Abstract
Vaccine‐induced human papillomavirus (HPV) antibodies originating from cervicovaginal secretions were recently shown to be detectable in first‐void (FV) urine. This presents a novel opportunity for noninvasive sampling to monitor HPV antibody status in women participating in large epidemiological studies and HPV vaccine trials. With a view towards method optimization, this study compared the measurement of HPV antibodies in FV urine using a multiplex L1/L2 virus‐like particles (VLP)‐based ELISA (M4ELISA) with previously reported results using a glutathione S‐transferase (GST)‐L1‐based immunoassay (GST‐L1‐MIA). We tested 53 paired FV urine and serum samples from 19‐ to 26‐year‐old healthy women, unvaccinated (n = 17) or vaccinated with either the bivalent or quadrivalent HPV‐vaccine during adolescence (n = 36). HPV6/11/16/18 antibodies were measured using M4ELISA and compared with GST‐L1‐MIA results. Inter‐assay and inter‐specimen correlations were examined using the Spearman's rank test (rs). As expected, lower HPV antibody concentrations were found in FV urine than in serum. Vaccinated women had significantly higher HPV6/11/16/18 antibody levels in both FV urine and serum compared with those unvaccinated (M4ELISA; FV urine P = .0003; serum P ≤ .0001). HPV antibody levels in FV urine and serum showed a significant positive correlation (M4ELISA anti‐HPV6/11/16/18, r s = 0.85/0.86/0.91/0.79, P ≤ .001). Despite assay differences, there was moderate to good correlation between M4ELISA and GST‐L1‐MIA (FV urine anti‐HPV6/11/16/18, r s = 0.86/0.83/0.89/0.53, P ≤ .0001; serum anti‐HPV6/11/16/18, r s = 0.93/0.89/0.94/0.75, P ≤ .0001). FV urine HPV antibody detection is comparable with both assays, further supporting this noninvasive sampling method as a possible option for HPV vaccine assessment. Approaches to improve the sensitivity and larger studies are warranted to determine the feasibility of FV urine for vaccine‐induced HPV antibody detection.
Keywords: HPV antibodies, HPV serology, HPV vaccines, human papillomavirus, urine
Highlights
First‐void urine HPV antibody detection is comparable with both assays.
Urine of vaccinees contain higher antibody levels as opposed to unvaccinated women.
Urinary HPV6/11/16/18 antibodies correlate significantly with paired sera.
Urine might offer a non‐invasive tool to assess serum antibody transudate.
Approaches to improve the sensitivity of vaccine‐induced HPV antibody detection in FV urine are required.
1. INTRODUCTION
Several studies examining human papillomavirus (HPV) antibody levels in female genital secretions following HPV vaccination have shown moderate to good correlation between titers in cervicovaginal secretions (CVS) and serum. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 It is postulated that these vaccine‐induced HPV‐specific antibodies (HPV‐Abs) are present in CVS due to transudation and exudation from blood into the cervical mucus where HPV virions are neutralized before infection. 13
Women's first‐void (FV) urine, the initial part of the urine flow, contains secretions and exfoliated cells from the vulva, vagina, and cervix. 14 The feasibility of using the genital “debris” included in this non‐invasively collected sample to monitor HPV vaccination impact through virologic endpoints (HPV DNA) has been reported. 15 , 16 , 17 Besides HPV DNA, a recent study conducted by our group confirmed that HPV‐Abs are also detectable in the FV urine of young women, albeit at low levels. 18 This study showed significant higher HPV antibody levels in vaccinated compared with unvaccinated women and positive correlations were observed between HPV6/11/16/18 antibodies in FV urine and paired sera using the glutathione S‐transferase‐L1 multiplex serology assay (GST‐L1‐MIA).
As current HPV vaccines target up to 9 HPV types, vaccine monitoring is facilitated with multiplexed assays for type‐specific HPV‐Ab detection. The GST‐L1‐MIA and the multiplex direct L1/L2 virus‐like particles (VLP)‐based ELISA (M4ELISA) are both high‐throughput assays that require minimal sample volume and thus have the potential for HPV‐Abs detection in FV urine. 19 , 20 , 21 , 22 , 23 The GST‐L1‐MIA detects antibodies against the L1 protein, thought to be assembled as pentamers, on fluorescent bead‐based liquid array (Luminex) platform. The M4ELISA utilizes intact VLP bound to multi‐spot wells in a direct binding assay with electrochemiluminescent (ECL) detection on the Meso Scale Discovery (MSD, Rockville, MD) platform. The carbon electrode multi‐spot MSD plate reduces background noise as each spot forms its own circuit measuring ECL signal bound only to the spot and not to the walls of the microwell. The separate binding surface also reduces competition between antibodies and detection reagent. 22 In settings with low HPV‐Ab titers, such as FV urine, M4ELISA could have advantages as low background allows detection of low signals.
