Abstract
Objective
Glucosylceramide (Glu‐Cer), a glycosylated form of ceramide, has been reported to have cytotoxic effects in the cells of various cancers. We previously reported that dietary Glu‐Cer from rice bran had inhibitory effects on human head and neck squamous cell carcinoma (HNSCC) in nonobese diabetes (NOD)/severe combined immunodeficiency (SCID) mice. In HNSCC, preventing recurrence and second primary cancer is required to improve prognosis. The purpose of the present study was to determine whether dietary Glu‐Cer had anticarcinogenic and antitumorigenic effects in a mouse model of HNSCC.
Methods
A total of 40 CB6F1‐Tg rasH2@Jcl mice were divided into two groups: control and Glu‐Cer. All mice were given 4‐nitroquinoline 1‐oxide for 24 weeks. Control group mice were fed the normal diet without Glu‐Cer. The Glu‐Cer group mice were given a mixture of the normal diet plus 0.25% Glu‐Cer for 24 weeks. Microscopic examination was performed to identify grossly visible preneoplasms and neoplasms in the mouth, pharynx, and esophagus. Epithelial regions were classified as normal tissue, carcinoma in situ (CIS), or SCC; and the number of each type of region was counted.
Results
Compared with the Glu‐Cer group mice, control group mice more frequently developed individual and multiple tumors of each type, including CIS and SCC, in the mouth, pharynx, or esophagus.
Conclusion
Tumor development was effectively inhibited by dietary Glu‐Cer derived from rice bran, indicating that this and related compounds show promise as prophylactic agents for human HNSCC.
Level of Evidence
NA Laryngoscope, 130:E593–E597, 2020
Keywords: Glucosylceramide, ceramide, tumorigenesis, carcinogenesis, head and neck squamous cell carcinoma
INTRODUCTION
Due to field cancerization, chronic exposure to carcinogens leads to development of several primary tumors in the upper aerodigestive tract.1 The risk factors for carcinogenesis in head and neck squamous cell carcinoma (HNSCC) are exposure of mucous membranes to tobacco smoke, viral infections with human papillomavirus or Epstein‐Barr virus, exposure to previous radiation, insufficient hygiene of the mouth cavity, prolonged mechanical irritation by improperly made dentures, and immunosuppression.2, 3, 4, 5 The risks of HNSCC among nondrinkers increased with amount smoked; conversely, the risks among nonsmokers increased with the level of alcohol intake.6 Furthermore, second primary tumors are known to occur at a rate of approximately 20% through the process of field cancerization and are the most common cause of treatment failure and death among early‐stage HNSCC patients.7 To improve the outcome of such patients, there is a need for both local and systemic forms of chemoprevention that can protect the upper aerodigestive areas. Chemoprevention of HNSCC involves the use of drugs or natural agents to hinder or delay cancer development. A large number of chemopreventive agents have been developed from dietary agents, natural plants, and synthetic chemicals, including retinoids,8 tea polyphenols,9 black raspberry extract,10 curcumin,11 limonoids,12 cyclooxygenase‐2 inhibitors,13 toona sinensis leaf,14 and epidermal growth factor receptor inhibitors.15 In the case of HNSCC, no chemopreventive agent thus far has demonstrated positive results in a phase III clinical trial. Tolerability of such agents is important, as is lack of toxicity.
