Abstract
Dermatomyositis is an inflammatory disorder involving muscle and skin. Similar to many other autoimmune diseases, environmental factors appear to trigger the onset of disease in some cases. Many drugs have been reported to be associated with dermatomyositis, and rarely infections have been described as potential triggering agents. Here we are describing a case of dermatomyositis that developed after doxycycline and levofloxacin use, who also had recent Epstein-Barr virus infection. Dermatomyositis associated with doxycycline or levofloxacin use has not yet been described in the literature, while reports of dermatomyositis after Epstein-Barr virus infection have been rare and limited to juvenile dermatomyositis or in association with cancer. It is important for clinicians to be aware of this rare association so that the diagnosis and treatment can be exercised promptly.
Key Indexing Terms: Dermatomyositis, Antibiotics associated dermatomyositis, EBV associated dermatomyositis
INTRODUCTION
Dermatomyositis (DM) is an autoimmune condition characterized by cutaneous rash and chronic inflammatory myopathy resulting in proximal muscle weakness. Autoimmune diseases can develop in genetically predisposed individuals exposed to specific environmental insults.1 Environmental triggers that have been associated with DM include UV radiation, viruses, emotional stress and drugs. Drug-induced DM is rare and impossible to predict. Although D-penicillamine, Non-steroidal Anti-inflammatory Drugs and statins are most associated with drug-induced DM, several other medications have been described to induce this phenomenon.2 Epstein-Barr virus (EBV) past exposure as been shown to be positively associated with juvenile dermatomyositis (JDM) in a study. The patients with JDM had a higher positive rate of nuclear and capsid antigen IgG as compared to the control group.3 In a study among patients with nasopharyngeal carcinoma, those with DM had higher viral capsid IgA titers compared to the patients without DM.4 We describe here a case of a woman who, following a recent EBV infection, developed DM after treatment with doxycycline and levofloxacin.
CASE PRESENTATION
A 33-year-old European American female with a history of hypertension presented to the hospital with a 3-week history of a diffuse painful rash, upper and lower proximal extremity weakness and odynophagia/dysphagia. The patient was diagnosed with community-acquired pneumonia 1 month prior to this presentation. She was treated with doxycycline for 3 days and subsequently switched to levofloxacin due to inadequate treatment response. On day 7, after starting levofloxacin, the patient began to notice difficulty climbing stairs and rising from a sitting position. Within 1 week of weakness onset, the patient developed odynophagia with dysphagia and a diffuse erythematous and painful rash on the face and neck region. She was admitted to an outside hospital where she was diagnosed with rhabdomyolysis and treated with IV fluids and steroids. She was discharged after transient improvement of symptoms but ultimately arrived at the current hospital due to worsening of weakness and rash. Due to her odynophagia on presentation, the patient was unable to eat solid food and was confined to liquids for 1 week. She was not able to ambulate due to lower extremity weakness and had difficulty moving her arms above the shoulder level. She had a family history significant for a mother with psoriasis. She was not on any medications prior to her admission. She had no known allergies to medications or food. She denied the use of tobacco, alcohol or other illicit substances. She did not have children and had no history of miscarriages.
On physical exam, the patient had an erythematous, macular rash with ill-defined borders, especially prominent on her eyelids, face including bilateral cheeks, neck, upper chest and upper back. Lesions on the eyelids had a purplish hue (Figure 1). Rashes on eyelids, neck and upper chest had painful erosions with crusting. Rashes extended to lower chest, lower back, arms and proximal thighs but were fainter without erosion or crusting. No rashes or papules were found on the skin overlying small joints of hands or knees. The patient described the rash as burning, painful and mildly itchy. She had no oral or genital ulcers on examination. Neurologic examination revealed 4/5 muscle strength in proximal upper extremities and 3/5 muscle strength in proximal lower extremities. Strength in distal extremities, including handgrips and ankle flexion/dorsiflexion, was intact. Her deep tendon reflexes were intact, and plantar reflexes were normal. Her cardiovascular, respiratory and abdominal examination was within normal limits.
FIGURE 1.
