Abstract
Aim
To evaluate the pattern, impact on quality of life and management of common functional gastrointestinal disorders (FGIDs) and related signs and symptoms in infants below 1 year of age in Africa.
Methods
Multicentre, cross‐sectional, observational study in 10 African countries. At the first paediatric consultation of children with gastrointestinal symptoms, the perception of paediatricians on FGIDs (infant colic, constipation and regurgitation) and gas/bloating, impact on infant quality of life and parental anxiety and patient management practices were evaluated by standardised questionnaires.
Results
Questionnaires were completed by 759 paediatricians for 10 812 infants. Overall, 49.9% of paediatricians reported ≥30% of first infant consultations each month for FGIDs or related symptoms. Infant colic was most commonly diagnosed (57.6% of infants), followed by gas/bloating (43.2%), regurgitation (39.7%) and constipation (31.4%). Overall, 53% presented >1 symptom. Mean scores for infant quality of life, sleep and parental anxiety were worse when children had multiple symptoms compared to children with a single symptom (P < .025). Prescription of medication was common (62.4%). There were no consistent differences between countries.
Conclusion
Functional gastrointestinal disorder occurrence in Africa was high with a gap between expert recommendation that emphasises parental reassurance and nutritional advice and daily practice, particularly prescription of medication.
Keywords: functional gastrointestinal disorder, infant, Africa, colic
Abbreviations
- FGID
functional gastrointestinal disorder
- QUALIN
quality of life in infants
Key notes.
The first large‐scale study on functional gastrointestinal disorders (FGIDs) in Africa, showing incidences in consultations of children with gastrointestinal complaints of infant colic (57.6%), gas/bloating (43.2%), regurgitation (39.7%) and constipation (31.4%), with 53% presenting more than one symptom.
Infant quality of life, quality of sleep and parental anxiety were adversely affected by FGIDs.
A gap was identified between FGID management practices and expert recommendations, particularly regarding over‐prescribed medication.
1. INTRODUCTION
It has been reported that the most frequent functional gastrointestinal disorders (FGIDs) (including regurgitation, infant colic and constipation) affect approximately 50% of all infants during the first year after birth. 1 , 2 In France, FGIDs account for approximately 25% of paediatric consultations of infants up to 4 months of age. 3 These FGIDs have been reported to be associated with an adverse impact on the quality of life of both the infant and the family. 3 , 4 , 5 , 6 , 7 , 8
Despite FGIDs frequently being individually reported, there is evidence towards the frequent occurrence of more than one FGID in the same infant. 1 , 9 , 10 The exact prevalence of FGIDs, as well as their impact on infants and their families remains poorly defined, especially at the primary healthcare level. 2 , 11 , 12
Patient management should focus on parental reassurance and nutritional advice. 11 , 13 , 14 , 15 , 16 However, several European surveys have suggested that paediatricians often address parental demands by adopting unnecessary practices such as performing extra investigations or prescribing medications. 17 , 18 , 19 , 20
In Africa, the occurrence and impact of FGIDs have been very poorly studied. This large‐scale, non‐interventional study was conducted to characterise the occurrence of regurgitation, infant colic, functional constipation and gas/bloating in paediatric consultations in African countries as well as to evaluate their impact on quality of life of the infant and parental anxiety. Patient management practices were also recorded.
2. MATERIALS AND METHODS
2.1. Study design and participants
This was a multicentre, observational study conducted in 10 countries in Africa (Algeria [182 paediatricians], Morocco [203], Tunisia [50], Mauritius [28], Madagascar [38], Senegal [15], Gabon [35], Congo [39], Ivory Coast [107] and Cameroon [62]). As a non‐interventional study, the visits and procedures were the community standard practice, and there were no procedures other than usual patient management and collection of specific data by questionnaires. The questionnaire was developed in consultation with African and international experts and was designed to be as clear as possible regarding the terms and language understood and used by African paediatricians.
Questionnaires were completed by the treating paediatricians following consultation of full‐term, and otherwise healthy infants (per clinical examination of the paediatrician) between 0 and 12 months of age presenting for the first time with functional gastrointestinal symptoms as the main complaint, and who were not known to be allergic to cow's milk protein. Paediatricians were instructed about FGIDs in advance of the study and questionnaires included definitions for 3 FGIDs (infant colic, constipation and regurgitation) and 1 symptom (gas/bloating). The questionnaires were used to collect data on professional practice experience of each in this patient population, and on the individual patient cases, including a description and characteristics of the FGID or gas/bloating by the paediatrician and the first intent patient management prescribed. The questionnaires were completed from June to November 2017.
