Abstract
Senescence-associated alterations in microglia may have profound impact on cerebral homeostasis and stroke outcomes. However, the lack of a transcriptome-wide comparison between young and aged microglia in the context of ischemia limits our understanding of aging-related mechanisms. Herein, we performed RNA sequencing analysis of microglia purified from cerebral hemispheres of young adult (10-week-old) and aged (18-month-old) mice five days after distal middle cerebral artery occlusion or after sham operation. Considerable transcriptional differences were observed between young and aged microglia in healthy brains, indicating heightened chronic inflammation in aged microglia. Following stroke, the overall transcriptional activation was more robust (>13-fold in the number of genes upregulated) in young microglia than in aged microglia. Gene clusters with functional implications in immune inflammatory responses, immune cell chemotaxis, tissue remodeling, and cell-cell interactions were markedly activated in microglia of young but not aged stroke mice. Consistent with the genomic profiling predictions, post-stroke cerebral infiltration of peripheral immune cells was markedly decreased in aged mice compared to young mice. Moreover, post-ischemic aged microglia demonstrated reduced interaction with neighboring neurons and diminished polarity toward the infarct lesion. These alterations in microglial gene response and behavior may contribute to aging-driven vulnerability and poorer recovery after ischemic stroke.
Keywords: Aging, Clec7a, flow cytometry, ischemic stroke, permanent cerebral ischemia, RNA sequencing
Introduction
Microglia are the principal resident immune cells of the central nervous system (CNS), accounting for 5–12% of total brain cells varying in different brain regions.1 Microglia possess highly specialized plasticity with the capability of adapting to different local microenvironments.2–6 Aging markedly increases CNS inflammatory signatures, implicating that aged microglia have a sensitized phenotype.7–9 Notably, exposure to such aging-afforded neuroinflammatory environment has been shown to change the transcriptome profile of microglia in both human and rodents.10,11 Several studies examining aging-associated transcriptional changes in microglia mainly focus on neurodegenerative diseases and demyelination disorders.12,13 However, few studies have explored senescence-associated alterations in microglia in the context of ischemic or hemorrhagic stroke.
Aging is not only an independent risk factor for stroke incidence14,15 but also a major negative determinant for stroke outcomes, as advanced ages are associated with poorer neurological recovery in stroke patients and in animal stroke models.16–18 Although known factors such as increased co-morbidities likely contribute to aging-driven poorer outcomes after ischemic stroke,19 senescence-associated alteration in microglial functions has emerged as a potential new mechanism.18,20,21 Studies examining inflammation-phenotypic (pro- vs. anti-inflammatory) markers in stroke models reveal a reduced anti-/pro-inflammatory ratio in microglia of aged mice compared to young adult mice,18 suggesting that aged microglia shift towards a pro-inflammatory and detrimental phenotype after cerebral ischemia. However, such data must be interpreted with great cautions. The assessment of one or several specific markers might not accurately recognize the overall inflammatory phenotypes of post-ischemic microglia. Moreover, microglia possess other functional properties such as production and release of trophic and angiogenic factors that could have important impact on cerebral homeostasis and stroke outcomes.22
To understand the aging effect on microglial genomic responses and its potential influence on stroke outcomes, we performed genome-wide transcriptional profiling of microglia purified from cerebral hemispheres of young adult and aged mice under both healthy and post-ischemic conditions. The current study aimed to determine: (1) senescence-associated alterations in microglial genomic profiles and their potential contribution to cerebral homeostasis in healthy aged mice; (2) age-specific genomic alterations in microglia after cerebral ischemia with functional implications of these cells in ischemic brain injury; (3) senescence-associated alterations in microglial genomic profiles and functional impairments that lead to worsened stroke recovery in aged mice.
Materials and methods
Methodological details beyond the descriptions below are provided in Supplementary Materials. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, performed in accordance with the Guide for the Care and Use of Laboratory Animals, and reported in accordance with the ARRIVE guidelines.
Male young adult (10 weeks old) and aged (18 months old) C57BL/6 mice were subjected to focal cerebral ischemia induced by permanent occlusion of the left distal middle cerebral artery (MCA) and left common carotid artery (CCA).18 To investigate age-related transcriptome differences in microglia in response to ischemic stroke, microglia were extracted from young and aged mouse brains by fluorescence-activated cell sorting (FACS) five days after brain ischemia or after sham procedures, and subjected to bulk RNA sequencing (RNA-seq). Infiltration of various peripheral immune cells into the post-stroke brain was assessed by flow cytometry.23 Interaction between microglia and neighboring cells was examined in young and aged mouse brains after stroke by immunostaining and image analyses with the Imaris software.
All RNA-seq data are deposited at GEO (GSE145265). All statistics are summarized in Supplementary Table 1.
Results
Young and aged microglia acquire distinct morphological features in response to ischemic stroke
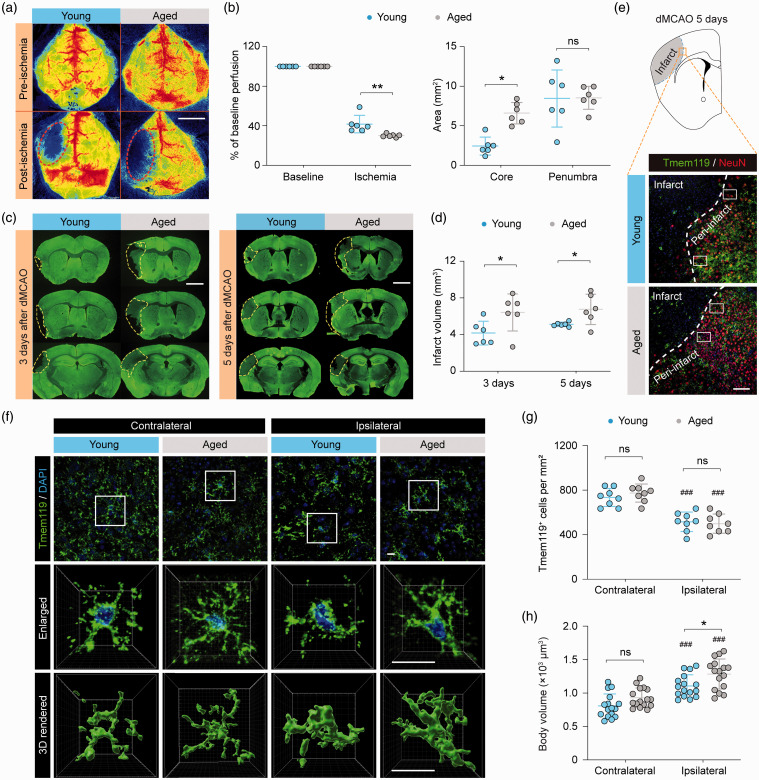
To induce ischemic stroke in both young adult (10 weeks old) and aged (18 months old) mice, we adopted a tandem occlusion model consisting of left distal middle cerebral artery occlusion (dMCAO) and left common carotid artery occlusion (referred to as “dMCAO” thereafter). dMCAO resulted in greater reduction of cortical cerebral blood flow (CBF) and a larger ischemic core in aged mice than in young mice (Figure 1(a) and (b)), consistent with previous reports.18 Furthermore, aged mice developed larger brain infarct than young mice as measured three and 5fivedays after dMCAO (Figure 1(c) and (d)). These data indicated that aged mice are more susceptible to dMCAO-induced ischemic brain injury.
Figure 1.
Morphological differences between young and aged microglia in response to ischemic stroke. Young adult (8–10 weeks old) and aged (18 months old) mice were subjected to dMCAO. (a,b) Cortical CBF was monitored by two-dimensional laser speckle imaging. (a) Representative laser speckle images show cortical CBF before (baseline) and 10 min after dMCAO. Dashed lines: the approximate boundaries of the ischemic area. (b) Summarized data on mean cortical CBF (left panel) and areas of the ischemic core (CBF reduction of >70% of baseline) and penumbra (CBF reduction of 50–70% of baseline; right panel). n = 6 mice per group. (c) Brain infarct volumes were assessed three and five days after dMCAO on MAP2 (green)-immunostained coronal brain sections. Dashed lines demarcate infarct in the left hemisphere. n = 6 mice per group. (e–g) Microglial morphology was assessed five days after dMCAO on Tmem119-immunostained brain sections. (e) Representative images of Tmem119 and NeuN double-label immunofluorescence depict the peri-infarct regions in the ipsilesional hemisphere (rectangles) where images in (f) were captured. Dashed line: the approximate infarct boundary defined by the dramatic reduction of NeuN immunofluorescence. (f) Representative images showing Tmem119 immunofluorescence in the ipsilesional peri-infarct areas and in the corresponding regions in the non-injured contralateral hemisphere five days after dMCAO. Cells were counterstained with DAPI for nuclear labeling. Rectangles: regions that were enlarged (2nd row) and 3D-rendered by Imaris (3rd row). (g,h) The numbers and body volumes of Tmem119+ cells were quantified. n = 8 mice per group (g) or 16 cells from 4 mice per group (h). ###P<0.001 vs. contralateral side. *P<0.05, **P<0.01, aged vs. young. ns, no significant difference. Scale bars: 4 mm (a), 2 mm (c), 100 μm (e), and 10 μm (f).
