Abstract
BACKGROUND:
Dexmedetomidine is increasingly used off-label in infants and children with cardiac disease during cardiopulmonary bypass (CPB) and in the postoperative period. Despite its frequent use, optimal dosing of dexmedetomidine in the setting of CPB has not been identified but is expected to differ from dosing in those not supported with CPB. This study had the following aims: (1) characterize the effect of CPB on dexmedetomidine clearance (CL) and volume of distribution (V) in infants and young children; (2) characterize tolerance and sedation in patients receiving dexmedetomidine; and (3) identify preliminary dosing recommendations for infants and children undergoing CPB. We hypothesized that CL would decrease, and V would increase during CPB compared to pre- or post-CPB states.
METHODS:
Open-label, single-center, opportunistic pharmacokinetics (PK) and safety study of dexmedetomidine in patients ≤36 months of age administered dexmedetomidine per standard of care via continuous infusion. We analyzed dexmedetomidine PK data using standard nonlinear mixed effects modeling with NONMEM software. We compared model-estimated PK parameters to those from historical patients receiving dexmedetomidine before anesthesia for urologic, lower abdominal, or plastic surgery; after low-risk cardiac or craniofacial surgery; or during bronchoscopy or nuclear magnetic resonance imaging. We investigated the influence of CPB-related factors on PK estimates and used the final model to simulate dosing recommendations, targeting a plasma concentration previously associated with safety and efficacy (0.6 ng/mL). We used the Wilcoxon rank sum test to evaluate differences in dexmedetomidine exposure between infants with hypotension or bradycardia and those who did not develop these adverse events.
RESULTS:
We collected 213 dexmedetomidine plasma samples from 18 patients. Patients had a median (range) age of 3.3 months (0.1–34.0 months) and underwent CPB for 161 minutes (63–394 minutes). We estimated a CL of 13.4 L/h/70 kg (95% confidence interval, 2.6–24.2 L/h/70 kg) during CPB, compared to 42.1 L/h/70 kg (95% confidence interval, 38.7–45.8 L/h/70 kg) in the historical patients. No specific CPB-related factor had a statistically significant effect on PK. A loading dose of 0.7 μg/kg over 10 minutes before CPB, followed by maintenance infusions through CPB of 0.2 or 0.25 μg/kg/h in infants with postmenstrual ages of 42 or 92 weeks, respectively, maintained targeted concentrations. We identified no association between dexmedetomidine exposure and selected adverse events (P = .13).
CONCLUSIONS:
CPB is associated with lower CL during CPB in infants and young children compared to those not undergoing CPB. Further study should more closely investigate CPB-related factors that may influence CL. (Anesth Analg 2019;129:1519–28)
Dexmedetomidine is used off-label in up to 30% of infants and children with cardiac disease during cardiopulmonary bypass (CPB) and in the postoperative period.1–5 As a central-acting, α−2 adrenergic agonist, dexmedetomidine has sedative and analgesic properties and may help attenuate harmful neuroendocrine and hemodynamic responses to surgical trauma and CPB.1,2,6 Unlike other sedatives, dexmedetomidine is not associated with respiratory depression. It is therefore an ideal choice to facilitate early tracheal extubation, especially in children for whom positive intrathoracic pressure due to mechanical ventilation can impede passive pulmonary blood flow.7,8
Although potentially beneficial in infants with complex congenital heart disease, administration of dexmedetomidine has potential to cause harm. Dexmedetomidine can cause hypotension and bradycardia, necessitating cessation of infusion or decreased infusion rate.1,9,10 Therefore, to maximize benefits and minimize harm of dexmedetomidine in infants and young children with complex heart disease, optimal dosing is imperative.
Optimal dosing relies on knowledge of drug pharmacokinetics (PK), most commonly obtained via clinical trial. Unfortunately, clinical trials are difficult to conduct in children.11,12 Techniques such as opportunistic sampling, population PK modeling, and dosing simulation can help overcome trial difficulties and provide vital information for off-label therapeutics such as dexmedetomidine.13 Accordingly, previous investigators characterized the PK of dexmedetomidine in children without CPB using a 2-compartment population PK model.3,14,15 Dexmedetomidine clearance (CL) increased with age until reaching adult values at 2 years of age.16–18
PK is expected to differ among infants undergoing CPB compared to those without CPB. CPB involves induced hypothermia, hemodilution of albumin, altered blood flow to target organs, potential adsorption of drug by CPB circuit materials, and marked inflammation, all of which are known to affect drug PK and pharmacodynamics.15,19–25 Accordingly, in a study of dexmedetomidine initiated in the post-CPB period for sedation of infants and young children, dexmedetomidine CL decreased and volume of distribution (V) increased with increasing duration of CPB.15 However, other investigators found no relationship between CPB and post-CPB CL or V.26 To date, dexmedetomidine PK during CPB has not been studied.
We present results of a pilot clinical trial conducted to do the following: (1) characterize the effect of CPB on dexmedetomidine CL and V in infants and young children; (2) detail experience regarding patient ability to tolerate dexmedetomidine and achieve sedation; and (3) derive preliminary dosing recommendations for infants and young children undergoing CPB. We hypothesized that CL would decrease, and V would increase during CPB compared to pre- or post-CPB states.
METHODS
Study Design
We conducted a single-center, open-label, opportunistic PK study of dexmedetomidine (Precedex; Hospira, Inc, Lake Forest, IL) administered to infants and young children (≤36 months of age) who were receiving the drug per standard of care and undergoing CPB with a blood-primed circuit. Based on US Food and Drug Administration guidance regarding sample size requirements for precision in pediatric population PK studies that use opportunistic sampling, we targeted a total sample size of 18.27 This sample provides sufficient data to estimate CL with a 95% confidence interval (CI) within 60%–140% of the geometric mean, assuming a total between-subject variability (coefficient of variation) up to 66%. We assumed each patient would provide at least 10 samples, with ≥5 during the on-CPB period. This strategy allowed each patient to serve as his/her own control for each phase (pre-CPB, on-CPB, post-CPB) and for us to collect a number of samples within the range of other similarly sized pediatric population PK studies.28,29 We excluded patients with postmenstrual age (PMA) of <38 weeks and allowed participation in the study once for 24 hours after CPB initiation. We obtained written informed consent for the study from the parents or guardians of all patients before CPB. The Duke University Institutional Review Board approved all study protocols and documents. We conducted our study from March 2014 to September 2014.
