Abstract
Microglia are key regulators of inflammatory response after stroke and brain injury. To better understand activation of microglia as well as their phenotypic diversity after ischemic stroke, we profiled the transcriptome of microglia after 75 min transient focal cerebral ischemia in 3-month- and 12-month-old male spontaneously hypertensive rats. Microglia were isolated from the brains by FACS sorting on days 3 and 14 after cerebral ischemia. GeneChip Rat 1.0ST microarray was used to profile the whole transcriptome of sorted microglia. We identified an evolving and complex pattern of activation from 3 to 14 days after stroke onset. M2-like patterns were extensively and persistently upregulated over time. M1-like patterns were only mildly upregulated, mostly at day 14. Younger 3-month-old brains showed a larger microglial response in both pro- and anti-inflammatory pathways, compared to older 12-month-old brains. Importantly, our data revealed that after stroke, most microglia are activated towards a wide spectrum of novel polarization states beyond the standard M1/M2 dichotomy, especially in pathways related to TLR2 and dietary fatty acid signaling. Finally, classes of transcription factors that might potentially regulate microglial activation were identified. These findings should provide a comprehensive database for dissecting microglial mechanisms and pursuing neuroinflammation targets for acute ischemic stroke.
Keywords: Microglia, ischemic stroke, transcriptome, phenotype, inflammatory response
Introduction
Microglia are resident immune cells of the central nervous system (CNS) and serve as critical sensors, effectors and regulators for CNS homeostasis during normal development and disease conditions.1–3 Microglia differ from macrophages that reside in other tissues based on their cell-specific gene expression signatures, distinct ontogeny and differential functions.1,4–8 In healthy brain, microglial activation is restricted and shows a more quiescent immunological profile than other tissue macrophages. They continually survey the environment and quickly respond to signs of injury and infection via phenotypic transitions.2,9–13 In the past, M1/M2 microglial polarization has been widely accepted to identify microglial activation states after stimulation, i.e. classically activated M1-like phenotype, which releases pro-inflammatory mediators to cause tissue damage, and alternatively activated M2-like phenotype, which is anti-inflammatory and involved in debris clearance and tissue regeneration.14–18 However, it is now increasingly recognized that microglia demonstrate tremendous heterogeneity beyond a simply dichotomous polarization, depending on different stages of brain development, brain regions, and response to the injury, etc.19–24
In the context of ischemic stroke, microglia participate in a multitude of pathways including complement activation and release of reactive oxygen species. During the process of stroke evolution, microglia will undergo highly dynamic phenotypic changes to regulate neuroinflammation as well as tissue regeneration.25–29 It is now clear that microglia play complex and multiphasic roles displaying both adverse and beneficial effects after ischemic stroke. However, given the diverse and complex microglial response, the molecular characterization and temporal phenotypic underpinnings of this complex process remain largely unknown. To address these questions, we performed a transcriptome analysis of microglial activation in a rat model of focal cerebral ischemia. We mapped microglial activation patterns at the acute stage (3 days) and the subacute stage (14 days) and further compared responses in young (3-month-old, 3-mon) versus middle-aged (12-month-old, 12-mon) rats. We focused on molecular and functional signatures associated with microglia activation, the phenotypic conversion from acute to subacute phases, and the influence of aging on microglia response to ischemic stroke. Importantly, we also asked whether novel microglia phenotypes other than classic M1/M2 dichotomy can be detected and the potential mediators that are responsible for the microglial phenotypic switching.
Methods
Rat cerebral ischemia
All experiments were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee following standard protocols according to the NIH Guidelines for the Care and Use of Animals in Biomedical Research as well as the ARRIVE guidelines. All studies and measurements were randomized and blinded. Transient focal cerebral ischemia was induced by 75 min monofilament occlusion of the right middle cerebral artery and reperfusion in 3-month- and 12-month-old male spontaneously hypertensive rats (SHRs) (Charles River Laboratories Wilmington, MA, USA) under 1.2% isoflurane anesthesia and with Laser Doppler monitoring. Sham animals underwent the same procedure excluding insertion of a monofilament. Rectal temperature was maintained at 37°C ± 1 with a thermostat-controlled heating pad. Right femoral arteries were cannulated to confirm that the blood pressure, pH and gases were all within normal range. We intentionally used hypertensive rats because it is now recognized that these rodent models should more closely mimic clinical stroke patients with vascular comorbidities.30–32
Isolation of microglia from the brains using FACS sorting
On day 3 and day 14 after cerebral ischemia, rats were transcardially perfused with ice-cold PBS. Brains were quickly removed, cerebrum was dissected and separated into ipsilateral and contralateral hemispheres, and then the tissue was cut into small pieces. A pre-warmed enzyme mix (Neural Tissue Dissociation Kit, Miltenyi Biotec) was added to the tissue pieces and incubated at 37°C for 15 min. The tissue was further mechanically dissociated by trituration and the suspension was applied to a 30 μm cell strainer. Myelin was removed using Myelin Removal Beads (Miltenyi Biotec). The single cells were stained with FITC-conjugated anti-CD11b and APC-conjugated anti-CD45 antibodies. The population of microglia (CD11b positive and CD45 intermediate positive) was sorted using FACS sorting (Supplementary Figure 1(a)). Microglia can be distinguished from other infiltrated cells, including macrophages, neutrophils, and dendritic cells, by CD45 expression level. CNS border-associated and monocyte-derived macrophages, neutrophils, and dendritic cells are CD45 bright positive (Supplementary Figure 1(a)). The purity of sorted microglia was determined by the abundance of expression of cell-specific genes (Supplementary Figure 1(b)). All the sorted cell samples were subjected to microarray analysis with three biological replications per group.
