Abstract
Age-dependent alterations in microglia behavior have been implicated in neurodegeneration and CNS injuries. Here, we compared the transcriptional profiles of young versus aged microglia during stroke recovery. CD45intermediateCD11b+ microglia were FACS-isolated from the brains of young (10-week-old) and aged (18-month-old) male mice with sham operation or 14 days after distal middle cerebral artery occlusion and subjected to RNA-sequencing analysis. Functional groups enriched in young microglia are indicative of upregulation in cell movement, cell interactions, inflammatory responses and angiogenesis, while aged microglia exhibited a reduction or no change in these features. We confirmed reduced chemoattractive capacities of aged microglia toward ischemic brain tissue in organotypic slide co-cultures, and delayed accumulation of aged microglia around dead neurons injected into the striatum in vivo. In addition, aging is associated with an overall failure to increase the expression of microglial genes involved in cell–cell interactions, such as CXCL10. Finally, impaired upregulation of pro-angiogenic genes in aged microglia was associated with a decline in neovascularization in aged mice compared to young mice after distal middle cerebral artery occlusion. This study provides a new resource to understand the mechanisms underlying microglial alterations in the aged brain milieu and sheds light on new strategies to improve microglial functions in aged stroke victims.
Keywords: Microglia, aging, cell movement, angiogenesis, transcriptome
Introduction
Microglia, the resident immune cells in the central nervous system (CNS), are important participants in brain physiology and pathology. Aside from their established roles as immune sentinels, microglia perform a range of homeostatic activities to maintain normal CNS functions.1,2 In an injured or diseased brain, resident microglia, as well as infiltrating macrophages, serve as the first line of defense and play diverse roles in debris clearance, inflammation induction and resolution, trophic factor release and neuroregeneration. Indeed, clinical studies reveal prompt and persistent activation of microglia up to months after ischemic stroke.3,4 The activation of microglia at the infarct site and along injured axonal tracts has been associated with long-term brain damage after stroke.4 A causal link between microglia activity and stroke outcomes is further underscored by animal studies showing that the depletion of microglia alters the progression of ischemic stroke. Depending upon the context, however, the depletion of microglia may generate opposing impacts—beneficial or detrimental—on post-stroke injury and repair.2,5–7 These collective observations highlight the dynamic and diverse roles of microglia in response to distinct microenvironmental cues in the ischemic brain.
Despite advances in the exploration of therapies to promote long-term recovery in experimental stroke models, no recovery-enhancing strategies have been successfully translated into clinical application. One of the reasons for this failure is that stroke mainly afflicts the elderly, while animal studies mostly rely on data generated from young adults.8 Indeed, aging is associated with profound alterations in brain structure and function, thereby influencing the incidence, progression, and treatment of age-related diseases, such as stroke. For example, aged mice exhibit deterioration of long-term functional outcomes after stroke, which might be linked to heightened pro-inflammatory tone and weakened anti-inflammatory microglial responses in the ischemic brain.9–11 In particular, microglia are primed to express specific levels of chemokine receptors and cytokines during the process of aging, which can result in dramatic immune responses upon a second stimulation.12 Consequently, senescent microglia acquire a distinct phenotype characterized by impaired phagocytosis and enhanced production of pro-inflammatory cytokines in comparison to young microglia.13–15 Therefore, an aged microglial phenotype may negatively affect brain function, exacerbate aging-related brain diseases and impair brain repair.16 However, the critical features of aged microglia that distinguish them from their young counterparts in the context of ischemic stroke, particularly during the stroke recovery phase, remains unclear.
In this study, we compared the genome-wide transcriptional profiles of young and aged microglia at chronic stages after ischemic stroke, to determine the impact of aging on microglia responses to ischemic brain injury and repair. Our data reveal prominent reductions in cell migration, cell–cell interaction, and repair capacities in aged microglia. This information can be leveraged to construct new hypotheses on microglia/macrophage aging and the mechanisms underlying age-dependent responses to pathological conditions, which may shed light on future therapeutic targets for stroke recovery.
Methods
Animal model
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, performed in accordance with the principles outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and reported in accordance with the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments). Young male C57/BL6 mice (10 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Aged male C57/BL6 mice (18 months old) were provided by the National Institute of Aging. Mice were randomly divided into sham or ischemic surgery groups using a lottery-drawing box. Mice were subjected to distal middle cerebral artery occlusion (dMCAO), as described previously.10 Cerebral blood flow (CBF) was measured using laser Doppler flowmetry. Mice with less than 70% reduction of blood flow in the ischemic core, or that died during surgery, were excluded from further analyses. Mice in the sham group were similarly anesthetized but not subjected to MCA cauterization. All efforts were made to minimize animal suffering. For microglia/macrophage depletion, PLX5622 (Plexxikon Inc, Berkeley, CA) was supplied to mice (9 weeks old, 25-30 g body weight) in the diet (Research Diets, New Brunswick, NJ) at 1200 PPM (1200 mg/kg of chow), starting seven days prior to surgery until the end of the experiment. All outcome endpoints were measured by investigators blinded to experimental group assignments.
BrdU injections
In order to label proliferating cells, animals were injected intraperitoneally with the thymidine analogue 5′-bromo-2′-deoxy-uridine (BrdU, 50 mg/kg, B9285, Sigma, St. Louis, MO) twice a day (with an interval of at least 8 h) for four consecutive days, beginning three days after MCAO.
Microglia harvest
Brain tissues were collected from the ischemic hemisphere 14 days after stroke or from the equivalent part of the brains from the sham animals. Brain homogenates were prepared with the Neural Tissue Dissociation Kit (T) (Miltenyi Biotec 130-093-231, Auburn, CA) using a gentle MACS octo dissociator with heaters (Miltenyi Biotec, Auburn, CA). Brain homogenates were passed through a cell strainer (70 µm, Fisher Scientific, Pittsburgh, PA). Single cell suspensions were separated from myelin and debris by Percoll (GE Healthcare 17-0891-01, Chicago, IL) gradient (30% and 70%) centrifugation (500 g, 30 min, 18°C). The mononuclear cells at the interface were collected and washed with HBSS (Sigma-Aldrich, St. Louis, MO) containing 1% FBS (Sigma-Aldrich, St. Louis, MO) and 2 mM EDTA (Sigma-Aldrich, St. Louis, MO). Cells were stained with antibody cocktails containing PerCP-Cy5.5 labeled anti-CD45 (Thermo Fisher eBioscience 45-0451-82, Pittsburgh, PA), Pacific blue labeled anti-CD11b (BD Biosciences 563015, San Jose, CA), FITC labeled anti-Gr1 (Invitrogen 4335848, Carlsbad, CA), and BV421 labeled anti-CD11c (BD Biosciences 562782, San Jose, CA). Cell sorting was performed using FACS ARIA (BD Biosciences, San Jose, CA). Data analyses were performed using FlowJo software (FlowJo, version 10.0, Ashland, OR). Microglia collected from five brains were pooled into one sample.
RNA preparation and RNA-sequencing
Total RNA was extracted from FACS-sorted microglia using RNeasy Plus micro/mini kits (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. RNA quality was assessed using an Agilent Bioanalyzer (Agilent, Santa Clara, CA). All RNA used had an RNA Integrity Number (RIN) >9.2. RNA sequencing was performed at the next-generation sequencing facility core at the University of California at Los Angeles. Libraries for RNA-Seq were prepared with the Clonetech SMARTer Stranded Total RNA-Seq (Pico) Kit. The workflow consists of first-strand synthesis, template switching, adaptor ligation, cleavage of ribosomal cDNA and PCR amplification. Different adaptors were used for multiplexing samples in one lane. Sequencing was performed on Illumina Hiseq3000 for a single end read 50-bp run. Data quality checks were performed on Illumina Sequencing Analysis Viewer (SAV). Demultiplexing was performed with Illumina Bcl2fastq2 v 2.17 program.
