Dear Editor,
We have recently reported on the CP‐GEP model to identify patients with primary cutaneous melanoma who may forgo the sentinel lymph node (SLN) biopsy procedure because of their low risk of nodal metastasis. 1 , 2 The CP‐GEP model combines clinicopathologic (CP) variables, Breslow thickness, patient age, and the expression of eight genes related to epithelial‐to‐mesenchymal transition 3 , 4 to categorize patients into two groups: low risk or high risk for nodal metastasis (Fig. 1). Here, we report on a feasibility study of cutaneous melanoma patients seen at Mayo Clinic (MC) between October and December of 2019 who had their diagnostic biopsy tissue tested by CP‐GEP in a CAP/CLIA‐certified laboratory in San Diego (operated by SkylineDx). The primary objective of this study was to evaluate the feasibility of standardized CP‐GEP testing in a certified laboratory in the United States and to evaluate and optimize the requisitioning logistics. This pilot study was conducted under the framework of the Falcon Melanoma R&D program, which aims to investigate the relevance of gene expression‐based testing in personalized healthcare. The CP‐GEP model for predicting nodal metastasis, which we here refer to as the Merlin test, is the first diagnostic test developed under this program. The human investigations performed in this study were completed after approval by the Mayo Clinic Institutional Review Board and in accordance with the requirements of the Department of Health and Human Services, where appropriate.
Figure 1.
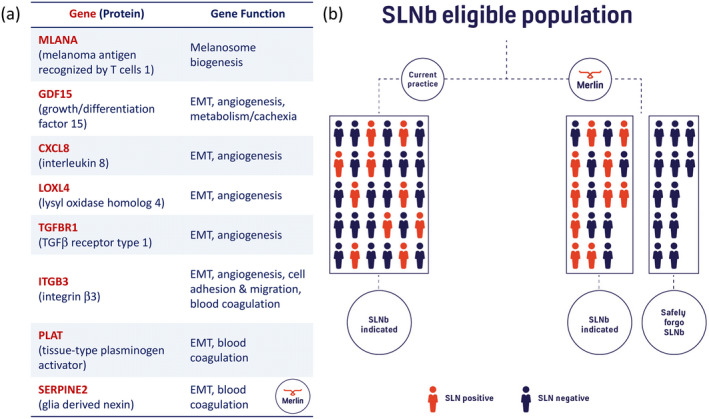
The CP‐GEP model, which we refer to as the Merlin test, identifies patients with primary cutaneous melanoma who may forgo the sentinel lymph node biopsy (SLNb) procedure because of their low risk of nodal metastasis. (a) The CP‐GEP model combines clinicopathologic variables, i.e. Breslow thickness and patient age, and the expression of eight genes. These genes serve biological functions in epithelial‐to‐mesenchymal transition (EMT) with specific roles in angiogenesis/hypoxia, coagulation, and melanosome biogenesis. (b) The Merlin test has the potential to reduce the sentinel lymph node biopsy rate by up to 80% for T1 disease and 42% for T2 disease. 1 In our pilot study, none of the negatively tested patients were found to have nodal metastasis.
Fifty consecutive patients were identified by daily reviews of pathology reports. Charts were checked for eligibility criteria (see below) and if met, we requested 50 micron tissue recuts either mounted on charged glass slides or as five times 10 micron tissue curls through the MC anatomic pathology or MC pathology research core laboratory. Eligibility was determined based on histopathology data derived from patient medical records and established by two or more board‐certified MC dermatopathologists. Patients were eligible for this study if they met criteria for an SLN biopsy by National Comprehensive Cancer Network guidelines. 4 Specifically, a patient was eligible if their melanoma was (i) tumor (T) stage 1a (Breslow thickness of less than 0.8 mm) with at least one of the following risk factors: ulceration, mitoses present, and patient age <40 years; or (ii) T1b to T3 melanoma (Breslow thickness of 0.8–4 mm). All patients were at least 18 years of age and received care at MC. Formalin‐fixed paraffin‐embedded shave, punch, or excisional biopsy material was acceptable. If the pathology case consisted of more than one paraffin block, an MC dermatologist selected the block with the greatest tumor involvement for molecular testing. Exclusion criteria were: T4 melanoma (Breslow thickness greater than 4 mm); macroscopic nodal involvement, distant metastasis within 90 days of diagnosis, insufficient primary tumor tissue, and denial of access to medical records for research purposes (per Minnesota State law).
