Abstract
The blood–brain barrier (BBB) is a critical regulator of CNS homeostasis. It possesses physical and biochemical characteristics (i.e. tight junction protein complexes, transporters) that are necessary for the BBB to perform this physiological role. Microvascular endothelial cells require support from astrocytes, pericytes, microglia, neurons, and constituents of the extracellular matrix. This intricate relationship implies the existence of a neurovascular unit (NVU). NVU cellular components can be activated in disease and contribute to dynamic remodeling of the BBB. This is especially true of microglia, the resident immune cells of the brain, which polarize into distinct proinflammatory (M1) or anti-inflammatory (M2) phenotypes. Current data indicate that M1 pro-inflammatory microglia contribute to BBB dysfunction and vascular “leak”, while M2 anti-inflammatory microglia play a protective role at the BBB. Understanding biological mechanisms involved in microglia activation provides a unique opportunity to develop novel treatment approaches for neurological diseases. In this review, we highlight characteristics of M1 proinflammatory and M2 anti-inflammatory microglia and describe how these distinct phenotypes modulate BBB physiology. Additionally, we outline the role of other NVU cell types in regulating microglial activation and highlight how microglia can be targeted for treatment of disease with a focus on ischemic stroke and Alzheimer’s disease.
Keywords: Alzheimer’s disease, blood–brain barrier, inflammation, ischemic stroke, microglia, neurovascular unit, oxidative stress, paracellular permeability, tight junctions
Introduction
The blood–brain barrier (BBB) is a physical and biochemical barrier that separates the central nervous system (CNS) from the peripheral circulation. This highly specialized barrier tissue is critical in maintaining the CNS microenvironment, a role that permits physiological functioning of neurons. Such homeostatic capabilities require specialized molecular features (i.e. tight junction protein complexes, transporters) in endothelial cells that line the cerebral microvasculature. Tight junction protein complexes are localized between apposing endothelial cells and involve transmembrane proteins (i.e. claudins, occludin, junctional adhesion molecules (JAMs), tricellulin) that are linked to the actin cytoskeleton by zonula occludens (ZO) family members (i.e. ZO-1, -2, -3).1–3 The essential role of claudin-5 at the BBB is supported by its established sealing function and known development of early postnatal brain edema and lethality in claudin-5 null mice.4 Occludin is also prominently involved in regulating BBB paracellular permeability; however, occludin knockout mice exhibit functionally intact tight junctions,5 an effect that may reflect compensation from other tight junction constituents. Tricellulin regulates paracellular permeability to macromolecules,1 while JAMs can contribute to endothelial barrier properties.3 Paracellular permeability is also controlled by adherens junctions, which are formed by interactions between transmembrane cadherin proteins and intracellular catenins.3 Additionally, brain microvascular endothelial cells express primary active transporters (i.e. P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), multidrug resistance proteins (MRPs)) that restrict the ability of endogenous and exogenous substances from accessing brain parenchyma.6,7 Most research on efflux transporters has been conducted on P-gp and high endothelial expression of this critical transporter is a BBB identifying feature.2,6 P-gp is known to limit brain permeability of hundreds of structurally diverse substances ranging in molecular weight from 100 to 4000 Da. An important concept in understanding efflux transport properties at the BBB is the fact that many P-gp substrates are also transported by BCRP, which functions in synergy with P-gp to limit CNS drug permeation.8 In contrast to P-gp and BCRP, MRPs participate in the brain-to-blood transport of a more limited set of compounds that are primarily anionic or their associated glucuronidated, sulfated, or glutathione-conjugated metabolites.8
A detailed understanding of the BBB requires an appreciation that this “barrier” is a dynamic interface between the systemic circulation and brain parenchyma. This is necessary due to the high metabolic demands of the CNS coupled with an inherent inability to store biological fuels for later use.9 As noted by Constantino Iadecola in his seminal article,9
… the brain receives energy substrates, primarily oxygen and glucose, “on the fly” through its blood supply. Considering the dynamic and regionally diverse energy requirements imposed by brain activity, blood flow needs to reach the brain at the right time and place, and in the right amount.
This requirement implies that the brain microvasculature must rapidly respond to environmental changes in an attempt to match cerebral blood flow to brain metabolic demands. It is also abundantly clear that the BBB phenotype can only be acquired through coordinated intercellular communication. The unique phenotype of the cerebral microvasculature implies the existence of a “neurovascular unit (NVU).” By emphasizing the symbiotic nature of cell–cell interactions between endothelial cells, glial cells, pericytes, and neurons as well as contributions from extracellular matrix components, the NVU concept led to a significant ideological shift in our understanding of neurological diseases.9 Instead of considering each cell type independently, it is now understood that NVU components communicate directly with the cerebral microvasculature. This enables endothelial cells to develop distinct barrier properties and allows for dynamic responses to pathological stressors.
Over the past several years, much work has focused on the role of astrocytes and pericytes in regulation of the BBB phenotype. For example, in vivo administration of the selective astrocyte toxin 3-chloropropanediol resulted in BBB disruption as indicated by enhanced paracellular “leak” of fluorescent 10-kDa dextran.10 This BBB injury was reversible as demarcated by astrocyte repopulation into lesioned brain tissue and subsequent reduction in 10-kDa dextran permeability that occurred six days post-3-chloropropanediol treatment.10 Such an observation indicates that astrocytes play a critical role in maintenance of the BBB phenotype. Several published studies have identified astrocyte-released effector molecules that can contribute to development of the BBB phenotype including members of the hedgehog family,11 angiotensin II,12 and/or apolipoprotein E (APOE)/lipoprotein receptor-related protein 1 (LRP1) signaling.13 Similarly, pericyte-deficient mice, caused by dysfunctional platelet-derived growth factor BB (PDGF-BB) and platelet-derived growth factor receptor-β (PDGFR-β) signaling, suffer from impaired BBB formation.14–16 More recently, Nikolakopoulou et al.17 demonstrated reduced pericyte coverage and BBB breakdown in the cerebral cortex, hippocampus, and thalamus in PdgfrβF7/F7 mice, which exhibit dysfunctional PDGFRβ signaling.17
In contrast to astrocytes and pericytes, the role of microglia in regulation of the BBB phenotype is only beginning to be defined. Microglia, the innate immune cells of the brain, were first described by the Spanish neuroanatomist del Rio-Hortega in 191918 and comprise approximately 10–15% of all cells that reside in the central nervous system (CNS).19 Microglia are myeloid cells, a lineage that also includes monocytes/macrophages, neutrophils, and platelets.20 The pathophysiological functions of central myeloid cells (i.e. microglia) are best exemplified in the setting of ischemic stroke where they have been shown to participate in initiation and maintenance of inflammation but also post-ischemic inflammatory resolution and tissue repair.20 To this end, physiological functions of microglia include participation in regulatory processes that control tissue development, maintenance of the neural environment, response to pathophysiological stressors, and promotion of neural repair.21 Under normal conditions, microglia exist in a quiescent state lacking endocytotic and phagocytotic activity. These microglia possess a ramified morphology characterized by a small (5–10 µm) cell body, exhibit little or no cellular movement, and possess multiple radial processes that extend from the cell body and screen the brain extracellular milieu for pathological mediators and/or toxicants.21 Evidence for the existence of cytoplasmic projections and protrusions in resting microglia have been derived from two-photon microscopy imaging.22 During disease or trauma, microglia rapidly transition to an activated phenotype. The degree of activation is directly correlated to the type and severity of brain injury. Throughout the scientific literature, activated microglia are often described as existing in two distinct states designated as M1 and M2. M1 microglia are defined by pro-inflammatory and pro-killing functions, whereas M2 microglia are involved in immunoregulation, control of inflammatory mechanisms, and repair/injury resolution.21 M2 microglia are also capable of phagocytosis, which contributes to the ability of M2-polarized microglia to remove cellular debris and contribute to neural repair.23 There are several exceptions to the M1/M2 classification system as has been shown in various studies on neurodegenerative diseases where considerable heterogeneity in microglial activation phenotypes have been described.24,25 Indeed, microglial activation and proliferation are implicated in neuronal cell death in CNS pathological states such as ischemic stroke and neurodegenerative diseases. Such activation correlates with dysfunction of the blood–brain barrier (BBB), which is an early event in stroke3 and Alzheimer’s disease.26 In this review, we will discuss direct effects of microglia activation on BBB functional integrity. We will also provide examples of neurological diseases where microglia activation causes BBB dysfunction and provide perspective on microglial molecular targets that can be exploited for the development of novel treatment paradigms.