With a view toward method optimization, we measured in present study HPV‐Ab levels in FV urine and serum of (un)vaccinated subjects using M4ELISA and directly compared the outcomes with previous published GST‐L1‐MIA results. 18 Extending previously published results by a direct comparison of M4ELISA with GST‐L1‐MIA will help to advance the use of FV urine for HPV‐Ab detection. This is, by our knowledge, the first‐time vaccine‐induced HPV‐Abs in FV urine were evaluated by M4ELISA and the first direct comparison of M4ELISA and GST‐L1‐MIA in both FV urine and serum.
2. MATERIALS AND METHODS
2.1. Study characteristics, sample collection, and pre‐analytical processing
Samples from earlier recruited study population (healthy women, 19‐26 years of age) were used, 18 trial registration ID: NCT02714114). Details regarding sample collection and processing has been described previously. 18 Briefly, HPV vaccinated (n = 38) and unvaccinated (n = 19) women were asked to collect an FV urine sample with the Colli‐Pee device (Novosanis, Belgium). Upon collection, FV urine samples were immediately placed on ice and aliquots stored at − 80°C. Blood samples were collected and allowed to clot for 30 to 60 minutes, centrifuged, and subsequently serum was divided in aliquots before storage at −80°C until antibody detection.
Pre‐analytical processing was the same as for previously reported GST‐L1‐MIA testing. 18 After thawing the urine aliquot for M4ELISA, one volume of urine conservation medium (UCM, UAntwerp, Belgium) was added to two volumes of FV urine, with a total volume of 4 mL, before centrifuging in an Amicon Ultra‐4 50 K filter device (Merck Millipore, Belgium). The concentrate retained on the filter was stored at − 80°C until antibody detection.
2.2. Multiplex VLP‐based IgG ELISA (M4ELISA)
The ECL detection based quantification of anti‐HPV6/11/16/18 antibodies in FV urine and serum was performed at the Centers for Disease Control and Prevention (CDC) as described previously with slight modifications. 22 Briefly, serum and FV urine samples were serially‐diluted 3.16 fold in assay diluent, 0.1X Diluent 100 in PBST (phosphate‐buffered saline supplemented with 0.1% Tween 20 [MSD]), using the Janus Automated Workstation (PerkinElmer, Waltham, MA). For each sample, a minimum of three dilutions were tested. Standards, controls, and test sera were examined starting at 1:100 or higher dilution, whereas FV urine samples were tested starting at neat (undiluted). VLPs (types 6, 11, 16, and 18) were coated at a concentration of 80 μg/mL for printing of 7‐spot MSD plates, as described previously. 22 Plates were blocked for 1 hour with 150 µL of 5% ECL Blocking Agent (GE Healthcare) in PBST at room temperature (24°C ± 2°C) on a lab rotator set at 650 rpm. All incubations for subsequent steps were at 37°C for 1 hour with shaking at 650 rpm. After each incubation, plates were washed four times with PBST using an automated plate washer (ELx405, Biotek, Winooski, VT). After removal of the blocking agent, 50 µL of each sample dilution was added to the plates and incubated. Next, 25 µL of Sulfo‐Tag labelled mouse anti‐human total IgG (Fc‐specific) (Biotrend‐MSD) with a concentration of 1 µg/mL in assay diluent was added and incubated. 150 µL of 1 × Read Buffer T was then added to each well. The plate was read on the MESO® Quickplex SQ120 (MSD). Relative light units (RLUs) for each spot with type‐specific VLP was exported to Microsoft Excel.
Raw RLUs were used in the determination of HPV‐Abs concentrations for each HPV type using the parallel line method (PLL) as described in the WHO HPV Labnet Manual, 24 WHO HPV Labnet, 2009. Samples with signal below the detection limit or with low signal that did not titrate in a linear fashion (therefore failing statistical conditions of the PLL analysis) were given a zero value. Antibody titers are reported in arbitrary units/mL (AU/mL) for HPV6 and 11, and International Units (IU/mL) for HPV16 and 18.
2.3. GST‐L1‐based multiplex immunoassay (GST‐L1‐MIA) and human IgG isotyping
The GST‐L1‐MIA method and results, and total human IgG measures have been previously published. 18
2.4. Statistical analysis
The Chi‐squared and Fisher's exact tests were used to assess the association of the study population characteristics between the vaccinated and unvaccinated cohort. The Mann‐Whitney U test and unpaired Student t test were used to test for differences in continuous measures. Inter‐assay and inter‐specimen correlation was calculated using Spearman's rank test (r s). The mean plus three standard deviations of type‐specific results of the unvaccinated group were used to estimate a threshold of HPV‐Ab values in FV urine to distinguish between vaccinated and unvaccinated groups. Given that at least some of the unvaccinated group had an antibody response to natural HPV infection, the cut‐off value (COV) for serum was determined at CDC using children's serum. For this analysis, serum samples were considered positive if they passed PLL conditions as well as were above median plus two standard deviations of the PLL/titer generated from the children sera tested. COV for HPV 6, 11, 16, and 18 were 0.5 AU/mL, 0.3 AU/mL, 1.4 IU/mL and 2.4 IU/mL, respectively. Statistical analyses were performed at a significance level of 5% using the statistical software JMP Pro 13.
