Abstract
Background and Aim
The association of fecal calprotectin (FC) and endoscopic response in inflammatory bowel disease patients during vedolizumab (VDZ) treatment is largely unknown. The aim of this study is to assess the diagnostic value of FC to predict endoscopic response.
Methods
Patients with active endoscopic disease at baseline were included. Endoscopies and FC tests were performed at baseline and week 16. Patients with a confirmed endoscopic response at week 16 continued VDZ maintenance therapy, and endoscopy and FC tests were performed at week 52. Endoscopic response was defined as endoscopic Mayo score reduction of ≥ 1, SES‐CD of ≥ 50%, or Rutgeerts' score of ≥ 1. Correlations were assessed using Spearman and receiver operating characteristic statistics.
Results
A total of 114 patients, 46 ulcerative colitis and 68 Crohn's disease patients (44 men, median age 40 years), were included after the start of VDZ; 85% was anti‐tumor necrosis factor alpha refractory. Endoscopic response was observed in 60 (53%) patients at week 16; the response sustained in 73% at week 52. FC decreased significantly from 819 at baseline to 154 μg/g at week 16. FC at weeks 16 and 52 were significantly correlated to (sustained) endoscopic response (r = −0.62 / r = −0.67, P < 0.001). FC < 200 μg/g indicates endoscopic response (area under the curve = 0.89, positive predictive value = 94%), whereas FC > 450 μg/g indicates endoscopic non‐response after induction (negative predictive value = 83%). An increase in FC level of > 400 μg/g after induction indicates endoscopic loss of response (area under the curve = 0.97, negative predictive value = 96%).
Conclusion
This prospective study demonstrates a significant correlation between FC and endoscopic response to VDZ. FC < 200 μg/g prognosticate endoscopic response, and FC > 450 μg/g endoscopic non‐response. An increase in FC of > 400 μg/g after induction indicates endoscopic loss of response. This simple FC algorithm may guide clinical decisions on the continuation and optimization of VDZ in inflammatory bowel disease patients.
Keywords: Clinical algorithm, Correlation, Endoscopy, Fecal calprotectin, Inflammatory bowel diseases, Predictive value, Vedolizumab
Introduction
Vedolizumab (VDZ) is a gut‐selective humanized monoclonal anti‐α4β7 integrin and an effective treatment option for ulcerative colitis (UC) and Crohn's disease (CD). 1 , 2 , 3 Although VDZ has yielded a manifest position in inflammatory bowel disease (IBD) treatment after registration, an important drawback for clinical decisions on the use of VDZ is the uncertainty on the objective efficacy of VDZ as induction strategy. The evaluation of the effect of VDZ induction therapy preferably combines objective response to substantiate subjective improvement of symptoms, as endoscopic remission is associated with improved prognosis. 4 , 5 , 6 , 7 , 8 Clinical data on the effect of VDZ induction therapy are available abundantly. For instance, the registration trials (GEMINI) reported response of 47% in UC and 31% in CD patients at week 6. 9 , 10 In addition, a meta‐analysis of real‐world studies showed clinical remission in 50% of anti‐tumor necrosis factor (TNF)‐naïve patients and 30% of anti‐TNF refractory patients at week 14. 11 Prospective data on objective parameters of response to VDZ with endoscopy and biochemistry (fecal calprotectin [FC]) are gradually emerging. Recently, important data on endoscopic and histological response to VDZ have been reported. 12 , 13 , 14 In these studies, endoscopic response to VDZ was 25–40% at week 26 and approximately 50% at week 52 with histological response in 24% at week 26 and 34% at week 52. 13 In current practice, clinical decisions on therapy continuation or optimization strategies are often scheduled after VDZ induction; therefore, more data on the objective response after induction in real‐world studies are required. In addition, non‐invasive markers such as FC to substitute endoscopy are preferred in these situations. In this prospective cohort study, we assessed the diagnostic value of FC to predict endoscopic response after VDZ induction at week 16 and to develop a simple algorithm for clinical practice. As secondary aim, the diagnostic value of FC to predict a sustained (endoscopic) response to VDZ will be assessed.
Methods
Study population
Adult patients with UC, IBD unclassified (IBD‐U), and CD who started VDZ between October 2014 and January 2019 at the Erasmus University Medical Center (EMC) with active disease at baseline endoscopy were included. Baseline clinical and demographic characteristics (age, gender, smoking status, disease characteristics, and treatment history) were collected.
Vedolizumab therapy
VDZ induction therapy consisted of four 300‐mg infusions for UC patients and was scheduled at week 0 (baseline), week 2, week 6, and week 14. CD patients received an additional VDZ infusion at week 10. For patients with an endoscopic response at week 16, VDZ maintenance infusions were scheduled every 8 weeks. Dose escalation of VDZ therapy was accepted and was left to the discretion of the treating physician.
Endoscopic assessment
Colonoscopy and FC tests were performed at baseline and week 16. Patients with a confirmed endoscopic response at week 16 continued VDZ maintenance therapy. In these patients, colonoscopy and FC tests were repeated at week 52. Endoscopic inflammation was determined using the scores as depicted in Table S1. Endoscopic remission was defined as an endoscopic Mayo score of 0, SES‐CD of ≤ 2, and a Rutgeerts' score of i0 or i1. Endoscopic response was defined as a decrease of 1 or more points in the endoscopic Mayo score, ≥ 50% decrease in SES‐CD score, or a decrease of 1 or more points in the Rutgeerts' score. In patients with an ileostomy, ileoanal pouch anastomosis, or ileorectal anastomosis, endoscopic disease activity was classified as no, mild, moderate, or severe as judged by the endoscopist. Remission was defined as “no endoscopic disease activity” and response as a decrease of ≥ 1 point on the above mentioned 4‐grade scale. Endoscopic inflammation was further categorized as shown in Table S1.