It is well known that normal aging induces chronic low-level inflammation and increases microglial reactivity in the homeostatic brain.24 However, whether young and aged microglia respond differently to ischemic brain injury remains poorly understood. Previous studies indicate that differences in microglial phenotypes between young and aged mice likely contribute to the deterioration of long-term stroke outcome in aged mice, under comparable chronic infarct sizes.18 We examined the responses of microglia in young and aged mouse brains five days after dMCAO, using immunofluorescence staining of a specific microglial marker Tmem119.25 In all brain sections double-labeled with Tmem119 and the neuronal marker NeuN, cortical infarct was easily identified based on the dramatically decreased density of NeuN immunofluorescence-positive cells (Figure 1(e)). Tmem119 immunofluorescence was readily detectable in all areas examined except within the infarct, where Tmem119 was drastically downregulated (Figure 1(e)). Therefore, Tmem119 immunofluorescence was analyzed mainly in the peri-infarct areas in all subsequent experiments. In the non-injured contralateral hemisphere, the numbers of Tmem119+ microglia were comparable between young and aged mice, and young and aged microglia had similar cell body volumes (Figure 1(f) to (h)). There were significantly less Tmem119+ cells in the ipsilesional peri-infarct regions than in the contralateral side in both young and aged mice (Figure 1(g)). A concomitant enlargement of cell body volume was also observed in the ipsilesional side (Figure 1(h)), indicating that these cells were activated in response to brain ischemia. Interestingly, the body volumes of aged microglia were significantly larger than young microglia five days after dMCAO (Figure 1(h)). This morphological difference indicated that aged microglia may have different functions from young microglia in the post-stroke brain, which could contribute to the previously reported worse long-term functional outcomes of aged mice after ischemic stroke.17,18
Aging primes microglia to a unique activation state in the homeostatic brain
To explore the intrinsic genomic differences between young and aged microglia, we used FACS to purify microglia (CD11b+CD45low cells) from the brains of young adult mice and aged mice five days after dMCAO or after sham operation, and performed bulk RNA-seq on isolated cells (Supplementary Figure 1(a) to (c)). FACS-purified cells expressed high levels of microglia signature genes, such as Itgam (encoding CD11b), Cx3cr1, Aif1 (encoding Iba1), and Tmem119 (Supplementary Figure 1(d)). In contrast, prototypic markers for neurons, astrocytes, oligodendrocytes, T cells, B cells, and granulocytes were all expressed at negligible levels in purified microglia in all groups (Supplementary Figure 1(e)). Markers specific to systemic macrophages (e.g. Mrc1 and Cd163)10 were also expressed at low levels (Supplementary Figure 1(e)), confirming that our extraction protocol generated microglia of high purity.
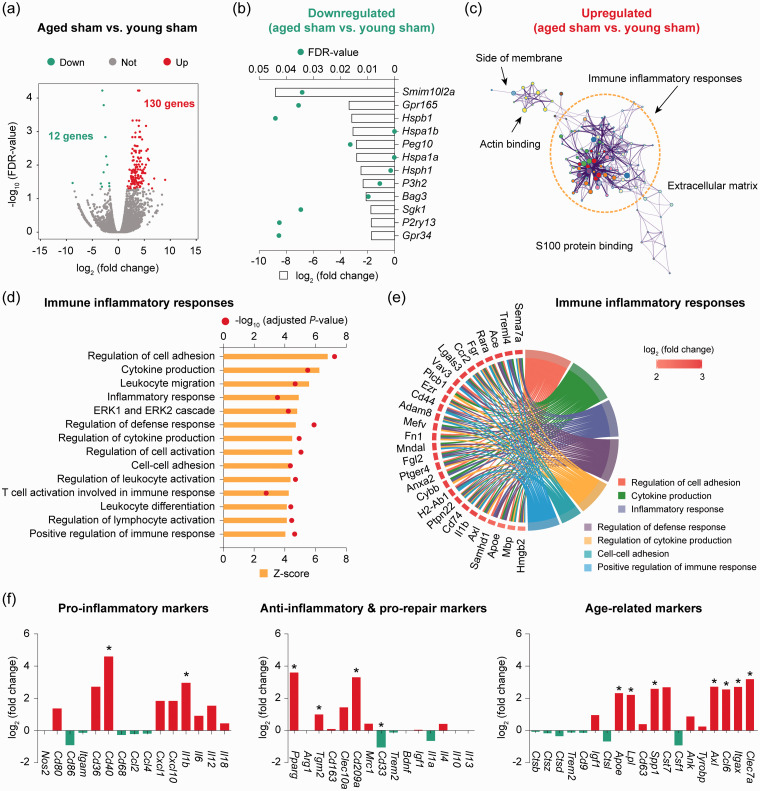
Next, differential expression analysis was performed on RNA-seq data to determine the transcriptomic changes induced by aging in homeostatic microglia from non-injured sham brains. We identified 142 differentially expressed genes (DEGs; defined as genes with a fold change > 2 or <−2 and a false discovery rate (FDR)<0.05) in microglia from aged sham mice compared to young sham mice (Figure 2(a) and Supplementary Table 2), representing a moderate alteration of the genome (0.6% of total ∼23,000 genes). Aging downregulated 12 genes in microglia, including genes encoding the G protein-coupled receptors Gpr165 and Gpr34, and the heat shock protein family members Hspb1, Hspa1a, Hspa1b, and Hsph1 (Figure 2(b)). On the other hand, 130 genes were upregulated in aged microglia compared to young microglia, the functional implications of which were further explored by gene ontology (GO) enrichment analysis. A total of 263 GO terms were significantly overrepresented (P < 0.01) by the upregulated DEGs (Supplementary Table 3), among which the largest functional cluster was associated with immune inflammatory responses (Figure 2(c)). This functional cluster consisted of a variety of biological processes such as regulation of cell adhesion, cytokine production, leukocyte migration, and regulation of defense response, all of which were predicted to be strongly activated (z-score > 4) in aged microglia (Figure 2(d)).
Figure 2.
RNA-seq reveals transcriptomic differences between young and aged microglia in the homeostatic brain. Microglia (CD11b+CD45low cells) were sorted from the brain of young adult and aged mice after sham operation and subjected to bulk RNA-seq. (a) Differential expression analysis was performed on RNA-seq data. Volcano plot shows the differentially expressed genes (DEGs; fold change > 2 or < −2, false discovery rate (FDR) < 0.05) in aged microglia compared to young microglia. (b) Expression profiles of the 12 downregulated DEGs in aged microglia compared to young microglia. (c) Gene ontology (GO) enrichment analysis was performed using Metascape on the 130 upregulated DEGs in aged microglia versus young microglia. The significantly overrepresented (P<0.01) GO terms were grouped into color-coded clusters based on their membership similarities and rendered as a network plot. Each node represents an enriched term, and one representative term is shown for each cluster. Terms with a similarity > 0.3 are connected by edges. The complete enrichment results are provided in Supplementary Table 3. (d) The activation status of biological processes related to immune inflammatory responses was assessed by calculating their activation z-scores using GOplot. Shown are the biological processes associated with immune inflammatory responses that were predicted to be strongly activated (z-score > 4). (e) Genes related to immune inflammatory responses were explored for their involvement in seven functional sub-categories. Shown are genes associated with at least three sub-categories, displayed as a Circos plot. (f) Expression profiles of representative pro-inflammatory, anti-inflammatory and pro-repair, and aging-related genes. Data were expressed as log2 transformation of fold changes in aged microglia vs. young microglia. n = 2 biological replicates per group. *P<0.05 aged vs. young.