Microglial activation: M1 pro-inflammatory phenotype and BBB injury
Microglia populate the brain prior to development of the cerebrovascular network and localize adjacent to brain microvasculature (i.e. perivascular microglia). Microglia distribution in brain tissue is not homogeneous as evidenced by studies in mouse brain tissue that described elevated microglia cell counts in basal ganglia, hippocampus, substantia nigra, and olfactory telencephalon.24 Using immunofluorescence microscopy, we have observed resting microglia in close proximity to cerebral microvessels in healthy Sprague-Dawley rats (Figure 1). This close physical association enables microglia to simultaneously monitor BBB integrity and influx of blood-derived solutes into brain parenchyma. Experimental evidence suggests that microglia also contribute to maintenance of the BBB phenotype. For example, co-culture of mouse brain endothelial cells (bEND3) with resting microglia resulted in enhanced endothelial expression of critical tight junction proteins occludin and ZO-1.27 More recently, in vivo studies demonstrated that perivascular microglia may directly supply critical proteins (i.e. claudin-5) to the BBB endothelium for assembly into tight junction complexes.28
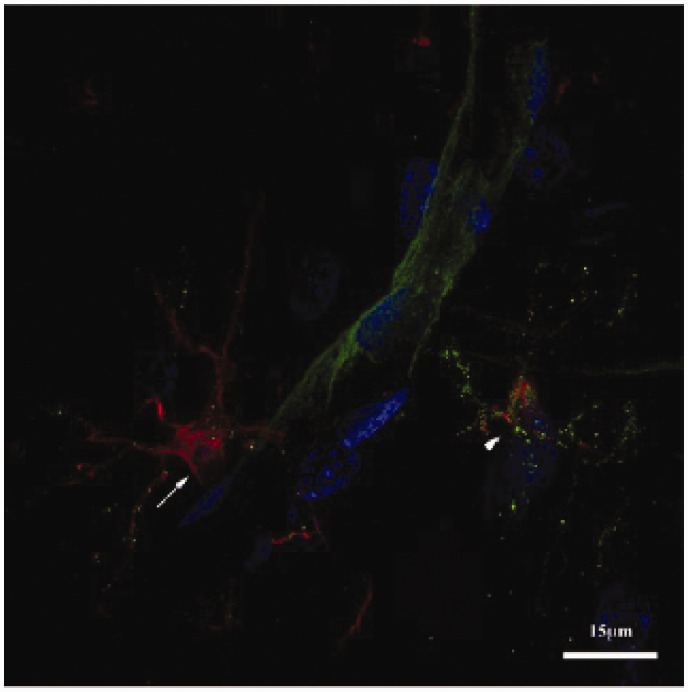
Figure 1.
Perivascular microglial cells. This image illustrates the proximity of microglia to cerebral capillaries in the adult rat hindbrain. A 30-µm rat brain section was stained for the microglial marker IBA1 (red) and major histocompatibility complex (MHC) II (green), which is upregulated in activated microglia but also stains endothelial cells. Nuclei were stained with 4ʹ,6-diamindino-2-phenylindole (DAPI; blue). The white arrow highlights a surveillance microglia and the arrowhead highlights an activated microglia that has increased expression of MHCII. Note the capillary between the two microglia. This image is a maximum intensity projection of a 10-µm segment of the brain slice and was processed for brightness, contrast, and RGB levels. Adapted from Herndon JM, Tome ME and Davis TP. Development and maintenance of the blood-brain barrier. In: LR Caplan, J Biller, MC Leary, et al. (eds) Primer on cerebrovascular diseases, 2nd ed. San Diego: Elsevier Academic Press, 2017.
Upon vascular exposure to pathophysiological stressors, there appears to be a very precise and tight spatiotemporal correlation between vascular activation, breakdown of the BBB, and transition of resting microglia to a M1 activated state.29,30 This concept was recently demonstrated by Haruwaka et al. in vivo using the Murphy Roths Large/lymphoproliferation (MRL/lpr) mouse model of systemic lupus erythematosus. In this study, an increased number of activated microglia were observed in MRL/lpr mice by increased immunohistochemical staining against ionized calcium binding adaptor molecule 1 (IBA-1).28 IBA-1 is a well-established protein marker of macrophages and microglia that is selectively upregulated in activated microglia. IBA-1-positive microglia were primarily localized in perivascular regions of the motor cortex and correlated with increased BBB leak to 10-kDa dextran.28 Similar effects on microvascular integrity were observed when microglia were activated following in vivo administration of lipopolysaccharide (LPS) for seven days.28 It is critical to point out that these observations are largely correlative and do not provide direct evidence that microglial transition from a resting to a M1 activated state is directly responsible for BBB disruption. Nonetheless, this study does provide evidence for a dual role of activated microglia involving both beneficial or detrimental roles at the NVU in a manner that depends upon the timing of activation. Specifically, CCR5-dependent migration of activated microglia to the perivascular space early in the course of inflammation protects the BBB phenotype, while sustained inflammation triggers activated microglia to induce BBB damage.28 In contrast, measurable disruption of rat brain endothelial tight junction protein complexes was observed following 6-h incubation with LPS as indicated by decreased transendothelial electrical resistance (TEER), reduced protein expression of claudin-5, occludin, and ZO-1, and increased paracellular transport of sodium fluorescein (NaF).31 Mice treated with the purinergic receptor P2Y G-protein coupled 12 (P2RY12) inhibitor clopidogrel, a drug that blocks microglia process motility, showed an impaired ability of microglia to promote repair of laser-induced BBB injury.32 This study also showed that ablation of juxtavascular microglia by focused laser radiation abolished BBB “leak” resolution.32 More recently, studies in spinal cord blood vessels demonstrated that microglial depletion caused increased vascular permeability to fibrinogen in the setting of chronic mild hypoxia.33 It is also noteworthy that increased BBB permeability can result from disruption of endothelial glycocalyx as has been demonstrated in a rodent model of cardiac arrest and subsequent cardiopulmonary resuscitation.34 Microglia are known to engage neuronal glycocalyx, a process that regulates microglial immune responses and phagocytosis.35 Forsberg et al.36 have recently shown that IBA-1-positive microglia are colocalized with the glycocalyx marker Ulex Europaeus Agglutinin 1 (UAE-1) in string vessels suggesting that activated microglia can interact with vascular glycocalyx and disrupt vessel integrity.
Production of pro-inflammatory mediators
Activation of microglia to produce and secrete proinflammatory mediators (M1 phenotype) requires signaling via toll-like receptor (TLR)-4,37 the interferon-gamma (IFN-γ) receptor complex,38 and/or the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor (Figure 2).39 Previous research has demonstrated that M1 microglia are involved in the production and secretion of proinflammatory cytokines and chemokines including TNF-α, IL-1β, IL-6, IL-12, CCL2 (also known as monocyte chemoattractant protein-1 (MCP-1)), and CXCL10.21,40 Significant injury to the NVU occurs following exposure to TNF-α, IL-1β, and IL-6.29,41,42 Inflammatory signaling in microglia may also involve cyclooxygenase-2 (COX2), which is inducible in response to pathological stressors43 or gonadal testosterone depletion44 and contributes to opening of the BBB. In neuroinflammation, COX2 activates sphingomyelinases leading to release of ceramides and subsequent activation of p38 mitogen-activated protein kinase (MAPK).45 Activation of the p38 MAPK pathway is associated with increased microglial synthesis of proinflammatory cytokines.46 This is supported by the observation that pharmacological treatment with SB203580, a selective p38 MAPK inhibitor, attenuates TNF-α secretion from primary cultures of rat microglia.46 Additionally, Forster et al.47 showed reduced protein expression of occludin and claudin-5, but no change in VE-cadherin, following 8-h exposure to TNF-α. These changes in tight junction constituent proteins correlated with a statistically significant reduction in TEER, thereby reflecting a possible increase in paracellular permeability.47 The ability of TNF-α to disrupt tight junctions and open a paracellular route between apposing endothelial cells at the BBB is related to the upregulation of matrix metalloproteinase (MMP) -2, -3 and -9 enzymes in the extracellular matrix.48,49 Similar modifications to BBB functional integrity have been reported following exposure to IL-1β,50 IL-6,51 and IL-12.51 Proinflammatory cytokines can also modulate expression of critical BBB transporters as shown in in vitro and ex vivo studies where brain microvascular endothelial cells or isolated brain microvessels were directly exposed to proinflammatory cytokines. Studies in the hCMEC/d3 cell line have shown suppression of MDR1 (i.e. the gene that encodes P-gp) mRNA and ABCG2 (i.e. the gene that encodes BCRP) mRNA following treatment with IL-6.52 In contrast, MDR1 mRNA was increased in hCMEC/d3 cells following cellular exposure to TNF-α.52 Despite the change in mRNA expression, P-gp protein levels were unchanged following TNF-α treatment in the hCMEC/d3 cell line; however, transport activity was significantly reduced by a mechanism involving actin filament-associated protein-1 (AFAP-1).53 Using an ex vivo microvessel transport assay, Hartz et al.54 showed that TNF-α treatment abolished P-gp-mediated efflux activity via the endothelin B (ETB) receptor. Interestingly, Bauer et al.55 showed that microvascular exposure to TNF-α at time points greater than 6 h resulted in increased P-gp functional expression. These results suggest that proinflammatory cytokines, such as those released from activated M1 microglia, can modulate transport kinetics at the BBB, alter brain permeability to circulating solutes, and render the brain accessible to potentially toxic substances. Despite their prominent role in the production of pro-inflammatory mediators, it is important to note that microglia are not the only source of such cytokines in the brain. Both astrocytes and pericytes are well known to release cytokines in response to pathophysiological stressors and, therefore, can also contribute to BBB injury under pathological stress conditions.56
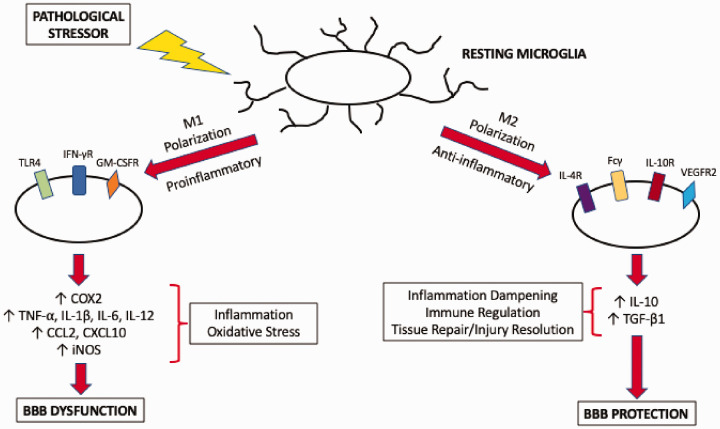
Figure 2.
