Abstract
Understanding how progenitor cell function is regulated in the mammary gland is an important developmental problem that has significant implications for breast cancer. Although it had been assumed that the expression the α6β4 integrin (β4) is restricted to the basal lineage, we report that alveolar progenitor cells in the mouse mammary gland also express this integrin based on analysis of single cell RNA-Seq data. Subsequent experiments using a mouse mammary epithelial cell line (NMuMG) confirmed this finding and revealed that β4 is essential for maintaining progenitor function as assessed by serial passage mammosphere assays. These data were substantiated by analyzing the alveolar progenitor population isolated from nulliparous mouse mammary glands. Based on the finding that the alveolar progenitor cells express Whey Acidic Protein (WAP), WAP-Cre mice were crossed with itgβ4flox/flox mice to generate conditional knock-out of β4 in alveolar progenitor cells. These itgβ4flox/flox WAP-Cre+ mice exhibited significant defects in alveologenesis and milk production during pregnancy compared to itgβ4flox/flox WAP-Cre− mice, establishing a novel role for the β4 integrin in alveolar progenitor function and alveologenesis.
Keywords: Integrin, Mammary Gland, Progenitor Cell, Alveologenesis, Lactation
Introduction
The lactating mammary gland is composed of a branched system of excretory ducts and secretory alveoli. The epithelium of ducts and secretory alveoli consists of two layers of cells: a layer of luminal cells, which is responsible for the synthesis and secretion of milk components, and a layer of myoepithelial (basal) cells that generate contractility for milk ejection (1, 2). Current evidence indicates that the ductal luminal, alveolar luminal and basal lineages are maintained by distinct progenitor cells (3). The nature of these progenitor cells is of considerable interest in understanding how the mammary gland develops in puberty and expands dramatically during pregnancy. Moreover, it is likely that these cells function as the cells of origin for the different sub-types of breast cancer (3-5).
Our interest is in understanding how integrins contribute to the different lineages and the function of progenitor cells in the mammary gland. Integrins have the capacity to influence many aspects of cell behavior including stem/progenitor cell function and survival in their role as signaling receptors for extracellular matrix proteins (6). Moreover, ligand-independent integrin functions can also affect cell behavior (6). We are particularly interested in the α6β4 integrin (referred to as β4 because there is only one β4 integrin heterodimer), which functions primarily as a receptor for laminins in the basement membrane (7, 8). The assumption has been that β4 expression is restricted to the basal lineage and that its major role is to anchor these cells to the basement membrane and contribute to the contractility needed for milk ejection, although definitive data to support these assumptions are lacking (9). Nonetheless, β4 expression has not been detected in luminal or alveolar cells and it is presumed that it does not contribute to this lineage.
Our perspective on the contribution of the β4 integrin to mammary gland biology was challenged by recent studies that used single cell RNA-seq and other approaches to profile the distinct lineages present in the developing and lactating mammary gland (10). Our analysis of these data revealed that expression of the β4 integrin is not restricted to basal cells and that it is also expressed in population of progenitor cells that give rise to alveolar luminal cells during pregnancy and lactation. These unexpected observations prompted us to assess a causal role for this integrin in the function of alveolar progenitor cells.
