Abstract
Background
Autonomic hyperarousal has been proposed as a dispositional risk factor for anxiety disorders (ADs). Therefore, we studied physiological arousal in offspring of fathers and mothers with and without ADs and whether infant hyperarousal predicts subsequent fearful temperament.
Methods
Infants (N = 128; age = 4 months) did a novel stimuli task (exposure to visual, olfactory, and acoustic stimuli and an unfamiliar male) and a habituation task (exposure to a repeated acoustic stimulus). Heart rate (HR) and heart rate variability (HRV) were measured during baseline, stimuli and post‐stimuli rest. Parents’ AD status and severity were measured using a diagnostic interview and their fearful temperament using a questionnaire. Child fearful temperament was measured at 4 months, 1 year and 2.5 years with observations during structured tasks.
Results
Parents’ fearful temperament (significant in the habituation task), AD status (significant in the novel stimuli task) and AD severity (significant in both tasks) predicted a higher HR in their infants. Infants’ higher HR reactivity to novel stimuli and diminished HR recovery at 4 months predicted a more fearful temperament during infancy and toddlerhood. Infants’ higher HR at 4 months predicted a more fearful temperament at 2.5 years.
Conclusions
Parental prenatal anxiety (disorders) predicted infants’ autonomic arousal, which in turn predicted later fearful temperament in children. Outcomes suggest that autonomic hyperarousal is a dispositional risk factor of ADs.
Keywords: Behavioural inhibition, fearful temperament, habituation, heart rate, heart rate variability
Introduction
Worldwide, anxiety disorders (ADs) are the most prevalent mental disorders, with annual prevalence rates ranging from 2% to 18% (WHO Mental Health Survey Consortium, 2004). Because of their adverse consequences on individual well‐being (e.g. poor health, impaired social relationships and impaired societal functioning; see Olatunji, Cisler, & Tolin, 2007, for a review) and high societal costs (e.g. Gustavsson et al., 2011), there is a strong need to understand causal pathways to ADs in order to improve early detection, prevention and treatment.
Anxiety disorders run in families, and the intergenerational transmission of ADs is attributable to various family factors (see Rapee, 2012, for an overview). Of these family factors, genetic factors are relevant as heritability estimates vary from 30% to 73% for ADs (Bolton et al., 2006; Hettema, Neale, & Kendler, 2001) and from 65% to 74% for anxiety symptoms (Ask et al., 2014). Several decades ago, autonomic hyperarousal was proposed as a marker of genetic vulnerability to ADs (Kagan, Reznick & Snidman, 1987). Given that fear learning is associated with an increase in autonomic arousal that is mediated by the limbic system in the brain (Olsson & Phelps, 2007), assessing autonomic hyperarousal as a predisposition of anxiety development may advance insight in aetiological pathways to ADs.
Autonomic hyperarousal is reflected in excessive sympathetic activation and/or diminished parasympathetic activation (Friedman, 2007). This deviant activation of the nervous system is thought to arise from deviant functioning of the limbic system, which is connected to peripheral sympathetic and parasympathetic nerves (Bradley & Lang, 2007; Cannon, 1914; Kaltsas & Crousos, 2007). A rise in sympathetic activity is usually reflected in a rise in heart rate (HR) and skin conductance, and diminished parasympathetic activation is indexed by reduced heart rate variability (HRV) in the high‐frequency domain (Berntson, Quickley, & Lozano, 2007; Dawson, Schell, & Filion, 2007).
Autonomic hyperarousal associated with fear or anxiety may manifest itself in various ways. According to Kagan, Reznick, and Snidman (1987), a chronic state of autonomic hyperarousal, a characteristic of a fearful temperament, stems from a lower threshold for limbic activation. Chronic, or tonic, autonomic hyperarousal is visible in a resting state (Kagan et al., 1987). Physiological hyperarousal may also become apparent as increased reactivity and/or delayed recovery in low threat situations (i.e. novel or challenging situations), that is phasic autonomic hyperarousal (Kagan, et al., 2007). Alternatively, autonomic hyperarousal may occur when physiological habituation to repetitive stimuli is impaired. Turner, Beidel, and Epstein (1991) proposed impaired physiological adaptation to the environment, which is reflected in impaired physiological habituation, as a risk factor for ADs.
