Abstract
Embalming fixatives such as formaldehyde and phenol have been associated with occupational health hazards. While anatomists aim at replacing these chemicals, this seems presently unfeasible in particular for formaldehyde. Furthermore, fixation protocols usually require well‐equipped facilities with highly experienced staff to achieve good fixation results in spite of only a minimal use of formaldehyde. Combining these aspects, a technique robust enough to be carried out by morticians is presented, resulting in durable tissues with minimal formaldehyde use. An embalming protocol involving phenoxyethanol was established, using concentrations of 7 and 1.5 Vol% of phenoxyethanol in the fixative and the conservation fluid, respectively. Visual, haptic, histological, and biomechanical properties and their perceived potential to positively influence student learning outcomes were compared to standard embalming techniques. The phenoxyethanol technique provides esthetic, durable, and odorless tissues. Bleaching is less pronounced compared to ethanol‐ or formaldehyde‐based protocols. The tissues remain pliable following the phenoxyethanol‐based embalming and can be used for biomechanical experiments to some extent. Phenoxyethanol‐fixed tissues are well suited for undergraduate teaching with perceived positive learning outcomes and partly for postgraduate training. Phenoxyethanol tissues provide the option to obtain well‐preserved histology samples, similar to those derived from formaldehyde. The provided protocol helps replace the use of phenol and formaldehyde for conservation purposes and minimizes the use of formaldehyde for the initial injection fixation. Phenoxyethanol‐based embalming forms an effective alternative to standard embalming techniques for human cadavers. It is simple to use, allowing fixation procedures to be carried out in less sophisticated facilities with non‐anatomy staff.
Keywords: cadaver embalming, cadaver fixation, dissection room teaching, formaldehyde reduction, gross anatomy education, laboratory teaching, learning outcomes, phenol replacement, phenoxyethanol
Introduction
Chemical fixation of human cadavers forms the mainstay in gross anatomy education (Drake, 2007; Drake et al., 2009; Ochs et al., 2012; Brenner, 2014). It aims at preserving tissues for longer durations, thereby allowing fine dissection of tissues without the risks of biological hazards, autolysis, or decay; it also provides optical and haptic tissue characteristics in a standardized manner, yet often incomparable to vital tissue from surgery or fresh cadaveric tissue known from routine autopsy (Ochs et al., 2012; Balta et al., 2015a,b). There is clear evidence in favor of cadaver‐based teaching to facilitate student learning. Cadaveric dissection forms a distinct and unmatched educational tool, allowing students in medicine and allied life sciences to appreciate the spatial three‐dimensional anatomy, concepts of form and related function, anatomical variation, and changes induced by age and pathology (Drake et al., 2009; Pabst, 2009; Eisma and Wilkinson, 2014).
In recent years, increasingly, health concerns were raised regarding the chemicals involved with the embalming of cadavers in the anatomy setting. These concerns apply in particular to formaldehyde, which has become classified as 1B carcinogenic. Both preparation of cadavers for the courses and cadaveric dissection potentially put at risk the prosectors, students, and teaching staff (Hauptmann et al., 2004, 2009; Goldstein, 2011).
The assessment of formaldehyde has led to a number of measures in order to minimize its use and exposure in the dissection room (Thullner et al., 2015; Waschke et al., 2019), and to the introduction of alternatives to the classic formaldehyde‐based embalming (Blum, 1896). Technical and administrative attempts were made to abandon formaldehyde from the dissection room at varying success rates. In a recent report, the use of an ethanol–glycerin‐based fixation technique with thymol conservation has been introduced, which has the potential to be applied without the use of formaldehyde (Hammer et al., 2011, 2012). This particular setting requires highly trained staff in order to result in optimal fixation (Hammer et al., 2012, 2015b). Highly sophisticated facilities and expert embalmers may however not necessarily be available for all anatomy facilities and settings. Likewise, the anatomy infrastructure or extremely large catchment areas may make it impossible to bring all cadavers to the site of the anatomy premises for embalming. The latter is the case for the South Island of New Zealand, with only one anatomical institute covering a catchment area similar to the size of the combined New England States. The sparsely populated country makes it necessary to transport cadavers over distances as much as 800 km to reach the anatomy facilities (Fig. 1). Consequently, cadavers are required to be embalmed close to the site of death, which can only happen by local funeral homes.
Figure 1.
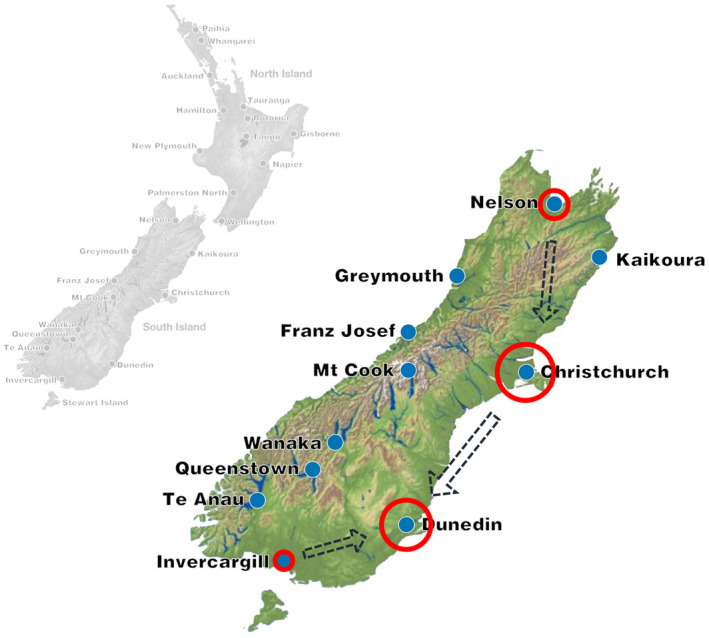
New Zealand map, showing centers and major catchment areas at the South Island, contracted funeral homes (red circles, size reflects number of donations) and transportation streams (interrupted arrows) for cadavers to the anatomy premises at the University of Otago, Dunedin (main campus). When considering the size of the South Island (150,000 km2) and its sparse population of only 1.1 million and a median age of only 37 years, it becomes evident that running a body donation system requires body transportation over large distances as indicated in the map to allow for cadaver‐based teaching in a university setting. This involves adjusted logistics with local funeral homes performing the embalming and a robust fixation protocol.
Considering these infrastructural challenges, this given work presents an 18 years' experience on a robust non‐commercial fixation protocol which, can be carried out by remote funeral home morticians with less experience in embalming cadavers for anatomy purposes. This protocol allows to store the fixed cadavers for months before being transported long distances, while at the same time providing high‐quality embalming results in spite of a minimal utilization of formaldehyde. A detailed protocol is given for the community of anatomists interested in approaches to lower the amount of formaldehyde and phenol in their fixatives. The results of the embalming protocol will be shown for tissues on a gross anatomical and histological level, and the suitability of this method is assessed by comparing macroscopic and histological features to three other embalming methods. Its potential and limitations will be discussed in detail, quantifying tissue quality and the potential to influence learning outcomes positively using Crosado‐embalmed tissues as a valuable learning resource.
Materials and Methods
The process of fixation, conservation, and storage is summarized as a step‐by‐step protocol (Table 1). Major chemicals and instruments are given in Table 2. Technical quality chemicals suffice the needs of this fixation protocol. The embalming mixtures are exclusively prepared in the anatomy premises in Dunedin and then shipped to the contracted funeral homes. This guarantees for a consistent quality of the fixatives and is more cost‐effective in the New Zealand setting compared to shipments of the individual chemicals. Exclusion criteria of bodies for embalming in general are as follows (if known): extensive surgery within a four‐week time frame prior to death, BMI ≥ 30 kg/m2, renal failure, sepsis, infectious diseases including Hepatitis B or C, active tuberculosis, Creutzfeldt–Jakob disease, those who are HIV positive, rapid onset of dementia, a diagnosis of ruptured aneurysm, and progressed autolysis and if the bodies had undergone a prior autopsy.
Table 1.
Step‐by‐Step Protocol and Summary of Fixatives, Final Concentrations, and Required Conditions
| Procedure | Amount of fixatives | Protocol, contents, and concentrations | Required conditions/instruments |
|---|---|---|---|
| Registration/cleaning |
|
||
| Cannulation of femoral artery |
|
No. 4 scalpel handle, No. 21 scalpel blades, Adson forceps, dressing forceps, blunt/blunt curved scissors, retractors, aneurysm hooks | |
| Body fixation | 20 L of injection solution (70 kg cadaver; needs to be decreased or increased depending on individual cadaver) |
|
Metal cannula, needle holders, curved needles, injection pump, suture material |
| Brain fixation (can be done as part of the initial fixation or at a later stage) | 20 ml formalin mixed with 100 ml injection solution |
|
No. 4 scalpel handle, No. 21 scalpel blades, 6‐mm drill, bee wax, syringe |
| Conservation and long‐term storage | Phenoxyethanol (1 Vol%), optional Arquad‐75 (0.1 Vol%) |
|
Storage at room temperature (warehouse 10‐25°C), polyethylene foil |
| Treatment of mold growth | Phenoxyethanol (2.4 Vol%, 120 ml), Arquad‐75 (0.25 Vol%, 1.25 ml) in 5 L water |
|
Table 2.
Agents Used for Fixation and Conservation
| Agent | Molecular formula | Hazard statements | Amount (Liters) | Price (US$/L) | Effective concentration in embalming fluid (%) | Tissue concentration (%) | Costs per cadaver (US$) |
|---|---|---|---|---|---|---|---|
| Fixation solution (20 L per cadaver) | |||||||
| Ethanol (95%) | C2H5OH | F, I | 40.00 | 1.72 | 57.4 | 12.7 | $20.81 |
| Formalin (37% formaldehyde) | CH2O | Ca, F, I, T | 1.25 | 27.96 | 1.9 | 0.4 | $10.59 |
| Glycerin | C3H5(OH)3 | n/a | 10.00 | 8.95 | 15.1 | 3.4 | $27.11 |
| Phenoxyethanol (90%) | C8H10O2 | Co, I, H, T | 5.00 | 1.69 | 6.8 | 1.5 | $2.56 |
| Water | H2O | n/a | 10.00 | 0.01 | 15.1 | $0.02 | |
| Total volume | 66.25 | ||||||
| Storage and moistening solution (5 L per cadaver) | |||||||
| Phenoxyethanol (90%) | C8H10O2 | Co, I, H, T | 0.15 | 1.69 | 1.3 | $0.13 | |
| Dimethyl di(hydrogenated tallow) ammonium chloride (Arquad 2HT) | R2N(CH3)2Cl | I | 0.04 | 3.29 | 0.4 | $0.07 | |
| Water | H2O | n/a | 10.00 | 0.01 | 98.1 | $0.03 | |
| Total volume | 10.19 | ||||||
| Brain fixation | |||||||
| Formalin mixed with fixation fluid (1:5 parts) | CH2O | Ca, F, I, T | 0.02 | 27.96 | 6.0 | 0.5 | $0.17 |
| Total cost | $61.49 |
Embalming Protocol
Following the arrival of the (unembalmed) bodies, documentation is checked. The body is placed in a supine position with the face and hand palms facing upwards. In cases of contractures, nylon plates may be utilized to achieve this. The body is then shaved and washed thoroughly.