Sustained effectiveness was defined as an endoscopic response at week 16 that sustained at week 52. Endoscopic loss of response (eLOR) was defined as an increase in Mayo endoscopic score of ≥ 1 point, SES‐CD increase of ≥ 50%, or Rutgeerts' score increase of ≥ 1 point in patients with an initial endoscopic response achieved at week 16 (induction).
Fecal calprotectin measurements
At baseline, weeks 16 and 52 FC was determined using a quantitative enzyme‐linked immunosorbent assay (ELISA) (Bühlmann Laboratories AG, Schönenbuch, Switzerland) or with the QuantOn Cal (QoC) (Preventis, Germany) FC home test. 15 During follow‐up, patients were offered the same FC measurement technique.
Statistical analysis
Descriptive statistics were used for baseline characteristics. Continuous data were presented as mean ± standard deviation (SD) or median with interquartile range (25th to 75th) according to the distribution. Categorical data were presented in percentages. IBD‐U patients were included in the UC group for analysis. Chi‐square test or Wilcoxon rank sum test was used to evaluate differences in categorical or continuous not‐normally distributed data, between UC and CD patients. The principal endpoint of interest was endoscopic response at week 16. A multivariable logistic regression analysis was performed to identify predictors of endoscopic response at week 16. Variables with significance level of < 0.2 on univariate analysis and based on literature were included in the multivariable model. Additional analyses were performed to evaluate the endpoints of sustained endoscopic response at week 52 and endoscopic remission at week 16 and at week 52. The correlation between week 16 and week 52 FC and (sustained) endoscopic response, endoscopic remission, and endoscopic disease activity was analyzed using a non‐parametric two‐tailed Spearman's rank correlation test. For FC, a delta (FC at baseline − FC at week 16/FC at week 16 − FC at week 52) and a % decrease were calculated. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FC were calculated on endoscopic response, endoscopic remission, and endoscopic disease activity (no/mild/moderate/severe) using receiver operating characteristic (ROC) statistics. The optimal cut‐off (highest clinical relevant PPV/NPV) was determined using the ROC curve for FC and endoscopic outcome. A two‐sided P‐value of < 0.05, and an area under the curve (AUC) of ≥ 0.80, was considered significant. A correlation coefficient of > 0.6 was considered as strong correlation. All statistical analyses were performed using IBM SPS Statistics version 24.0 (IBM Corp. Released 2013, IBM Corp, Armonk, NY). The study protocol was approved by the Medical Ethics Committee (METC) Rotterdam, The Netherlands, MEC‐2018‐1187.
Results
In total, 134 patients received VDZ during the study period, of whom 20 patients were excluded due to various reasons (Fig. 1). The study population comprised 114 patients (38 UC [33%], 8 IBD‐U [7%], and 68 CD patients [60%]) with a median age of 40 years (Table 1). VDZ was initiated after a median disease duration of 14 years (8 ‐ 20). In total, 104/114 patients (91%) were exposed to one or more anti‐TNF drugs prior to the start of VDZ, of whom 88/104 (85%) were anti‐TNF refractory, defined as clinical primary failure or secondary loss of response and 16/104 (15%) stopped anti‐TNF due to side effects.
Figure 1.
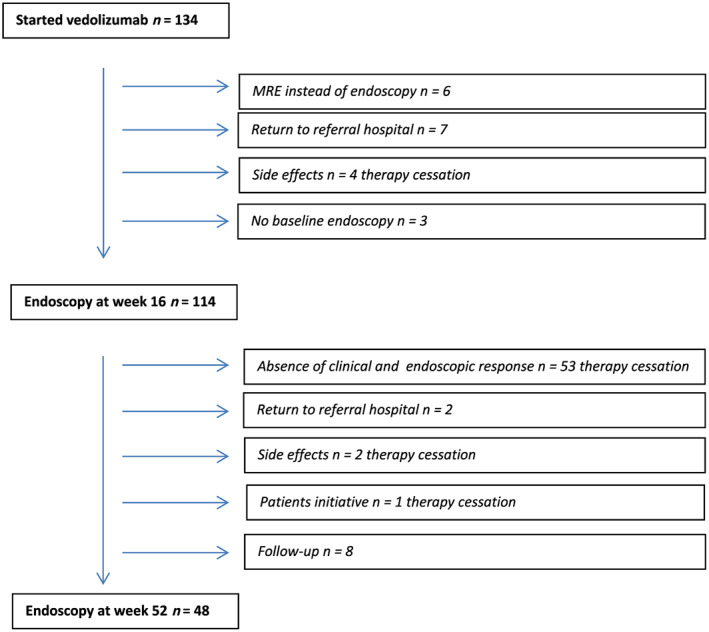
Flowchart of patient inclusion. MRE, magnetic resonance enterography. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Baseline patient characteristics
| N = 114 | |
|---|---|
| Male, n (%) | 44 (38.6) |
| Median age (years) (25th to 75th) | 39.8 (31.0–49.7) |
| Smoking, n (%) | |
| Yes | 22 (21.2) |
| No | 82 (78.8) |
| Median disease duration (years) (25th to 75th) | 13.8 (7.8–20.1) |
| Diagnosis, n (%) | |
| UC | 38 (33.3) |
| IBD‐U | 8 (7) |
| CD | 68 (59.6) |
| + Perianal disease | 19 (27.9) |
| CD disease location, n (%) | |
| L1 ileal | 6 (8.8) |
| L2 colonic | 13 (19.1) |
| L3 ileocolonic | 49 (72.1) |
| + L4 upper GI disease | 6 (8.8) |
| CD disease behavior, n (%) | |
| B1 | 29 (42.6) |
| B2 | 30 (44.1) |
| B3 | 9 (13.2) |
| UC disease location, n (%) | |
| E2 | 17 (37.0) |
| E3 | 29 (63.0) |
| Previous IBD‐related surgery, n (%) † | 34 (29.8) |
| Previous exposure to anti‐TNFα therapy, n (%) | |
| 0 | 10 (8.8) |
| 1 | 40 (35.1) |
| 2 | 59 (51.7) |
| 3 | 5 (4.4) |
| Anti‐TNFα refractory disease, n (%) | 88 (84.6) |
| Primary non‐responders | 36 (40.9) |
| Secondary loss of response | 52 (59.1) |
| Concomitant steroid therapy, n (%) | |
| Prednisolone | 42 (36.8) |
| Budesonide | 32 (28.1) |
| Concomitant IBD medication, n (%) | |
| Immunomodulator | 27 (23.7) |
| Thiopurines ‡ | 20 (17.5) |
| Tacrolimus | 7 (6.1) |
| 5‐ASA | 14 (12.3) |
Five patients with ileostomy, ileoanal pouch anastomosis, or ileorectal anastomosis (the remainder received; 26 ileocolonic, 2 small intestine, and 1 colonic resections).
