Abstract
BACKGROUND
Admission to hospital provides the opportunity to review patient medications; however, the extent to which the safety of drug regimens changes after hospitalization is unclear.
OBJECTIVE
To estimate the number of potentially inappropriate medications (PIMs) prescribed to patients at hospital discharge and their association with the risk of adverse events 30 days after discharge.
DESIGN
Prospective cohort study.
SETTING
Tertiary care hospitals within the McGill University Health Centre Network in Montreal, Quebec, Canada.
PARTICIPANTS
Patients from internal medicine, cardiac, and thoracic surgery, aged 65 years and older, admitted between October 2014 and November 2016.
MEASURES
Abstracted chart data were linked to provincial health databases. PIMs were identified using AGS (American Geriatrics Society) Beers Criteria®, STOPP, and Choosing Wisely statements. Multivariable logistic regression and Cox models were used to assess the association between PIMs and adverse events.
RESULTS
Of 2,402 included patients, 1,381 (57%) were male; median age was 76 years (interquartile range [IQR] = 70‐82 years); and eight discharge medications were prescribed (IQR = 2‐8). A total of 1,576 (66%) patients were prescribed at least one PIM at discharge; 1,176 (49%) continued a PIM from prior to admission, and 755 (31%) were prescribed at least one new PIM. In the 30 days after discharge, 218 (9%) experienced an adverse drug event (ADE) and 862 (36%) visited the emergency department (ED), were rehospitalized, or died. After adjustment, each additional new PIM and continued community PIM were respectively associated with a 21% (odds ratio [OR] = 1.21; 95% confidence interval [CI] = 1.01‐1.45) and a 10% (OR = 1.10; 95% CI = 1.01‐1.21) increased odds of ADEs. They were also respectively associated with a 13% (hazard ratio [HR] = 1.13; 95% CI = 1.03‐1.26) and a 5% (HR = 1.05; 95% CI = 1.00‐1.10) increased risk of ED visits, rehospitalization, and death.
CONCLUSIONS
Two in three hospitalized patients were prescribed a PIM at discharge, and increasing numbers of PIMs were associated with an increased risk of ADEs and all‐cause adverse events. Improving hospital prescribing practices may reduce the frequency of PIMs and associated adverse events. J Am Geriatr Soc 68:1184–1192, 2020.
Keywords: adverse drug events, hospital discharge, potentially inappropriate medications
Short abstract
See related editorial by Donna M. Fick in this issue.
While any medication may have adverse effects, potentially inappropriate medications (PIMs) are defined as those that have a greater risk of harm than benefit, particularly in patients older than 65 years.1 Consequently, PIMs may offer an opportunity to improve care through deprescription or, if required, may be replaced by a safer alternative. The prevalence of PIMs in older adults ranges between 20% and 60%, depending on the healthcare setting (eg, community vs hospital) or criteria used to define inappropriate prescribing (eg, the AGS (American Geriatrics Society) Beers Criteria® vs STOPP criteria).2, 3, 4, 5, 6, 7, 8, 9 Their use may not be benign, as PIM use by community‐dwelling patients has been associated with a 10% to 30% increased risk of hospitalization,9, 10, 11, 12, 13 as well as increased risk of adverse drug events (ADEs), emergency department (ED) visits, and poor quality of life.14, 15, 16, 17
Several studies have demonstrated that when older adults are hospitalized, they are often discharged on substantially different medication regimens than at admission.16, 17, 18 Changes to patient drug regimens can occur for several reasons, including: new health conditions (either permanent or transient), ineffective treatment in the community, in response to ADEs, or new medications started to treat transient hospital‐related symptoms (eg, pain, nausea, sleeplessness, or delirium) that are continued at discharge. Hospitalization can provide an opportunity to review and optimize a patient's medication regimen with the potential to reduce unplanned ED visits and readmissions by preventing drug interactions and adverse effects.19, 20 However, while some studies report a decrease in PIMs at discharge,5, 6 it can also be an environment where new PIMs are prescribed.9 Although a few clinical trials have evaluated the impact of in‐hospital interventions that have the potential to improve medication appropriateness (medication reconciliation, medication review, or addition of clinical pharmacists to the hospital‐based team21, 22, 23), no study has prospectively evaluated the impact of PIMs prescribed at discharge on subsequent adverse events in the posthospitalization period. To address this paucity of evidence, we followed a large cohort of medical and surgical patients to evaluate the association of PIMs, both continued from the community and newly prescribed at discharge, with the risk of ADEs, ED visits, readmission, and death after discharge.
METHODS
Study Design and Setting
This prospective cohort study was conducted in the province of Quebec, Canada, where comprehensive data are available on all medical visits, ED visits, hospitalizations, and deaths. To evaluate the study question, we linked individual data from a completed hospital‐based randomized trial of medication reconciliation with comprehensive provincial data for consenting patients.24, 25 The study took place at two tertiary care academic hospitals at the McGill University Health Centre (MUHC). Ethics approval was provided by the MUHC Research Ethics Board, and patients provided informed consent.
Participants
Patients admitted from the community to medical or surgical units at the study hospitals between October 1, 2014, and November 1, 2016, who were 18 years and older and covered under the provincial public drug plan for the year before admission and after discharge were eligible to be included in the original cluster randomized controlled trial (RCT).24 For the current cohort study, we selected patients who were 65 years and older and were discharged alive from one of the study units on one or more medications (Figure 1). The province of Quebec provides health insurance for all provincial residents and drug insurance to all those 65 years and older.26 Patients were followed up until death or 30 days after discharge, whichever came first.
Figure 1.