The femoral artery on one side is the primary site of the cannulation and injection; there is no preferred side for the injection, but it should be varied by the embalmers to enable the students to dissect either side in different cadavers. When dissected carefully, femoral cannulation causes visible but acceptable damage to the femoral triangle, which may still be used for later dissection performed by students, in particular of the deeper structures. Otherwise, this step is also necessary for routine formaldehyde or ethanol embalming. Standard dissection instruments and an explosion‐proof environment are required for this step (Table 1). Following a longitudinal skin incision into the femoral triangle of 80 to 120 mm length, the fascia lata is exposed and transected in the same plane as the skin. After identifying the femoral artery in the femoral triangle, it is freed from its surrounding tissues at a length of 30 to 50 mm and sutures are placed but not fixed on both the proximal and distal ends of the artery. Then, the femoral artery is opened carefully at a length of approximately 10 mm.
Cannulation then takes place as described by Hammer et al. (2012). It is recommended to begin with the distal site of the cannulation, placing the needle inferiorly. After completion of the injection of the tissues distal to the cannulation, the distal femoral artery is closed using the prepared sutures, and the cannula is placed superiorly. The process is then repeated after the completion of the superior injection site and the fascia and skin are closed using a continuous suture. Venous drainage of the blood does not usually take place but forms an option.
The composition of the embalming primarily consists of ethanol, glycerin, water, and phenoxyethanol (PE). Given the PE forms the differentiating feature to other embalming fluids, it is here referred to as PE‐based fixative in spite of its relatively small amount in the solution. A total of 20 liters is required for a standard (70 kg) cadaver, but the volumes may alter depending on body constitution. The embalming fluid is injected at a flow rate of 0.3‐0.6 L/min with a standard injection pump. The injection should end once the peripheral tissues become firm, and foam starts forming from the mouth and nose as a sign that the fluid has reached and filled the airways. Another commonly observed feature for sufficient peripheral fixation is the appearance of goose‐pimpled skin. The injection takes approximately 4 hours to complete, usually in two 2‐hour sessions over 24 hours.
Different sites of fixation may be used in case of previous surgery to the femoral region, severe atherosclerosis, or in case, perfusion attempts have failed. Here, the carotid artery seems to be the next choice. Using trocars to inject additional volumes peripherally in case of ineffective perfusion will also be conducted frequently, as the subsequent conservation protocol relies on a thorough initial fixation. If nylon plates are used to reinforce any joint position, care must be taken that this does not impair the embalming as a consequence of vessel kinking, compression, or postmortem rupture. For on‐site storage at the funeral homes, the bodies are wiped down with the fixation fluid and sealed in heavy clear polyethylene bags. The fixatives draining from the airways and skin form a base layer from which fumes evaporate, resulting in the fixation of the outermost skin layers. Each injection is concluded with a detailed embalming report. This ensures that all steps are taken even by less experienced morticians and allows for further alterations of the protocol based on their feedback or clarification in case something went wrong during these steps.
Brain Fixation
The brain is embalmed using a volume of 10 ml formalin (37% formaldehyde) mixed with 50 ml of the fixation embalming fluid per side. For this purpose, bilateral burr holes are drilled 10 to 15 mm lateral to the median sagittal plane of the skull using a 6‐mm drill. Following this, a needle is placed anteromedially at a depth of 20 mm. This step has led to the most effective brain embalming results using minimal amounts of formaldehyde. The burr hole should ideally be closed with a wax (Modern Materials® Utility Wax Strips, Large, Kulzer Australia Pty Ltd, Homebush, NSW, Australia) or resin, and the scalp should then be closed by sufficient sutures to prevent leakage of the formaldehyde. This step of brain fixation can be done alongside with the fixation. While the quality of the brain fixation is more suited for prosections or plastination if done immediately, the desired hardening of the tissues is still sufficient if the injection is done at a later stage as the PE‐based embalming.
An option to decrease the (ca. 0.4‐Vol%) formalin content prior to the craniotomy laboratories is to drain the fluid within the cranial cavity with a syringe via the existing burr holes and then add 50 ml of 25% formalin neutralizer (e.g., Formalex GREEN, Newcomer Supply, Middleton, WI) diluted with water and 1.5‐Vol% PE. This is usually done two to seven days prior to the craniotomy.
Transportation of the Embalmed Bodies
The undissected cadavers are transported in batches to the anatomy department, usually in a 1‐ to 3‐month time frame, enclosed in body bags and protected by one additional transportation coffin for each cadaver. For the University of Otago, a local freight company has been contracted to carry out this uncooled land transport. This approach is feasible according to the New Zealand Human Tissue Act (Human Tissue Act, 2008) in conjunction with the New Zealand Land Transport Act (Land Transport Act, 1998). In return, the premixed fixatives are shipped to the funeral homes. Given the concentrations of the ethanol exceeding 50 Vol%, these transports are labeled as dangerous goods.
Cadaver Storage and Conservation Protocol
Once the bodies are received in the anatomy premises, they are re‐registered and re‐cleaned. For this purpose, the cadavers are removed from their body bags, examined for the quality of the embalming, and re‐wrapped in polyethylene bags before placed in racks. Transparent polyethylene foil allows to check the condition of the cadavers at any stage, which renders particularly helpful if in doubt about the results of the fixation. Storage of the cadavers on site in anatomy is done at a temperature of 10‐25 °C in an unheated warehouse before being moved into the anatomy department and being placed on dissection tables.
A PE‐based agent is used for conservation and long‐term storage purposes of prosections. For this purpose, an aqueous 1.5‐Vol% PE, 0.04‐Vol% dihydrogenated tallow dimethylammonium chloride (Arquad 2HT; Merck KGaA, Darmstadt, Germany) is used (Table 1). Prosections and opened cadavers are moistened using this solution but not entirely immersed in the solution. Damp towels are used for unused prosections during the courses to prevent drying of the tissues.
Treatment if Tissues are Suspected of Being Contaminated
One of the challenges of this fixation is the potential development of mold or bacterial growth under conditions of extensive use or extreme storage conditions involving higher temperatures. In these cases, Trigene (Ceva Animal Health Pty Ltd, Glenorie, NSW, Australia) can be used as an effective quaternary ammonium disinfectant with cationic surfactant properties. In any case, microbiological assessment of the samples is recommended.
Examples from Gross Anatomy and Histology
A number of specimens were retrieved from cadavers embalmed with the PE‐based mixture. While alive, all body donors gave their informed consent to the donation of their postmortem tissues for research and teaching. The tissues were used in compliance with the Human Tissues Act 2009 governing all processes related to body donation in New Zealand. Examples of prosections were chosen for the face and neck region (91‐year‐old male), muscles of the arm and hand (65‐year‐old male), the viscera (78‐year‐old female), and the central nervous system (98‐year‐old female) and are presented in Figure 2. Histology samples of PE‐based tissues were retrieved from a 78‐year‐old female and a 77‐year‐old male, paraffin embedded, sectioned at 5 and 20 μm, and stained using hematoxylin–eosin (H&E), Luxol fast blue‐cresyl violet, Masson trichrome, reticulin, and with silver staining (see Fig. 3).
Figure 2.
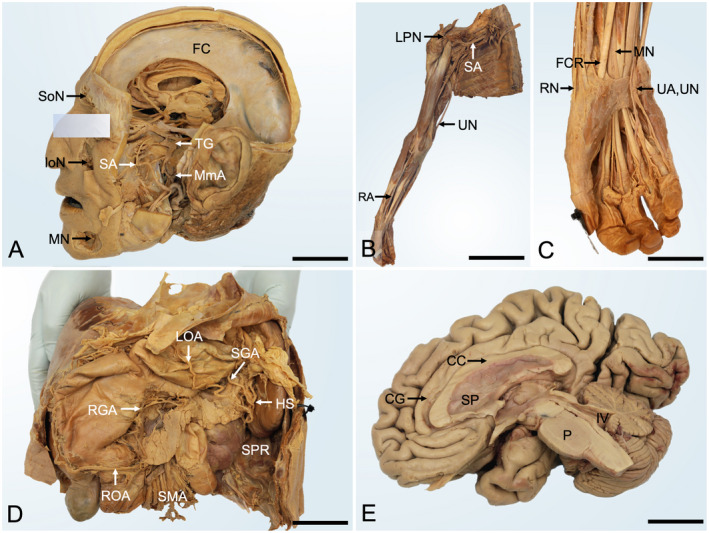
Gross anatomy samples of phenoxyethanol‐based embalming prosections. A, Head dissection from a 91‐year‐old male (5 years postfixation). The neurocranium and parts of the zygomatic bone, mandible, and left cerebral hemisphere were removed to show the trigeminal nerve and its branches. Note the reflected parotid duct. FC, falx cerebri; IoN, infraorbital nerve; MmA, middle meningeal artery; MN, mental nerve; SoN, supraorbital nerve; SA, sphenopalatine artery; TG, trigeminal ganglion; scale bar 45 mm. B, Right upper limb dissection of a 65‐year‐old male (7 years postfixation) shows an overview of the upper limb. LPN, lateral pectoral nerve; RA, radial artery; SA, subclavian artery; UA, ulnar artery; scale bar 280 mm. C, the magnified wrist and hand dissection of the upper limb shown in panel B. FCR, flexor carpi radialis; MN, median nerve; RN, radial nerve; UA, ulnar artery; UN, ulnar nerve; scale bar 45 mm. D, Upper abdominal area prosection from a 78‐year‐old female (4 years postfixation). The liver, stomach pancreas, and spleen are shown to be supplied by branches of the celiac trunk and superior mesenteric artery. HS, hilum of the spleen; LOA, left gastro‐omental artery; RGA, right gastric artery; ROA, right gastro‐omental artery; SGA, short gastric arteries; SMA, superior mesenteric artery; SPR, spleno‐phrenic recess; scale bar 100 mm. E, Right sagittal dissection of the brain from a 98‐year‐old female (2 years postfixation) after removal of the arachnoid layer. CC, corpus callosum; CG, cingulate gyrus; P, pons; SP, septum pellucidum; IV, fourth ventricle; scale bar 30 mm.
