Summary
Background
Scabies is a common parasitic skin condition that causes considerable morbidity globally. Clinical and epidemiological research for scabies has been limited by a lack of standardization of diagnostic methods.
Objectives
To develop consensus criteria for the diagnosis of common scabies that could be implemented in a variety of settings.
Methods
Consensus diagnostic criteria were developed through a Delphi study with international experts. Detailed recommendations were collected from the expert panel to define the criteria features and guide their implementation. These comments were then combined with a comprehensive review of the available literature and the opinion of an expanded group of international experts to develop detailed, evidence‐based definitions and diagnostic methods.
Results
The 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies include three levels of diagnostic certainty and eight subcategories. Confirmed scabies (level A) requires direct visualization of the mite or its products. Clinical scabies (level B) and suspected scabies (level C) rely on clinical assessment of signs and symptoms. Evidence‐based, consensus methods for microscopy, visualization and clinical symptoms and signs were developed, along with a media library.
Conclusions
The 2020 IACS Criteria represent a pragmatic yet robust set of diagnostic features and methods. The criteria may be implemented in a range of research, public health and clinical settings by selecting the appropriate diagnostic levels and subcategories. These criteria may provide greater consistency and standardization for scabies diagnosis. Validation studies, development of training materials and development of survey methods are now required.
What is already known about this topic?
The diagnosis of scabies is limited by the lack of accurate, objective tests. Microscopy of skin scrapings can confirm the diagnosis, but it is insensitive, invasive and often impractical.
Diagnosis usually relies on clinical assessment, although visualization using dermoscopy is becoming increasingly common.
These diagnostic methods have not been standardized, hampering the interpretation of findings from clinical research and epidemiological surveys, and the development of scabies control strategies.
What does this study add?
International consensus diagnostic criteria for common scabies were developed through a Delphi study with global experts.
The 2020 International Alliance for the Control of Scabies (IACS) Criteria categorize diagnosis at three levels of diagnostic certainty (confirmed, clinical and suspected scabies) and eight subcategories, and can be adapted to a range of research and public health settings.
Detailed definitions and figures are included to aid training and implementation. The 2020 IACS Criteria may facilitate the standardization of scabies diagnosis.
Short abstract
What is already known about this topic?
The diagnosis of scabies is limited by the lack of accurate, objective tests. Microscopy of skin scrapings can confirm the diagnosis, but it is insensitive, invasive and often impractical.
Diagnosis usually relies on clinical assessment, although visualization using dermoscopy is becoming increasingly common.
These diagnostic methods have not been standardized, hampering the interpretation of findings from clinical research and epidemiological surveys, and the development of scabies control strategies.
What does this study add?
International consensus diagnostic criteria for common scabies were developed through a Delphi study with global experts.
The 2020 International Alliance for the Control of Scabies (IACS) Criteria categorize diagnosis at three levels of diagnostic certainty (confirmed, clinical and suspected scabies) and eight subcategories, and can be adapted to a range of research and public health settings.
Detailed definitions and figures are included to aid training and implementation. The 2020 IACS Criteria may facilitate the standardization of scabies diagnosis.
1.
Scabies is a contagious skin disease caused by Sarcoptes scabiei var. hominis, a human‐specific ectoparasite of approximately 0·4 mm in size that is invisible to the naked eye.1, 2 Scabies is estimated to affect around 150–200 million people globally3 with an estimated 455 million annual incident cases,4 although the accuracy of these estimates is limited by a paucity of epidemiological data.5 Scabies infestation exists in all countries, but with a higher burden in low‐income settings and tropical areas, and among infants, children and adolescents.6 Outbreaks are common in institutions and enclosed communities in both high‐income and low‐income settings, particularly where crowding occurs. Outbreaks impose considerable health and economic burden, and are often difficult to control.7, 8
Scabies causes a rash, which may cause stigma, as well as itch that can lead to sleep disruption, difficulty with concentration and absenteeism from education and employment. Scabies predisposes to superficial bacterial skin infection (due mainly to Staphylococcus aureus and Streptococcus pyogenes),9 which in turn can lead to serious complications including severe skin and soft‐tissue infections, sepsis, glomerulonephritis and likely acute rheumatic fever.10, 11 Although the immune response is incompletely understood, infestation does not confer complete immunity and protection on further exposure.12 Therefore, recurrent episodes, especially in children, are common in areas of high transmission.13, 14
The course of a scabies infestation begins when a fertilized female mite burrows into the skin of an uninfected individual. Following primary infestation, individuals are usually asymptomatic for the incubation period of 4–6 weeks. Symptoms develop much more rapidly (hours to days) with subsequent infestations.15 Itch and skin lesions, most commonly small scattered papules, often with excoriation, develop as a result of hypersensitivity to mites and their products.1 Burrows may be found in some, but not all, cases. This pattern of symptoms and signs is known as ‘common scabies’ (also described as classical, typical, ordinary, standard, usual or normal scabies). In the most obvious cases, scabies may be readily recognized based on clinical presentation.16, 17 However, scabies can manifest with a wide spectrum of clinical signs and variable severities, making clinical diagnosis challenging.
Current approaches to the diagnosis of common scabies, including clinical assessment and laboratory tests, have been assessed in two systematic reviews.18, 19 These reviews identified the inconsistent and varied approaches to diagnosis, and the absence of a gold standard. Scabies can be confirmed by microscopy of skin scrapings; however, this method has low sensitivity20, 21 and requires specialized equipment, operator training and time, making it unsuitable for use in the low‐income settings where the highest scabies burden persists.22
The need for standardized diagnostic criteria
The World Health Organization now includes scabies within the portfolio of neglected tropical diseases, and is working with global and local partners, such as the International Alliance for the Control of Scabies (IACS), to devise effective strategies for population‐level control.10, 23, 24 These strategies require a greater understanding of the global and regional epidemiology of the disease. Therefore, standardized diagnostic case definitions and validated methods for undertaking prevalence surveys are a high priority.10, 25
The development of simplified, standardized diagnostic methods has enabled the successful mapping and surveillance of other neglected tropical diseases, thereby progressing the control and elimination agendas for those diseases.26 Therefore, the IACS led a project to develop consensus criteria for the diagnosis of common scabies that could be implemented in a variety of clinical, research and public health settings.
Development and intent of standardized criteria
We conducted a modified Delphi study of 34 international experts to develop the 2020 IACS Consensus Criteria for the Diagnosis of Scabies, herein referred to as the 2020 IACS Criteria (Table 1).27 In this current report, we aimed to develop detailed, evidence‐based definitions and diagnostic methods to support the adoption and implementation of the 2020 IACS Criteria (detailed methods are included in Appendix S1; see Supporting Information).