Empirical evidence for autonomic hyperarousal as a characteristic of a fearful temperament has been accumulating (see Fox, Henderson, Marshall, Nichols & Ghera, 2005, for a review), as is the evidence for autonomic hyperarousal as a risk factor for developing ADs (e.g. Schmitz, Krämer, Tuschen‐Caffier, Heinrichs, & Blechert, 2011; Schmitz, Tuschen‐Caffier, Wilhelm, & Blechert, 2013). However, the majority of studies have cross‐sectional designs and/or included children who had already developed an AD, making them unsuitable to distinguish between the potential predisposing versus maintaining roles of autonomic hyperarousal in ADs. Studies using a high‐risk or a prospective design deliver stronger results, as they relate premorbid characteristics to either ADs in parents or presence of an AD later in life in the child itself.
So far, several studies using a high‐risk design found evidence for autonomic hyperarousal as a genetic vulnerability to develop ADs (Grillon, Dierker, & Merikangas, 1997; Merikangas, Avenevoli, Dierker, & Grillon, 1999; Turner, Beidel, & Roberson‐Nay, 2005). Turner et al. (2005) reported evidence for higher tonic sympathetic activity in offspring of parents with ADs, as they showed more spontaneous galvanic skin responses (GSRs) during baseline than offspring of parents without ADs (total N = 90, age: 7–12 years). Grillon et al. (1997) and Merikangas et al. (1999), reporting on partly overlapping samples, found evidence for higher phasic arousal (i.e. larger sympathetic reactivity) in offspring at risk for ADs, as they showed a larger startle reflex (total N = 66; age: 10–17 years) and a larger GSR to bursts of white noise (total N = 192; age: 10–17 years) than offspring of parents without ADs.
Concerning impaired physiological adaptation, two studies (one using a high‐risk design and one using a prospective design) found support for this marker as a genetic vulnerability to developing ADs. Turner et al. (2005) found that offspring of parents with ADs were less likely to habituate with skin conductance to visual and auditory mildly threatening stimuli (a picture of a snake and a 100 dB tone) than offspring of parents without ADs (total N = 90, age: 7–12 years). Moehler et al. (2006) showed that impaired autonomic habituation of HR responses to repeated 75 dB pink noises in 2‐week‐old infants (N = 101) predicted more behavioural inhibition; a core feature of a fearful temperament reflected in fearfulness and avoidance of novelty (e.g. Clauss & Blackford, 2012; Kagan & Snidman, 1999), at 14 months. However, null findings in high‐risk studies have also been reported, as Grillon et al. (1997) and Merikangas et al. (1999) found no evidence for impaired startle habituation in offspring of parents with ADs.
To summarize, despite some inconsistent findings, high‐risk studies and Moehler et al.’s (2006) prospective study generally point towards autonomic hyperarousal, reflected in increased tonic sympathetic activity, increased phasic sympathetic activity and/or impaired habituation, as a predisposition for ADs. Whether the various manifestations of autonomic hyperarousal are expressions of one latent hyperarousal construct has not been clarified yet. Research suggests that trait fear and trait anxiety are different constructs which are mediated by different neural pathways and physiologically expressed in different ways (Sylvers, Lilienfeld, & LaPrairie, 2011). Both trait fear and trait anxiety appear to be associated with autonomic arousal during fear and anxiety states. However, trait fear seems to be characterized by impaired extinction of the fear response (i.e. impaired recovery, an index of deviant phasic hyperarousal and/or impaired physiological adaptation), whereas trait anxiety seems to be characterized by tonic hyperarousal.
The studies discussed above have several shortcomings. Children in the high‐risk studies were older than 6 years and thus already had an extensive fear learning history. Moreover, samples also included children that already developed ADs themselves. Since the premorbid situations were unknown (except for the study of Turner et al., 2005), these results cannot be interpreted as unequivocal support for autonomic hyperarousal as predisposing to ADs; they may well indicate that autonomic hyperarousal is a ‘side effect’ of ADs. While Moehler et al. (2006) did demonstrate the link between autonomic hyperarousal and a fearful temperament before ADs can develop, they did not address the intergenerational association between ADs and autonomic hyperarousal.