RNA sequence data analysis
Raw data from high-throughput sequencing was stored in FASTQ format files, which contained the read sequence and corresponding base quality. All files are available from the GEO database (access number GSE142361). After cleaning up barcode and adapter contaminations and low-quality regions, we used Tophat2 (http://tophat.cbcb.umd.edu) to align the trimmed reads to mouse reference genome sequences (mm10) and to annotate the reads. Cufflinks (2.2.1) was then applied to estimate gene and isoform abundance. The abundance of mapped reads is expressed as Fragment Per Kilobase of transcript per Million mapped reads (FPKM) format. In addition, we applied HTSeq (version: 0.11.2) to quantify the gene expression by number of read counts. We used R software (version: 3.6.0) with several packages for the bioinformatics analysis. Differential expression analysis was performed with DESeq2 (version: 1.24.0).17 A level with log2 (fold change) < −1 or >1 and adjusted p-value <0.1 was set to filter differential expression genes (DEGs). Heatmaps were generated by using pheatmap package (version: 1.012). In order to predict the putative biological processes the DEGs involved, gene ontology enrichment (GO) analyses were carried out with ClusterProfiler (version: 3.12.0)18 in R with an adjusted p value (p-adj) <0.05 (Benjamini-Hocherg correction). For visualization of bubble plots, a reduction function was used to delete all those redundant terms with gene overlaps equal to or greater than 75% with GO Plot package (version: 1.0.2).19 The Z-score was calculated with the number of up-regulated genes minus down-regulated genes and then divided by the square root of the total number of DEGs assigned to each specific term. A website tool, Metascape (http://metascape.org), was also used for the enrichment analysis. Terms with p-values <0.01, min-overlap = 4 and an enrichment factor >1.5 were regarded as significantly involved terms. The p-value was calculated based on the accumulative hypergeometric distribution, and a correction was made with Benjamin-Hochberg method for multiple comparison tests. Clustering was based on similarity (similarity >0.3), and the most statistically significant terms were presented within a cluster. Kappa scores were used to cluster the enriched terms. Function enrichment and pathway analysis was performed in ingenuity pathway analysis software (IPA, QIAGEN). The methods of Z-score calculation can be found in causal analysis approaches in IPA.20
Dead neuron labeling
Primary cortical neuronal cultures were prepared from C57/BL6 mouse E17 embryos. Neurons (3 × 105 cells per well) were seeded on poly-d-lysine (Sigma P6704, St. Louis, MO) coated 24-well plates (Thermo Fisher Scientific, Pittsburgh, PA) with neurobasal medium (Gibco, Gaithersburg, MD) containing B27 (Invitrogen, Carlsbad, CA) and GlutaMax (Gibco; Gaithersburg, MD). In vitro ischemia was initiated at 10d in vitro with 120 min-long oxygen glucose deprivation (OGD). Briefly, two-thirds of the culture medium was replaced four times with serum- and glucose-free medium, resulting in a final glucose concentration of <1 mm. The glucose-deprived cultures were then placed in a Billups-Rothenberg modular incubator chamber, which was flushed for 5 min with 95% argon and 5% CO2, and then sealed. The chamber was maintained at 37°C for 2 h and then returned to 95% air, 5% CO2, and glucose-containing medium for 24 h.21 Neurons were collected and labeled with 1 μg/mL propidium iodide (Sigma P4170, St. Louis, MO) in FBS-free neurobasal medium (1 mL PI staining medium to every 107 neurons) and incubated at 37°C for 10 min. Cells were washed before using stereotactic injection.
Stereotactic injections
After mice were anesthetized, 2 µL suspended OGD neurons (∼100,000 cells, prepared as above mentioned) were bilaterally injected into the brain (AP = +0.1 mm, ML = ±2.0 mm, z = −2.5 mm relative to Bregma) with a microsyringe pump controller (UMP3-4, WPI, Sarasota, FL) at a rate of 200 nL/min. After completion of the infusion, the needle was left for an additional 10 min in the brain before withdrawal. After recovery from the surgery, mice were returned to their home cages and sacrificed three or seven hours after the injection.
Immunohistochemistry
Immunohistochemistry staining was performed as described before.21 All photographs were captured under the same settings and analyzed in parallel with ImageJ software. Primary antibodies used in this study include rabbit anti-Iba1 (Wako Chemicals 019-19741, Richmond, VA), goat anti-CXCL10 (R&D AF-466-NA, Minnesota, USA), mouse anti-BrdU (BD, 555627, Franklin Lakes, NJ, USA), and rat anti-CD31 (BD, 550274, Franklin Lakes, NJ, USA). Two fields in the peri-infarct area or peri-injection area were randomly chosen for imaging and further quantification. Recorded images were loaded onto Image J (NIH) and were quantified by two independent observers blinded to grouping.
Organotypic slice cultures and ex vivo migration assay
Organotypic cultures were prepared as described.22 Briefly, brain slices from young (10 weeks old) and aged (18 months old) mice were coronally sectioned at 300 μm on a McIlwain tissue chopper, and plated onto Millipore-Millicel-CM mesh inserts (Thermo Fisher Scientific, Pittsburgh, PA) in 6 well culture plates with media containing 50% basal medium Eagle (Sigma B1522, St. Louis, MO), 25% heat-inactivated horse serum (GIBCO 26050-070), 25% Earle’s balanced salt solution (GIBCO 24020-117), 6.5 mg/mL glucose (Sigma G8769, St. Louis, MO), 1% penicillin-streptomycin, and 1% Glutamax. On the next day, brain slices were collected from CX3CR1-GFP mice at five days after dMCAO and added into the inserts with intact young or aged brain slices. Slices were fixed in 4% PFA for 1 h at 10 days after co-culture. Goat anti-Iba1 (Abcam ab5076, Cambridge, MA) and mouse anti-GFP antibodies (Invitrogen A-11122, Cambridge, MA) were applied for 48 h at 4°C. Fluorescence-conjugated secondary antibodies were applied overnight at 4°C. Slices were mounted onto glass slides and coverslipped with Fluoromount-G. Images were captured with confocal microscopy (Fluoview FV1000; Olympus, Tokyo, Japan). The migration distance was measured by drawing a perpendicular line between the migratory cell and the border line of intact brain slice in ImageJ.
Statistical analyses
Results are all presented as mean ± standard deviation (SD). GraphPad Prism software (version 8.0, La Jolla, CA) was used for statistical analyses. The Student’s t test was used for comparison of two groups for continuous variables with normal distributions. The Mann-Whitney U rank sum test was used for continuous variables with non-normal distributions. One-way or two-way ANOVA was used for comparison of multiple groups. Pair-wise comparisons between means were tested by post hoc Bonferroni tests. In all analyses, p < 0.05 was considered statistically significant.
Results
Isolation of microglia for RNAseq analysis
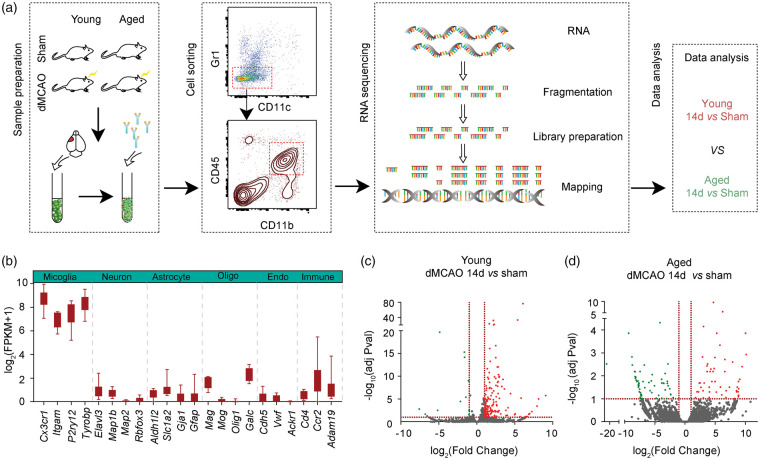
Cerebral ischemia was induced by permanent distal middle cerebral artery occlusion (dMCAO) in young (10 weeks old) and aged (18 months old) mice. Immunostaining showed that similar amount of Iba1+ cells were accumulated in the ischemic core and peri-infarct areas 14 days after dMCAO in young and aged mice (Figure S1A). Brain samples were then collected from young and aged mice with sham operation (YSham and ASham, respectively) or 14 days after dMCAO (YMCAO and AMCAO, respectively). Microglia were isolated using FACS, based on the expression of a combination of cell-specific markers (Figure 1(a)). There was a comparable increase in the number of CD45intCD11b+ microglia in both young and aged brains 14 days after dMCAO (Figure S1(B) to (C)). Low numbers of CD45highCD11b+ macrophages were detected in the ischemic mouse brain 14 days after dMCAO (Figure S1(B)). CD45int CD11b+ microglia sorted from five brains were pooled into one sample for RNA preparation, and each group included two pools of samples. The transcriptome was investigated by RNA sequencing (RNA-seq). Cell purity was confirmed by expression of microglia lineage-specific genes (Cx3cr1, Itgam, P2ry12, and Tyrobp). In contrast, the expression of markers for neurons (Elavl3, Map1b, Map2 and Rbfox3), astrocytes (Aldh1l2, Slc1a2, Gja1, and Gfap), oligodendrocytes (Mag, Mog, Olig1 and Galc), endothelial cells (Cdh5, Vwf and Ackr1), and other immune cells (Cd4, Ccr2 and Adam19) was low, confirming that our sorted populations were highly enriched in microglia, without substantial cross-contamination (Figure 1(b)).