Study Procedures
Dexmedetomidine Administration.
At our center, infants undergoing cardiac surgery are initiated on dexmedetomidine via continuous infusion at 0.5 μg/kg/min, without an initial loading dose, unless already receiving dexmedetomidine before the operating room. Adjustment of dose occurs only after successful separation from CPB and when the patient is eligible for transfer to the pediatric cardiac intensive care unit for postoperative care.
Cardiopulmonary Bypass.
CPB equipment included Medtronic DLP arterial cannulas (8–10F; Medtronic, Minneapolis, MN), Edwards Thin-Flex venous cannulas (10–18F; Edwards Lifesciences Corp, Irvine, CA), and a CAPIOX FX 05 oxygenator (Terumo Cardiovascular Group, Ann Arbor, MI) with a hard-shell reservoir, integrated arterial filter, and biocompatible amphiphilic polymer surface coating (Xcoating; Terumo Cardiovascular Group, Ann Arbor, MI). Oxygenators for patients <5 and 5–12 kg had surface areas of 0.22/0.5 and 0.38/0.61 m2, respectively (Supplemental Digital Content, Table 1, http://links.lww.com/AA/C532).
Blood-primed circuits contained Normosol R (Hospira, Inc), packed red blood cells, heparin, sodium bicarbonate, 25% albumin, mannitol, Solu-Medrol (Pfizer, New York, NY), aminocaproic acid, fresh frozen plasma, and calcium gluconate according to the size of the circuit tubing and specific bicarbonate and calcium needs (Supplemental Digital Content, Table 2, http://links.lww.com/AA/C532). All patients experienced hypothermia and modified ultrafiltration during CPB. Only 2 patients underwent regional lowflow perfusion, and 1, deep hypothermic cardiac arrest.
Biologic Sample Collection.
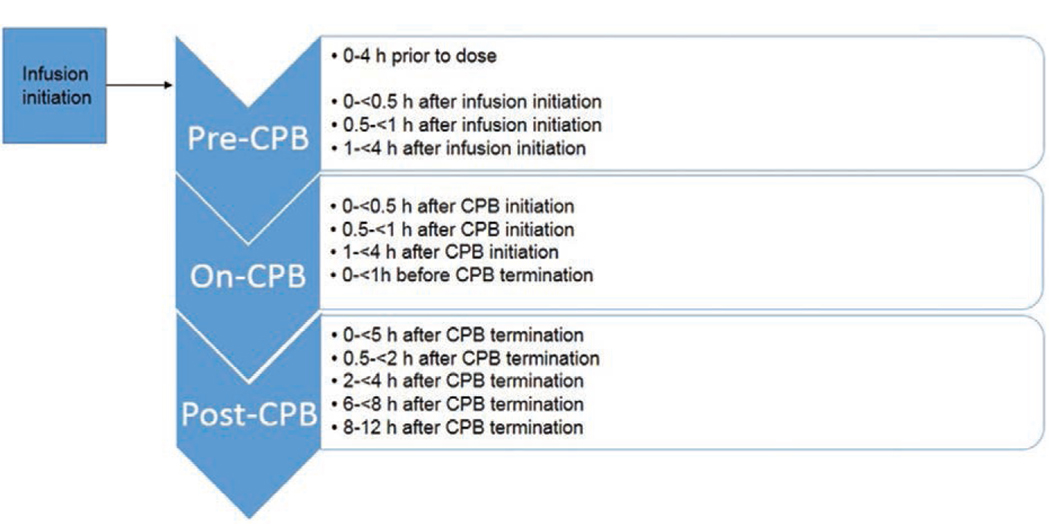
We obtained blood samples from the patient via an indwelling catheter other than the site of dexmedetomidine infusion. Each sample was at least 0.2 mL of whole blood, and we attempted to align sample collection with routine blood draws within scheduled time intervals relative to dexmedetomidine initiation and CPB initiation and discontinuation (Figure 1). We collected all samples in human EDTA plasma tubes and centrifuged the samples at 3000g for 10 minutes within 1 hour of the blood draw. We then transferred the plasma into a second polypropylene tube and immediately froze specimens at −20°C. Specimens were transferred to a −80°C freezer within 8 hours. The maximum sample storage time at −80°C was 1 year and 7 months. Dexmedetomidine is stable in human EDTA plasma for >2 years under these storage conditions (M. Scheinin, University of Turku, Finland, personal communication, March 3, 2018; data on file).
Figure 1.
Scheduled dexmedetomidine sampling times. CPB indicates cardiopulmonary bypass.
Biologic Sample Analysis.
Concentrations of dexmedetomidine in plasma were determined with reversed-phase high-performance liquid chromatography with tandem mass spectrometric detection (Sciex API4000 System; Sciex, Concord, ON, Canada), with solid-phase extraction and deuterium-labeled dexmedetomidine as the internal standard.30 Calibration standards and quality control samples were prepared in drug-free human EDTA plasma. The linear concentration range of dexmedetomidine (base form) was from 0.02 to 5.0 ng/mL. The lower limit of quantitation (LLOQ) was 0.05 ng/mL, and the limit of detection was 0.005 ng/mL. The intra-assay accuracies of the quality control samples (0.06, 0.15, 1.0, and 4.0 ng/mL) ranged from 98.5% to 108.1%.