To overcome the shortcomings of previous studies, we studied autonomic hyperarousal already during early infancy using a high‐risk prospective design, thereby shortening the fear learning history and ruling out the confounding role of presence of ADs in children themselves. More specifically, we tested (a) the intergenerational link between both parents’ anxiety and infant autonomic activity, and (b) the link between infant autonomic hyperarousal and their own fearful temperament during infancy and toddlerhood. We expected that higher levels of anxiety, presence of an AD, and a higher severity of ADs in parents, measured before the child was born, would predict autonomic hyperarousal in their infants (age 4 months). In turn, infants’ autonomic hyperarousal at 4 months was expected to predict more temperamental fearfulness, a main risk factor for developing ADs (Fox et al., 2005), in infancy and toddlerhood. Since the various indices of autonomic hyperarousal are differentially linked to either trait fear or trait anxiety (Sylvers, et al., 2011), they may operate independently in the intergenerational transmission of ADs. Therefore, they were examined independently. In addition, we tested specificity of the association between parents’ anxiety and infant autonomic hyperarousal by also relating presence of parental depression to infant hyperarousal. Grounded in the empirically supported tripartite model of anxiety and depression defined by Clark and Watson (1991), which identifies physiological hyperarousal as a characteristic specific to anxiety, we hypothesized physiological hyperarousal to be specifically related to parents’ ADs and not to parents’ depressive disorder.
Methods
Participants
Participants were 137 couples (at inclusion M age fathers = 33.67, SD = 5.43; M age mothers = 30.66 years, SD = 4.26) and their firstborn infant (77 girls [56%]) who participated in the longitudinal study the Social Development of Children (Majdandžić, de Vente, Colonnesi, & Bögels, 2018). For details on recruitment and sample characteristics, see de Vente, Majdandžić, Colonnesi and Bögels (2011). The Department of Psychology’s ethics committee at the University of Amsterdam approved the study, and written informed consent was obtained. Children’s mean age was 18.01 weeks (SD = 1.39) at the 4‐month measurement; 11.99 months (SD = 0.96) at the 1‐year measurement and 29.65 months (SD = 0.56) at the 2.5‐years measurement.
Procedure
At the prenatal assessment, couples filled out questionnaires and completed a diagnostic interview. At the postnatal assessments, mothers and fathers visited the laboratory separately with their child and home visits were made. For a description of measurements, see de Vente et al. (2011). The present study focused on (a) parents’ anxiety (questionnaire; diagnostic interview) measured prenatally, (b) infants’ autonomic arousal measured during exposure to novel stimuli (visit with mother) and to repeated acoustic stimuli (visit with father) at 4 months, and (c) child fearful temperament observed at 4 months, 1 year, and 2.5 years.
Measures
Infants’ autonomic hyperarousal
Infants’ physiological activity was measured by an ECG using a Lead II configuration during two tasks: (a) a novel stimuli task consisting of nonsocial stimuli using the paradigm of Kagan and Snidman (1991) and a social stimulus based on the Laboratory Temperament Assessment Battery (Lab‐TAB; Goldsmith & Rothbart, 1996); and (b) an auditory habituation task, using the paradigm of Moehler et al. (2006). During the tasks, the infant was seated in a stable baby rocker on a table. Data were recorded and processed using Vsrrp software (Molenkamp, 2011). Data acquisition was performed by a National Instruments NI6224 data acquisition card sampling at a rate of 200S/s per channel. R‐peaks were detected and corrected for artifacts. HR was calculated as the number of R‐peaks per minute, and HRV as the square root of the mean squared successive differences (RMSSD) in interbeat intervals (IBIs). The RMSSD in IBIs reflects high‐frequency variations in HR indicative of parasympathetic activation (Pumprla, Howorka, Groves, Chester & Nolan, 2002).
The novel stimuli task started with a 2‐minute baseline, during which an ECG was taken while the mother quietly interacted with her infant (Figure 1). Subsequently, the mother was seated behind the infant and the infants’ physiological reactivity was measured during presentation of unknown stimuli, consisting of, (a) mobiles; (b) butanol smell; and (c) white noise, and (d) a stranger approach task. Between the white noise and the stranger approach task, the infant had a 1‐min. resting period. For further details about the procedure, see Majdandžić et al. (2018). After the male stranger left the room, a 1‐min resting period started.