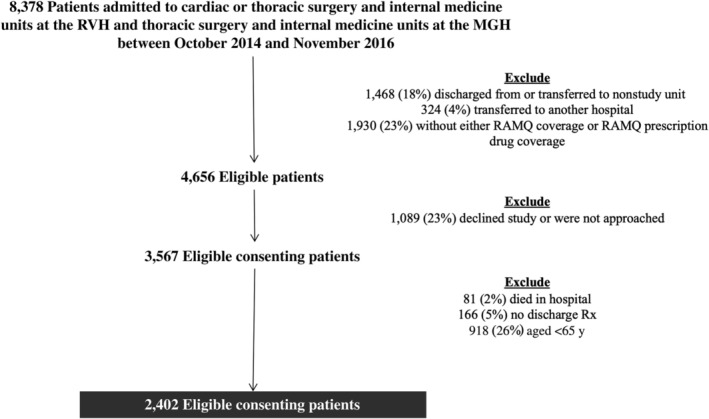
Flowchart of patient exclusions. MGH, Montreal General Hospital; RAMQ, Regiel'assurance maladie; RVH, Royal Victoria Hospital; Rx, prescription.
Data Sources
For each patient, we obtained demographic, healthcare service use, and prescription claims data from the Quebec provincial healthcare administrative database (acquired for the year before hospital admission and the year after discharge). Beneficiary medical billing and pharmacy claims data have been widely validated and are frequently used for health services and epidemiologic research.27, 28, 29, 30
Information pertaining to the patientʼs hospital stay (including the discharge prescription) was abstracted from the medical chart by a trained research assistant with a clinical background. Health problems were coded using the International Classification of Diseases, Tenth Revision (ICD‐10), and medications were classified according to drug molecule and the American Hospital Formulary Service System. Results of laboratory tests (hemoglobin A1c, creatinine, potassium, calcium, and sodium) ordered during the hospital stay were abstracted from the laboratory information system.
The Australian adverse reaction and drug event report was used to collect patient self‐reported information within 25 to 30 days after discharge and was administered by telephone by a trained research assistant.31 Patients were asked whether they had stopped taking any medications since discharge as a result of an adverse reaction to that medication as well as whether any new health problem or changes in their condition had occurred since discharge. Caregivers could also be contacted to provide this information if indicated during the consent process for the original randomized trial.
STUDY MEASURES
Community Medications at Admission and Medications Prescribed at Discharge
Community medications at the time of admission were determined using the pharmacy claims database based on dispensations within 3 months of admission and calculated supply. In Quebec, more than 95% of prescriptions have a daysʼ supply of 30 or fewer days.27 Medications that were likely not active at admission (ie, short courses of antibiotics) were excluded from community medications.
Medications prescribed at hospital discharge were obtained using the discharge prescription found in the patientʼs chart. New medications were defined as drugs prescribed at discharge that were not dispensed in the 3 month before admission, while medications continued from the community were defined as those that were dispensed before admission and prescribed at discharge. Medications that were dispensed before admission and prescribed at discharge, but at a different dose than before admission, were grouped into the continued community medication category. Medications that were dispensed before admission but were indicated as stopped at discharge or were left off the discharge prescription were not included in our list of medications prescribed at discharge.
PIMs were identified based on medications found in the AGS Beers Criteria® and STOPP criteria, drugs listed by Choosing Wisely Canada,32, 33, 34 and new evidence of harm published after the most recent versions of the AGS Beers Criteria® and STOPP lists35 (see Supplementary Material S1). Patient health conditions were assigned based on diagnoses abstracted from the provincial database, the patientʼs hospital chart, as well as the results of in‐hospital laboratory tests. We considered both existing patient conditions and new diagnoses that arose during the hospitalization.
To evaluate the independent impact of PIMs prescribed at discharge, we needed to consider the characteristics and number of all prescribed medications. Thus, each medication prescribed at discharge was classified into one of four categories: (1) medications continued from the community that were not PIMs; (2) PIMs continued from the community; (3) new medications that were not PIMs; and (4) new medications that were PIMs prescribed at discharge (Figure 2). Additionally, for prescribed medications to have an impact on patient health outcomes, patients needed to visit the pharmacy and fill them. A previous study from our team suggested that up to 30% of new medications prescribed at discharge are not filled in the 30 days after discharge.36 Consequently, we inspected postdischarge pharmacy claims data to calculate the cumulative number of newly prescribed medications that had been filled for each patient on each day of follow‐up, according to whether they were a PIM. Community medications (including dose changes) had been dispensed before admission; thus, we assumed the patient had a supply on hand and would be taking these medications as prescribed after discharge. Only medications that were prescribed at hospital discharge were considered; newly occurring PIMs based on postdischarge dispensing data alone were not included as part of the PIM measure.
Figure 2.
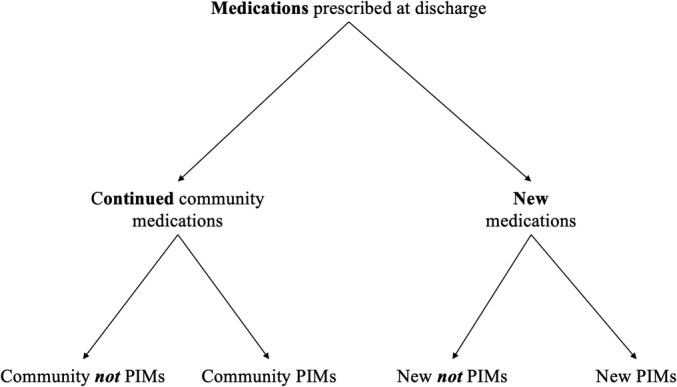
Classification of medications prescribed at discharge. PIM indicates potentially inappropriate medication.
Adverse Health Outcomes After Discharge
The primary outcome was the occurrence (yes/no) of an ADE in the 30 days after discharge. This was defined as either the patient stopping a medication due to an adverse effect or the patient reporting a new health problem, ED visit, or rehospitalization that was specifically adjudicated as drug related by a clinical panel using the Leape‐Bates method (see Supplementary Material S1).37, 38, 39
The secondary composite outcome was defined as time to the first all‐cause ED visit, rehospitalization, or death in the 30 days after discharge. ED visits and hospital readmissions were determined using medical service claims that require physicians to record the date and location where the service was delivered, to receive payment. Date of death was established using the health insurance beneficiary demographic file.