Polarization of microglia in response to pathological stressors. In the absence of pathological mediators, microglia maintain a resting phenotype where they perform surveillance of the brain extracellular milieu. In the presence of a stressor, microglia are activated and can assume one of two activation states. Polarization to an M1 state is mediated by TLR4, IFN-γ receptors, or GM-CSF receptors and leads to increased production of proinflammatory cytokines and chemokines as well as increased expression of COX2 and iNOS. This results in increased inflammation and oxidative stress, processes that cause dysfunction of the BBB. In contrast, M2 microglia perform inflammation dampening, immune regulation, and tissue repair/injury resolution functions. Polarization of microglia to a M2 activation state involves IL-4 receptors, Fcγ, IL-10 receptors, or VEGFR2. M2 microglia secrete anti-inflammatory mediators such as IL-10 and TGF-β1 and help to protect the BBB in the setting of neurological disease.
Promotion of immune cell trafficking
M1 microglia secrete chemokines (i.e. CCL2, CXCL10) that promote BBB disruption and increased trafficking of immune cells into brain parenchyma. Deleterious effects on BBB integrity have been shown following intracerebroventricular (ICV) injection of murine recombinant CCL2, which caused a reduction in vascular immunostaining of occludin, claudin-5, ZO-1, and ZO-2.57 These changes in tight junction molecular composition were associated with an increase in brain uptake of fluorescein isothiocyanate (FITC)-albumin, a fluorescently labeled plasma protein that is not capable of permeating the intact BBB.57 The effect of CCL2 on cerebral microvascular permeability is dependent upon its ability to bind to its receptor CCR2. This mechanism was demonstrated in CCR2-null mice where the increase in FITC-albumin extravasation following ICV injection of CCL2 was ameliorated as compared to age-matched CCR2-positive animals.57 Additionally, increased CCL2 secretion promotes migration of monocytes and macrophages across the BBB, an effect that further exacerbates NVU injury and neuronal cell loss.58–60 Microglia signaling via CCR2 (as well as CX3CR1) may also play a significant role in neurovascular damage in younger individuals as recently demonstrated in a mouse model of childhood arterial ischemic stroke.61 In contrast, CCL2 gene expression was downregulated in microglia isolated from aged brain tissue.62 CXCL10 (also referred to as IP-10) is produced by microglia in response to the proinflammatory mediator interferon (IFN)-γ. Similar to CCL2, CXCL10 acts as a chemoattractant protein that facilitates monocyte and macrophage migration across the BBB. This role for CXCL10 has been observed in ischemic stroke63 and elevation of this chemokine in circulating blood is associated with poor neurological outcomes following hemorrhagic stroke.64 Increased CXCL10 and associated monocyte/macrophage migration into brain tissue have also been observed in Alzheimer’s disease65,66 and traumatic brain injury.67 Taken together, these studies demonstrate a critical role for microglial chemokines in exacerbating NVU injury.
Microglia and oxidative stress
In addition to cytokines and chemokines, opening of the BBB during disease can be triggered by production of reactive oxygen species (ROS) and subsequent oxidative stress. We have shown that occludin is redox-sensitive and oxidative stress can cause collapse of occludin oligomeric assemblies and BBB dysfunction.68 In microglia that are polarized to an M1 phenotype, ROS generation is related to increased expression and activity of inducible nitric oxide synthase (iNOS).69 Increased iNOS activity in the brain results in enhanced production of nitric oxide (NO) that can be conjugated with superoxide anion (O2−) to form the potent cytotoxic molecule peroxynitrite (ONOO−). Peroxynitrite causes extensive damage to neurons and cerebral microvessels through lipid peroxidation, consumption of endogenous antioxidants (i.e. glutathione), DNA fragmentation, and induction of mitochondrial failure.70 Peroxynitrite can also induce cellular injury due to its ability to nitrosylate tyrosine residues, thereby inducing modifications to critical proteins.71 Breakdown of peroxynitrite into nitrogen dioxide and hydroxyl radicals also contributes to endothelial cell dysfunction and BBB disruption.72 Both NO and peroxynitrite can increase MMP-mediated opening of the BBB. Pharmacological inhibition of iNOS with the non-selective NOS inhibitor L-NG-nitro-arginine-methyl-ester (L-NAME) reduced MMP-2/MMP-9 activity at the NVU and prevented BBB disruption in a rodent model of cerebral ischemia/reperfusion injury.73 More recently, BBB opening following middle cerebral artery occlusion (MCAO) in male Sprague-Dawley rats was attenuated by baicalin, a flavone glycoside and potent antioxidant, in a dose-dependent manner.74 In the study by Chen et al.,74 baicalin was shown to exert BBB protective effects by direct scavenging of peroxynitrite and inhibition of MMP-9 activity. Other signaling pathways that can be targeted by peroxynitrite and promote BBB dysfunction include high mobility group box 1 (HMGB1), TLR-2 and 4, poly(ADP ribose) polymerase, Src, Rho-associated protein kinase (ROCK), and glycogen synthase kinase (GSK)3β.75 Of these pathways, the link between Src signaling and BBB opening is particularly intriguing. This connection is supported by a previous study where imatinib, a drug commonly used to treat chronic myeloid leukemia, decreased CNS expression of phosphorylated Src, reduced MMP-9 activity, and reduced Evan’s blue-albumin extravasation in a rodent model of subarachnoid hemorrhage (SAH).76 Indeed, allosteric binding sites have been identified for imatinib on Src that cause reduced kinase activity upon drug binding.77 These observations are in contrast to a more recent study in the setting of intracerebral hemorrhage, where increased Src signaling following activation by macrophage stimulating protein (MSP) was shown to preserve BBB integrity.78 Src signaling interacts with multiple downstream pathways and may be associated with variable cross-talk between other intracellular signaling cascades when pathologies differ, which may explain the conflicting observations between these two studies. It is noteworthy that these studies also showed involvement of different downstream pathways that are linked to Src signaling. Specifically, Zhan et al.76 showed reduced phosphorylation of JNK following imatinib treatment, while Lu et al.78 demonstrated that increased phosphorylation of Src resulted in enhanced nuclear translocation of β-catenin. Clearly, these observations point towards a need for detailed molecular studies to elucidate the exact role of Src signaling in the regulation of BBB functional integrity.
Microglia activation: M2 anti-inflammatory phenotype and BBB protection
Activation of microglia is commonly associated with BBB breakdown and deleterious processes including inflammation, oxidative stress, and immune cell trafficking; however, positive effects such as immune regulation, inflammation dampening, and repair/injury resolution have also been reported.21 Morphologically, microglia that participate in BBB protection and neural repair have enlarged cell bodies and produce anti-inflammatory cytokines, extracellular matrix proteins, glucocorticoids, and other substances.21 Transition from a resting state to a protective M2 phenotype is mediated by signaling via the IL-4 receptor, the FCγ receptor, or the IL-10 receptor.21 Vascular endothelial growth factor receptor 2 (VEGFR2) signaling may also be involved in polarizing microglia towards a protective role. This mechanism was shown by Esposito et al.79 where increased VEGF production in the setting of ischemic preconditioning resulted in increased VEGFR2 expression in microglia and polarization of IBA-1-positive microglia to a ramified morphology with increased expression of M2 markers. The role of VEGFR2 was confirmed using a Flk-1 inhibitor, which blocks VEGFR2 kinase and attenuated microglial polarization.79 Activation of the IL-4 receptor triggers phagocytotic mechanisms that are associated with tissue repair. For example, studies in a mouse model of intracerebral hemorrhage showed that IL-4 administration promoted microglial polarization to an anti-inflammatory phenotype, an effect that reduced brain infarction and edema and improved neurological outcomes.80 Fcγ receptors fuse with TLRs, which then enables the receptor complex to bind IgG.21 Following IgG binding, microglia secrete IL-10, increase CD86 expression on their cell surface, and increase expression of MHC-II.21 This mechanism is critical to promoting migration of regulatory T-cells in an effort to ameliorate brain injury as has been demonstrated in the setting of hemorrhagic stroke.81 Microglial functions mediated by the IL-10 receptor promote dampening of inflammation and injury repair.21 Increased brain levels of IL-10 activate both IL-10R1 and IL-10R2, which triggers JAK1 and promotes translocation of STAT3 to the microglial cell nucleus.82 This mechanism suppresses production of proinflammatory cytokines and ensures microglial polarization to an M2 phenotype.83 Recently, microglia treated with tetramethylpyrazine, a drug that promotes M2 microglial polarization, were shown to preserve functional integrity of the blood–spinal cord barrier in a murine model of experimental autoimmune encephalomyelitis (EAE).84 This is the first study to directly show that microglial cells with an anti-inflammatory phenotype can protect brain barriers against pathological stressors.