PK Analysis and Model Development
To integrate PK parameter variability into the modeling process and provide tools to explain these variations based on different biological characteristics, we used nonlinear mixed effects modeling with NONMEM (version 7.2; Icon Solutions, Ellicott City, MD) and the first-order conditional estimation with interaction algorithm to analyze concentration data from our study cohort. We first attempted to develop a “de novo” population PK model with standard investigation of 1- and 2-compartment structural PK models, evaluation of bias introduced by below quantification limit (BQL) samples, and assessment of interindividual and residual variability for PK model parameters using standard techniques.31,32 We assessed interindividual variability using an exponential relationship and explored proportional, additive, and proportional plus additive residual error models.33
Parameter estimates from the de novo population PK model lacked adequate precision, presumably due to few quantifiable concentrations shortly after dexmedetomidine initiation and variability in sampling during CPB. We therefore identified a 2-compartment population PK model from the literature that was developed in infants and children in whom dexmedetomidine was initiated before anesthesia for urologic, lower abdominal, or plastic surgery; after low-risk cardiac or craniofacial surgery; or during bronchoscopy or nuclear magnetic resonance imaging.14 Using the literature model as a base model, we fixed the following population parameter values to estimates in the literature-based population: (1) drug CL in a healthy 70-kg adult (CL = 42.1 L/h); (2) maturation function (TM50=44.5 weeks) and its slope parameter (Hill = 2.56); (3) central V (Vc = 56.3 L); (4) CL (69 L/h); and (5) peripheral V (Vp = 78.3 L).14
We then used the literature model with fixed parameters to predict concentrations at specified time points and to compare literature-predicted concentrations with our actual data. Literature-predicted concentrations were dissimilar to our data and showed a systematic bias, indicating differences in PK between the literature population and our population. To account for these apparent differences, we performed a covariate analysis, including a fixed-effect parameter, Finf (scaling factor accounting for difference in Vc between CPB patients from our study and non-CPB patients from the pooled analysis), whose final value was estimated based on its ability to improve fit between literature-predicted concentrations and our data. To identify the impact of the on-CPB period compared to the pre- and post-CPB periods, we included the presence or absence of CPB as a covariant in our model. Subsequently, we evaluated the impact of CPB-related covariates on model fit and PK parameter precision. Evaluated covariates included temperature, flow rate, total time on CPB, and total time of aortic cross-clamp, on model fit and PK parameter precision.
We used forward inclusion and backward elimination to assess final model covariates, included covariates in the multivariable model that reduced the objective function value (OFV) by >3.84 (P ⪅ .05), and retained covariates in the final model that reduced the OFV by >6.64 (P ⪅ .01).34 We also evaluated models based on successful minimization, goodness-of-fit plots, plausibility of parameter estimates, and precision of parameter estimates. We evaluated the precision of the final model using bootstrapping.35 During this process, 1000 datasets are randomly created based on the entire range of possible values of PK parameters as dictated by interindividual variability. The datasets are used to generate median and 95% CI for the PK parameter estimates.35 We generated visual predictive check plots to assess the degree of overlap between observed and predicted data.36 To evaluate the relationship between parameter estimates in our population and those in infants and children from whom the base model was obtained, we numerically compared parameter estimates and their CI between the 2 populations. Finally, to further evaluate relationships between parameter estimates for CL and CPB-related factors, we used a 2-sample Wilcoxon rank sum test. For this analysis, we dichotomized CPB-related factors based on the median values of temperature, flow rate, and total time of aortic cross-clamp within our study population. We dichotomized CPB duration at 200 minutes based on evidence of increased inflammation in children associated with CPB duration >200 minutes.37
Dosing Simulation.
Using the final PK model and parameter estimates, we simulated dosing in 2 typical patients of our cohort: (1) PMA of 42 weeks and 4 kg; and (2) PMA of 92 weeks (≈2 years) and 10.3 kg. We defined PMA as the sum of gestational and postnatal ages. We chose our typical patients based on median age and weight in our cohort among neonates and infants, respectively. Based on simulated concentrations, we determined the optimal loading dose and maintenance dose for the pre-CPB, on-CPB, and post-CPB periods. We targeted a plasma concentration of 0.6 ng/mL based on prior safety and efficacy data14,38,39 and aimed to attain the target concentration by 5 hours after dexmedetomidine initiation, which correlated with the mean time from dexmedetomidine initiation to CPB initiation for patients in our study.
Evaluation of Sedation and Patient Ability to Tolerate Dexmedetomidine
We used the COMFORT Behavior (COMFORT-B) scale to evaluate sedation after dexmedetomidine administration.40 The COMFORT-B scale is a 6-question, 30-point scale used to assess alertness, agitation, respiratory response or cry, physical movement, muscle tone, and facial tension. We obtained COMFORT-B scores before surgery, on arrival to the pediatric cardiac intensive care unit, and 12 hours after discontinuation of CPB in patients who were not receiving neuromuscular blockers. The same investigator conducted all assessments and was not blinded. To evaluate patient ability to tolerate dexmedetomidine, we identified adverse events of special interest, including bradycardia or hypotension in the 24 hours after study drug initiation. We defined bradycardia as a decrease in heart rate >30% of patient’s median baseline heart rate or requiring intervention, including administration of atropine, epinephrine, chest compressions, or decreased dose or discontinuation of dexmedetomidine infusion. We defined hypotension as decreased systolic or mean arterial blood pressure >30% from patient’s median baseline blood pressure or requiring intervention, including administration of a fluid bolus, or initiation or increase in vasopressor therapy (epinephrine, norepinephrine, vasopressin, dopamine, etc). Baseline heart rate and blood pressure were determined from the 6 hours before initiation of dexmedetomidine. Because patients were critically ill and subject to labile hemodynamics, we classified each adverse event as definitely, probably, possibly, or not related to dexmedetomidine administration. The primary investigator (K.O.Z.) adjudicated events based on review of the patient’s medical chart and discussion with the patient’s primary medical provider.
RESULTS
Patient Characteristics
The 18 enrolled patients had a median age of 3.3 months (range: 0.1–34.0 months) and dosing weight of 4.8 kg (2.5–15.3 kg). Patients required CPB for a variety of conditions, including stage 2 palliation for single ventricle physiology, repair of ventricular septal defect, and heart transplantation (Table 1). Patients underwent CPB for 161 minutes (63–394 minutes), and all patients except 1 received a dexmedetomidine starting dose of 0.5 μg/kg/h without a loading dose. One patient received dexmedetomidine for 48 hours before CPB, including 0.8 μg/kg/h for 12 hours before presentation to the operating room. Dexmedetomidine infusion rate did not change while on CPB. After CPB, dexmedetomidine infusion rates ranged from 0.2 to 2.0 μg/kg/h.