Table 1.
Summary of the 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies
| A. Confirmed scabies |
| At least one of: |
| A1: Mites, eggs or faeces on light microscopy of skin samples |
| A2: Mites, eggs or faeces visualized on an individual using a high‐powered imaging device |
| A3: Mite visualized on an individual using dermoscopy |
| B. Clinical scabies |
| At least one of: |
| B1: Scabies burrows |
| B2: Typical lesions affecting male genitalia |
| B3: Typical lesions in a typical distribution and two history features |
| C. Suspected scabies |
| One of: |
| C1: Typical lesions in a typical distribution and one history feature |
| C2: Atypical lesions or atypical distribution and two history features |
| History features |
| H1: Itch |
| H2: Positive contact history |
Diagnosis can be made at one of the three levels (A, B or C). A diagnosis of clinical or suspected scabies should only be made if other differential diagnoses are considered less likely than scabies.
The 2020 IACS Criteria are intended to standardize the diagnosis of common scabies, and therefore to facilitate communication and comparison of epidemiological and clinical findings. These criteria may also provide a basis for the development of teaching and training tools for scabies diagnosis.28 The 2020 IACS Criteria are not intended for use in the diagnosis of variant or atypical presentations of scabies, such as crusted scabies; bullous scabies;29, 30 scabies in immunocompromised individuals; or scabies in elderly, cognitively impaired or bedridden individuals.7, 31, 32, 33 Previously administered treatments, including topical or systemic corticosteroids, often modify the symptoms and signs and confound diagnosis.34, 35 These criteria are intended for initial diagnosis rather than assessment of the resolution of infestation or outcome of treatment. The criteria are not intended to replace the judgement of experienced clinicians, nor to be used to determine who should be treated with antiscabetic medications. The target audiences of the guidelines are clinicians, educators, and research and public health professionals.
Diagnostic criteria
The 2020 IACS Criteria comprise three levels, representing degrees of diagnostic certainty. Confirmed scabies (level A) is the most specific level, requiring direct visualization of the mite or its products. Clinical scabies (level B) and suspected scabies (level C) are expected to be more sensitive but less specific, relying on clinical assessment of signs and symptoms. Each level may be appropriate to use in making the diagnosis of scabies, depending on the clinical, public health or research setting. For example, level A diagnoses might be used for clinical trials of new therapeutics, but are unlikely to be practical to implement in most field studies. Levels B and C may be most appropriate for clinical settings and field surveys, particularly in low‐income settings. We recommend that research studies and surveys report breakdown of diagnoses by level and subcategory (e.g. A1, B3, etc.; Table 1) to allow clearer interpretation of the clinical syndromes described.
Level A: confirmed scabies
The diagnosis of confirmed scabies can be made through identification of the scabies mite (adult or immature stages), eggs (ova) or faecal pellets (scybala).19, 36 This can be achieved through definitive visualization of: (i) the mite or mite products through microscopic examination of skin samples (subcategory A1); (ii) the mite or mite products using noninvasive, high‐magnification devices (A2); or (iii) the mite using dermoscopy (A3).
A1. Microscopy
The most well‐established approach to confirming the diagnosis of scabies is to visualize mites, eggs or faecal pellets through optical (light) microscopy of material taken from lesional skin (Figure 1).37 The accuracy of microscopy depends on the expertise of the operator, particularly in finding burrows and extracting the relevant material (Table 2). While a positive test confirms the diagnosis of scabies, a negative test does not exclude it, as microscopy is frequently negative in patients with clinically diagnosed scabies.18, 21 Detailed methods for microscopy are provided in Appendix S2 (see Supporting Information).
Figure 1.
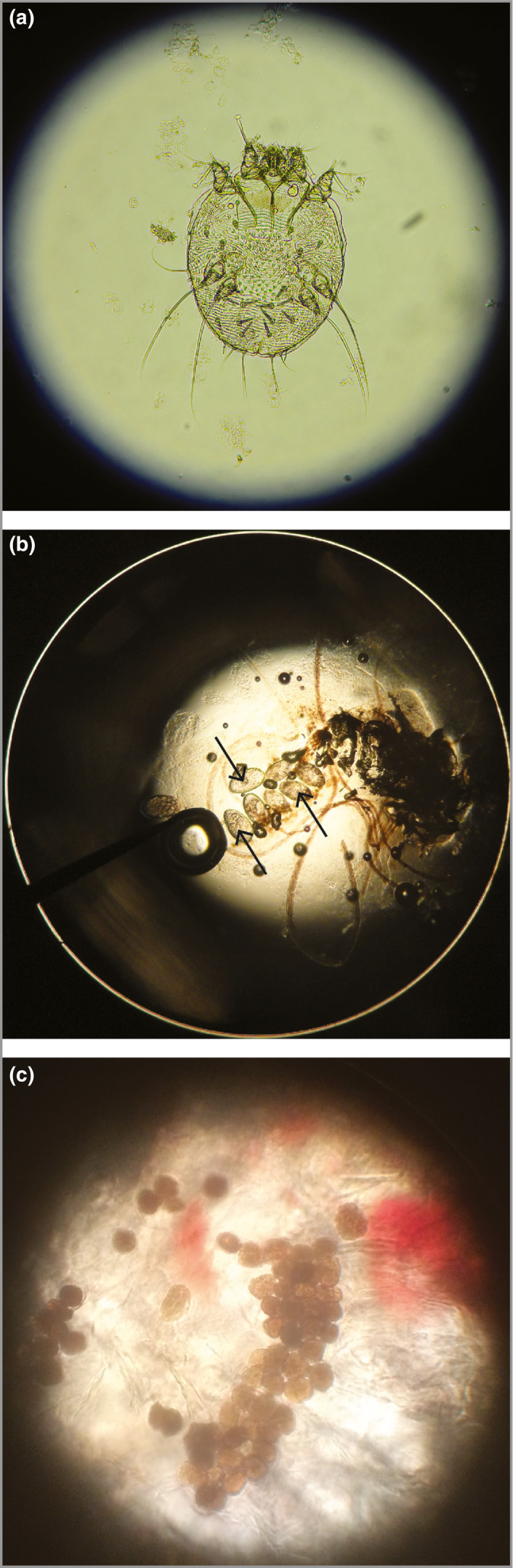
Optical microscopy of skin scrapings for diagnosis of scabies. (a) Female scabies mite, magnification × 200. (b) Eggs of a scabies mite, magnification × 200. (c) Faecal pellets (scybala) are seen as small oval structures, magnification × 400.