Figure 1.
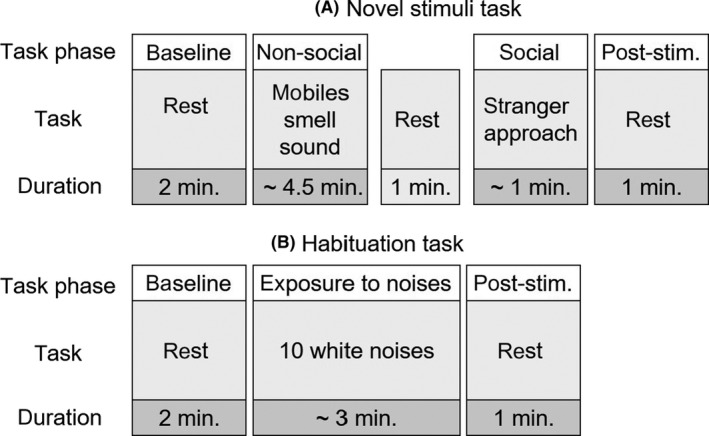
Schematic overview of the task phases of the novel stimuli task (A) and the habituation task (B). Note: post‐stim. = post‐stimuli rest phase
The habituation task started with a 2‐min. baseline, followed by a series of 10 white noises (75 dB, duration: 1 s – volume was monthly checked), followed by a 1‐min. resting period (Figure 1). The noises were presented by two 15 cm speakers placed at 2.5 m. from the infant.
For both tasks, mean HR and RMSSD were computed per task phase (Figure 1). Then, for the novel stimuli task, mean HR and RMSSD were calculated for the nonsocial stimuli (mobiles, smell and noise). For the habituation task, mean HR during the noises was calculated by averaging mean HR during the three 5‐s periods after the first three noises. Because no reliable RMSSD can be calculated during 5‐s intervals, no mean RMSSD was calculated during the stimuli of the habituation task.
Three indices of hyperarousal were defined. First, tonic arousal operationalized as mean baseline HR and RMSSD (calculated separately for each task). Second, phasic arousal operationalized as reactivity and recovery (calculated separately for each task). Reactivity was defined as a HR increase and a RMSSD reduction during the stimuli in either task, as compared to baseline. In both tasks, recovery was defined as HR and RMSSD during the post‐stimuli rest phase relative to the baseline level (i.e. less return to baseline indexes persistent arousal). As a third index of autonomic hyperarousal, physiological adaptation was operationalized as physiological habituation. A habituation index was calculated for HR only (again, since no reliable RMSSD can be calculated during 5‐s intervals) by subtracting mean HR reactivity (5‐s HR post‐stimulus minus 5‐s HR prestimulus) to the first three noises from mean HR reactivity to the last three noises, similar to Moehler et al. (2006). In this manner, a negative value indicates habituation, while a positive value indicates sensitization.
Parents’ fearful temperament
Parents’ fearful temperament was measured using the fear subscale of the short form Adult Temperament Questionnaire (ATQ; Evans & Rothbart, 2007), which consists of seven items assessing various fears rated on 7‐point Likert scales. An example is ‘Sometimes I feel a sense of panic or terror for no apparent reason’. Reliability in the present sample was .66 for mothers and .52 for fathers, which can be considered sufficient given the independency of specific fears such as fear of heights and claustrophobia. To relate parents’ fearful temperament to children’s physiological activity, fathers’ and mothers’ fear scores were averaged.