Results
The β4 integrin is expressed in alveolar progenitor cells and contributes to their function:
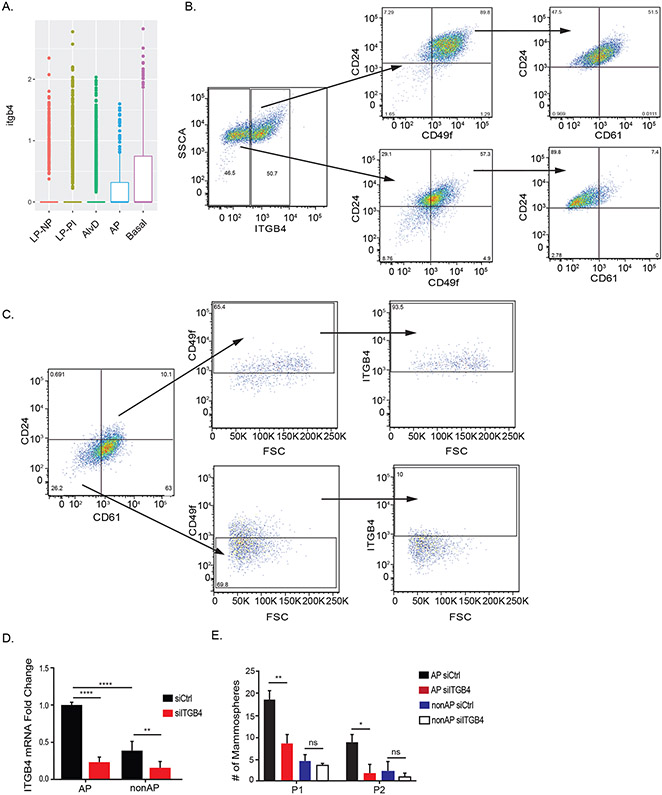
This project was initiated by our analysis of data reported in a study that used single cell RNA-seq to profile mouse mammary epithelial cells at different stages of development: nulliparous, gestation, lactation and involution (10). These cells were grouped into populations using unsupervised and hierarchical clustering based on gene expression, and cell populations were identified based on known markers. Although it had been assumed that the expression of the β4 integrin is restricted to the basal lineage in the mammary gland, our analysis of these data revealed that this integrin is also expressed in a population of alveolar progenitor cells but not in either luminal progenitor or differentiated alveolar cells (Figure 1A). Further analysis of these data revealed that expression of the β4 integrin is not significantly different among sub-clusters within the AP cluster (data not shown).
Figure 1: Alveolar progenitor cells express the β4 integrin and it contributes to their function.
A) Single cell RNAseq analysis of ITGB4 expression in differentiated luminal, alveolar progenitor, and differentiated basal mouse mammary cell populations based on reference (10). B) NMuMG cells were sorted based on β4 surface expression into two distinct populations (β4high and β4 low) and these populations were analyzed by flow cytometry for alveolar progenitor cell surface markers (CD24, CD49f, and CD61). C) NMUMG cells were analyzed by flow cytometry for expression of CD24, CD49f, CD61, and β4. D) NMuMG cells were sorted into alveolar progenitor (CD24high/CD49fhigh/CD61high) and non-alveolar progenitor (CD24low/CD49flow/CD61low) populations. Expression of the β4 integrin was diminished in these populations using siRNA, and their progenitor potential was assessed using serial passage mammosphere assays (E).
Based on the data described above, we undertook our own analysis of the expression and function of the β4 integrin in mammary epithelial cells. Our initial experiments used NMuMG cells, an immortalized mammary epithelial cell line with a luminal phenotype. These cells were sorted based on β4 surface expression into β4high and β4low populations and these populations were analyzed for expression of alveolar progenitor cell surface markers (CD24, CD49f, and CD61) (3). As shown in Figure 1B, these markers were enriched in the β4high compared to the β4low populations. To assess the relative expression of β4 on alveolar progenitor cells, parental NMuMG cells were analyzed by flow cytometry for CD24, CD49f, CD61 and β4 expression. This analysis revealed that the alveolar progenitor population (CD24high/CD49fhigh/CD61high) was 93.5% positive for β4 surface expression and that the non- alveolar progenitor population (CD24low/CD49flow/CD61low) was only 10% positive (Figure 1C). Also, β4 mRNA expression was significantly higher in the alveolar progenitor population compared to the non-alveolar progenitor population (Figure 1D). These data confirm that the NMuMG cell line is luminal and indicate that it is enriched in progenitor cells.
Serial passage mammosphere assays were used to evaluate the potential contribution of the β4 integrin to AP function. As expected, the CD24high/CD49fhigh/CD61high population had robust mammosphere forming ability that was lacking in the CD24low/CD49flow/CD61low population (Figure 1E). Diminishing β4 expression in the CD24high/CD49fhigh/CD61high population using siRNA resulted in a significant decrease in serial mammosphere formation compared to cells treated with control siRNA (Figure 1E) supporting the hypothesis that this integrin contributes to the pluripotency of alveolar progenitor cells.