Azahiorpine/mercaptopurine/thioguanine.
B, behavior; CD, Crohn's disease; E, extent; IBD‐U, inflammatory bowel disease unclassified; L, location; N, numbers; TNFα, tumor necrosis factor alpha; UC, ulcerative colitis; 5‐ASA, 5‐aminosalicylic acid.
In 74/114 patients (65%), initiation of VDZ was combined with corticosteroid induction therapy (prednisolone in 42/114 patients [37%]; budesonide in 32/114 patients [28%]) and was completely tapered at week 16 in 50/74 (68%) patients, without statistical difference between UC and CD patients. In total, 27/114 patients (24%) were on concomitant immunomodulator (IM) therapy.
Endoscopic response
Endoscopic response was observed in 60/114 (53%) patients at week 16 (28/46 UC and 32/68 CD, P = 0.18). Endoscopic remission at week 16 was observed in 23/114 (20%) patients (10/46 UC and 13/68 CD, P = 0.81) (Fig. S1). Twenty‐one patients used corticosteroids at week 16, of whom 15/21 (71%) were endoscopic non‐responders (P = 0.071). Patients on concomitant IM therapy at baseline showed lower endoscopic response rates as compared with patients without concomitant IM therapy (9/27, 33% vs 51/87, 59%; P = 0.03), whereas no significant difference was observed for endoscopic remission (2/27, 7% vs 21/87, 24%; P = 0.10). Only 2/27 (7%) patients on concomitant IM therapy received dose optimization of thiopurine treatment before week 16; both patients were endoscopic non‐responders. No patients started new (concomitant) immunomodulatory therapy during 16 weeks of VDZ treatment.
The mean SES‐CD decreased from 12 (SD 5.1) at baseline to 7.7 points (SD 5.9) at week 16 (P < 0.001). The Rutgeerts' score decreased from 3.2 (SD 0.7) at baseline to 2.5 points (SD 1.3) at week 16 (P = 0.003). Mayo endoscopic score decreased from 2.3 (SD 0.7) at baseline to 1.5 points (SD 1.1) at week 16 (P < 0.001) (Fig. S2). Sixty patients with an endoscopic response at week 16 continued VDZ; a total of 48/60 (80%) patients underwent an endoscopy at week 52 (Fig. 1). A sustained endoscopic response at week 52 was observed in 35/48 (73%) patients (14/23 UC and 21/25 CD patients, P = 0.07), of whom endoscopic remission was observed in 22/48 (46%) patients (9/23 UC and 13/25 CD, P = 0.37). Mean SES‐CD decreased to 3.0 points (SD 2.9) at week 52, Rutgeerts' score decreased to 1.5 points (SD 1.2), and the Mayo endoscopic score decreased to 1.2 points (SD 1.2). eLOR at week 52 was observed in 13/48 (27%) patients (Fig. S2) (UC 9/23 [39%] and CD 4/24 [17%], P = 0.111). A total of 10/48 patients needed VDZ dose escalation, to every 6 weeks in five patients and to every 4 weeks in five patients. After a median follow‐up time of 24 weeks (20 – 33), 1/10 (10%) patient recaptured clinical response to VDZ and continued treatment.
Clinical predictors of endoscopic response at week 16
Univariable and multivariable analysis showed no significant associations between clinical parameters and endoscopic response at week 16 (Table 2). As depicted in the multivariable analysis, there was no additional effect on the endoscopic response for the use of corticosteroids at baseline (prednisolone and budesonide), when corrected for endoscopic severity and IBD type.
Table 2.