Confounder Adjustment
To account for potential confounding by patient health severity, we first used multivariable logistic regression to calculate a propensity score for being prescribed at least one PIM at discharge (Supplementary Material S1). This continuous score was adjusted for in all models. In addition to the propensity score, we also adjusted for covariates measured at hospital discharge, including discharge unit (cardiac surgery, thoracic surgery, or internal medicine), discharge destination (home to the community, rehabilitation center, convalescence facility, or long‐term care facility), as well as day of week and month of discharge.40
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize the prevalence and characteristics of PIMs prescribed to patients at discharge from hospital. The crude risk for the outcome of drug‐related adverse events was calculated as was the crude incidence rate for the combined outcome of ED visit, readmission, or death.
Four separate models were constructed to assess the potential association between PIMs prescribed at discharge and adverse health outcomes. Models 1 and 2 were multivariable logistic regression models, and the binary outcome was the occurrence of at least one drug‐related adverse event in the 30 days after discharge. Models 3 and 4 were multivariable Cox models, and the outcome was time to first ED visit, rehospitalization, and death up to 30 days after discharge. The presence of at least one community PIM and at least one new PIM was modeled as two separate binary variables in models 1 and 3, and the number of community PIMs and new PIMs prescribed at discharge was included in models 2 and 4 as two separate, continuous variables. The number of community non‐PIMs and new non‐PIMs were adjusted for as separate continuous variables in each of the four models.
Since we expected that the risk associated with new medications would be modified by whether patients actually went to the pharmacy to fill their prescriptions, two different interaction terms were included in model 4 between the continuous number of newly prescribed medications and the time‐varying cumulative number of new medications that were filled (centered around the mean) according to PIM status: (1) new PIMs prescribed × new PIMs filled and (2) new non‐PIMs prescribed × new non‐PIMs filled. The main effect is reported as the percentage increase in risk associated with the number of newly prescribed PIMs for a person with the average number of newly filled PIMs41, 42 (Supplementary Material S1). Since we assumed that patients would have a supply on hand for continued community medications, we did not fit an interaction term for these variables.
Sensitivity Analyses
In sensitivity analyses, we conducted subgroup analyses according to the service the patient was discharged from (medicine vs surgery) to understand potential differences in the incidence of prescribed PIMs and their impact on adverse events.
RESULTS
Overall, 8,378 patients were admitted to the four medical and surgical study units during the enrollment period; 1,729 (22%) were discharged from a nonstudy unit, and 1,930 (23%) did not have public drug coverage and thus were not included in the original cluster RCT. Of eligible patients, 1,089 (23%) declined to participate. Of the remaining 3,567 patients, 81 (2%) died in hospital, 166 (5%) did not have a discharge prescription for one or more medications, and 918 (26%) were younger than 65 years and were excluded. Thus, 2,402 patients were included in our analyses (Figure 1), and their demographics are shown in Table 1.
Table 1.
Patient Characteristics Overall and Stratified According to Being Prescribed Any PIM, at Least One PIM Continued From the Community, or at Least One Newly Prescribed PIM
| Variable | Overall (n = 2,402) | At Least One PIM Prescribed at Discharge (n = 1,576) | At Least One Community PIM at Discharge (n = 1,176) | At Least One New PIM at Discharge (n = 755) |
|---|---|---|---|---|
| Demographics | ||||
| Age at admission, y | ||||
| 65‐74 | 1,055 (43.9) | 694 (44.0) | 490 (41.7) | 360 (47.7) |
| 75‐84 | 908 (37.8) | 596 (37.8) | 462 (39.3) | 262 (34.7) |
| ≥85 | 439 (18.3) | 286 (18.2) | 224 (19.1) | 133 (17.6) |
| Median (IQR) | 76 (70‐82) | 76 (70‐82) | 76 (71‐83) | 75 (70‐81) |
| Sex | ||||
| Females | 1,021 (42.5) | 665 (42.2) | 508 (43.2) | 305 (40.4) |
| Males | 1,381 (57.5) | 911 (57.8) | 668 (56.8) | 450 (59.6) |
| Preadmission healthcare use | ||||
| ED visits | ||||
| No | 707 (29.4) | 444 (28.2) | 303 (25.8) | 236 (31.3) |
| Yes | 1,695 (70.6) | 1,132 (71.8) | 873 (74.2) | 519 (68.7) |
| Hospitalizations | ||||
| No | 1,255 (52.3) | 803 (51.0) | 601 (51.1) | 380 (50.3) |
| Yes | 1,147 (47.8) | 773 (49.1) | 575 (48.9) | 375 (49.7) |
| Ambulatory care visits | ||||
| 0 | 113 (4.7) | 64 (4.1) | 38 (3.2) | 36 (4.8) |
| 1‐8 | 1,077 (44.8) | 676 (42.9) | 482 (41.0) | 341 (45.2) |
| 9‐15 | 665 (27.7) | 447 (28.4) | 342 (29.1) | 221 (29.3) |