We then annotated the relationship between specific genes and biological processes in the immune inflammatory responses functional cluster (Figure 2(e)). Numerous key genes upregulated in aged microglia, including Adam8, Ccr2, Cd74, Il1b, Ptpn22, Hmgb2, Rara, Ezr, Fgl2, Lgals3, Ptger4 and Sema7a, were clustered in at least three immune and inflammatory functions such as cytokine production, regulation of cell adhesion and inflammatory response (Figure 2(e)). Adam8 encodes a metalloprotease that can be induced by tumor necrosis factor (TNF)-α in CNS cells including activated microglia, and can contribute to neurodegeneration by facilitating glia–neuron interactions.26 CD74 is a macrophage migration inhibitory factor (MIF) receptor associated with pro-inflammatory M1 polarization of microglia and macrophages.27 PTPN22, a protein tyrosine phosphatase linked to many autoimmune diseases such as Crohn’s disease, is also known to regulate macrophage polarization.28 High-mobility group box protein 2 (HMGB2) is expressed in microglial nucleus and its cytoplasmic translocation mediates the release of pro-inflammatory cytokines.29 A number of upregulated genes encode chemokine receptors (e.g. CCR2) and other cell-surface proteins, such as Cd44, Ezr, Fgl2, Ptger4 (encoding prostaglandin E receptor 4) and Lgals3, which are involved in cell–cell interactions, cell adhesion and migration.30–34 These gene products may be important in the recruitment of mononuclear phagocytes, microglia, and leukocytes at sites of inflammation. Finally, activated microglia can directly contribute to neurovascular inflammation after cerebral ischemia by releasing a number of soluble factors such as the pro-inflammatory cytokines interleukin (IL)-1β and TNF-α.35
We also analyzed the transcriptional signature genes defining pro-inflammatory, anti-inflammatory, or senescent microglial populations (Figure 2(f)). Two out of 15 pro-inflammatory genes (Cd40, Il1b), 3 out of 15 anti-inflammatory genes (Pparg, Tgm2, Cd209a), and 7 out of 19 senescent genes (Apoe, Lpl, Spp1, Axl, Ccl6, Itgax, Clec7a) were significantly elevated in aged microglia compared to young microglia (Figure 2(f)). Apoe is implicated in microglial dysfunction associated with neurodegenerative diseases.36 Increased expression of Lpl (encoding lipoprotein lipase) is linked to enhanced innate immunity of reactive microglia in the mouse brain.37 Axl expression has been found to be upregulated in brain microglia in inflammatory environment and its function is required for microglial phagocytosis.38 Notably, CD33 (or Siglec3), a transmembrane receptor expressed on cells of myeloid lineage39 and involved in the regulation of phagocytosis,40 was significantly downregulated in aged microglia (Figure 2(f)). Taken together, these results suggest that microglia in healthy aged mice are in an inflammation-active state compared to young microglia, exhibiting upregulation of transcriptomic pathways with implications in immune inflammatory response. This aging-associated microglial transcriptome may help maintain a healthy homeostatic brain environment.
Ischemic stroke induces distinct transcriptomic alterations in young and aged microglia
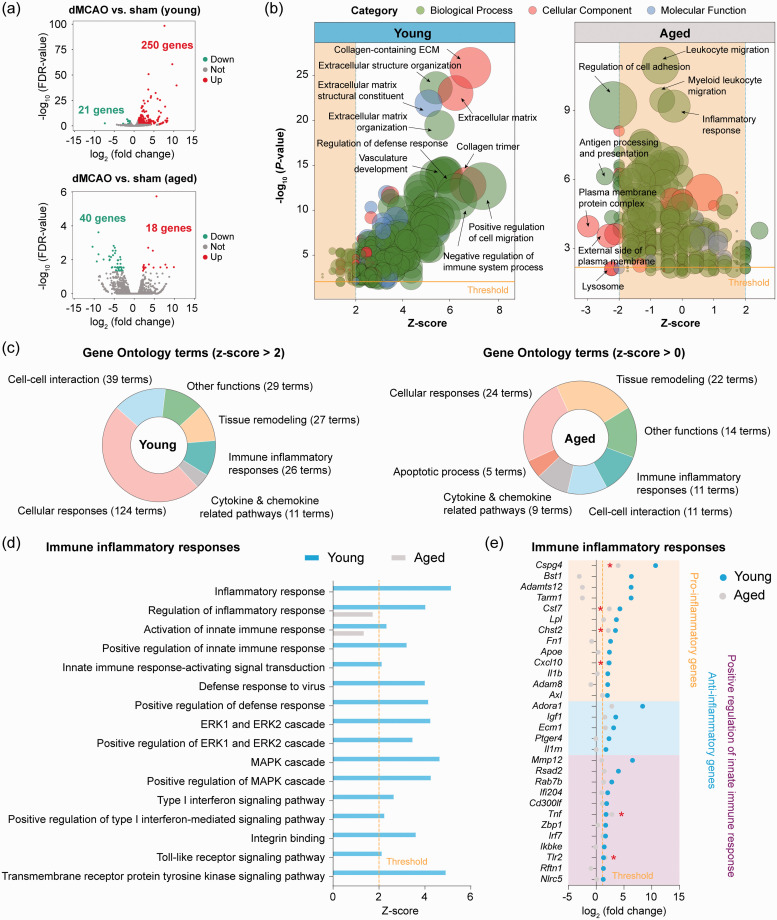
We sought to explore the differences in microglial gene expression profiles between young adult and aged mice in response to ischemic stroke by comparing their transcriptome five days after dMCAO relative to sham operation. We chose five days after dMCAO for microglial gene profiling as microglia are maximally activated at this time point after dMCAO.18 In young microglia, dMCAO upregulated 250 genes and downregulated 21 genes (Figure 3(a) and Supplementary Table 4). Aged microglia showed only 18 upregulated genes and 40 downregulated genes after dMCAO (Figure 3(a) and Supplementary Table 5). Functionally, GO enrichment analysis of these DEGs predicted that 256 GO terms were activated (z-score> 2, P < 0.01) in young microglia after ischemia (Figure 3(b) and Supplementary Table 6), from which six major functional clusters were yielded (Figure 3(c)). In contrast, the number of enriched GO terms (P < 0.01) in aged microglia was much less than in young microglia, and none of the enriched GO terms was predicted to be activated in aged microglia (0<z-score < 2; Supplementary Table 7). These analyses revealed that aged microglia, although chronically activated in a homeostatic brain, were less responsive to an acute ischemic insult than young microglia. Moreover, several potentially important biological functions related to immune cell recruitment (cell adhesion), immune responses (antigen presenting, response to interferon-gamma), and cellular homeostasis (lysosome, lytic vacuole) were even predicted to be inhibited (z-score<−2) in aged mice after stroke (Supplementary Figure 2).
Figure 3.
Transcriptional differences between young and aged microglia in response to ischemic stroke. Differential expression analysis was performed on RNA-seq data obtained from young and aged microglia five days after dMCAO or after sham operation. (a) Volcano plots show the DEGs (fold change > 2 or < −2, FDR < 0.05) in microglia from dMCAO brain versus sham brain in young adult (upper panel) and aged (lower panel) mice. (b) GO enrichment analysis was performed using Metascape on all dMCAO-induced DEGs in young and aged microglia. GO terms in the three categories (biological process, cellular component, and molecular function) that were significantly overrepresented (P<0.01) were presented as bubble plots, where the sizes of the bubbles reflect the number of genes under each term. (c) Shown are the numbers of significantly overrepresented GO terms in young (z-score > 2; left panel) and aged (z-score > 0; right panel) microglia in response to dMCAO. Six major clusters of functions were identified from 256 GO terms with a z-score > 2 in young microglia. None of these functional clusters was activated in aged microglia (0 < z-score < 2). (d) Shown are the activation z-scores of biological functions related to immune inflammatory responses that were predicted to be strongly activated (z-score > 2) in young microglia in response to dMCAO. Only two of these functions were significantly enriched (P< 0.01) in aged microglia, and neither of them was predicted to be activated in aged microglia (0 < z-score < 2). (e) Expression profiles of upregulated DEGs (fold change > 2, FDR < 0.05) in young microglia related to immune inflammatory responses are shown with comparison to their expression in aged microglia. *P<0.05 aged dMCAO vs. aged sham.
We further compared individual biological processes in the immune inflammatory responses functional cluster between young and aged microglia after dMCAO (Figure 3(d)). Of the 16 GO terms that were predicted to be activated (z-score > 2) in young microglia after dMCAO, none was predicted to be activated in aged microglia (0<z-score < 2; Figure 3(d)). When we extracted dMCAO-induced DEGs in young mice that were involved in these biological processes, only six of them were significantly upregulated (P < 0.05) in aged mice (Figure 3(e)). Among the 13 pro-inflammatory genes, only four genes were significantly upregulated in both ages of mice after dMCAO (Figure 3(e)), including Cspg4, Cst7, Chst2, and Cxcl10.41–43 Five of the pro-inflammatory genes (II1b, Adam8, Apoe, Lpl, Axl) were among the elevated genes in healthy aged microglia versus young microglia (Figure 2), and these genes were not further increased in aged mice after dMCAO. Moreover, four out of nine pro-inflammatory genes (Bst1, Adamts12, Tarm1, Fn1) were not significantly altered in either healthy aged mice or aged mice after dMCAO (Figure 3(e)). Bst1 (CD157) is expressed on the surface of circulating neutrophils and plays an important role in mediating neutrophil adhesion and migration;44 its expression and role in microglia has not been reported thus far. The ADAMTS family of secretary metalloproteases, including Adamts12, cleaves certain extracellular matrix proteins such as versican, brevican, and neurocan during inflammation, and are involved in CNS repair through its ability to degrade neurocan.45 Tarm1 encodes the T cell-interacting activating receptor on myeloid cells 1 protein, which can promote pro-inflammatory cytokine secretion by neutrophils and myeloid cells under certain inflammatory conditions.46 Interestingly, 5 anti-inflammatory genes (Adora1, Igf1, Ecm1, Ptger4, II1rn) were significantly upregulated (>2-fold, FDR < 0.05) in microglia of young mice but not aged mice after dMCAO (Figure 3(e)).