Table 1.
Patient Demographics
| ID | Age (mo) | Race/Ethnicity | Sex | Diagnosis | Procedure | CPB Duration (min) |
|---|---|---|---|---|---|---|
| 1 | 19 | African American | M | Multiple aortopulmonary collaterals | Unifocallzation | 155 |
| 2 | 2 | Caucasian | M | Aortic valve stenosis; hypoplastic arch | Ross Konno procedure; arch reconstruction | 257 |
| 3 | 4 | American Indian | F | Ventricular septal defect | Ventricular septal defect repair | 207 |
| 4 | 0.10 | Caucasian | F | D-transposition of the great arteries | Arterial switch | 325 |
| 5 | 4 | Caucasian | F | Atrioventricular septal defect | Stage 2 palliation (Glenn) | 63 |
| 6 | 2 | Caucasian | M | Ventricular septal defect | Ventricular septal defect repair | 87 |
| 7 | 0.03 | Caucasian | M | Transposition of the great arteries with intact ventricular septum | Arterial switch | 262 |
| 8 | 3 | African American | F | Hypoplastic left heart syndrome variant | Heart transplant | 394 |
| 9 | 3 | African American | F | Extracardiac tumor | Removal of extracardiac tumor | 100 |
| 10 | 0.5 | Caucasian | F | Coarctation of the aorta | Coarctation repair | 212 |
| 11 | 0.10 | Caucasian | M | Coarctation of the aorta | Coarctation repair | 129 |
| 12 | 1 | Caucasian | F | Anomalous left coronary artery off the pulmonary artery | Reimplantation of coronary arteries | 221 |
| 13 | 6 | Multiracial | M | Double outlet right ventricle | Stage 2 palliation (Glenn) | 100 |
| 14 | 1 | Caucasian | F | Atrioventricular septal defect; pulmonary valvar stenosis | Repair of atrioventricular septal defect and valvar pulmonary stenosis | 143 |
| 15 | 0.25 | African American | M | Hypoplastic right ventricle, malposed great arteries, dextrocardia, atrioventricular septal defect, total anomalous pulmonary venous return | Repair of total anomalous pulmonary venous return; pulmonary artery banding | 167 |
| 16 | 6 | Caucasian | F | Hypoplastic right heart syndrome | Stage 2 palliation (Glenn) | 202 |
| 17 | 33 | Caucasian | M | Hypoplastic left heart syndrome | Stage 3 single ventricle palliation (Fontan) | 150 |
| 18 | 7 | Asian | F | Supravalvar pulmonary stenosis | Repair of pulmonary valve stenosis | 73 |
Abbreviations: CPB, cardiopulmonary bypass; F, female; ID, identifier; M, male.
We collected 213 dexmedetomidine plasma samples from study patients, including samples pre-, on-, and post-CPB. We excluded 1 concentration due to concern that it was collected from the line with dexmedetomidine infusion. Eighteen samples (8%) were of insufficient quantity for analysis, and 28 samples (13%) were below the LLOQ, including 6 samples below the limit of detection. The median number of samples per patient was 10 (range: 4–14), and dexmedetomidine concentration for non-BQL samples was 0.437 ng/mL (0.058–2.4 ng/mL) (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/C532).
For most patients, BQL samples occurred in the pre-CPB period and in the first few hours after initiation of CPB. The first sample above the LLOQ occurred at a median of 1.9 hours (range: 0.17–6.7 hours) after drug initiation and 0.5 hours (0.11–4.9 hours) after initiation of CPB. BQL samples in the post-CPB period reflected discontinuation of dexmedetomidine in the hours before sampling.
PK Model and Effect of CPB on Dexmedetomidine Exposure
During univariable analysis of potential covariates for our base model for CL, variables for the on-CPB and post-CPB periods each resulted in a significant decrease in OFV. On multivariable analysis for CL, simultaneous inclusion of variables for the on- and post-CPB periods resulted in a significant drop in OFV and precise parameter estimates. The effect of the on-CPB period and post-CPB period on other parameters, including Vc and Q, was tested but not included in the model due to high relative standard error in parameter estimates. No other CPB-related covariates (eg, flow rate, induced hypothermia) resulted in a significant drop in the OFV. After backward elimination, we retained only the variable for the on-CPB period. The OFV for the final model was 15.4 points lower compared to the base model. The interindividual variability of CL and Vc changed 5.6% and −4.1% from the base model to the final model. Residual variability dropped 2.5% from base to final model, while proportional residual error dropped 2.5%. In the final model, eta shrinkage for CL and Vc was acceptable at 26% and 7%, respectively. The between-subject variability for CL and Vc was 41.8% and 51.0%, respectively. The proportional and additive within-subject variability was 31.6% and 0.03 ng/mL, respectively.
The final model was as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where preCPB, postCPB, and onCPB in Equations 1 and 3 are variables (yes = 1, no = 0) that indicate time before CPB, after CPB, and during CPB, respectively. CLi is individual plasma CL, Wti is individual dosing weight in kilograms, PMAi is individual PMA, TM50 (Equation 1) is the maturation factor to account for changes in CL with maturation, Vci (Equation 2) is individual Vc, Vpi (Equation 4) is individual Vp, Qi is individual intercompartmental CL, and Vssi (Equation 5) is individual total V at steady state; ηCLi (Equation 1) is a normally distributed random variable, with zero mean and variance ωCL2, accounting for the difference between the individual CLi and typical parameter estimate for CL in the population; ηVci is a normally distributed random variable, with zero mean and variance ωVc2, accounting for the difference between the individual Vci and typical parameter estimate for Vc in the population.