Univariable and multivariable logistic regression analysis of factors associated with vedolizumab endoscopic response at week 16
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Baseline characteristics |
Response, yes n = 60 (%) |
Response, no n = 54 (%) |
P‐value | OR [95% CI] | P‐value |
| Sex | |||||
| Male | 20 (33) | 24 (44) | 0.25 | — | — |
| Smoking | |||||
| Yes | 14 (23) | 8 (15) | 0.23 | — | — |
| IBD type | |||||
| CD | 32 (53) | 36 (67) | 0.18 | — | — |
| UC | 28 (47) | 18 (33) | 0.52 [0.24–1.15] | 0.11 | |
| Prior surgery for CD | 17 (28) | 17 (32) | 0.84 | — | — |
| Steroid induction | 35 (58) | 40 (74) | 0.11 | 1.87 [0.83–4.23] | 0.13 |
| Anti‐TNFα refractory | 48 (81) | 45 (83) | 0.81 | — | — |
| Endoscopic disease severity at baseline | |||||
| Mild | 8 (13) | 8 (15) | 0.12 † | — | — |
| Moderate | 36 (60) | 24 (44) | — | — | |
| Severe | 16 (27) | 22 (41) | 1.99 [0.87–4.55] | 0.10 † | |
| Disease location CD | |||||
| Ileal | 3 (9) | 3 (8) | 0.55 ‡ | — | — |
| Colonic | 5 (16) | 8 (22) | — | — | |
| Ileocolonic | 24 (75) | 25 (69) | — | — | |
| Upper GI | 4 (6) | 2 (4) | — | — | |
| Disease location UC | |||||
| Proctitis | 0 (0) | 0 (0) | 0.36 | — | — |
| Left‐sided | 12 (43) | 5 (28) | — | — | |
| Pancolitis | 16 (57) | 13 (72) | — | — | |
| Perianal disease | |||||
| Yes | 6 (10) | 13 (24) | 0.18 | — | — |
Severe endoscopic disease activity versus no severe endoscopic disease activity.
Colonic versus ileal and ileocolonic disease.
Univariable and multivariable analysis showed no significant associations between clinical baseline parameters and endoscopic response. CD, Crohn's disease; CI, confidence interval; GI, gastrointestinal disease activity; IBD, inflammatory bowel disease; N, numbers; OR, odds ratio; TNFα, tumor necrosis factor alpha; UC, ulcerative colitis.
Fecal calprotectin
FC at baseline and week 16 were available in 76/114 (67%) patients. FC at week 52 was available in 35/60 (58%) patients. In 90/114 (79%) patients, FC was analyzed using the ELISA, and 15/114 (13%) patients used the FC home test at all time points. Only 9/114 (8%) patients intended to use the FC home test but switched to the ELISA. The overall median FC decreased significantly from 843.0 μg/g (403.0 – 1800.0) at baseline to 256.5 μg/g (116.0 – 897.0) at week 16 (P < 0.001). No statistical differences were observed between UC and CD.
Correlation between fecal calprotectin and endoscopy
Median FC decreased significantly only in patients with an endoscopic response at week 16 from 819.0 μg/g (243.0 – 1800.0) at baseline to 154.0 μg/g (49.0 – 254.5) at week 16 (P < 0.001). In patients without an endoscopic response at week 16, median FC was 974.50 μg/g (543.00 – 1748.50) at baseline and 833 μg/g at week 16 (P = 0.60).
Within the group of patients with an endoscopic response at week 16, a statistically non‐significant increase in median FC was observed from 154.0 μg/g (49.0 – 254.5) at week 16 to 224.0 μg/g (45.0 – 786.0) at week 52 (P = 0.41). No statistical differences were observed between UC and CD.
Baseline FC was not correlated to endoscopic response, endoscopic remission, nor endoscopic inflammation at week 16 (r = −0.08, r = −0.18, and r = 0.16).
FC at week 16, as well as ∆FC (FC at baseline − FC at week 16) and percentage decrease in FC from baseline to week 16, correlated significantly with endoscopic response, endoscopic remission, and endoscopic inflammation (no/mild/moderate/severe) at week 16. Correlation coefficients (r) are mentioned in Table 3. No statistical differences were observed between UC and CD, except for the correlation between ∆FC and endoscopic remission, which reached only statistical significance for UC patients (r = 0.407, P = 0.021) as compared with CD (r = 0.095, P = 0.538).
Table 3.
Correlation coefficients between fecal calprotectin (μg/g) and endoscopy after induction (at week 16) and sustained endoscopic response (at week 52)
| Week 16 | Week 52 | |||||
|---|---|---|---|---|---|---|
| FC | ∆FC † | % decrease FC ‡ | FC | ∆FC § | % decrease FC ¶ | |
| Endoscopic response | −0.62 ** | 0.40 ** | 0.52 ** | −0.67 ** | 0.69 ** | 0.61 ** |
| Endoscopic remission | −0.47 ** | 0.25 * | 0.41 ** | −0.63 ** | 0.52 * | 0.45 * |
| Endoscopic inflammation | 0.60 ** | −0.36 * | −0.53 ** | 0.71 ** | −0.59 * | −0.52 * |
P < 0.05.
P < 0.001.
∆FC indicates the absolute change between baseline and week 16.
% decrease indicates the relative change between baseline and week 16.
∆FC indicates the absolute change between week 16 and week 52.
% decrease indicates the relative change between week 16 and week 52.
Spearman's correlation between fecal calprotectin and endoscopic response, endoscopic remission, and endoscopic inflammation (no/mild/moderate/severe) at week 16 and week 52. FC at week 16, n = 97/114 (both time points, n = 76) is correlated to endoscopy at week 16. FC at week 52, n = 35/60 (both time points, n = 30) is correlated with endoscopy at week 52. FC, fecal calprotectin (μg/g).
Week 16 FC was not correlated with a sustained endoscopic response/eLOR at week 52 (r = −0.291, P = 0.076); no differences were observed between UC (r = −0.30, P = 0.226) and CD patients (r = −0.24, P = 0.311).
FC at week 52, FC (FC at week 16 − FC at week 52), and percentage decrease in FC from week 16 to week 52 correlated significantly with sustained endoscopic response/eLOR endoscopic remission and endoscopic inflammation (no/mild/moderate/severe) at week 52. The correlation coefficients (r) are mentioned in Table 3.