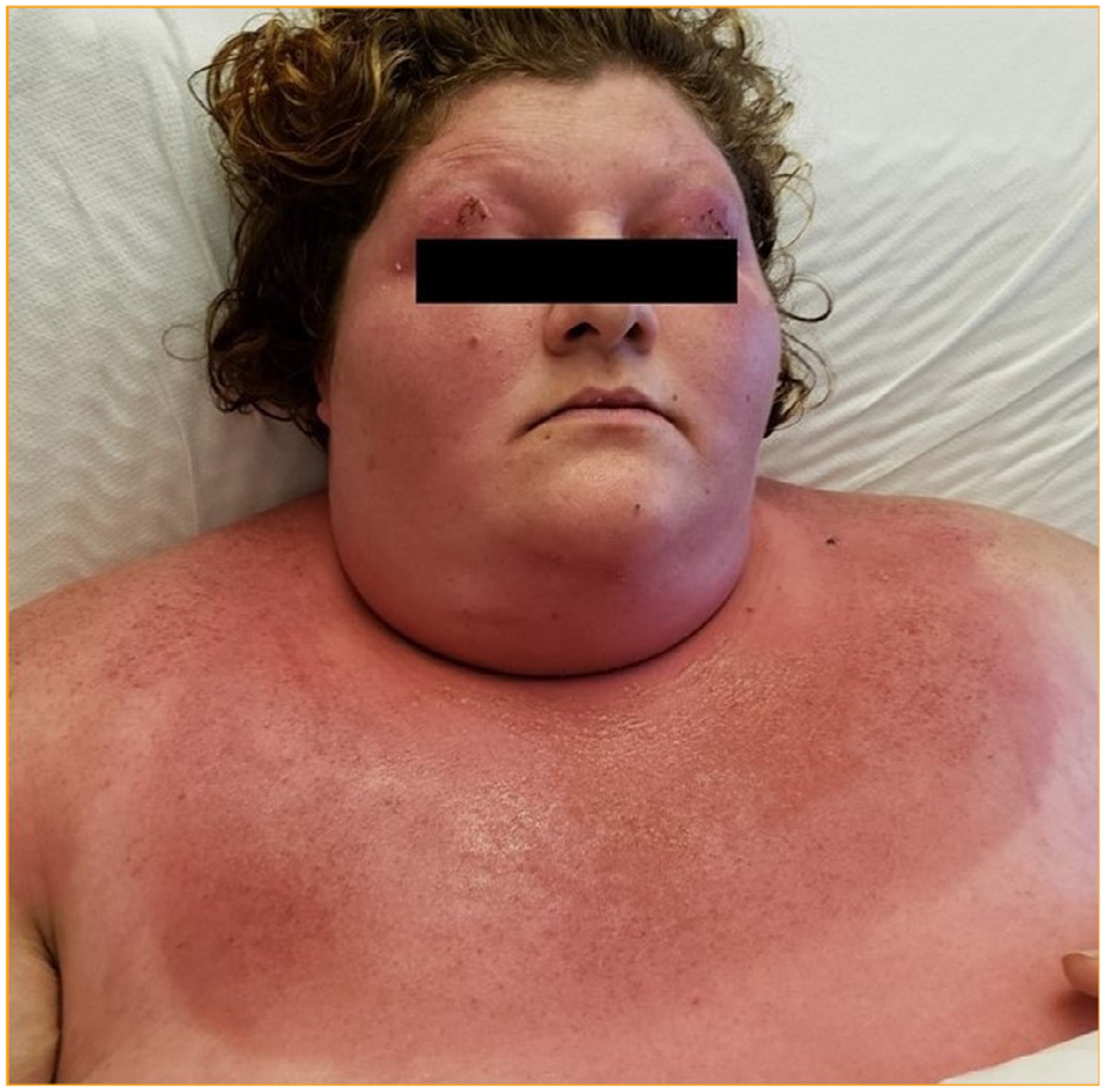
Skin manifestation. Voilaceous rash around the eyelids and face. Rash on the neck and upper chest (Shawl Sign).
Laboratory parameters were significant for elevated aspartate transaminase of 281 IU/L (range 15–46) and alanine transaminase of 99 IU/L (range 10–26). CBC showed thrombocytopenia of 110 × 103/mcl (range 150–450 × 103) with WBC and hemoglobin at normal ranges. The patient had creatine phosphokinase (CPK) of 8500 Unit/L (range 22–269) and aldolase of 31.5 Unit/L (range 3.3–10.3). TSH was within the normal range. With the presence of normal bilirubin and alkaline phosphatase, absence of an immediate cause of liver injury and presence of high CPK and aldolase, the elevation of aspartate transaminase and alanine transaminase were considered to originate from muscle source. Serum studies for antinuclear antibodies, extractable nuclear antibodies (dsDNA Abs, Anti-smith Abs, RNP Abs, Anti-SSA, Anti-SSB, SCL 70 Abs and Anti-Centromere), and the antiphospholipid antibodies were negative. Myositis specific antibodies (Jo-1 Ab, PL-7 Ab, PL-12 Ab, EJ Ab, OJ Ab, SRP Ab, Mi-2 Ab, MDA-5 Ab, TIF-1y Ab and NXP-2 Ab) were negative as well. The 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibody was also negative. Of note, patient had elevated EBV titers (viral capsid antigen [VCA] IgM = 56.2; VCA IgG = 347; EBV nuclear antigen [EBNA] IgG = 300). The punch biopsy of the skin rash featured an interface dermatitis characterized by a sparse superficial perivascular infiltrate of lymphocytes joined by vacuolar alteration along the dermo-epidermal junction and scattered individually necrotic keratinocytes, along with the presence of abundant interstitial mucin within the reticular dermis; findings consistent with DM (Figure 2). The muscle biopsy of the left quadriceps revealed inflammatory infiltrate with minimal fiber necrosis.