| ≥16 | 547 (22.8) | 389 (24.7) | 314 (26.7) | 157 (20.8) |
| No. of unique prescribers | ||||
| 0 | 114 (4.8) | 60 (3.8) | 0 (0) | 60 (8.0) |
| 1‐2 | 549 (22.7) | 318 (20.2) | 201 (17.1) | 182 (24.1) |
| 3‐4 | 700 (29.1) | 460 (29.2) | 334 (28.4) | 231 (30.6) |
| ≥5 | 1,039 (43.3) | 738 (46.8) | 641 (54.5) | 282 (37.4) |
| No. of chronic conditions | ||||
| 0 | 27 (1.1) | 13 (0.8) | 5 (0.4) | 9 (1.1) |
| 1‐3 | 663 (27.6) | 352 (22.3) | 226 (19.2) | 176 (23.3) |
| 4‐6 | 1,139 (47.4) | 758 (48.1) | 549 (46.7) | 390 (51.7) |
| ≥7 | 573 (23.9) | 453 (28.7) | 396 (33.7) | 181 (24.0) |
| Characteristics measured at discharge | ||||
| No. of discharge medications | ||||
| 1‐4 | 417 (17.4) | 137 (8.7) | 65 (5.5) | 83 (11.0) |
| 5‐6 | 515 (21.4) | 292 (18.5) | 191 (16.2) | 143 (18.9) |
| 7‐8 | 481 (20.0) | 318 (20.2) | 222 (18.9) | 158 (20.9) |
| 9‐11 | 591 (24.6) | 471 (29.9) | 384 (32.7) | 210 (27.8) |
| ≥12 | 398 (16.6) | 358 (22.7) | 314 (26.7) | 161 (21.3) |
| Median (IQR) | 8 (5‐10) | 9 (6‐11) | 9 (7‐12) | 8 (6‐11) |
| No. of new medications | ||||
| 0 | 239 (10.0) | 155 (9.8) | 155 (13.4) | 0 (0) |
| 1‐3 | 1,445 (60.2) | 911 (57.8) | 731 (62.2) | 385 (51.0) |
| ≥4 | 718 (29.9) | 510 (32.4) | 290 (24.7) | 370 (49.0) |
| Unit discharged from | ||||
| Cardiac surgery | 637 (26.5) | 395 (26.1) | 262 (22.3) | 240 (31.8) |
| Thoracic surgery | 478 (19.0) | 321 (20.4) | 228 (19.4) | 347 (46.0) |
| Internal medicine | 1,287 (53.6) | 860 (54.6) | 686 (58.3) | 168 (22.3) |
| Discharge destination | ||||
| Home to community | 2,003 (83.4) | 1,306 (82.9) | 982 (83.6) | 599 (79.3) |
| Long‐term care | 114 (4.8) | 72 (4.6) | 47 (4.0) | 42 (5.6) |
| Rehabilitation center | 261 (10.8) | 179 (11.4) | 133 (11.3) | 105 (13.9) |
| Convalescence facility | 23 (1.0) | 18 (1.1) | 13 (1.1) | 9 (1.2) |
Note: Data are given as number (percentage), unless otherwise indicated.
Abbreviations: ED, emergency department; IQR, interquartile range; PIM, potentially inappropriate medication.
Overall, included patients had a median age of 76 years (interquartile range [IQR] = 70‐82 years), had a median of 5 medical diagnoses (IQR = 3‐6), and were prescribed a median of 8 medications at discharge (IQR = 2‐8). A total of 1,287 (53.6%) were discharged from internal medicine, 637 (26.9%) were discharged from cardiac surgery, and the remaining 478 (19.9%) were discharged from thoracic surgery.
In total, 1,576 (66%) patients were prescribed at least one PIM at discharge (including both new PIMs and/or those continued from the community), and the median number of PIMs prescribed per patient was 1 (IQR = 0‐2). A total of 1,176 (75.0%) patients were represcribed at least one of their community PIMs, and 755 (47.9%) were prescribed at least one new PIM. The most common newly prescribed PIMs were benzodiazepines in patients without epilepsy or anxiety (7.3% of 1,576 patients prescribed at least one PIM), proton pump inhibitors in patients without gastrointestinal hemorrhage or peptic ulcer (4.1%), cyclooxygenase‐2 inhibitors in patients with hypertension (3.8%), selective α‐1 adrenergic blocking agents in patients with hypertension without benign prostatic hyperplasia (3.5%), opioids in patients with delirium (2.0%), and atypical antipsychotics in patients with delirium who did not have a diagnosis of schizophrenia or bipolar affective disorder (2.0%) (Table 2).
Table 2.
Characteristics and Prevalence of Most Common PIMs Among Patients Who Were Prescribed at Least One PIM at Discharge (n = 1,576)
| PIM | Medication(s) Flagged in Study Patients | Evidence Source(s) | No. (%) of Patients With Any Prescription at Discharge | No. (%) of Patients With New Prescription at Discharge |
|---|---|---|---|---|
| Benzodiazepines in patients without epilepsy or anxiety | Diazepam, oxazepam, lorazepam, bromazepam, alprazolam, flurazepam, nitrazepam, temazepam | AGS Beers Criteria®, STOPP, Choosing Wisely | 408 (25.9) | 114 (7.2) |
| Proton pump inhibitors in patients without gastrointestinal hemorrhage or peptic ulcer not taking anticoagulant agents | Omeprazole, pantoprazole, lansoprazole, rabeprazole, esomeprazole, dexlansoprazole | AGS Beers Criteria®, STOPP, Choosing Wisely | 131 (8.3) | 64 (4.1) |
| Cyclooxygenase‐2 inhibitors in patients with hypertension | Celecoxib | STOPP | 88 (5.6) | 61 (3.9) |
| Selective α‐1‐adrenergic blocking agents in patients with hypertension without prostatic hypertrophy | Alfuzosin, tamsulosin, silodosin | STOPP, AGS Beers Criteria®, Choosing Wisely | 88 (5.6) | 56 (3.6) |
| Opioids in patients with delirium without cancer | Codeine, fentanyl, hydromorphone, morphine, oxycodone | AGS Beers Criteria®, STOPP, Choosing Wisely | 47 (3.0) | 31 (2.0) |
| Atypical antipsychotics in patients with delirium without schizophrenia or bipolar affective disorder | Aripiprazole, olanzapine, quetiapine, risperidone | AGS Beers Criteria®, STOPP, Choosing Wisely | 42 (2.7) | 32 (2.0) |
Abbreviations: AGS, American Geriatrics Society; PIM, potentially inappropriate medication.