We also extracted genes participating in the positive regulation of inflammatory responses (Figure 3(e)), which revealed 12 genes that were significantly upregulated (>2-fold, FDR < 0.05) in microglia of young adult mice. Several of these genes are well characterized for their regulatory roles in inflammation, including Cd300lf,47 Ifi204,48 and Ikbke.49 Only two genes (TIr2, Tnf) in this pathway had a P-value less than 0.05 in aged microglia after dMCAO (Figure 3(e)). We also examined the genes and GO terms in the “cellular responses” cluster (Figure 3(c)) and found similar reduced responsivity to ischemic stroke in aged microglia compared to young microglia (Supplementary Figure 3).
Age-afforded decline in immune cell chemotaxis after ischemic stroke
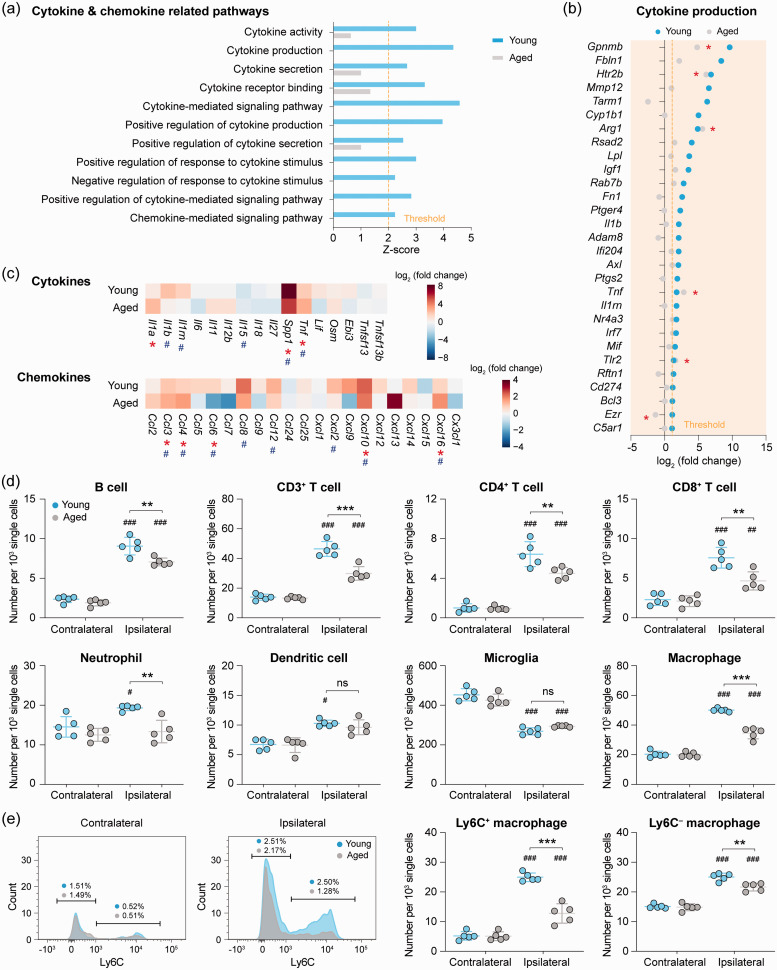
We analyzed post-stroke gene expression profiles for pathways involved in cytokine and chemokine production in young and aged microglia. Eleven GO terms in these pathways were predicted to be activated (z-score > 2, P < 0.01) in young microglia after dMCAO, but none of them was activated in aged mice (Figure 4(a)). Young microglia upregulated 29 genes regulating cytokine production after dMCAO, whereas only 5 of them were upregulated in aged mice (Figure 4(b)). We also examined dMCAO-induced gene expression changes of a panel of cytokines and chemokines in young and aged microglia (Figure 4(c)), of which four cytokine-encoding genes (II1b, II1rn, Spp1, Tnf) and eight chemokine-encoding genes (Ccl3, Ccl4, Ccl6, Ccl8, Ccl12, Cxcl2, Cxcl10, Cxcl16) were significantly upregulated in young microglia (Figure 4(c)). In contrast, aged microglia showed significant upregulation (P < 0.05) of only three cytokine-encoding genes (Il1a, Spp1, Tnf) and four chemokine-encoding genes (Ccl3, Ccl4, Cxcl10, Cxcl16), with a concomitant downregulation of Ccl6. Collectively, these data suggested that cytokine and chemokine production by microglia in the post-ischemic brain is suppressed in aged mice.
Figure 4.
Reduction of cerebral immune cell chemotaxis in aged mice after ischemic stroke. (a) GO enrichment analysis was performed by Metascape on all dMCAO-induced DEGs in young and aged microglia. Shown are the z-scores of biological functions on cytokine and chemokine-related pathways predicted to be strongly activated (z-score > 2) in young microglia after dMCAO. Only four of these functions were significantly enriched (P<0.01) in aged microglia, and none of them was predicted to be activated (0 < z-score < 2). (b) Expression profiles of DEGs (fold change > 2, FDR < 0.05) in young microglia related to cytokine and chemokine pathways are shown with comparison to their expression in aged microglia. *P<0.05 aged dMCAO vs. aged sham. (c) Heatmaps showing the expression profiles of genes encoding cytokines and chemokines. Data were expressed as log2 transformation of fold changes in dMCAO vs. sham in young microglia (1st row) and aged microglia (2nd row). #P<0.05 dMCAO vs. sham in young microglia. *P<0.05 dMCAO vs. sham in aged microglia. (d,e) Young adult and aged mice were subjected to dMCAO. The infiltration of peripheral immune cells into the brain was assessed by flow cytometry five days after dMCAO (for gating strategies see Supplementary Figure 4). (d) The numbers of CD19+ B cells, CD3+ T cells (further gated into CD4+ and CD8+), Ly6G+ neutrophils, CD11c+ dendritic cells, CD11b+CD45low microglia and CD11b+CD45high macrophages in the ipsilateral brain hemisphere and the non-injured contralateral hemisphere were quantitatively compared between young and aged mice. (e) Macrophages were further gated into pro-inflammatory macrophages (Ly6C+) and non-inflammatory macrophages (Ly6C–) according to the expression level of Ly6C. n = 5 mice per group. #P<0.05, ##P<0.01, ###P<0.001 ipsilateral vs. contralateral. **P<0.01, ***P<0.001 aged vs. young. ns: no significant difference.
After ischemic stroke, a variety of chemokines are produced in the brain, attracting blood immune cells to enter the ischemic brain parenchyma.50 Since our RNA-seq data showed that aging impairs stroke-induced upregulation of chemokine-coding genes in microglia, we hypothesized that such decline of chemokine gene expression may negatively impact immune cell infiltration after brain ischemia in aged mice. To test this hypothesis, we quantified the various immune cell populations in the brain of young and aged mice using flow cytometry five days after dMCAO (Supplementary Figure 4) as a means for functional verification of this impaired chemotaxis in aged microglia. Compared to the non-ischemic hemispheres contralateral to stroke, as expected there were robust increases in the amount of infiltrating immune cells in the ischemic hemisphere of young mice, including B cells, CD3+ T cells (both CD4+ and CD8+),51 neutrophils, dendritic cells, and macrophages52,53 (both Ly6C+ and Ly6C–; Figure 4(d) and (e)). In contrast, indeed the infiltration of neutrophils and dendritic cells was completely abolished in post-stroke aged mice (P > 0.05 vs. contralateral controls), and the infiltration of all other immune cells were significantly suppressed in aged mice (P < 0.05 aged vs. young; Figure 4(d) and (e)).
Activation of tissue remodeling pathways is diminished in aged microglia after ischemic stroke
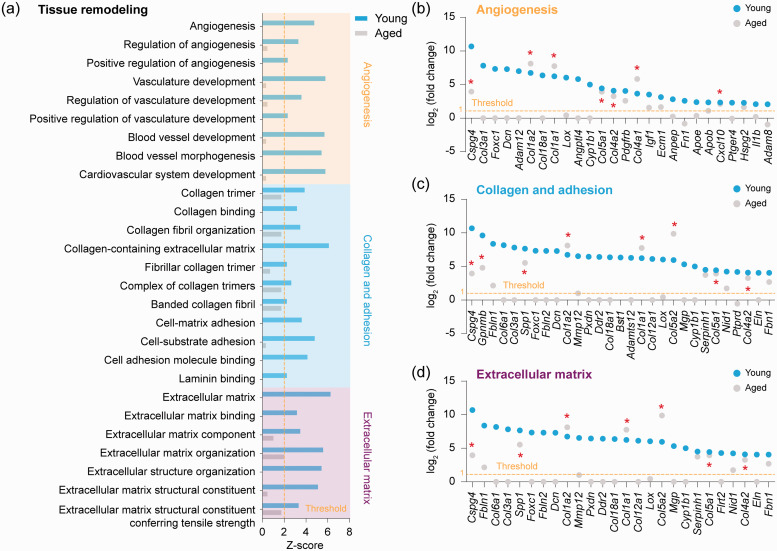
We analyzed gene expression profiles for functional pathways involved in tissues remodeling. Twenty-seven GO terms in these pathways were predicted to be activated (z-score > 2, P < 0.01) in young microglia after dMCAO, none of which was significantly activated in aged microglia (Figure 5(a)). Based on their functional implications, the 27 GO terms were grouped into 3 clusters, including 9 GO terms related to angiogenesis, 11 GO terms related to collagen and adhesion, and 7 GO terms related to extracellular matrix (Figure 5(a)). Genes belonging to these three clusters were further extracted and analyzed (Figure 5(b) to (d)). In the angiogenesis cluster, 26 genes were significantly upregulated (FDR < 0.05, >2-fold) in young microglia, but only 7 of them (Cspg4, Col1a2, Col1a1, Col5a1, Col4a2, Col4a1, Cxcl10) were significantly upregulated (P < 0.05) in aged microglia after dMCAO (Figure 5(b)). In the collagen adhesion cluster, 29 genes were significantly upregulated in young microglia, among which 8 genes (Cspg4, Gpnmb, Spp1, Col1a2, Col1a1, Col5a2, Col5a1, Col4a2) were also significantly upregulated (P < 0.05) in aged microglia after dCMAO (Figure 5(c)). In the extracellular matrix cluster, 26 genes were dMCAO-induced DEGs in young microglia, and 7 of them were also significantly upregulated (P < 0.05) in microglia from aged mice (Figure 5(d)). The age-dependent decline in the activation of tissue remodeling-related genes may help explain, but does not prove, the poorer stroke recovery observed in aged mice.