Parents’ anxiety and depression diagnoses
Parents’ diagnostic status was determined using the Anxiety Disorders Interview Schedule for adults (ADIS‐A; Brown, Barlow, & Di Nardo, 1994), a semistructured interview assessing DSM‐IV diagnoses. Three well‐trained interviewers with a master’s degree in Psychology or Educational Sciences administered the ADIS and rated disorder severity (Clinician Severity Rating [CSR]; 0 = not at all, 8 = very much). Diagnoses with ratings ≥4 are considered clinical. Interobserver reliability for diagnostic classification was good (range 90%–100%, mean Cohen κ = .88; Aktar, Colonnesi, de Vente, Majdandžić, & Bögels, 2017). Past and current AD diagnoses were combined into a lifetime AD diagnosis per parent. Subsequently, a categorical AD score was created per couple: ‘AD 0’ = none of the parents with an AD (reference category); ‘AD 1’ = one parent with an AD; and ‘AD 2’ = both parents with an AD. The child was considered at risk for ADs when one or both parents had a lifetime AD. Likewise, past and current diagnoses of major depression (MD) were combined into a lifetime MD diagnosis per parent and a categorical MD score was created per couple, with ‘MD 0’ as reference category indicating that none of the parents had a diagnosis MD, ‘MD 1’ indicating that one parent had a diagnosis MD, and ‘MD 2’ indicating that both parents had a diagnosis MD. Additionally, a dimensional approach was adopted, and AD and MD severity ratings of mothers and fathers of lifetime ADs and MD were combined into a total severity score of couples’ AD and MD, respectively.
Child fearful temperament
Infants’ negative reactivity at 4 months, which is considered a precursor of behavioural inhibition and fearful temperament (Kagan & Snidman, 1991), was coded during the novel stimuli task described above. In each time interval of all stimulus presentations, the following behavioural indices were coded: negative facial expression (e.g. frowning, a fearful/crying face), protest (e.g. whining, fussing), crying, motor activity and arching. These behaviours were averaged across time intervals, standardized and aggregated to obtain a negative reactivity score. Interobserver reliability was good: intraclass correlation = 0.85. For further information on tasks and coding, see Majdandžić et al. (2018).
At 1 and 2.5 years, child fearful temperament was assessed using 7 tasks and 8 tasks, respectively, from well‐known standard laboratory protocols to observe behavioural inhibition. During the tasks, the parent was sitting behind the child and instructed to remain neutral. At 1 year, the child was sitting in a high chair, except in the truck task in which it was positioned on the floor. The tasks were as follows: (1) unpredictable mechanical toy, (2) Stranger approach, (3) Masks, all three from the Lab‐TAB (Goldsmith & Rothbart, 1996); (4) Truck (Calkins, Fox, & Marshall, 1996); (5) Buzzing animal, (6) Ambulance, and (7) Horse, all three modelled after Rothbart (1988). At 2.5 years, the child was seated in a child‐sized chair behind a small table. The tasks were as follows: (1) unpredictable mechanical toy and (2) Stranger approach, both from the Lab‐TAB (Goldsmith & Rothbart, 1996); (3 and 4) two different versions of Risk room (Durbin, Klein, Hayden, Buckley, & Moerk, 2005); (5) Clown (Buss, 2011); 6) Dino; (7) Bug and (8) Parrot, all three modelled after Rothbart (1988). At both ages, the following behaviours were coded: facial, bodily and verbal expressions of fear, latency to first fear response, latency to touch the stimulus, escape, freeze and distress vocalizations (Goldsmith & Rothbart, 1996). Coded behaviours were averaged across coding intervals within each task, standardized and averaged per task. Then, task scores were averaged into a single score for fearful temperament at 1 year and 2.5 years. Mean interobserver reliability (intraclass correlation) of coded variables across tasks was good: 0.91 at 1 year and 0.83 at 2.5 years. For detailed descriptions, see: Majdandžić et al. (2018) and Aktar et al. (2017).
Background variables
Family’s socioeconomic status (SES) was measured through a question about the education level of the parent on an 8‐point scale from 1 – primary education, to 8: university. A mean family level of SES was calculated by averaging father’s and mother’s education level. Child gender was measured through a question about the sex of the child. Child age was calculated as the mean age at the three measurement occasions (father laboratory visit, mother laboratory visit and home visit) of the 4 months measurement. Child gestational age was calculated by adding 40 (estimated pregnancy duration in weeks) to the difference of the actual birth date and the estimated birth date (at 40 weeks).