Compared to the base model for CL, incorporation of CPB improved the model fit without evidence of constant or proportional bias (Figure 2). The median of bootstrap fixed-effects parameter estimates was within 4% of population estimates from the original dataset for all parameters. The final model described the observed data adequately as indicated by 8.7% of observed concentrations outside of the 90% prediction interval in visual predictive check (Supplemental Digital Content, Figure 2, http://links.lww.com/AA/-C532). Our results for the median of bootstrap parameter estimates and observed concentrations compared to the prediction interval meet criteria commonly used to assess model fit.
Figure 2.
Final population pharmacokinetic model diagnostic plots: observed versus population prediction (A) and individual prediction (B), conditional weighted residuals versus population predictions (C), and time after last dose (D). The solid line in A and B is the line of identity. The solid line in C and D is a reference line at y = 0. The dashed lines in A, B, C, and D are smooth lines. A dose is defined as administration of a bolus dose of dexmedetomidine or change in infusion rate.
Based on the final population PK model, the median empirical Bayes estimate (EBE) of CL for the pre- and post-CPB periods for our study population was 0.80 L/kg/h (range: 0.32–1.44 L/kg/h). The EBE for CL on CPB was approximately 70% lower, 0.25 L/kg/h (0.10–0.46 L/kg/h). The population CL parameter during CPB was 13.4 L/h/70 kg (95% CI, 2.6–24.2 L/h/70 kg) (Table 2), compared to 42.1 L/h/70 kg (95% CI, 38.7–45.8 L/h/70 kg) in historical patients. The EBE for Vss was 4.42 L/kg (3.39–15.32 L/kg). When we evaluated CL estimates according to dichotomous values in temperature, flow rate, CPB duration, and cross-clamp time, we identified no statistically significant differences (Table 3; Supplemental Digital Content, Figure 3, http://links.lww.com/AA/C532).
Table 2.
Population Pharmacokinetics Parameters
| Bootstrap CI | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | RSE (%) | 2.5% | Median | 97.5% |
| Structural PK model | |||||
| CLpre_postCPB (L/h/70 kg) | 42.1a | … | … | … | … |
| CLonCPB (L/h/70 kg) | 13.4 | 41 | 3.58 | 13.70 | 27.15 |
| Qpre_post CPB (L/h/70 kg) | 78.3a | … | … | … | … |
| Vc (L/70 kg) | 56.3a | … | … | … | … |
| Vp (L/70 kg) | 69a | … | … | … | … |
| Hill coefficient | 2.56a | … | … | … | … |
| TM50 (wk) | 44.5a | … | … | … | … |
| Finf | 4.94 | 22 | 3.65 | 4.96 | 7.24 |
| Interindividual variability (%CV) | … | … | … | … | … |
| CL IIV | 41.8 | 39 | 20.30 | 42.28 | 57.03 |
| Vc IIV | 51.0 | 57 | 27.26 | 51.02 | 75.85 |
| Residual variability | … | … | … | … | … |
| Proportional error (%) | 31.6 | 18 | 26.16 | 32.02 | 39.25 |
| Additive error (ng/mL) | 0.03 | 54 | 0.013 | 0.030 | 0.046 |
Abbreviations: CI, confidence interval; CL, clearance (L/h); CLonCPB, clearance during CPB; CLpre_postCPB, clearance before and after CPB; CPB, cardiopulmonary bypass; %CV, coefficient of variation; Finf, scaling factor accounting for difference in Vc between CPB patients from our study and non-CPB patients from the pooled analysis; Hill coefficient, slope parameter for the sigmoidal maturation model; IIV, interindividual variability; on_CPB, during CPB procedure = 1, otherwise = 0; PK, pharmacokinetics; post_CPB, after CPB procedure = 1, otherwise = 0; pre_CPB, before start of CPB = 1, otherwise = 0; Q, distribution clearance (L/h); Qpre_post_CPB, distribution clearance before and after CPB; RSE, relative standard error; TM50, maturation half-life; Vc, volume of distribution for central compartment (L); Vp, volume of distribution for peripheral compartment (L).
Values fixed to values in the literature.
Table 3.
Individual Empirical Bayesian Post Hoc Parameter Estimates of Clearance in Infants Stratified by CPB-Related Factors
| Variable | N | CL (L/kg/h) | CL (L/h/70 kg) |
|---|---|---|---|
| Temperature (°C) | |||
| ≥32 | 11 | 0.29 (0.18–0.46) | 11.27 (6.61–17.53) |
| <32 | 7 | 0.23 (0.10–0.45) | 7.70 (3.51–15.65) |
| P value | .14 | .11 | |
| Median flow rate (L/min/kg) | |||
| ≥0.135 | 9 | 0.25 (0.17–0.45) | 8.05 (5.62–17.35) |
| <0.135 | 9 | 0.26 (0.10–0.46) | 11.27 (3.51–17.53) |
| P value | .57 | .51 | |
| Total time on CPB (min) | |||
| ≥200 | 8 | 0.24 (0.10–0.45) | 8.25 (3.51–15.65) |
| <200 | 10 | 0.27 (0.20–0.46) | 11.31 (6.86–17.53) |
| P value | .21 | .21 | |
| Total time on CPB (min) | |||
| ≥161 | 9 | 0.25 (0.10–0.45) | 7.77 (3.51–15.65) |
| <161 | 9 | 0.29 (0.20–0.46) | 11.59 (6.86–17.53) |
| P value | .17 | .10 | |
| Total time on cross-clamp (min) | |||
| ≥68 | 9 | 0.25 (0.10–0.45) | 8.73 (3.51–17.35) |
| <68 | 9 | 0.26 (0.20–0.46) | 11.02 (6.86–17.53) |
| P value | .63 | .63 |
Data are median (range).
Abbreviations: CL, clearance (L/h); CPB, cardiopulmonary bypass.
Preliminary Dosing Recommendations
According to dosing simulations for an infant with PMA of 42 weeks, a loading dose of 0.7 μg/kg over 10 minutes in the pre-CPB period followed by infusion of 0.2 μg/kg/h through CPB led to target concentrations (0.6 ng/mL) by 5 hours. On discontinuation of CPB, a maintenance infusion of 0.4 μg/kg/h was required. In an infant with PMA of 92 weeks, the same loading dose was required, followed by infusion of 0.25 μg/kg/h during CPB and 0.6 μg/kg/h after CPB (Table 4).