Fecal calprotectin's optimal cut‐off value predicting endoscopic response
At week 16, FC < 200 μg/g predicted endoscopic response after induction (AUC = 0.860, PPV = 94%, NPV = 67%, sensitivity = 64%, specificity = 94%, Fig. 2). FC > 450 μg/g at week 16 predicted an endoscopic non‐response after induction (AUC = 0.858, NPV = 83%, PPV = 87%, sensitivity = 87%, specificity = 83%, Fig. 2). The specific diagnostic accuracies for UC and CD are mentioned in Table 4.
Figure 2.
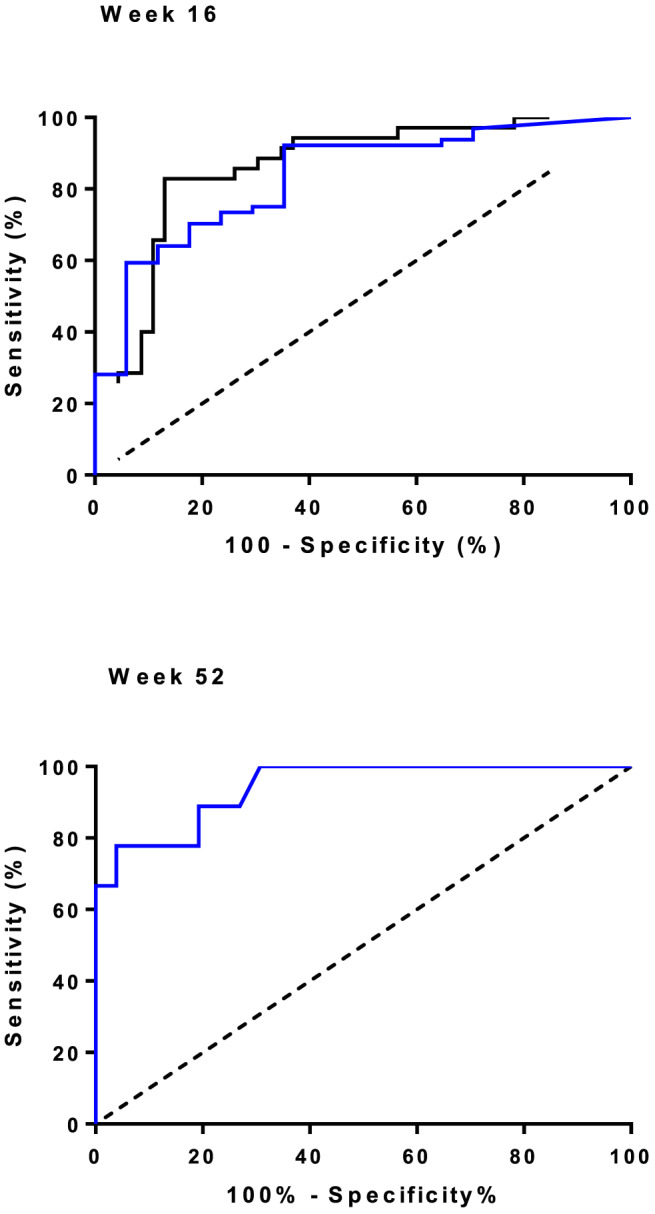
Association between fecal calprotectin (μg/g) and endoscopic response to vedolizumab at weeks 16 and 52. Receiver operating characteristic curves for the optimal fecal calprotectin cut‐off value to predict endoscopic response after vedolizumab induction at week 16 and week 52. The area under the curve for endoscopic response at week 16 = 0.860, for endoscopic remission at week 16 = 0828, and for sustained endoscopic response at week 52 = 0.942. Upper panel:  , endoscopic response;
, endoscopic response; , endoscopic remission. Lower panel:
, endoscopic remission. Lower panel:  , sustained endoscopic response. [Color figure can be viewed at wileyonlinelibrary.com]
, sustained endoscopic response. [Color figure can be viewed at wileyonlinelibrary.com]
Table 4.
. Diagnostic accuracy for fecal calprotectin (μg/g) to predict endoscopic response after vedolizumab induction at week 16, specified for ulcerative colitis and Crohn's disease
|
FC < 200 μg/g UC Week 16 |
FC < 200 μg/g CD Week 16 |
FC > 450 μg/g UC Week 16 |
FC > 450 μg/g CD Week 16 |
|
|---|---|---|---|---|
| AUC | 0.874 | 0.877 | 0.874 | 0.872 |
| Sensitivity (%) | 61.9 | 66.7 | 81.0 | 91.7 |
| Specificity (%) | 100.0 | 91.7 | 100.0 | 75.0 |
| PPV (%) | 100.0 | 88.0 | 100.0 | 78.6 |
| NPV (%) | 57.9 | 73.3 | 73.3 | 90.0 |
Receiver operating characteristics for the optimal fecal calprotectin (FC) cut‐off value to predict endoscopic response at week 16, n = 32 UC / 48 CD. AUC, area under the curve; CD, Crohn's disease; NPV, negative predictive value; PPV, positive predictive value; UC, ulcerative colitis.
No optimal cut‐off value could be determined for the prediction of endoscopic remission at week 16.
At week 52, FC < 200 μg/g predicted sustained endoscopic response after 1 year (AUC = 0.942, PPV = 100%, NPV = 50% sensitivity = 65%, specificity = 100%, Fig. 2). At week 52, FC > 450 μg/g predicted eLOR (AUC = 0.942, NPV = 62%, PPV = 96%, sensitivity = 81%, specificity = 89%). The cut‐off value for ∆FC (FC at week 16 − FC at week 52) of > 400 μg/g is most strongly correlated with an eLOR (AUC = 0.969, NPV = 96%, PPV = 86%, sensitivity = 86%, specificity = 96%).