Transcriptome analysis
Total RNAs were extracted using miRNeasy kit (Qiagen) according to the manufacturer’s instructions. All RNA samples had initial RIN scores above 7.0. Biotinylated cDNA was prepared according to the standard Affymetrix protocol from 50 ng total RNA using amplification kit NuGEN Ovation Pico WTA System V2, followed by labeling 5 μg of cDNA using kit NuGEN Encore Biotin Module. Following fragmentation, 5 μg of cDNA were hybridized for 16 h at 45°C on GeneChip Rat 1.0 ST Array in an Affymetrix GeneChip Hybridization Oven 645. GeneChips were washed and stained in the Affymetrix Fluidics Station 450, and scanned using the Affymetrix GeneChip Scanner 3000 7 G running Affymetrix Gene Command Console ver 3.2. The data were analyzed with Affymetrix Expression Console using RMA algorithm. Raw data were processed by RMA algorithm. Microarray data are accessible from Gene Expression Omnibus database (GSE148350). Expression data were quantile normalized and probes with mean log-transformed intensity less than three were removed. With this, a total of 17,344 genes were identified and quantified by the maximum intensity of the probes assigned to each gene. Gene expression change was determined using one-way ANOVA followed by multiple testing corrections with false discovery rate (FDR) method. Genes with FDR adjusted p-value < 0.01 were considered to be differentially expressed. Post hoc Dunnett’s test was performed by comparing each sample with Sham control. Genes with p-value < 0.05 and fold change >1.5 were considered to have significant change.
Gene set enrichment analysis
Functional analysis was performed using Gene Set Enrichment Analysis (GSEA) with all the genes identified as input data. Gene ontology (GO) database and Kyoto Encyclopedia of Genes and Genomics (KEGG) database were used for annotation. Gene sets with FDR q-value <0.05 were considered as significantly changed.
Real-time PCR
Quantitative real-time PCR was used to measure the gene expression of sorted microglia isolated from ischemic and contralateral hemispheres after cerebral ischemia. Total RNAs were reverse transcribed into cDNA using high-capacity RNA-to-cDNA kit (Thermo) according to the manufacturer’s instructions. Quantitative expression of C3, C4a, Irf7, Parp8, Lgals3bp, Cxcr4, Dapp1, Lgals3, Gpnmb, Mki67, Plgs2, and Fabp4 genes were measured using gene-specific TaqMan Gene Expression Assays in triplicates (ABI 7500HT, Applied Biosystems). Fold-changes in gene expression were determined using the 2−ΔΔCt method with normalization to hypoxanthine phosphoribosyltransferase 1 and β-2-microglobulin. All real-time PCRs were performed in triplicates.
Transcriptome datasets
Transcriptome data of macrophages triggered by 28 different stimuli are obtained from GSE46903.33 Microglia transcriptome data in normal condition were obtained for mouse, rat and human from GSE77986, GSE79060, GSE115757, GSE84113, GSE119904, GSE63506 and GSE76734.34–38
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics (version 25.0). Data are presented as mean ± standard deviation (SD). Kolmogorov–Smirnov test was used for normality test. Normally distributed data were analyzed with Student’s t test, while non-normally distributed data were analyzed with Mann–Whitney U rank sum test. Z-score was calculated with the number of up-regulated genes minus the number of down-regulated genes divided by the square root of the total number of differentially expressed genes involved in each specific term. Z-scores greater than 2 or smaller than −2 were considered significant.39
Results
Transcriptome changes of microglia in response to ischemic stroke
Using FACS sorting, microglia were isolated from the ipsilateral hemisphere (ipsi) and the contralateral hemisphere (Contra) of young (3-mon) and middle-aged (12-mon) rats at the acute (3d) and the subacute (14d) stages post MCAO. Microglia isolated from brains of untreated rats were used as controls (Sham). Isolated microglia were then subjected to transcriptome analyses with three biological replicates for each group. Compared with markers for other brain and immune cells, microglia specific markers were detected at high levels in all sample tested, indicating high purity of sorted microglia (Supplementary Figure 1(b)). Under normal condition (sham group), small but significant effects of age in normal microglia were detectable, consistent with existing literature.40,41 Expression levels of complement component C3 and C4a were up-regulated with age (Figure 1(a) and (b)), and an overall age-related activation of complement cascade was observed (Figure 1(c)).39
Figure 1.
Transcriptome changes of microglia in response to stroke. (a) Genes with significant baseline difference (fold change > 1.5, FDR < 0.05) between 3-mon and 12-mon microglia. (b) Real-time PCR validation of the differential C3 and C4a expression between 3-mon and 12-mon microglia in Sham controls. (c) The expression changes of complement components in 12-mon microglia compared to 3-mon in Sham controls. (d) Principal component analysis (PCA) of transcriptome data for 3-mon and 12-mon microglia. (e) Unsupervised hierarchical clustering of differentially expressed genes. Log-transformed genes expression was compared to the mean value of that gene over all samples (row). (f) The numbers of up- and down-regulated genes induced by ischemic stroke as compared to Sham control. (g) Pearson’s correlation of gene expression change between 3-mon and 12-mon microglia isolated from ipsilateral hemisphere on 3d and 14d. Data present mean ± SD of three biological replicates. *p < 0.05; **p < 0.01; ***p < 0.001.
Principal component analysis (PCA) of transcriptome data for both 3-mon and 12-mon microglia showed high similarity between replicates within each group but high heterogeneity amongst different groups (Figure 1(d)), which are fully mirrored by unsupervised hierarchical clustering of the samples (Figure 1(e)). As shown in Supplementary Figure 2, global gene expression of the contralateral microglia was highly correlated with that of shams, while more dramatic changes were observed in the ipsilateral samples, consistent with the presence of focal ischemia. In the ipsilateral hemisphere, compared to Sham, both 3-mon and 12-mon microglia showed extensive gene expression changes on 3d post stroke, that were then gradually recovered by 14d (Figure 1(f)). The greater microglia response at 3d was consistent with patterns observed in the PCA and heatmap analyses, where transcriptome profiles appeared to partially renormalize by 14d (Figure 1(d) and (e)).