Table 2.
Visualization methods for scabies
| Technique | Advantages | Disadvantages |
|---|---|---|
| Optical microscopy (standard, ×4–400) |
|
|
| Videodermoscopy (×70–1000) |
|
|
| Low‐cost videomicroscopy (×70–1000) |
|
|
| Reflectance confocal microscopy (×30–400) |
|
|
| Handheld dermoscopy (×10) |
|
|
Approximate costs are provided in US dollars.
A2. High‐powered imaging
High‐powered imaging devices are tools that allow noninvasive, detailed visualization of scabies mites in vivo. These devices allow magnification of at least × 70 (often much higher) and include videodermoscopy, low‐cost videomicroscopy and reflectance confocal microscopy (Table 2 and Figure 2c, d).38 Further details are provided in Appendix S3 (see Supporting Information).
Figure 2.
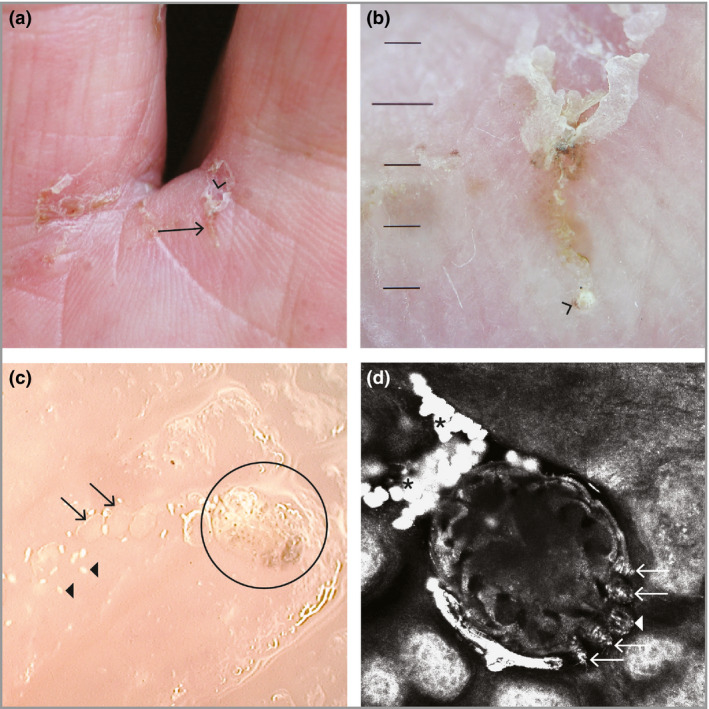
Direct visualization of a scabies mite. (a) Scabies burrow on the finger web space (arrow), visible with the naked eye. The V‐shaped scale (‘wake sign’) is visible at the top (arrowhead). (b) Visualization of the scabies burrow from (a) using dry dermoscopy (magnification × 10). The open portion of the ‘V’ points to the intact entrance of the burrow. The female scabies mite is seen at the distal end of the burrow as a brown triangular spot (arrowhead). (c) Videodermoscopy image of a burrow (magnification × 200). The oval body of the female scabies mite (circle), its eggs (arrows) and its faecal pellets (arrowheads) are visible. (d) In vivo reflectance confocal microscopy image (field of view 0·75 × 0·75 mm) of the female mite. The oval body is visible within the epidermis (upper stratum granulosum), along with its head (arrowhead), anterior legs (arrows) and faecal pellets (asterisks).
A3. Dermoscopy
Dermoscopy can confirm the diagnosis of scabies through the identification of mites (Figure 2).21, 39, 40 To meet the diagnostic level of confirmed scabies (subcategory A3), a mite must be definitively visualized. If only a burrow is visualized, the diagnosis of clinical scabies can be made (subcategory B1, see below). Detailed methods for dermoscopy are provided in Appendix S4 (see Supporting Information).
Other tests
Several diagnostic techniques that do not require visualization of the mite are under investigation, but none are currently available for routine use in humans.41 Examples of these methods include polymerase chain reaction, matrix‐assisted laser desorption/ionization–time of flight mass spectroscopy, and an antigen detection system.42, 43, 44, 45, 46, 47 Well‐designed, well‐conducted studies are needed to determine the accuracy and utility of these methods.
Levels B and C: clinical and suspected scabies
The diagnosis of clinical scabies (level B) or suspected scabies (level C) is reliant on clinical assessment, including features of the patient's history and skin examination. Where these features meet the criteria considered to be adequately specific for scabies, a diagnosis of clinical scabies can be made. Where these features are less specific, a diagnosis of suspected scabies can be made.
Patients should be examined with adequate lighting. Ideally, as much of the individual's body surface as possible should be examined. In infants, the entire body surface, including the head, may be involved. In immunocompetent adults, it is advisable to examine the scalp in those reporting scalp itch, even though scalp involvement is uncommon. In some circumstances it may not be appropriate to examine sensitive areas such as external genitalia and breasts due to issues of personal and cultural modesty. Where full examination is not feasible, for instance in field surveys, examination of all four limbs may suffice as a minimal level of exposure.48 Arms should be examined from at least the mid‐upper arm to the fingertips, and legs should be examined from at least the mid‐thigh to toes. In such cases, the diagnostic assessment would not include subcategory B2 (male genital lesions). It would be expected that the vast majority of cases diagnosed by clinical assessment, especially in field survey settings, would be classified as subcategories B3 or C1 (typical features), with far fewer classified as B1 (burrows) or C2 (atypical features).
History features
H1. Itch
Itch (pruritus) is a very common feature of scabies, but is not universal.40, 49, 50, 51, 52, 53, 54 Individuals typically do not develop itch for 4–6 weeks after initial infestation.1 Individuals with cognitive impairment are less likely to report itch.7, 55 For some, itch is extremely severe and profoundly affects quality of life, whereas for others itch may be a minor complaint.54, 56, 57 Itch is typically described as more severe at night,58, 59 although this pattern is shared by many pruritic conditions and is not useful for diagnosis.57, 60 Itch may be localized to the site of visible scabies lesions, or generalized to other body parts. Itch may be attenuated (e.g. by corticosteroids) or exacerbated (e.g. by benzyl benzoate) by treatments.