Figure 5.
Transcriptional regulation of tissue remodeling-related genes in aged and young microglia after ischemic stroke. (a) GO enrichment analysis was performed by Metascape on all dMCAO-induced DEGs in young and aged microglia. Shown are the z-scores of biological functions related to tissue remodeling predicted to be strongly activated (z-score > 2) in young microglia after dMCAO. None of them was predicted to be activated in aged microglia (0 < z-score < 2). Enriched biological processes were further classified into three clusters: angiogenesis-related, collagen and adhesion-related, and extracellular matrix-related. (b–d) Shown are the expression profiles of DEGs (fold change > 2, FDR < 0.05) in young microglia which were associated with angiogenesis (b), collagen and adhesion (c), and extracellular matrix (d), with comparison to their expression in aged microglia. *P<0.05 aged dMCAO vs. aged sham.
Suppression of genes and pathways involved in cell–cell interactions in aged microglia after stroke
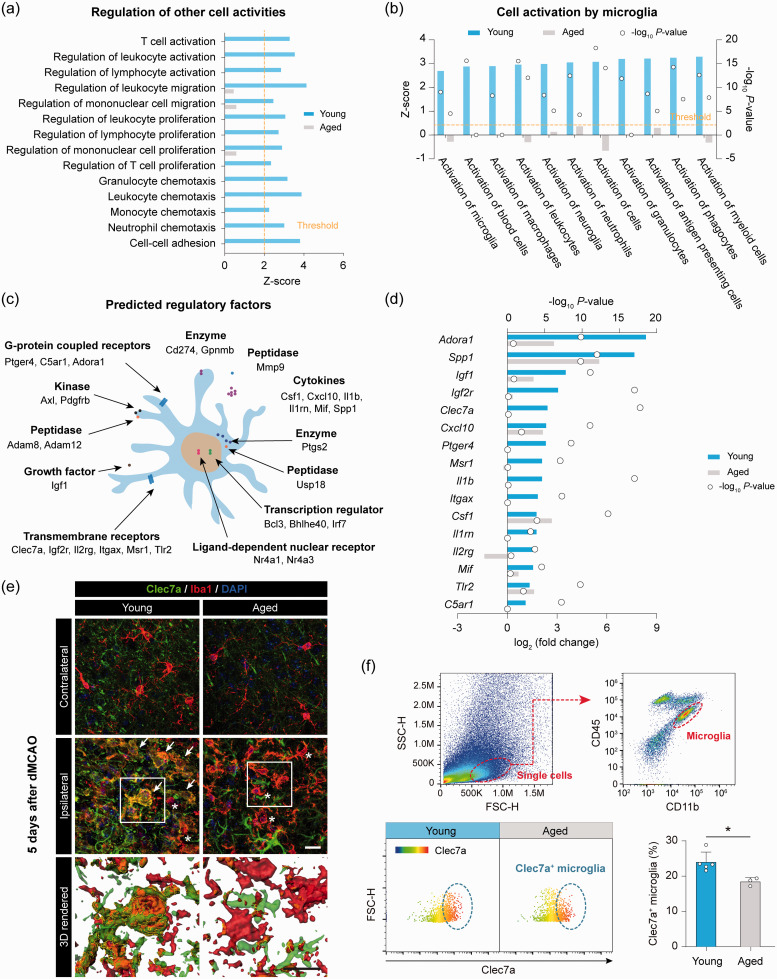
Following ischemic brain injury, activated microglia influence the activities of surrounding cells through cell–cell interactions,54 and direct (cell contact) or indirect (via release of soluble factors) interactions by microglia may be essential for microglia to protect against ischemic neuronal injury.55 Therefore, we analyzed gene expression profiles for functional pathways engaged in cell–cell interactions. Fourteen GO terms in these pathways were predicted to be activated (z-score > 2) in young microglia after dMCAO, but none of them was significantly activated in aged mice (Figure 6(a)). We also assessed the overall activation status of microglia by ingenuity pathway analysis (IPA), which was based not only on the gene expression profiles in our dataset but also prior biological knowledge in the ingenuity database.56 IPA identified a panel of biological functions that were predicted to be significantly activated (z-score > 2, P < 0.01) in young microglia after dMCAO (Figure 6(b)), all of which are related to cell activation. These results suggested that microglia are activated after ischemia with enhanced interactions with other CNS resident cells and infiltrating peripheral immune cells; such cell–cell interaction abilities of microglia substantially decline in aged mice.
Figure 6.
Impaired transcriptional regulation of cell–cell interaction molecules by aged microglia after ischemic stroke. (a) GO enrichment analysis was performed by Metascape on all dMCAO-induced DEGs in young and aged microglia. Shown are the z-scores of biological functions related to cell–cell interactions predicted to be strongly activated (z-score > 2) in young microglia after dMCAO. Only three of these functions were significantly enriched (P< 0.01) in aged microglia, and none of them was predicted to be activated (0 < z-score < 2). (b) The DEGs induced by dMCAO in young and aged microglia were further explored for their functional implications using ingenuity pathway analysis (IPA). Shown are the biological functions relevant to the activation of cells by microglia predicted to be strongly activated (z-score > 2, P<0.01) in young microglia. None of them was predicted to be activated in aged microglia. (c) The predicted regulatory molecules involved in the cell–cell interaction signaling network were manually annotated according to the type and subcellular localization of gene products. (d) Expression profiles of regulatory molecules whose gene products are active in the plasma membrane or extracellular space. (e,f) The expression of Clec7a in microglia of young and aged mice was assessed five days after dMCAO by immunostaining (e) and flow cytometry (f). (e) Representative images showing the immunosignal of Clec7a double-labeled with Iba1 in the ipsilesional peri-infarct area and the corresponding area in the non-injured contralateral hemisphere. Cells were counterstained with DAPI for nuclear labeling. Arrow: Iba1 and Clec7a double-positive cell. Asterisk: Iba1+Clec7a– cell. Rectangles: regions enlarged and 3D-rendered by Imaris in the 3rd row. Scale bar: 20 µm. (f) The expression of Clec7a in CD11b+CD45low microglia was examined in the ipsilesional hemisphere of young adult and aged mice by flow cytometry. The percentage of Clec7a+ microglia was significantly lower in aged mice than in young mice after dMCAO. n = 3–5 mice per group. *P<0.05 aged vs. young.
We next examined the genes in these pathways whose products are active in the plasma membrane or extracellular space and may therefore play a regulatory role on other cells, such as Cd274, Clec7a, Cxcl10, Ptger4, Serpine1, Csf1, Il1b, Tnf and Axl (Figure 6(c)). Approximately 50% of these regulatory genes, including Clec7a, Ptger4, II1b, Itgax, II1m, II2rg and C5ar1, were significantly upregulated in young microglia but not in aged microglia (Figure 6(d)). These data offer insights into the molecular basis underlying the diminished capability of aged microglia to interact with neighboring cells after stroke.
We verified whether the age-dependent changes of gene expression at the RNA level led to changes at the protein level after stroke, focusing on Clec7a. Clec7a encodes Dectin-1, a pattern-recognition receptor expressed by myeloid phagocytes (macrophages, microglia, and dendritic cells).57 We performed double-label immunofluorescence staining for Iba1 and Clec7a, and found that Clec7a immunofluorescence was readily detectable in Iba1+ cells in peri-infarct area five days after dMCAO in young mice but not in aged mice (Figure 6(e)). Furthermore, we performed flow cytometry to detect Clec7a in brain microglia (CD11b+CD45low cells) five days after dMCAO. The percentage of Clec7a+ microglia relative to total CD11b+CD45low cells was significantly lower in aged mice than in young mice five days after dMCAO (Figure 6(f)). These results exemplified the RNA-seq findings at the protein level.