We then evaluated the influence of age on microglia activation after stroke. In general, three-month brains showed a greater reaction to cerebral ischemia, with larger numbers of genes being altered in the ipsilateral hemisphere, especially during the acute stage at 3d (Figure 1(d) to (f)). This greater response in younger brains can also be observed in the contralateral hemisphere. However, in 12-month-old brains, almost no response was detected in the contralateral side (Figure 1(f) and (g)). This effect of age on the microglia response mostly occurred at three days (Figure 1(f) and (g)).
Functional changes of microglia in response to ischemic stroke
Functional analysis revealed that microglial alterations in response to ischemic stroke were mainly involved in four major categories comprising energy generation, immune response, cell cycle regulation, and RNA metabolism. Energy generation processes were consistently activated in 3-mon and 12-mon microglia on both 3d and 14d (Figure 2(a)). Genes involved in oxidative phosphorylation and PPAR signaling were coordinately up-regulated (Figure 2(b)), which may fulfill energy and biosynthetic requirements during microglial activation.
Figure 2.
Functional alterations in response to stroke. (a) Functional alterations in response to stroke in 3-mon and 12-mon ipsi microglia on 3d and 14d. (b) Heatmaps of representative pathways involved in energy generation (oxidative phosphorylation, PPAR signaling), immune response (complement cascade, interferon response, chemokine-chemokine receptor) and cell cycle. Gene expressed was log-transformed and compared to the mean value across all the samples (row). (c) GSEA analysis shows the concordant differences in cell cycle and inflammation gene expression between 3-mon and the 12-mon microglia isolated from ipsilateral hemisphere. (d) Real-time PCR validation of distinct gene expression between 3-mon and 12-mon microglia on 3d and 14d post stroke. (e) Real-time PCR validation of the persistent upregulation genes involved in PPAR signaling (Fabp4) and microglia activation (Gpnmb, Lgals3) as well as the delayed increase of complement components (C3, C4a) and interferon response regulator (Irf7) along with its downstream transcriptional targets (Lgals3bp, Parp8, Dapp1). Data present mean ± SD of three biological replicates. *p < 0.05; **p < 0.01; ***p < 0.001.
Unlike the relatively uniform activation of energy processes, changes in immune response and cell cycle were more complex, with both time-dependent and age-dependent effects in different pathways. Complement cascades and interferon responses appeared to be delayed, with activation occurring at 14d but not at 3d (Figure 2(a) and (b)). Chemokine-related immune pathways were extensively upregulated at 3d, but mostly in 3-mon rather than in 12-mon old brains (Figure 2(a) and (b)). Overall, functional analyses of immune response suggested that younger 3-mon brains might have a higher inflammation index than older 12-mon brains. Cell cycle processes showed an opposite age effect. A greater activation of these pathways was observed in 12-mon versus 3-mon microglia, especially at the early stage on day 3 (Figure 2(a) and (b)). These results were further confirmed by Gene Set Enrichment Analysis (GSEA), which revealed a higher expression of inflammation genes and lower expression of cell cycle genes in 3-mon compared to 12-mon microglia at 3 d (Figure 2(c)). Differential expression of representative inflammation regulators (Cxcr4, COX-2) and a cell proliferation marker (Mki67) between 3-mon and 12-mon microglia on 3 d were confirmed by real-time PCR (Figure 2(d)). Real-time PCR was also used to validate the responses in representative genes related to PPAR pathway (Fabp4), microglia activation (Gpnmb, Lgals3), complement cascade (C3, C4a), and regulator of interferon response and its downstream targets (Irf7, Lgals3bp, Parp8, Dapp1) (Figure 2(e)).
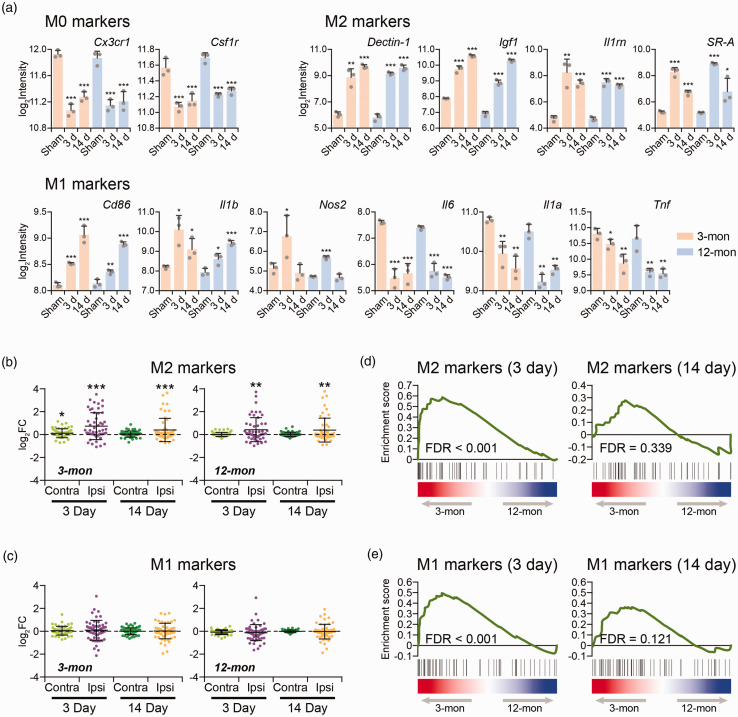
Changes in M1-like and M2-like markers in response to ischemic stroke
Since classically activated M1-like and alternatively activated M2-like phenotypes of microglia are still widely used to describe microglial activation, we first surveyed transcriptome profiles of well-known M1-like and M2-like phenotypic markers in ipsilateral microglia after focal cerebral ischemia (Table S1). In line with microglial activation, the expression of representative resting microglia (M0) markers Cx3cr1 and Csf1r was reduced after ischemic stroke (Figure 3(a)). Representative M2-like markers (Dectin-1, Igf1, Il1rn, SR-A) were up-regulated (Figure 3(a)). Representative M1-like markers were changed in an inconsistent manner; Cd86, Il1b and Nos2 were upregulated, while the production of Il6, Il1a and Tnf was suppressed (Figure 3(a)). Altogether, this analysis demonstrated that ischemic stroke induced a rapid and persistent M2-like polarization of microglia at 3d and 14d, occurring in both 3-mon and 12-mon old brains (Figure 3(b)), whereas no significant overall induction of M1-like polarization (Figure 3(c)). Moreover, a comparison between 3-mon and 12-mon samples revealed significantly higher expression of both M1-like and M2-like markers in the 3-mon samples compared to the 12-mon samples on 3d rather than on 14d (Figure 3(d) and (e)), indicating a greater overall activation of microglia during the acute stage in younger brains. This was consistent with the higher inflammation response in the three-month microglia (Figure 2(c)).