Sphingolipids are a group of structural and functional derivatives that have a long‐chain (sphingoid) base backbone and exert a variety of biological activities that mediate cell growth, differentiation, and apoptosis.16, 17, 18 Ceramide, an important sphingolipid that forms in response to treatment with chemotherapeutic reagents and cell‐death ligands, such as Fas ligand and tumor necrosis factor‐alpha, has been implicated in mediating apoptosis in cancer cells.19, 20, 21 Moreover, treatment with exogenous ceramides has been shown to introduce apoptosis in human cells both in vitro and in vivo.20, 22
Glucosylceramide (Glu‐Cer), a glycosylated form of ceramide, has been reported to have cytotoxic effects in the cells of various cancers, for example liver, skin, and lung.23 We previously reported that dietary Glu‐Cer from rice bran had inhibitory effects on human HNSCC in nonobese diabetic/ severe combined immunodeficiency mice.24 Further, dietary Glu‐Cer suppressed tumor growth in a mouse xenograft model of HNSCC by inhibiting angiogenesis through an increase in ceramide, and dietary Glu‐Cer was synthesized by a de novo pathway after digestion absorption, thus raising the blood level of ceramide.25
The inhibitory effect of Glu‐Cer on colon tumorigenesis has previously been reported. Inamine et al. showed that dietary Glu‐Cer had potential chemopreventive effects in short‐term colon carcinogenesis bioassays in rats, inhibiting the formation of preneoplastic lesions such as aberrant crypt foci and β‐catenin‐accumulated crypts.22 However, the inhibitory effect of carcinogenesis on HNSCC has not yet been reported.
The purpose of the present study was to determine whether dietary Glu‐Cer had anti‐carcinogenic and anti‐tumorigenic effects in a mouse model of HNC.
MATERIALS AND METHODS
Materials
Male and female CB6F1‐Tg rasH2@Jcl mice (Tg mice) bred by CLEA Japan (Tokyo, Japan) were obtained at 10 weeks of age and maintained in plastic cages in an experimental room controlled at 23 ± 2°C temperature, 50% ± 10% humidity, and a 12‐hour light–dark cycle. The mice were all allowed free access to a powdered basal diet (CLEA Japan) and to tap water containing 20 parts per million (ppm) 4‐nitroquinoline 1‐oxide (4‐NQO) (98% pure, CAS No. 56‐57‐5, Wako Pure Chemical Ind., Osaka, Japan). The powered basal diet of mice in the experimental group contained 0.25 Glu‐Cer (1‐O‐β‐glucosyl‐N‐2’‐hydrox‐yarachidoyl‐4,8‐sphingadienine). Glu‐Cer was extracted from rice bran at Nippon Flour Mills Co., Ltd. (Tokyo, Japan). The purity of the Glu‐Cer was 78%. 4‐NQO was used to induce oral cavity, pharyngeal, and esophageal tumors in this study. A 4‐NQO solution was prepared in 1,2‐propanediol (> 99.0% pure, code no. 24‐6170‐5, Sigma‐Aldrich Japan, Tokyo, Japan) at 5 mg/mL. The experiments were conducted according to the Guidelines for Animal Experiments of Tottori University.
Treatment
A total of 40 Tg mice (male 20, female 20) were transferred to the experimental room after a 1‐week quarantine. The mice were divided into two groups, control (n = 20) and Glu‐Cer (n = 20), and were monitored daily after the experiment was initiated. The protocols were approved by the Animal Research Committee of Tottori University.
All mice were given 4‐NQO (20 ppm in drinking water) for 24 weeks. Control group mice received no further treatment and were fed the normal diet without Glu‐Cer. Glu‐Cer group mice were given a mixture of the normal diet plus 0.25% Glu‐Cer for 24 weeks. This percentage of Glu‐Cer was chosen because it achieved a daily oral dose of 300 mg/kg Glu‐Cer, which in our previous study safely inhibited the growth of HNSCC in vivo.
The body weight of all mice was measured once per week. The mice were killed at 24 weeks by exsanguination under deep pentobarbital anesthesia, and macroscopic observation was performed to determine the occurrence of grossly visible preneoplasms and neoplasms in the mouth, pharynx, and esophagus. Both cancerous and noncancerous tissues were excised parallel to the esophagus to facilitate histopathological examination after fixation in 10% buffered formalin. Tissues were embedded in paraffin blocks, and the histopathological sections were stained with hematoxylin and eosin. Epithelial regions were classified as normal tissue, dysplasia, or carcinoma; and the number of each type of region was counted.
Survival Evaluation
Animal survival was evaluated from the day of initiation of 4‐NQO or Glu‐Cer until death. Mice were examined daily for signs of distress, and body weight was measured once a week. To minimize animal suffering, mice were euthanized when they became moribund as defined by any of the following predefined criteria: weight loss (> 20%), inability to ambulate, and inability to drink or feed.