Aged microglia demonstrate less interaction with neighboring cells in the post-stroke brain
Given the functional importance of cell–cell interactions between microglia and surrounding cells, and since our RNA-seq data suggested that there are remarkable differences between young and aged microglia in their cell–cell communication behavior after stroke, we sought to explore such differences at the cellular level in the peri-infarct area (as depicted in Figure 1(e)) using double-label immunofluorescence staining followed by Imaris-assisted image analysis. To this end, we performed immunostaining using the microglial marker Tmem119 and the neuron marker NeuN, the astrocyte maker GFAP, or the oligodendrocyte marker APC to examine microglial interactions with neighboring cells in both young and aged mice five days after dMCAO (Figure 7 and Supplementary Figure 5).
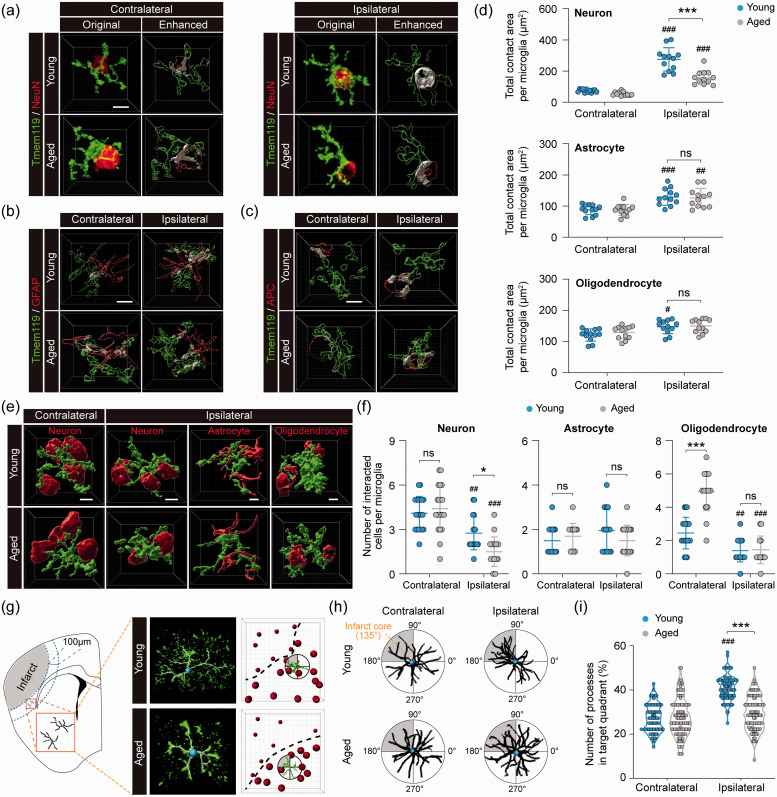
Figure 7.
Aged microglia demonstrate reduced interactive responses to ischemic neurons and infarct in the post-stroke brain. Young adult and aged mice were subjected to dMCAO. The interaction between microglia and neighboring brain cells was examined by immunofluorescence staining five days after dMCAO in the ipsilesional peri-infarct regions and corresponding regions in the non-injured contralateral hemisphere. (a) Original images from confocal microscopy and Imaris-enhanced images showing the cell–cell contact areas between Tmem119+ microglia and NeuN+ neurons in white color. (b,c) Imaris-enhanced images showing the cell–cell contact areas between Tmem119+ microglia and GFAP+ astrocytes (b) or APC+ oligodendrocytes (c). See Supplementary Figure 5(a) to (c) for all original immunofluorescence images. (d) Summarized data of total contact area with neurons, astrocytes and oligodendrocytes per microglia. n = 12 cells from four mice per group. (e) Representative immunofluorescence images that were surface rendered by Imaris illustrate the cell–cell interaction between microglia (green) and neurons, astrocytes or oligodendrocytes (red). See Supplementary Figure 5(d) for original immunofluorescence images. (f) Summarized data on the numbers of neurons, astrocytes and oligodendrocytes each microglia interacted with. n = 20 cells from four mice per group. (g) The polarity of Tmem119+ microglia in the ipsilateral peri-infarct region (0–100 µm from the infarct border) was assessed in an arbitrary polar coordinate system. The direction from the microglia cell body to the infarct core was defined as 135°, and the quadrant from 90° to 180° was defined as the target quadrant toward the infarct core. (h) Tmem119 immunofluorescence was converted into binary signals to illustrate the direction of cellular processes. Shown is the overlay of three representative cells in each group. (i) The number of processes in the target quadrant was quantified and expressed as percentage of total processes in all four quadrants. n = 100 cells from four mice per group. #P<0.05, ##P<0.01, ###P<0.001 ipsilateral vs. contralateral. *P<0.05, ***P<0.001 aged vs. young. ns: no significant difference. Scale bars: 5 µm.
Microglia can communicate with neighboring cells via direct cell–cell contact. Hence, cell–cell surface contact area is a rational parameter for cell–cell interactions. In the non-injured contralesional hemisphere, microglia formed contact with neurons, astrocytes, and oligodendrocytes, with no significant differences in the total cell–cell contact area per microglia between young and aged mice (Figure 7(a) and (b)). The total contact areas per microglia were significantly larger in the ipsilesional peri-infarct area than in the contralateral side for all three types of cells in both young and aged mice (Figure 7(a) and (b)), suggesting enhanced cell–cell engagement between microglia and neighboring cells after ischemia. Such changes were the most robust in microglia–neuron interactions after stroke, exhibiting approximately 3-fold and 1.8-fold increases in total contact area per microglia in young and aged mice, respectively (P < 0.001 aged vs. young), compared to contralateral controls (Figure 7(a) and (b)). Furthermore, the numbers of neurons and oligodendrocytes that interacted with each of microglia were significantly decreased in both young and aged mice after stroke compared to contralateral controls (Figure 7(e) and (f)), which further increased the calculated average of contact area per neuron or oligodendrocyte interacting with microglia (data not shown). Consistent with these observations, RNA-seq data demonstrated dMCAO-induced upregulation of a panel of cytoskeleton and cell adhesion regulators in young microglia (e.g. the ERM protein ezrin, and α5 and β1 integrins), suggesting active cytoskeleton reorganization and enhanced cell–cell interactions. Such genomic changes were abolished in aged microglia (Supplementary Figure 6). Taken together, these results suggested that ischemia induces robust interactions between microglia and neighboring neurons, astrocytes, and oligodendrocytes, and that the induced interactions between microglia and neurons decline with aging.
Microglia can respond to brain injury by extending their processes towards the injury site, a reaction termed “polarity”.58 To study microglial polarity after stroke, we used Imaris to analyze Tmem119+ cells in the peri-infarct area by quantifying their processes that were present in the quadrant proximate to the infarct (Figure 7(g) and (h)). In non-injured contralateral cortex of young and aged mice, microglial processes were evenly distributed in all four quadrants (Figure 7(h) and (i)). In the peri-infarct area of young mice, dMCAO significantly increased microglial polarity towards the infarct (Figure 7(h) and (i)). In contrast, such increased microglial polarity was not detectable in aged mice (Figure 7(i)). These results further supported our RNA-seq findings that aged microglia exhibit decreased communications with other cells, including injured or dying cells close to the infarct.
Discussion
The data presented in this report provide, for the first time, genome-wide transcriptional profiling of microglia purified from the brain of young adult and aged mice under both homeostatic and post-ischemic conditions. Major findings of our study include that (i) there are considerable transcriptional differences between young and aged microglia in the homeostatic brain, implicating a heightened chronic inflammation state in aged microglia; (ii) stroke-induced transcriptional activation was substantially suppressed in aged microglia compared to young microglia, especially those gene clusters relevant to immune inflammatory responses, immune cell chemotaxis, tissue remodeling and repair, and cell–cell interactions; (iii) consistent with the transcriptional profiling predictions, post-stroke cerebral infiltration of peripheral immune cells and microglial interactions with neighboring cells were markedly decreased in aged mice compared to young mice.