Figure 3.
Expression changes of the M1 and M2 phenotypic markers in response to stroke. (a) Expression levels of representative M0, M1 and M2 markers. (b) The expression change of M2 markers in each sample versus the Sham control in 3-mon and 12-mon microglia. (c) The expression change of M1 markers in each sample versus the Sham control in 3-mon and 12-mon microglia. Each dot in (b) and (c) represents a particular M1 or M2 marker gene as listed in Supplementary Table 1. The p values were determined by comparing the expression of all the marker genes in a specific sample to Sham control with paired Student’s t-test. (d) GSEA analysis shows the differences in M2 marker expression between the ipsi microglia obtained from the 3-mon and the 12-mon rats on 3d and 14d. (e) GSEA analysis shows the differences in M1 marker expression between the 3-mon and the 12-mon microglia on 3d and 14d. Data present mean ± SD of three biological replicates. *p < 0.05; **p < 0.01; *** p < 0.001.
CIBERSORT dissection of stroke-induced microglia response beyond M1/M2
Recently, growing evidence has revealed that microglia activation in pathological conditions is extremely diverse, and a variety of intermediate phenotypes beyond the traditional M1 versus M2 dichotomy exist to modulate neuroinflammation.42 To get a broader and unbiased understanding of stroke-induced microglia activation, we compared our data with the transcriptome data of macrophages activated by 28 different stimuli, including various ligands, cytokines, and metabolic cues that are all highly linked to stroke.33 CIBERSORT analysis43 was then performed to enumerate the proportion of microglia subsets subjected to each individual stimulus in the mixed population.
Consistent with stroke-induced microglia activation, a dramatic loss of resting microglia (M0) signals was observed in the ipsilateral but not in the contralateral microglia (Figure 4(a)). Moreover, both down-regulated (IFN-γ, sLPS+IC) and up-regulated (sLPS, sLPS+IFN-γ) signals activated by M1-type stimuli in ipsilateral microglia were identified (Figure 4(a)). When grouping the stimuli together, overall M1-like signals showed no significant changes (Figure 4(b)). In contrast, signals activated by M2-type stimuli (IL13, IL10) were up-regulated in both 3-mon and 12-mon ipsilateral microglia on 3d and 14d (Figure 4(a)), indicating an overall and persistent activation of M2 phenotype (Figure 4(b)). Of note, 3-mon microglia showed higher proportion of both M1 and M2-like phenotypes than 12-mon microglia on 3d (Figure 4(b)), which was in line with the observation that younger brains showed more enhanced microglia activation after stroke (Figure 3). Taken together, the dynamics of M0, M1 and M2 microglia quantified by CIBERSORT is highly consistent with that measured by standard microglia markers in Figure 3, suggesting the accuracy of CIBERSORT enumeration.
Figure 4.
Traditional M0, M1 and M2-like microglia phenotypes as enumerated by CIBERSORT. (a) The heatmap shows the changes in the proportion of resting microglia (M0) and microglia phenotypes activated by classic M1 and M2 stimuli, as enumerated by CIBERSORT, in each sample versus the Sham control. (b) The proportions of the microglia subtypes (M0, M1 and M2) in different samples are shown. The data present mean values of replicates within the same group. *p < 0.05; **p < 0.01; ***p < 0.001.
Although CIBERSORT confirmed clear patterns in M1-like and M2-like microglia after stroke, the largest proportion of microglia exhibits phenotypes other than traditional M1 and M2 (Figure 5(a)). These included phenotypes activated by the Tlr2 ligand nPam3CSK4 (P3C) and various dietary fatty acids, such as lauric acid (LA), stearic acid (SA), oleic acid (OA), linoleic acid (LiA), which accounted for a large fraction of microglia even in normal brain in the Sham control group (Figure 5(b)). The importance of these non-M0/M1/M2 populations was noted by the overexpression of signature genes induced by P3C and dietary fatty acid stimuli33 compared to the whole transcriptome of microglia (Figure 5(c) and Table S2). To further dissect these microglia groups, we examined publicly available microglia transcriptome databases from normal mouse, rat and human brain.34–38 CIBERSORT analysis showed that subsets activated by Tlr2 ligand and dietary fatty acids were found to exist in a large proportion across all mouse, rat and human species (Figure 5(d)).
Figure 5.
Microglia polarization states other than traditional M1/M2 phenotypes as enumerated by CIBERSORT. (a) The predicted composition of resting M0-like microglia and microglia triggered by M1-like, M2-like and other stimuli in each replicate is shown. (b) The proportion of each phenotype detected in Sham control for 3-mon and 12-mon microglia. (c) The expression level of microglia transcriptome and signature genes specific to P3C (Tlr2 ligand) and dietary fatty acids (LA, SA, OA, LiA, PA) in Sham controls. (d) The proportion of each microglia phenotype in different species (mouse, rat and human) at normal condition. (e) The heatmap shows the changes of other microglia phenotypes in each sample versus the Sham control, as enumerated by CIBERSORT. (f) The proportion of Tlr2 ligand and dietary fatty acids-activated microglia subtypes as well as the expression of representative signature genes in different samples. *p < 0.05; **p < 0.01; ***p < 0.001.