Data analyses plan
We assessed hypothesis 1, on the relation of parents’ anxiety (temperament, disorder and disorder severity) and indices of infants’ autonomic arousal (HR and RMSSD), and hypothesis 2, on the relation of indices of infants’ autonomic arousal (HR and RMSSD) and child fearful temperament (observed fearful temperament), using hierarchical linear mixed model analyses. For hypothesis 1, in which we studied the relation of parents’ anxiety with indices of infants’ autonomic arousal that were repeatedly measured, two‐level models (task phases [Figure 1] within individual) using maximum likelihood estimation were tested for HR and RMSSD separately. Separate models were run for each predictor: (a) couple’s temperamental fear score, (b) couples’ AD (0/1/2) and (c) couples’ AD severity, resulting in testing six models per task (novel stimuli task and habituation task). To predict infant phasic arousal, we tested the models with the predictor (i.e. parents’ anxiety index), task phase (dummy coded with baseline as a reference) and the predictor*task‐phase interaction included. A significant predictor*task‐phase interaction implies different associations between the predictor and the various task phases, which points towards an association between the predictor and deviant physiological reactivity and/or recovery. In case interactions were statistically nonsignificant (suggesting that no significant associations between parents’ anxiety index and infant physiological reactivity and/or recovery are presented), main effects were interpreted as reflecting tonic arousal and were reported. This analytic strategy, rather than interpreting associations with baseline as tonic arousal, is more parsimonious and analyses have more power. To predict infants’ physiological adaptation, associations between predictors (i.e. parents’ anxiety indices) and infants’ habituation index were analysed using linear regression analyses. To test specificity of the association between parents’ anxiety and infant hyperarousal, we repeated the analyses with depression (disorder and disorder severity) as a covariate. In this manner, we tested whether the parents’ anxiety – infant hyperarousal associations still hold, when accounting for parental depression.
For hypothesis 2, in which we assessed the relation of infants’ autonomic arousal (HR and RMSSD) with child fearful temperament (observed fearful temperament at 4 months, 1 year and 2.5 years), two‐level models (repeated fearful temperament observations within individual) using maximum likelihood estimation were tested for the hyperarousal indices (baseline, reactivity, recovery, physiological adaptation) separately. To assess tonic arousal as a predictor of child fearful temperament, baseline HR and RMSSD were predictors (in separate analyses). To study phasic arousal as a predictor of fearful temperament, separate models were run for physiological reactivity and recovery. First, to assess physiological reactivity predicting child fearful temperament, mean HR during the stimuli and baseline HR were added to the model (i.e. mean HR stimuli value corrected for baseline HR). We did the same analysis for RMSSD. Second, to assess physiological recovery predicting child fearful temperament, HR during the post‐stimuli resting phase and baseline HR were predictors in the model (i.e. HR during post‐stimuli rest corrected for baseline HR). We did the same analysis for RMSSD. Hence, we tested six models per task (novel stimuli task and habituation task). Robustness of the findings of hypotheses 1 and 2 was tested by repeating the analyses, adjusting for the covariates family SES, child gender, child age and gestational age at birth. All analyses were performed with IBM SPSS, version 25.
Results
Preliminary analyses
Information on dropout can be found in de Vente et al. (2011) and Majdandžić et al. (2018). Physiological data during the novel stimuli task were available for 120 infants (17 missing). For nine infants data were missing because no mother laboratory visit took take place; for two infants because of equipment problems; for six infants, data were too noisy. Parents of infants with versus without missing data during this task did not differ on couples’ anxiety measures; all p‐values >.10. Physiological data during the habituation task were available for 114 infants (23 missing). For eight infants, data were missing because no father lab visit took place; for 12 infants because of equipment problems; for three infants, data were too noisy. Parents of infants with versus without missing physiological data during the habituation task did not differ on couples’ anxiety measures; all p‐values >.10. For the habituation index, data were available for 72 infants (42 missing); 20 infants did not have data due to equipment problems during the repeated noises; for three infants, the signal during the repeated noises was too noisy; for 19 infants, the task was terminated due to infant distress. Parents of infants with missing habituation index data due to technical problems or due to infant crying (analysed separately) did not differ from parents of infants with present data on couples’ anxiety measures; all p‐values >.10. Data of at least one parental anxiety measure or one child temperament measure and at least one infant physiological measure were available for 128 infants and were analysed in this study.