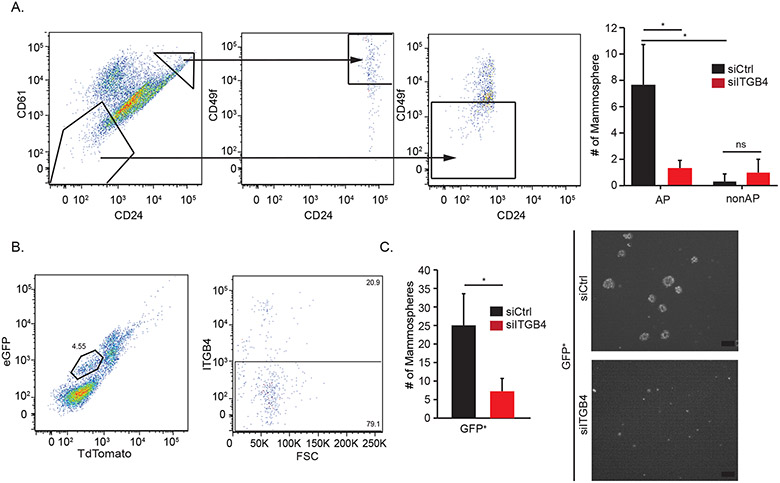
Based on the data we obtained with NMuMG cells, we investigated the expression and function of the β4 integrin in alveolar progenitor cells in the nulliparous mouse mammary gland. Initially, we sorted mammary epithelial cells harvested from nulliparous C57/B6 mice into alveolar progenitor and non-alveolar progenitor populations based on the expression of CD24/CD49f/CD61 (Figure 2A). Knock-down of β4 in the alveolar progenitor population using siRNA reduced mammosphere forming ability significantly compared to the control cells (Figure 2A). These results suggest that β4 contributes to the progenitor potential of alveolar progenitor cells in nulliparous female mice.
Figure 2: Alveolar progenitor cells in the nulliparous mouse mammary gland express the β4 integrin and it contributes to their function.
A) Alveolar progenitor and non-alveolar progenitor cells were isolated from the mammary fat pads of nulliparous C57/B6 mice based on expression of CD24/CD49f/CD61 surface markers. Expression of the β4 integrin was diminished in these populations using siRNA, and their progenitor potential was assessed using serial passage mammosphere assays. B) WAP-Cre+/ROSAmT/mG virgin glands were isolated and analyzed by flow cytometry for GFP and TdTomato. C) Alveolar progenitor cells were isolated from virgin WAP-Cre+/ROSAmT/mG mice by sorting for WAP+ (GFP+) cells. Expression of the β4 integrin was diminished in these populations using siRNA, and their progenitor potential was assessed using serial passage mammosphere assays (Scale bar = 200μm).
Another approach taken to investigate the contribution of β4 to alveolar progenitor cells was based on a lineage tracing study using WAP-Cre mice, which revealed a distinct population of WAP+ cells in the virgin mammary gland with alveolar progenitor function (5, 11). Based on this observation, we used a similar reporter system (WAP-Cre/ROSAmT/mG) to isolate the small population of WAP+ alveolar progenitors (GFP+) in the nulliparous mammary gland (Figure 2B)(12). Flow cytometry revealed that ~20% of these GFP+ cells expressed β4 and they exhibited β4-dependent mammosphere formation (Figure 2C). These data support the hypothesis that β4 is expressed in alveolar progenitor cells and contributes to their function.
