;30:172-9. 10.1177/1062860614525989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matthey PW, Finegan BA, Finucane BT. The public’s fears about and perceptions of regional anesthesia. Reg Anesth Pain Med 2004;29:96-101. 10.1016/j.rapm.2003.10.017 [DOI] [PubMed] [Google Scholar]
- 71. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia [table of contents.]. Anesth Analg 2004;98:1486-90. 10.1213/01.ANE.0000113549.98873.B1 [DOI] [PubMed] [Google Scholar]
- 72. Pelinka LE, Pelinka H, Leixnering M, Mauritz W. Why patients choose regional anesthesia for orthopedic and trauma surgery. Arch Orthop Trauma Surg 2003;123:164-7. 10.1007/s00402-003-0479-y [DOI] [PubMed] [Google Scholar]
- 73. Shevde K, Panagopoulos G. A survey of 800 patients’ knowledge, attitudes, and concerns regarding anesthesia. Anesth Analg 1991;73:190-8. 10.1213/00000539-199108000-00013 [DOI] [PubMed] [Google Scholar]
- 74. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 75. Shulman MA, Myles PS, Chan MT, McIlroy DR, Wallace S, Ponsford J. Measurement of disability-free survival after surgery. Anesthesiology 2015;122:524-36. 10.1097/ALN.0000000000000586 [DOI] [PubMed] [Google Scholar]
- 76. Cook TM, Counsell D, Wildsmith JA, Royal College of Anaesthetists Third National Audit Project Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102:179-90. 10.1093/bja/aen360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables e1-e7 and fig e1