Transcriptomic comparison between young and aged microglia in the homeostatic brain identified 142 DEGs, representing a moderate alteration of the genome (0.6% of total ∼23,000 genes). The largest cluster of biological functions overrepresented by these DEGs is associated with immune inflammatory responses, including regulation of cell adhesion, cytokine production, leukocyte migration, and regulation of defense response. These results suggest that microglia in healthy aged mice are in an enhanced pro-inflammatory state compared to young microglia. This finding is strikingly consistent with previously reports, in which production of pro-inflammatory cytokines is found to be upregulated in microglia during aging.10,59,60 While the mechanism underlying such senescence-associated chronic inflammation remains elusive, emerging evidence supports the longstanding postulation of an infectious etiology of brain aging and age-prone neurodegenerative disorders, such as Alzheimer’s disease.61 The “microbial hypothesis” suggests that a number of pathogens, including latent viruses, gut bacteria, periodontal bacteria, and pulmonary bacteria, may invade the CNS via the trigeminal nerve or the oral-olfactory route, or from systemic circulation by crossing the compromised blood–brain barrier during aging.62–65 More recent studies have emphasized the potentially important role of aging-associated increases in gut permeability and activation of the microbiota–gut–brain axis in chronic systemic inflammation, brain senescence and neurodegeneration.66,67
The major objective of this study is to explore aging-associated alterations in microglial transcriptome five days after ischemic stroke, as several key biological processes of neuroinflammation, including innate immunity responses and cerebral infiltration of circulating immune cells, peak at this sub-acute injury stage.68,69 Our data reveal that cerebral ischemia induces a considerable transcriptional activation in young microglia, upregulating approximately 1.1% of the transcriptome (250 of total ∼23,000 genes). Unsurprisingly, many of the functional gene-clusters which were predicted to be strongly activated in young microglia are associated with immune inflammatory responses (26 GO terms) and cytokine and chemokine pathways (11 GO terms). In contrast, ischemic stroke induced merely a modest transcriptional activation in aged microglia (18 genes upregulated), and none of the aforementioned neuroinflammation-relevant GO terms was activated in aged microglia. These results suggest that the immune inflammatory responses are severely suppressed in aged microglia after ischemic stroke compared to young microglia. As the transcriptional gene profile for chemokines predicts negative regulation on chemotaxis activity in aged microglia after ischemia, we quantified post-stroke cerebral infiltration of various types of immune cells in young and aged mice. Consistent with the gene profiling predictions, the infiltration of all types of examined immune cells was found to be diminished in aged mice compared to young mice after ischemic stroke. The suppressed immune inflammatory responses and brain infiltration of circulating immune cells seem to contradict to the poorer stroke outcomes in aged mice, as the general notion indicates that neuroinflammation exacerbates ischemic brain injury. However, this concept has been reversed by many recent studies, which suggest: (1) certain cytokines, such as IL-4, IL-10, IL-33 and TGFα are neuroprotective against ischemic brain injury;70–74 (2) certain infiltrating immune cells, such as bone-marrow monocytes-derived macrophages and regulatory T cells (Tregs), confer neuroprotection and/or are directly involved in neurovascular remodeling after ischemic stroke;69,75,76 (3) certain pro-inflammatory molecules, such as MMP-9, may be important for post-injury brain repair.77
Another novel finding of this study is that several gene-clusters with functional implications in brain repair are robustly activated in young microglia, but not in aged microglia, after ischemic stroke. These gene-clusters include angiogenesis and vascular development (9 GO terms), collagen and adhesion (11 GO terms), and extracellular matrix organization (7 GO terms), all of which have strong relevance in post-stroke brain tissue remodeling and functional recovery. The suggestive involvement of microglia in promoting angiogenesis in post-ischemic brain is unexpected, yet it is a legitimate assumption. Our recent study has explored a similar functional role for infiltrating monocyte-derived macrophages using the dMCAO model.69 In the latter study, PPARγ was identified as the key upstream regulatory molecule for the pro-angiogenesis gene-cluster in the myeloid-cell lineage, as conditional knockout of PPARγ selectively from these cells led to impaired angiogenesis in post-stroke brain.69 Thus, our studies point to a novel source of molecular signals that contribute to neurovascular remodeling after ischemic stroke. This mechanism may be particularly significant for aging-associated impairment of stroke recovery. Among the post-stroke brain repair processes, diminished angiogenesis is strongly correlated with poorer stroke recovery in aged mice.17
Microglia are highly dynamic cells that interact with other cells via direct (physical cell–cell touch) or indirect (release of soluble molecules) mechanisms.22,78 Our transcriptional profiling data suggest that the capabilities to interact and activate surrounding cells are remarkably dampened in aged microglia after cerebral ischemia. Gene clusters with functional implications for cellular activation are strongly activated in young microglia, but not in aged microglia, after ischemic stroke. Further analysis of the GO terms revealed that post-ischemic young microglia not only activate brain residential cells, such as neuroglia and microglia, but also regulate the migration, proliferation, and chemotaxis of infiltrating immune cells, such as neutrophils, lymphocytes and phagocytes. Our follow-up experiments partially confirmed the remarkable differences between young and aged microglia in their cell–cell communication behavior after stroke. First, we demonstrated that the protein expression levels for Clec7a, a pattern-recognition receptor on microglia that likely mediates cell–cell interactions,57 are decreased in aged mice after stroke. Second, we found that the cell–cell physical contacts between microglia and neurons are suppressed in aged mice after stroke. Third, we showed that, in the peri-infarct brain regions, microglial polarity towards the infarct is nearly lost in aged mice after stroke, as compared to young mice. These results suggest, albeit do not prove, that microglia may influence stroke outcomes via functional cell–cell interactions. Future studies that elucidate the specific molecular signaling pathways underlying distinct microglial behaviors may help uncover senescence mechanisms. The permanent focal cerebral ischemia model that we used in this study involves occlusion of the distal MCA and ipsilateral CCA. This model has high clinical relevance, as the majority of stroke patients do not receive reperfusion therapy, especially for distal branch occlusions that are more common and remain beyond the reach of endovascular thrombectomy. In future studies, conclusions drawn from this study should be tested in other stroke models of ischemia reperfusion injury after proximal MCAO, which are applicable to the recent advances in clinical reperfusion therapies for a significant minority of stroke patients.16
Several limitations of the present study should be noted. First, the RNA-seq profiling was performed using purified microglia from cerebral hemispheres. The CD11b+CD45low-based cell sorting approach, while ensuring high level of cell purity, would not include microglia that express high levels of CD45. Upon activation after cerebral ischemia, a subpopulation of microglia may shift to the CD11b+CD45high population,70 and the latter cell populations were excluded for genomic profiling in this study. Second, although the bulk RNA-seq technology offers the advantage of deep gene profiling (∼23,000 genes), the identified DEGs were the result of averaging gene expression-level changes in the entire biological sample (approximately 1.0 × 107 cells per sample in this study). Thus, a caveat with bulk RNA-seq is that this approach would miss out the subtle changes in gene expression, especially when such changes occur only in small subpopulations of cells within the biological sample. The approach combining of bulk and single-cell RNA-seq would help overcome both roadblocks. Third, the DEGs resulting from RNA-seq do not always dictate molecular alterations at either the protein expression or functional levels. In the current study, we have confirmed several changes at protein and functional levels as predicted by RNA-seq, including Clec7a protein expression, immune cell infiltration, and cell–cell interactions in young and aged microglia after stroke. Nevertheless, future studies are warranted to further understand the functional significances of transcriptional alterations characterized in this report. Overall, our study used data-driven analytic approaches to provide unbiased genome-level characterization of age-associated differences in microglial transcriptome under homeostatic and ischemic conditions. The datasets generated by this study are useful resources for future studies to screen for potential regulators to modulate microglial functions in aged subjects. However, solid conclusions on whether certain regulators are causatively connected to stroke outcomes must be obtained with mechanism-oriented approaches, such as specific gain or loss-of-function manipulations.