Overall, 218 (9.1%) patients experienced an ADE and 862 (36%) visited the ED, were readmitted to hospital, and/or died in the 30 days after discharge. With respect to the composite end point, 656 patients (76.1% of all events) had a first event that was an ED visit, 194 (22.5%) were rehospitalizations, and 12 (1.4%) were deaths. The incidence rate for the composite outcome was 1.51 events/100 person‐days.
With respect to ADEs, we found that 120 (10.2%) patients prescribed at least one PIM continued from the community vs 98 (8.0%) not prescribed a community PIM experienced an ADE. For those prescribed a new PIM, 82 (10.9%) patients experienced an ADE compared to 136 (8.3%) patients who were not. After adjustment for the propensity score and confounders related to hospital discharge, being prescribed at least one community PIM was associated with a 32% increased odds of ADE compared to those not prescribed any community PIMs (adjusted odds ratio [aOR] = 1.32; 95% confidence interval [CI] = 0.98‐1.90), while being prescribed at least one new PIM was associated with a 41% increase in the odds of ADE (aOR = 1.41; 95% CI = 1.05‐1.90). Consistent with these findings, each additional community PIM and newly prescribed PIM was associated with a 10% (aOR = 1.10; 95% CI = 1.01‐1.21) and a 21% (aOR = 1.21; 95% CI = 1.01‐1.45) increased odds of ADE, respectively (Table 3).
Table 3.
Medications Prescribed at Discharge According to Appropriateness and Their Impact on Drug‐Related Adverse Events and Rehospitalizations, ED Visits, and Death in 30 Days After Discharge
| Outcome | Model | Medications Prescribed at Discharge | Risk of Outcome, No. (%) | Unadjusted OR (95% CI) | Adjusted OR(95% CI)a |
|---|---|---|---|---|---|
| Adverse drug event | 1 | Binary | |||
| No community PIMs | 98 (8.0) | Reference | Reference | ||
| At least one community PIM | 120 (10.2) | 1.31 (0.99‐1.73) | 1.32 (0.98‐1.80) | ||
| No new PIMs | 136 (8.3) | Reference | Reference | ||
| At least one new PIM | 82 (10.9) | 1.35 (1.01‐1.81) | 1.41 (1.05‐1.90) | ||
| 2 | Continuous | ||||
| Community non‐PIMs | 1.00 (0.96‐1.04) | 0.96 (0.91‐1.02) | |||
| Community PIMs | 1.10 (1.01‐1.20) | 1.10 (1.01‐1.21) | |||
| New non‐PIMs | 1.04 (0.97‐1.11) | 1.06 (0.98‐1.14) | |||
| New PIMs | 1.19 (1.00‐1.41) | 1.21 (1.01‐1.45) | |||
| Outcome | Medications Prescribed at Discharge | Incidence Rate (95% CI)a | Unadjusted HR (95% CI) | Adjusted HR(95% CI)a | |
|---|---|---|---|---|---|
| Time to ED visit, rehospitalization, or death | 3 | Binary | |||
| No community PIMs | 1.36 (1.23‐1.50) | Reference | Reference | ||
| At least one community PIM | 1.67 (1.52‐1.83) | 1.17 (1.05‐1.32) | 1.11 (0.97‐1.30) | ||
| No new PIMs | 1.45 (1.33‐1.58) | Reference | Reference | ||
| At least one new PIM | 1.64 (1.46‐1.84) | 1.12 (0.98‐1.29) | 1.22 (1.00‐1.49) | ||
| 4 | Continuous | ||||
| Community non‐PIMs | 1.02 (1.00‐1.04) | 0.99 (0.96‐1.02) | |||
| Community PIMs | 1.07 (1.03‐1.12) | 1.05 (1.00‐1.10) | |||
| New non‐PIMs | 1.04 (1.01‐1.07) | 1.05 (1.01‐1.10) | |||
| New PIMs | 1.11 (1.03‐1.19) | 1.13 (1.03‐1.26) | |||
Abbreviations: CI, confidence interval; ED, emergency department; HR, hazard ratio; OR, odds ratio; PIM, potentially inappropriate medication.
Adjusted for conditional probability of being prescribed at least one PIM, discharge unit, and destination.
Per 100 person‐days.
Patients who were prescribed at least one community PIM had a higher incidence rate of ED visit, rehospitalization, or death in the 30 days after discharge (1.67 events/100 person‐days) compared to patients who were not prescribed any community PIMs (1.36 events/100 person‐days), as did patients who were prescribed at least one new PIM vs none (1.64 vs 1.45 events/100 person‐days). After adjustment for the propensity score and additional discharge characteristics, the prescription of at least one community PIM was associated with an 11% increased risk of ED visit, rehospitalization, or death in 30 days (adjusted hazard ratio [aHR] = 1.11; 95% CI = 0.97‐1.30) and receiving at least one new PIM prescription was associated with a 22% increase in risk (aHR = 1.22; 95% CI = 1.00‐1.49). When the number of medications prescribed at discharge was modeled as continuous variables, we found that each additional PIM continued from the community was associated with a 5% increase in risk (aHR = 1.05; 95% CI = 1.00‐1.10) and each additional new PIM 13% (aHR = 1.13; 95% CI = 1.03‐1.26) (Table 3).
When we evaluated medical and surgical patients separately, we found that 686 (53%) patients from medicine and 490 (44%) patients from surgery were prescribed a PIM continued from the community, while 347 (27%) patients from medicine and 408 (37%) patients from surgery were prescribed at least one new PIM. The impact of PIMs on ED visits, rehospitalization, and death in 30 days was similar between medical and surgical patients (Supplementary Material S1 and Supplementary Tables S1 and S2); however, there was a stronger associated between new PIMs and ADEs in surgical patients compared to medical patients (aOR for new PIMS in medicine = 1.13 [95% CI = 0.88‐1.46]; aOR for new PIMS in surgery = 1.32 [95% CI = 1.03‐1.71) (Supplementary Material S1 and Supplementary Tables S3 and S4).