3. RESULTS
3.1. Study population characteristics
For the present study, test results from 53 women (19‐26‐year old) were included for statistical analysis; whereof 36 were vaccinated (Figure A, appendix). Three FV urine samples were excluded due to insufficient volume and one due to blood contamination (>200 erythrocytes/µL). Study population characteristics have been published in detail 18 and are shown in the appendix (Table A). Briefly, most vaccinees received three doses of the 4vHPV vaccine (n = 31 of 36; 86%), four received three doses of the 2vHPV vaccine (4 of 36, 11%) and one woman received a combination of both vaccines (1 × 2vHPV, 2 × 2vHPV vaccine).
3.2. M4ELISA measured HPV antibody levels
No matrix effects with UCM were observed allowing the FV sample to be tested neat. Depending on the HPV type, antibody titers in FV urine and serum varied between 0 and 2.8 IU(AU)/mL and from 0 to 569 IU(AU)/mL, respectively. For all four HPV types median antibody levels are given by sample type and according to vaccination status in Table 1. HPV‐Ab levels in FV urine were 0.03% of values in serum.
Table 1.
Median human papillomavirus (HPV) antibody levels in first‐void (FV) urine and serum according to vaccination status
| Antibody type | Total | According to vaccination status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Not vaccinated | P value* FV urine | P value* serum | ||||||
| FV urine, median (IQR) | Serum, median (IQR) | Ratio urine/serum, % (IQR) a | FV urine, median (IQR) | Serum, median (IQR) | FV urine, median (IQR) | Serum, median (IQR) | |||
| Total Ig, µg/mL | |||||||||
| Human IgG | 36.31 | 10 641 | 0.35 | 40.19 | 10 851 | 30.29 | 9448 | .11 | .26 |
| (22.53‐72.74) | (8293‐12 649) | (0.20‐0.68) | (26.64‐76.55) | (9043‐12762) | (17.39‐66.63) | (8041‐12235) | |||
| HPV specific Ig | |||||||||
| M4ELISA (log10 AU/mL for HPV 6/11, and IU/mL for HPV16/18) | |||||||||
| HPV6‐Ig b | 0.65 × 10−3 | 0.91 | 0.029 | 3.03 × 10−3 | 1.36 | 0.06 × 10−3 | 0.09 | <.0001* | <.0001* |
| (0.10‐3.39 × 10−3) | (0.14‐1.41) | (0.01‐0.08) | (0.66‐12.9 × 10−3) | (0.98‐1.60) | (0‐0.29 × 10−3) | (0.02‐0.25) | |||
| HPV11‐Ig b | 1.41 × 10−3 | 1.08 | 0.027 | 3.46 × 10−3 | 1.44 | 0 | 0.06 | <.0001* | <.0001* |
| (0.06‐4.14 × 10−3) | (0.10‐1.54) | (0.01‐0.10) | (1.79‐12.9 × 10−3) | (1.20‐1.69) | (0‐0.25 × 10−3) | (0.02‐0.14) | |||
| HPV16‐Ig | 3.08 × 10−3 | 1.63 | 0.028 | 12.1 × 10−3 | 1.96 | 0 | 0.28 | <.0001* | <.0001* |
| (0.31‐24.6 × 10−3) | (0.71‐2.03) | (0.02‐0.08) | (2.99‐56.3 × 10−3) | (1.61‐2.10) | (0‐0.33 × 10−3) | (0‐‐0.77) | |||
| HPV18‐Ig | 2.51 × 10−3 | 1.13 | 0.034 | 5.03 × 10−3 | 1.37 | 0.30 × 10−3 | 0.32 | .0003* | <.0001* |
| (0‐8.58 × 10−3) | (0.48‐1.64) | (0.003‐0.07) | (1.29‐13.8 × 10−3) | (1.03‐1.79) | (0‐2.01 × 10−3) | (0.09‐0.93) | |||
| GST‐L1‐MIA (103 MFI) | |||||||||
| HPV6‐Ig b | 0.16 | 45.5 | 0.31 | 0.26 | 100 | 0.02 | 7.23 | <.001* | <.001* |
| (0.03‐0.43) | (8.63‐112) | (0.15‐0.83) | (0.16‐0.87) | (68.5‐137) | (0‐0.06) | (2.78‐12.9) | |||
| HPV11‐Ig b | 0.10 | 29.8 | 0.55 | 0.23 | 60.1 | 0.05 | 4.38 | <.001* | <.001* |
| (0.05‐0.27) | (5.1‐72.8) | (0.26‐1.19) | (0.09‐0.57) | (34.8‐100) | (0.01‐0.10) | (1.73‐6.18) | |||
| HPV16‐Ig | 0.16 | 55.9 | 0.38 | 0.26 | 96.9 | 0.02 | 1.73 | <.001* | <.001* |
| (0.03‐0.49) | (6.28‐117.1) | (0.20‐0.92) | (0.14‐1.05) | (55.1‐161) | (0‐0.04) | (0.33‐6.28) | |||
| HPV18‐Ig | 0.08 | 10.48 | 0.66 | 0.10 | 18.3 | 0.06 | 0 | .028* | <.001* |
| (0.03‐0.13) | (0.95‐24.5) | (0.45‐2.04) | (0.04‐0.15) | (4.30‐28.3) | (0.02‐0.09) | (0‐2.35) | |||
Note: Median total human IgG (µg/mL), M4ELISA HPV Log 10 antibody levels (arbitrary units/mL [AU/mL] for HPV6 and 11, and International Units [IU/mL] for HPV16 and 18), GST‐L1‐MIA HPV antibody levels (103 median fluorescence intensity [MFI]) and interquartile ranges (IQR, Q25‐Q75) are displayed for the total cohort (n = 53), vaccinated (n = 36/53), and not vaccinated (n = 17/53) women.