Discussion
In this prospective cohort with a high percentage of anti‐TNF‐exposed IBD patients, an objective endoscopic response at week 16 to VDZ is observed in approximately half of patients. Median FC levels decreased significantly after the start of VDZ in patients with an endoscopic response. FC levels and endoscopic response at week 16 were significantly correlated: FC level of < 200 μg/g at week 16 predicts endoscopic response with a diagnostic accuracy of 85% (PPV = 93%, NPV = 66%), and FC > 450 μg/g at week 16 predicts endoscopic non‐response with a diagnostic accuracy of 85% (PPV = 86%, NPV = 83%). Approximately 75% of patients with an endoscopic response at week 16 had a sustained endoscopic response at week 52. FC level of < 200 μg/g predicts a sustained endoscopic response at week 52 with a diagnostic accuracy of 94% (PPV = 50%, NPV = 100%), and FC level increase of > 400 μg/g predicts eLOR with high diagnostic accuracy of 97% (PPV = 96%, NPV = 86%).
In this study, we propose an FC algorithm after VDZ induction to predict endoscopic response as depicted in Figure 3. In this cohort, an accurate correlation between FC levels and endoscopic disease activity was shown for both UC and CD. FC levels of < 200 μg/g at week 16 are indicative of endoscopic response; VDZ can be continued after induction without the need for routine endoscopic confirmation of the response and thus save unnecessary burden to patients and costs. When FC levels are between 200 and 450 μg/g after induction, we suggest to first explore the presence of possible confounders (e.g. infections and NSAIDs). An endoscopy after induction is required to evaluate the objective response. When FC levels are > 450 μg/g after induction, an endoscopic response is unlikely, and decisions have to be made on individual patient's level. In addition, FC level of < 200 μg/g at week 52 indicates a sustained endoscopic response, in both UC and CD. An increase in FC level of > 400 μg/g at week 52 (as compared with FC levels at week 16) indicates loss of endoscopic response, and in these cases, decisions have to be made on individual patient's level.
Figure 3.
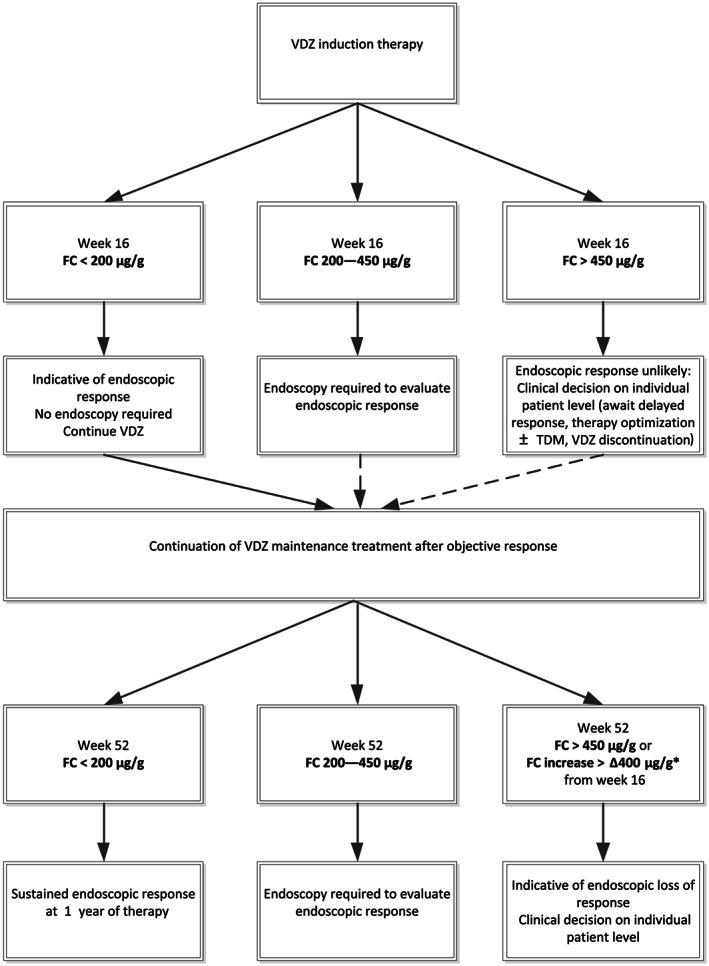
Fecal calprotectin (μg/g) algorithm to evaluate response to vedolizumab. VDZ, vedolizumab; FC, fecal calprotectin; TDM, therapeutic drug monitoring. *FC increase of > ∆400 μg/g from week 16 to week 52 is most strongly correlated with an endoscopic loss of response. Preferably use FC increase of > ∆400 μg/g from week 16 for objective response evaluation.
Decisions on the individual patient level in the absence of an endoscopic response at week 16 or loss of endoscopic response at week 52 may include empiric or trough level‐guided dose optimization. In these situations, endoscopic evaluation may be postponed. Recent data support early trough levels to predict mucosal healing and response to VDZ. 16 , 17 , 18 , 19 Other biomarkers such as undetectable soluble mucosal addressin cell adhesion molecule 1 are potential predictors. 20 An advanced algorithm integrating therapeutic drug monitoring and FC levels may further improve (early) optimization strategies during VDZ induction and maintenance treatment.
In previous studies, analyzing data from the GEMINI UC registration trial on the ability of FC in combination with clinical data on week 6 to predict endoscopic remission have shown only moderate predictive value. 21 , 22 A possible explanation for the discrepancy between these findings and the accurate correlation of FC and endoscopy in our study may be that in the VDZ registration trials the evaluation of the response to VDZ induction have been scheduled too early.