Figure 3.
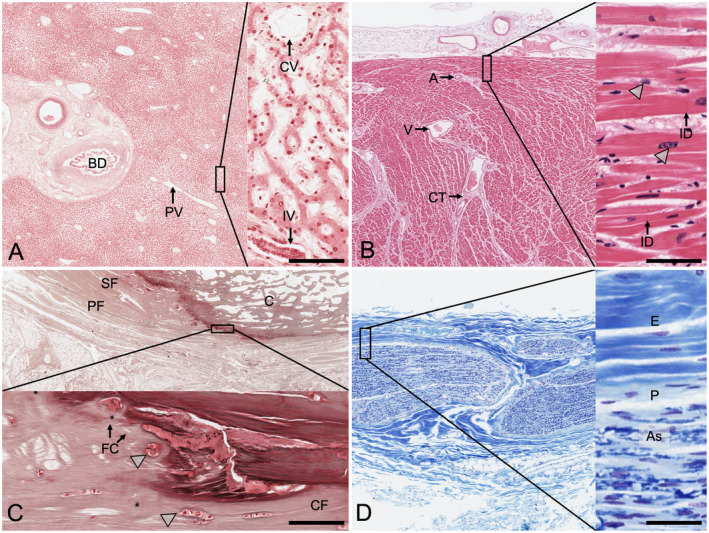
Histological samples showing the results from phenoxyethanol‐based embalming following a 10‐month duration of the embalmed tissues in the dissection course. The highlighted squares on the left are magnified as inserts on the right side of each image set. A, Liver (reticulin stain). The classical arrangement of hepatic lobules can be seen, with the central veins (CV) and the periportal space. The staining shows well‐preserved tissues, but the fibers appear to mask the fixative. BD, bile duct; IV, interlobular vein; PV, branch of portal vein; scale bar 40 μm. B, Cardiac muscle (H&E stain). This tissue sample from the left ventricle shows intact cardiac myocytes and at higher magnifications intercalated disks (ID). The arrowheads indicate the nuclei of the cardiac myocytes. A, arteriole; CT, connective tissue; V, vein; scale bar 25 μm. C, Bone, calcaneus (silver stain). The calcaneal insertion of the plantar fascia (PF) with the adjacent superficial foot muscle layer (SF) is presented in sagittal orientation. The arrowheads point at the chondrocytes forming the fibrocartilage (FC) at the transitional zone of the collagen fiber (CF) insertions. Only remnants of osteocytes can be seen within the osteon structure of the calcaneus (C). No canaliculi become visible following the staining; scale bar 25 μm. D, Peripheral nerve (Luxol fast blue/cresyl violet stain). The myelin is stained in the various bundles of axons in this sample of the lateral cutaneous nerve of the thigh. Axons (As) and nuclei of Schwann cells are visible. E, epineurium, P, perineurium; scale bar 50 μm.
Quantified Assessment of Tissue Suitability for the Gross Anatomy and Histology Course
The suitability of PE‐embalmed tissues and staining was assessed by academic staff for both gross anatomy (14 assessors, 12 from a university and 2 from a non‐university setting, mean age 38.1 ± 13.8 years) and histology (18 assessors, 16 from a university and 2 from a non‐university setting, mean age 37.8 ± 15.3 years) comparing the findings to formaldehyde, ethanol–glycerin, and Thiel‐embalmed tissues as typical examples for alternative embalming methods. For this purpose, further tissues were retrieved from cadavers embalmed with the respective techniques after the dissection course (mean age at death 82.8 ± 5.7 years, range 75 to 88 years, 3 females, 2 males). Gross anatomy, tissue preservation, colorfastness, and tissue pliability were surveyed from anatomists, all with experience in dissection‐based teaching and experience in the abovementioned techniques.
Microscopically, tissue preservation, colorfastness, and tissue suitability for histological diagnosis were surveyed. For this purpose, specimens were retrieved following the dissection course from the following organs: liver, stomach/jejunum, skeletal muscle, tendon, bone, heart, lung, central nervous system, and peripheral nerve. Furthermore, the perceptions of the raters were assessed regarding tissue suitability to influence student learning outcomes positively. A subgroup of three anatomists/pathologists assessed the quality of the various staining techniques for histology.
A five‐point Likert scale was used for the assessment (1, perfect/well suited/fully agree to 5, poor/completely unsuitable/fully disagree). Furthermore, qualitative statements from the raters are incorporated in Table 3 alongside with recent publications on the topic. Ethical approval for this study was obtained from the Ethics Committee of the University of Leipzig, Germany (348/19‐ek).
Table 3.
Comparison of Phenoxyethanol‐Based Fixation with Other Contemporary Embalming Techniques
| Characteristics | Formaldehyde | Ethanol | Phenoxyethanol | Thiel |
|---|---|---|---|---|
| Visual characteristics | ||||
| Overall appearance | wrinkled | wrinkled | welled | lifelike |
| Skin, subcutaneous fat | yellow‐brown | pallid | yellow | pale, oily |
| Bones | unaltered | slightly bleached | unaltered, yellow coloration | unaltered, white coloration |
| Cartilage | unaltered, bleached | in vivo‐like | unaltered, green coloration | unaltered, yellow coloration |
| Ligaments | bleached | dehydrated, bleached | welled | unaltered |
| Muscles | gray‐yellow color | bleached | welled, bleached | intense red to brown |
| Vessels | colorfast | bleached | bleached | unaltered |
| Central nervous system | blue‐gray, bleached | shrinked, browned | blue‐gray, bleached* | shrinked, liquified |
| Peripheral nerves | bleached, gray | bleached | welled | lifelike |
| Intestine, glands | bleached | colorfast | pink‐gray appearance | colorfast |
| Haptic characteristics | ||||
| Overall appearance | stiffened | stiffened | pliable, soft | in vivo‐like |
| Skin, subcutaneous fat | strongly indurated | slightly indurated | pliable | softened |
| Bones | brittle | in vivo‐like | in vivo‐like | brittle |
| Cartilage | in vivo‐like | in vivo‐like | in vivo‐like | in vivo‐like |
| Ligaments | severely stiffened | stiffened | pliable, dissolved appearance | slightly softened |
| Joint mobility | strongly decreased | strongly decreased | moderately decreased | moderately increased |
| Muscles | strongly indurated | slightly indurated | pliable, elastic | severely softened and liquified |
| Vessels | stiffened | stiffened, blood clots | elastic, blood clots | in vivo‐like, easily collapsible |
| Central nervous system | rigid | softened | soft | unsuitable |
| Peripheral nerves | rigid | flexible, slightly stiffened | elastic | in vivo‐like |
| Intestine, glands | stiffened | flexible | rubber‐like | softened |
| Odor | intrusive | none (ethanol) | minimal nutty odor | intrusive |
| Potential health effects | toxic, carcinogenic | none described | toxic and carcinogenic effects minimized due to formaldehyde reduction | toxic, carcinogenic, teratogenic |
| Usability for histology/immunohistochemistry | well suited | suitable for histology, limited use for immunohistochemistry | suitable for histology, vastly limited use for immunohistochemistry | unsuitable |
| Usability for biomechanical experiments | unsuitable | limited use (musculoskeletal soft tissues, bone) | limited use (musculoskeletal soft tissues, bone) | partly usable (extracellular‐rich tissues) |
| Bone | unusable (Hammer et al., 2014) | usable after rinsing (Hammer et al., 2014) | usable after rinsing (Tomlinson et al., 2016) | usable (Tomlinson et al., 2016) |
| Joints | strongly reduced mobility (Balta et al., 2019a) | strongly reduced mobility | moderately reduced mobility | moderately increased mobility (Wilke et al., 2011; Balta et al., 2019a) |
| Ligaments, tendons | unusable (Steinke et al., 2012) | usable after rinsing (Steinke et al., 2012) | usable after rinsing (Stewart et al., 2018) | partly usable (Fessel et al., 2011; Hohmann et al., 2019; Zwirner et al., 2019; Balta et al., 2019a) |
| Skin | unusable | dried | dried | usable (Zwirner et al., 2019) |
| Costs per body in US$ | $10.53 | $66.78 | $62.49 | $491.90 |
| Costs per body in € | 9.55 € | 60.70 € | 55.81 € | 437.24 € |
| (excludes transportation of chemicals and cadavers) | ||||
Statistical Analyses
Prism software, version 8 (GraphPad, San Diego, CA), and SPSS statistical package, version 23.0 (IBM Corp., Armonk NY), were used for statistical analyses of the rating data. Normal distribution of the data was determined using the Shapiro–Wilk test. Comparison of gross anatomical and histological tissue characteristics and suitability between the four embalming techniques was done using a Kruskal–Wallis test for multiple comparisons. Inter‐rater reliability was assessed using the intraclass correlation coefficient (ICC).
Results
This PE‐based fixation helped with preserving the postmortem tissues in a stable condition, resulting in a consistent tissue appearance. So far, more than 750 cadavers have been embalmed with this protocol at the University of Otago, 30‐60 cadavers per annum. Cadavers are used after a minimum of six months postfixation, with up to three years in storage. The use of entire cadavers and prosected tissues fixed with this protocol includes first year (health sciences) teaching, second to fifth‐year medical teaching, forensic science, physiotherapy, and physical education as undergraduate courses, and presently a dissection‐based diploma in surgical anatomy, obstetrics, and gynecology teaching.
Quantification of formaldehyde exposure levels was performed twofold. First, staff exposed to the cadavers during teaching sessions were assessed using a sampling pump. Second, static area samples were measured in tubes placed at breathing height. Measured personal exposure levels ranged between < 0.001 and 0.03 mg/m3, resulting in a time‐weighted average for eight hours of < 0.001 to 0.02 mg/m3. The static measurements resulted in levels of 0.002 mg/m3. None of the measurements reached the 0.41 mg/m3 (0.33 ppm), 0.37 mg/m3, and the 0.75 ppm threshold of formaldehyde exposure for New Zealand (WorkSafe, 2018), Europe (Thullner et al., 2015), and the United States (NIH, 2011), respectively.