Antibody levels in FV urine divided by serum levels, denoted as median %, plus IQR.
For HPV6/11 antibodies; women (n = 4) previously vaccinated with the bivalent vaccine were considered not vaccinated (n = 32/53 vaccinated; n = 21/53 not vaccinated).
P values (Mann‐Whitney U test) indicated by an asterisk indicates a significant difference between median antibody yield between vaccinated and not vaccinated women.
3.3. M4ELISA comparison between FV urine and serum
For all four HPV genotypes, median log HPV‐Ab levels for serum were significantly higher compared with paired FV urine (Table 1). A significant difference is observed in median HPV antibody levels between vaccinated and unvaccinated women. The median HPV antibody levels for all genotypes are higher in vaccinated compared to unvaccinated women (log difference 0.003‐0.012 in FV urine and 1.05‐1.67 in serum samples, Figure 1).
Figure 1.
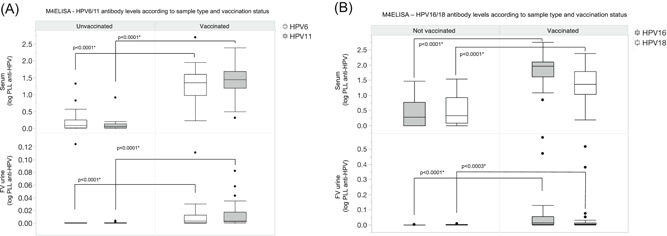
M4ELISA median log10(x + 1) transformed antibody levels for HPV6 and 11 (AU/mL) (A), 16 and 18 (IU/mL) (B) are visualized according to vaccination status for serum and first‐void (FV) urine. For HPV6/11 antibodies, women previously vaccinated with the bivalent vaccine were considered not vaccinated (n = 32/53 vaccinated; n = 21/53 not vaccinated). P values indicated by an asterisk indicate a significant difference in median antibody levels between vaccinated and not vaccinated women (Mann‐Whitney U test)
We used the correlation between serum and FV urine HPV antibody levels in paired samples to assess how well FV urine antibodies reflect serum antibodies. As illustrated in Figure 2, Spearman's correlation was 0.85 (P < .001) for HPV6; 0.86 (P < .001) for HPV11, 0.91 (P < .001) for HPV16 and 0.79 (P < .001) for HPV18. Excluding the extreme HPV‐Ab values did not affect the Spearman's correlation (Table B, Appendix). All reported results were not normalized to total IgG because this did not alter findings (data not shown).
Figure 2.
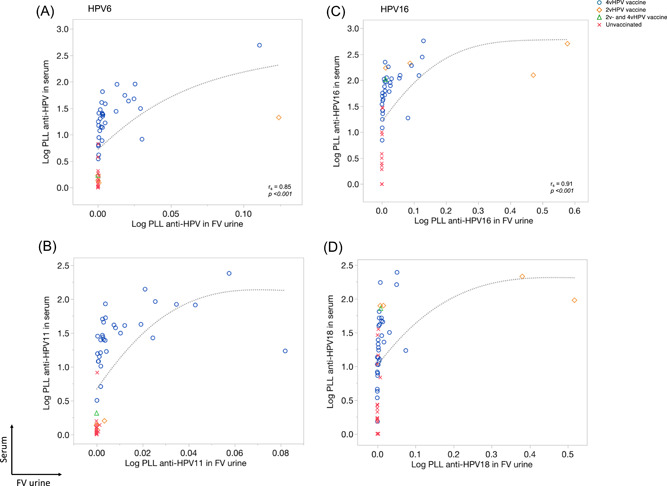
M4ELISA correlation plots of human papillomavirus 6 (HPV6) (A), 11 (B), 16 (C), and 18 (D) between serum and first‐void (FV) urine according to vaccination status. Log10(x + 1) transformed HPV antibody levels for serum and FV urine are plotted on the y‐ and x‐axis, respectively. Markers are used to visualize women vaccinated with the quadrivalent (4vHPV; blue circles), bivalent (2vHPV; orange squares), a combination of both vaccines (2vHPV and 4vHPV; green triangle), and not vaccinated women (red crosses). Spearman's rank correlation coefficients (r s) are displayed for each HPV type. A sensitivity analysis that excluded high values was performed to determine their influence on the Spearman's correlations. The analyses showed no differences in correlations (Appendix Table 3)
Higher HPV16/18 antibody responses were seen in the FV urine and serum in four women vaccinated with the 2vHPV compared with those vaccinated with 4vHPV. While those women receiving 2vHPV were considered unvaccinated for HPV6 and 11, one woman receiving 2vHPV had a high HPV6 antibody response in both FV urine and serum, perhaps due to previous exposure to HPV6 (Figure 2, 3, 4).