Production of anti-inflammatory mediators
Following activation of cell surface receptors, anti-inflammatory microglia can be primed to release mediators that facilitate BBB protection. These substances include IL-10 and transforming growth factor-β1 (TGF-β1). Evidence for protective effects of IL-10 on BBB functional integrity is provided by a previous study where the antioxidant resveratrol (3,5,4ʹ-trihydroxy-trans-stilbene) prevented EAE-induced loss of tight junction proteins (i.e. claudin-5, occludin, ZO-1), thereby attenuating Evan’s blue-albumin extravasation.85 This effect was associated with increased brain levels of IL-10 and arginase-1, a marker of microglia and macrophages.85 Of particular note, resveratrol also increased ICAM-1 and VCAM-1 expression in endothelial cells, an effect that promoted migration of macrophages into brain parenchyma.85 Secretion of TGF-β1 is also likely to have significant positive effects on BBB functional integrity in health and disease. Although astrocytes are the primary source of TGF-β1 in the brain, M2 microglia have been shown to produce quantities of this cytokine.86 It is critical to point out that TGF-β1 production by microglia may decrease in association with chronological age since this is a primary pathway that is perturbed in response to age-related changes in the brain.62 Indeed, receptors for TGF-β1 are functionally expressed at the BBB and play a critical role in maintenance of barrier integrity. Members of the TGF-β superfamily of signaling ligands, which include bone morphogenetic proteins, activins, and TGF-βs, bind to two types of serine-threonine kinase receptors known as type I and type II receptors. At the brain microvascular endothelium, two distinct type I receptors have been identified that are designated as activin receptor-like kinase (ALK) -1 and -5.6 These receptors phosphorylate distinct R-Smad proteins, which leads to physiological differences between ALK-1 receptors and ALK-5 receptors. Specifically, ALK-1 phosphorylates Smad1, Smad5, and Smad8, while ALK-5 propagates intracellular signals via phosphorylation of Smad2 and Smad3. Our laboratory has demonstrated that signaling mediated by the ALK-5 receptor can modulate tight junction protein expression and BBB “leak”.87 Specifically, we demonstrated that exogenous administration of TGF-β1 attenuated the increase in paracellular permeability to sucrose, a vascular marker that does not permeate the intact BBB, observed in the setting of peripheral inflammatory pain.87 Furthermore, pharmacological inhibition of the ALK-5 receptor by the selective inhibitor SB431542 in both pained and non-pained experimental animals resulted in dysregulation of tight junction integrity.87 Furthermore, administration of SB431542 enhanced microvascular permeability to sucrose, suggesting a critical role for TGF-β/ALK-5 signaling in the regulation of BBB functional integrity.87
Dynamic transition between microglia activation states
Microglia are highly dynamic cells that are capable of transitioning between activation states. In fact, the transition of microglial polarization from a M1 pro-inflammatory phenotype to a M2 anti-inflammatory state is believed to improve outcomes following CNS exposure to pathological stressors and to restore cerebral homeostasis.21 One factor that may be critical for the microglial transition between activation states is suppressor of cytokine signaling 3 (SOCS3). In vitro studies have shown that upregulation of SOCS3 by cinnamic acid suppressed expression of iNOS, TNF-α, IL-1β, and IL-6 in LPS-triggered mouse BV-2 microglia.88 Studies in LysMCre-SOCS3fl/fl mice, which lack SOCS3 expression in myeloid lineage cells, demonstrated enhanced polarization of microglia towards a pro-inflammatory state as demarcated by increased production and secretion of TNF-α, IL-1β, IL-6, CCL3, CCL4, and CXCL11.89 Histone H3K27me3 demethylase Jumonji domain containing 3 (Jmjd3) also plays a pivotal role in microglial polarization towards an anti-inflammatory phenotype.90 Several mediators have been reported to promote this phenotypic shift in microglia including, but not limited to, IL-10, glatiramer acetate, beta interferons, and peroxisome proliferator-activated receptor (PPAR) -γ activators.21 Development of therapeutic approaches that can favor M2 polarization are likely to provide beneficial effects by protecting the NVU. Further research in this area will facilitate discovery of new molecular targets and development of novel strategies to control microglial activation states. As noted by Subramaniam and Federoff in their seminal review, this is a particularly unique opportunity because microglia can transition between M1 and M2 activation states, while peripheral immune cells cannot.21
Microglia activation: Involvement of other NVU cell types
Astrocytes
Astrocytes are the most abundant cell type in the brain and are critical in the development and/or maintenance of BBB characteristics.10,91,92 They are localized between neuronal cell bodies and endothelial cells and ensheath over 99% of cerebral capillaries with their end-feet.71 For example, studies using porcine brain endothelial cells showed improved BBB properties when co-cultured with an immortalized rat astrocyte cell line (CTX TNA2), which implies that astrocytes secrete trophic factors critical to the BBB phenotype.91 Astrocytes derived from induced pluripotent stem cells (iPSC) were shown to enhance TEER, reduce passive permeability, and improve tight junction continuity in iPSC-derived brain endothelial cells.92 Astrocytes may be involved in transient regulation of cerebral microvascular permeability, in particular via Ca2+ signaling between astrocytes and the endothelium via gap junctions and purinergic transmission.71 Other key roles for astrocytes include regulation of water and ion exchange across the microvascular endothelium93,94 as well as removal of excess synaptic glutamate and contribute to maintenance of excitatory neurotransmitter concentrations in the brain.71 Elevated brain levels of glutamate may lead to a pathological condition known as excitotoxicity, which has been implicated in neuronal damage in stroke.95 Additionally, astrocytes are known to express volume-regulated anion channels (VRACs). These channels are involved in Ca2+-independent release of anionic amino acids (i.e. glutamate, aspartate, taurine) during conditions that cause cellular swelling such as cerebral hypoxia.96
In the setting of CNS pathology, astrocytes contribute to neuroinflammation through the release of pro-inflammatory cytokines.97,98 Additionally, crosstalk between microglia and perivascular astrocytes is critical in controlling activity of other cellular components of the NVU such as microglia. In response to stressors, astrocytes can also secrete large quantities of IL-17A.99 IL-17A receptors (IL-17AR) are typically expressed at low levels in the CNS; however, in the setting of neurological disease, signaling via these receptors is upregulated and contributed to the polarization of microglia towards a M1 proinflammatory state.100 For example, pharmacological inhibition of IL-17A/IL-17AR signaling with neutralizing antibodies prevented microglial transition to an activated pro-inflammatory state, a mechanism that reduced cognitive impairment in a murine model of sepsis-associated encephalopathy.101 Interestingly, IL-17A signaling contributes to BBB dysfunction as indicated by decreased claudin-5 and occludin protein expression and increased MMP-2/MMP-9 activity.102 Astrocytes also participate in a positive feedback loop with microglia that can further exacerbate BBB injury and neuroinflammation. For example, pro-inflammatory cytokines such as IL-1β that are released from activated microglia can suppress astrocytic production of sonic hedgehog and increase secretion of CCL2, CCL20, and CXCL2.103 This is a critical finding due to the key role played by sonic hedgehog in the maintenance of BBB functional integrity.11
Pericytes
Pericytes play a crucial role in maintenance of BBB homeostasis.16,104,105 Pericytes are localized to the basement membrane of small blood vessels including capillaries, pre-capillary arterioles, and post-capillary venules.106,107 They are flat, contractile mural cells that communicate with other cell types of the NVU.104 Pericytes also attempt to maintain contacts with microvascular endothelial cells as demonstrated by single-cell pericyte ablation experiments that result in extension of cytoplasmic processes from adjacent pericytes to restore contacts with uncovered regions of the endothelium.108 Contractile properties of pericytes are evidenced by the observation that several contractile and cytoskeletal proteins including α-smooth muscle actin (α-SMA), neural/glial antigen-2 (NG-2), vimentin, desmin, myosin, and nestin are expressed by pericytes.106,109 Of particular note, pericytes vary in terms of their α-SMA content, which implies the presence of different pericyte populations (i.e. ensheathing pericytes, mesh pericytes, thin-strand pericytes) with different morphology.107 Additionally, pericytes express multiple cell surface proteins such as PDGFR-β, aminopeptidases A and N (CD13), regulator of G-protein signaling-5 (RGS5), and CD146.106 When using biochemical approaches to identify pericytes in the cerebral microvasculature, it is important to appreciate that many cell surface antigens expressed by pericytes are also expressed by vascular smooth muscle cells. Therefore, it is critical that multiple marker proteins be evaluated in an effort to establish a “pericyte profile” before making inferences regarding pericyte localization and/or function at the NVU. Secretion of angiopoetin by pericytes induces expression of occludin at the BBB, an observation that suggest that pericytes are directly involved in induction and/or maintenance of barrier properties.110 Using mouse mutant strains with reduced mural cell densities, Armulik et al.14 demonstrated that the degree of microvascular pericyte coverage is inversely proportional to paracellular leak.14 That is, mice with increased vascular pericyte coverage showed reduced extravasation of Evan’s blue-albumin, BSA-Alexa Fluor-555, IgG-DyLight 549, and horseradish peroxidase.14 Increased BBB leak following pericyte loss is region-specific with the highest permeability observed in cortex, striatum, and hippocampus.111 Although the study by Villasenor et al. indicated that these regional differences in leak were not due to tight junction dysfunction, they did not perform a comprehensive evaluation of tight junction protein expression, localization, or oligomerization in the pericyte-deficient Pdgf-bret/ret mouse model.111 Furthermore, BBB permeability was assessed via extravasation of IgG, a large molecule with a molecular weight of 150-kDa. Therefore, it is impossible to conclude from this study that reduced microvascular pericyte coverage did not result in tight junction dysfunction, particularly because a range of paracellular permeability markers of variable molecular weights were not utilized.