Conditional deletion of the β4 integrin in WAP+ cells supports its functional role in alveologenesis:
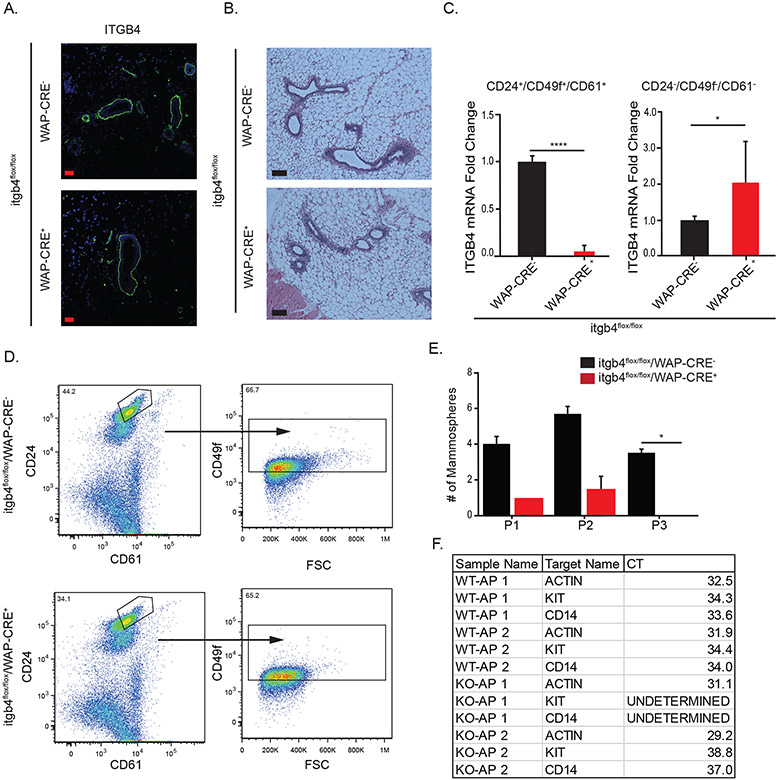
The fact that the alveolar progenitor population expresses WAP (5) provided an opportunity to delete β4 expression in this population specifically by crossing WAP-Cre mice with itgβ4flox/flox mice to generate itgβ4flox/flox WAP-Cre− (WAP-Cre−) and itgβ4flox/flox WAP-Cre+ (WAP-Cre+) mice (11, 13). We observed no significant change in basal β4 expression (Figure 3A) or gland morphology (Figure 3B) in WAP-Cre− nulliparous mice compared to WAP-Cre+ mice. However, the alveolar progenitor population isolated from the WAP-Cre+ mammary glands exhibited loss of β4 mRNA expression as assessed by qPCR (Figure 3C) and reduced mammosphere forming ability compared to the alveolar progenitor population from WAP-Cre− glands (Figure 3D and 3E). Interestingly, we did not see a significant change in the number of AP cells isolated from the WAP-Cre− and WAP-Cre+ nulliparous glands based on expression of the surface markers CD24/CD49f/CD61 (Figure 3D). We did find, however, that the expression of other known AP markers (c-kit and CD14) (1) was diminished in the WAP-Cre+ AP cells compared to WAP-Cre− AP cells (Figure 3F), suggesting that the contribution of the β4 integrin to gene expression in AP cells is selective.
Figure 3: Conditional knock out of β4 in AP cells in the nulliparous mammary gland does not alter morphology but does impair progenitor function in vivo.
WAP-Cre+ and WAP-Cre− virgin mammary tissue was isolated at 4-6 weeks. A) β4 surface expression was analyzed by immunofluorescence microscopy (Scale bar = 20μm) and B) gland morphology was analyzed by H&E staining (Scale bar = 200μm). C) Alveolar progenitor cells were isolated from nulliparous WAP-Cre+ and WAP-Cre− mice, and knock-out of β4 was confirmed by qPCR. D, E) Alveolar progenitor cells isolated from nulliparous WAP-Cre+ and WAP-Cre− mice were assayed for progenitor potential using mammosphere assays. Mammospheres ≥ 40 μm in diameter were counted using ImageJ. F) Alveolar progenitor cells were isolated from nulliparous WAP-Cre+ and WAP-Cre− mice and expression of c-kit and CD14 was quantified by qPCR. CT values from two biological replicates of each genotype are shown.