In order to fulfil this criterion, an individual (or carer) may report either generalized or localized itch. Signs of excoriations and behaviours including scratching affected skin, particularly in children, also meet the definition. Itch that is considered to be more likely due to another cause (e.g. localized to arthropod bites)61 does not fulfil this criterion.
H2. Contact history
Scabies is transmitted by skin‐to‐skin contact.20 Transmission via fomites such as clothing or bedding is rare for common scabies, but may occur with crusted scabies.62 The risk of transmission for common scabies is related to the frequency, duration and surface area of skin contact,63 and therefore is highest among people sharing the same bed and between young children and those who carry them. The minimum duration of skin contact required to transmit mites has been estimated to be 20 min,64, 65 but it remains largely unknown. In practice, a positive contact history may be defined as per the definitions in Table 3.28, 66 Gathering a history of scabies in an individual's contacts may be aided by showing photos of typical scabies lesions (Figure 3).
Table 3.
Definitions for contact history for scabies transmission
| Positive contact history: all of the following are considered high risk for scabies transmission– |
| Any contact with an individual diagnosed with crusted scabies |
| Close contact with an individual diagnosed with scabies |
| Close contact with an individual with itch that is not accounted for by another condition |
| Close contact with an individual with typical scabies lesions in a typical distribution that are not accounted for by another condition. |
| Close contacts are defined as any of: |
| Individuals who sleep in the same dwelling |
| Individuals who share a bed (including sexual partners) |
| Children in the same classroom or who play closely together |
| Adults with known skin‐to‐skin contacta |
Examples of skin‐to‐skin exposures include occupational exposures (healthcare workers, residential care workers, carers and educators of children) and recreational exposures (e.g. contact sports such as wrestling).
Figure 3.
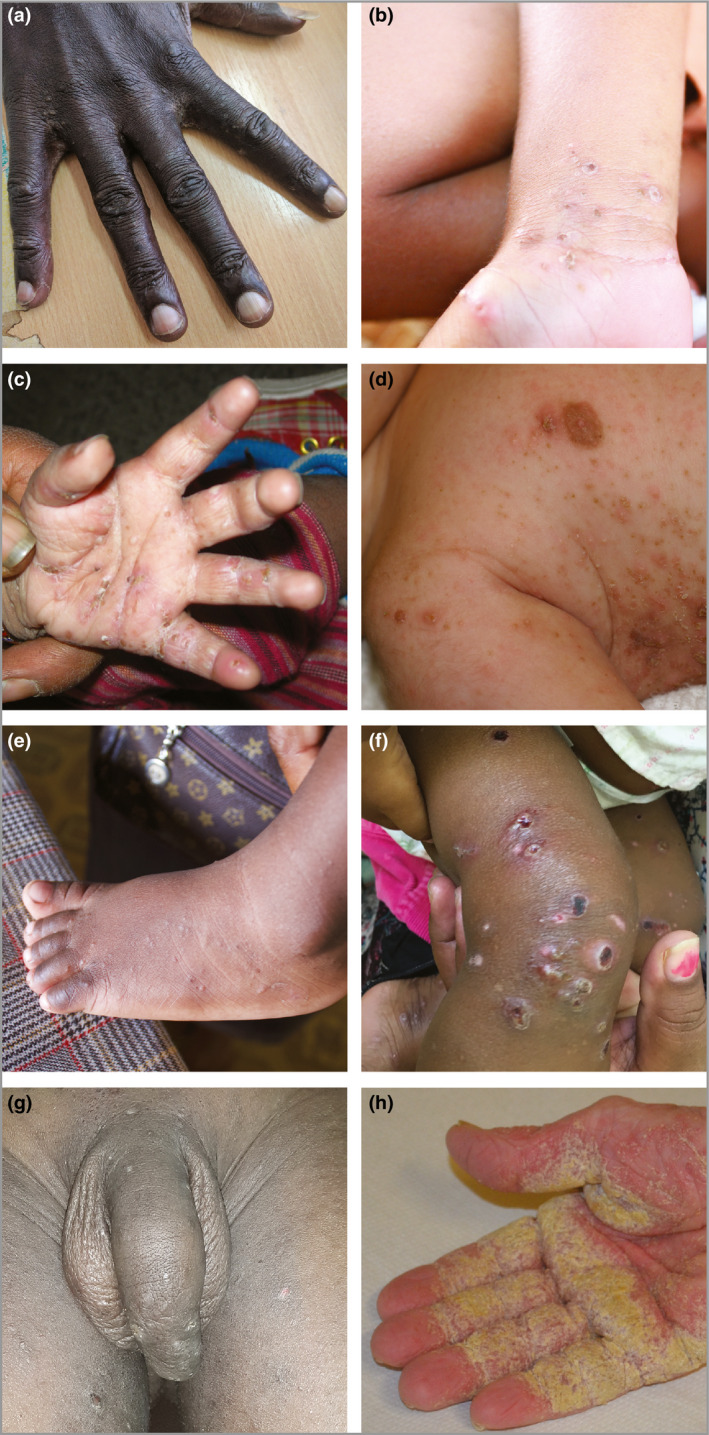
Skin examination findings of scabies. (a) Papules over the fingers, finger web spaces and back of hand of an adult. (b) Papules and vesicles with excoriation on the volar wrist of a child. (c) Papules, vesicles and pustules with excoriations over the palm and fingers of an infant. (d) Widespread scabies rash in an infant. Larger nodules are seen on the torso, axilla and shoulder. (e) Papules over the toes, feet and ankle of an infant. (f) Ulcers, pustules and crust representing impetiginization (secondary bacterial infection) of scabies lesions on the legs of a child. (g) Papules and nodules on the scrotum and penis. Lesions are also seen on the groin and inner thighs. (h) Crusted scabies with thick, yellowish scale of the right hand.
Examination features
1. Burrows
Burrows are highly specific signs of common scabies. However, burrows are not seen in many individuals with clinical scabies, so to meet this criterion the burrow must be definitively identified. A burrow is created by a fertilized female mite as she tunnels to the bottom layer of the stratum corneum (superficial layer of the skin), laying eggs behind her. The female mite favours certain areas of the skin, perhaps due to the presence of lipids, humidity, few hair follicles or other site‐specific factors.1, 67 The most common places to find burrows are the creases of the palms and soles, volar aspects of the wrists, ankles, between the fingers and toes, and the sides of the hands and feet. Burrows are seen less frequently in the genital region, breasts, buttocks and axillae.1, 68 Burrows may be harder to visualize in deeply pigmented skin. In individuals with darker skin types, it may be possible to appreciate burrows on paler skin areas such as the wrists, fingers, palms and soles.