Table 4.
Preliminary Dosing Recommendations
| Postmenstrual Age (wk) | ||
|---|---|---|
| Phase of CPB | 42 | 92 |
| Loading dose (μg/kg) | 0.7 | 0.7 |
| Maintenance pre-CPB (μg/kg/h) | 0.7 | 0.8 |
| Maintenance: on-CPB (μg/kg/h) | 0.2 | 0.25 |
| Maintenance: post-CPB (μg/kg/h) | 0.4 | 0.6 |
Abbreviation: CPB, cardiopulmonary bypass.
Ability of Patients to Tolerate Dexmedetomidine and Achieve Sedation
The majority of the patients (11/18, 61%) did not undergo post-CPB COMFORT-B assessments due to receipt of neuromuscular blockade. In the remaining 7 patients, scores ranged from 8 to 13 (COMFORT-B: ≤10, deep sedation; ≥23, inadequate sedation).
Eight of 18 patients (44%) developed study-defined hypotension in the postoperative period. For 3 of the 8 patients (38%), 2 had increase in epinephrine infusion and 1 received a fluid bolus. The remaining 5 patients with hypotension had epinephrine infusion increase. No episodes of hypotension were attributed to dexmedetomidine administration according to study adjudication criteria, with infusion rates maintained or increased for all patients with hypotension.
Three of 18 patients (18%) had bradycardia. In 1 patient, bradycardia progressed to pulseless electrical activity arrest requiring extracorporeal membrane oxygenation. Providers attributed bradycardia to the patient’s cardiac defect and recent surgical intervention. In a second patient, providers attributed bradycardia to primary graft failure and sinus node dysfunction but discontinued dexmedetomidine during persistent bradycardia.41 In the 2 nontransplant patients with bradycardia, dexmedetomidine infusion rate was maintained or increased after the event. There was no difference in median maximum post-CPB concentrations of dexmedetomidine in those who developed hypotension or bradycardia (N = 8) compared to those who did not (N = 10): 0.59 ng/mL (range: 0.03–2.4 ng/mL) vs 0.94 ng/mL (0.54–1.9 g/mL), P = .13.
DISCUSSION
In our small cohort of infants and young children undergoing palliation or repair of cardiac disease, we identified markedly decreased CL and increased V during CPB compared to estimates from historical infants and children who had dexmedetomidine initiated in the perisurgical or procedural periods.
Reasons for lower CL during CPB are likely related to CPB-associated altered hepatic blood flow, hypothermia, and inflammation, and the CPB circuit. Dexmedetomidine is moderately extracted by the liver (extraction ratio = 0.71); therefore, drug CL depends on both hepatic blood flow and drug-metabolizing enzyme activity.22 CPB provides nonpulsatile blood flow, which can reduce hepatic blood flow by 20%–50% depending on the concurrent presence of hypothermia or low CPB flow.24,25 Hypothermia helps facilitate protection of organs during CPB, and inflammation results from hemolysis, ischemia, reperfusion, and exposure to foreign pump surface material. Both processes lead to decreased drug-metabolizing CYP2A6 activity and associated reduction in CL.42–44 Consistent with what is known about the effects of blood flow, temperature, and CPB on drug CL, we would expect that higher temperatures and flow rates during CPB and shorter durations of CPB and aortic cross-clamp would result in increased CL. In our cohort, observed trends failed to achieve statistical significance.
In a pooled PK analysis of 4 trials of dexmedetomidine administered to those ≤15 years of age, CL estimates among those post-CPB (CL = 1.07 L/kg/h; 95% CI, 1.04– 1.11 L/kg/h) (N = 81) were similar to those who did not undergo CPB (CL = 1.1 L/kg/h; 95% CI, 1.05–1.13 L/kg/h) (N = 43).26 The lack of CPB effect on CL in this trial differs from our findings. Reasons for these differing conclusions could relate to different patient demographics (ie, average age), timing of dexmedetomidine initiation (ie, before versus after CPB), patient diagnoses that are known to influence drug PK, or comparison populations (ie, elective surgical population [present study] versus intensive care unit population [pooled analysis]).14–18 Dosing and drug interactions are unlikely to contribute to observed differences. In the studies, patients were administered similar dosing; PK of dexmedetomidine is linear in this dosing range, indicating that CL is a dose-independent parameter14,15; patients received similar concomitant drugs; and dexmedetomidine has no well-established PK drug interactions.38 Although neither analysis included patients with severe renal or hepatic dysfunction, more subtle differences in disease state and severity of illness between the populations may exist.
Patients in our study had a Vc that was approximately 5 times that of infants and children from Potts’ pooled analysis that served as the base population PK model for our study14 and >3 times that in studies of infants with dexmedetomidine initiated after CPB15,45 (Supplemental Digital Content, Table 3, http://links.lww.com/AA/C532). Because our estimate of Vc is derived largely from on-CPB concentrations, such differences in Vc between our study and previous studies are consistent with a CPB effect. Specifically, CPB-induced inflammation and capillary leak,46 adsorption of dexmedetomidine by the CPB circuit,47,48 and (to a lesser extent) addition of pump prime19 can contribute to increased Vc.