Visual and Haptic Appearance of the Tissues
The outer appearance of the cadavers is consistently yellow‐pale even over a long duration of the tissues in the dissection course when moistened frequently with the PE‐based conservative. This appearance is a desired outcome of the embalming procedure. The tissues remain pliable, allowing for restricted joint movement in an undissected state mimicking rigor mortis in lesser intensity. Body parts removed during the dissection course usually do not require any additional chemical treatment. These tissues are placed in labeled boxes or plastic bags, which remain with the cadaver until being cremated. The development of odors from the cadavers is less intrusive compared to other fixation techniques. No formaldehyde scent is observed. In some cases of cadavers with history of renal or multi‐organ failure (which was unknown primarily), a uremic scent can be observed.
Figure 2 illustrates examples of dissections of a variety of anatomical regions. Table 3 summarizes the tissue features compared to other standard anatomical fixations. Bones and cartilage remained in a condition close to the unfixed natural state, though a slight yellow coloration can be observed, especially in obese or sarcopenic cadavers. Ligaments and muscles remain relatively pliable, and joint motion increases over time, likely as a long‐term effect of conservation. Bleaching and yellowing can be observed in particular for the neurovascular structures and subcutaneous fat. Fat is retained in the tissues more effectively than with ethanol–glycerin or Thiel embalming. Veins remain filled with blood clots; this feature facilitates identifying the vessel type alongside with coloration effects (hemoglobin imbibition) making venous valves visible to the blunt eye. Central nervous system appearance largely depends on the extent of (additional) formaldehyde embalming. If fixed exclusively with the PE‐based fixative, the brain parenchyma shrinks, has a reddish appearance, and is much softer compared to standard formaldehyde fixation. Isolated islands of unfixed areas are likely to be related to (microangio‐) atherosclerosis with this fixation type predominantly depending on vascular perfusion. Adding the recommended amount of formaldehyde to enhance the brain fixation results in a similar appearance as would be seen if the cadaver was perfused with higher concentrations of formaldehyde. The eyeballs tend to shrink to the base of the orbital cavity. This process can be circumvented by injecting 10‐Vol% industrial quality gelatin following the fixation, combined with 1.5‐Vol% PE. The condition of the thoracic and abdominal viscera largely depends on the postmortem interval between the onset of death and the start of the embalming. An ideal time frame is less than 36 hours under cooled conditions. The heart and lungs remain pliable and more elastic compared to formaldehyde‐ or ethanol‐based fixatives. The liver, spleen, and kidneys are elastic with only minimal tendency to dry out. This feature allows to examine the abdominal pouches similar to cadavers embalmed with the Thiel method. The celiac trunk branches can usually be accessed without damaging the parenchyma of the aforementioned organs. Stomach and intestine fixation are considered less effective compared to formaldehyde or ethanol fixation but provides sufficient results for medical education purposes.
Phenoxyethanol‐embalmed tissues are a suitable basis for silicone‐ and epoxy‐resin based plastination. It was noted, however, that the duration of PE‐fixed tissues in the acetone for the purpose of dehydration and degreasing was longer than with formaldehyde‐fixed tissues, usually 4‐6 weeks.
Quantitative macroscopic comparison of PE to other embalming protocols yielded that tissue preservation gave similar results (2.0 ± 0.9, good) as formaldehyde‐based (1.9 ± 1.0, good) and ethanol–glycerin (2.1 ± 0.9, good)‐based fixation techniques (P ≥ 0.054), being non‐significantly better assessed than Thiel embalming (2.8 ± 1.2, partly suitable; P > 0.05). Figure 4 summarizes these results. Colorfastness tended to be non‐significantly better for PE (2.4 ± 1.0, good) compared to formaldehyde (3.2 ± 1.3, partly suitable) and ethanol (3.0 ± 1.2, partly suitable), but was significantly less lifelike for formaldehyde than for Thiel embalming (2.2 ± 1.2, good; P = 0.023). Similar observations were made for tissue pliability (P = 0.007). When assessing perceived learning outcomes based on study using the four embalming techniques, no significant difference was found (P > 0.50); however, embalming based on ethanol–glycerin (2.0 ± 0.7, good) and PE (2.1 ± 0.7, good) gave less variable results than formaldehyde (2.2 ± 0.9, good) and Thiel (2.5 ± 1.6, good to partly suitable) embalming when considering the extremes of the assessment.
Figure 4.
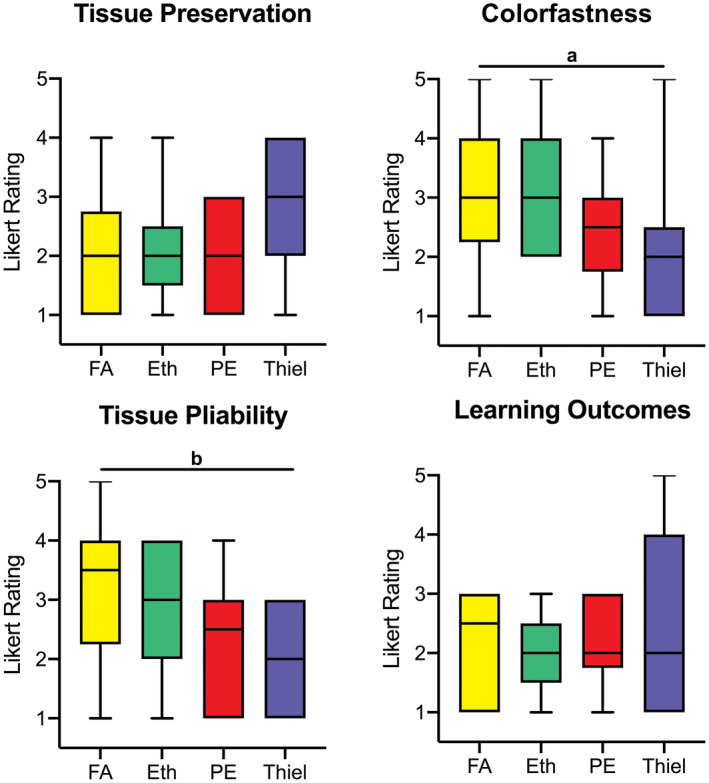
Survey on gross anatomical tissue quality. Tissue preservation, colorfastness, the pliability of the tissues, and their potential to improve student learning outcomes when used as study material was assessed for formaldehyde (FA), ethanol–glycerin (Eth), phenoxyethanol (PE), and Thiel‐embalmed tissues. Significant difference (a P ≤ 0.05, b P < 0.001) was found especially when comparing FA‐ and Thiel‐embalmed tissues. Likert scale rating: 1 = perfect/well suited/fully agree and 5 = poor/completely unsuitable/fully disagree.
Suitability for Biomechanical Experiments and Cadaver‐Based Professional Workshops
Joint motion and pliability are reduced evidently compared to the fresh condition or to Thiel‐fixed tissues, in line with the results presented in Figure 4. This appears to be related to the effects of the formaldehyde and the ethanol in the embalming fluid, causing crosslinking of the proteins' secondary and tertiary structures to some degree (Romeis, 1989; Werner et al., 2000) (Table 3). It can be observed that following partial resection of the muscles or longer term conservation with the PE solution, joint motion increases. While musculoskeletal tissues are generally used for preliminary biomechanical tests providing useful data (Scholze et al., 2018; Stewart et al., 2018), thoracic and visceral soft tissue mechanical properties render these areas unsuitable for obtaining data similar to the unfixed condition (own unpublished results). Phenoxyethanol‐embalmed tissues appear well suited for morphometric analyses in the context of surgical research given tissue shrinkage is less marked than in formaldehyde‐fixed tissues (Trowbridge et al., 2017; Becker et al., 2019).
The suitability of the tissues for cadaver‐based workshops seems to follow the pattern of their biomechanical properties. Phenoxyethanol‐fixed tissues are frequently utilized for postgraduate workshops such as the “Postgraduate Diploma in Surgical Anatomy” (Stringer and Lyall, 2012), and a number of small focused workshops for specialist audience including emergency medicine and gynecology. Trials for trauma and orthopedics workshops have rendered these tissues unsuitable in favor of establishing the Thiel method at the University of Otago. Here, Thiel has clear advantages concerning tissue pliability, joint range of motion, and the colorfastness of the tissues, especially for the course called “Surgical Exposures in Orthopedic and Trauma Surgery” and for the “Advanced Anatomy” course for more advanced medical students with particular interest in surgical topics (Hammer et al., 2015a,b; Klima et al., 2017) and intensivist training including (rescue) airway management.
Suitability of Tissues for Histology
The ICCs for histological tissue assessment ranged between 0.85 and 0.88. The assessment of sample quality from a variety of organs revealed that the PE‐fixed tissues result in a tissue quality adequate to perform standard histology. The staining yielded largely intact tissues including both the cells and extracellular matrix, as can be seen in Figures 3, 5 and Table 3. The best staining results were obtained from solid organs, especially the liver and heart, using routine staining methods such as hematoxylin–eosin (H&E). Nerve tissues from the brain and periphery showed minor variability regarding their colorfastness. Skeletal muscle samples were seen most often without their typical cross‐striping irrespective of the staining method used. Intestinal mucosa was decomposed in most examples as it is also known from routine histology slides of fresh cadaveric tissues. Further, some tissues showed cellular hydropic swelling, which is known to be a sign of hypoxia in autopsy histology. The quality of the tissues here seemed similar to ethanol–glycerin fixation.
Figure 5.