The observed endoscopic remission rate of 20% in both UC and CD at week 16 in our cohort is slightly lower as compared with other real‐world studies. 11 , 23 , 24 , 25 For instance, the US VICTORY consortium showed endoscopic remission, defined as the absence of ulcers and/or erosions, in 35% in UC and 20% of CD patients at 6 months. 26 In addition, a recent prospective cohort study showed endoscopic remission (defined as SES‐CD of ≤ 3) in 33% of CD patients at week 26. 12 In our study, definition for endoscopic remission was more strict as compared with the aforementioned studies that explains the lower endoscopic remission rates, as a definition of a endoscopic Mayo 0/1 and SES‐CD of ≤ 3 in our cohort would result in clearly higher remission rates, that is, 59% in UC and 25% in CD. An additional explanation for the differences in endoscopic remission is the timing of endoscopy in our study (week 16 vs week 26). A delayed response to VDZ has been suggested previously. 27 , 28 However, additional objective data on this issue are eagerly awaited. Week 52 sustained endoscopic response was not comparable with the formerly described real‐world studies, because in our study sustained response was objectified. In addition, eLOR is not previously described, 29 and clinical data on loss of response to VDZ showed loss of response in 54% of UC and 48% of CD patients per 100 person‐years of follow‐up. 30
Major strengths of our study are the prospective inclusion of a real‐world cohort of anti‐TNF‐exposed patients with standardized evaluation of FC levels and endoscopy at baseline, week 16 after induction, and after 1 year of therapy. Objective response evaluation after VDZ induction phase is highly relevant for therapeutic decisions in daily clinical practice, for example, continuation of drug therapy and optimization strategies. Furthermore, based on objective response evaluation, our study may provide insight in the timing of endoscopy, and the provided practical algorithm may aid in individual clinical decision making and avoid routine endoscopy procedures.
Nevertheless, some limitations of our study need to be considered, mostly attributed to the real‐world design of this study in a tertiary referral center. Firstly, FC levels can be influenced by several factors such as stool composition, NSAIDs, blood admixture, storage temperature, bowel movements, and infections. These factors were not mentioned in our inclusion and exclusion criteria and could therefore affect the outcomes. Secondly, the endoscopists were not blinded, nor were the endoscopies read centrally. However, the inter‐observer variability is considered low because all endoscopic results (images and video material if available) were scored by one researcher. Finally, as only patients with a confirmed endoscopic response at week 16 continued with VDZ as maintenance therapy, the week 52 analyses concern a selection of the baseline population, and the sample size at week 52 was relatively small. In addition, patients who stopped VDZ before week 16 were not analyzed in this study (per protocol analysis). For this, we performed a sensitivity analysis, and no differences in outcomes were observed (data not shown).
In conclusion, this prospective study demonstrates that as high as half of IBD patients reach endoscopic response with VDZ therapy at week 16, and approximately 75% of this patients had a sustained endoscopic response after 1 year of therapy. A significant correlation with FC levels and endoscopic response and loss of response was demonstrated. FC levels of < 200 μg/g can be used to prognosticate endoscopic response after induction and long‐term sustained response after 1 year of therapy. FC levels of > 450 μg/g can be used to prognosticate endoscopic non‐response after induction, and an increase in FC level of > 400 μg/g after induction indicates eLOR. This simple FC algorithm may guide clinical decisions on the continuation of VDZ in IBD patients.
Conference presentations
Poster presentation at the 12th congress of ECCO—Inflammatory Bowel Diseases 2017. (February 15–18, Barcelona, Spain). “P447 Vedolizumab induces significantly higher endoscopic remission rates at week 16 in ulcerative colitis as compared to Crohn's disease.”
Oral presentation at the Dutch Digestive Days (Veldhoven) 2017 “Vedolizumab induces significantly higher endoscopic remission rates at week 16 in ulcerative colitis as compared to Crohn's disease.”
Poster presentation at the 13th congress of ECCO—Inflammatory Bowel Diseases 2018 (February 14–17, Vienna, Austria), “P433 Fecal calprotectin is correlated to endoscopic disease activity at Week 16 in IBD patients on vedolizumab therapy.”
Supporting information
Figure S1. Endoscopic response and remission rates at week 16 and week 52.
Figure S2. Endoscopic scores at week 0, week 16 and week 52.
Table S1. Schematic overview of categorization of endoscopic scores
Pauwels, R. W. M. , de Vries, A. C. , and van der Woude, C. J. (2020) Fecal calprotectin is a reliable marker of endoscopic response to vedolizumab therapy: A simple algorithm for clinical practice. Journal of Gastroenterology and Hepatology, 35: 1893–1901. 10.1111/jgh.15063.
Declaration of conflict of interest: R. W. M. Pauwels has nothing to disclose. A. C. de Vries has participated in advisory board and/or received financial compensation from the following companies: Janssen, Takeda, Abbvie, and Tramedico. C. J. van der Woude received grant support from Falk Benelux and Pfizer; received speaker fees from AbbVie, Takeda, Ferring, Dr. Falk Pharma, Hospira, and Pfizer; and served as a consultant for AbbVie, MSD, Takeda, Celgene, Mundipharma, and Janssen. The authors have no financial relationships relevant to this article to disclose.
Financial support: QuantOn Cal home‐based fecal calprotectin tests were funded by Tramedico.