Figure 1.
Isolation of microglia for RNAseq analysis. Young (10 weeks old) or aged (18 months old) mice were subjected to distal middle cerebral artery occlusion (dMCAO) or sham operation. Microglia were purified from sham brains and brains 14 days after dMCAO using FACS. (a) Schematic experimental design to compare gene profiles between young and aged microglia in ischemic brains. (b) Gene expression of cell-specific markers in purified microglia. Specific markers for microglia, neurons, astrocytes, oligodendrocytes (Oligo), endothelial cells (Endo) and peripheral immune cells (Immune) were compared. Data are expressed as log2(FPKM + 1). (c–d) Volcano plot of gene expression profiles in microglia collected after dMCAO or after sham operation in young (c) and aged (d) group, showing distribution of significance [−log10(adjusted Pvalue)] vs. fold change [log2(fold change)] for all genes. The green dots indicate genes down regulated (fold change <−2, adjusted Pval <0.1), the red dots indicate genes upregulated (fold change >2, adjusted Pval <0.1), and the gray dots indicate genes with no significant change after dMCAO. Horizontal dashed lines indicate adjusted p-value = 0.1. Vertical dashed lines indicate expression fold change equal to −2 and 2, respectively.
Differential gene expression in microglia from ischemic and sham brains of young and aged mice
Comparison of gene profiles of microglia from late-stage ischemic brain and age-matched sham brains yielded profound differences in both young and aged mice. Genes were identified as differentially expressed genes (DEGs) only when the fold difference between two cell populations was greater than 2 and the adjusted p values was lower than or equal to 0.1. Volcano plots graphically highlight the DEGs that were significantly up (red) or down (green) regulated in response to stroke in young (Figure 1(c)) and aged mice (Figure 1(d)). In YMCAO microglia, 172 transcripts were differentially expressed compared to YSham microglia, among which 159 genes (92.44%) were upregulated and 13 genes (7.56%) were downregulated (Figure 2(a) and Table S1). A total of 111 DEGs were identified between AMCAO and ASham microglia, of which 60 transcripts were downregulated and 51 were upregulated (Figure 2(b) and Table S2). Out of the total DEGs, 96.40% (107 out of 111) were differentially expressed by at least 5-fold in AMCAO microglia vs ASham microglia (Figure 1(d) and Table S2), while 33.72% (58 out of 172) were differentially expressed by at least five-fold in YMCAO microglia versus YSham microglia (Figure 1(c) and Table S1). These data revealed a prominent upregulation of gene expression in young microglia and more dramatic changes in gene expression levels in aged microglia in response to ischemic injury.
Figure 2.
Gene ontology enrichment analysis of DEGs (dMCAO vs. sham) identifies key biological processes in young and aged stroke mice. (a–b) Heatmap plot of DEGs between dMCAO and sham microglia in young and aged groups (left). The bubble plots illustrate the highest differentially regulated biological processes, graphically displayed according to Z scores and significance [−log10(adjusted Pvalue)]. Each circle indicates a term. Larger size indicates more DEGs involved in that term. Reduction of the bubble allowed better visualization (see methods). (c–d) The enrichment functional analysis was performed in IPA for young (c) and aged (d) groups. Top enriched functions were clustered into five groups: cell movement, cell to cell signaling and interaction, cardiovascular development and function, inflammatory response, and cell death/survival/function. The bars indicate z-score calculated in IPA, with a z-score >2 suggests activation and a z-score <−2 suggests inhibition. The black dots indicate statistical significance [–log10(P value)] for each term.
Gene ontology (GO) analysis of microglia from ischemic brains and sham brains of young and aged mice
To further reveal functional categories of DEGs, Gene Oncology (GO) enrichment analysis was performed. The top 25 significantly upregulated and downregulated biological processes in YMCAO microglia are listed in Table S3. The bubble plot in Figure 2(a) illustrates an upregulation (z score >2) of biological processes enriched by the DEGs in YMCAO microglia, which are mainly involved in cytokine production and signaling, cell adhesion/migration and inflammatory/defense responses (Figure 2(a)). The top GO terms in aged microglia revealed enrichments in similar functional categories related to cell adhesion/migration and inflammatory responses (Figure 2(b) and Table S3). Interestingly, these functions showed a trend toward downregulation or lower degrees of upregulation in AMCAO microglia versus sham controls, compared to upregulations of the same terms in YMCAO microglia versus sham controls (Figure 2(a) and (b)). Of note, genes related to tissue remodeling were upregulated in young stroke mice (z score = 3.0) (Figure 2(a)), while no association with tissue remodeling was found in the DEGs in aged mice (Figure 2(b)).
The DEGs in young and aged microglia were further scrutinized using Ingenuity Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com). The activation z-scores and p-values were used as parameters to identify the most salient effects. A positive z-score indicates increased functional activity and a negative z-score indicates decreased functional activity in YMCAO or AMCAO microglia relative to YSham or ASham microglia, respectively. Similar to GO analysis, DEGs in YMCAO microglia vs YSham microglia displayed significant upregulations in function/disease categories that are associated with cell movement, cell–cell signaling and interaction, cardiovascular system development and function, inflammatory responses and cell death/survival/function (Figure 2(c) and Table S4). In contrast, weak negative associations with cell movement, cell-cell signaling and interaction, and inflammatory responses were found in the DEGs in AMCAO versus ASham microglia (Figure 2(d) and Table S5). IPA analysis also identified six activated pathways, including Trem1 signaling, neuroinflammation signaling, and activation of IRF by cytosolic pattern recognition receptors, based on DEGs (z score > 2) (Figure S2(a)). In aged microglia, the LXR/RXR signaling pathway was significantly elevated, consistent with alterations in lipid metabolism and its potential influence on microglia activity (Figure S2(b)).
Age-dependent transcriptional profiles and alterations after stroke
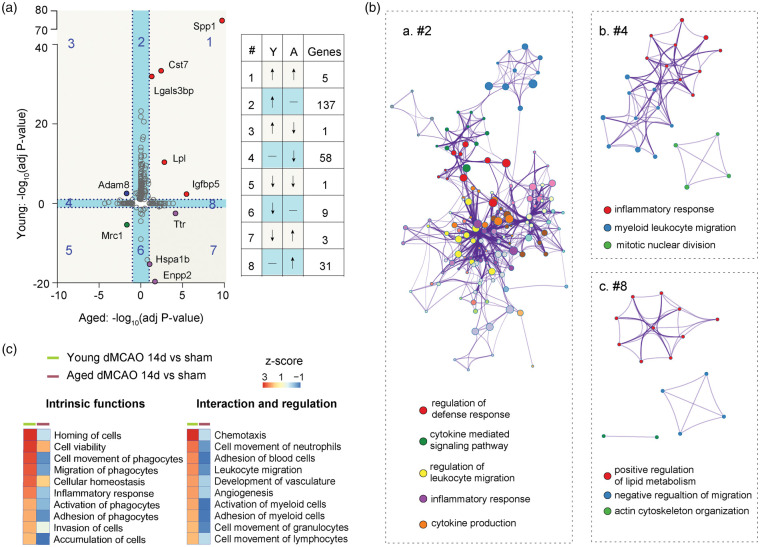
Next, we compared the gene expression profiles in young versus aged microglia after stroke. DEGs in young versus aged microglia are displayed in a scatterplot of adjusted p values. All DEGs were divided into eight groups (Figure 3(a)). Group 2 includes 137 genes that were significantly upregulated only in young microglia 14 days after stroke (Figure 3(a)). This DEG set distinctive to young microglia was associated with regulation of defense responses, leukocyte migration, and cytokine production and signaling, highlighting age-dependent disparities in these particular functions (Figure 3(Ba)). The 58 genes in group 4 were selectively downregulated in aged microglia and are implicated in myeloid cell migration and inflammatory responses (Figure 3(Bb)). The 31 genes in group 8 were upregulated in aged microglia and are associated with positive regulation of lipid metabolism, negative regulation of cell migration, and actin cytoskeleton reorganization (Figure 3(Bc)). These results underscore an enhanced cell movement function in young microglia in ischemic brains, which appears to be impaired in aged microglia.