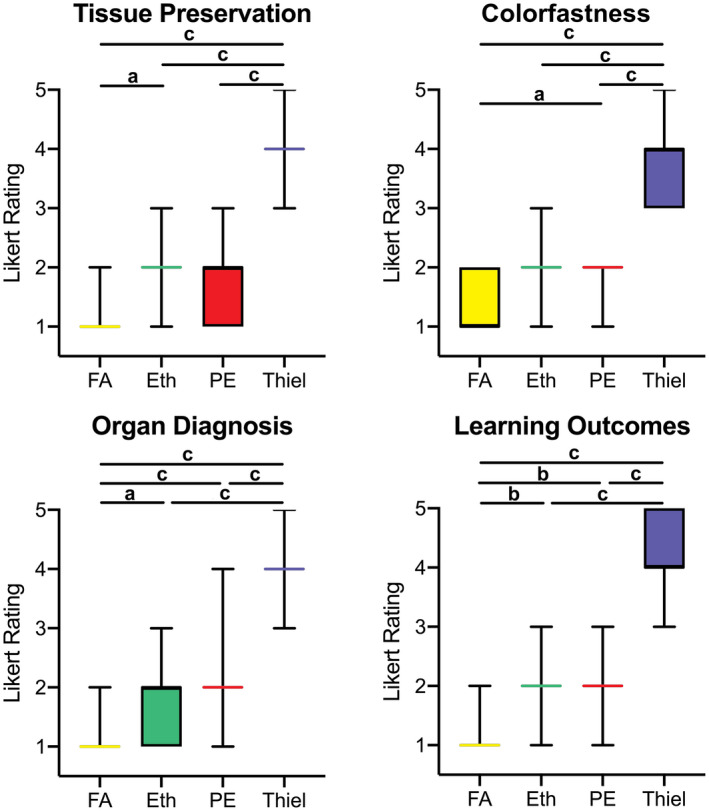
Survey on histological anatomical tissue quality derived from human cadavers embalmed with formaldehyde (FA), ethanol–glycerin (Eth), phenoxyethanol (PE), and Thiel. Tissue preservation, colorfastness, their suitability to make a proper organ diagnosis and their potential to improve student learning outcomes when used as study material were assessed. Significant difference (a P ≤ 0.05, b P < 0.001, c P < 0.0001) was found throughout the different embalming techniques, showing superiority of formaldehyde embalmed compared the other techniques especially for organ diagnosis and student learning outcomes compared to PE, and PE being superior to Thiel yet similar to Eth for most aspects. Likert scale rating: 1 = perfect/well suited/fully agree and 5 = poor/completely unsuitable/fully disagree.
Quantitative histological assessment of tissues retrieved from PE‐embalmed tissues yielded significantly better tissue preservation (1.7 ± 0.6, suitable; Fig. 5), colorfastness (1.9 ± 0.4, suitable), and suitability to diagnose organs (2.1 ± 0.7, good) compared to Thiel embalming (all 3.8 ± 0.6 to 4.2 ± 0.6, unsuitable; P < 0.001), but less favorable results than formaldehyde tissues regarding color fastness (P = 0.003). Similarly, perceived learning outcomes of histology sections were better for tissues originating from PE‐based (1.9 ± 0.6, suitable) compared to Thiel (4.2 ± 0.6, unsuitable; P < 0.001)‐based tissues but significantly inferior compared to formaldehyde‐based tissues (1.1 ± 0.4, perfectly suitable; P = 0.008).
Further detailed assessment from staining of PE‐embalmed tissues with Masson trichrome was assessed as giving good to excellent results (1.6), followed by H&E (2.0, suitable), silver staining (2.3, suitable), cresyl violet (2.8, partly suitable), and reticulin (2.9, partly suitable). Attempts to apply sufficient immunohistochemistry however rendered unsuccessful (no results given). Tissues stained with reticulin were graded significantly lower compared to H&E (P = 0.027) and Masson's trichrome (P = 0.014). Similar, tissues stained with cresyl violet were stained significantly lower compared to H&E (P = 0.022) and Masson's trichrome (P = 0.013).
Challenges of Tissue Quality—Drying and Bacterial–Fungal Contamination
Dehydration of hands and feet can be seen in cases with poorer fixation or in cadavers with prolonged positioning of the tissues in the body bags. Here, the fixative appears to evaporate most quickly from the distal extremities, resulting in a brownish transparent discoloration. In these cases, the tissues may not be recovered by moistening. A second aspect is the onset of bacterial or fungal growth. Though its onset only occurs under extreme storage conditions, for example, direct sun exposure of storage cabinets with condensing water washing out chemicals. Here, cases with Micrococcus and Cladosporium species have been observed, which appear to be contaminations introduced by normal skin commensal micro‐organisms of the users or air‐borne fungi. Tissue susceptibility to fungal contamination appears to be similar to the Dodge and Genelyn fixation (Jaung et al., 2011). Here, improvements in storage temperature, thorough cleaning with Trigene and PE/Arquad‐75, have been successful to treat and prevent contamination.
Discussion
Phenoxyethanol‐based embalming (“Crosado” technique) has shown to give reliable and reproducible results for the anatomical fixation over the period of nearly two decades of use at the University of Otago. This technique is suitable for embalming human tissues for a variety of dissection‐based courses and prosections, providing a suitable basis for plastination and histology. A major advantage of the technique is its potential to reduce the amount of formaldehyde to a minimal content, while at the same time being robust enough to be performed outside of the anatomy setting. The specimens resulting from PE‐based fixation are durable when used in the dissection course and provide a level of pliability to position the cadaver for dissection. The mixture of chemicals does not result in an unpleasant or intrusive smell (Frølich et al., 1984) as formaldehyde based or Thiel embalming may cause, and the tissues remain esthetic even after longer use as prosections. The PE‐based fixation complements existing embalming techniques (Thiel, 1992; Whitehead and Savoia, 2008; Messmer et al., 2010; Hammer et al., 2012; Brenner, 2014; Hammer et al., 2015b; Wedel et al., 2019) and provides an alternative to the use of hazardous chemicals or minimizes its application.
Rationale for the Use of Phenoxyethanol
Phenoxyethanol is a glycol ether with known bactericidal and antifungal properties (Lowe and Southern, 1994), with an oily colorless appearance and a characteristic pleasant odor. It is a widely used preservative to prevent bacterial and fungal contamination, especially in cosmetic industry and for pharmaceutical products. Phenoxyethanol is relatively inexpensive and acts as a softener (Brenner, 2014). Though PE is combustible, it must be preheated before ignition can occur (NPFA Fire Rating 1) and a harmful contamination of the air will not or only very slowly be reached on evaporation of this substance at room temperature. Concerning potential health risks, its use has been attributed to irritation, sensitization, and allergic contact dermatitis at low incidence rates in large cohort studies (Cheng et al., 2014; Horev et al., 2015). Further case reports exist on PE causing pain, headache, tremor, and central nervous system depression when swallowed intentionally or inhaled in large quantities (Toxnet, 2019). Consequently, standard safety precautions must be followed including room ventilation, protective gloves, and clothing and safety goggles when handling PE (ILO, 2019). Three studies by Frølich et al. (1984), Wineski and English (1989), and Tandon et al. (2014) highlight the potential of PE in lowering the exposure to formaldehyde and phenol. They describe that soft tissue pliability can be recovered by partly removing the formaldehyde and phenol from their fixatives (Frølich et al., 1984; Wineski and English, 1989). Their findings are in large agreement with the results presented here, where increasing pliability is found when tissues are exposed to the PE‐based conservation fluid. It remains unclear in the named studies (Frølich et al., 1984; Wineski and English, 1989) how much of the initial formaldehyde and phenol is trapped within the tissues. One may hypothesize that some of these fixatives are necessary to retain the desired bactericidal and fungicidal effects. Waschke et al. (2019) mention that PE forms part of their embalming protocol and that PE is being used for humidifying cadavers without the additional use of formaldehyde. This recommendation confirms the experience in Otago for conservation purposes over the last two decades. Further to their findings, it could be shown that even for the fixation fluid, a mixture involving PE may help reducing the amount of formaldehyde from 1.2 liters (Waschke et al., 2019) to less than 0.4 liters per body without necessarily adding further toxic biocides.
An area of uncertainty remains regarding the amount of PE used. A study by Rumph and Williams (1988) has shown inverse relationship between the concentration of PE and the propensity of the immersion fluids to effectively reduce the formaldehyde in the tissues. They suggest that PE may decrease the potential of the water to remove the formaldehyde from the tissues. Their data appear contradictory to the findings from Owen and Steedman (1956, 1958) and Frølich et al. (1984). In light of this given protocol using low concentrations of formaldehyde, these retention effects of PE might be beneficial in maintaining the tissues fixed. This might especially be true for the formaldehyde, which is structurally bound to the tissues' proteins.
Gross Anatomical and Histological Tissue Assessment and Perceived Impact on Student Learning Outcomes
There is a paucity of literature directly comparing the quality of tissues resulting from different embalming methods to date. In a recent series of studies, a group from the University of Dundee, Scotland, compared student (Balta et al., 2015b) and staff perceptions (Balta et al., 2017), tissue haptic properties (Kennel et al., 2018; Balta et al., 2019a), and antimicrobial properties (Balta et al., 2019b) of formaldehyde and Thiel‐based embalming techniques. They found that Thiel embalming made undergraduate students feel more uncomfortable than formaldehyde‐fixed tissues as they looked more lifelike (Balta et al., 2015b). Anatomists viewed that formaldehyde‐fixed tissues do more accurately resemble the features of the human body, whereas soft embalming techniques including Thiel (1992) and Genelyn (Balta et al., 2015b) more accurately resembled the features of the human body (Balta et al., 2017). These findings are in line with groups assessing professional perceptions on Thiel and other fixation techniques (Eisma et al., 2011; Yiasemidou et al., 2017) with high tissue pliability (Wedel et al., 2019) for postgraduate use.
A recent study by Kennel et al. (2018) assessed student perceptions and learning outcomes on learning gain following arm dissections of Thiel and formaldehyde/phenol fixed tissues in two distinct groups. They found that learning with Thiel‐embalmed tissues made students feel more confident in recognizing anatomy in the living and that they found it easier to identify anatomical structures. These perceptions were however not substantiated when assessing for functional anatomy knowledge between the two cohorts, indicating that the link between perceived and actual learning gain is more complex. The results of this study indicate that in spite of some differences in gross anatomical tissue preservation, colorfastness, and tissue pliability, the technique seems to result in similar properties as ethanol–glycerin fixation, sitting in between the extremes of formaldehyde and Thiel embalming for most features (Fig. 4). Similar, histological samples derived from PE tissues appear to have similar preservation, colorfastness, and distinguishable features to form a proper tissue diagnosis, being inferior to formaldehyde as a gold standard, similar to ethanol–glycerin, and much superior to Thiel embalming (Fig. 5). Consequently, perceived learning outcomes from PE‐embalmed tissues on the gross anatomical and histological scale seem to make this a suitable and versatile technique. It could further be shown that a significant relation appears to exist for PE‐embalmed tissues regarding the preservation quality between organ groups, in line with the findings of Rae et al. (2018).