Pericytes are cells that are in a constant state of differentiation. Therefore, they have the capability to transition to a microglia-like phenotype in response to pathophysiological stressors.112 Using reporter Rgs5gfp/+ mice, pericytes were shown to undergo a morphological and functional change into microglia following permanent MCAO.113 Further evidence for pericyte differentiation into microglia was obtained by Sakuma et al.114 who showed presence IBA1-positive and PDGFR-β-positive microglia in perivascular regions of ischemic brain tissue in mice subjected to permanent MCAO. Additionally, pericytes themselves can be activated by pro-inflammatory mediators and chemokines secreted from microglia. Pericyte exposure to cytokines such as TNF-α and IL-1β triggers production and release of IL-6 and MMP-9, factors that can lead to BBB disruption.115 Pro-inflammatory cytokines can also increase adhesion molecule expression in pericytes, an event that can facilitate monocyte trafficking across the BBB in the presence of chemokines such as CCL2.116
Targeting microglia for treatment of neurological diseases
Ischemic stroke
Each year in the United States, there are approximately 795,000 new incidences of stroke.117 Ischemic stroke comprises 87% of all strokes and involves a pathophysiology related to reduced oxygen and glucose delivery to an affected brain region. This results in an irreversibly damaged infarct core as well as potentially salvageable surrounding tissue known as the penumbra. Treatment of the ischemic core is virtually impossible due to rapid development of necrosis; however, the penumbra is a primary target for therapeutic intervention due to slower cell degradation.3,118,119 Recombinant tissue plasminogen activator (r-tPA, alteplase) is the only drug approved for clinical use as a stroke therapeutic by the Food and Drug Administration (FDA) of the United States. Therapy with r-tPA is limited by its narrow therapeutic window (i.e. 4.5 h) and/or risk of intracerebral bleeding complications.118 More recently, reperfusion therapy via mechanical endovascular thrombectomy (EVT) has provided considerable benefits to stroke patients including a marked improvement in reperfusion;120 however, many patients who receive EVT remain severely disabled.120,121 Therapy with r-tPA and EVT are the only two measures that are currently available as immediate therapeutic strategies to treat ischemic stroke. Both r-tPA and EVT involve recanalization (i.e. reperfusion) of ischemic brain tissue, a process that can exacerbate neuronal injury. CNS damage following recanalization ranges in severity from ischemic core enlargement to development of edema or fatal hemorrhaging, a critical component of ischemia/reperfusion (I/R) injury.122,123 Pathophysiological processes associated with I/R injury include enhanced cerebrovascular permeability and leakage, activation of cell death mechanisms (i.e. apoptosis, autophagy, necrosis), and increased production of ROS.3,8,123
The frequent use of reperfusion therapies highlights a requirement for novel stroke treatment modalities that can protect neuronal tissue from further ischemic damage or promote neuronal repair following I/R injury. Discovery and development of new therapeutic approaches for stroke should incorporate BBB protection since microvascular leakage and tight junction dysfunction are primary causes of vasogenic edema and subsequent mortality. This unmet clinical need points towards targeting of microglia as a potential option in development of such therapeutic strategies. There has been considerable interest in minocycline, a tetracycline antibiotic, as a therapeutic that selectively targets M1 microglia. Minocycline can permeate the BBB, has a good safety profile, and a delayed therapeutic window thus rendering it an ideal candidate drug for treatment of ischemic stroke.71,124 Experimental evidence in animal stroke models indicates that minocycline may limit BBB dysfunction and reduce vasogenic edema. For example, Yenari et al.125 reported that minocycline reduced infarction volume and neurological deficits as well as prevented BBB disruption and hemorrhage in male C57/BL6 mice subjected to transient MCAO. Adult spontaneously hypertensive rats subjected to transient MCAO exhibited reduced microglial production of TNF-α and IL-1β following treatment with minocycline.126Of particular note, minocycline increased production and secretion of TGF-β1 and IL-10 by microglia, suggesting that this therapeutic does not inhibit microglia polarized to a M2 phenotype.126 More recently, minocycline was shown to prevent tight junction protein complex disruption in a rat model of progressive white matter disease.127 These in vivo observations have been extended to the clinic where minocycline has shown promise as a neuroprotective agent in stroke patients.128 Taken together, these studies emphasize the utility of targeting microglia in development of effective therapeutic strategies for ischemic stroke.
TLRs are widely expressed in the human CNS, particularly by microglia. Targeting these receptors has emerged as a promising goal for therapeutic control of ischemic stroke, primarily due to the fact that TLRs are involved in all aspects of BBB dysfunction and NVU injury in the setting of ischemia.129 For example, administration of the experimental TLR4 inhibitor eritoran (E5564) reduced microglial production of pro-inflammatory mediators, reduced infarction volume, attenuated BBB breakdown, and improved neurological outcomes in mice subjected to transient MCAO.130 Additionally, regulation of TLR4 activity by a novel microRNA (i.e. miR-1906) was shown to reduce ischemic damage and improve functional outcomes following experimental stroke in mice.131 Pinocembrin, a flavanone with antioxidant properties, was shown to block the activity of M1 microglia in mice subjected to collagenase-induced intracerebral hemorrhage, an effect that reduced brain injury volume and improved performance in the wire hanging test and in the corner turn test as compared to animals that did not receive this compound.132 Interestingly, the effect of pinocembrin was abolished in TLR4lps-del mice, demonstrating that pharmacological effects of this flavanone are related to inhibition of TLR4 signaling.132 Taken together, these studies suggest that modification of microglial cell pathology is a viable approach for the development of novel strategies to treat ischemic stroke; however, much work needs to be done on understanding pharmacokinetics, TLR ligand selectivity, and off-target effects/systemic toxicity of TLR-targeting drugs before such stroke therapeutics can reach evaluation in randomized controlled clinical trials.
Administration of annexin isoforms is another compelling therapeutic strategy. Recently, Li et al.133 showed that human recombinant annexin A2 (ANXA2) ameliorated hypoxia/IL-1β changes in cultured human brain endothelial cells. In vivo, ANXA2 gene knockout resulted in decreased expression of critical BBB proteins (i.e. claudin-5, occludin, VE-cadherin) and increased BBB permeability to 10 kDa dextran.133 The BBB protective effect of ANXA2 was shown to be mediated by the Robo4-paxillin-ADP-ribosylation factor 6 (ARF6) pathway.133 ANXA2 may also control the pro-inflammatory response in resident microglia by modulating IL-17 signaling as well as ROS production.134 Additionally, annexin A1 (ANXA1) was shown to be a regulator of microglia activation in the BV-2 microglia cell line under oxygen/glucose deprivation conditions.135 In this study, the effect of ANXA1 on microglia was shown to be mediated by formyl peptide receptors (FPRs).135 Annexins have been shown to exert protective effects in experimental stroke models such as MCAO136 or focal embolic stroke.137 Indeed, the potential of annexins as stroke therapeutics, particularly with respect to their ability to control of microglial activation, is intriguing and requires more detailed analysis.
Alzheimer’s disease
Alzheimer’s disease and associated dementias are an escalating global health challenge as evidenced by 40–50 million people living with dementia worldwide.138 The pathological features of Alzheimer’s disease are neurofibrillary tangles and the formation of neurite plaques that may be focal or diffuse. Neurofibrillary tangles are primarily comprised of the microtubule-associated protein tau. Tau is a soluble protein; however, insoluble aggregates are produced during formation of neurofibrillary tangles, a process that disrupts neuronal structure and function. These insoluble aggregates are comprised of hyperphosphorylated tau, which reduces tau interactions with microtubules and promotes the impairment of both long-term and short-term synaptic plasticity.139 The principal component of neurite plaques are aggregates of amyloid-beta (Aβ) protein. These aggregates result from abnormally folded Aβ peptides comprised of 40 (Aβ40) or 42 (Aβ42) amino acids. Aβ42 is more abundant within neurite plaques because it has a higher rate of fibrillization and insolubility.140 Elevated brain levels of Aβ42 are associated with impaired learning and recognition memory in mice.141 Accumulation of Aβ plaques can induce inflammatory processes characterized by astrocytosis and microgliosis. Activated glial cells secrete neurotoxic mediators that can directly cause neuronal cell injury and death. Neuronal cell death in Alzheimer’s disease may result in clinical manifestations, including impaired memory as well as cognitive and behavioral dysfunction.142 As elegantly described in recent work from Berislav Zlokovic’s laboratory, microvascular injury is the initial insult in Alzheimer’s disease, which leads to BBB dysfunction, reduced cerebral blood flow and, subsequently, Aβ accumulation in the brain and neuronal injury.143 Alzheimer’s disease neurological and cerebrovascular pathology is accelerated by genetic risk factors (i.e. carriage of the ε4 allele of apolipoprotein E (APOE4)), vascular risk factors such as hypertension, diabetes mellitus, or dyslipidemia, and chronic exposure to environmental toxicants such as air pollution.143,144
Microglia are key players in neuroinflammation, a hallmark of Alzheimer’s disease. As a result, preclinical studies have examined efficacy of targeting microglia in an effort to reduce production and secretion of pro-inflammatory mediators. Similar to ischemic stroke, minocycline has been examined in preclinical studies for its ability to inhibit activation of M1 microglia, counteract neuroinflammation, and protect against further neuronal cell loss.145,146 Despite these positive observations, minocycline has not been successful in patients as evidenced by a recent randomized controlled trial where 24 months of minocycline treatment did not delay progression of cognitive impairment in the setting of symptomatic Alzheimer’s disease.147 This observation suggests that microglia-targeted therapies will need to focus on different molecular targets for different neurological diseases. Perhaps a more viable approach for Alzheimer’s disease is targeting phagocytotic mechanisms in microglia, which have been shown to facilitate Aβ clearance and limit plaque formation.148,149 The ability of microglia to phagocytose Aβ is dependent, in part, upon triggering receptor expressed on myeloid cells (TREM)1 and 2 (Figure 3).148,150 Aβ oligomers, as well as APOEs, are ligands for TREM receptors. The role of TREM1 in phagocytosis was demonstrated by the observation that knockdown of this receptor in the brains of APP/PSEN1 mice increased Aβ42 concentrations, while overexpression of TREM1 on microglia protected against Aβ neuropathology and promoted cognitive improvements.148 TREM1 gene expression was reported to be reduced in aged brain tissue;62 however, the significance of this observation with respect to Alzheimer’s disease requires further investigation. TREM2 deficient microglia also show reduced phagocytosis of Aβ peptides.150 At the microglial plasma membrane, TREM1 and TREM2 form protein complexes with DAP12, which enables intracellular signal transduction and activation of downstream targets.151 Targeting microglia to stimulate phagocytosis of Aβ is a therapeutic approach likely to show neuroprotection as well as BBB (i.e. vascular protection). In fact, several studies have shown that Aβ plaque formation and/or elevated cerebral Aβ concentrations are associated with vascular impairment in Alzheimer’s disease and related dementias. This concept has been demonstrated in multiple studies using transgenic mouse models of Alzheimer’s disease that exhibit abnormal cleavage of amyloid precursor protein by β-secretase and, therefore, has elevated cerebral Aβ concentrations. For example, Paul et al.152 demonstrated age-related BBB breakdown triggered by Aβ that resulted in enhanced Evan’s blue-albumin extravasation.152 Biron et al.153 demonstrated that transgenic Alzheimer’s disease mice that overproduce Aβ have reduced levels of occludin and ZO-1 in their cerebral microvasculature. Additionally, Aβ40 has been shown to decrease expression of critical tight junction proteins (i.e. claudin-1, claudin-5) and increase MMP-2/MMP-9 expression in cerebral microvasculature.154 Interestingly, Hartz et al.154 reported that 5% of patients with a positive diagnosis of cerebral amyloid angiopathy (CAA) in their study group had asymptomatic BBB leakage and/or posterior reversible encephalopathic syndrome. Overall, these studies indicate a critical need to evaluate, in detail, mechanisms of microglial phagocytosis, particularly with respect to the ability of these cells to clear Aβ from the brain. Development of a microglial cell-targeted therapeutic could make a significant impact in the pharmacotherapy of Alzheimer’s disease, particularly due to the fact that enhanced Aβ deposition in the brain causes significant BBB impairment and this vascular impairment is an early biomarker of Alzheimer’s disease.26
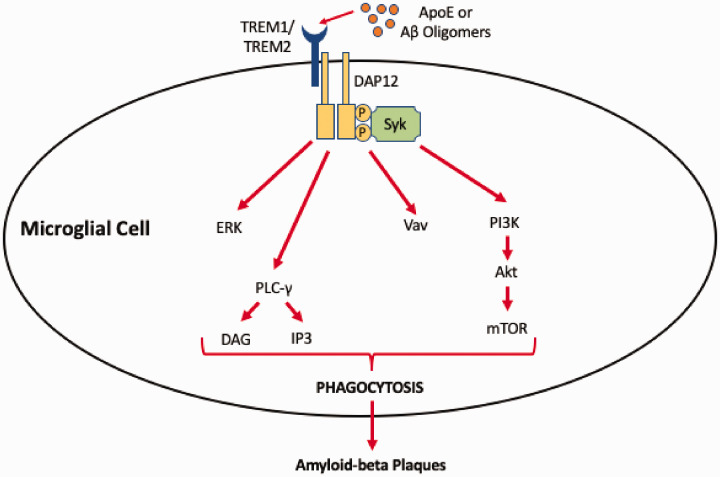
Figure 3.