Burrows are slightly raised, silvery‐grey, white or light brown, thread‐like lesions. They are short, ranging in length from about 3 to 7 mm, and approximately 0·4 mm wide.69, 70 They are linear and often curved or sinuous. To the naked eye, the superficial end (representing the original entrance of the mite) may be scaly in appearance and easier to see. The entrance may become ‘V’ shaped, sometimes described as the ‘wake sign’71 (Figure 2a, b). Excoriations, scratch marks and secondary bacterial infection may modify or mask the appearance of burrows,54 which may explain why burrows are uncommonly observed in tropical areas where secondary infection is common.20, 28, 58, 72
In addition to dermoscopy, the ‘ink test’ can assist the correct identification of scabies burrows. This involves rubbing the suspected burrow with ink from a fountain or surgical marking pen, then removing excess ink with an alcohol wipe. The tracking of ink into the stratum corneum can indicate the presence of a burrow.19, 73
2. Male genital lesions
Discrete papules or larger nodules found on the penis (shaft, corona, glans, prepuce) and/or scrotum are highly specific for scabies.34 Their surface may be smooth or rough depending on how vigorously the patient has been scratching, but are not scaly. Typically, several lesions are present at one time in an affected individual (Figure 3g).74, 75 These lesions are often very itchy, but in some individuals they may not be itchy at all. Genital lesions are reported to occur in up to 10–30% of male patients with scabies, but they may be more frequent in adult male individuals in temperate climates.58, 76
3. Typical lesions
The clinical signs of common scabies vary widely in terms of the appearance of individual lesions; appearance of grouped or clustered lesions; and severity and degree of secondary changes such as excoriation, impetiginization, eczematization and lichenification (thickening of skin due to scratching and rubbing). In the absence of the highly specific signs of burrows or male genital lesions, the decision whether or not other observed skin lesions are typical for scabies is a critical determination in diagnosing clinical scabies or suspected scabies. For lesions to be considered typical, examiners must consider both morphology and number.
In morphology, typical scabies lesions are small, elevated and easily palpated. The most common lesions are solid and 2–3 mm in diameter (papules).77 Larger, nodular lesions, usually 5–10 mm and occasionally > 10 mm, are more likely to be seen in certain body areas (groin and genitalia, buttocks, axillae, breasts of women and torsos of infants)78, 79 and may persist for several months, even after mites are successfully eradicated. The colour is usually erythematous (pink to red) but may be hyperpigmented in darker‐skinned individuals.
Vesicles (small, circumscribed, fluid‐filled lesions) and pustules (small, circumscribed, yellow or white lesions containing neutrophils) may also be present in infants, especially on the palms and soles (Figure 3c),80 but they are less commonly seen in adults. Diagnoses other than scabies should also be considered if the dominant lesions are vesicles, larger blisters or pustules (Table 4).
Table 4.
Differential diagnoses for scabies
| Differential diagnoses for typical lesions of common scabies | Differential diagnoses for specific scabies signs and variant presentations |
|---|---|
| Arthropod bites | Burrows |
| Atopic dermatitis | Cutaneous larva migrans |
| Avian mites | Larva currens |
| Contact dermatitis, irritant or allergic | Infantile scabies |
| Delusionary parasitosis | Infantile acropustulosis |
| Dermatitis herpetiformis | Urticaria pigmentosa |
| Dyshidrotic eczema (pompholyx) | Bullous scabies |
| Erythroderma (exfoliative eczema) | Bullous arthropod bites |
| Fiberglass dermatitis | Bullous drug eruptions |
| Folliculitis | Bullous impetigo |
| Impetigo | Bullous pemphigoid |
| Langerhans cell histiocytosis | Incontinentia pigmenti (inflammatory stage) |
| Lice: body and pubic | Pemphigus vulgaris |
| Lichen planus | Crusted scabies |
| Nummular (discoid) eczema | Atopic dermatitis |
| Molluscum contagiosum | Contact dermatitis |
| Mycosis fungoides | Darier disease |
| Onchocerciasis (acute and chronic papular onchodermatitis) | Erythrodermic mycosis fungoides or Sézary syndrome |
| Papular urticaria | Palmoplantar keratoderma |
| Pityriasis rosea | Pityriasis rubra pilaris |
| Prurigo nodularis | Psoriasis |
| Secondary syphilis | Seborrhoeic dermatitis |
| Tinea (corporis, manuum or pedis) | |
| Transient acantholytic dermatosis | |
| Verrucas (warts) | |
| Varicella zoster (chickenpox, shingles) | |
| Viral exanthems |
Lesions that are secondarily infected with S. aureus or S. pyogenes develop a different appearance. These impetiginized lesions may have signs of inflammation, with redness, ulceration, yellow crusting or scattered minute pustules. Multiple secondarily infected lesions may form larger lesions, and it may be difficult to discern the individual underlying scabies lesions (Figure 3f).
Lesions usually appear in groups, crops or clusters on the same body area (Figure 3a,b,e), but they may be widespread (Figure 3d). Uninfected lesions are discrete and scattered. The total number of observed lesions in common scabies can range from three to 10 in milder infestations, through to several hundred in severe infestations.81, 82 For scabies to be classified as typical, there should be at least three lesions on the same body area (e.g. left hand, right upper arm), or within an area of approximately 10–20 cm in diameter.
4. Atypical lesions
Lesions without typical morphology, or that number less than three in any body area, are classified as atypical lesions. Lesions with an appearance more suggestive of another condition should not be classified as either typical or atypical (see Differential diagnoses, below).
5. Typical distribution
In common scabies, lesions are found frequently in some body areas, and rarely in others (Figure 4). In many cases, multiple body surfaces are involved,16 particularly in more severe infestations, and the regions containing lesions are roughly symmetrical across the left and right sides of the body. The reasons for this distribution are incompletely understood, but are likely to involve local differences in the skin.29, 75 In contrast to the burrows, typical scabies lesions are caused by a hypersensitivity response to mite products, and possibly by temporary excavations made by immature mites.67, 68 Further details are provided in Appendix S5 (see Supporting Information).
Figure 4.

Typical distribution of scabies lesions. (a) Children aged > 2 years and adults. (b) Infants aged < 2 years.