Statistical Analysis
All values are presented as means ± standard error. We used Prism 6 for Mac to analyze the data. The Mann‐Whitney U test was used to evaluate differences in both the incidence of lesions and in the food and water intake between the control and Glu‐Cer groups.
RESULTS
General Observations
All Tg mice showed good tolerance for 4‐NQO exposure in their drinking water. Food intake was greater in Glu‐Cer group mice than in control group mice, but the two groups did not differ significantly in terms of total drinking water volume (Fig. 1). Body weight was not significantly different between the two groups.
Figure 1.
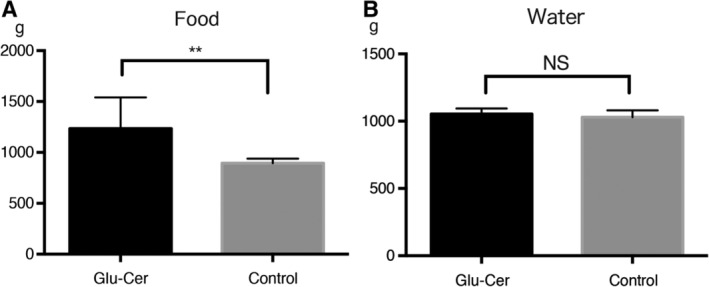
Feed dosage and water supply. Glu‐cer group mice consumed more food than control group mice (A). However, there was no significant difference between the two groups in the total volume of drinking water consumed (B). Glu‐cer = glucosylceramide; NS = nonsignificant.
Tumor Development
Tumors of any type developed in the mouth, pharynx, or esophagus in 16 control mice (80%) and eight Glu‐Cer group mice (40%), indicating a significantly higher incidence in the control group. Also, control group mice developed multiple tumors significantly more frequently than Glu‐Cer group mice (6.15 ± 1.51 vs. 0.9 ± 0.28 per mouse, respectively). These results are shown in Figures 2 and 3.
Figure 2.
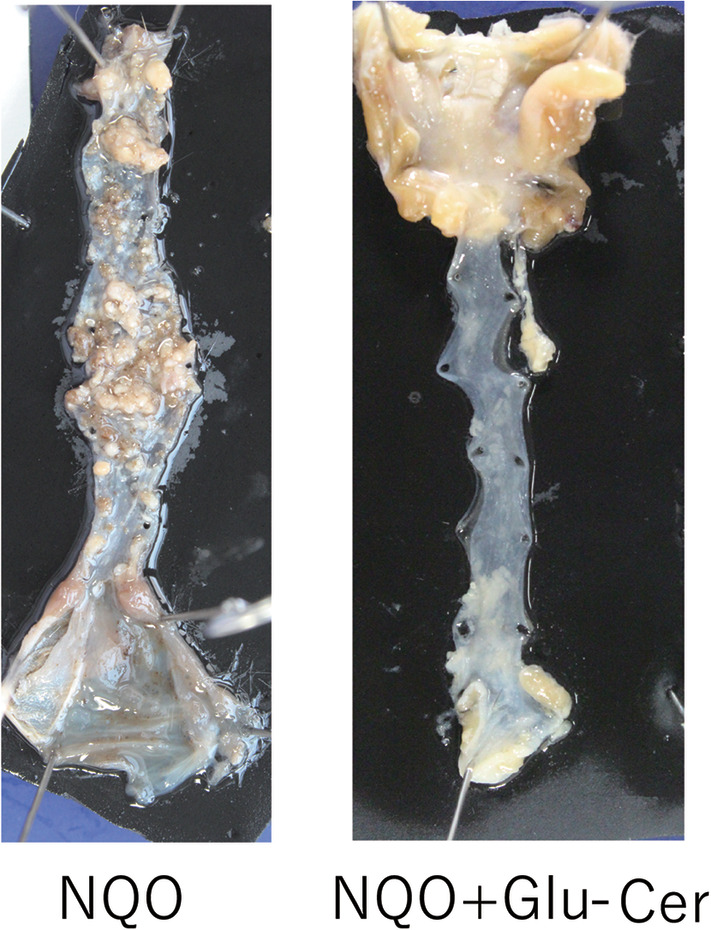
Morphological observation of mouse tongue, pharynx, and esophagus. Multiple tumors formed on the tongue, pharynx, and esophagus of a control group mouse (A). Few tumors were observed in these regions in a Glu‐cer group mouse (B). 4‐NQO = 4‐nitroquinoline 1‐oxide; Glu‐cer = glucosylceramide.