2.2. Study assessments
The study objectives were to collect the perception of paediatricians on regurgitation, infant colic, functional constipation and gas/bloating and their consequences, to evaluate their relative frequency and their impact on family quality of life, and to describe their management in several African countries.
Paediatricians reported their clinical experience for cases of regurgitation, infant colic, functional constipation and gas/bloating as well as the real‐life characteristics associated with the symptom, including quality of life, treatment and patient management.
Regurgitation and infant colic were assessed according to adapted Rome IV criteria for clinical research. 11 A certified French translation of the description of infant colic and regurgitation based on the ROME criteria was used (Parléclair, France). Regurgitation was defined as occurring at least twice per day for at least 3 weeks without nausea, haematemesis, retching, apnoea, failure to thrive, difficulty in swallowing or feeding or abnormal posture (Sandifer syndrome) in otherwise healthy infants aged 3 weeks to 12 months. Infant colic was defined as paroxysms of irritability, agitation or crying without any obvious cause that lasted ≥3 hours per day and occurred ≥3 times in 1 week, were unpredictable and inconsolable and were present before 5 months of age, without failure to thrive or fever. Functional constipation was assessed differently from the Rome IV criteria according to a definition for newborns as infrequent or difficult bowel movements for more than 2 weeks without any organic cause. 13 , 21 Information on gas/bloating was recorded using parental reports of symptoms.
Quality of life and sleep assessments were completed for infants during the consultation using a scale extracted from the Quality of Life in Infants (QUALIN) questionnaire validated for paediatricians. 22 Additionally, parental anxiety was assessed using a visual analogue scale.
2.3. Statistical analyses
Continuous variables were described by the number of observations, the mean, standard deviation, median, inter‐quartile range, minimum and maximum. Categorical variables were described using the frequency of infants by category. Missing data were not replaced and were not imputed for inclusion in the data analyses. An arbitrary sample size of 10 000 infants was targeted, to be included by approximately 900 paediatricians with each paediatrician having a 2‐month period to include 12 infants. Statistical comparisons were performed using two‐sided chi‐square tests with a type I error and a significance level of α = .05. All statistical analyses were performed by Groupe Axonal‐Biostatem, France, using SAS® version 9.4.
3. RESULTS
3.1. Participants studied
Questionnaires were completed by 759 paediatricians for a total of 10 812 infants. Overall, 49.9% of paediatricians reported that ≥30% of first infant consultations each month were for functional gastrointestinal symptoms.
There was a similar number of male (50.5%) and female (49.5%) infants. The overall mean ± SD age at onset of symptom(s) was 6.6 ± 9.1 weeks and varied between countries being lowest in Cameroon (4.8 ± 6.5 weeks) and highest in Congo (13.1 ± 13.0 weeks) (Figure 1). Overall, mean ± SD age at the time of consultation was longest for constipation (16.4 ± 13.5 weeks), being shorter for gas/bloating (12.2 ± 11.4 weeks), regurgitation (12.0 ± 10.8 weeks) and infant colic (10.5 ± 10.1 weeks). Mean ± SD weight at the time of consultation was 5.4 ± 1.7 kg, and 65% of infants were delivered vaginally.
FIGURE 1.
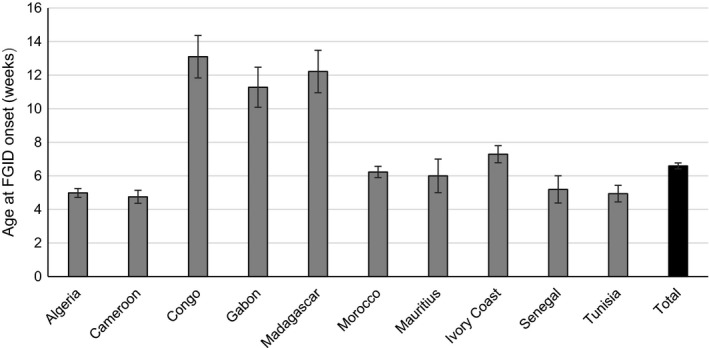
Infant age (mean ± SD) at onset of FGID by country (N = 9864). FGID, functional gastrointestinal disorder
Overall, 90.8% of infants had been breast‐fed. At the time of evaluation for functional gastrointestinal symptoms, 27.2% of infants were still exclusively breast‐fed, 47.7% were fed breast milk and infant formula, 19.4% were fed infant formula exclusively, while 5.7% were fed either cow's milk, other animal milk or a vegetable‐based drink exclusively or as a supplement to breast milk and/or infant formula.