6. Atypical distribution
Lesions are infrequently seen on the head, scalp and neck of older children and adults, and diagnoses other than common scabies should be considered. In elderly individuals, or those who are bedridden, the distribution may be asymmetrical and the neck, scalp and areas on which the individual has been lying may be involved.7
Variant presentations of scabies
Variant presentations of scabies will not be accurately diagnosed using the 2020 IACS Criteria. Their recognition is likely to be beyond the ability of clinical examiners other than dermatologists or experienced physicians. A brief description is included in Appendix S6 (see Supporting Information).
Differential diagnoses
The diagnosis of clinical or suspected scabies (levels B or C) requires that conditions other than scabies are considered less likely. Given the nonspecific nature of itch and the morphologically varied lesions of scabies, there are numerous differential diagnoses. A comprehensive list, including common and rare conditions, is shown in Table 4, and further detail is given in Appendix S7 (see Supporting Information). The ability to recognize these conditions will depend on the expertise of the examiner. Dermoscopy may also be useful for differentiating causes.
Future priorities
The development of the 2020 IACS Criteria is a major step towards standardizing the diagnosis of scabies. Materials for training examiners to use the 2020 IACS Criteria can now be developed to support diagnosis of common scabies. Training could be expanded also to include assessment of impetigo, which may be important to document during prevalence surveys. Broader training of other neglected tropical diseases and common skin conditions may be helpful for clinicians as these diseases frequently coexist.25, 83 Teledermatology has been successfully utilized and could be further developed for training and support of clinicians in remote areas.83, 84 In addition to the images shown in this manuscript, we have collated an online library of high‐quality images and videos, which may be useful for developing training materials (www.controlscabies.org/media-library). A concise summary of the 2020 IACS Criteria is provided as AppendixS8 (see Supporting Information).
Methods for conducting prevalence surveys now need to be developed and standardized, including selection of the target population (e.g. school‐attending children or community wide), sampling frame (e.g. cluster randomized or convenience) and appropriate level of diagnostic certainty (e.g. level A, B and/or C). Similarly, implementing standardized definitions and approaches to diagnosis would be helpful for clinical trials of scabies treatments. Additional criteria may need to be developed for diagnosis of scabies in the elderly and bed‐bound patients.7
The 2020 IACS Criteria now require evaluation and validation of diagnostic accuracy. Such research would ideally be conducted in a range of settings, with differing prevalences of scabies and other skin conditions, and different manifestations of scabies infestation, for example in areas with low and high levels of secondary impetiginization. The positive and negative predictive values of the 2020 IACS Criteria are likely to vary according to the local prevalences of scabies and other skin conditions. Assessing the diagnostic accuracy of different cadres of examiners, from dermatologists through to frontline health workers, is also required.
In parallel with efforts to standardize and improve diagnosis using existing tools, there is a need to develop further diagnostic tests for scabies.10 The 2020 IACS Criteria could be used as a reference standard for studies of new diagnostics. A point‐of‐care diagnostic test would be of great benefit, particularly for atypical cases and in areas where access to experienced clinicians is limited. The IACS Criteria will require updating to incorporate new evidence obtained through validation studies and when new diagnostic tests become available. We plan to undertake a review and update after 5 years (2025), or earlier if considered necessary based on progress in novel diagnostics. We believe the structure of the criteria allows for such updates.
Conclusions
The 2020 IACS Criteria represent a global attempt to develop a pragmatic, yet robust set of diagnostic features. It is hoped these criteria will provide greater consistency and standardization for scabies diagnosis in field and clinical settings.
Supporting information
Appendix S1 Development of standardized criteria.
Appendix S2 Microscopy for the diagnosis of scabies.
Appendix S3 High‐powered imaging for the diagnosis of scabies.
Appendix S4 Dermoscopy for the diagnosis of scabies.
Appendix S5 Typical distribution of scabies.
Appendix S6 Variant presentations of scabies.
Appendix S7 Differential diagnoses.
Appendix S8 Concise summary of the 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies.
Acknowledgments
We acknowledge the contributions of Erin Amerson, Raul Cabrera, Roberto Estrada, Giaconda Gaudiano, Jorg Heukelbach, Janet Hickman, Richard Keller, Arum Krismi, Stefania Lanza, Kieron Leslie, Harvey Liu and Fatimata Ly to the expert panel. Hilary Bruce assisted with the figures.
Funding sources D.E., A.C.B., L.R. and A.C.S. are supported by fellowships from the Australian National Health and Medical Research Council. The International League of Dermatological Societies supported the International Alliance for the Control of Scabies to undertake this work.
Conflicts of interest C.B. reports receiving research support from Bioderma Laboratoire Dermatologique and Codexial Dermatologie. W.R. has served on advisory boards for Cipher, Pfizer, LEO Pharma and Sanofi Genzyme in the past 2 years. All of the other authors declare no conflicts.
The author affiliations can be found in the Appendix.
References
- 1. Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology 2009; 35:197–206. [Google Scholar]
- 2. Heilesen B. Studies on Acarus scabiei and Scabies . Acta Derm Venereol 1946; 26:370. [Google Scholar]
- 3. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karimkhani C, Colombara DV, Drucker AM et al The global burden of scabies: a cross‐sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romani L, Steer AC, Whitfeld MJ et al Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis 2015; 15:960–7. [DOI] [PubMed] [Google Scholar]
- 7. Cassell JA, Middleton J, Nalabanda A et al Scabies outbreaks in ten care homes for elderly people: a prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect Dis 2018; 18:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mounsey KE, Murray HC, King M et al Retrospective analysis of institutional scabies outbreaks from 1984 to 2013: lessons learned and moving forward. Epidemiol Infect 2016; 144:2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swe PM, Reynolds SL, Fischer K. Parasitic scabies mites and associated bacteria joining forces against host complement defence. Parasite Immunol 2014; 36:585–93. [DOI] [PubMed] [Google Scholar]
- 10. Engelman D, Cantey PT, Marks M et al The public health control of scabies: priorities for research and action. Lancet 2019; 394:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis 2012; 25:145–53. [DOI] [PubMed] [Google Scholar]
- 12. Bhat SA, Mounsey KE, Liu X, Walton SF. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors 2017; 10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kearns T, Clucas D, Connors C et al Clinic attendances during the first 12 months of life for Aboriginal children in five remote communities of Northern Australia. PLOS ONE 2013; 8:e58231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMeniman E, Holden L, Kearns T et al Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australas J Dermatol 2011; 52:270–3. [DOI] [PubMed] [Google Scholar]
- 15. Pollack RJ, Engelman D, Steer AC et al Ectoparasites In: The International Encyclopedia of Public Health (Quah SR, Cockerham WC, eds), 2nd edn Oxford: Academic Press, 2017; 417–28. [Google Scholar]
- 16. Mahe A, Faye O, N'Diaye HT et al Definition of an algorithm for the management of common skin diseases at primary health care level in sub‐Saharan Africa. Trans R Soc Trop Med Hyg 2005; 99:39–47. [DOI] [PubMed] [Google Scholar]
- 17. Steer AC, Tikoduadua LV, Manalac EM et al Validation of an Integrated Management of Childhood Illness algorithm for managing common skin conditions in Fiji. Bull World Health Organ 2009; 87:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson MJ, Engelman D, Gholam K et al Systematic review of the diagnosis of scabies in therapeutic trials. Clin Exp Dermatol 2017; 42:481–7. [DOI] [PubMed] [Google Scholar]
- 19. Leung V, Miller M. Detection of scabies: a systematic review of diagnostic methods. Can J Infect Dis Med Microbiol 2011; 22:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev 2007; 20:268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walter B, Heukelbach J, Fengler G et al Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource‐poor setting. Arch Dermatol 2011; 147:468–73. [DOI] [PubMed] [Google Scholar]
- 22. Morgan MS, Rider SD Jr, Arlian LG. Identification of antigenic Sarcoptes scabiei proteins for use in a diagnostic test and of non‐antigenic proteins that may be immunomodulatory. PLOS Negl Trop Dis 2017; 11:e0005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Neglected tropical diseases. Available at: https://www.who.int/neglected_diseases/diseases/en (last accessed 18 February 2020).