Moreover, in response to ischemic stroke, down-regulated signals were observed for Tlr2 ligand-activated phenotype in both 3-mon and 12-mon microglia (Figure 5(e)). Down-regulation of Tlr2 signaling was confirmed by a decreased expression of the Tlr2 gene as well as reduction of the signature genes specific to P3C stimulation (Figure 5(f)). In contrast, microglia subsets activated by dietary fatty acids showed inconsistent patterns after stroke. Signals for saturated fatty acid (lauric acid, LA)-induced phenotype were decreased in both 3-mon and 12-mon microglia, particularly on 14d, while unsaturated fatty acid (oleic acid, OA)-activated microglia showed decreased signal in 3-mon microglia but was up-regulated in 12-mon sample on 3d (Figure 5(e)). Altogether, ischemic stroke was associated with a general loss of fatty acid-induced microglia, and genes involved in saturated and unsaturated fatty acid processing, such Hmgcs1, Acsl3, Alox5 and Degs1, showed decreased expression in both 3-mon and 12-mon microglia (Figure 5(f)).
Transcription factors analysis
Transcription factors (TFs) regulate gene expression. Therefore, we systematically analyzed TF families that were significantly changed in microglia after ischemic stroke. The TF targets database (Molecular Signature Database, MSigDB) was used to identify TFs for all differentially expressed genes in ipsilateral hemispheres. TFs enriched with differentially expressed targets were connected with each other based on their correlations, determined as the percentage of common targets shared by two TFs (Figure 6(a)). Based on the z-scores calculated with the number of up- and down-regulated targets, the changes of transcriptional activity were also determined for each TF (Figure 6(b)). Four clusters of TFs were found to regulate gene expression in ischemic stroke (Figure 6(a) and (b)). TFs in Cluster I comprised members of the E2F family, which are responsible for regulating the expression of genes involved in cell cycle regulation and synthesis of DNA. TFs in Clusters II and IV were involved in regulating diverse biological functions (Figure 6(a) and (b)). For example, in Cluster II, MYC drives cell proliferation and regulates cell growth, differentiation and apoptosis, while ARNT may modulate cellular response to hypoxia. In addition, HNF3 in Cluster IV is responsible for modulating glucose and lipid metabolism, and ELK is expressed at high level in brain and plays a crucial role in depression, long-term memory, drug addiction and Alzheimer’s disease.
Figure 6.
Transcription factors (TFs) analysis. (a) Correlation network of four clusters of TFs responsible for regulating microglial phenotypes in ischemic stroke. Edge width stands for the percentage of shared targets between each TF pair; (b) Hierarchical clustering of TFs based on the z-scores of their transcriptional targets; (c) The exact z-score changes of IRFs in cluster III TFs; (d) The expression change of Irf7; (e) The expression change of Irf7 transcriptional targets.
In terms of inflammation, Cluster III may be important since it comprised interferon-related TFs (ISRE, IRFs) (Figure 6(a)), which were increased on day 14 compared with day 3 after cerebral ischemia (Figure 6(b) and (c)). Among Cluster III, Irf7 showed the most significant change. The expression of IRF7 was not significantly affected by stroke on day 3 but was largely increased on day 14 (Figure 6(d)) and an identical changing pattern was also found for its transcriptional activity (Figure 6(c)). On day 3 after ischemia, around 45% of IRF7 transcriptional targets were significantly up-regulated, which were offset by the similar level (around 55%) of the down-regulated targets in both 3-month and 12-month old rats (Figure 6(e)). However, on day 14, an increase of up-regulated targets was identified in both 3- and 12-month old rats (81.0% for 3 month and 78.9% for 12 month), indicating the elevated transcriptional activity of IRF7 (Figure 6(c) and (e)). Further dissection of TF response networks may enable alternate ways to interpret microglial response in the future.
Discussion
Neuroinflammation is a major part of stroke pathophysiology and microglia play a central role in this process.25–29 Therefore, a comprehensive mapping of microglial responses is essential for any effort to dissect stroke mechanisms and develop stroke therapeutics. In this study, we performed a transcriptomic mapping of microglial responses in a rat model of focal cerebral ischemia. Our data show that (i) acute cerebral ischemia induces a complex activation of microglia that evolves from 3 to 14 days after onset; (ii) microglia activation is more dramatic in younger 3-month compared to older 12-month brains; (iii) M2-like patterns are extensively and persistently upregulated early (3 days) and late (14 days), whereas M1-like patterns are only mildly upregulated at day 14; and (iv) most microglia are activated towards novel polarized states beyond M1/M2 dichotomy, especially in pathways related to TLR2 and dietary fatty acid signaling. The present findings should provide a comprehensive database that can be potentially leveraged for future hypothesis-testing investigations into mechanisms and therapeutic targets for stroke.
In aging, the activities of microglia become dysregulated, and dysregulated microglia are implicated in the pathophysiology of neurodegenerative diseases. Aged microglia display increased immune vigilance and reduced phagocytic capacity.44 On the one hand, microglial priming and increased immune sensitivity to stimuli engage in excessive synaptic pruning that causes loss of viable synaptic connections. On the other hand, microglia exhibit insufficient phagocytic activity toward apoptotic bodies, protein aggregates and myelin. Both insufficient phagocytic activity and excessive synaptic pruning contribute to age-related cognitive impairments. Recent study revealed complement-dependent synapse elimination by microglia as an underlying mechanism of the forgetting of remote memories.45 Inhibition of microglial phagocytosis by inhibiting complement pathways prevented forgetting and the dissociation of engram cells. Our study demonstrated an overall activation of complement cascade in aged microglia (12 month) compared with the young microglia (3 month), consistent with dysregulated microglial functions with aging.
The pattern of stroke-induced microglia response appeared to fall within four categories including energy generation, immune response, cell cycle regulation, and RNA metabolism. Some pathways were closely connected. For example, oxidative phosphorylation and PPAR signaling are known to be the major energy generation pathways for M2 polarization.46 Therefore, a significant upregulation in oxidative phosphorylation and PPAR signaling may be consistent with the prolonged activation of M2 phenotype in microglia from 3 to 14 days in both young and older brains. Conversely, some pathways may be disconnected. For example, the early three-day activation pattern differs in young versus older brains. Cell cycle pathways show a larger response in older 12-month brains, whereas overall inflammation pathways show a larger response in younger 3-month brains. Overall, the pattern of microglial activation after cerebral ischemia was highly complex, evolving with time after onset in age-dependent ways.