Infants' families represented a range of socio‐economic backgrounds. Most fathers were employed (97%) compared with 49% of mothers. For both fathers and mothers, the highest percentage worked as employees (33% and 18%), followed by executive level employment (21% and 13%), and artisans (19% and 8%). Most infants were from urban areas, with only 5% of fathers and 1% of mothers working as farmers.
3.2. Characteristics of functional gastrointestinal symptoms
The data for each country are shown in Figure 2 as well as for the overall population for each symptom. Overall, infant colic was most commonly reported (57.6% of infants), followed by regurgitation (39.7%) and constipation (31.4%; Figure 2). Gas/bloating was reported for 43.2% of the infants. Despite certain country specificities (eg regurgitation was slightly more frequent in Gabon, Madagascar and Mauritius; colic was slightly more frequent in Algeria, Cameroon, Senegal and Tunisia; constipation was slightly more frequent in Congo, Gabon and Ivory Coast; and gas/bloating was slightly more frequent in Tunisia), there were no consistent trends.
FIGURE 2.
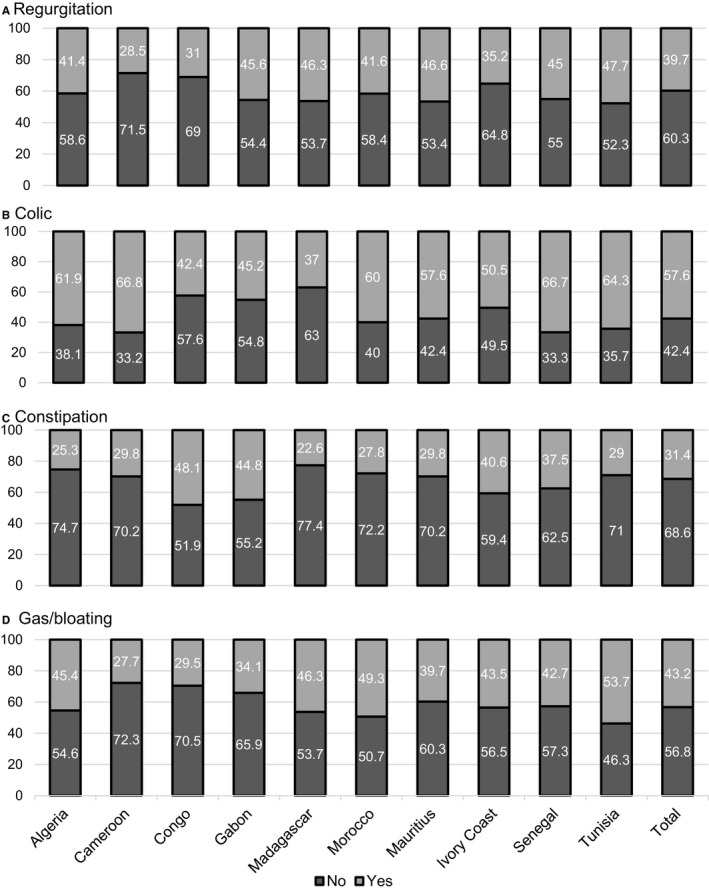
Percentage of patients with FGID symptoms (regurgitation, colic and constipation) and gas/bloating by country (N = 10 458). FGID, functional gastrointestinal disorder
More than one FGID, or a combination of FGIDs with gas/bloating, were reported for 53% of infants. Only regurgitation, infant colic, constipation and gas/bloating were reported for 26.7%, 34.1%, 25.2% and 14.0% of infants, respectively. For those with more than one FGID, or a combination of FGIDs with gas/bloating, the three most frequent combinations were colic and gas/bloating (24.4%), colic and regurgitation (14.9%) and regurgitation, colic and gas/bloating (14.2%), as reported in Table 1.
TABLE 1.