FIGURE 2.
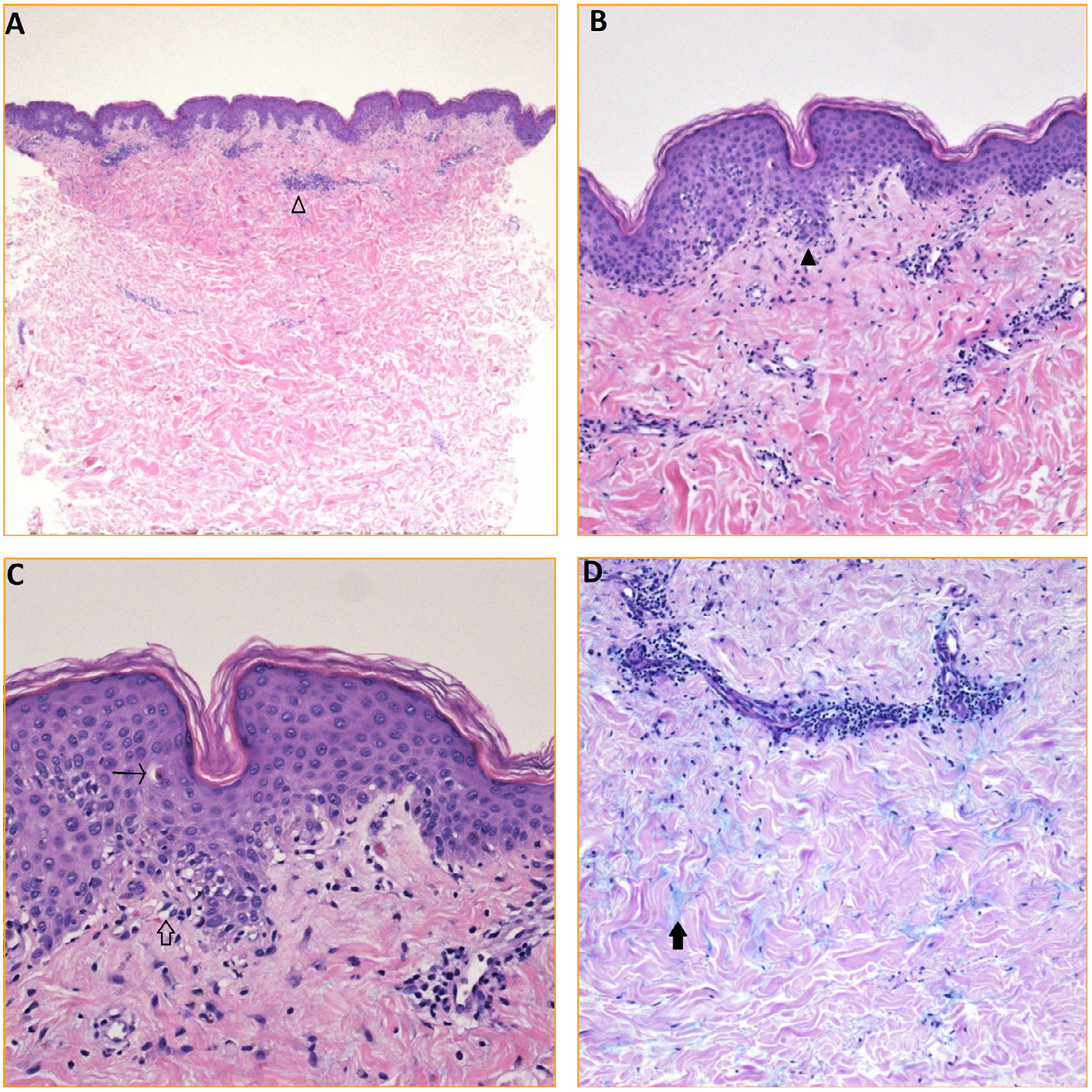
Skin biopsy findings. (A) H&E (20×): punch biopsy of skin shows a superficial perivascular infiltrate of entirely lymphocytes (empty arrowhead). (B) H&E (100×): subtle interface change characterized by vacuolar alteration along the dermo-epidermal junction (black arrowhead). (C) H&E (200×): obvious vacuolization along the dermo-epidermal junction (empty arrow) with rare individually necrotic keratinocytes (arrow). (D) Alcian blue (100×): copious mucin (black arrow) situated between bundles of collagen within the reticular dermis.