DISCUSSION
In this prospective cohort study of discharged medical and surgical patients, 65 years and older, we found that two in three were prescribed a PIM overall at discharge and one in three was prescribed at least one new PIM. After adjustment for confounding by severity of patient health status, other medications prescribed at discharge, and whether newly prescribed medications had been filled, we found that increasing numbers of both community PIMs and new PIMs were associated with an increased odds of having an ADE within 30 days as well as the risk of ED visits, hospital readmission, and death. The strength of these associations was consistently highest for new PIMs.
Our study found that 66% of patients were prescribed PIMs overall at discharge, which is similar to the findings of two recent studies reporting prevalence between 42% and 71%.43, 44 Additionally, we found that the most commonly prescribed PIMs included benzodiazepines and proton pump inhibitors, which has also been reported previously.9, 43, 45 Our study is novel in that it is the first to report the risk of adverse events after discharge that are specifically associated with new PIMs and those continued from the community. Importantly, we were able to demonstrate that PIMs were associated with both all‐cause and drug‐related adverse events. Our finding that new PIMs conferred a higher risk of adverse events compared to community PIMs is not surprising given that if a patient had experienced a major adverse event associated with a PIM he/she was already taking, it may have already been discontinued. That said, even if an ADE has not already occurred, it seems plausible there would be merit in a comprehensive assessment of all medication use during hospitalization among at‐risk older adults, particularly with respect to preventing new PIMs during hospitalization from being continued into the community.
The high prevalence of PIM prescribing suggests that the intended outcomes of campaigns such as Choosing Wisely and other deprescribing initiatives, are not yet being fully realized. Some clinicians may continue legacy PIMs due to fears of diminished credibility, potential for litigation, and potential conflict with other prescribers and health professionals46, 47, 48 as well as concerns over withdrawal syndrome or symptom relapse.49, 50 Prescribers may also lack the necessary decision support within their healthcare setting to enable them to easily and efficiently identify inappropriate prescribing and/or communicate this to other members of patientsʼ healthcare teams. Standing order sets for medications in hospital may also inadvertently increase potentially inappropriate prescribing. For example, the administration of benzodiazepines for sleep during hospitalization is unfortunately standard practice in many institutions, as is the administration of proton pump inhibitors within intensive care units for gastroprotection or the use of antipsychotics for delirium or sleep. However, these medications may be inadvertently continued once patients leave the hospital.51, 52, 53
Given the increased risk of adverse health outcomes observed for patients prescribed PIMs at discharge, it is important to consider potential solutions for this issue. Effecting change likely requires a multifaceted approach. Accurate medication reconciliation is a first step to ensure that all medications, including PIMs, can be appropriately identified. An important next step is to consider each of the medications and its role in the context of the individual patient. Although the published criteria used to identify PIMs in this study could be used as reference guides for medication review within the inpatient setting, it has proved difficult to integrate these recommendations widely into everyday clinical practice. Engaged providers and teams or expert consultation can facilitate medication rationalization and deprescribing for individual patients. However, on a larger scale, the process of cross‐referencing multiple medications and medical conditions to highlight instances of potentially inappropriate prescribing requires significant investments of human resources. Electronic decision support tools for deprescribing, such as MedSafer, can help facilitate the identification of PIMs and target them for consideration for deprescribing.35 In a feasibility pilot study, MedSafer increased the proportion of patients with one or more PIMs deprescribed from 46.9% to 54.7%, with an estimated number needed to treat of 12. A multisite, national Canadian RCT is currently underway to determine whether use of the MedSafer tool is associated with a decreased risk of ADEs in the 30 days following hospital discharge as well as potential cost savings.54 If sufficiently automated, this could be a scalable intervention to address inappropriate prescribing across a range of healthcare settings with the potential to improve patient health outcomes.
Strengths and Limitations
An important strength of this study was the analysis of a large cohort of patients for whom we had detailed clinical information (including the results of laboratory tests) linked to administrative health data. Therefore, not only were we able to determine which medications patients were prescribed at discharge, we were also able to measure whether patients went to the pharmacy to fill their prescriptions. However, we could not determine whether filled medications were actually taken by patients. Indeed, several studies have suggested that differences between filling a medication and its actual use are not trivial.55 Additionally, we excluded prescribed medications that were not covered under the public provincial drug plan; thus, PIMs, including these medications, were likely underestimated (eg, over‐the‐counter nonsteroidal anti‐inflammatory drugs used in combination with platelet aggregation inhibitors).
CONCLUSION
In conclusion, the incidence of PIM prescribing attributed to hospitalization is high, and this is associated with an increase in ADEs, ED visits, rehospitalizations, and death within 30 days of discharge. These findings may provide impetus not only for further research into the risks associated with continuing PIMs at hospital discharge but also for the support of quality improvement initiatives to optimize prescribing in older hospitalized adults.
Conflict of Interest
Drs McDonald and Lee own the intellectual property rights to MedSafer in conjunction with McGill University.
Author Contributions
All authors conceived and designed the study. D.L.W. and R.T. acquired the data. D.L.W. did the statistical analyses. All authors interpreted the data. D.L.W. wrote the manuscript, and all authors critically revised it. All authors approved the final version of the manuscript and agree to be accountable for the accuracy of the work.
Sponsorʼs Role
The original study was funded by a foundation scheme grant from the Canadian Institutes of Health Research. The sponsor had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. D.L.W. is the recipient of doctoral fellowships from the Fonds de Recherche du Québec–Santé and the Canadian Institutes of Health Research.