Figure 3.
Microglia demonstrate age-dependent transcriptional profiles and functional alterations 14 days after stroke. (a) Scatterplot shows comparison of −log10(adjusted Pval) for DEGs in young and aged microglia at 14 days after stroke. The DEG distributions within each subgroup (1–8) are listed in a table on the right. Quadrants 1, 3, 5, and 7 (with light grey background) indicate DEGs in both young and aged microglia. Quadrants 2, 4, 6, and 8 with light blue background indicate genes significantly changed in either young or aged group. (b) Metascape enrichment analysis for genes in second (a), fourth (b) and eighth (c) quadrants. Each node indicates a term. Clustering was made based on similarity (similarity > 0.3). (c) Function enrichment analysis performed by IPA identifies differences in intrinsic functions (left) and regulatory functions (right) between young and aged microglia. Functional terms were sorted according to z-score (high to low) in the young microglia.
Among the total transcripts detected, 10 genes were differentially expressed after stroke in both young and aged microglia (Figure S3(a)). These 10 DEGs overlapping between young and aged cells were distributed in four corners of the scatterplot in Figure 3(a). Five genes in group 1 (Spp1, Igfbp5, Lpl, Cst7 and Lgals3bp) were upregulated independent of age, in both young and aged stroke mice (Figures 3(a) and S3(B)). The reported biological functions of these five genes are related to tissue remodeling, chemoattraction and cell migration, cell–cell and cell–matrix interactions and inflammatory responses (Figure S3(B)).23–25 Group 5 has only one gene (Mrc1), which was age-independently downregulated in both young and aged stroke mice (Figures 3(a) and S3(B)). The fold decrease of Mrc1 was more dramatic in aged stroke mice versus young stroke mice (−11.4 vs. −2.7). The Mrc1 encodes CD206 protein, a well-known marker of anti-inflammatory microglia.26 Three genes (Ttr, Enpp2 and Hspa1b) in group 7 were upregulated in aged mice, but downregulated in young mice (Figures 3(a) and S3(B)). The protein products of these genes are functionally related to protein folding and lipid signaling,27,28 which are known to alter with aging and neurological diseases. Adam8, a gene encodes a membrane-anchored protein implicated in cell–cell and cell-matrix interactions,29 was significantly upregulated in young microglia but decreased in aged microglia (group 3, Figures 3(a) and S3(B)).
Finally, young–aged comparative analysis using IPA highlights differences in intrinsic cell functions and regulatory functions on other cells (Figure 3(c)). Aged microglia displayed a downregulation of functions related to cell movement, inflammatory response, cell viability, and cell homeostasis. In addition, senescent microglia appeared to lose their regulatory functions and display fewer interactions with other cells (Figure 3(c)).
Aged microglia suffer impaired cell migration after stimulation
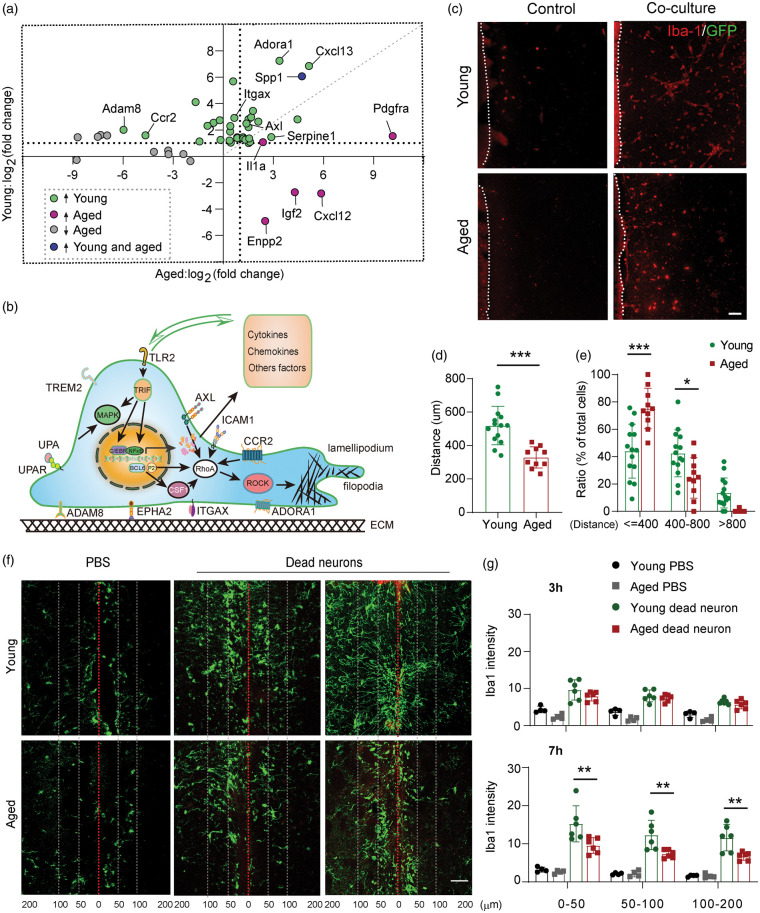
As the innate immune cells of the brain, microglia are the first cells to respond to external stimulation or internal cell damage.1 Our DEG and functional enrichment analysis of young versus aged microglia suggest prominent differences in phagocyte chemotaxis and migration (Figures 2 and 3). We then further analyzed the differences in cell migration between young and aged microglia. The scatterplot in Figure 4(a) is based on the fold changes of DEGs important for chemotaxis and cell migration between Ymcao microglia versus Amcao compared to their corresponding controls (adjusted P < 0.1) and reveals 35 unique genes changed in young microglia, 14 distinctive genes in Amcao microglia, and only three genes (Spp1, Enpp2 and Adam8) shared between the two populations (Figure 4(a)). Functionally, some genes that were distinctively upregulated in young microglia after stroke encode surface receptors for chemoattractants or for other signals involving cell recruiting (Ccr2, Plaur, Tlr2, Axl, and Itgax) (Figure 4(b)). Genes that encode cell-surface anchored proteins and important for cell–matrix interactions, such as Epha2, Adam8, and Icam, were also upregulated in young microglia. Some DEGs in YMCAO microglia are known to activate the Rho/ROCK signaling important for actin polymerization and depolymerization (Figure 4(b)). In addition, young microglia upregulate genes involving NFκB signaling pathways and the production of cytokines, chemokines, and other factors, which can in turn influence their own migration as well as the migratory ability of other cells in the ischemic brain (Figure 4(b)). In contrast, more than half of migration-relation DEGs in aged mice were downregulated after stroke, suggesting reduced migratory capacities (Figure 4(a)).
Figure 4.