The patient was diagnosed with DM, likely secondary to infectious trigger and antibiotics use. The patient was treated with prednisone 60 mg daily and 1 course of intravenous immunoglobulin at the dose of 2g/Kg administered over 3 days. Her muscle strength improved rapidly, odynophagia resolved and CPK normalized. She was able to ambulate with a walker. The skin rash, however, did not improve and posed a significant challenge in treatment. She was treated with topical steroids and emollient with a mild relief. She received 1 dose of IV rituximab 1,000 mg and was eventually discharged with prednisone 60 mg daily, mycophenolate mofetil 1 gram twice daily and hydroxychloroquine 200 mg twice daily. On follow-up examination 2 weeks later in an outpatient office, the patient noted a significant improvement in muscle weakness; however, the cutaneous lesions persisted with little improvement. Routine cancer screening revealed no underlying malignancy. The patient was encouraged to avoid sun exposure. She was continued on mycophenolate mofetil 1.5 gram BID, hydroxychloroquine 200 mg BID, the tapering prednisone, and the monthly intravenous immunoglobulin infusions. At the 3-month follow-up appointment, there was a significant improvement in the cutaneous lesions, the muscle weakness had fully resolved and CPK had normalized.
DISCUSSION
Dermatomyositis is an autoimmune disorder characterized by skin rash and muscle weakness secondary to an inflammatory process. A genome-wide association study has shown certain genetic predispositions for DM.5 Genetically predisposed individuals might be more likely to develop DM after single- or multiple environmental triggers. In DM, like many autoimmune disorders, drugs have been proposed as a possible trigger for autoimmunity.
In a published review of drug-induced DM of 70 cases, D-penicillamine, Non-steroidal Anti-inflammatory Drugs and statins were among the most common medications associated with DM.2 The mechanism of drug-induced inflammatory myositis or DM has not been well studied. To our knowledge, there have been no case reports published that have described the development of DM secondary to fluoroquinolone or doxycycline use. Fluoroquinolones have been associated with various muscle-related disorders, including tendinitis, tendinosis, tendon rupture, arthralgia, myalgia and rhabdomyolysis.6 Fluoroquinolones work by inhibiting DNA gyrase and topoisomerase IV, 2 enzymes essential for DNA replication and transcription within the bacterial cell.7 The mechanism behind fluoroquinolone-associated muscle disorders is not well understood, but different hypotheses have been postulated. For example, it has been proposed that fluoroquinolone-mediated disruption of human topoisomerases IIa and IIb, which are present in the mitochondria, could be contributing to the drug’s adverse effects. There are studies that have shown that high doses of fluoroquinolones can inhibit human topoisomerases IIa or IIb, but these observations were not seen with clinically relevant drug concentrations.8,9 Fluoroquinolones have also been shown to chelate bivalent cations and proteins, generate oxidative stress and reduce mitochondrial membrane potential, all of which could contribute to their toxicity.10 It is possible that fluoroquinolone-dependent damage of muscle components is involved in the breakage of immune tolerance, which could eventually lead to the inflammatory myositis seen in our patient. We now know a great detail about statin-induced muscle disorders, ranging from myalgia and rhabdomyolysis to immune-mediated necrotizing myositis. The cause of inflammatory myositis after statin exposure has been attributed to the upregulation of HMGCR expression in muscle, which can result in excess antigen presentation, breakage of self-tolerance and generation of autoantibodies to HMGCR.11 Similar mechanisms might play a role in the pathogenesis of the spectrum of muscle disorders associated with fluoroquinolones. The development of specific autoantibodies, however, has yet to be observed. Our patient had 3 days of use of doxycycline as well before Levaquin use. Doxycycline has been associated with inhibition of matrix metalloproteinase-2 and −9, and has been shown to inhibit smooth muscle migration after vascular injury.12 Also, tetracycline has shown to influence various cellular functions like apoptosis of macrophages and reduce matrix synthesis by chondrocytes. In a study involving mouse model of congenital muscular dystrophy, minocycline or doxycycline increased the median lifespan of laminin-alpha2-null mice from approximately 32 days to approximately 70 days, showing its protective effect in neuromuscular function.13 Despite these studies of doxycycline’s overall effects in muscles, there is no known understanding of any role it can play in development of inflammatory myositis. There has been no reports of doxycycline induced DM until the date.