Figure 3.
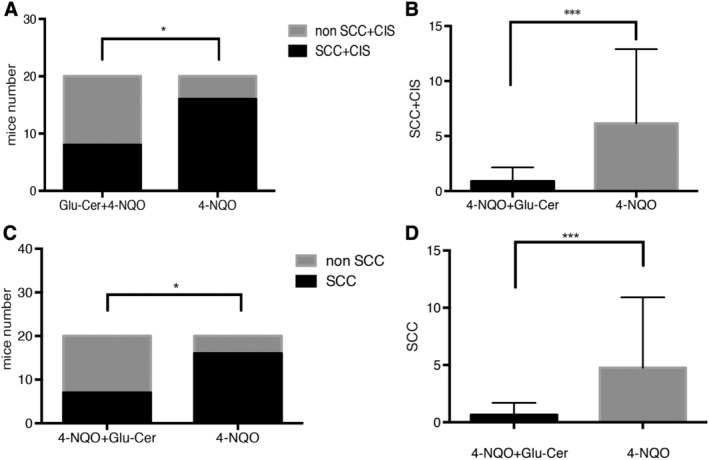
Development of tongue, pharyngeal, and esophageal tumors (SCC and carcinoma in situ). Tumors of any type developed in 16 control group mice (80%) and eight Glu‐Cer group mice (40%), indicating a significantly higher incidence in the control group (A). Control group mice developed multiple tumors significantly more frequently than Glu‐Cer group mice (6.15 ± 1.51 vs. 0.9 ± 0.28 per mouse, respectively) (B). Tumors classified as SCC developed in 16 control group mice (80%) and in seven Glu‐Cer group mice (35%), indicating a significantly higher incidence in the control group (C). Control group mice developed multiple tumors significantly more frequently than Glu‐Cer group mice (4.75 ± 1.38 vs. 0.65 ± 0.23 per mouse, respectively) (D). 4‐NQO = 4‐nitroquinoline 1‐oxide; CIS = carcinoma in situ; Glu‐cer = glucosylceramide; SCC = squamous cell carcinoma.
Tumors classified as dysplasia developed in the mouth, pharynx, or esophagus in nine control group mice (45%) and three Glu‐Cer group mice (15%), indicating a significantly higher incidence in the control group. Also, control group mice developed multiple dysplastic tumors significantly more frequently than Glu‐Cer group mice (1.4 ± 0.70 vs. 0.25 ± 0.14 per mouse, respectively).
Tumors classified as SCC developed in the mouth, pharynx, or esophagus in 16 control group mice (80%) and seven Glu‐Cer group mice (35%), indicating a significantly higher incidence in the control group. Also, control group mice developed multiple SCCs significantly more frequently than Glu‐Cer group mice (4.75 ± 1.38 vs. 0.65 ± 0.23 per mouse, respectively). These results are shown in Figure 3. There was no difference between the two groups in the proportion of SCC and carcinoma in situ.
Survival
The median survival period was 181 days in the control group and 159 days in the Glu‐Cer group, indicating no significant difference (Fig. 4).
Figure 4.
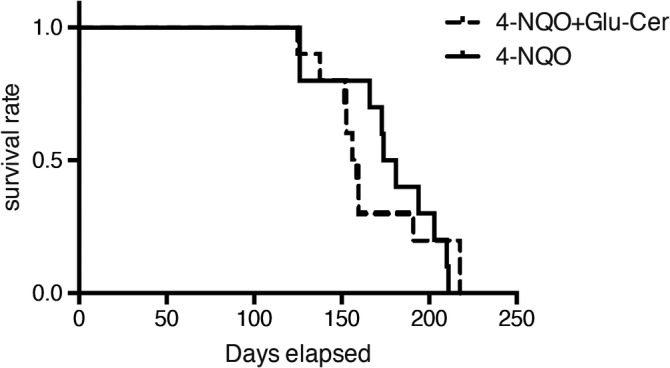
Overall survival. The median survival period was 181 days in control group mice and 159 days in Glu‐cer group mice, indicating no significant difference between the two groups. 4‐NQO = 4‐nitroquinoline 1‐oxide; Glu‐cer = glucosylceramide.