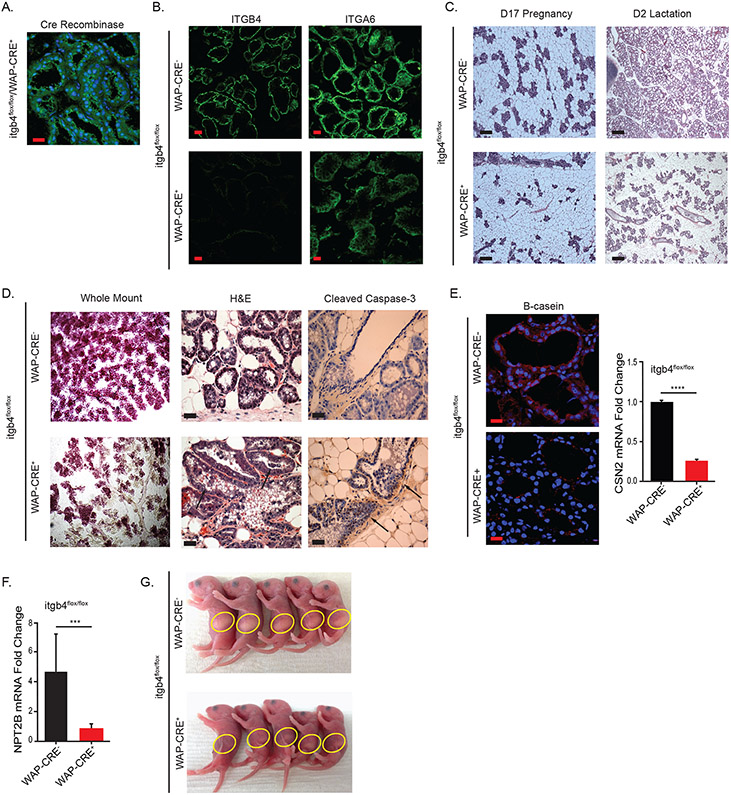
Our findings that the β4 integrin is expressed on alveolar progenitor cells and is necessary for their progenitor function infer that the WAP-Cre+ mice should exhibit deficiency in alveologenesis and lactation. As expected, the WAP-Cre+ glands expressed Cre Recombinase during lactation (Figure 4A). Interestingly, immunostaining of the β4 and α6 subunits revealed that expression of the β4 was significantly reduced in the WAP-Cre+ compared to WAP-Cre− glands, while the expression of the α6 subunit was maintained (Figure 4B). This result indicates that the WAP-Cre+ glands express the α6β1 integrin. To assess whether loss of the β4 integrin affects alveologenesis, we compared the mammary glands isolated from lactating (2 days post birth) WAP-Cre− and WAP-Cre+ mice by whole-mount analysis and hematoxylin and eosin (H&E) staining. This analysis revealed that the development of the alveolar structures and their density in WAP-Cre+ glands are significantly reduced compared to WAP-Cre− glands (Figures 4C & 4D). Also, the WAP-Cre+ glands had significantly more apoptotic cells, including apoptotic cells and cellular debris present in their lumens, as assessed by cleaved caspase 3 staining (Figure 4D). These data prompted us to assess the impact of β4-deletion on β-casein expression and milk production. Indeed, β-casein expression was reduced substantially in WAP-Cre+ glands compared to WAP-Cre− glands (Figure 4E). Also, expression of the Na-Pi type IIb co-transporter (Npt2b), which is a known marker of secretory function in the mammary gland (14), was significantly reduced in WAP-Cre+ glands compared to WAP-Cre− glands (Figure 4F). The functional consequences of decreased β-casein expression were assessed by analyzing the milk content of lactating pups of WAP-Cre+ and WAP-Cre− females. As shown in Figure 4G, the pups of WAP-Cre+ females had little milk in their stomachs compared to pups of WAP-Cre− females and they had a high rate of mortality presumably because of a lack of milk (data not shown).
Figure 4: Conditional β4 depletion results in impaired alveologenesis and milk production in vivo.
A) Immunostaining of Cre Recombinase in WAP-Cre+ glands from mice at day 2 of lactation. B) Immunostaining of ITGB4 and ITGA6 WAP-Cre+ and WAP-Cre− glands from mice at day 2 of lactation. (Scale bar = 20μm) C) H&E staining of WAP-Cre+ and WAP-Cre− glands from mice at day 17 of pregnancy and day 2 of lactation (Scale bar = 500μm). D) Whole-mount, H&E, and cleaved caspase 3 staining of WAP-Cre+ and WAP-Cre− glands from mice at day 2 of lactation. (Scale bar = 100μm) E) β-casein protein expression was analyzed using immunostaing and mRNA expression was quantified by qPCR in WAP-Cre+ and WAP-Cre− glands at day 2 of lactation. (Scale bar = 20μm) F) Npt2b expression was quantified by qPCR in WAP-Cre+ and WAP-Cre− glands at day 2 of lactation. G) Milk uptake in pups born to WAP-Cre+ and WAP-Cre− mice was compared at 2 days post birth.