Age-dependent changes in microglia migration after dMCAO. (a) Scatterplot shows comparison of log2(fold change) for DEGs in young and aged microglia at 14 days after stroke. Green dots are DEGs that are selectively upregulated in young mice. Purple dots are DEGs upregulated distinctively in aged mice. Grey dots are DEGs downregulated in aged mice. Spp1 (blue dot) was upregulated in both young and aged mice. The horizontal and vertical dashed lines indicate expression fold change equal to 2. (b) Schematic illustration showing cellular distribution of some migration related DEGs in young microglia and their functions in cell migration. (c) Representative images showing migration of Iba1+GFP− microglia out of young and aged brain slices cultured alone or together with CX3CR1-GFP ischemic brain at 11 DIV. Scale bar, 100 μm. (d) Quantification of cell migration distance for Iba1+GFP− microglia. Totally 372 cells from 14 young brain slices and 178 cells from 10 aged brain slices were analyzed. Aged microglia migrated much shorter distances compared to young microglia. ***p < 0.001 Student’s t test. (e) The distribution of Iba1+GFP− cells was quantified as the percentage of cells located within 400 µm, 400–800 µm, and beyond 800 µm from their original young or aged brain slices. ***p < 0.001 *p < 0.05, two-way ANOVA followed by Bonferroni post hoc. (f) Representative images showing migration of Iba1+ microglia (green) towards injected dead neurons in young and aged mouse brains. Red line indicates needle track (injection channel). White dashed lines indicate 50 µm and 100 µm distance marks from the injection site. Scale bar, 50 μm. (g) Quantification of Iba1 staining intensity within 50 µm, 50–100 µm, and 100–200 µm from the injection track. **p < 0.01 one-way ANOVA followed by Bonferroni post hoc. There are significant differences between dead neuron injection groups and PBS injection groups at both time points and in all areas.
In order to confirm the functional consequences of the abovementioned changes in gene expression, we compared the migratory capacities of young and aged microglia using an ex vivo assay. Organotypic brain slices collected from young (10-week-old) and aged (18-month-old) mice were co-cultured with ischemic brain slices collected from CX3CR1-GFP mice. This assay allows the quantitative comparison of chemotactic migration of young and aged microglia towards injured tissue (Figure 4(c) to (e)). Cell migration was analyzed after 10 days of coculture. Microglia from intact brains were visualized using Iba1 staining, while microglia in the ischemic brain were identified by GFP as the microglia reporter (Figure 4(c)). Young or aged microglia barely moved out of their origin brain slices without any chemotactic signals. In both young and aged brain slices, juxtaposing an injured brain slice markedly increased the numbers of migrating microglia, which were Iba1+GFP− (Figure 4(c)). Almost all Iba1+GFP+ cells stayed within the ischemic brains (not shown), resulting in a lack of GFP signal in the imaging space between two co-cultured slices (Figure 4(c)). The mean cell migratory distance of Iba1+GFP− cells was significantly longer in young slice cultures compared to those in the aged slice culture (Figure 4(d)). Larger portion of Iba1+GFP− microglia stay within 400 µm away from the aged brain sections, while more Iba1+GFP− microglia migrate into the zone 400 µm–800 µm away from the young brain sections (Figure 4(e)).
Next, we measured in vivo the ability of microglia to migrate towards apoptotic neurons injected into the brain. AlexaFluor‐593‐labelled apoptotic neurons were stereotactically injected into the striatum of young (10-week) and aged (18-month) mice. Brain sections were collected 3 h and 7 h after injection and stained with Iba1. Both young and aged microglia were attracted to the injected dead neurons as early as 3 h post-injection and continued to accumulate around the injection track (Figure 4(f)). A greatly retarded migration and accumulation pattern was observed in the aged brain (Figure 4(f)), resulting in a significant reduction in microglial staining intensity within 50 µm, 50–100 µm, and 100–200 µm away from the injection track, at 7 h after injection (Figure 4(g)). These experiments confirm significant impairments in microglial chemotaxis and migration with aging.
Aged microglia lose functional capacities for cell-cell interaction and regulation
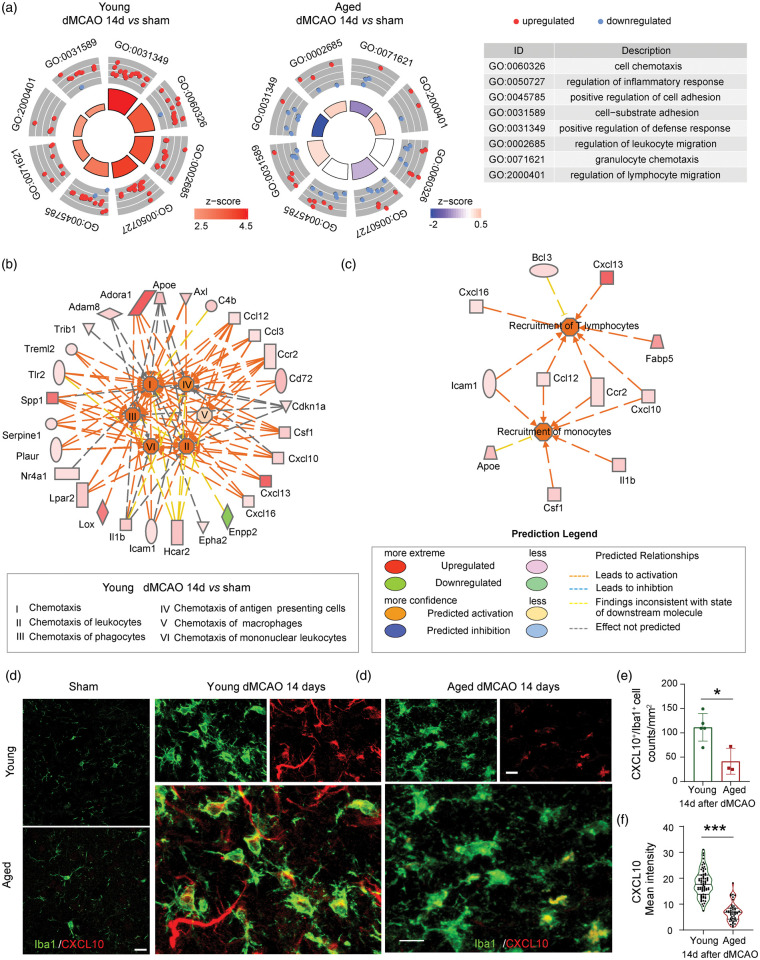
Another prominent functional difference between young and aged microglia is their immunoregulatory capacity (Figure 3(c)). The circular scatterplots in Figure 5(a) showed the DEGs involved in specific GO terms covering the regulation of cell chemotaxis, inflammatory responses, cell adhesion, defense response, and leukocyte migration in young and aged microglia 14 days after stroke. The upregulated or downregulated genes are illustrated as red or blue dots, respectively. The areas of the inner trapezoid are proportional to the significance of each term, and the color represents the overall direction of change in expression of each term. These terms showed an overall upregulation in young microglia, whereas half of the genes in each category were downregulated in aged microglia. The lack of induction of regulatory genes and cell–cell interactions in aged microglia suggested diminished regulatory functions of these immune cells. IPA analyses suggested a positive effect of young microglia on the chemotaxis of a variety of other immune cells at 14 days after stroke (Figure 5(b)). In contrast, the chemotaxis of other immune cells was mostly inhibited or did not change in aged microglia (Figure S4). In particular, multiple cytokines upregulated in young microglia 14 days after stroke were predicted to be involved in the recruitment of T lymphocytes and monocytes (Figure 5(c)). Immunostaining confirmed that the expression of CXCL10, a chemokine important for T cell and monocyte recruitment, was elevated in Iba1+ microglia in both young and aged mice 14 days after stroke. However, the number of CXCL10+Iba1+ cells was significantly higher in microglia of young animals compared to those in aged (Figure 5(d) to (e)). The intensity of CXCL10 staining was also higher in young than in aged microglia (Figure 5(d) and (f)).
Figure 5.