Figure 3.
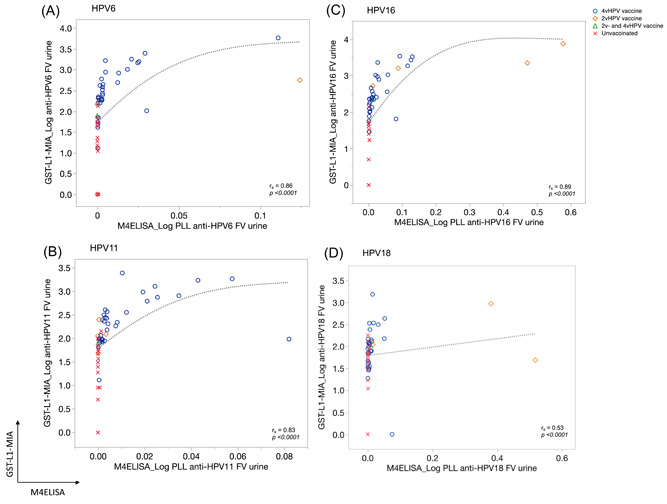
First‐void (FV) urine correlation plots for human papillomavirus 6 (HPV6) (A), 11 (B), 16 (C), and 18 (D) between M4ELISA (x axis) and GST‐L1‐MIA (y axis) according to vaccination status. Log10(x + 1) transformed HPV antibody levels for FV urine are plotted. Markers are used to visualize women vaccinated with the quadrivalent (4vHPV; blue circles), bivalent (2vHPV; orange squares), a combination of both vaccines (2vHPV and 4vHPV, green triangle), and not vaccinated women (red crosses). A sensitivity analysis that excluded high values was performed to determine their influence on the Spearman's correlation. The analyses showed no differences in correlations (Table 6, Appendix). Spearman rank correlation coefficients (r s) are displayed for each HPV type. Note differing scales for x‐ and y‐axis, as assays use different scales for measurement
Figure 4.
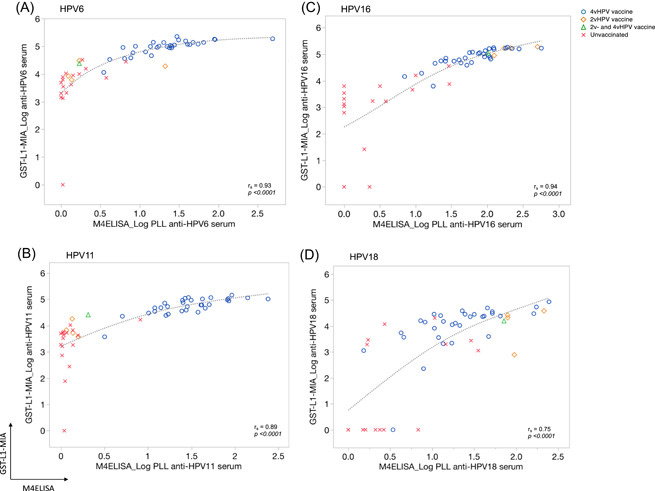
Serum correlation plots for HPV6 (A), 11 (B), 16 (C), and 18 (D) between M4ELISA (x axis) and GST‐L1‐MIA (y axis) according to vaccination status. Log10(x + 1) transformed HPV antibody levels for serum are plotted. Markers are used to visualize women vaccinated with the quadrivalent (4vHPV; blue circles), bivalent (2vHPV; orange squares), a combination of both vaccines (2vHPV and 4vHPV; green triangle), and not vaccinated women (red crosses). Spearman's rank correlation coefficients (r s) are displayed for each HPV type. Note differing scales for x‐ and y‐axis, as assays use different scales for measurement
3.4. Comparison between M4ELISA and GST‐L1‐MIA
Both assays produced valid results for HPV‐Abs detection in all FV urine and sera samples, and both found significant differences in HPV‐Abs levels according to vaccination status for all four HPV types (Table 1). The proportion of serum HPV‐Abs detected in FV urine range from 0.027% to 0.034% and 0.31‐0.66% by M4ELISA and GST‐L1‐MIA, respectively (Table 1). The tenfold difference in this ratio between the assays may be explained by different upper limits of quantification in serum HPV‐Abs.
Correlations between assays for FV urine and serum samples are shown in Figures 3 and 4, respectively. The inter‐assay correlations for all types in FV urine were significant (<.0001), with the highest value for HPV16 (0.89) and lowest for HPV18 (0.53). For serum the Spearman's correlations for all HPV types were also significant (<.0001), highest for HPV16 (0.94) and lowest for HPV18 (0.75). Excluding the extreme HPV‐Ab values did not affect these correlations (Table C, appendix).