The vast majority of tissue samples tested, i.e. 46 of 50, was from shave biopsies. Of the remaining four samples, two were from punch and two from excisional biopsies. Twenty‐four samples were received according to specifications defined in the tissue request form of which 16 arrived in presupplied containers and eight on glass slides. All samples and forms were received within 48 hours after sending. Turnaround time from sample receipt to test reporting was five working days during the peak phase of the study. On average more than two micrograms of total RNA could be extracted from samples. However, for three of the 50 samples, RNA yield did not meet prespecified quality control criteria, and the Merlin test was not performed. Of the 47 remaining patients, 34 underwent successful SLN biopsy within 90 days of diagnosis whereas 13 were without known SLN status because (i) SLN biopsy was not requested for T1a disease with risk factors (n = 9); (ii) SLN biopsy was attempted but failed (n = 2); (iii) patient no‐showed for the procedure (n = 1). A summary of patient and tumor characteristics of all patients (n = 50) and patients with Merlin test results and known SLN status (n = 34) is shown in Table 1. One of 13 (7.7%) T1 patients, four of 13 (30.1%) T2 patients, and one of eight (12.5%) T3 patients were SLN positive. All SLN positive patients were correctively identified as high risk by the Merlin test. Metastatic tumor volume ranged from individual tumor cells to cell clusters 26 mm in diameter.
Table 1.
Patient and tumor characteristics stratified by sentinel lymph node biopsy outcome
| Characteristic | Total samples (N = 50) | Merlin tested (N = 34) |
|---|---|---|
| Male gender, n (%) | 30/50 (60%) | 19/34 (55.9%) |
| Age at SLN (years), mean (SD) | 56.59 (18.48) | 56.76 (17.11) |
| Biopsy location, n (%) | ||
| Head and neck | 8/50 (16%) | 5/34 (14.7%) |
| Trunk | 22/50 (44%) | 13/34 (38.2%) |
| Upper extremity | 13/40 (26%) | 11/34 (32.4%) |
| Lower extremity | 5/50 (10%) | 3/34 (8.8%) |
| Acral | 2/626 (4%) | 2/34 (5.9%) |
| Breslow thickness (mm), n (%) | ||
| 0.5–1 (T1) | 25/50 (50%) | 13/34 (38.2%) |
| 1.1–2 (T2) | 14/50 (28%) | 13/34 (38.2%) |
| 2.1–4 (T3) | 11/50 (22%) | 8/34 (23.5%) |
| Mitotic rate type, n (%) | ||
| Absent | 13/50 (26%) | 8/34 (23.5%) |
| 1–6 | 32/50 (64%) | 23/34 (67.7%) |
| >6 | 5/50 (10%) | 3/34 (8.8%) |
| Ulceration, n (%) | 12/50 (12%) | 8/34 (8.8%) |
| Histologic type, n (%) | ||
| Superficial spreading | 31/50 (62%) | 18/34 (52.9%) |
| Nodular | 10/50 (20%) | 9/34 (26.5%) |
| Desmoplastic | 2/50 (4%) | 1/34 (2.9%) |
| Acral lentiginous | 1/50 (2%) | 1/34 (2.9%) |
| Spitzoid | 2/50 (4%) | 2/34 (5.9%) |
| Nevoid | 1/50 (2%) | 1/34 (2.9%) |
| Unclassifiable | 3/50 (6.0%) | 2/34 (5.9%) |
This study was a feasibility study and not intended for test validation. Merlin test development and initial validation data has been published. 1 , 5 Additional validation studies are ongoing as part of the Merlin Study Initiative under the Falcon R&D Program. We conclude from our pilot study that Merlin testing is feasible as a send‐out test using primary melanoma diagnostic biopsy tissue which is routinely obtained in patient care.
Conflict of interest: None.
Funding source: This work was funded by the National Cancer Institute (grant CA215105) with additional support from the Mayo Clinic and SkylineDx B.V.
References
- 1. Bellomo D, Arias‐Mejias SM, Ramana C, et al A model combining tumor molecular and clinicopathologic risk factors predicts sentinel lymph node metastasis in primary cutaneous melanoma. JCO Precis Oncol 2020; 4: 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meves A, Nikolova E, Heim JB, et al Tumor cell adhesion as a risk factor for sentinel lymph node metastasis in primary cutaneous melanoma. J Clin Oncol 2015; 33: 2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonso SR, Tracey L, Ortiz P, et al A high‐throughput study in melanoma identifies epithelial‐mesenchymal transition as a major determinant of metastasis. Cancer Res 2007; 67: 3450–3460. [DOI] [PubMed] [Google Scholar]
- 4. Arias‐Mejias SM, Warda KY, Quattrocchi E, et al The role of integrins in melanoma: a review. Int J Dermatol 2020; 59: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulder EEAP, Dwarkasing JT, Hollestein D, et al Validation of a clinicopathological and gene expression profile (CP‐GEP) model for sentinel lymph node metastasis in primary cutaneous melanoma. Ann Oncol 2019; 30: v533–v563. [DOI] [PMC free article] [PubMed] [Google Scholar]