Occupation Exposure Related Considerations
When first introduced in 2000 at the University of Otago, the aim of using the PE‐based embalming protocol was to minimize the use of formaldehyde as a fixative and to replace phenol for conservation purposes in foresight of the health hazards related to the application of these chemicals. Formaldehyde has been classified as carcinogen category 1B later, with presumed carcinogenic effect on humans (National Toxicology Program, 2010). A number of publications make attempt to correlate work exposure to formaldehyde with cancer, including cutaneous, nasopharyngeal, lymphatic, and hematopoietic malignancies found in embalmers and funeral directors (Coggon et al., 1984; Hayes et al., 1990; Hauptmann et al., 2004, 2009; Dreyfuss, 2010; Goldstein, 2011). Additional irritating (Muzi et al., 2004; Takahashi et al., 2007; Viegas et al., 2010; Wolkoff and Nielsen, 2010), cell proliferation altering (Hester et al., 2003), and neurophysiological effects (Marceaux et al., 2008) have been described. Phenol has shown toxic and irritating effects (Murray et al., 2007; Thullner et al., 2015). As a direct consequence of the carcinogenic effects of formaldehyde, the European Committee on Hazardous Substances has decreased the formaldehyde exposure limits to 0.37 mg/m3 with the long‐term aim to completely abandon it. Yet, much higher formaldehyde exposure levels are frequently found for staff exposed to embalmed cadavers (Ryan et al., 2003; Ohmichi et al., 2006; Vimercati et al., 2010). At the same time, banning formaldehyde seems to be a simplistic but unfeasible approach, and those working with chemicals should legitimately request to get provided with safe working conditions with the necessary infrastructural investments to achieve this.
As a consequence, the German Anatomical Society has established a “Working Group for Reduction of Formaldehyde Exposure in Dissection Courses.” Their consensus was that it is presently impracticable to completely abandon formaldehyde in the gross anatomy setting (Waschke et al., 2019). The group formalized recommendations to amend the composition of the chemicals aiming at reducing formaldehyde and suggested structural measures such as ventilation systems and room temperature adaptations. These recommendations include not to exceed formaldehyde concentrations of 4% in the embalming fixatives.
The given PE‐based protocol has an effective 1.9‐Vol% formalin dilution, equaling a 0.7 Vol% formaldehyde dilution. Consequently, neither the typical eye irritation symptoms (threshold 0.5 mg/m3), nor formaldehyde odor (0.1 mg/m3) are perceived with the fixture and peak exposure levels of 0.03 mg/m3, quantified in own recent measurements.
Tissue Quality and Suitability and Costs
A variety of anatomical fixation techniques exists, aiming at accentuating various tissue properties‐optical, haptic, or mechanical (Brenner, 2014). Regarding the tissues provided for undergraduate education, it has been shown to be advantageous to introduce a level of artificiality concerning tissue color and stiffness (Balta et al., 2015b; Hammer et al., 2015b). While formaldehyde fixation results in stiffer, unpliable tissues with a bleached appearance, other techniques such as light embalming (Messmer et al., 2010) or Thiel embalming (Thiel, 1992; Benkhadra et al., 2009; Jaung et al., 2011; Balta et al., 2015a; Hammer et al., 2015b; Balta et al., 2019a) result in colorfast and elastic tissues with particular suitability for postgraduate education and specialist workshops. The deliquescent tissue behavior of Thiel embalming is extremely helpful for (simulated) surgical interventions, which require a realistic range of tissue mobility (Groscurth et al., 2001; Eisma et al., 2011; Jaung et al., 2011; Eisma et al., 2013; Hammer et al., 2015a,b; Tomlinson et al., 2016; Balta et al., 2019a). Such tissues however have limitations for dissecting neurovascular structures or muscles, which then lack the extent of inherent stability and durability necessary for prosections. The here proposed PE‐based embalming method sits between these extremes of formaldehyde‐based and Thiel embalming regarding its optical and haptic properties: Soft tissues are pliable enough to be reverted. The PE‐based fixation may be considered well suited for undergraduate teaching and partly suited for postgraduate education. The use of PE‐based tissues for biomechanical experiments should be limited to pilot trials on bones and ligaments, with unembalmed fresh tissues giving clearly superior results (Scholze et al., 2018; Lozano et al., 2019) as even small quantities of ethanol and formaldehyde are known to impact negatively on the load‐deformation behavior of bone (Hammer et al., 2014; Trowbridge et al., 2017; Becker et al., 2019) and soft tissue (Steinke et al., 2012; Hammer et al., 2016).
Further to these considerations for gross anatomy education, it could be shown that the chemical fixation of the tissues introduced by PE fixation suffices for histological investigations. This can be done even after completion of a (one‐year) dissection course, which renders particularly helpful in case of suspected pathology, anatomical variation, or for the use for research projects where histological proof of a certain tissue quality is essential. Thiel embalming would not provide such opportunity (Hammer et al., 2015b), which has been substantiated in the given study. The results presented here are in line with two previous smaller‐scale investigations on PE‐fixed tissues for histology (Frølich et al., 1984; Nicholson et al., 2005). As in their investigations, the here presented study observed that histological tissue preservation results in agreement with an optimal PE fixation in a 24‐ to 36‐hour time frame postmortem. This applied to a variety of tissues and staining techniques that have been quantified in extent here. Limitations were seen for the preservation of bone and intestines, with disintegration of subcellular structures. These were likely related to hypoxia, enzymatic, and bacterial digestion. However, these effects are unlikely to be related to the PE and rather to the general setting of fixation. In order to achieve better results, shorter postmortem delays are necessary until fixation starts. Intestinal mucosa could alternatively best be prepared and stained from samples retrieved from living patients undergoing diagnostic procedures with histology obtained from biopsies.
Loftspring et al. (2008) in their study observed PE‐related effects on differentiating white from gray brain matter using Prussian blue stain, indicating that it may have similar effects as hot phenol in partly dissolving lipids and creating a layer which minimizes the accessibility of the white matter tissues by the staining colors. While this effect of PE seems advantageous for brain slices, it is less optimal for immunohistochemistry. Here, the technique is inferior to standard formaldehyde fixation at comparable postmortem delays. Likewise, the effects of the glycerin to immunohistochemistry are unclear. Here, the glycerin in the fixative may also be responsible for the lacking dyeability, as it masks the proteins' epitopes. The combination of PE and glycerin may also explain why longer durations of the embalmed tissues are required to give optimal results in epoxy and silicone plastination.
When considering the financial expenses of the embalming, PE may be considered costlier than formaldehyde‐based fixatives but slightly cheaper than ethanol‐based fixation (Hammer et al., 2012) and vastly cheaper than Thiel embalming (Hammer et al., 2015b) as summarized in Table 2. The PE only adds a small contribution to the overall cost of the embalming. Beyond the costs of the chemicals, further aspects with impact on the cost of fixation need to be considered. First, the fixatives are shipped twice, once to the anatomy facilities for the mixtures, and second to the funeral homes for the injection. These shipments are conducted as dangerous goods given the high concentrations of the ethanol in the mixture. An explosion‐proof storage and injection setting are furthermore required. This adds significantly to the overall cost of body fixation in the New Zealand setting. Furthermore, contracting and training funeral homes for undertaking the fixation incurs additional costs, which are only partly absorbed by staff reductions on the anatomy premises.
Limitations
Future studies may help assess student learning outcomes in a standardized setting, directly comparing the different embalming types. Moreover, the suitability of the various embalming techniques may be assessed for postgraduate training and clinical anatomical as well as biomechanical research, to substantiate the best suiting fixation techniques for all fields of cadaver‐based research and teaching. Future aims may also involve looking into further alternatives to glycerol as a suspected source of mold growth. This may also positively impact on the usability of the tissues for immunohistochemistry and plastination.
Conclusions
The here presented nearly 20‐year experience using a PE‐based embalming protocol shows that it is particularly suitable for the New Zealand environment with large catchment areas and long transport distances. It offers a robust embalming while at the same time being robust and requiring minimal amounts of formaldehyde and making the use of phenol redundant. The application of PE in the dissection room allows to deploy cadaveric tissues over a broad range of applications, including the dissection course, postgraduate training, histology, and to limited extent (preliminary) biomechanical tests.
Decreasing the amount of ethanol in the fixation composition may facilitate the storage and transportation requirements without explosion precautions. Ultimately, long‐term results on the (potential) health effects of PE in mixture with other chemicals are pending.
Notes on Contributors
BRYNLEY CROSADO is a prosector in the Department of Anatomy at the University of Otago, Dunedin, New Zealand. He has committed most of his work life to refining techniques of anatomical fixation and dissection.
SABINE LÖFFLER, D.M.D., P.D. Dr. Habil., M.M.E., is an academic prosector in the Institute of Anatomy at the University of Leipzig, Faculty of Medicine, Leipzig, Germany. She teaches anatomy and histology to first‐ and second‐year medical students and clinical anatomy to graduate students.
BENJAMIN ONDRUSCHKA, M.D., P.D. Dr. Habil., is a senior forensic pathologist and researcher in the Institute of Legal Medicine at the University of Leipzig, Faculty of Medicine, Leipzig, Germany. He teaches forensic medicine to medical and forensic students and to graduate students.
MING ZHANG, M.D., Ph.D., is an anatomist in the Department of Anatomy at the University of Otago, Dunedin, New Zealand. He teaches gross anatomy to undergraduate students and clinical anatomy to graduate students. His research is focused on plastination, the scull base, and fascia.
JOHANN ZWIRNER, M.D., Dr. med., is a research fellow in the Department of Anatomy at the University of Otago, Dunedin, New Zealand. His research focuses on surgical anatomy of the pelvis and head biomechanics.
NIELS HAMMER, M.D., Dr. Habil., is a professor and Chair of the Department of Clinical and Macroscopic Anatomy at the Medical University of Graz, Graz, Austria. He is further affiliated with the Department of Orthopedic and Trauma Surgery at the University of Leipzig, Leipzig, Germany, and the Medical Branch of the Fraunhofer IWU, Dresden, Germany. He teaches gross and clinical anatomy and histology to undergraduate students, and to graduate students and surgeons. His research focuses on surgical anatomy, biomechanics, and teaching in clinical anatomy.