References
- 1. Lobaton T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti‐adhesion therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2014; 39: 579–594. [DOI] [PubMed] [Google Scholar]
- 2. Engel T, Ungar B, Yung DE, Ben‐Horin S, Eliakim R, Kopylov U. Vedolizumab in IBD—lessons from real‐world experience; a systematic review and pooled analysis. J. Crohns Colitis 2018; 12: 245–257. [DOI] [PubMed] [Google Scholar]
- 3. De Vos M, Dhooghe B, Vermeire S et al Efficacy of vedolizumab for induction of clinical response and remission in patients with moderate to severe inflammatory bowel disease who failed at least two TNF antagonists. United Eur. Gastroenterol J. 2018; 6: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long‐term outcomes of patients with ulcerative colitis: a systematic review and meta‐analysis. Clin. Gastroenterol. Hepatol. 2016; 14: 1245–1255.e8. [DOI] [PubMed] [Google Scholar]
- 5. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta‐analysis: mucosal healing is associated with improved long‐term outcomes in Crohn's disease. Aliment. Pharmacol. Ther. 2016; 43: 317–333. [DOI] [PubMed] [Google Scholar]
- 6. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61: 1619–1635. [DOI] [PubMed] [Google Scholar]
- 7. Romkens TE, Gijsbers K, Kievit W, Hoentjen F, Drenth JP. Treatment targets in inflammatory bowel disease: current status in daily practice. J. Gastrointestin. Liver Dis. 2016; 25: 465–471. [DOI] [PubMed] [Google Scholar]
- 8. Chudy‐Onwugaje KO, Christian KE, Farraye FA, Cross RK. A state‐of‐the‐art review of new and emerging therapies for the treatment of IBD. Inflamm. Bowel Dis. 2019; 25: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feagan BG, Rutgeerts P, Sands BE et al Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Feagan BG, Rutgeerts P et al Vedolizumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 11. Schreiber S, Dignass A, Peyrin‐Biroulet L et al Systematic review with meta‐analysis: real‐world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J. Gastroenterol. 2018; 53: 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowenberg M, Vermeire S, Mostafavi N et al Vedolizumab induces endoscopic and histologic remission in patients with Crohn's disease. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Sandborn WJ, Colombel JF et al Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn's disease. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 14. Tursi A, Mocci G, Faggiani R et al Vedolizumab is effective and safe in real‐life treatment of inflammatory bowel diseases outpatients: a multicenter, observational study in primary inflammatory bowel disease centers. Eur. J. Intern. Med. 2019; 66: 85–91. [DOI] [PubMed] [Google Scholar]
- 15. de Jong MJ, Roosen D, Degens J et al Development and validation of a patient‐reported score to screen for mucosal inflammation in inflammatory bowel disease. J. Crohns Colitis 2019; 13: 555–563. [DOI] [PubMed] [Google Scholar]
- 16. Dreesen E, Verstockt B, Bian S et al Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2018; 16: 1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 17. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state‐of‐the‐art overview. BMC Med. 2019; 17: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yacoub W, Williet N, Pouillon L et al Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment. Pharmacol. Ther. 2018; 47: 906–912. [DOI] [PubMed] [Google Scholar]
- 19. Williet N, Boschetti G, Fovet M et al Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin. Gastroenterol. Hepatol. 2017; 15: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 20. Paul S, Williet N, Di Bernado T et al Soluble mucosal addressin cell adhesion molecule 1 and retinoic acid are potential tools for therapeutic drug monitoring in patients with inflammatory bowel disease treated with vedolizumab: a proof of concept study. J. Crohns Colitis 2018; 12: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 21. Reinisch W, Bressler B, Curtis R et al Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a post hoc analysis of GEMINI 1. Inflamm. Bowel Dis. 2019; 25: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waljee AK, Liu B, Sauder K et al Predicting corticosteroid‐free endoscopic remission with vedolizumab in ulcerative colitis. Aliment. Pharmacol. Ther. 2018; 47: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amiot A, Serrero M, Peyrin‐Biroulet L et al One‐year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2017; 46: 310–321. [DOI] [PubMed] [Google Scholar]
- 24. Vivio EE, Kanuri N, Gilbertsen JJ et al Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J. Crohns Colitis 2016; 10: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ylisaukko‐Oja T, Aaltonen J, Nuutinen H et al High treatment persistence rate and significant endoscopic healing among real‐life patients treated with vedolizumab—a Finnish Nationwide Inflammatory Bowel Disease Cohort Study (FINVEDO). Scand. J. Gastroenterol. 2018; 53: 158–167. [DOI] [PubMed] [Google Scholar]
- 26. Dulai PS, Singh S, Jiang X et al The real‐world effectiveness and safety of vedolizumab for moderate‐severe Crohn's disease: results from the US VICTORY consortium. Am. J. Gastroenterol. 2016; 111: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 27. Kopylov U, Verstockt B, Biedermann L et al Effectiveness and safety of vedolizumab in anti‐TNF‐naive patients with inflammatory bowel disease—a multicenter retrospective European study. Inflamm. Bowel Dis. 2018; 24: 2442–2451. [DOI] [PubMed] [Google Scholar]
- 28. Plevris N, Chuah CS, Allen RM et al Real‐world effectiveness and safety of vedolizumab for the treatment of inflammatory bowel disease: the Scottish vedolizumab cohort. J. Crohns Colitis 2019; 13: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 29. Pauwels RWM, de Vries AC, van der Woude CJ. Comment on “Predictors and management of loss of response to vedolizumab in inflammatory bowel disease”. Inflamm. Bowel Dis. 2018; 25: e59‐e. [DOI] [PubMed] [Google Scholar]
- 30. Peyrin‐Biroulet L, Danese S, Argollo M et al Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn's disease or ulcerative colitis: a systematic review and meta‐analysis. Clin. Gastroenterol. Hepatol. 2019; 17: 838–846 e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Endoscopic response and remission rates at week 16 and week 52.
Figure S2. Endoscopic scores at week 0, week 16 and week 52.
Table S1. Schematic overview of categorization of endoscopic scores