The data reported in this study highlight a novel role for the β4 integrin in alveolar progenitor function and alveologenesis that differs from its presumed function in anchoring basal cells to the basement membrane. Such a role is not indicated by immunofluorescent staining of this integrin in the mammary gland because the staining is concentrated in the basal layer, e.g., (15). The power of single cell RNA-Seq, however, revealed that the relatively small population of alveolar progenitor cells, but not other progenitor cells or differentiated luminal cells, expresses β4. Although it is not known whether alveolar progenitor cells are in direct contact with the basement membrane, our results suggest that the function and signaling properties of β4 may differ between differentiated basal cells and alveolar progenitor cells. Interestingly, our data also indicate that the α6β1 integrin, which is expressed in the WAP-Cre+ glands, cannot compensate for the contribution of α6β4 to alveologenesis.
It is worth speculating on the potential connection between β4 expression and function in alveolar progenitor cells and the genesis of specific breast cancer sub-types because there is evidence that cells with alveolar progenitor characteristics can serve as cells of origin of mammary tumors with basal differentiation (5). Moreover, we reported previously that β4 expression in human breast tumors is associated primarily with tumors of the basal sub-type (16).
The findings reported here build upon our past work demonstrating that the β4 integrin contributes to the elongation of the mammary bud during the early stages of mammary gland development based on our analysis itgb4flox/flox MMTV-Cre− and itgb4flox/flox MMTV-Cre+ mice (17). Together, our studies support distinct roles for this integrin in mammary bud development and alveologenesis.
Materials and Methods
Cell culture and animal models:
NMuMG cells were purchased from ATCC (CRL-1636). C57BL/6 itgβ4flox/flox mice were generated as described (13). C57BL/6 WAP-Cre mice (11) and B6.129 ROSA26mT/mG mice (12) were obtained from Ingolf Bach and Jaime Rivera, respectively at the University of Massachusetts Medical School (11, 12). All of the animal breeding and procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Antibodies:
FITC anti-mouse CD24, APC anti-human/mouse CD49f, PE anti-mouse/rat CD61, Biotin anti-mouse β4 (346–11A), and Brilliant Violet 421 Streptavidin were purchased from BioLegend, Inc. Rat anti-human integrin α6 (GoH3) was purchased from BD Biosciences. Rat anti-mouse integrin β4 (346–11A-3C3) and mouse anti-β-casein (H-4) were purchased from Santa Cruz Biotechnology, Inc.. Rabbit anti-Cre recombinase (ab137240) was purchased from Abcam. Rabbit anti-cleaved caspase-3 (D175) was purchased from Cell Signaling Technology, Inc.. Donkey anti-rabbit, Alexa Fluor 488 and donkey anti-mouse, Alexa Fluor 555 were purchased from Invitrogen. Goat anti-rat conjugated APC, donkey anti-rat conjugated 488 and goat anti-rabbit conjugated 488 were obtained from Jackson Immune Research Laboratories, Inc.
Dissociation and analysis of mammary epithelial cells:
The fourth inguinal mammary glands were dissected and either processed for histological analysis or dissociated into single cells for flow cytometry and mammosphere assays. For histological analysis, the glands were embedded in either paraffin or Optimal Cutting Temperature (OCT) compound, and cut into 5um (paraffin) or 12um (OCT) sections. Hematoxylin and eosin (H&E) and immunofluorescence (IF) staining were performed as described (18). Lactating mammary tissue for whole mount analysis was fixed using a whole mount fixing solution (25% glacial acetic acid, 75% ethanol) for one hour and stained using a whole mount stain (0.2% (w/v) carmine, 0.5% (w/v) aluminum potassium sulfate in distilled water) overnight. Tissues were dehydrated in a series of washes: 70%, 95%, and 100% ethanol for 15 minutes each at room temperature. Tissues were cleared in xylene for 1 hour and mounted on a slide for analysis.
For separation into single cells, the glands were washed in 1X PBS and incubated in a digestion medium consisting of Advanced DMEM/F12 (Gibco), 1X GlutaMAX (Gibco), 10mM HEPES (Gibco), insulin (Sigma), 5mg/ml collagenase A (Sigma), 1X Trypsin-EDTA (Gibco), penicillin-streptomycin (Gibco), fetal bovine serum (HyClone) and gentamicin (Gibco) at 37°C for 2 hours. These samples were vortexed every 30 minutes, and filtered through a 40-μm nylon strainer with two successive washes in PBS.
Flow cytometry:
Antibody staining and flow cytometry were accomplished as described (19). Flow cytometry and data analysis were performed by Sony SH800 sorter and FlowJo software.