The incidence of stroke increases with age, so our comparisons of young versus older microglia may be informative. In normal sham controls, slightly higher M1-like signals were already detectable in both 3-month and 12-month samples. This may be because hypertensive rats were used in order to closely mimic stroke patient,30–32 which may reflect a higher neuroinflammatory baseline in hypertensive individuals.47 However, somewhat surprisingly, inflammation pathways show a larger response in younger compared to older brains after stroke. At first glance, these data may seem counter-intuitive because neuroinflammation and injury severity are worse in older patients and animal models. A closer look at our data may offer a potential explanation. M1 patterns were mildly activated only by day 14. In contrast, M2 patterns showed a strong and persistent upregulation at both 3 and 14 days. The early activation of M2 microglia and delayed increase of M1 microglia are consistent with previous studies in stroke,48 suggesting the overall activation of microglia after stroke, at least in the present animal model, appears to comprise potentially beneficial pro-recovery responses. Hence, a larger response in younger brains may be related to improved CNS remodeling and recovery.49–51
Although the M1–M2 dichotomy has long served as the most common paradigm for microglia activation,29,48,52 perhaps the most important finding in our study is the observation that the vast majority of microglial phenotypes after cerebral ischemia fall outside of M1/M2 categories. We used CIBERSORT to enumerate different phenotypes of activated microglia by using a training dataset of macrophages triggered by 28 different stimuli,33 including classic M1 (IFNγ, LPS, TNF) and M2 (IL4, IL10, IL13) stimuli, as well as many other stimuli, including Tlr2 ligand (P3C), dietary fatty acid (LA, OA, LiA, SA), lipid compounds (HDL) and stimuli associated with chronic inflammation (TPP). Our analysis thus provided a conceptual framework which revealed the heterogeneity of microglia after cerebral ischemia and identified various phenotypes of activated microglia extending beyond the M1-like/M2-like phenotypic transition model. Among the non-M1/M2 phenotypes of microglia, we found that Tlr2 ligand and dietary fatty acids activated microglia existed at high prevalence, even in normal conditions. These novel categories of microglia activation showed dramatic and dynamic changes in response to stroke. Recently, dietary fatty acids have been increasingly recognized as a crucial regulator of normal brain function and are implicated in various neurological disorders.53–57 Diet rich in saturated fatty acid, such as palmitic acid, has been found to promote inflammation and produce neuronal stress in part, by activating microglia, and these mechanisms have been implicated in the pathogenesis of many brain disorders, such as Alzheimer’s disease, traumatic brain injury.56–59 In contrast, unsaturated fatty acids, such as omega-3, linoleic acid, oleic acid, have showed potential to attenuate inflammation and exert neuroprotective function.55–57 In the context of cerebral ischemia, dietary fatty acid oleic acid was found to exert neuroprotective function by activating PPARγ signaling pathway and inhibiting the production of COX-2, iNOS and TNFα.60 Omega-3 polyunsaturated fatty acids can also protect against ischemic brain injury by potentiating microglial M2 activation of microglia and suppressing NF-κB-mediated inflammation in microglia.61,62 All the findings suggest the functional importance of dietary fatty acids in regulating microglia activation and neuroinflammation. More importantly, since dietary composition of fatty acids can be easily modified through diet, understanding the underlying mechanism may provide novel and simple methods to prevent stroke and many other neurological diseases and promote functional recovery in patients.
Another novel non-M1/M2 microglial phenotype identified by CIBERSORT is associated with decreased Tlr2 signaling after ischemia. Toll-like receptors (TLRs) belong to a class of pattern recognition receptors that play an important role in host defense against pathogens and in responses to non-infectious inflammation.63 Microglial TLR2 plays a role in mediating pathological processes in many neurological diseases. It was reported that early minor stimulation of microglial TLR2 receptor attenuates cognitive deficit by regulating the inflammatory and anti-inflammatory phenotypes of microglia and enhancing microglial ability to remove Abeta from the brain parenchyma.64 However, other studies showed that inhibiting TLR2 activation decreased microglial activation, reduced Abeta accumulation, and improved cognitive performance.65,66 Anti-TLR2 antibody can restore phagocytic function by triggering oxidative phosphorylation and reducing inflammasome activation in microglia.67 Therefore, TLR2-mediated inflammation is complex and dependent on context. Furthermore, there may be overlap between TLR2 pathways and classically formulated M1/M2 pathways. For example, Tlr signaling is known to mediate microglia polarization,68,69 and Tlr2 activation is capable of attenuating the anti-inflammatory potential of M2-like macrophages.70 Our data showed that overall Tlr2 signaling is decreased after cerebral ischemia, thus potentially allowing for an increase in beneficial M2-like responses. However, this Tlr2 downregulation is smaller in older 12-month brains. Lower Tlr2 responses in older brains may therefore lead to a dampened M2-like response that in turn may be related to less remodeling and recovery after stroke. How these new microglial phenotypes modulate the activity of microglia, which in turn, lead to protective or deleterious effects on neurons or other cells, is unclear. Further functional studies are necessary to elucidate the role of diversity of microglia in the stroke and other neurological diseases.