Aged microglia show reduced capacities for cell–cell interaction and regulation compared to young counterparts 14 days after stroke. (a) Circle plot shows eight cell-cell interaction related GO terms in young (left) and aged (middle) group. The outer circle shows a scatterplot for each term with log2(fold change) of assigned genes. Red dots indicate upregulated genes, and blue dots indicate downregulated genes. The color of the inner circle displays the z-score, with the area of trapeziform indicating the p value of each term. The table shows the description of each term. (b) IPA analysis identified chemotaxis-related functions enriched in young microglia. The color of the outer circle nodes indicates the expression of assigned genes. The color of the inner circle nodes indicates the z-scores of enriched functions. The color of segments between gene nodes or function nodes implies gene activation or inhibition. Red indicates upregulation and green indicates downregulation. (c) Genes related to recruitment of T lymphocyte and monocytes were upregulated in young microglia 14 days after stroke. (d) Immunostaining of CXCL10 (red) and Iba1 (green). The left panels (bar = 20 µm) show the staining in young and aged sham. The middle and right panels (bar = 10 µm) show the staining in young and aged mice, respectively, 14 days after stroke. (e) Quantification of the number of CXCL10+Iba1+ microglia in young and aged mice 14 days after stroke. n = 5 for young and n = 3 for aged. *p < 0.05, Student’s t test. (f) Quantification of the mean intensity of CXCL10 signal in Iba1+ microglia. Violin plots are shown, with n = 71 cells for young and n = 47 cells for aged. ***p < 0.001. Mann-Whitney U test.
Senescent microglia are associated with impaired angiogenesis in aged brain
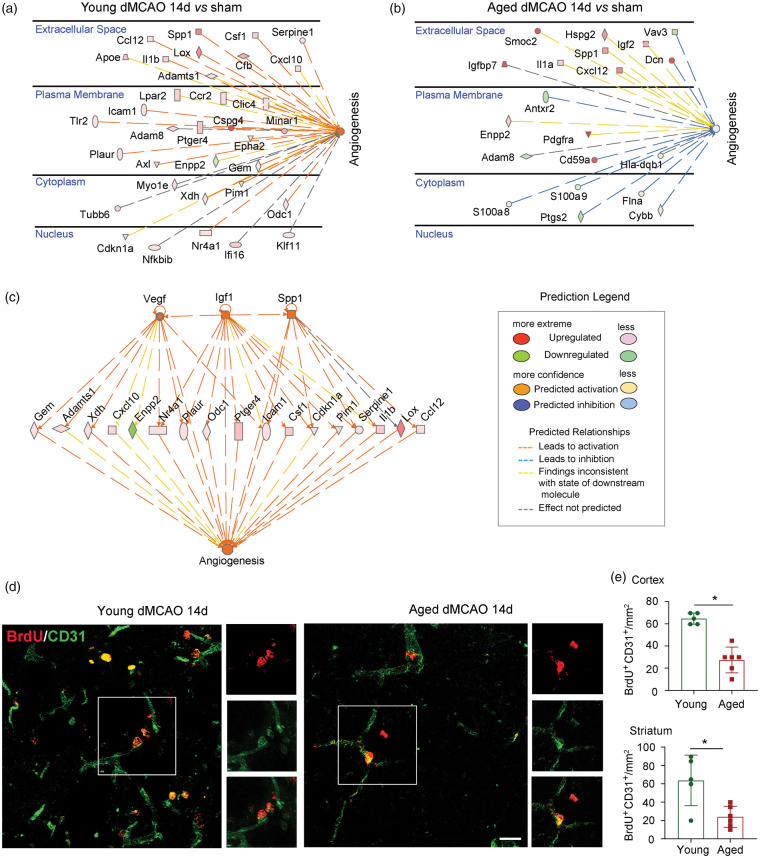
The IPA analysis reveals a difference in angiogenesis between young and aged microglia in the ischemic brain (Figure 3(c)). The DEGs related to angiogenesis in young and aged microglia after dMCAO are listed in Figure 6(a) and (b). More specific changes in angiogenesis-related terms are displayed in Figure S5(A), where GO biological process terms involving angiogenesis, including regulation of angiogenesis, endothelial cell migration/proliferation/differentiation, vascular-associated smooth muscle cell migration/proliferation and tissue remodeling demonstrated an overall upregulation in young mice 14 days after dMCAO. In contrast, a smaller number of angiogenesis-related genes were altered in aged microglia at 14 days after dMCAO (Figure 6(b)), and were associated with neutral effects on angiogenesis (Figure S5A).
Figure 6.
Senescent microglia are associated with impaired angiogenesis in the aged brain after stroke. (a,b) DEGs related to angiogenesis were explored in both young (a) and aged (b) groups, respectively, in IPA. The genes were indicated by nodes and scatter plotted in different parts of cells. The color of the nodes indicates the expression levels of genes, and the dashed line indicates the predicated activation or inhibition of genes. (c) IPA analysis in young microglia showed that Vegf, Igf1 and Spp1 were predicted as upstream regulators of genes involved in angiogenesis. (d) BrdU (red) and CD31 (green) double staining was performed in both young and aged group 14 days after dMCAO. (e) BrdU+CD31+ cells were quantified in peri-infarct areas in both cortex and striatum.
As most significant differences in angiogenesis terms were in YMCAO microglia versus YSham microglia, further functional analysis of IPA was performed on this comparison. Upstream regulator analysis predicted the activation of several genes encoding pro-angiogenic factors (Vegf, Igf1 and Spp1), and established the connections between these genes and angiogenesis-related DEGs (Figure 6(c)).
Angiogenesis is an important brain repair process necessary for histological and neurological improvements after stroke.30,31 Previous studies reveal the importance of microglia in developmental angiogenesis32 and angiogenesis in retinal microvasculature.33 To confirm whether microglia/macrophages play critical roles in post-stroke angiogenesis, we used PLX5622, a small-molecule inhibitor of the CSF1 receptor, to deplete microglia in vivo. We have shown recently that dietary supplement of PLX5622 beginning seven days prior to ischemic surgery dramatically reduced the number of microglia/macrophages in the brain.22 Notably, we discovered that PLX5622-fed mice with significant microglia/macrophage depletion exhibited impairments in angiogenesis, as manifested by double-label immunostaining of BrdU and CD31 (a marker for endothelial cells of microvessels). The numbers of BrdU+CD31+ cells were significantly reduced in the cortical peri-infarct area and showed a trend toward reduction in striatal peri-infarct areas in PLX5622 fed stroke mice compared to microglia/macrophage-competent stroke mice at 14 days after 60 min MCAO (Figure S5B). It is therefore conceivable that the transcriptomic differences between young and aged microglia profoundly influence angiogenic functions after stroke. In line with this assumption, we observed an impairment in post-stroke neovascularization in aged mice. Significantly higher numbers of BrdU+CD31+ cells were observed in the cortical and striatal peri-infarct areas in young mice compared to aged mice after dMCAO (Figure 6(d) to (e)). Juxtaposition of Iba1+ microglia/macrophages with the BrdU+CD31+ vessels was observed in both young and aged brains (Figure S6).
Discussion
An increasing number of research reports support age-dependent alterations in immune cell behavior. Microglia are long-lived immune cells, and their senescence is implicated in the pathogenesis of neurological disorders.34 We previously reported that aged mice exhibited greater brain lesions and worse long-term functional recovery after stroke.10 Although the underlying mechanisms are unknown, we observed a proinflammatory shift in the microglia/macrophage response to injury in aged mice, and a correlation between microglial phenotype and post-stroke functional performance, suggesting that microglia/macrophage phenotypic variance is critical for age-related deterioration in long-term stroke outcomes. The current study extends the exploration of age-dependent functional divergences in microglia and their contributions to brain recovery by deciphering the transcriptomic differences between young and aged microglia during the recovery phase after stroke. Although an increasing number of specific markers have been discovered to identify microglia,35,36 the expression of these markers is usually down regulated when microglia are activated during CNS injury or disease, making them undetectable as markers for microglia sorting in stroke brains. Therefore, the traditional markers, CD45intCD11b+, were used to sort out microglia in this study.