Incidence of combined FGID symptoms (regurgitation, infant colic and constipation) and gas/bloating (N = 5532)
| Symptoms | % |
|---|---|
| Two symptoms | |
| Infant colic, gas/bloating | 24.4 |
| Infant colic, regurgitation | 14.9 |
| Infant colic, constipation | 10.0 |
| Gas/bloating, regurgitation | 9.6 |
| Gas/bloating, constipation | 7.0 |
| Regurgitation, constipation | 3.6 |
| Three symptoms | |
| Infant colic, gas/bloating, regurgitation | 14.2 |
| Infant colic, gas/bloating, constipation | 7.4 |
| Infant colic, regurgitation, constipation | 2.3 |
| Gas/bloating, regurgitation, constipation | 1.3 |
| Four symptoms | |
| Infant colic, gas/bloating, regurgitation, constipation | 5.3 |
Data are % for the subpopulation of infants for whom more than 1 symptom was reported.
Abbreviation: FGID, functional gastrointestinal disorder.
3.3. Infant quality of life and sleep and parental anxiety
The overall mean ± SD score for infant quality of life was 6.1 ± 2.0 on a scale of 1 (very bad) to 10 (excellent), with 23.4% having a score ≤ 4 (bad or very bad). The mean ± SD score was worse for infants with more than one FGID symptom than for those with a single symptom (5.9 ± 2.0 vs 6.3 ± 2.1; P < .001; Table 2). The infants' quality of sleep scores were similar to the quality of life scores, with an overall mean ± SD score of 6.2 ± 2.2 and being worse for infants with more than one FGID, or a combination of FGIDs with gas/bloating (5.9 ± 2.1 v 6.5 ± 2.2; P < .001; Table 2).
TABLE 2.
Patient quality of life, sleep and parental anxiety
| 1 symptom | >1 symptom | Overall | P value* | |
|---|---|---|---|---|
| Infant quality of life | 6.3 ± 2.1 | 5.9 ± 2.0 | 6.1 ± 2.0 | <.001 |
| Infant quality of sleep | 6.5 ± 2.2 | 5.9 ± 2.1 | 6.1 ± 2.2 | <.001 |
| Parental anxiety | 6.1 ± 2.2 | 6.2 ± 2.2 | 6.1 ± 2.2 | .022 |
Data are mean ± SD for the subpopulation of infants for whom at least 1 symptom was reported.
Student's t test for the comparison of 1 symptom vs >1 symptom.
The overall mean ± SD parental anxiety score was 6.1 ± 2.2 on a scale of 1 (very low) to 10 (very high), with 30.9% of parents having a score ≥8. Parental anxiety was lower for infants with one FGID symptom than for those with more than one symptom (6.1 ± 2.2 vs 6.2 ± 2.2; P = .022; Table 2).
3.4. Patient management and treatment
The most common overall patient management practice for infants with FGID symptom(s) was parental reassurance (reported by 95.4% of paediatricians), followed by advice to parents (87.6%). Prescription of medication was, however, a very common practice (61.8%). Infant formula was changed in 43.5% of cases (data not available for the detail of the change of formula). In each case, the percentage of paediatricians adopting each patient management practice (ie parental reassurance, advice, prescribed medication and change of formula) was higher for infants with more than one FGID symptom (N = 5532) than for those with a single symptom (N = 4926) for parental reassurance (96.1% vs 94.7%), advice to parents (89.6% vs 85.3%), medication (68.7% vs 54.1%) and change of infant formula (48.1% vs 38.3%) (P < .001; Figure 3A). Of those who were prescribed medication, 42% were prescribed more than one treatment, and the number of prescribed treatments was higher for infants with more than one FGID symptom (mean ± SD 1.5 ± 0.7 treatments) than for infants with a single FGID (mean ± 1.2 ± 0.5 treatments; P < .001). The most commonly prescribed drugs included antispasmodics (43.9% of patients), laxatives (28.0%), prokinetics (13.2%), antacids (5.6%) and combinations of these and other prescription medications. Table 3 shows the distribution of medication by FGID or gas/bloating. Antacids, protein‐pump inhibitors, and prokinetics and anti‐H2s were most commonly used for regurgitation; antispasmodics were most commonly used for infant colic; treatment against intestinal gas was most commonly used for gas/bloating; and oral laxatives and laxative enemas were most commonly used for constipation.
FIGURE 3.