Acknowledgments
The authors would like to thank Robbie McPhee for drawing the image. Christine Hammer took photographs of the tissues and typeset the figures. Aqeeda Singh proofread the paper as a native speaker. Benjamin Ondruschka was funded by the German Academic Exchange Service (Deutscher Akademischer Austauschdienst) for his University‐borne activities in New Zealand in 2019.
Mr. Crosado and Dr. Löffler contributed equally to this work.
Literature Cited
- Balta JY, Cronin M, Cryan JF, O'Mahony SM. 2015a. Human preservation techniques in anatomy: A 21st century medical education perspective. Clin Anat 28:725–734. [DOI] [PubMed] [Google Scholar]
- Balta JY, Lamb C, Soames RW. 2015b. A pilot study comparing the use of Thiel‐ and formalin‐embalmed cadavers in the teaching of human anatomy. Anat Sci Educ 8:86–91. [DOI] [PubMed] [Google Scholar]
- Balta JY, Cronin M, Cryan JF, O’Mahony SM. 2017. The utility of cadaver‐based approaches for the teaching of human anatomy: A survey of British and Irish anatomy teachers. Anat Sci Educ 10:137–143. [DOI] [PubMed] [Google Scholar]
- Balta JY, Twomey M, Moloney F, Dugan O, Murphy KP, O’Connor OJ, Cronin M, Cryan JF, Maher MM, O’Mahony SM. 2019a. A comparison of embalming fluids on the structures and properties of tissue in humans. Anat Histol Embryol 48:64–73. [DOI] [PubMed] [Google Scholar]
- Balta JY, Cryan JF, O’Mahony SM. 2019b. The antimicrobial capacity of embalming solutions: A comparative study. J Appl Microbiol 126:764–770. [DOI] [PubMed] [Google Scholar]
- Becker D, Lopez‐Marambio FA, Hammer N, Kieser D. 2019. How to avoid posterior interosseous nerve injury during single‐incision distal biceps repair drilling. Clin Orthop Relat Res 477:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkhadra M, Faust A, Ladoire S, Trost O, Trouilloud P, Girard C, Anderhuber F, Feigl G. 2009. Comparison of fresh and Thiel's embalmed cadavers according to the suitability for ultrasound‐guided regional anesthesia of the cervical region. Surg Radiol Anat 31:531–535. [DOI] [PubMed] [Google Scholar]
- Blum F. 1896. Über Wesen und Wert der Formolhärtung. Anat Anz 11:718–727. [Google Scholar]
- Brenner E. 2014. Human body preservation ‐ Old and new techniques. J Anat 224:316–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Leow YH, Goh CL, Goon A. 2014. Contact sensitivity to preservatives in Singapore: Frequency of sensitization to 11 common preservatives 2006‐2011. Dermatitis 25:77–82. [DOI] [PubMed] [Google Scholar]
- Coggon D, Pannett B, Acheson ED. 1984. Use of job‐exposure matrix in an occupational analysis of lung and bladder cancers on the basis of death certificates. J Natl Cancer Inst 72:61–65. [DOI] [PubMed] [Google Scholar]
- Drake RL. 2007. A unique, innovative, and clinically oriented approach to anatomy education. Acad Med 82:475–478. [DOI] [PubMed] [Google Scholar]
- Drake RL, McBride JM, Lachman N, Pawlina W. 2009. Medical education in the anatomical sciences: The winds of change continue to blow. Anat Sci Educ 2:253–259. [DOI] [PubMed] [Google Scholar]
- Dreyfuss JH. 2010. Occupational formaldehyde exposure linked to increased risk of myeloid leukemia and death. CA Cancer J Clin 60:135–136. [DOI] [PubMed] [Google Scholar]
- Eisma R, Lamb C, Soames RW. 2013. From formalin to Thiel embalming: What changes? One anatomy department's experiences. Clin Anat 26:564–571. [DOI] [PubMed] [Google Scholar]
- Eisma R, Mahendran S, Majumdar S, Smith D, Soames RW. 2011. A comparison of Thiel and formalin embalmed cadavers for thyroid surgery training. Surgeon 9:142–146. [DOI] [PubMed] [Google Scholar]
- Eisma R, Wilkinson T. 2014. From “silent teachers” to models. PLoS Biol 12:e1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel G, Frey K, Schweizer A, Calcagni M, Ullrich O, Snedeker JG. 2011. Suitability of Thiel embalmed tendons for biomechanical investigation. Ann Anat 193:237–241. [DOI] [PubMed] [Google Scholar]
- Frølich KW, Andersen LM, Knutsen A, Flood PR. 1984. Phenoxyethanol as a nontoxic substitute for formaldehyde in long‐term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec 208:271–278. [DOI] [PubMed] [Google Scholar]
- Goldstein BD. 2011. Hematological and toxicological evaluation of formaldehyde as a potential cause of human leukemia. Hum Exp Toxicol 30:725–735. [DOI] [PubMed] [Google Scholar]
- Groscurth P, Eggli P, Kapfhammer J, Rager G, Hornung JP, Fasel JD. 2001. Gross anatomy in the surgical curriculum in Switzerland: Improved cadaver preservation, anatomical models, and course development. Anat Rec 265:254–256. [DOI] [PubMed] [Google Scholar]
- Hammer N, Hepp P, Löffler S, Schleifenbaum S, Steinke H, Klima S. 2015a. Teaching surgical exposures to undergraduate medical students: An integration concept for anatomical and surgical education. Arch Orthop Trauma Surg 135:795–803. [DOI] [PubMed] [Google Scholar]
- Hammer N, Löffler S, Bechmann I, Steinke H, Hädrich C, Feja C. 2015b. Comparison of modified Thiel embalming and ethanol‐glycerin fixation in an anatomy environment: Potentials and limitations of two complementary techniques. Anat Sci Educ 8:74–85. [DOI] [PubMed] [Google Scholar]
- Hammer N, Löffler S, Feja C, Bechmann I, Steinke H. 2011. Substitution of formaldehyde in cross anatomy is possible. J Natl Cancer Inst 103:610–611. [DOI] [PubMed] [Google Scholar]
- Hammer N, Löffler S, Feja C, Sandrock M, Schmidt W, Bechmann I, Steinke H. 2012. Ethanol‐glycerin fixation with thymol conservation: A potential alternative to formaldehyde and phenol embalming. Anat Sci Educ 5:225–233. [DOI] [PubMed] [Google Scholar]
- Hammer N, Schröder C, Schleifenbaum S. 2016. On the suitability of Thiel‐fixed samples for biomechanical purposes: Critical considerations on the articles of Liao et al. “Elastic properties of thiel‐embalmed human ankle tendon and ligament” and Verstraete et al. “Impact of drying and Thiel embalming on mechanical properties of Achilles tendons”. Clin Anat 29:424–425. [DOI] [PubMed] [Google Scholar]
- Hammer N, Voigt C, Werner M, Hoffmann F, Bente K, Kunze H, Scholz R, Steinke H. 2014. Ethanol and formaldehyde fixation irreversibly alter bones' organic matrix. J Mech Behav Biomed Mater 29:252–258. [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am J Epidemiol 159:1117–1130. [DOI] [PubMed] [Google Scholar]
- Hauptmann M, Stewart PA, Lubin JH, Beane Freeman LE, Hornung RW, Herrick RF, Hoover RN, Fraumeni JF Jr, Blair A, Hayes RB. 2009. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Canc Inst 101:1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RB, Blair A, Stewart PA, Herrick RF, Mahar H. 1990. Mortality of U.S. embalmers and funeral directors. Am J Ind Med 18:641–652. [DOI] [PubMed] [Google Scholar]
- Hester SD, Benavides GB, Yoon L, Morgan KT, Zou F, Barry W, Wolf DC. 2003. Formaldehyde‐induced gene expression in F344 rat nasal respiratory epithelium. Toxicology 187:13–24. [DOI] [PubMed] [Google Scholar]
- Hohmann E, Keough N, Glatt V, Tetsworth K, Putz R, Imhoff A. 2019. The mechanical properties of fresh versus fresh‐frozen and preserved (Thiel and formalin) long head of biceps tendons: A cadaveric investigation. Ann Anat 221:186–191. [DOI] [PubMed] [Google Scholar]
- Horev L, Isaksson M, Engfeldt M, Persson L, Ingber A, Bruze M. 2015. Preservatives in cosmetics in the Israeli market conform well to the EU legislation. J Eur Acad Dermatol Venereol 29:761–766. [DOI] [PubMed] [Google Scholar]
- Human Tissue Act . 2008. Public Act No 28. Wellington, New Zealand: New Zealand Government; 74 p. URL: http://www.legislation.govt.nz/act/public/2008/0028/latest/DLM1152940.html [accessed 11 November 2019]. [Google Scholar]
- ILO . 2019. International Labour Organization. Ethylene glycol monophenyl ether: ICSC 0538. United Nations, International Labour Organization, Geneva, Switzerland: URL: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0538 [accessed 19 October 2019]. [Google Scholar]
- Jaung R, Cook P, Blyth P. 2011. A comparison of embalming fluids for use in surgical workshops. Clin Anat 24:155–161. [DOI] [PubMed] [Google Scholar]
- Kennel L, Martin DMA, Shaw H, Wilkinson T. 2018. Learning anatomy through Thiel‐ vs. formalin‐embalmed cadavers: Student perceptions of embalming methods and effect on functional anatomy knowledge. Anat Sci Educ 11:166–174. [DOI] [PubMed] [Google Scholar]
- Klima S, Hepp P, Loffler S, Cornwall J, Hammer N. 2017. A novel phased‐concept course for the delivery of anatomy and orthopedics training in medical education. Anat Sci Educ 10:372–382. [DOI] [PubMed] [Google Scholar]
- Land Transport Act . 1998. Public Act 1998 No 110. Parliamentary Counsel Office, New Zealand Legislation, Wellington, New Zealand: URL: http://www.legislation.govt.nz/act/public/1998/0110/166.0/DLM433613.html [accessed 27 December 2019]. [Google Scholar]
- Lewis RJ Sr. 2007. Hawley's Condensed Chemical Dictionary. 15th Ed. New York, NY: John Wiley & Sons, Inc; 530 p. [Google Scholar]
- Loftspring MC, Smanik J, Gardner C, Pixley SK. 2008. Selective gray matter staining of human brain slices: Optimized use of cadaver materials. Biotech Histochem 83:173–177. [DOI] [PubMed] [Google Scholar]
- Lowe I, Southern J. 1994. The antimicrobial activity of phenoxyethanol in vaccines. Lett Appl Microbiol 18:115–116. [DOI] [PubMed] [Google Scholar]