Using results in the unvaccinated cohort to establish a threshold for the determination of vaccine status was somewhat successful. Results for HPV6/11/16 in the vaccinated group were distinguished from the unvaccinated group by M4ELISA and GST‐L1‐MIA in 72%‐97% and 59%‐86% of FV urine samples and 81%‐100% and in 91%‐97% of serum samples, respectively (Table 2). HPV18 results among those vaccinated were distinguished from unvaccinated by M4ELISA and GST‐L1‐MIA in 58% and 19% in FV urine and 42% and 75% of serum samples, respectively. Based on the threshold determined at CDC using children's serum, 97%‐100% vaccinated women were seropositive for HPV6/11/16/18 compared with 29%‐43% of unvaccinated women (Table 2).
Table 2.
Antibody positivity against human papillomavirus 6/11/16 and 18 for serum and first‐void (FV) urine
| Serum | FV urine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV type | COV | Vaccinated n/total (%) | Not vaccinated n/total (%) | Threshold titer | HPV type | COV | Vaccinated n/total (%) | Not vaccinated n/total (%) | Threshold titer |
| HPV6 | M4ELISA COV1 | 31/32 (97) | 3/21 (14) | 1.31 AU/mL | HPV6 | M4ELISA COV1 | 23/32 (72) | 1/21 (5) | 0.002 AU/mL |
| M4ELISA COV2 | 32/32 (100) | 7/21 (33) | 0.5 AU/mL | M4ELISA COV2 | NA | ||||
| GST‐L1‐MIA | 29/32 (91) | 0/21 (0) | 356 MFI | GST‐L1‐MIA | 25/32 (78) | 3/21 (14) | 33 MFI | ||
| HPV11 | M4ELISA COV1 | 32/32 (100) | 1/21 (5) | 0.66 AU/mL | HPV11 | M4ELISA COV1 | 31/32 (97) | 5/21 (24) | 0.0004 AU/mL |
| M4ELISA COV2 | 32/32 (100) | 9/21 (43) | 0.3 AU/mL | M4ELISA COV2 | NA | ||||
| GST‐L1‐MIA | 31/32 (97) | 1/21 (5) | 169 MFI | GST‐L1‐MIA | 19/32 (59) | 1/21 (5) | 41 MFI | ||
| HPV16 | M4ELISA COV1 | 29/36 (81) | 0/17 (0) | 32.57 IU/mL | HPV16 | M4ELISA COV1 | 29/36 (81) | 1/17 (6) | 0.006 IU/mL |
| M4ELISA COV2 | 36/36 (100) | 7/17 (41) | 1.4 IU/mL | M4ELISA COV2 | NA | ||||
| GST‐L1‐MIA | 35/36 (97) | 2/17 (12) | 101 MFI | GST‐L1‐MIA | 31/36 (86) | 1/17 (6) | 20 MFI | ||
| HPV18 | M4ELISA COV1 | 15/36 (42) | 0/17 (0) | 36.82 IU/mL | HPV18 | M4ELISA COV1 | 21/36 (58) | 1/17 (6) | 0.009 IU/mL |
| M4ELISA COV2 | 35/36 (97) | 5/17 (29) | 2.4 IU/mL | M4ELISA COV2 | NA | ||||
| GST‐L1‐MIA | 27/36 (75) | 2/17 (12) | 40 MFI | GST‐L1‐MIA | 7/36 (19) | 0/17 (0) | 51 MFI | ||
Note: M4ELISA cut‐off value 1 (COV1) and GST‐L1‐MIA are based on the mean plus three standard deviations of the not vaccinated group. M4ELISA cut‐off value 2 (COV2) is determined at CDC using serum samples from children human. For HPV6/11 antibodies; women (n = 4) previously vaccinated with the bivalent vaccine were considered not vaccinated (n = 32/53 vaccinated; n = 21/53 not vaccinated). Arbitrary units/mL (AU/mL); international Units (IU/mL); median fluorescence intensity (MFI).
4. DISCUSSION
A recent study confirmed that HPV‐Abs originating from CVS are detectable in FV urine of young women, albeit at low levels. 18 With a view towards method optimization we compared M4ELISA with GST‐L1‐MIA for the measurement of HPV‐Abs in FV urine and paired serum.
A moderate to good correlation was observed between the two assays in FV urine (r s, 0.53‐0.89) and serum (r s, 0.75‐0.94). Hence, despite assay differences, FV urine HPV‐Abs detection is comparable with both assays, further supporting this noninvasive sampling method as an option for HPV vaccine assessment. Correlation differences observed between types, especially for HPV18, indicate that the assays seem to have a lower sensitivity for post‐vaccine HPV18 antibodies, potentially because the vaccines induce a lower HPV18‐Ab response. It is reported that HPV antibodies may decline to become undetectable over time, most remarkable for HPV18, despite continued vaccine efficacy in preventing infections. 25 Notable as well is the difference in antigens (VLPs vs L1) used in the two assays that could contribute to the differences in antibody titers in serum and FV urine samples.