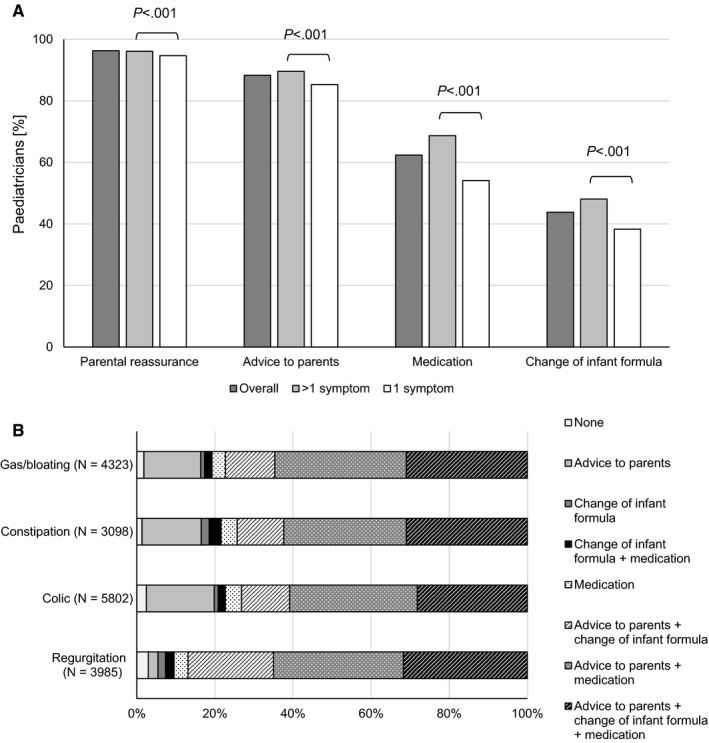
Clinical management practices overall (A) in addition to parental reassurance by FGID symptom (regurgitation, colic and constipation) and gas/bloating (B). FGID, functional gastrointestinal disorder
TABLE 3.
Medication used for FGID symptoms (regurgitation, infant colic and constipation) and gas/bloating
|
Regurgitation (N = 2367) |
Infant colic (N = 3996) |
Constipation (N = 2271) |
Gas/bloating (N = 3114) |
Total (N = 6545) |
|
|---|---|---|---|---|---|
| Antacids | 11.8 | 4.5 | 2.0 | 4.3 | 5.6 |
| Protein‐pump inhibitors & anti‐H2 | 7.8 | 2.8 | 1.2 | 2.6 | 3.6 |
| Prokinetics | 28.4 | 10.7 | 8.9 | 10.8 | 13.2 |
| Antispasmodics | 42.7 | 56.9 | 36.0 | 49.7 | 43.9 |
| Treatment against intestinal gas | 40.9 | 50.3 | 27.9 | 62.8 | 52.1 |
| Oral laxative | 4.6 | 8.0 | 34.1 | 8.3 | 13.1 |
| Laxative enema | 5.5 | 9.7 | 37.6 | 10 | 14.9 |
| Other | 8.6 | 7.0 | 5.9 | 6.2 | 7.0 |
Data are percentage of infants receiving the medication.
Abbreviation: FGID, functional gastrointestinal disorder.
The patient management practices not including parental reassurance were similar for each type of FGID symptom, and combined practices were more common than single practices (Figure 3B).
4. DISCUSSION
This was the first large‐scale study of the characterisation and management of FGIDs in Africa. The study results confirm the occurrence of FGIDs in developing countries in Africa and showed FGIDs to be a very frequent cause for consultation of infants aged 0‐12 months in these countries. Notably, even in the country with the oldest cohort (Congo, mean age 13.1 weeks), infant colic was still diagnosed in approximately 40% of patients. About half the paediatricians reported that at least one out of three consultations were linked to FGIDs and related signs and symptoms. This is in agreement with studies in Europe and the US, which have shown a similar incidence of FGIDs of 40%‐60% in infants aged 0‐12 months. 1 , 14 , 21 , 23 Often more than one FGID symptom occurred together in the African infants in the present study, which again is in line with pre‐existing data from other global regions, 1 , 10 , 24 and the most common combination of symptoms was infant colic with gas/bloating which accords with a previous study conducted in France. 3
This study found an adverse impact of FGIDs on infant quality of life, quality of sleep and parental anxiety, all of which were worse for infants with more than one FGID symptom. This reinforces the importance of the paediatrician not only diagnosing and treating the infant but also taking into account parental anxiety 11 and including this as a consideration in the overall management practice.