- 24. Engelman D, Kiang K, Chosidow O et al Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies. PLOS Negl Trop Dis 2013; 7:e2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engelman D, Fuller LC, Solomon AW et al Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol 2016; 32:843–54. [DOI] [PubMed] [Google Scholar]
- 26. Solomon AW, Pavluck AL, Courtright P et al The Global Trachoma Mapping Project: methodology of a 34‐country population‐based study. Ophthalmic Epidemiol 2015; 22:214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engelman D, Fuller LC, Steer AC et al Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLOS Negl Trop Dis 2018; 12:e0006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osti MH, Sokana O, Gorae C et al The diagnosis of scabies by non‐expert examiners: a study of diagnostic accuracy. PLOS Negl Trop Dis 2019; 13:e0007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chosidow O. Scabies and pediculosis. Lancet 2000; 355:819–26. [DOI] [PubMed] [Google Scholar]
- 30. Luo ZY, Zeng M, Gao Q et al Case report: bullous scabies in two children below 10 years. Am J Trop Med Hyg 2017; 97:1746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shafi Dar M, Pandith AA, Sameer AS et al hs‐CRP: a potential marker for hypertension in Kashmiri population. Indian J Clin Biochem 2010; 25:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson MMG, Philpott CD, Breer WA. Atypical presentation of scabies among nursing home residents. J Gerontol A Biol Sci Med Sci 2001; 56:M424–7. [DOI] [PubMed] [Google Scholar]
- 33. Tsutsumi M, Nishiura H, Kobayashi T. Dementia‐specific risks of scabies: retrospective epidemiologic analysis of an unveiled nosocomial outbreak in Japan from 1989–90. BMC Infect Dis 2005; 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall A. Scabies In: Atlas of Male Genital Dermatology (Hall A, ed.). Cham: Springer International Publishing, 2019; 103–6. [Google Scholar]
- 35. Estrada‐Chavez G, Estrada R, Engelman D et al Cushing syndrome due to inappropriate corticosteroid topical treatment of undiagnosed scabies. Trop Med Infect Dis 2018; 3:E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chouela E, Abeldano A, Pellerano G et al Diagnosis and treatment of scabies: a practical guide. Am J Clin Dermatol 2002; 3:9–18. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention . Scabies. Diagnosis. Available at: https://www.cdc.gov/parasites/scabies/diagnosis.html (last accessed 18 February 2020).
- 38. Micali G, Lacarrubba F, Verzi AE et al Scabies: advances in noninvasive diagnosis. PLOS Negl Trop Dis 2016; 10:e0004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fox G. Diagnosis of scabies by dermoscopy. BMJ Case Rep 2009; 2009:bcr06.2008.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dupuy A, Dehen L, Bourrat E et al Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol 2007; 56:53–62. [DOI] [PubMed] [Google Scholar]
- 41. Delaunay P, Herisse AL, Hasseine L et al Scabies polymerase chain reaction with standardized dry swab sampling: an easy tool for cluster diagnosis of human scabies. Br J Dermatol 2020; 182:197–201. [DOI] [PubMed] [Google Scholar]
- 42. Naz S, Desclozeaux M, Mounsey KE et al Characterization of Sarcoptes scabiei tropomyosin and paramyosin: immunoreactive allergens in scabies. Am J Trop Med Hyg 2017; 97:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong SS, Poon RW, Chau S et al Development of conventional and real‐time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol 2015; 53:2095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arlian LG, Feldmeier H, Morgan MS. The potential for a blood test for scabies. PLOS Negl Trop Dis 2015; 9:e0004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hahm JE, Kim CW, Kim SS. The efficacy of a nested polymerase chain reaction in detecting the cytochrome c oxidase subunit 1 gene of Sarcoptes scabiei var. hominis for diagnosing scabies. Br J Dermatol 2018; 179:889–95. [DOI] [PubMed] [Google Scholar]
- 46. Fraser TA, Carver S, Martin AM et al A Sarcoptes scabiei specific isothermal amplification assay for detection of this important ectoparasite of wombats and other animals. Peer J 2018; 6:e5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu J, Huang X, Dong X et al Serodiagnostic potential of alpha‐enolase from Sarcoptes scabiei and its possible role in host–mite interactions. Front Microbiol 2018; 9:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marks M, Engelman D, Romani L et al Exploration of a simplified clinical examination for scabies to support public health decision‐making. PLOS Negl Trop Dis 2018; 12:e0006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beeres DT, Ravensbergen SJ, Heidema A et al Efficacy of ivermectin mass‐drug administration to control scabies in asylum seekers in the Netherlands: a retrospective cohort study between January 2014 – March 2016. PLOS Negl Trop Dis 2018; 12:e0006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ugbomoiko US, Oyedeji SA, Babamale OA et al Scabies in resource‐poor communities in Nasarawa State, Nigeria: epidemiology, clinical features and factors associated with infestation. Trop Med Infect Dis 2018; 3:E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson A, Heukelbach J, Feldmeier H. Transmission of scabies in a rural community. Braz J Infect Dis 2007; 11:386–7. [DOI] [PubMed] [Google Scholar]
- 52. Boralevi F, Diallo A, Miquel J et al Clinical phenotype of scabies by age. Pediatrics 2014; 133:e910–16. [DOI] [PubMed] [Google Scholar]