DISCUSSION
In this study, we demonstrated that Tg mice were highly susceptible to a genotoxic carcinogen, 4‐NQO, administered in drinking water for 24 weeks. This substance caused the development of dysplastic tumors, carcinoma in situ (CIS), and SCC in the mouth, pharynx, and esophagus of the mice in this experiment. This animal carcinogenesis model also showed that the dietary sphingolipid Glu‐Cer, derived from rice bran, effectively inhibited this tumor development. The anti‐carcinogenic efficacy of sphingolipids has previously been reported, for example, in colorectal cancer; however, this has not yet been demonstrated in HNC. Because there was no significant difference in the amount of drinking water intake between the control and Glu‐Cer groups, and all mice therefore received similar doses of 4‐NQO, it is thought that the carcinogenic conditions were the same in the two groups.
Several carcinogenesis‐prevention studies have utilized animal models. The systemic application of 4‐NQO via drinking water readily induces tongue tumors in rats and mice.26, 27, 28, 29, 30 Appropriate animal models are essential for investigating the transition of oral squamous epithelium from normal through dysplastic states and ultimately into SCC. In this regard, Ras and H‐ras mutations have been implicated in human and murine oral carcinogenesis.31, 32, 33 Transgenic rats carrying the human c‐Ha‐ras proto‐oncogene are highly susceptible to 4‐NQO administered to the tongue.34 CB6F1‐Tg rasH2@Jcl mice (Tg mice), developed by Satoh et al. to evaluate the association of chemically induced transgene expression and tumor induction,35 were used in this study.
The development of pharyngeal cancer is a multistep process characterized by hyperplasia, dysplasia, and finally neoplasm (benign and malignant).26 In this study, mice that received Glu‐Cer developed SCC and CIS at similar rates, suggesting that this compound does not influence the multistep process of carcinogenesis but instead affects only the first step of tumorigenesis. It is well known that natural killer T (NKT) cells play important roles in both tumor rejection and the defense against infection. Inafuku et al. reported that β‐Glu‐Cer treatment activated invariant NKT cells, thus resulting in the inhibition of tumor metastasis.36 Furthermore, β‐Glu‐Cer alleviates immunologically distinct disorders and may be associated with the fine‐tuning of immune responses through changes in the plasticity of NKT cells. Therefore, in the present research Glu‐Cer may have activated NKT cells and immunologically inhibited the first step of tumorigenesis.
In this study, Glu‐Cer was administered orally in a mixture with food pellets. There was a significant difference between the control and Glu‐Cer groups in terms of the amount of food consumed; control group mice had more tumors, which may have prevented oral intake due to severe oropharyngeal or esophageal stenosis. This was a limitation of our experimental system. However, tumor‐related stenosis probably did not markedly influence the amount of water consumed. Thus, future studies should use water‐soluble rather than fat‐soluble Glu‐Cer or similar compounds in order to maintain constant dose levels.
In this study, dietary Glu‐Cer inhibited carcinogenesis and tumorigenesis but did not significantly prolong overall survival. It is possible that the administered Glu‐Cer and not carcinogenesis affected survival times. In our previous study, we investigated the maximum tolerated dose and optimal treatment dose of dietary Glu‐Cer and found that 300 mg/kg of Glu‐Cer was safe in mice.24 This was the dose used in the present study. Thus, systematic disturbances caused by 4‐NQO are suggested as the reason for the lack of prolonged overall survival.
Our study revealed the potential of dietary Glu‐Cers as prophylactic agents for human HNSCC. Furthermore, these compounds can be purified from rice bran and therefore are organic agents and have been certified to be safe in vivo. Thus, it is expected that dietary Glu‐Cers can be used in practice. However, the exact molecular mechanisms whereby they exert chemopreventive effects remain unknown. To fully understand the biological activity of dietary Glu‐Cers, further studies are required.