All interventions were more common for infants with more than one FGID, or a combination of FGIDs with gas/bloating. Other than parental reassurance, clinical management more often involved two or more approaches rather than a single practice and was similar for each FGID although information on extra investigations was not collected in this survey. In particular, medication was prescribed frequently (in >60% of cases where more than one symptom was present), constituting a deviation from expert guidance which recommends minimal use of medication for the management of FGIDs 13 , 14 , 16 , 21 , 25 , 26 and suggests that parental reassurance and nutritional advice constitute the most effective management practice for FGIDs. 16 It can be hypothesised that prescribing medication for infants is used at least in part as an effort to manage parental anxiety. However, the prescription of medication in this way is not only unnecessary and has no supportive evidence, but can also be associated with side effects and can have an impact on healthcare expenditure. 27
Limitations of this study included that participating paediatricians were not selected using a specific methodology and therefore may not be fully representative of the wider population. Only questions regarding regurgitation, infant colic and functional constipation as the putatively most common FGIDs were included. Other FGIDS, such as functional diarrhoea or dyschezia, were not evaluated to avoid the risk of misdiagnosis of these less common symptoms, which could have introduced the risk of less accurate data into the analyses. This approach served to foster compliance with the study procedures and therefore maximise the robustness of the data collected. Also, although there was an adverse impact of regurgitation, infant colic, functional constipation and gas/bloating on infant quality of life, quality of sleep and parental anxiety, no comparator group was included to provide information on these criteria in otherwise healthy infants nor the prevalence in the general paediatric population. The type of prescribed medications (eg the types of antispasmodic, laxative, prokinetic and antacid) was not recorded. A further potential limitation was that most infants were from urban areas since areas of higher literacy were preferred. Data on feeding type are not evaluated since the study was not designed for this comparison and the populations were not balanced in this regard. As a pilot study to characterise a large and relevant gap in the assessment of FGIDs and their consequences in infants in Africa, this study should be followed up to address these limitations by more stringent, prospective studies which utilise the complete Rome IV parental diagnostic questionnaire.
In conclusion, this is the first time that the occurrence, characteristics and management of FGID in Africa has been reported on a large scale. These aspects are well aligned with previously reported data from other global regions, showing a high incidence of FGID in the first 12 months after birth and a gap between management practices and recommended guidance, particularly regarding the prescription of largely unnecessary medication.
CONFLICTS OF INTEREST
Fanny Krumholz and Thomas Ludwig are employees of Danone Nutricia Africa & Overseas and Danone Nutricia Research Singapore. The other authors have no conflict of interests to declare.
ACKNOWLEDGEMENTS
The authors thank the doctors, participants and their families for their generous contribution to advancing the knowledge infant functional gastrointestinal disorders in Africa. Additionally, Dr Fadila Benhassine (Bologhine Hospital, Alger) provided valuable input in the development of the questionnaire, study design and coordination. Dr Andrew Lane (Lane Medical Writing) provided medical writing assistance in the preparation and development of the manuscript in accordance with the European Medical Writers Association guidelines and Good Publication Practice and was funded by Danone.
Bellaiche M, Ategbo S, Krumholz F, et al. A large‐scale study to describe the prevalence, characteristics and management of functional gastrointestinal disorders in African infants. Acta Paediatr. 2020;109:2366–2373. 10.1111/apa.15248
Funding information
The study was funded by Danone Nutricia Africa & Overseas, Limonest, France.