Based on population PK model and existing adult data regarding the dexmedetomidine exposure–safety relationships, preliminary dosing recommendations suggest the need for a loading dose followed by maintenance dosing that varies depending on patient PMA and whether the patient is pre-, on-, or post-CPB. The derived loading and maintenance doses are well within the range of doses previously administered to critically ill infants post-CPB, but differ from prior standard-of-care dosing at our institution, which led to a high quantity of patients not achieving quantifiable concentrations at the time of bypass initiation.8,14 Notably, dexmedetomidine was well tolerated in our population despite the majority of patients (12/18, 67%) exceeding our proposed dosing recommendations and concentration thresholds previously associated with safety events in adults. Such findings suggest that doses higher than proposed may be safely used in this population. These findings should be interpreted with caution given our relatively small sample size. However, the absence of definitive drug-related hemodynamic changes is consistent with several previous investigations that did not identify dexmedetomidine-related hemodynamic changes in excess of those routinely expected in the post-CPB period.8,49,50
Our trial is limited by its relatively small sample size that precluded extensive evaluation of covariates and identification of drug exposure–response relationships. In addition, some patients had fewer samples collected during CPB than anticipated, related to variable duration of CPB and the opportunistic sampling scheme. This variation in sampling resulted in wider confidence limits for CL than originally projected by our sample size calculation. Further, we were unable to adequately assess sedation in all patients or quantify changes in Vc and Vp during the transition from pre- to on-CPB periods. A larger PK trial is needed to better estimate the effect of CPB on Vc and Vp, to validate dosing recommendations, to better quantify the influence of CPB-related variables, and to further investigate the association between dexmedetomidine exposure and pharmacodynamic end points in this population.
Supplementary Material
KEY POINTS.
Question: What is the effect of cardiopulmonary bypass (CPB) on dexmedetomidine clearance (CL) and volume of distribution (V) in infants and young children?
Finding: We estimated a CL of 13.4 L/h/70 kg (95% confidence interval, 2.6–24.2 L/h/70 kg) during CPB, compared to 42.1 L/h/70 kg (95% confidence interval, 38.7–45.8 L/h/70 kg) in historical patients.
Meaning: CPB is associated with lower CL during CPB in infants and young children compared to estimates in those not undergoing bypass; such findings may have implications for dexmedetomidine dosing during CPB.
ACKNOWLEDGMENTS
The authors thank Duke Perfusion and Duke Pediatric Cardiac Anesthesia, who assisted with sample collection in the operating room and enabled the completion of the clinical trial.
Funding: K.O.Z. is funded by grant KL2TR001115-03 from the Duke Clinical and Translational Science Awards and K23 grant HD091398 from the National Institutes of Health (NIH). H.W. received salary support from grant K23HD0785891 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for completion of this project. M.L. receives support from the US government for work in neonatal clinical pharmacology, clinical trials, and cohort studies including Food and Drug Administration (FDA) R01 FD005101, Prinicipal Investigator (PI) M.L.; National Heart, Lung and Blood Institute (NHLBI) R34 HL124038, PI M.L.; the NIH Office of the Director, Environmental Influences on Child Health Outcomes (ECHO) Coordinating Center U2C OD023375, PI P.B.S., Duke University School of Medicine; the NICHD Pediatric Trials Network Government Contract HHSN267200700051C, PI Daniel Benjamin Jr, Duke University School of Medicine; and as the satellite site PI for the NICHD Neonatal Research Network NICHD U10 HD040492, PI C. Michael Cotten, Duke University School of Medicine. R.G.G. receives salary support for research from the NIH training grants (5T32HD043029-13), NIH awards (HHSN 275201000003I, HHSN 272201300017I), and from the FDA (HHSF223201610082C). P.B.S. receives salary support for research from the NIH (NIH-1R21HD08060601a1) and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NICHD (HHSN275201000003I), and the FDA (1R18-FD005292-01). C.P.H. receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117) and the US government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Daniel Benjamin Jr under the Best Pharmaceuticals for Children Act). M.C.-W. receives support for research from the NIH (1R01-HD076676-01A1), the National Institute of Allergy and Infectious Disease (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), and industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). K.M.W. receives support from the Pediatric Critical Care and Trauma Scientist Development Program (5K12HD047349) and the NICHD (1K23HD075891) for his work in pediatric clinical pharmacology.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Reprints will not be available from the authors.
The authors declare no conflicts of interest.
REFERENCES
- 1.Chrysostomou C, Di Filippo S, Manrique AM, et al. Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med. 2006;7:126–131. [DOI] [PubMed] [Google Scholar]
- 2.Chrysostomou C, Sanchez-de-Toledo J, Wearden P, et al. Perioperative use of dexmedetomidine is associated with decreased incidence of ventricular and supraventricular tachyarrhythmias after congenital cardiac operations. Ann Thorac Surg. 2011;92:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts AL, Warman GR, Anderson BJ. Dexmedetomidine dis-position in children: a population analysis. Paediatr Anaesth. 2008;18:722–730. [DOI] [PubMed] [Google Scholar]
- 4.Achuff BJ, Nicolson SC, Elci OU, Zuppa AF. Intraoperative dexmedetomidine reduces postoperative mechanical ventilation in infants after open heart surgery. Pediatr Crit Care Med. 2015;16:440–447. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz LI, Twite M, Gulack B, Hill K, Kim S, Vener DF. The perioperative use of dexmedetomidine in pediatric patients with congenital heart disease: an analysis from the Congenital Cardiac Anesthesia Society-Society of Thoracic Surgeons Congenital Heart Disease Database. Anesth Analg. 2016;123:715–721. [DOI] [PubMed] [Google Scholar]
- 6.Mukhtar AM, Obayah EM, Hassona AM. The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg. 2006;103:52–56. [DOI] [PubMed] [Google Scholar]
- 7.Morales DL, Carberry KE, Heinle JS, McKenzie ED, Fraser CD Jr, Diaz LK. Extubation in the operating room after Fontan’s procedure: effect on practice and outcomes. Ann Thorac Surg. 2008;86:576–581. [DOI] [PubMed] [Google Scholar]