In conclusion, this study performed transcriptome-wide comparisons between young and aged microglia under both homeostatic and post-stroke conditions. The alterations in microglial gene response and behavior may contribute, at least in part, to aging-afforded vulnerability and poorer recovery after ischemic stroke. Future elucidation of the microglia-dependent cellular and molecular pathways may help uncover novel therapeutic targets to boost functional recovery of older stroke patients.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-2-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-3-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-4-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-5-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-6-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-7-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The RNA sequencing data analysis in this work used Ingenuity Pathway Analysis software licensed through the Molecular Biology Information Service of the Health Sciences Library System, University of Pittsburgh. We thank Patricia Strickler for administrative support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Pittsburgh Medical Center (UPMC) Immune Transplant and Therapy Center grant (to JC, LRW, XH and YS) and the American Heart Association grant 17SDG33630130 (to YS). JC is the Richard King Mellon Professor of Neurology at the University of Pittsburgh and is also supported by the Senior Research Career Scientist Award and Merit Review grants (BX002495 and BX003377) from the U.S. Department of Veterans Affairs. LRW is the Henry B Higman Professor of Neurology at the University of Pittsburgh.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: JC and YS designed the research. LS, WZ, MJ, SL, QY, SHH, LL, MNA and JX performed the experiments. LS, YS, MR, LRW and JC analyzed and/or interpreted the data. JL mapped the RNA sequencing data. LS, JC and YS wrote the paper. MR, XH and LRW critically revised the paper.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Marcelo Rocha https://orcid.org/0000-0002-4503-5827
Yejie Shi https://orcid.org/0000-0001-7502-9201
References
- 1.Lawson LJ, Perry VH, Dri P, et al. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990; 39: 151–170. [DOI] [PubMed] [Google Scholar]
- 2.Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell 2014; 158: 15–24. [DOI] [PubMed] [Google Scholar]
- 3.Shibata M, Suzuki N. Exploring the role of microglia in cortical spreading depression in neurological disease. J Cereb Blood Flow Metab 2017; 37: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluge MG, Abdolhoseini M, Zalewska K, et al. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J Cereb Blood Flow Metab 2019; 39: 2456–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Hamanaka G, Lo EH, et al. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther 2019; 25: 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab 2018; 38: 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014; 10: 217–224. [DOI] [PubMed] [Google Scholar]
- 8.Erdo F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 2017; 37: 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M, Wang MM, Gao Y, et al. The effect of age-related risk factors and comorbidities on white matter injury and repair after ischemic stroke. Neurobiol Dis 2019; 126: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickman SE, Kingery ND, Ohsumi TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 2013; 16: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galatro TF, Holtman IR, Lerario AM, et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci 2017; 20: 1162–1171. [DOI] [PubMed] [Google Scholar]
- 12.Grabert K, Michoel T, Karavolos MH, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci 2016; 19: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safaiyan S, Kannaiyan N, Snaidero N, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 2016; 19: 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: a report From the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 15.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006; 296: 2939–2946. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Suenaga J, Pu H, et al. Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: efficacy declines with aging. Neurobiol Dis 2019; 126: 62–75. [DOI] [PubMed] [Google Scholar]
- 18.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousufuddin M, Bartley AC, Alsawas M, et al. Impact of multiple chronic conditions in patients hospitalized with stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2017; 26: 1239–1248. [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Cho S. Microglia and monocyte-derived macrophages in stroke. Neurotherapeutics 2016; 13: 702–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu MY, Lin YY, Zhang BJ, et al. Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci Ther 2019; 25: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 2015; 11: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Mu H, Liu L, et al. Transient selective brain cooling confers neurovascular and functional protection from acute to chronic stages of ischemia/reperfusion brain injury. J Cereb Blood Flow Metab 2019; 39: 1215–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh A, Imai SI, Guarente L. The brain, sirtuins, and ageing. Nat Rev Neurosci 2017; 18: 362–374. [DOI] [PubMed] [Google Scholar]
- 25.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016; 113: E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlomann U, Rathke-Hartlieb S, Yamamoto S, et al. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): implications for neuron-glia interactions during neurodegeneration. J Neurosci 2000; 20: 7964–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeiner PS, Preusse C, Blank AE, et al. MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1-polarized immune milieu and prolonged patient survival in gliomas. Brain Pathol 2015; 25: 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang HH, Miaw SC, Tseng W, et al. PTPN22 modulates macrophage polarization and susceptibility to dextran sulfate sodium-induced colitis. J Immunol 2013; 191: 2134–2143. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Nam Y, Koo JY, et al. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol 2014; 10: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 30.Lewis ND, Hill JD, Juchem KW, et al. RNA sequencing of microglia and monocyte-derived macrophages from mice with experimental autoimmune encephalomyelitis illustrates a changing phenotype with disease course. J Neuroimmunol 2014; 277: 26–38. [DOI] [PubMed] [Google Scholar]
- 31.Bates EE, Fournier N, Garcia E, et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol 1999; 163: 1973–1983. [PubMed] [Google Scholar]
- 32.Gould KL, Bretscher A, Esch FS, et al. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J 1989; 8: 4133–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta 2006; 1760: 616–635. [DOI] [PubMed] [Google Scholar]
- 34.Woodward DF, Jones RL, Narumiya S. International union of basic and clinical pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev 2011; 63: 471–538. [DOI] [PubMed] [Google Scholar]
- 35.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 2017; 47: 566–581.e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y, Vidal-Itriago A, Kalsbeek MJ, et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep 2017; 20: 3034–3042. [DOI] [PubMed] [Google Scholar]
- 38.Fourgeaud L, Traves PG, Tufail Y, et al. TAM receptors regulate multiple features of microglial physiology. Nature 2016; 532: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnache-Ottou F, Chaperot L, Biichle S, et al. Expression of the myeloid-associated marker CD33 is not an exclusive factor for leukemic plasmacytoid dendritic cells. Blood 2005; 105: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L. CD33 in Alzheimer’s disease – biology, pathogenesis, and therapeutics: a mini-review. Gerontology 2019; 65: 323–331. [DOI] [PubMed] [Google Scholar]
- 41.Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat 2005; 207: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton G, Colbert JD, Schuettelkopf AW, et al. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J 2008; 27: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Takeda-Uchimura Y, Foyez T, et al. Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer’s pathology. Proc Natl Acad Sci U S A 2017; 114: E2947–E2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funaro A, Ortolan E, Ferranti B, et al. CD157 is an important mediator of neutrophil adhesion and migration. Blood 2004; 104: 4269–4278. [DOI] [PubMed] [Google Scholar]
- 45.Fontanil T, Mohamedi Y, Moncada-Pazos A, et al. Neurocan is a new substrate for the ADAMTS12 metalloprotease: potential implications in neuropathies. Cell Physiol Biochem 2019; 52: 1003–1016. [DOI] [PubMed] [Google Scholar]
- 46.Radjabova V, Mastroeni P, Skjodt K, et al. TARM1 is a novel leukocyte receptor complex-encoded ITAM receptor that costimulates proinflammatory cytokine secretion by macrophages and neutrophils. J Immunol 2015; 195: 3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EJ, Lee SM, Suk K, et al. CD300a and CD300f differentially regulate the MyD88 and TRIF-mediated TLR signalling pathways through activation of SHP-1 and/or SHP-2 in human monocytic cell lines. Immunology 2012; 135: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir M, Tolosa L, Asensio VJ, et al. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J Neuroimmunol 2008; 204: 101–109. [DOI] [PubMed] [Google Scholar]
- 49.Moser CV, Moller M, Fleck SC, et al. Inhibition of the protein kinase IKKepsilon attenuates neuropathic pain in mice. Neuropharmacology 2019; 146: 198–211. [DOI] [PubMed] [Google Scholar]
- 50.An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol 2014; 115: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie L, Li W, Hersh J, et al. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab 2019; 39: 2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaud JP, Pimentel-Coelho PM, Tremblay Y, et al. The impact of Ly6Clow monocytes after cerebral hypoxia-ischemia in adult mice. J Cereb Blood Flow Metab 2014; 34: e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X, Liou AK, Leak RK, et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol 2014; 119-120: 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 56.Kramer A, Green J, Pollard J, Jr., et al. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014; 30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodridge HS, Reyes CN, Becker CA, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011; 472: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor S, Mehina E, White E, et al. Suppressing interferon-gamma stimulates microglial responses and repair of microbleeds in the diabetic brain. J Neurosci 2018; 38: 8707–8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho SH, Chen JA, Sayed F, et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci 2015; 35: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sierra A, Gottfried-Blackmore AC, McEwen BS, et al. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007; 55: 412–424. [DOI] [PubMed] [Google Scholar]
- 61.Panza F, Lozupone M, Solfrizzi V, et al. Time to test antibacterial therapy in Alzheimer’s disease. Brain 2019; 142: 2905–2929. [DOI] [PubMed] [Google Scholar]
- 62.Fulop T, Witkowski JM, Bourgade K, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front Aging Neurosci 2018; 10: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dando SJ, Mackay-Sim A, Norton R, et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev 2014; 27: 691–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh V, Sadler R, Heindl S, et al. The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab 2018; 38: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellegrini C, Antonioli L, Colucci R, et al. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 2018; 136: 345–361. [DOI] [PubMed] [Google Scholar]
- 67.Lin L, Zheng LJ, Zhang LJ. Neuroinflammation, gut microbiome, and Alzheimer’s disease. Mol Neurobiol 2018; 55: 8243–8250. [DOI] [PubMed] [Google Scholar]
- 68.Hu X, Leak RK, Thomson AW, et al. Promises and limitations of immune cell-based therapies in neurological disorders. Nat Rev Neurol 2018; 14: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang R, Liu Y, Ye Q, et al. RNA sequencing reveals novel macrophage transcriptome favoring neurovascular plasticity after ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 720–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai W, Dai X, Chen J, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019; 4: 131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Y, Liu H, Zhang H, et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci 2017; 37: 4692–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab 2020; 40: 639–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaceb A, Ozen I, Padel T, et al. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab 2018; 38: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab 2018; 38: 1276–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W, Zhao J, Wang R, et al. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther 2019; 25: 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Xia Y, Ye Q, et al. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J Neurosci 2018; 38: 10168–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006; 12: 441–445. [DOI] [PubMed] [Google Scholar]
- 78.Xie D, He M, Hu X. Microglia/macrophage diversities in central nervous system physiology and pathology. CNS Neurosci Ther 2019; 25: 1287–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-2-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-3-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-4-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-5-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-6-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-xlsx-7-jcb-10.1177_0271678X20925655 for Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia by Ligen Shi, Marcelo Rocha, Wenting Zhang, Ming Jiang, Sicheng Li, Qing Ye, Sulaiman H Hassan, Liqiang Liu, Maya N Adair, Jing Xu, Jianhua Luo, Xiaoming Hu, Lawrence R Wechsler, Jun Chen and Yejie Shi in Journal of Cerebral Blood Flow & Metabolism