REFERENCES
- 1. Iacono G, Merolla R, D'Amico D, et al. Gastrointestinal symptoms in infancy: a population‐based prospective study. Dig Liver Dis. 2005;37(6):432‐438. [DOI] [PubMed] [Google Scholar]
- 2. Vandenplas Y, Abkari A, Bellaiche M, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015;61(5):531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellaïche M, Oozeer R, Gerardi‐Temporel G, Faure C, Vandenplas Y. Multiple functional gastrointestinal disorders are frequent in formula‐fed infants and decrease their quality of life. Acta Paediatr. 2018;107(7):1276‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akman I, Kuscu K, Ozdemir N, et al. Mothers' postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91(5):417‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown M, Heine RG, Jordan B. Health and well‐being in school‐age children following persistent crying in infancy. J Paediatr Child Health. 2009;45(5):254‐262. [DOI] [PubMed] [Google Scholar]
- 6. Indrio F, Di Mauro A, Riezzo G, Cavallo L, Francavilla R. Infantile colic, regurgitation, and constipation: an early traumatic insult in the development of functional gastrointestinal disorders in children? Eur J Pediatr. 2015;174(6):841‐842. [DOI] [PubMed] [Google Scholar]
- 7. Partty A, Kalliomaki M, Salminen S, Isolauri E. Infant distress and development of functional gastrointestinal disorders in childhood: is there a connection? JAMA Pediatr. 2013;167(10):977‐978. [DOI] [PubMed] [Google Scholar]
- 8. Raiha H, Lehtonen L, Korhonen T, Korvenranta H. Family functioning 3 years after infantile colic. J Dev Behav Pediatr. 1997;18(5):290‐294. [DOI] [PubMed] [Google Scholar]
- 9. Vandenplas Y, Ludwig T, Bouritius H, et al. Randomised controlled trial demonstrates that fermented infant formula with short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 2017;106(7):1150‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Tilburg MA, Hyman PE, Walker L, et al. Prevalence of functional gastrointestinal disorders in infants and toddlers. J Pediatr. 2015;166(3):684‐689. [DOI] [PubMed] [Google Scholar]
- 11. Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016;150(6):1443‐1455.e2. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira‐Maia AP, Matijasevich A, Wang YP. Epidemiology of functional gastrointestinal disorders in infants and toddlers: a systematic review. World J Gastroenterol. 2016;22(28):6547‐6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence‐based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258‐274. [DOI] [PubMed] [Google Scholar]
- 14. Vandenplas Y, Benninga M, Broekaert I, et al. Functional gastro‐intestinal disorder algorithms focus on early recognition, parental reassurance and nutritional strategies. Acta Paediatr. 2016;105(3):244‐252. [DOI] [PubMed] [Google Scholar]
- 15. Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498‐547. [DOI] [PubMed] [Google Scholar]
- 16. Salvatore S, Abkari A, Cai W, et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018;109(9):1512‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCracken M. Gastroesophageal reflux guidelines: the European experience. J Pediatr Gastroenterol Nutr. 2014;58(4):395‐396. [DOI] [PubMed] [Google Scholar]
- 18. Quitadamo P, Miele E, Alongi A, et al. Italian survey on general pediatricians' approach to children with gastroesophageal reflux symptoms. Eur J Pediatr. 2015;174(1):91‐96. [DOI] [PubMed] [Google Scholar]
- 19. Quitadamo P, Papadopoulou A, Wenzl T, et al. European pediatricians' approach to children with GER symptoms: survey of the implementation of 2009 NASPGHAN‐ESPGHAN guidelines. J Pediatr Gastroenterol Nutr. 2014;58(4):505‐509. [DOI] [PubMed] [Google Scholar]
- 20. Quitadamo P, Urbonas V, Papadopoulou A, et al. Do pediatricians apply the 2009 NASPGHAN‐ESPGHAN guidelines for the diagnosis and management of gastroesophageal reflux after being trained? J Pediatr Gastroenterol Nutr. 2014;59(3):356‐359. [DOI] [PubMed] [Google Scholar]
- 21. Vandenplas Y, Alarcon P, Alliet P, et al. Algorithms for managing infant constipation, colic, regurgitation and cow's milk allergy in formula‐fed infants. Acta Paediatr. 2015;104(5):449‐457. [DOI] [PubMed] [Google Scholar]
- 22. Manificat S, Dazord A, Langue J, et al. Evaluation of the quality of life of infants and very young children: validation of a questionnaire. Multicenter European study. Arch Pediatr. 2000;7(6):605‐614. [DOI] [PubMed] [Google Scholar]
- 23. Robin SG, Keller C, Zwiener R, et al. Prevalence of pediatric functional gastrointestinal disorders utilizing the Rome IV criteria. J Pediatr. 2018;195:134‐139. [DOI] [PubMed] [Google Scholar]
- 24. Bellaïche M. Approche de première intention des troubles digestifs bénins associés du nourrisson de moins de 6 mois: résultats de l'observatoire ADAN [French]. Médecine & Enfance. 2014;34(7 suppl.):1‐12. [Google Scholar]
- 25. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vandenplas Y, Alarcon P. Updated algorithms for managing frequent gastro‐intestinal symptoms in infants. Benef Microbes. 2015;6(2):199‐208. [DOI] [PubMed] [Google Scholar]
- 27. Mahon J, Lifschitz C, Ludwig T, et al. The costs of functional gastrointestinal disorders and related signs and symptoms in infants: a systematic literature review and cost calculation for England. BMJ Open. 2017;7(11):e015594. [DOI] [PMC free article] [PubMed] [Google Scholar]