There are a few caveats in the present study. First, our data indicated that microglial cells comprised heterogeneous populations after activation in response to cerebral ischemia. Although we separated the cerebrum into ipsilateral and contralateral hemispheres and compared the difference of microglial responses after cerebral ischemia between the hemispheres in the present study, the microglial activation may be different in brain regions differently affected by the ischemia, especially between the infarct core, peri-infarct, and the relatively healthy tissue far from the infarct core. Subdividing the microglial responses according to the brain regions will provide further information to better understand the roles of microglia after ischemic stroke. Second, we used spontaneous hypertensive rats instead of normotensive rats in the present study because hypertension is one of the major risk factors for stroke. Evidence suggests that interplay between bone marrow, microglia and immune mediators underlies the development of arterial hypertension.71 Hypertension may affect the status of microglia under normal condition as well as the microglial responses to the insults. Future studies that compare the difference between hypertensive and normotensive animals will allow us to comprehensively understand the influences of hypertension on neuroinflammation after stroke. Third, our findings of microglial transcriptomic profiling after ischemic stroke are obtained from the animal models. However, human microglia possess unique features as compared to rodent microglia at molecular and functional levels as well as the responses to the insults.72,73 Further comparisons of the different responses of microglia in humans versus rodent models are needed to elucidate the roles of microglia in stroke patients. Fourth, although we verified the changes of some key genes using real-time PCR, we did not quantify protein or functional responses. Further studies are warranted to rigorously map the functional heterogeneity of microglia in both physiological and pathological conditions. Finally, it will be important to look for connections between ischemic profiles described in this study and microglial signatures that are being proposed for neurodegeneration in general.74
Microglia are key mediators of the neuroinflammatory pathophysiology after stroke. Our study provides a comprehensive database of various phenotypes of activated microglia in a rat model of focal cerebral ischemia. Taken together, these findings suggest that a rigorous dissection of activation pathways in this database may offer clues for nuanced approaches to modulate rather completely inhibit microglia for therapeutic gain.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20932870 for Transcriptomic characterization of microglia activation in a rat model of ischemic stroke by Wenjun Deng, Emiri Mandeville, Yasukazu Terasaki, Wenlu Li, Julie Holder, Aaron TT Chuang, Mingming Ning, Ken Arai, Eng H Lo and Changhong Xing in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-2-jcb-10.1177_0271678X20932870 for Transcriptomic characterization of microglia activation in a rat model of ischemic stroke by Wenjun Deng, Emiri Mandeville, Yasukazu Terasaki, Wenlu Li, Julie Holder, Aaron TT Chuang, Mingming Ning, Ken Arai, Eng H Lo and Changhong Xing in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the NIH, AHA, and the Rappaport Foundation.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: CX, EHL, and WD designed the research; EM, YT, CX, and WL performed the experiments; WD, CX, EHL, JH, ATC, KA, and MN analyzed data; WD, CX and EHL and wrote the paper.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014; 15: 300–312. [DOI] [PubMed] [Google Scholar]
- 2.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007; 10: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318. [DOI] [PubMed] [Google Scholar]
- 4.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13: 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butovsky O, Siddiqui S, Gabriely G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 2012; 122: 3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kierdorf K, Erny D, Goldmann T, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 2013; 16: 273–280. [DOI] [PubMed] [Google Scholar]
- 8.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012; 336: 86–90. [DOI] [PubMed] [Google Scholar]
- 9.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–758. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 11.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci 2008; 31: 653–659. [DOI] [PubMed] [Google Scholar]
- 12.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009; 27: 119–145. [DOI] [PubMed] [Google Scholar]
- 13.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature 2010; 468: 253–262. [DOI] [PubMed] [Google Scholar]
- 14.Barone FC, Arvin B, White RF, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 1997; 28: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 15.Lambertsen KL, Meldgaard M, Ladeby R, et al. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2005; 25: 119–135. [DOI] [PubMed] [Google Scholar]
- 16.Minami M, Kuraishi Y, Yabuuchi K, et al. Induction of interleukin-1 beta mRNA in rat brain after transient forebrain ischemia. J Neurochem 1992; 58: 390–392. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest 1997; 100: 2648–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green SP, Cairns B, Rae J, et al. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab 2001; 21: 374–384. [DOI] [PubMed] [Google Scholar]
- 19.Matcovitch-Natan O, Winter DR, Giladi A, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016; 353: aad8670. [DOI] [PubMed] [Google Scholar]
- 20.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 2016; 19: 987–991. [DOI] [PubMed] [Google Scholar]
- 21.Silvin A, Ginhoux F. Microglia heterogeneity along a spatio-temporal axis: more questions than answers. Glia 2018; 66: 2045–2057. [DOI] [PubMed] [Google Scholar]
- 22.Hammond TR, Dufort C, Dissing-Olesen L, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 2019; 50: 253–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Cheng Z, Zhou L, et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 2019; 101: 207–223.e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda T, Sankowski R, Staszewski O, et al. Microglia heterogeneity in the single-cell era. Cell Rep 2020; 30: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 25.Lalancette-Hebert M, Gowing G, Simard A, et al. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 2007; 27: 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura Y, Takata K, Inden M, et al. Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci 2004; 94: 203–206. [DOI] [PubMed] [Google Scholar]
- 27.Imai F, Suzuki H, Oda J, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab 2007; 27: 488–500. [DOI] [PubMed] [Google Scholar]
- 28.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci 2008; 9: 481–493. [DOI] [PubMed] [Google Scholar]
- 29.Zhao SC, Ma LS, Chu ZH, et al. Regulation of microglial activation in stroke. Acta Pharmacol Sin 2017; 38: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan J, Esposito E, Ayata C, et al. Different effects of normobaric oxygen in normotensive versus hypertensive rats after focal cerebral ischemia. Stroke 2018; 49: 1534–1537. [DOI] [PubMed] [Google Scholar]