As previously reported with GST‐L1‐MIA, 18 significantly higher HPV6/11/16/18 antibody levels were observed in vaccinated compared with unvaccinated women in FV urine and serum with M4ELISA. Furthermore, FV urine HPV‐Ab levels correlated with paired sera levels for all investigated HPV genotypes. The moderate to good correlation indicates that vaccine‐induced HPV‐Abs in CVS and FV urine are likely due to transudation/exudation from the blood.
In agreement with previous studies in CVS and FV urine, 5 , 8 , 18 , 26 we did not observe alterations in the level of correlations between paired samples when HPV antibody levels were normalized to total human IgG. Therefore, further options for normalization need to be evaluated.
Notably, HPV antibody levels in FV urine were around the detection limit for both the M4ELISA and GST‐L1‐MIA, which complicates the distinction between uronegative and low positive results. Expected better sensitivity for M4ELISA, because of a lower background signal, was not observed. Therefore, alternative optimization strategies to improve sensitivity, such as novel sample concentration and antibody purification techniques, will be required as well as an increase in the sensitivity of the assays used for FV urine.
Future validation in larger studies will be necessary to determine the suitability of these assays in conjunction with FV urine samples for epidemiological studies and the monitoring of HPV vaccines. Furthermore, studies are warranted that provide data on storage conditions and their impact on the observed antibody levels.
Non‐invasively, self‐collected FV urine may reduce the need for gynecological examinations and/or blood draws in clinical trials and vaccine follow‐up programs. In addition, assessment of both virologic (HPV DNA) and immunological (HPV antibody) end points in FV urine could provide major logistical and financial benefits to epidemiological studies to assess vaccine uptake, vaccination impact, and follow‐up of HPV vaccination.
Limitations of the study design were reported earlier. 18 Briefly, the largest limitation of this study is the relatively small sample size and the restraint on the unvaccinated cohort to establish a threshold for the determination of vaccine status. In addition, all figures should be interpreted cautiously since this study was set up as a hypothesis‐generating study without formal power calculations for sample size. Notwithstanding, results can be informative for other researchers to calculate more reliable sample size estimates.
In conclusion, our study demonstrates a good correlation between HPV6/11/16/18‐antibodies in FV urine and paired sera, as well as between both assays. Significantly higher HPV‐antibody levels were also observed with both assays in FV urine and sera of vaccinated as opposed to unvaccinated women, though additional larger studies will be required to further optimize and validate the detection of HPV‐Abs in FV urine and to test differences driven by different HPV vaccines and schedules. Nonetheless, these results demonstrate that FV urine might offer an alternative, noninvasive tool to assess vaccine‐induced antibodies at the cervicovaginal site in the future.
CONFLICT OF INTERESTS
PVD and AV are co‐founders and former board members of Novosanis (Belgium), a spin‐off company of the University of Antwerp. The University of Antwerp received unrestricted education grants from Sanofi Pasteur MSD, Merck, and GSK. PVD and AV participated in advisory board meetings of Merck.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
AUTHOR CONTRIBUTIONS
JP: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, writing—original draft, writing—review and editing. GP: methodology, resources, supervision, writing—review and editing. MW‐F: methodology, writing—review and editing. SVK: investigation, writing—review and editing. LT: investigation, writing—review and editing. WAAT: funding acquisition, writing—review and editing. ZP: writing—review and editing. PVD: funding acquisition, resources, writing—review and editing. EU: methodology, resources, supervision, writing—review and editing. AV: conceptualization, funding acquisition, methodology, project administration, resources, supervision, writing—review and editing.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
We thank all women who participated in the study and thank the clinical trial unit from the Center for the Evaluation of Vaccination (CEV) for their practical assistance. We furthermore thank S. Biesmans, A. De Smet (CEV), H. Pathak (CDC) and B. Aengeneyndt (DKFZ) for the laboratory analysis and N. Hens (CHERMID) for his support with the statistical analysis. This study was supported by the Industrial Research Fund of the University of Antwerp, Belgium (PS ID 32387). JP is supported by a PhD fellowship of the Royal Belgian Academy of Medicine (GSK grant). SVK is supported by a junior postdoctoral fellowship of the Research Foundation Flanders (1240220N).