CONCLUSION
4‐NQO caused the development of dysplasia, CIS, and SCC in the mouth, pharynx, and esophagus of Tg mice. Tumor development was effectively inhibited by dietary Glu‐Cers derived from rice bran, indicating that this and related compounds show promise as prophylactic agents for human HNSCC.
Editor's Note: This Manuscript was accepted for publication on November 16, 2019.
This study was funded by a Grant‐in‐Aid for Scientific Research C of the Japan Society for the Promotion of Science. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Hasina R, Martin LE, Kasza K, Jones CL, Jalil A, Lingen MW. ABT‐510 is an effective chemopreventive agent in the mouse 4‐nitroquinoline 1‐oxide model of oral carcinogenesis. Cancer Prev Res (Phila) 2009;2:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindsay C, Seikaly H, Biron VL. Epigenetics of oropharyngeal squamous cell carcinoma: opportunities for novel chemotherapeutic targets. J Otolaryngol Head Neck Surg 2017;46:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 4. Wright G, Morgan MY. Alcohol and tobacco misuse: Reducing aerodigestive cancer risk. World J Hepatol 2013;5:452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009; 18:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988; 48:3282–3287. [PubMed] [Google Scholar]
- 7. Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: the overshadowing threat for patients with early‐stage disease. Int J Radiat Oncol Biol Phys 1989;17:691–694. [DOI] [PubMed] [Google Scholar]
- 8. Hong WK, Endicott J, Itri LM, et al. 13‐cis‐retinoic acid in the treatment of oral leukoplakia. N Engl J Med 1986;315:1501–1505. [DOI] [PubMed] [Google Scholar]
- 9. Chandra Mohan KV, Devaraj H, Prathiba D, Hara Y, Nagini S. Antiproliferative and apoptosis inducing effect of lactoferrin and black tea polyphenol combination on hamster buccal pouch carcinogenesis. Biochim Biophys Acta 2006;1760:1536–1544. [DOI] [PubMed] [Google Scholar]
- 10. Han C, Ding H, Casto B, Stoner GD, D'Ambrosio SM. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr Cancer 2005;51:207–217. [DOI] [PubMed] [Google Scholar]
- 11. Manoharan S, Balakrishnan S, Menon VP, Alias LM, Reena AR. Chemopreventive efficacy of curcumin and piperine during 7,12‐dimethylbenz[a]anthracene‐induced hamster buccal pouch carcinogenesis. Singapore Med J 2009; 50:139–146. [PubMed] [Google Scholar]
- 12. Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs 2010;28:392–401. [DOI] [PubMed] [Google Scholar]
- 13. Nishimura N, Urade M, Hashitani S, et al. Increased expression of cyclooxygenase (COX)‐2 in DMBA‐induced hamster cheek pouch carcinogenesis and chemopreventive effect of a selective COX‐2 inhibitor celecoxib. J Oral Pathol Med 2004;33:614–621. [DOI] [PubMed] [Google Scholar]
- 14. Wang WC, Chen CY, Hsu HK, Lin LM, Chen YK. Chemopreventive effect of Toona sinensis leaf extract on 7,12‐dimethylbenz[a]anthracene‐induced hamster buccal pouch squamous cell carcinogenesis. Arch Oral Biol 2016; 70:130–142. [DOI] [PubMed] [Google Scholar]
- 15. Sun Z, Sood S, Li N, et al. Chemoprevention of 7,12‐dimethylbenz[a]anthracene (DMBA)‐induced oral carcinogenesis in hamster cheek pouch by topical application of a dual inhibitor of epidermal growth factor receptor (EGFR) and ErbB2 tyrosine kinases. Oral Oncol 2008;44:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 1996;274:1855–1859. [DOI] [PubMed] [Google Scholar]
- 17. Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL‐60 cells. Role in cell differentiation. J Biol Chem 1989;264:19076–19080. [PubMed] [Google Scholar]
- 18. Vesper H, Schmelz EM, Nikolova‐Karakashian MN, Dillehay DL, Lynch DV, Merrill AH, Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr 1999;129:1239–1250. [DOI] [PubMed] [Google Scholar]