Young and old microglia exhibit distinct transcriptomes during the stroke recovery phase
Previous studies34 and our recent data10 suggest that microglia in the normal, aged brain are functionally primed into a proinflammatory modality and express higher levels of inflammatory factors. These primed immune cells are resistant to anti-inflammatory regulation and linked to persistent neuroinflammation and neurodegeneration.37 However, the behavior of primed microglia during the recovery phase after brain injuries remains elusive. The present findings reveal age-specific alterations of a significant number of genes in microglia at 14 days after stroke. Young microglia exhibited a strong trend toward upregulation of gene expression after stroke. Functional annotations of these DEGs were related to induction of a wide variety of biological processes, including cell chemotaxis, cell–cell interactions, inflammatory responses, cell death/survival, and vascular remodeling. In contrast, the DEG profile in aged microglia demonstrated largely retarded or suppressed functions at late stages after stroke, which seems contrary to the reported sensitization of aged microglia in neurodegenerative diseases.37 It is recently reported that a single inflammatory stimulus induces acute immune training in microglia and exaggerates immune responses, while multiple doses of inflammatory stimuli result in immune tolerance in microglia and dampened inflammatory responses after stroke.38 This observation reveals an immune memory within microglia, which might also transpire during aging, preventing further amplification of immune responses in stroke, thereby resulting in relative immune suppression. An in vitro model of microglia senescence showed reduced responses of aged microglia to Aβ stimulation.39 Aged microglia also respond in a slower and more sustained manner to laser-induced focal retina injury.40 Therefore, microglial aging may involve transitions through changing gene expression profiles and functional features. Further studies are warranted to dissect the functional consequences of various microglial phenotypes during physiological and pathological aging.
With recent advances in single cell technologies, gene signatures for aged microglia and disease-associated microglia were defined. Notably, some DEGs (Spp1, Lpl, Cst7, and Lgals3bp) that were distinctively upregulated in aged microglia at late stages after stroke were signature genes known to be induced in senescent microglia41 and specific microglia clusters associated with Alzheimer disease41 or other types of neurodegeneration.42 It is possible that these genes are commonly expressed in response to chronic stimulation and can serve as markers for primed microglia.
Aged microglia display inferior migratory and angiogenic capacity
A striking discovery of the present study is a dramatic difference between young and aged microglia in migratory capacity. Microglia from young stroke mice expressed numerous genes involved in phagocyte chemotaxis and migration. For example, genes encoding surface receptors binding to chemoattractants or other stimulants [CCR2 (Ccr2),43 Adenosine A1 receptor (Adora1), Toll like receptor 2 (Tlr2), Triggering receptor expressed on myeloid cells like 2 (Treml2),44 Tyrosine-protein kinase receptor (Axl), CD11c (Itgax), Urokinase receptor (Plaur)45] and genes associated with cell surface proteins involving cell–matrix interaction [Intercellular adhesion molecule 1 (Icam), ephrin type-A receptor 2 (Epha2), and Adam8] were expressed more highly in young microglia from stroke mice compared to young microglia from sham mice. Thus, young microglia appear to actively respond to external signals and migrate to the site of brain injury and repair. Axl, Icam, Cdkn1a, Bcl6, and Csf1 are known to regulate the RhoA/Rock signaling pathways and actin cytoskeleton, which are important for cell motility.46–49 In addition, the activation of Trib through Tlr2 engagement may activate the NFκB or C/EBP pathway,50 which, in turn, upregulates the production of a plethora of chemokines, cytokines and other inflammatory factors. These factors further modulate the movement of microglia and other cells. Collectively, the transcriptome profile identified an upregulation of genes related to phagocyte movement in Ymcao microglia. In contrast, the gene profiles of aged microglia suggest inhibition of cell movement 14 days after stroke. Consistent with the transcriptomics, ex vivo and in vivo experiments confirmed a reduction in the migratory speeds of aged microglia toward ischemic brains or toward dying neurons, compared to their young counterparts. Other studies have also shown that microglia aging in culture demonstrate reduced migration over time.51
Microglia movement, including baseline surveillance process extension and directed chemotactic cell body migration, is a well-established feature of microenvironment surveillance by microglia, scavenger functions, and inflammation resolution under homeostatic and pathological conditions.52–54 In addition, microglial migration might play critical roles in neurovascular remodeling during brain recovery. The dynamic interactions between microglia and the synapse regulate neural circuitry formation and brain wiring during development and are important for learning and memory in adulthood.52,55 Significantly longer contacts between microglia and synapses have been observed in the ischemic brain, and this is frequently followed by the disappearance of the presynaptic bouton.56 It has therefore been suggested that the dynamic motility of microglial processes toward synapses in the ischemic areas contribute to increased turnover of synaptic connections and subsequent neuronal circuit remodeling.56 Microglia migration toward vascular endothelial cells in neovascular sprouts has also been shown to enhance angiogenesis in the retina.57 Reductions in microglia mobility may therefore play a role in impaired angiogenesis after stroke in the aged brain, as suggested by our study. Transcriptome analysis suggested activation of pro-angiogenic trophic factors, such as Vegf,58 Igf159 and Spp160 in young but not aged microglia after stroke. Collectively, the reduced mobility of aged microglia and impaired activation of proangiogenic cascades may impair angiogenesis in aged brain.
Aged microglia produce less chemoattractants and exhibit reduced cell–cell interactions
The present findings suggest that aged microglia display a reduced potential to interact with other immune cells and regulate their functions. Specifically, aged microglia release lower levels of chemoattractants that recruit immune cells at late phases after stroke. Recent research highlights the functions of late infiltrated immune cells in the recovery from CNS injuries.61 For example, infiltrated peripheral monocytes are known to assume anti-inflammatory phenotypes and promote long-term functional performance after stroke62 and spinal cord injury.63 It has been reported that the number of regulatory T cells are increased and maintained at high levels up to at least one month after stroke.64 The accumulation of regulatory T cells is important in inhibiting astrogliosis and promoting functional recovery. Microglia are one of the major sources of chemoattractants that recruit immune cells from the periphery. Here, we found reduced expression of chemokines for T lymphocyte and monocytes. The decreased capacity of aged microglia to recruit monocytes and T lymphocytes may negatively alter the immune environment and slow or stymie functional improvement after stroke.
Limitations
In addition to resident CD45intCD11b+ microglia, infiltrated and perivascular CD45highCD11b+ macrophages from the periphery also play important roles in ischemic brain injury and recovery.65 The biochemical and functional overlap between infiltrating macrophages, perivascular macrophages, and microglia have complicated our understanding of brain innate immune responses in injury and disease.66,67 The current study focused on CD45intCD11b+ microglia, as this population dominates the innate immune cell family at late stages after stroke. However, we also observed a small number of CD45highCD11b+ macrophages in the ischemic brain 14 days after stroke (Figure S1). Fate-mapping studies or studies at single-cell resolution are warranted to further elucidate age-associated changes in distinct microglia/macrophage subpopulations and their functional specificities in the ischemic brain. An encouraging recent study showed that the acute, noninvasive elimination of aged microglia using a CSF1 receptor inhibitor followed by drug withdrawal and repopulation of new microglia in the aged brain reversed cognitive, synaptic, and neuronal deficits in mice.68 Thus, adjusting microglia responses in an aged brain may be a strategy to promote long-term recovery in stroke as well as other age-related diseases.
Conclusions
The current study documents genomic alterations in aged microglia in the context of ischemic stroke. These changes are linked to age-dependent differences in cellular functions and stroke outcomes. The transcriptomic dissection of genomic differences between young and aged microglia will further elucidate mechanisms underlying age-related changes in microglial behavior and shed light on new strategies to improve microglial functions in aged stroke victims.