Involvement of triggering receptor expressed on myeloid cells (TREM) signaling on microglial phagocytosis in Alzheimer’s disease. Amyloid-beta (Aβ) oligomers or apolipoprotein E (APOE) isoforms can activate TREM1 or TREM2 at the microglia cell surface. Activation of TREM receptors results in formation of heterologous protein complex with DAP12, thereby enabling recruitment of Syk protein tyrosine kinase and subsequent signal transduction. Downstream effectors of the TREM/DAP12 complex include ERK, phospholipase C-gamma (PLC-γ), the Vav signaling pathway, and phosphoinositide 3-kinase (PI3K). These pathways activate phagocytotic mechanisms in microglia, thereby enabling these cells to participate in clearance of Aβ plaques from the brain.
Summary and conclusion
The field of NVU biology has rapidly advanced over the past 20 years. It is now well-established that tight junction protein complexes are dynamic in nature and can organize and reorganize in response to neurological disease challenge. These changes in tight junction protein complexes can cause increased BBB permeability to circulating substances. Many recent studies have implicated microglia activation as a critical determinant of BBB dysfunction in several disease states such as ischemic stroke and Alzheimer’s disease. Despite these exciting advances, we still have a long road before we understand the exact role of microglia in regulation of BBB functional integrity and how drugs can be developed to control microglial activity. This challenge extends beyond examining putative intracellular signaling pathways in microglia and other NVU constituents. Rather, a critical deficiency in understanding BBB changes induced by microglia is the experimental design of tight junction/barrier permeability studies. While several studies have focused on tight junction protein modifications and associated changes in paracellular “leak”, much of this work has not considered size selectivity of tracer molecule “leak”/diffusion across the BBB. This is exemplified by the fact that many publications have reported altered BBB permeability using only large-molecular weight tracers such as Evan’s blue-albumin, IgG, fibrinogen, or fluorescently labeled albumin. The concern with exclusively using large molecular weight tracers is that subtle improvements in BBB integrity will cause a dramatic reduction in extravasation; however, the cerebral microvasculature may remain permeable/“leaky” to small molecules, a fact that can cause continued cerebral injury and/or toxicity. Therefore, it is essential that future studies utilize multiple tracers of variable molecular weights to determine the magnitude of BBB protection incurred by targeting microglia. The experimental approach of using multiple paracellular permeability markers has been well demonstrated by our group155 and by others.28,156 In these experiments on paracellular permeability, selection of an appropriate small molecule tracer is an important consideration. While sucrose is an effective marker of paracellular permeability because it is not transported and does not permeate the intact BBB,87,157,158 NaF is less effective because it is a substrate for multiple BBB transporters (i.e. organic cation transporter 3, MRP2).159 Since these BBB transporters function in the brain-to-blood direction, NaF as a marker of paracellular permeability can greatly underestimate the magnitude of BBB “leak.” Additionally, there is little published information as to how microglial activation can modulate functional expression of transporters at the BBB/NVU. Transporters that are expressed in brain microvascular endothelial cells are established determinants of CNS disposition of endogenous and exogenous solutes.6,7,119 Altered expression of transporters can dramatically affect the ability of such substances to access molecular targets in the brain, even in pathological settings where tight junction protein complexes are simultaneously modulated. Our group has demonstrated this concept in a rodent model of peripheral inflammatory pain where P-gp expression and activity are increased and tight junction protein complexes are disrupted.160 In this study, brain uptake of the P-gp substrate drug morphine was reduced, an observation that suggests that transcellular transport mechanisms at the BBB can overcome paracellular “leak”.160 Similarly, we have shown that blood-to-brain uptake of drugs is primarily mediated by organic anion transporting polypeptide 1a4 in the setting of peripheral inflammatory pain161 and cerebral hypoxia,162 conditions where tight junction integrity is known to be compromised. Since previous studies have shown that microglial polarization to M1 or M2 phenotypes can have significant effects on BBB functional integrity, it stands to reason that microglia can regulate localization and functional expression of transporters as well. Furthermore, understanding regulation of BBB transporters by activated microglia is required to determine drug delivery kinetics of therapeutics developed for treatment of neurological diseases, including those designed to target microglia themselves. Innovative advances that will aid in meeting the challenges described above are novel in vitro tools that have been recently developed and characterized.163 Of particular note, systems comprised of human fetal brain endothelial cells164 or utilizing stem cell-derived brain-like endothelial cells grown on silicon nanomembranes165 are well positioned to advance the study of cell-cell interactions at the BBB interface and can be applied to the evaluation of microglial effects on paracellular “leak” and transporter expression/activity. Additionally, discovery of novel PET tracers that target activated microglia provides the ability to simultaneously image microglia and the BBB in order to provide state-of-the-art information on how different microglial phenotypes can regulate neurovascular integrity and function.166 Indeed, the identification and characterization of novel molecular targets on microglia provide a unique opportunity for novel therapeutic approaches to meet the challenge of successfully treating neurological diseases. Future work will continue to provide more insight on the interplay of tight junction protein complexes, transporters, and microglial activation states and how these can be effectively targeted. Ultimately, data derived from these studies will facilitate discovery and development of novel strategies to treat neurological diseases such as ischemic stroke and Alzheimer’s disease.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work is funded by grants from the National Institutes of Health to PTR (R01 NS084941) and to TPD and PTR (R01 DA051812) and by a grant from the American Heart Association to PTR (19TPA34910113).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Haseloff RF, Dithmer S, Winkler L, et al. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 2015; 38: 16–25. [DOI] [PubMed] [Google Scholar]
- 2.Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 2018; 135: 311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol 2018; 315: C343–C356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11: 4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdullahi W, Davis TP, Ronaldson PT. Functional expression of P-glycoprotein and organic anion transporting polypeptides at the blood-brain barrier: understanding transport mechanisms for improved CNS drug delivery? AAPS J 2017; 19: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris ME, Rodriguez-Cruz V, Felmlee MA. SLC and ABC transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J 2017; 19: 1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams EI, Betterton RD, Davis TP, et al. Transporter-mediated delivery of small molecule drugs to the brain: a critical mechanism that can advance therapeutic development for ischemic stroke. Pharmaceutics 2020; 12: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis CL, Nolan CC, Reith SN, et al. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia 2004; 45: 325–337. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011; 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 12.Wosik K, Cayrol R, Dodelet-Devillers A, et al. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 2007; 27: 9032–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 15.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolakopoulou AM, Zhao Z, Montagne A, et al. Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-beta signaling. PLoS One 2017; 12: e0176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sierra A, de Castro F, Del Rio-Hortega J, et al. The “Big-Bang” for modern glial biology: translation and comments on Pio del Rio-Hortega 1919 series of papers on microglia. Glia 2016; 64: 1801–1840. [DOI] [PubMed] [Google Scholar]
- 19.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017; 23: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab 2018; 38: 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson's disease. Front Aging Neurosci 2017; 9: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 23.Akhmetzyanova E, Kletenkov K, Mukhamedshina Y, et al. Different approaches to modulation of microglia phenotypes after spinal cord injury. Front Syst Neurosci 2019; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachiller S, Jimenez-Ferrer I, Paulus A, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 2018; 12: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Hamanaka G, Lo EH, et al. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther 2019; 25: 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrabadi AR, Korolainen MA, Odero G, et al. Poly(ADP-ribose) polymerase-1 regulates microglia mediated decrease of endothelial tight junction integrity. Neurochem Int 2017; 108: 266–271. [DOI] [PubMed] [Google Scholar]
- 28.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019; 10: 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen AQ, Fang Z, Chen XL, et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 2019; 10: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowyer JF, Sarkar S, Tranter KM, et al. Vascular-directed responses of microglia produced by methamphetamine exposure: indirect evidence that microglia are involved in vascular repair? J Neuroinflammation 2016; 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumi N, Nishioku T, Takata F, et al. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol 2010; 30: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lou N, Takano T, Pei Y, et al. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A 2016; 113: 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halder SK, Milner R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc Natl Acad Sci U S A 2019; 116: 26029–26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Li X, Yin J, et al. Glycocalyx degradation leads to blood-brain barrier dysfunction and brain edema after asphyxia cardiac arrest in rats. J Cereb Blood Flow Metab 2018; 38: 1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnartz B, Neumann H. Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia 2013; 61: 37–46. [DOI] [PubMed] [Google Scholar]