- 19. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139–150. [DOI] [PubMed] [Google Scholar]
- 20. Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science 1993;259:1769–1771. [DOI] [PubMed] [Google Scholar]
- 21. Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol 1998;60:643–665. [DOI] [PubMed] [Google Scholar]
- 22. Inamine M, Suzui M, Morioka T, et al. Inhibitory effect of dietary monoglucosylceramide 1‐O‐beta‐glucosyl‐N‐2'‐hydroxyarachidoyl‐4,8‐sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2‐dimethylhydrazine in F344 rats. Cancer Sci 2005;96:876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oku H, Wongtangtintharn S, Iwasaki H, Inafuku M, Shimatani M, Toda T. Tumor specific cytotoxicity of glucosylceramide. Cancer Chemother Pharmacol 2007;60:767–775. [DOI] [PubMed] [Google Scholar]
- 24. Fujiwara K, Kitatani K, Fukushima K, et al. Inhibitory effects of dietary glucosylceramides on squamous cell carcinoma of the head and neck in NOD/SCID mice. Int J Clin Oncol 2011;16:133–140. [DOI] [PubMed] [Google Scholar]
- 25. Yazama H, Kitatani K, Fujiwara K, et al. Dietary glucosylceramides suppress tumor growth in a mouse xenograft model of head and neck squamous cell carcinoma by the inhibition of angiogenesis through an increase in ceramide. Int J Clin Oncol 2015;20:438–446. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka T. Chemoprevention of oral carcinogenesis. Oral Oncol 1995;31b:3–15. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka T, Kojima T, Okumura A, Yoshimi N, Mori H. Alterations of the nucleolar organizer regions during 4‐nitroquinoline 1‐oxide‐induced tongue carcinogenesis in rats. Carcinogenesis 1991;12:329–333. [DOI] [PubMed] [Google Scholar]
- 28. Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen‐treated mice. Clin Cancer Res 2004;10:301–313. [DOI] [PubMed] [Google Scholar]
- 29. van Oijen MGCT, Slootweg PJ. Oral field cancerization: carcinogen‐induced independent events or micrometastatic deposits? Cancer Epidem Biomar 2000;9:249–256. [PubMed] [Google Scholar]
- 30. Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen‐treated mice. Clin Cancer Res 2004;10:301–313. [DOI] [PubMed] [Google Scholar]
- 31. Saranath D, Chang SE, Bhoite LT, et al. High frequency mutation in codons 12 and 61 of H‐ras oncogene in chewing tobacco‐related human oral carcinoma in India. Br J Cancer 1991;63:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sathyan KM, Nalinakumari KR, Abraham T, Kannan S. Influence of single nucleotide polymorphisms in H‐ras and cyclin D1 genes on oral cancer susceptibility. Oral Oncol 2006;42:607–613. [DOI] [PubMed] [Google Scholar]
- 33. Vairaktaris E, Papageorgiou G, Derka S, et al. Expression of ets‐1 is not affected by N‐ras or H‐ras during oral oncogenesis. J Cancer Res Clin Oncol 2007;133:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki R, Kohno H, Suzui M, et al. An animal model for the rapid induction of tongue neoplasms in human c‐Ha‐ras proto‐oncogene transgenic rats by 4‐nitroquinoline 1‐oxide: its potential use for preclinical chemoprevention studies. Carcinogenesis 2006;27:619–630. [DOI] [PubMed] [Google Scholar]
- 35. Saitoh A, Kimura M, Takahashi R, et al. Most tumors in transgenic mice with human C‐Ha‐ras gene contained somatically activated transgenes. Oncogene 1990;5:1195–1200. [PubMed] [Google Scholar]
- 36. Inafuku M, Li C, Kanda Y, et al. Beta‐glucosylceramide administration (i.p.) activates natural killer T cells in vivo and prevents tumor metastasis in mice. Lipids 2012;47:581–591. [DOI] [PubMed] [Google Scholar]