Supporting information
Supplementary Material S1: PIM descriptives and analyses according to discharge service
Supplementary Table S1: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Rehospitalizations, Emergency Department Visits, and Death in the 30 Days After Discharge in Patients 65 Years and Older From Internal Medicine (n = 1,287)
Supplementary Table S2: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Rehospitalizations, Emergency Department Visits, and Death in 30 Days After Discharge in Patients 65 Years and Older From Surgical Units (n = 1,115)
Supplementary Table S3: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Drug‐Related Adverse Events in 30 Days After Discharge in Patients 65 Years and Older Discharged From Internal Medicine (n = 1,287)
Supplementary Table S4: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Drug‐Related Adverse Events in 30 Days After Discharge in Patients 65 Years and Older Discharged From Surgical Units (n = 1,115)
ACKNOWLEDGMENTS
See related editorial by Donna M. Fick in this issue.
The copyright line for this article was changed on 14 October 2020 after original online publication.
REFERENCES
- 1. Amann U, Schmedt N, Garbe E. Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Ärztebl Int. 2012;109:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91‐102. [DOI] [PubMed] [Google Scholar]
- 3. Poudel A, Peel NM, Nissen L, Mitchell C, Gray LC, Hubbard RE. Potentially inappropriate prescribing in older patients discharged from acute care hospitals to residential aged care facilities. Ann Pharmacother. 2014;48:1425‐1433. [DOI] [PubMed] [Google Scholar]
- 4. Morgan SG, Hunt J, Rioux J, Proulx J, Weymann D, Tannenbaum C. Frequency and cost of potentially inappropriate prescribing for older adults: a cross‐sectional study. CMAJ Open. 2016;4:E346‐E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laroche M‐L, Charmes J‐P, Nouaille Y, Fourrier A, Merle L. Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use. Drugs Aging. 2006;23:49‐59. [DOI] [PubMed] [Google Scholar]
- 6. Komagamine J. Prevalence of potentially inappropriate medications at admission and discharge among hospitalised elderly patients with acute medical illness at a single centre in Japan: a retrospective cross‐sectional study. BMJ Open. 2018;8:e021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clyne B, Smith SM, Hughes CM, et al. Sustained effectiveness of a multifaceted intervention to reduce potentially inappropriate prescribing in older patients in primary care (OPTI‐SCRIPT study). Implement Sci. 2016;2:11‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y‐C, Hwang S‐J, Lai H‐Y, et al. Potentially inappropriate medication for emergency department visits by elderly patients in Taiwan. Pharmacoepidemiol Drug Saf. 2009;18:53‐61. [DOI] [PubMed] [Google Scholar]
- 9. Pérez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Der Stelt CA, Windsant‐van den Tweel AV, Egberts AC, et al. The association between potentially inappropriate prescribing and medication‐related hospital admissions in older patients: a nested case control study. Drug Saf. 2016;39:79‐87. [DOI] [PubMed] [Google Scholar]
- 11. Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43:767‐773. [DOI] [PubMed] [Google Scholar]
- 12. Moriarty F, Bennett K, Cahir C, Kenny RA, Fahey T. Potentially inappropriate prescribing according to STOPP and START and adverse outcomes in community‐dwelling older people: a prospective cohort study: potentially inappropriate prescribing and adverse outcomes in older people. Br J Clin Pharmacol. 2016;82:849‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau DT, Kasper JD, Potter DEB, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165:68‐74. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton H, Gallagher P, Ryan C, Byrne S, OʼMahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171:1013‐1019. [DOI] [PubMed] [Google Scholar]
- 15. Hill‐Taylor B, Sketris I, Hayden J, Byrne S, OʼSullivan D, Christie R. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360‐372. [DOI] [PubMed] [Google Scholar]
- 16. Viktil KK, Blix HS, Eek AK, Davies MN, Moger TA, Reikvam A. How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2:e001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochrane RA, Mandal AR, Ledger‐Scott M, Walker R. Changes in drug treatment after discharge from hospital in geriatric patients. BMJ. 1992;305:694‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9:150‐153. [DOI] [PubMed] [Google Scholar]
- 19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Craen K, Braes T, Wellens N, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta‐analysis. J Am Geriatr Soc. 2010;58:83‐92. [DOI] [PubMed] [Google Scholar]
- 21. Renaudin P, Boyer L, Esteve M‐A, Bertault‐Peres P, Auquier P, Honore S. Do pharmacist‐led medication reviews in hospitals help reduce hospital readmissions? a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:1660‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bullock B, Donovan P, Mitchell C, Whitty JA, Coombes I. The impact of a pharmacist on post‐take ward round prescribing and medication appropriateness. Int J Clin Pharmacol. 2019;41:65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravn‐Nielsen LV, Duckert M‐L, Lund ML, et al. Effect of an in‐hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178:375‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamblyn R, Huang AR, Meguerditchian AN, et al. Using novel Canadian resources to improve medication reconciliation at discharge: study protocol for a randomized controlled trial. Trials. 2012;13:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamblyn R, Winslade N, Lee TC, et al. Improving patient safety and efficiency of medication reconciliation through the development and adoption of a computer‐assisted tool with automated electronic integration of population‐based community drug data: the RightRx project. J Am Med Inform Assoc. 2018;25:482‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prescription Drug Insurance | RAMQ [Internet]. http://www.ramq.gouv.qc.ca/en/life-events/retirement/Pages/prescription-drug-insurance.aspx. Accessed February 15, 2019.