- 36.Forsberg KME, Zhang Y, Reiners J, et al. Endothelial damage, vascular bagging and remodeling of the microvascular bed in human microangiopathy with deep white matter lesions. Acta Neuropathol Commun 2018; 6: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Zhang JD, Duan L, et al. Microglia activation mediated by toll-like receptor-4 impairs brain white matter tracts in rats. J Biomed Res 2018; 32: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moritz KE, McCormack NM, Abera MB, et al. The role of the immunoproteasome in interferon-gamma-mediated microglial activation. Sci Rep 2017; 7: 9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponomarev ED, Shriver LP, Maresz K, et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol 2007; 178: 39–48. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Das A, Ray SK, et al. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 2012; 87: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritzel RM, Patel AR, Grenier JM, et al. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation 2015; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddick PC, Larson JL, Rathore N, et al. A common variant of IL-6R is associated with elevated IL-6 pathway activity in Alzheimer's disease brains. J Alzheimers Dis 2017; 56: 1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011; 42: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atallah A, Mhaouty-Kodja S, Grange-Messent V. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab 2017; 37: 3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akundi RS, Candelario-Jalil E, Hess S, et al. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005; 51: 199–208. [DOI] [PubMed] [Google Scholar]
- 46.Bhat NR, Zhang P, Lee JC, et al. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 1998; 18: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 2008; 586: 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Na W, Shin JY, Lee JY, et al. Dexamethasone suppresses JMJD3 gene activation via a putative negative glucocorticoid response element and maintains integrity of tight junctions in brain microvascular endothelial cells. J Cereb Blood Flow Metab 2017; 37: 3695–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonetti NR, Diaz-Canestro C, Liberale L, et al. Tumour necrosis factor-alpha inhibition improves stroke outcome in a mouse model of rheumatoid arthritis. Sci Rep 2019; 9: 2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards DN, Salmeron K, Lukins DE, et al. Integrin alpha5beta1 inhibition by ATN-161 reduces neuroinflammation and is neuroprotective in ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond AD, Diaz P, Chevelon S, et al. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neurovirol 2016; 22: 129–139. [DOI] [PubMed] [Google Scholar]
- 52.Poller B, Drewe J, Krahenbuhl S, et al. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol 2010; 30: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoshi Y, Uchida Y, Tachikawa M, et al. Actin filament-associated protein 1 (AFAP-1) is a key mediator in inflammatory signaling-induced rapid attenuation of intrinsic P-gp function in human brain capillary endothelial cells. J Neurochem 2017; 141: 247–262. [DOI] [PubMed] [Google Scholar]
- 54.Hartz AM, Bauer B, Fricker G, et al. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol 2006; 69: 462–470. [DOI] [PubMed] [Google Scholar]
- 55.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 2007; 71: 667–675. [DOI] [PubMed] [Google Scholar]
- 56.Banks WA, Kovac A, Morofuji Y. Neurovascular unit crosstalk: pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J Cereb Blood Flow Metab 2018; 38: 1104–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamatovic SM, Shakui P, Keep RF, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab 2005; 25: 593–606. [DOI] [PubMed] [Google Scholar]
- 58.Inose Y, Kato Y, Kitagawa K, et al. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology 2015; 35: 209–223. [DOI] [PubMed] [Google Scholar]
- 59.Saresella M, Marventano I, Calabrese E, et al. A complex proinflammatory role for peripheral monocytes in Alzheimer's disease. J Alzheimers Dis 2014; 38: 403–413. [DOI] [PubMed] [Google Scholar]
- 60.Abe N, Choudhury ME, Watanabe M, et al. Comparison of the detrimental features of microglia and infiltrated macrophages in traumatic brain injury: a study using a hypnotic bromovalerylurea. Glia 2018; 66: 2158–2173. [DOI] [PubMed] [Google Scholar]
- 61.Faustino J, Chip S, Derugin N, et al. CX3CR1-CCR2-dependent monocyte-microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke. J Cereb Blood Flow Metab 2019; 39: 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olah M, Patrick E, Villani AC, et al. A transcriptomic atlas of aged human microglia. Nat Commun 2018; 9: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Gao Z, Wang D, et al. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. J Neuroinflammation 2014; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landreneau MJ, Mullen MT, Messe SR, et al. CCL2 and CXCL10 are associated with poor outcome after intracerebral hemorrhage. Ann Clin Transl Neurol 2018; 5: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krauthausen M, Kummer MP, Zimmermann J, et al. CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer's disease model. J Clin Invest 2015; 125: 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guedes JR, Lao T, Cardoso AL, et al. Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer's disease-associated amyloid-beta and tau pathologies. Front Neurol 2018; 9: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Israelsson C, Bengtsson H, Lobell A, et al. Appearance of Cxcl10-expressing cell clusters is common for traumatic brain injury and neurodegenerative disorders. Eur J Neurosci 2010; 31: 852–863. [DOI] [PubMed] [Google Scholar]
- 68.Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, et al. Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol 2012; 302: H582–H593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jian YP, Dong SJ, Xu SS, et al. MicroRNA-34a suppresses neuronal apoptosis and alleviates microglia inflammation by negatively targeting the Notch pathway in spinal cord injury. Eur Rev Med Pharmacol Sci 2020; 24: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 70.Thompson BJ, Ronaldson PT. Drug delivery to the ischemic brain. Adv Pharmacol 2014; 71: 165–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des 2012; 18: 3624–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 2005; 39: 51–70. [DOI] [PubMed] [Google Scholar]
- 73.Gu Y, Zheng G, Xu M, et al. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem 2012; 120: 147–156. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Guan B, Chen X, et al. Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurological outcome in ischemic stroke rats with delayed t-PA treatment: involvement of ONOO(-)-MMP-9 pathway. Transl Stroke Res 2018; 9: 515–529. [DOI] [PubMed] [Google Scholar]
- 75.Chen H, Chen X, Luo Y, et al. Potential molecular targets of peroxynitrite in mediating blood-brain barrier damage and haemorrhagic transformation in acute ischaemic stroke with delayed tissue plasminogen activator treatment. Free Radic Res 2018; 52: 1220–1239. [DOI] [PubMed] [Google Scholar]
- 76.Zhan Y, Krafft PR, Lekic T, et al. Imatinib preserves blood-brain barrier integrity following experimental subarachnoid hemorrhage in rats. J Neurosci Res 2015; 93: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsutsui Y, Deredge D, Wintrode PL, et al. Imatinib binding to human c-Src is coupled to inter-domain allostery and suggests a novel kinase inhibition strategy. Sci Rep 2016; 6: 30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu T, Wang Z, Prativa S, et al. Macrophage stimulating protein preserves blood brain barrier integrity after intracerebral hemorrhage through recepteur d'origine nantais dependent GAB1/Src/beta-catenin pathway activation in a mouse model. J Neurochem 2019; 148: 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Esposito E, Hayakawa K, Ahn BJ, et al. Effects of ischemic post-conditioning on neuronal VEGF regulation and microglial polarization in a rat model of focal cerebral ischemia. J Neurochem 2018; 146: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y, Gao Y, Zhang Q, et al. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience 2020; 437: 161–171. [DOI] [PubMed] [Google Scholar]
- 81.Zhou K, Zhong Q, Wang YC, et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J Cereb Blood Flow Metab 2017; 37: 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Barrett JP, Henry RJ, Villapol S, et al. NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J Neuroinflammation 2017; 14: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michell-Robinson MA, Touil H, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain 2015; 138: 1138–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Lu X, Gong L, et al. Tetramethylpyrazine protects blood-spinal cord barrier integrity by modulating microglia polarization through activation of STAT3/SOCS3 and inhibition of NF-small ka, CyrillicB signaling pathways in experimental autoimmune encephalomyelitis mice. Cell Mol Neurobiol. Epub ahead of print 18 May 2020. DOI: 10.1007/s10571-020-00878-3. [DOI] [PMC free article] [PubMed]
- 85.Wang D, Li SP, Fu JS, et al. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol 2016; 116: 2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor RA, Chang CF, Goods BA, et al. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 2017; 127: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, et al. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab 2009; 29: 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chakrabarti S, Jana M, Roy A, et al. Upregulation of suppressor of cytokine signaling 3 in microglia by cinnamic acid. Curr Alzheimer Res 2018; 15: 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin H, Holdbrooks AT, Liu Y, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 2012; 189: 3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang Y, Li T, Li J, et al. Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson's disease. Cell Death Differ 2014; 21: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cantrill CA, Skinner RA, Rothwell NJ, et al. An immortalised astrocyte cell line maintains the in vivo phenotype of a primary porcine in vitro blood-brain barrier model. Brain Res 2012; 1479: 17–30. [DOI] [PubMed] [Google Scholar]
- 92.Canfield SG, Stebbins MJ, Morales BS, et al. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem 2017; 140: 874–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathiisen TM, Lehre KP, Danbolt NC, et al. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 2010; 58: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 94.Ren Z, Iliff JJ, Yang L, et al. ‘Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab 2013; 33: 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 2014; 115: 157–188. [DOI] [PubMed] [Google Scholar]