This case is unique due to the presence of 2 imminent environmental triggers for the development of DM: infection and antibiotics use. The respiratory infection our patient had before she was started on antibiotics appears to be viral and likely had been caused by EBV, as evident by VCA IgM positivity. Several studies have linked EBV infection with various autoimmune diseases like systemic lupus erythematosus and multiple sclerosis, and cutaneous manifestations such as hydroa vacciniforme, a photosensitivity dermatosis of childhood.14–16 Prior EBV exposure in JDM patients as compared to control subjects, as well as increased previous EBV exposure on patients with DM associated with nasopharyngeal carcinoma, has been shown in studies.3,4 Pathogenesis of EBV-related DM has not been well studied. EBV-infected cells, upon apoptosis, initiate an innate and adaptive immune response against released cellular antigens and EBV antigens. This results in the production of autoantibodies and EBV antibodies in an effort to control virus-induced inflammation, as well as activation of both autoreactive and EBV-reactive T cells. This response from the immune system is postulated to cause organ and tissue damage leading to the development of systemic lupus erythematosus.17 We can speculate that EBV-induced DM might share a similar immune-pathogenesis.
Our patient had a likely EBV infection and had the exposure to antibiotics prior to the development of DM, thereby making it impossible to tell if 1 or more factors were implicated in the pathogenesis of the disease. We believe it is important for physicians to be wary of these rare associations of DM so that they can quickly spot and treat this condition if it may arise in a patient.
Footnotes
All the authors declare no conflict of interests.
The authors have no financial interest to disclose.
REFERENCES
- 1.Vojdani A. A potential link between environmental triggers and autoimmunity. Autoimmune Dis. 2014;2014: 437231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidler AM, Gottlieb AB. Dermatomyositis induced by drug therapy: a review of case reports. J Am Acad Dermatol. 2008;59:872–880. [DOI] [PubMed] [Google Scholar]
- 3.Huang PY, Zhong ZL, Luo DH, et al. Paired study of 172 cases of nasopharyngeal carcinoma with or without dermatomyositis. Acta Otolaryngol. 2014;134:824–830. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Q, Zhu K, Gao CN, et al. Prevalence of Epstein-Barr virus infection and characteristics of lymphocyte subsets in newly onset juvenile dermatomyositis [e-pub ahead of print]. World J Pediatr. 2019. 10.1007/s12519-019-00314-7. Accessed June 10, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang Q, Wu Z, et al. Genetic association study of TNFAIP3, IFIH1, IRF5 polymorphisms with polymyositis/dermatomyositis in Chinese Han population. PLoS One. 2014;9: e110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall MM, Finnoff JT, Smith J. Musculoskeletal complications of fluoroquinolones: guidelines and precautions for usage in the athletic population. PM R. 2011;3:132–142. [DOI] [PubMed] [Google Scholar]
- 7.Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin Infect Dis. 2001;32(Suppl 1):S9–S15. [DOI] [PubMed] [Google Scholar]
- 8.Hangas A, Aasumets K, Kekalainen NJ, et al. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018;46:9625–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fief CA, Hoang KG, Phipps SD, et al. Examining the impact of antimicrobial fluoroquinolones on human DNA topoisomerase IIalpha and IIbeta. ACS Omega. 2019;4:4049–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalak K, Sobolewska-Wlodarczyk A, Wlodarczyk M, et al. Treatment of the fluoroquinolone-associated disability: the pathobiochemical implications. Oxid Med Cell Longev. 2017;2017: 8023935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohassel P, Mammen AL. Anti-HMGCR myopathy. J Neuromuscul Dis. 2018;5:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendeck MP, Conte M, Zhang M, et al. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girgenrath M, Beermann ML, Vishnudas VK, et al. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann Neurol. 2009;65:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James JA, Neas BR, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44:1122–1126. [DOI] [PubMed] [Google Scholar]
- 15.Lossius A, Johansen JN, Torkildsen O, et al. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis-association and causation. Viruses. 2012;4:3701–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwatsuki K, Satoh M, Yamamoto T, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol. 2006;142:587–595. [DOI] [PubMed] [Google Scholar]
- 17.Draborg AH, Duus K, Houen G. Epstein-Barr virus and systemic lupus erythematosus. Clin Dev Immunol. 2012;2012: 370516. [DOI] [PMC free article] [PubMed] [Google Scholar]