Supplemental Material
Supplemental material, JCB902542 Supplemental Material1 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material2 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material3 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material4 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material5 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material6 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is supported by University of Pittsburgh Medical Center Immune Transplant and Therapy Center (ITTC) grant (to JC, LRW and XH). XH is supported by a VA Merit Review grant (grant no. I01 BX003651). JC is supported by VA grants (grant nos I01 BX002495 and I01 BX003377). JC is a recipient of the VA Senior Research Career Scientist Award.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contribution: LJ, HM, FX, WS, DX, and ZS performed experiments and data analysis. LJ, HM, and XH wrote the manuscript. LJ, HM, SL, and JL analyzed the RNAseq data. RKL and YS revised the manuscript. XH, JC, and LRW designed the study.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Yejie Shi https://orcid.org/0000-0001-7502-9201
References:
- 1.Hu X, Liou AK, Leak RK, et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Progress Neurobiol 2014; 119–120: 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie D, He M, Hu X. Microglia/macrophage diversities in central nervous system physiology and pathology. CNS Neurosci Ther 2019; 25: 1287–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhard A, Schwarz J, Myers R, et al. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage 2005; 24: 591–595. [DOI] [PubMed] [Google Scholar]
- 4.Thiel A, Radlinska BA, Paquette C, et al. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J Nucl Med 2010; 51: 1404–1412. [DOI] [PubMed] [Google Scholar]
- 5.Lalancette-Hebert M, Gowing G, Simard A, et al. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 2007; 27: 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J Neuroinflammation 2016; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin WN, Shi SX, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab 2017; 37: 2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Sun L, Wang H, et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol 2007; 81: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 10.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu MY, Lin YY, Zhang BJ, et al. Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci Ther 2019; 25: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niraula A, Sheridan JF, Godbout JP. Microglia priming with aging and stress. Neuropsychopharmacology 2017; 42: 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Wu Y, Shi XQ, et al. Characteristics of spinal microglia in aged and obese mice: potential contributions to impaired sensory behavior. Immunity Ageing: I & A 2015; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babcock AA, Ilkjaer L, Clausen BH, et al. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav Immunity 2015; 48: 86–101. [DOI] [PubMed] [Google Scholar]
- 15.Lourbopoulos A, Erturk A, Hellal F. Microglia in action: how aging and injury can change the brain’s guardians. Frontiers Cellular Neurosci 2015; 9: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deczkowska A, Matcovitch-Natan O, Tsitsou-Kampeli A, et al. Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nature Commun 2017; 8: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012; 16: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter W, Sanchez-Cabo F, Ricote M. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics 2015; 31: 2912–2914. [DOI] [PubMed] [Google Scholar]
- 20.Kramer A, Green J, Pollard J, Jr., et al. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014; 30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. Epub ahead of print 5 March 2019. DOI: 10.1177/0271678X19830791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Zhu W, Xu F, et al. The interleukin-4/PPARgamma signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol 2019; 17: e3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab 2014; 3: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce KD, Gorkhali S, Given K, et al. Lipoprotein lipase is a feature of alternatively-activated microglia and may facilitate lipid uptake in the CNS during demyelination. Front Mol Neurosci 2018; 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorrell AM, Shand JH, Tonner E, et al. Insulin-like growth factor-binding protein-5 activates plasminogen by interaction with tissue plasminogen activator, independently of its ability to bind to plasminogen activator inhibitor-1, insulin-like growth factor-I, or heparin. J Biol Chem 2006; 281: 10883–10889. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 27.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 2005; 62: 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saraiva MJ. Transthyretin mutations in health and disease. Hum Mutat 1995; 5: 191–196. [DOI] [PubMed] [Google Scholar]
- 29.Schlomann U, Rathke-Hartlieb S, Yamamoto S, et al. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): implications for neuron-glia interactions during neurodegeneration. J Neurosci 2000; 20: 7964–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Chang S, Li W, et al. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res Ther 2018; 9: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H, Ma Y, Jiang L, et al. Hypoxia response element-regulated mmp-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther 2017; 25: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rymo SF, Gerhardt H, Wolfhagen S F, et al. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One 2011; 6: e15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding X, Gu R, Zhang M, et al. Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol 2018; 18: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spittau B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front Aging Neurosci 2017; 9: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016; 113: E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki Y, Hoshi M, Akazawa C, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 2003; 44: 242–250. [DOI] [PubMed] [Google Scholar]
- 37.Li JW, Zong Y, Cao XP, et al. Microglial priming in Alzheimer’s disease. Ann Transl Med 2018; 6: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendeln AC, Degenhardt K, Kaurani L, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018; 556: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldeira C, Cunha C, Vaz AR, et al. Key aging-associated alterations in primary microglia response to beta-amyloid stimulation. Front Aging Neurosci 2017; 9: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damani MR, Zhao L, Fontainhas AM, et al. Age-related alterations in the dynamic behavior of microglia. Aging Cell 2011; 10: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 2017; 169: 1276–1290 e17. [DOI] [PubMed] [Google Scholar]
- 42.Sousa C, Golebiewska A, Poovathingal SK, et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep 2018; 19: e46171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faustino J, Chip S, Derugin N, et al. CX3CR1-CCR2-dependent monocyte-microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke. J Cereb Blood Flow Metab 2019; 39: 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurisu K, Zheng Z, Kim JY, et al. Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J Cereb Blood Flow Metab 2019; 39: 1906–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon H, Kim JH, Kim JH, et al. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation 2012; 9: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pixley FJ, Xiong Y, Yu RY, et al. BCL6 suppresses RhoA activity to alter macrophage morphology and motility. J Cell Sci 2005; 118: 1873–1883. [DOI] [PubMed] [Google Scholar]
- 47.Lessey-Morillon EC, Osborne LD, Monaghan-Benson E, et al. The RhoA guanine nucleotide exchange factor, LARG, mediates ICAM-1-dependent mechanotransduction in endothelial cells to stimulate transendothelial migration. J Immunol 2014; 192: 3390–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park HJ, Baen JY, Lee YJ, et al. The TAM-family receptor Mer mediates production of HGF through the RhoA-dependent pathway in response to apoptotic cells. Mol Biol Cell 2012; 23: 3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gadepalli R, Kotla S, Heckle MR, et al. Novel role for p21-activated kinase 2 in thrombin-induced monocyte migration. J Biol Chem 2013; 288: 30815–30831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Liu YH, Tan KA, Morrison IW, et al. Macrophage migration is controlled by Tribbles 1 through the interaction between C/EBPbeta and TNF-alpha. Vet Immunol Immunopathol 2013; 155: 67–75. [DOI] [PubMed] [Google Scholar]
- 51.Caldeira C, Oliveira AF, Cunha C, et al. Microglia change from a reactive to an age-like phenotype with the time in culture. Front Cell Neurosci 2014; 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smolders SM, Kessels S, Vangansewinkel T, et al. Microglia: Brain cells on the move. Prog Neurobiol 2019; 178: 101612. [DOI] [PubMed] [Google Scholar]
- 53.Franco-Bocanegra DK, McAuley C, Nicoll JAR, et al. Molecular mechanisms of microglial motility: changes in ageing and Alzheimer’s disease. Cells 2019; 8: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kluge MG, Abdolhoseini M, Zalewska K, et al. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J Cereb Blood Flow Metab 2019; 39: 2456–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyamoto A, Wake H, Ishikawa AW, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun 2016; 7: 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wake H, Moorhouse AJ, Jinno S, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 2009; 29: 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unoki N, Murakami T, Nishijima K, et al. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci 2010; 51: 3362–3371. [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu W, Fan Y, Frenzel T, et al. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 2008; 39: 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009; 28: 3412–3422. [DOI] [PubMed] [Google Scholar]
- 61.Xie L, Li W, Hersh J, et al. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab 2019; 39: 2268--2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wattananit S, Tornero D, Graubardt N, et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci 2016; 36: 4182–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 2009; 6: e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito M, Komai K, Mise-Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019; 565: 246–250. [DOI] [PubMed] [Google Scholar]
- 65.Pedragosa J, Salas-Perdomo A, Gallizioli M, et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun 2018; 6: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 2018; 48: 380–395 e6. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Luo Y, Yao Y, et al. Silencing the lncRNA Maclpil in pro-inflammatory macrophages attenuates acute experimental ischemic stroke via LCP1 in mice. J Cereb Blood Flow Metab. Epub ahead of print 21 March 2019. DOI: 10.1177/0271678X19836118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmore MRP, Hohsfield LA, Kramar EA, et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 2018; 17: e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB902542 Supplemental Material1 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material2 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material3 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material4 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material5 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, JCB902542 Supplemental Material6 for Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery by Lu Jiang, Hongfeng Mu, Fei Xu, Di Xie, Wei Su, Jing Xu, Zeyu Sun, Silvia Liu, Jianhua Luo, Yejie Shi, Rehana K Leak, Lawrence R Wechsler, Jun Chen and Xiaoming Hu in Journal of Cerebral Blood Flow & Metabolism