- Lozano PF, Scholze M, Babian C, Scheidt H, Vielmuth F, Waschke J, Ondruschka B, Hammer N. 2019. Water‐content related alterations in macro and micro scale tendon biomechanics. Sci Rep 9:7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceaux JC, Dilks LS, Hixson S. 2008. Neuropsychological effects of formaldehyde use. J Psychoactive Drugs 40:207–210. [DOI] [PubMed] [Google Scholar]
- Messmer C, Kellogg RT, Zhang Y, Baiak A, Leiweke C, Marcus JR, Levin LS, Zenn MR, Erdmann D. 2010. A technique to perfuse cadavers that extends the useful life of fresh tissues: The Duke experience. Anat Sci Educ 3:191–194. [DOI] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Castranova V, Kommineni C, Gunther MR, Shvedova AA. 2007. Phenol‐induced in vivo oxidative stress in skin: Evidence for enhanced free radical generation, thiol oxidation, and antioxidant depletion. Chem Res Toxicol 20:1769–1777. [DOI] [PubMed] [Google Scholar]
- Muzi G, dell'Omo M, Murgia N, Abbritti G. 2004. Chemical pollution of indoor air and its effects on health. G Ital Med Lav Ergon 26:364–369. [PubMed] [Google Scholar]
- National Toxicology Program . 2010. Final report on carcinogens background document for formaldehyde. Rep Carcinog Backgr Doc 2010:i‐512. [PubMed] [Google Scholar]
- Nicholson HD, Samalia L, Gould M, Hurst PR, Woodroffe M. 2005. A comparison of different embalming fluids on the quality of histological preservation in human cadavers. Eur J Morphol 42:178–184. [DOI] [PubMed] [Google Scholar]
- NIH . 2011. National Institutes of Health. Formaldehyde and Cancer Risk. National Cancer Institute at the National Institutes of Health, Bethesda, MD: URL: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/formaldehyde/formaldehyde-fact-sheet [accessed 19 October 2019]. [Google Scholar]
- Ochs M, Mühlfeld C, Schmiedl A. 2012. Präparierkurs: Grundlage ärztlichen Handelns. Dtsch Arztebl 109:2126–2127. [Google Scholar]
- Ohmichi K, Komiyama M, Matsuno Y, Takanashi Y, Miyamoto H, Kadota T, Maekawa M, Toyama Y, Tatsugi Y, Kohno T, Ohmichi M, Mori C. 2006. Formaldehyde exposure in a gross anatomy laboratory. Personal exposure level is higher than indoor concentration (5 pp). Environ Sci Pollut Res 13:120–124. [DOI] [PubMed] [Google Scholar]
- Owen G, Steedman HF. 1956. Preservation of animal tissues, with a note on staining solutions. J Cell Sci 97:319–321. [Google Scholar]
- Owen G, Steedman HF. 1958. Preservation of molluscs. J Molluscs Stud 33:101–103. [Google Scholar]
- O'Neil MJ. (Editor). 2006. The Merck Index An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th Ed. Whitehouse Station, NJ: Merck and Co., Inc; 2564 p. [Google Scholar]
- Pabst R. 2009. Anatomy curriculum for medical students: What can be learned for future curricula from evaluations and questionnaires completed by students, anatomists and clinicians in different countries? Ann Anat 191:541–546. [DOI] [PubMed] [Google Scholar]
- Rae G, Newman WP, McGoey R, Donthamsetty S, Karprinski AC, Green J. 2018. The histopathologic reliability of tissue taken from cadavers within the gross anatomy laboratory. Anat Sci Educ 11:207–214. [DOI] [PubMed] [Google Scholar]
- Romeis B. 1989. Mikroskopische Technik. 17th Ed. München, Germany: Urban & Schwarzenberg; p 556. [Google Scholar]
- Rumph PF, Williams JC. 1988. Efficiency of phenoxyethanol at removing formaldehyde from immersion fixed muscle tissue. Anat Histol Embryol 17:226–231. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Burroughs GE, Taylor K, Kovein RJ. 2003. Video exposure assessments demonstrate excessive laboratory formaldehyde exposures. Appl Occup Environ Hyg 18:450–457. [DOI] [PubMed] [Google Scholar]
- Scholze M, Singh A, Lozano PF, Ondruschka B, Ramezani M, Werner M, Hammer N. 2018. Utilization of 3D printing technology to facilitate and standardize soft tissue testing. Sci Rep 8:11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke H, Lingslebe U, Bohme J, Slowik V, Shim V, Hädrich C, Hammer N. 2012. Deformation behavior of the iliotibial tract under different states of fixation. Med Eng Phys 34:1221–1227. [DOI] [PubMed] [Google Scholar]
- Stewart R, Kieser DC, Scholze M, Hammer N, Stone B, Hooper G. 2018. Preliminary biomechanical results of a novel pin configuration for external fixation of vertical shear pelvic fractures. ANZ J Surg 88:1051–1055. [DOI] [PubMed] [Google Scholar]
- Stringer MD, Lyall P. 2012. Design, implementation, and evaluation of a postgraduate diploma in surgical anatomy. Anat Sci Educ 5:48–54. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tsuji K, Fujii K, Okazaki F, Takigawa T, Ohtsuka A, Iwatsuki K. 2007. Prospective study of clinical symptoms and skin test reactions in medical students exposed to formaldehyde gas. J Dermatol 34:283–289. [DOI] [PubMed] [Google Scholar]
- Tandon A, Bhatnagar R, Pokhrel R, Solanke K. 2014. Phenoxetol as a formaldehyde‐removing agent for long‐term preservation: Our experience. Eur J Anat 18:267–272. [Google Scholar]
- Thiel W. 1992. Die Konservierung ganzer Leichen in natürlichen Farben. Ann Anat 174:185–195. [PubMed] [Google Scholar]
- Thullner I, Stockmann R, Hohenberger L. 2015. Formaldehyd in der vorklinischen medizinischen Ausbildung (Anatomie). Gefahrenstoffe, Reinhaltung der Luft 76:219–228. URL: https://www.dguv.de/medien/ifa/de/pub/grl/pdf/2016_123.pdf [accessed 31 May 2019]. [Google Scholar]
- Tomlinson JE, Yiasemidou M, Watts AL, Roberts DJ, Timothy J. 2016. Cadaveric spinal surgery simulation: A comparison of cadaver types. Global Spine J 6:357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxnet . 2019. Toxicology Data Network. HSDB: 2‐Phenoxyethanol. U.S. National Library of Medicine, Bethesda, MD: URL: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+122-99-6 [accessed 19 October 2019]. [Google Scholar]
- Trowbridge S, Vidakovic H, Hammer N, Kieser DC. 2017. A case of anomalous flexor carpi radialis brevis muscle and its clinical significance. Int J Anat Var 10:91–93. [Google Scholar]
- Viegas S, Ladeira C, Nunes C, Malta‐Vacas J, Gomes M, Brito M, Mendonca P, Prista J. 2010. Genotoxic effects in occupational exposure to formaldehyde: A study in anatomy and pathology laboratories and formaldehyde‐resins production. J Occup Med Toxicol 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimercati L, Carrus A, Martino T, Galise I, Minunni V, Caputo F, Dell'erba A, Assennato G. 2010. Formaldehyde exposure and irritative effects on medical examiners, pathologic anatomy post‐graduate students and technicians. Iran J Public Health 39:26–34. [PMC free article] [PubMed] [Google Scholar]
- Waschke J, Bergmann M, Bräuer L, Brenner E, Buchhorn A, Deutsch A, Dokter M, Egu DT, Ergun S, Fassnacht U, Fietz D, Gundlach S, Heermann S, Hirt B, Kugelmann D, Müller‐Gerbl M, Neiss W, Nimtschke U, Plendl J, Pretterklieber M, Redies C, Scaal M, Schmidt MH, Schmiedl A, Schnittler HJ, Schomerus C, Sebestény T, Spittau B, Steiniger B, Tschernig T, Unverzagt A, Viebahn C, Voigt E, Weigner J, Weyers I, Winkelmann A, Winkler M, Paulsen F. 2019. Recommendations of the working group of the Anatomische Gesellschaft on reduction of formaldehyde exposure in anatomical curricula and institutes. Ann Anat 221:179–185. [DOI] [PubMed] [Google Scholar]
- Wedel T, Ackermann J, Hagedorn H, Mettler L, Maass N, Alkatout I. 2019. Educational training in laparoscopic gynecological surgery based on ethanol‐glycerol‐lysoformin‐preserved body donors. Ann Anat 221:157–164. [DOI] [PubMed] [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H. 2000. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol 24:1016–1019. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Savoia MC. 2008. Evaluation of methods to reduce formaldehyde levels of cadavers in the dissection laboratory. Clin Anat 21:75–81. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Werner K, Häussler K, Reinehr M, Böckers TM. 2011. Thiel‐fixation preserves the non‐linear load–deformation characteristic of spinal motion segments, but increases their flexibility. J Mech Behav Biomed Mater 4:2133–2137. [DOI] [PubMed] [Google Scholar]
- Wineski LE, English AW. 1989. Phenoxyethanol as a nontoxic preservative in the dissection laboratory. Acta Anat (Basel) 136:155–158. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Nielsen GD. 2010. Non‐cancer effects of formaldehyde and relevance for setting an indoor air guideline. Environ Int 36:788–799. [DOI] [PubMed] [Google Scholar]
- WorkSafe . 2018. Workplace Exposure Standards and Biological Exposure Indices. 10th Ed. Wellington, New Zealand: New Zealand Government, WorkSafe New Zealand; 68 p. URL: https://worksafe.govt.nz/dmsdocument/923-workplace-exposure-standards-and-biological-exposure-indices [accessed 31 May 2019]. [Google Scholar]
- Yiasemidou M, Roberts D, Glassman D, Tomlinson J, Biyani S, Miskovic D. 2017. A multispecialty evaluation of Thiel cadavers for surgical training. World J Surg 41:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirner J, Scholze M, Ondruschka B, Hammer N. 2019. Tissue biomechanics of the human head are altered by Thiel embalming, restricting its use for biomechanical validation. Clin Anat 32:903–913. [DOI] [PubMed] [Google Scholar]