Pattyn J, Panicker G, Willhauck‐Fleckenstein M, et al. Comparison of a VLP‐based and GST‐L1‐based multiplex immunoassay to detect vaccine‐induced HPV‐specific antibodies in first‐void urine. J Med Virol. 2020;92:3774–3783. 10.1002/jmv.25841
REFERENCES
- 1. Draper E, Bissett SL, Howell‐Jones R, et al. A randomized, observer‐blinded immunogenicity trial of cervarix((R)) and gardasil((R)) human papillomavirus vaccines in 12‐15 year old girls. PLOS One. 2013;8(5):e61825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18‐45 years. Hum Vaccin. 2009;5(10):705‐719. [DOI] [PubMed] [Google Scholar]
- 3. Einstein MH, Baron M, Levin MJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)‐16/18 vaccine and HPV‐6/11/16/18 vaccine: follow‐up from months 12‐24 in a phase III randomized study of healthy women aged 18‐45 years. Hum Vaccin. 2011;7(12):1343‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Einstein MH, Levin MJ, Chatterjee A, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)−16/18 AS04‐adjuvanted vaccine and HPV‐6/11/16/18 vaccine in healthy women aged 18‐45 years: follow‐up through Month 48 in a Phase III randomized study. Hum Vaccin Immunother. 2014;10(12):3455‐3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kemp TJ, García‐Piñeres A, Falk RT, et al. Evaluation of systemic and mucosal anti‐HPV16 and anti‐HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29‐30):3608‐3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nardelli‐Haefliger D, Wirthner D, Schiller JT, et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus‐like particles. J Natl Cancer Inst. 2003;95(15):1128‐1137. [DOI] [PubMed] [Google Scholar]
- 7. Petäjä T, Pedersen C, Poder A, et al. Long‐term persistence of systemic and mucosal immune response to HPV‐16/18 AS04‐adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129(9):2147‐2157. [DOI] [PubMed] [Google Scholar]
- 8. Scherpenisse M, Mollers M, Schepp RM, et al. Detection of systemic and mucosal HPV‐specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Human VaccinImmunother. 2013;9(2):314‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwarz T, Spaczynski M, Kaufmann A, et al. Persistence of immune responses to the HPV‐16/18 AS04‐adjuvanted vaccine in women aged 15‐55 years and first‐time modelling of antibody responses in mature women: results from an open‐label 6‐year follow‐up study. BJOG. 2015;122(1):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarz TF, Galaj A, Spaczynski M, et al. Ten‐year immune persistence and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in females vaccinated at 15‐55 years of age. Cancer Med. 2017;6(11):2723‐2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwarz TF, Kocken M, Petäjä T, et al. Correlation between levels of human papillomavirus (HPV)−16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV‐16/18 AS04‐adjuvanted vaccine. Hum Vaccin. 2010;6(12):1054‐1061. [DOI] [PubMed] [Google Scholar]
- 12. Schwarz TF, Spaczynski M, Schneider A, et al. Immunogenicity and tolerability of an HPV‐16/18 AS04‐adjuvanted prophylactic cervical cancer vaccine in women aged 15‐55 years. Vaccine. 2009;27(4):581‐587. [DOI] [PubMed] [Google Scholar]
- 13. Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(suppl 3):S3/106‐S3/10113. [DOI] [PubMed] [Google Scholar]
- 14. Vorsters A, Van Damme P, Clifford G. Urine testing for HPV: rationale for using first void. BMJ. 2014;349:g6252. [DOI] [PubMed] [Google Scholar]
- 15. Cuschieri K, Nandwani R, McGough P, et al. Urine testing as a surveillance tool to monitor the impact of HPV immunization programs. J Med Virol. 2011;83(11):1983‐1987. [DOI] [PubMed] [Google Scholar]
- 16. Franceschi S, Chantal Umulisa M, Tshomo U, et al. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int J Cancer. 2016;139(3):518‐526. [DOI] [PubMed] [Google Scholar]
- 17. Vorsters A, Van Keer S, Van Damme P. The use of urine in the follow‐up of HPV vaccine trials. Hum Vaccin Immunother. 2015;11(2):350‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Keer S, Willhauck‐Fleckenstein M, Pattyn J, et al. First‐void urine as a non‐invasive liquid biopsy source to detect vaccine‐induced human papillomavirus antibodies originating from cervicovaginal secretions. J Clin Virol. 2019;117:11‐18. [DOI] [PubMed] [Google Scholar]
- 19. Robbins HA, Li Y, Porras C, et al. Glutathione S‐transferase L1 multiplex serology as a measure of cumulative infection with human papillomavirus. BMC Infect Dis. 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robbins HA, Waterboer T, Porras C, et al. Immunogenicity assessment of HPV16/18 vaccine using the glutathione S‐transferase L1 multiplex serology assay. Hum Vaccin Immunother. 2014;10(10):2965‐2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ‐purified glutathione s‐transferase fusion proteins. Clin Chem. 2005;51(10):1845‐1853. [DOI] [PubMed] [Google Scholar]
- 22. Panicker G, Rajbhandari I, Gurbaxani BM, Querec TD, Unger ER. Development and evaluation of multiplexed immunoassay for detection of antibodies to HPV vaccine types. J Immunol Methods. 2015;417:107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17(1):67‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grabowska K, Wang X, Jacobsson A, Dillner J. Evaluation of cost‐precision rations of different strategies for ELISA measurement of serum antibody levels. J Immunol Methods. 2002;271(1‐2):1‐15. [DOI] [PubMed] [Google Scholar]
- 25. Joura EA, Kjaer SK, Wheeler CM, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26(52):6844‐6851. [DOI] [PubMed] [Google Scholar]
- 26. Bontkes HJ, de Gruijl TD, Walboomers JM, et al. Immune responses against human papillomavirus (HPV) type 16 virus‐like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV‐16. J Gen Virol. 1999;80(Pt 2):409‐417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