- 53. Nair PA, Vora RV, Jivani NB et al A study of clinical profile and quality of life in patients with scabies at a rural tertiary care centre. J Clin Diagn Res 2016; 10:WC01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Worth C, Heukelbach J, Fengler G et al Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int J Dermatol 2012; 51:275–82. [DOI] [PubMed] [Google Scholar]
- 55. Engelman D, Steer AC. Diagnosis, treatment, and control of scabies: can we do better? Lancet Infect Dis 2018; 18:822–3. [DOI] [PubMed] [Google Scholar]
- 56. Puza CJ, Suresh V. Scabies and pruritus – a historical review. JAMA Dermatol 2018; 154:536. [DOI] [PubMed] [Google Scholar]
- 57. Jannic A, Bernigaud C, Brenaut E et al Scabies itch. Dermatol Clin 2018; 36:301–8. [DOI] [PubMed] [Google Scholar]
- 58. Jackson A, Heukelbach J, Filho AF et al Clinical features and associated morbidity of scabies in a rural community in Alagoas, Brazil. Trop Med Int Health 2007; 12:493–502. [DOI] [PubMed] [Google Scholar]
- 59. Shin K, Jin H, You HS et al Clinical characteristics of pruritus in scabies. Indian J Dermatol Venereol Leprol 2017; 83:492–3. [DOI] [PubMed] [Google Scholar]
- 60. Brenaut E, Garlantezec R, Talour K et al Itch characteristics in five dermatoses: non‐atopic eczema, atopic dermatitis, urticaria, psoriasis and scabies. Acta Derm Venereol 2013; 93:573–4. [DOI] [PubMed] [Google Scholar]
- 61. Bernardeschi C, Le Cleach L, Delaunay P et al Bed bug infestation. BMJ 2013; 346:f138. [DOI] [PubMed] [Google Scholar]
- 62. Mellanby K. Transmission and prevention of scabies. Public Health 1941; 55:150–1. [Google Scholar]
- 63. Ministry of Health Malaysia . Guideline for management of scabies in adults and children Malaysia 2015. Available at: http://www.moh.gov.my/index.php/file_manager/dl_item (last accessed 18 February 2020).
- 64. Arlian LG, Runyan RA, Achar S et al Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis . J Am Acad Dermatol 1984; 11:210–15. [DOI] [PubMed] [Google Scholar]
- 65. Mellanby K. Transmission of Scabies . Br Med J 1941; 2:405–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health 2019; 11:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors 2017; 10:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mellanby K. Biology of the parasite In: Scabies and pediculosis (Orkin M, Maibach HI, Parish LC, Schwartzman RM, eds). Philadelphia, PA: J.B. Lippincott, 1977; 8–16. [Google Scholar]
- 69. Executive Committee of Guideline for the Diagnosis and Treatment of Scabies . Guideline for the diagnosis and treatment of scabies in Japan (third edition): Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. J Dermatol 2017; 44:991–1014. [DOI] [PubMed] [Google Scholar]
- 70. Goldsmith LA, Katz SI, Gilchrest BA et al Fitzpatrick's Dermatology in General Medicine, 8th edn New York: McGraw‐Hill, 2012. [Google Scholar]
- 71. Yoshizumi J, Harada T. ‘Wake sign’: an important clue for the diagnosis of scabies. Clin Exp Dermatol 2009; 34:711–14. [DOI] [PubMed] [Google Scholar]
- 72. Salavastru CM, Chosidow O, Boffa MJ et al European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31:1248–53. [DOI] [PubMed] [Google Scholar]
- 73. Woodley D, Saurat JH. The burrow ink test and the scabies mite. J Am Acad Dermatol 1981; 4:715–22. [DOI] [PubMed] [Google Scholar]
- 74. Hengge UR, Currie BJ, Jager G et al Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis 2006; 6:769–79. [DOI] [PubMed] [Google Scholar]
- 75. Chosidow O. Clinical practices. Scabies. N Engl J Med 2006; 354:1718–27. [DOI] [PubMed] [Google Scholar]
- 76. Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ 2005; 331:619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nast A, Griffiths CE, Hay R et al The 2016 International League of Dermatological Societies’ revised glossary for the description of cutaneous lesions. Br J Dermatol 2016; 174:1351–8. [DOI] [PubMed] [Google Scholar]
- 78. Suh KS, Han SH, Lee KH et al Mites and burrows are frequently found in nodular scabies by dermoscopy and histopathology. J Am Acad Dermatol 2014; 71:1022–3. [DOI] [PubMed] [Google Scholar]
- 79. Orkin M. Special forms of scabies In: Scabies and pediculosis (Orkin M, Maibach HI, Parish LC, Schwartzman RM, eds). Philadelphia, PA: J.B. Lippincott, 1977; 23–30. [Google Scholar]
- 80. Prendiville JS. Scabies and lice In: Harper's Textbook of Pediatric Dermatology (Irvine AD, Hoeger PH, Yan AC, eds). Chichester: Wiley‐Blackwell, 2011; Chapter 72. [Google Scholar]
- 81. Romani L, Whitfeld MJ, Koroivueta J et al The epidemiology of scabies and impetigo in relation to demographic and residential characteristics: baseline findings from the Skin Health Intervention Fiji Trial. Am J Trop Med Hyg 2017; 97:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mason DS, Marks M, Sokana O et al The prevalence of scabies and impetigo in the Solomon Islands: a population‐based survey. PLOS Negl Trop Dis 2016; 10:e0004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mitja O, Marks M, Bertran L et al Integrated control and management of neglected tropical skin diseases. PLOS Negl Trop Dis 2017; 11:e0005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Faye O, Bagayoko CO, Dicko A et al A teledermatology pilot programme for the management of skin diseases in primary health care centres: experiences from a resource‐limited country (Mali, West Africa). Trop Med Infect Dis 2018; 3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Development of standardized criteria.
Appendix S2 Microscopy for the diagnosis of scabies.
Appendix S3 High‐powered imaging for the diagnosis of scabies.
Appendix S4 Dermoscopy for the diagnosis of scabies.
Appendix S5 Typical distribution of scabies.
Appendix S6 Variant presentations of scabies.
Appendix S7 Differential diagnoses.
Appendix S8 Concise summary of the 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies.