Mammosphere assays:
Cells were plated in UltraLow attachment six-well plates in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with B27, epidermal growth factor, and fibroblast growth factor as previously described (20). The number of mammospheres per well were counted 5–7 days after plating. Briefly, images of all spheres were taken before each passage and measured using ImageJ. Spheres over the 40μm in diameter were counted as mammospheres.
RNAi:
For β4 small interfering RNA (siRNA) knockdown, cells were transfected using Dharmafect 4 (Dharmacon). Cells were processed for qPCR 48 hours after transfection. β4 (sc-35679) and control siRNA were purchased from Santa Cruz Biotechnology.
Real-time quantitative (q)PCR:
RNA extraction was performed using an RNA isolation kit (BS88133, Bio Basic Inc.). cDNAs were produced using an AzuraQuant cDNA synthesis kit (AzuraGenomics) and AzuraQuant Green Fast qPCR Mix LoRox (AzuraGenomics) was used as the qPCR master mix. Experiments were performed in triplicate and normalized to 18S ribosomal RNA (18S rRNA). qPCR primer sequences were obtained from the Massachusetts General Hospital/Harvard Medical School PrimerBank (http://pga.mgh.harvard.edu/primerbank/). In mouse tissue, qPCR was performed as previously described (20). Experiments were performed in triplicate and normalized to β-actin. qPCR primers for CD14 (Mm00438094_g1), c-kit (Mm00445212_m1) β4 (Mm01266844_m1), Npt2b (Mm01215846_m1) and β-actin (Mm02619580) were purchased from ThermoFisher Scientific.
Single Cell RNA-seq Analysis:
Analysis of the single cell RNA-seq data in Bach et al (2017) was performed as previously described (10).
Highlights.
Alveolar progenitor cells in the mouse mammary gland express the β4 integrin
The β4 integrin contributes to alveolar progenitor function
Targeted deletion of β4 in nulliparous glands impedes alveologenesis and milk production
Acknowledgements:
This work was supported by National Institutes of Health (NIH) grants CA168464, CA203439 (A.M.M) and CA221780 (H.L.G.).
References:
- 1.Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes & development. 2014;28(11):1143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nature Reviews Molecular Cell Biology. 2005;6(9):715–25. [DOI] [PubMed] [Google Scholar]
- 3.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes & development. 2009;23(22):2563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, Reis-Filho JS, Smalley MJ. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell stem cell. 2010;7(3):403–17. [DOI] [PubMed] [Google Scholar]
- 5.Tao L, van Bragt MP, Li Z. A Long-Lived Luminal Subpopulation Enriched with Alveolar Progenitors Serves as Cellular Origin of Heterogeneous Mammary Tumors. Stem Cell Reports. 2015;5(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–40. Epub 2015/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(22):8970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee EC, Lotz MM, Steele GD Jr., Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. The Journal of Cell Biology. 1992;117(3):671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisticò P, Di Modugno F, Spada S, Bissell MJ. β1 and β4 integrins: from breast development to clinical practice. Breast Cancer Research. 2014;16:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach K, Pensa S, Grzelak M, Hadfield J, Adams DJ, Marioni JC, Khaled WT. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat Commun. 2017;8(1):2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25(21):4323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis (New York, NY : 2000). 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 13.Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. α6β4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci. 2008;28(26):6714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shillingford JM, Miyoshi K, Robinson GW, Bierie B, Cao Y, Karin M, Hennighausen L. Proteotyping of Mammary Tissue from Transgenic and Gene Knockout Mice with Immunohistochemical Markers: a Tool To Define Developmental Lesions. J Histochem Cytochem. 2003;51(5):555–65. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Pursell B, Lu S, Chang TK, Mercurio AM. Regulation of β4 integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J Cell Sci. 2009;122(Pt 14):2473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin β4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14(4):1050–8. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Sun H, Feltri ML, Mercurio AM. Integrin β4 regulation of PTHrP underlies its contribution to mammary gland development. Developmental Biology. 2015;407(2):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. The Journal of clinical investigation. 2011;121(12):4655–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479(7372):189–93. [DOI] [PubMed] [Google Scholar]
- 20.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, Norum JH, Toftgard R, Shaw LM, Mercurio AM. Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Reports. 2014;7(3):747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]