- 8.Su F, Nicolson SC, Zuppa AF. A dose-response study of dexmedetomidine administered as the primary sedative in infants following open heart surgery. Pediatr Crit Care Med. 2013;14:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkenbosch JW, Tobias JD. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4:203–205. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Schmidt U, Wain JC, Bigatello L. Bradycardia leading to asystole during dexmedetomidine infusion in an 18 year-old double-lung transplant recipient. J Clin Anesth. 2010;22:45–49. [DOI] [PubMed] [Google Scholar]
- 11.Laughon MM, Benjamin DK Jr, Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine of the National Academies, Committee on Clinical Research Involving Children In: Field MJ, Berman RE, eds. The Ethical Conduct of Clinical Research Involving Children. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 13.Manolis E, Osman TE, Herold R, et al. Role of modeling and simulation in pediatric investigation plans. Paediatr Anaesth. 2011;21:214–221. [DOI] [PubMed] [Google Scholar]
- 14.Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care–a pooled analysis. Paediatr Anaesth. 2009;19:1119–1129. [DOI] [PubMed] [Google Scholar]
- 15.Su F, Nicolson SC, Gastonguay MR, et al. Population pharmacokinetics of dexmedetomidine in infants after open heart surgery. Anesth Analg. 2010;110:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tateishi T, Nakura H, Asoh M, et al. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci. 1997;61:2567–2574. [DOI] [PubMed] [Google Scholar]
- 17.Coughtrie MW, Burchell B, Leakey JE, Hume R. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol. 1988;34:729–735. [PubMed] [Google Scholar]
- 18.Díaz SM, Rodarte A, Foley J, Capparelli EV. Pharmacokinetics of dexmedetomidine in postsurgical pediatric intensive care unit patients: preliminary study. Pediatr Crit Care Med. 2007;8:419–424. [DOI] [PubMed] [Google Scholar]
- 19.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–417. [DOI] [PubMed] [Google Scholar]
- 21.Petroz GC, Sikich N, James M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–1110. [DOI] [PubMed] [Google Scholar]
- 22.Dutta S, Lal R, Karol MD, Cohen T, Ebert T. Influence of cardiac output on dexmedetomidine pharmacokinetics. J Pharm Sci. 2000;89:519–527. [DOI] [PubMed] [Google Scholar]
- 23.Utley JR, Wachtel C, Cain RB, Spaw EA, Collins JC, Stephens DB. Effects of hypothermia, hemodilution, and pump oxygenation on organ water content, blood flow and oxygen delivery, and renal function. Ann Thorac Surg. 1981;31:121–133. [DOI] [PubMed] [Google Scholar]
- 24.Mori A, Watanabe K, Onoe M, et al. Regional blood flow in the liver, pancreas and kidney during pulsatile and nonpulsatile perfusion under profound hypothermia. Jpn Circ J. 1988;52:219–227. [DOI] [PubMed] [Google Scholar]
- 25.Mathie RT, Ohri SK, Batten JJ, Peters AM, Keogh BE. Hepatic blood flow during cardiopulmonary bypass operations: the effect of temperature and pulsatility. J Thorac Cardiovasc Surg. 1997;114:292–293. [DOI] [PubMed] [Google Scholar]
- 26.Nallani SC, Brar SS, Bhattaram VA, Xu Y. Clinical Pharmacology Review: NDA 21–038 S21 S222013. Available at: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM362466.pdf. Accessed May 20, 2018.
- 27.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012; 52:1601–1606. [DOI] [PubMed] [Google Scholar]
- 28.Piper L, Smith PB, Hornik CP, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg RG, Wu H, Laughon M, et al. Population pharmacokinetics of dexmedetomidine in infants. J Clin Pharmacol. 2017;57:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iirola T, Ihmsen H, Laitio R, et al. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth. 2012;108:460–468. [DOI] [PubMed] [Google Scholar]
- 31.Keizer RJ, Jansen RS, Rosing H, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3:e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. [DOI] [PubMed] [Google Scholar]
- 33.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wählby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci. 2002;4:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thai HT, Mentré F, Holford NH, Veyrat-Follet C, Comets E. Evaluation of bootstrap methods for estimating uncertainty of parameters in nonlinear mixed-effects models: a simulation study in population pharmacokinetics. J Pharmacokinet Pharmacodyn. 2014;41:15–33. [DOI] [PubMed] [Google Scholar]
- 36.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Abraham R, Weinbroum AA, Lotan D, et al. Interleukin-8 secretion following cardiopulmonary bypass in children as a marker of early postoperative morbidity. Paediatr Anaesth. 2002;12:156–161. [DOI] [PubMed] [Google Scholar]
- 38.US National Library of Medicine. DailyMed. Available at: https://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=dexmedetomidine. Accessed February 3, 2017.
- 39.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. [DOI] [PubMed] [Google Scholar]
- 40.Ista E, van Dijk M, Tibboel D, de Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Pediatr Crit Care Med. 2005;6:58–63. [DOI] [PubMed] [Google Scholar]
- 41.Heinz G, Kratochwill C, Buxbaum P, et al. Long-term intrinsic pacemaker function in patients paced for sinus node deficiency after cardiac transplantation. Pacing Clin Electrophysiol. 1992;15:2061–2067. [DOI] [PubMed] [Google Scholar]
- 42.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94:2209–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60:225–230. [DOI] [PubMed] [Google Scholar]
- 44.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–S720. [DOI] [PubMed] [Google Scholar]
- 45.Su F, Gastonguay MR, Nicolson SC, DiLiberto M, Ocampo-Pelland A, Zuppa AF. Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Anesth Analg. 2016;122:1556–1566. [DOI] [PubMed] [Google Scholar]
- 46.Sinclair DG, Haslam PL, Quinlan GJ, Pepper JR, Evans TW. The effect of cardiopulmonary bypass on intestinal and pulmonary endothelial permeability. Chest. 1995;108:718–724. [DOI] [PubMed] [Google Scholar]
- 47.Wagner D, Pasko D, Phillips K, Waldvogel J, Annich G. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion. 2013;28:40–46. [DOI] [PubMed] [Google Scholar]
- 48.Park J, Shin DA, Lee S, et al. Investigation of key circuit constituents affecting drug sequestration during extracorporeal membrane oxygenation treatment. ASAIO J. 2016;63:293–298. [DOI] [PubMed] [Google Scholar]
- 49.Klamt JG, Vicente WV, Garcia LV, Ferreira CA. Hemodynamic effects of the combination of dexmedetomidine-fentanyl versus midazolam-fentanyl in children undergoing cardiac surgery with cardiopulmonary bypass. Rev Bras Anestesiol. 2010;60:350–362. [DOI] [PubMed] [Google Scholar]
- 50.Chrysostomou C, Sanchez De Toledo J, Avolio T, et al. Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009;10:654–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.