- 96.Kimelberg HK, Goderie SK, Higman S, et al. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 1990; 10: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol 2006; 70: 1087–1098. [DOI] [PubMed] [Google Scholar]
- 98.Rutkowska A, Shimshek DR, Sailer AW, et al. EBI2 regulates pro-inflammatory signalling and cytokine release in astrocytes. Neuropharmacology 2018; 133: 121–128. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Zhou F, Yang Y, et al. MiR-409-3p and MiR-1896 co-operatively participate in IL-17-induced inflammatory cytokine production in astrocytes and pathogenesis of EAE mice via targeting SOCS3/STAT3 signaling. Glia 2019; 67: 101–112. [DOI] [PubMed] [Google Scholar]
- 100.Das Sarma J, Ciric B, Marek R, et al. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 2009; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye B, Tao T, Zhao A, et al. Blockade of IL-17A/IL-17R pathway protected mice from sepsis-associated encephalopathy by inhibition of microglia activation. Mediators Inflamm 2019; 2019: 8461725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ni P, Dong H, Wang Y, et al. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice. J Neuroinflammation 2018; 15: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Jin S, Sonobe Y, et al. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One 2014; 9: e110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol 2011; 686: 49–68. [DOI] [PubMed] [Google Scholar]
- 105.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab 2016; 36: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grant RI, Hartmann DA, Underly RG, et al. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 2019; 39: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berthiaume AA, Grant RI, McDowell KP, et al. Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep 2018; 22: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dore-Duffy P, Katychev A, Wang X, et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 2006; 26: 613–624. [DOI] [PubMed] [Google Scholar]
- 110.Hori S, Ohtsuki S, Hosoya K, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 2004; 89: 503–513. [DOI] [PubMed] [Google Scholar]
- 111.Villasenor R, Kuennecke B, Ozmen L, et al. Region-specific permeability of the blood-brain barrier upon pericyte loss. J Cereb Blood Flow Metab 2017; 37: 3683–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rustenhoven J, Jansson D, Smyth LC, et al. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 2017; 38: 291–304. [DOI] [PubMed] [Google Scholar]
- 113.Ozen I, Deierborg T, Miharada K, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol 2014; 128: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakuma R, Kawahara M, Nakano-Doi A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation 2016; 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herland A, van der Meer AD, FitzGerald EA, et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS One 2016; 11: e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Persidsky Y, Hill J, Zhang M, et al. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab 2016; 36: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 118.Manning NW, Campbell BC, Oxley TJ, et al. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 2014; 45: 640–644. [DOI] [PubMed] [Google Scholar]
- 119.Brzica H, Abdullahi W, Ibbotson K, et al. Role of transporters in central nervous system drug delivery and blood-brain barrier protection: relevance to treatment of stroke. J Cent Nerv Syst Dis 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 122.Pan J, Konstas AA, Bateman B, et al. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007; 49: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol 2013; 1: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fagan SC, Cronic LE, Hess DC. Minocycline development for acute ischemic stroke. Transl Stroke Res 2011; 2: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yenari MA, Xu L, Tang XN, et al. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 2006; 37: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 126.Yang Y, Salayandia VM, Thompson JF, et al. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation 2015; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang Y, Kimura-Ohba S, Thompson JF, et al. Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury. Neurobiol Dis 2018; 114: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malhotra K, Chang JJ, Khunger A, et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol 2018; 265: 1871–1879. [DOI] [PubMed] [Google Scholar]
- 129.Downes CE, Crack PJ. Neural injury following stroke: are Toll-like receptors the link between the immune system and the CNS? Br J Pharmacol 2010; 160: 1872–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parada E, Casas AI, Palomino-Antolin A, et al. Early toll-like receptor 4 blockade reduces ROS and inflammation triggered by microglial pro-inflammatory phenotype in rodent and human brain ischaemia models. Br J Pharmacol 2019; 176: 2764–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu X, Wen Z, Zhao N, et al. MicroRNA-1906, a novel regulator of toll-like receptor 4, ameliorates ischemic injury after experimental stroke in mice. J Neurosci. 2017; 37: 10498–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lan X, Han X, Li Q, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav Immun 2017; 61: 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li W, Chen Z, Yuan J, et al. Annexin A2 is a Robo4 ligand that modulates ARF6 activation-associated cerebral trans-endothelial permeability. J Cereb Blood Flow Metab 2019; 39: 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu N, Jiang Y, Chung JY, et al. Annexin A2 deficiency exacerbates neuroinflammation and long-term neurological deficits after traumatic brain injury in mice. Int J Mol Sci 2019; 20: 6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu S, Gao Y, Yu X, et al. Annexin-1 mediates microglial activation and migration via the CK2 pathway during oxygen-glucose deprivation/reperfusion. Int J Mol Sci 2016; 17: 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gavins FN, Dalli J, Flower RJ, et al. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J 2007; 21: 1751–1758. [DOI] [PubMed] [Google Scholar]
- 137.Fan X, Jiang Y, Yu Z, et al. Annexin A2 plus low-dose tissue plasminogen activator combination attenuates cerebrovascular dysfunction after focal embolic stroke of rats. Transl Stroke Res 2017; 8: 5495–59. [DOI] [PubMed] [Google Scholar]
- 138.Collaborators GBDD. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naseri NN, Wang H, Guo J, et al. The complexity of tau in Alzheimer's disease. Neurosci Lett 2019; 705: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol 2018; 25: 59–70. [DOI] [PubMed] [Google Scholar]
- 141.Jaeger LB, Dohgu S, Hwang MC, et al. Testing the neurovascular hypothesis of Alzheimer's disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis 2009; 17: 553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ballard C, Gauthier S, Corbett A, et al. Alzheimer's disease. Lancet 2011; 377: 1019–1031. [DOI] [PubMed] [Google Scholar]
- 143.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hanson AJ, Craft S, Banks WA. The APOE genotype: modification of therapeutic responses in Alzheimer's disease. Curr Pharm Des 2015; 21: 114–120. [DOI] [PubMed] [Google Scholar]
- 145.Seabrook TJ, Jiang L, Maier M, et al. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 2006; 53: 776–782. [DOI] [PubMed] [Google Scholar]
- 146.Ferretti MT, Allard S, Partridge V, et al. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology. J Neuroinflammation 2012; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Howard R, Zubko O, Bradley R, et al. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol 2019; 77: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang T, Zhang YD, Gao Q, et al. TREM1 facilitates microglial phagocytosis of amyloid beta. Acta Neuropathol 2016; 132: 667–683. [DOI] [PubMed] [Google Scholar]
- 149.Rubio-Araiz A, Finucane OM, Keogh S, et al. Anti-TLR2 antibody triggers oxidative phosphorylation in microglia and increases phagocytosis of beta-amyloid. J Neuroinflammation 2018; 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yeh FL, Wang Y, Tom I, et al. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 2016; 91: 328–340. [DOI] [PubMed] [Google Scholar]
- 151.Konishi H, Kiyama H. Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci 2018; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med 2007; 204: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Biron KE, Dickstein DL, Gopaul R, et al. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS One 2011; 6: e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hartz AM, Bauer B, Soldner EL, et al. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012; 43: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab 2010; 30: 1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dithmer S, Staat C, Muller C, et al. Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery. Ann N Y Acad Sci 2017; 1397: 169–184. [DOI] [PubMed] [Google Scholar]
- 157.Huber JD, Witt KA, Hom S, et al. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 2001; 280: H1241–H1248. [DOI] [PubMed] [Google Scholar]
- 158.Witt KA, Mark KS, Sandoval KE, et al. Reoxygenation stress on blood-brain barrier paracellular permeability and edema in the rat. Microvasc Res 2008; 75: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hawkins BT, Ocheltree SM, Norwood KM, et al. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett 2007; 411: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seelbach MJ, Brooks TA, Egleton RD, et al. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem 2007; 102: 1677–1690. [DOI] [PubMed] [Google Scholar]
- 161.Ronaldson PT, Finch JD, Demarco KM, et al. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther 2011; 336: 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thompson BJ, Sanchez-Covarrubias L, Slosky LM, Zhang Y, Laracuente ML, Ronaldson PT. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab 2014; 34: 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sivandzade F, Cucullo L. In-vitro blood-brain barrier modeling: a review of modern and fast-advancing technologies. J Cereb Blood Flow Metab 2018; 38: 1667–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andrews AM, Lutton EM, Cannella LA, et al. Characterization of human fetal brain endothelial cells reveals barrier properties suitable for in vitro modeling of the BBB with syngenic co-cultures. J Cereb Blood Flow Metab 2018; 38: 888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mossu A, Rosito M, Khire T, et al. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood-brain barrier under flow. J Cereb Blood Flow Metab 2019; 39: 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cumming P, Burgher B, Patkar O, et al. Sifting through the surfeit of neuroinflammation tracers. J Cereb Blood Flow Metab 2018; 38: 204–224. [DOI] [PMC free article] [PubMed] [Google Scholar]