- 31.Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab 2018; 38: 2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan J, Guo S, Shi J, et al. Effects of hypertension and exercise in the brain and heart vasculome of rats. Condition Med 2019; 2: 63–74. [Google Scholar]
- 33.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40: 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arumugam TV, Manzanero S, Furtado M, et al. An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab 2017; 37: 2098–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoller T, Schneider A, Kleimeyer C, et al. Silencing of TGFbeta signalling in microglia results in impaired homeostasis. Nat Commun 2018; 9: 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Li YJ, Wu XY, et al. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J Neurochem 2015; 132: 713–723. 2014/12/30. DOI: 10.1111/jnc.13021. [DOI] [PubMed] [Google Scholar]
- 37.Healy LM, Perron G, Won SY, et al. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J Immunol 2016; 196: 3375–3384. [DOI] [PubMed] [Google Scholar]
- 38.Owens R, Grabert K, Davies CL, et al. Divergent neuroinflammatory regulation of microglial TREM expression and involvement of NF-kappaB. Front Cell Neurosci 2017; 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer A, Green J, Pollard J, Jr., et al. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014; 30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho K. Emerging roles of complement protein C1q in neurodegeneration. Aging Dis 2019; 10: 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zabel MK, Kirsch WM. From development to dysfunction: microglia and the complement cascade in CNS homeostasis. Age Res Rev 2013; 12: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol 2019; 4: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen B, Khodadoust MS, Liu CL, et al. Profiling tumor infiltrating immune cells with CIBERSORT. Meth Mol Biol 2018; 1711: 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deczkowska A, Amit I, Schwartz M. Microglial immune checkpoint mechanisms. Nat Neurosci 2018; 21: 779–786. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Yue H, Hu Z, et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020; 367: 688–694. [DOI] [PubMed] [Google Scholar]
- 46.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol 2014; 5: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marvar PJ, Lob H, Vinh A, et al. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol 2011; 11: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 49.Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature 2004; 429: 883–891. [DOI] [PubMed] [Google Scholar]
- 50.Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron 2013; 80: 729–741. [DOI] [PubMed] [Google Scholar]
- 51.Sophie Su YR, Veeravagu A, Grant G. Neuroplasticity after traumatic brain injury In: Laskowitz D and Grant G (eds) Translational research in traumatic brain injury. Boca Raton, FL: CRC Press, 2016, pp.163–178. [Google Scholar]
- 52.Ma Y, Wang J, Wang Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol 2017; 157: 247–272. [DOI] [PubMed] [Google Scholar]
- 53.Tracy LM, Bergqvist F, Ivanova EV, et al. Exposure to the saturated free fatty acid palmitate alters BV-2 microglia inflammatory response. J Mol Neurosci 2013; 51: 805–812. [DOI] [PubMed] [Google Scholar]
- 54.Kim SM, McIlwraith EK, Chalmers JA, et al. Palmitate induces an anti-inflammatory response in immortalized microglial BV-2 and IMG cell lines that decreases TNFalpha levels in mHypoE-46 hypothalamic neurons in co-culture. Neuroendocrinology 2018; 107: 387–399. [DOI] [PubMed] [Google Scholar]
- 55.Tu TH, Kim H, Yang S, et al. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem Biophys Res Commun 2019; 513: 201–206. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Chen C, Fan S, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation 2018; 15: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chausse B, Kakimoto PA, Caldeira-da-Silva CC, et al. Distinct metabolic patterns during microglial remodeling by oleate and palmitate. Biosci Rep 2019; 39: BSR20190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Liu D, Wang F, et al. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr 2012; 107: 229–241. [DOI] [PubMed] [Google Scholar]
- 59.Valdearcos M, Robblee MM, Benjamin DI, et al. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 2014; 9: 2124–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song J, Kim YS, Lee DH, et al. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci Rep 2019; 9: 10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Pu H, Hu X, et al. A post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl Stroke Res 2016; 7: 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W, Hu X, Yang W, et al. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke 2010; 41: 2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayward JH, Lee SJ. A decade of research on TLR2 discovering its pivotal role in glial activation and neuroinflammation in neurodegenerative diseases. Exp Neurobiol 2014; 23: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pourbadie HG, Sayyah M, Khoshkholgh-Sima B, et al. Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer’s disease-related cognitive deficit in rats: behavioral, molecular, and electrophysiological evidence. Neurobiol Aging 2018; 70: 203–216. [DOI] [PubMed] [Google Scholar]
- 65.McDonald CL, Hennessy E, Rubio-Araiz A, et al. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav Immun 2016; 58: 191–200. [DOI] [PubMed] [Google Scholar]
- 66.Costello DA, Carney DG, Lynch MA. alpha-TLR2 antibody attenuates the Abeta-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav Immun 2015; 46: 70–79. [DOI] [PubMed] [Google Scholar]
- 67.Rubio-Araiz A, Finucane OM, Keogh S, et al. Anti-TLR2 antibody triggers oxidative phosphorylation in microglia and increases phagocytosis of beta-amyloid. J Neuroinflammation 2018; 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 2005; 175: 4320–4330. [DOI] [PubMed] [Google Scholar]
- 69.Fiebich BL, Batista CRA, Saliba SW, et al. Role of microglia TLRs in neurodegeneration. Front Cell Neurosci 2018; 12: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quero L, Hanser E, Manigold T, et al. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther 2017; 19: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calvillo L, Gironacci MM, Crotti L, et al. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat Rev Cardiol 2019; 16: 476–490. [DOI] [PubMed] [Google Scholar]
- 72.Xu R, Li X, Boreland AJ, et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun 2020; 11: 1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du Y, Deng W, Wang Z, et al. Differential subnetwork of chemokines/cytokines in human, mouse, and rat brain cells after oxygen-glucose deprivation. J Cereb Blood Flow Metab 2017; 37: 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell 2017; 169: 1276–1290. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20932870 for Transcriptomic characterization of microglia activation in a rat model of ischemic stroke by Wenjun Deng, Emiri Mandeville, Yasukazu Terasaki, Wenlu Li, Julie Holder, Aaron TT Chuang, Mingming Ning, Ken Arai, Eng H Lo and Changhong Xing in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-2-jcb-10.1177_0271678X20932870 for Transcriptomic characterization of microglia activation in a rat model of ischemic stroke by Wenjun Deng, Emiri Mandeville, Yasukazu Terasaki, Wenlu Li, Julie Holder, Aaron TT Chuang, Mingming Ning, Ken Arai, Eng H Lo and Changhong Xing in Journal of Cerebral Blood Flow & Metabolism