- 27. Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999‐1009. [DOI] [PubMed] [Google Scholar]
- 28. Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131‐141. [DOI] [PubMed] [Google Scholar]
- 29. Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160:441‐450. [DOI] [PubMed] [Google Scholar]
- 30. Tamblyn R, Poissant L, Huang A, et al. Estimating the information gap between emergency department records of community medication compared to on‐line access to the community‐based pharmacy records. J Am Med Inform Assoc. 2014;21:391‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell AS, Henry DA, Hennrikus D, OʼConnell DL. Adverse drug reactions: can consumers provide early warning? Pharmacoepidemiol Drug Saf. 1994;3:257‐264. [Google Scholar]
- 32. OʼMahony D, OʼSullivan D, Byrne S, OʼConnor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227‐2246. [DOI] [PubMed] [Google Scholar]
- 34. Choosing Wisely . Choosing Wisely Canada Recommendations [Internet]. 2018. https://choosingwiselycanada.org/recommendations/. Accessed December 2017.
- 35. EG MD, Wu PE, Rashidi B, et al. The MedSafer study: a controlled trial of an electronic decision support tool for deprescribing in acute care. J Am Geriatr Soc. 2019;67(9):1843‐1850. [DOI] [PubMed] [Google Scholar]
- 36. Weir DL. Medications Prescribed, Stopped and Modified at Hospital Discharge and Filled Medications in the Community: Factors Associated With Failure to Follow Hospital Medication Changes 30‐Days Post Discharge. Toronto: Canadian Association for Health Services and Policy Research Conference; 2017. [Google Scholar]
- 37. Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. JAMA. 1995;274:35‐43. [PubMed] [Google Scholar]
- 38. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29‐34. [PubMed] [Google Scholar]
- 39. Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinicianʼs guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795. [DOI] [PubMed] [Google Scholar]
- 40. Lapointe‐Shaw L, Austin PC, Ivers NM, Luo J, Redelmeier DA, Bell CM. Death and readmissions after hospital discharge during the December holiday period: cohort study. BMJ. 2018;363:k4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Afshartous D, Preston RA. Key results of interaction models with centering. J Stat Educ. 2011;19 10.1080/10691898.2011.11889620. [DOI] [Google Scholar]
- 42. Aguinis H, Gottfredson RK. Best‐practice recommendations for estimating interaction effects using moderated multiple regression. J Organ Behav. 2010;31:776‐786. [Google Scholar]
- 43. Gutiérrez‐Valencia M, Izquierdo M, Malafarina V, et al. Impact of hospitalization in an acute geriatric unit on polypharmacy and potentially inappropriate prescriptions: a retrospective study. Geriatr Gerontol Int. 2017;17:2354‐2360. [DOI] [PubMed] [Google Scholar]
- 44. Pardo‐Cabello AJ, Manzano‐Gamero V, Zamora‐Pasadas M, Gutiérrez‐Cabello F, Esteva‐Fernández D, Luna‐Del Castillo J de D, Jiménez‐Alonso J. Potentially inappropriate prescribing according to STOPP‐2 criteria among patients discharged from internal medicine: prevalence, involved drugs and economic cost. Arch Gerontol Geriatr. 2018;74:150–154. [DOI] [PubMed] [Google Scholar]
- 45. Wauters M, Elseviers M, Vaes B, et al. Too many, too few, or too unsafe? impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community‐dwelling oldest old. Br J Clin Pharmacol. 2016;82:1382‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Britten N, Brant S, Cairns A, et al. Continued prescribing of inappropriate drugs in general practice. J Clin Pharm Ther. 1995;20:199‐205. [DOI] [PubMed] [Google Scholar]
- 47. Moen J, Norrgård S, Antonov K, Nilsson JLG, Ring L. GPsʼ perceptions of multiple‐medicine use in older patients. J Eval Clin Pract. 2010;16:69‐75. [DOI] [PubMed] [Google Scholar]
- 48. Schuling J, Gebben H, Veehof LJG, Haaijer‐Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs: a qualitative study. BMC Fam Pract. 2012;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iliffe S, Curran HV, Collins R, Yuen Kee SC, Fletcher S, Woods B. Attitudes to long‐term use of benzodiazepine hypnotics by older people in general practice: findings from interviews with service users and providers. Aging Ment Health. 2004;8:242‐248. [DOI] [PubMed] [Google Scholar]
- 50. Dickinson R, Knapp P, House AO, et al. Long‐term prescribing of antidepressants in the older population: a qualitative study. Br J Gen Pract. 2010;60:144‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bell CM, Fischer HD, Gill SS, et al. Initiation of benzodiazepines in the elderly after hospitalization. J Gen Intern Med. 2007;22:1024‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villamañán E, Ruano M, Lara C, et al. Reasons for initiation of proton pump inhibitor therapy for hospitalised patients and its impact on outpatient prescription in primary care. Rev Esp Enferm Dig. 2015;107:652‐658. [DOI] [PubMed] [Google Scholar]
- 53. Lee TC, Desforges P, Murray J, Saleh RR, McDonald EG. Off‐label use of quetiapine in medical inpatients and postdischarge. JAMA Intern Med. 2016;176:1390‐1391. [DOI] [PubMed] [Google Scholar]
- 54.Reducing Post‐Discharge Potentially Inappropriate Medications Among Older Adults ‐ Full Text View ‐ ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT02918058. Accessed November 18, 2019.
- 55. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487‐497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1: PIM descriptives and analyses according to discharge service
Supplementary Table S1: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Rehospitalizations, Emergency Department Visits, and Death in the 30 Days After Discharge in Patients 65 Years and Older From Internal Medicine (n = 1,287)
Supplementary Table S2: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Rehospitalizations, Emergency Department Visits, and Death in 30 Days After Discharge in Patients 65 Years and Older From Surgical Units (n = 1,115)
Supplementary Table S3: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Drug‐Related Adverse Events in 30 Days After Discharge in Patients 65 Years and Older Discharged From Internal Medicine (n = 1,287)
Supplementary Table S4: Medications Prescribed at Discharge, According to Appropriateness, and Their Impact on Drug‐Related Adverse Events in 30 Days After Discharge in Patients 65 Years and Older Discharged From Surgical Units (n = 1,115)