RMSSD, the index of HRV, was non‐normally distributed (standardized skewness and kurtosis> |3.29|39) and was therefore log‐transformed (ln). After transformation, standardized skewness and kurtosis were < |3.29|. There was one outlier in the habituation index (>M ± 3.29 SD), which was excluded from the analyses.
Descriptive information
Of the 128 couples, 33 (25.8%) couples received no lifetime AD diagnosis, 51 (39.8%) couples had one parent diagnosed with at least one lifetime AD, and 44 (34.4%) couples had both parents diagnosed with at least one lifetime AD. Regarding MD, 82 (64.0%) couples received no lifetime MD diagnosis, 41 (32.0%) couples had one parent diagnosed with lifetime MD, and 5 (4.0%) couples had both parents diagnosed with lifetime AD. Other descriptive information is presented in Table 1. Descriptive information on infant HR and HRV per category of couples’ diagnostic status is presented in Figure 2A–D. A correlation matrix of the main variables is provided in Table S1. A correlation matrix including the covariates SES, child gender, child age and gestational age at birth and the infant hyperarousal measures is provided in Table S2. We found no infant gender differences in arousal indices (all p‐values >.10).
Table 1.
Descriptives of couples’ continuous anxiety measures, child fearful temperament measures and infant habituation index
| M (SD) | n | |
|---|---|---|
| Couples’ anxiety | ||
| ATQ fear scale | 3.05 (0.60) | 125 |
| AD severity (CSR score) | 7.18 (7.09) | 128 |
| Couples’ depression | ||
| MD severity (CSR score) | 1.61 (1.87) | 128 |
| Child fearful temperament | ||
| 4 months | −0.01 (0.41) | 123 |
| 1 year | −0.02 (0.40) | 120 |
| 2.5 years | 0.01 (0.44) | 117 |
| Infant habituation | ||
| Habituation index | −2.14 (13.71) | 71 |
AD, anxiety disorder; ATQ, Adult Temperament Questionnaire; CSR, Clinician Severity Rating; MD, major depression.
Figure 2.
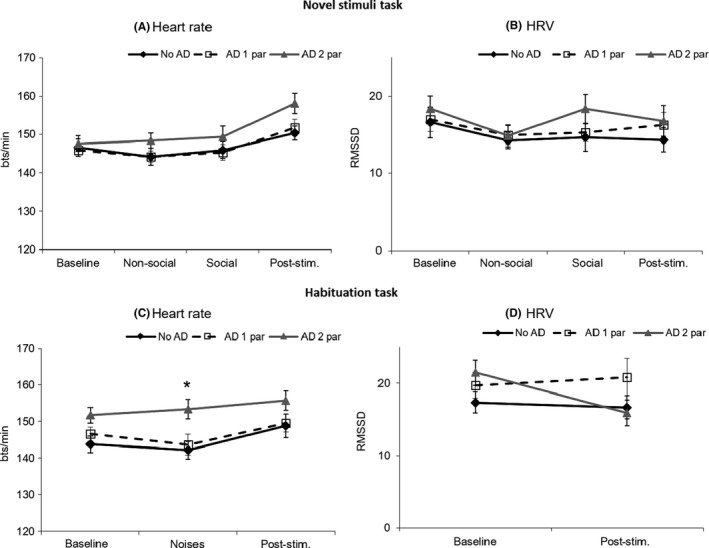
Descriptives of infant heart rate and infant HRV (untransformed values) during the novel stimuli task (A, B) and the habituation task (C, D) separately for infants of couples with no AD (no AD), of couples with one parent with an AD (AD 1 par) and of couples with both parents with ADs (AD 2 par). Note: post‐stim. = post‐stimuli rest phase. *Infants with two parents with an AD showed a higher HR during the habituation task, main effect: B = 7.53, SE = 2.88, β = .51, p = .010
Parents’ anxiety predicting infant autonomic arousal and habituation
All parental anxiety index*task‐phase interactions were statistically nonsignificant, providing no support for the presence of an association between parents’ anxiety and deviant reactivity or recovery, which would be indicative of an association between parental anxiety and phasic hyperarousal. Therefore, main effects were reported as an index of infant tonic hyperarousal (see Table S3). Parents’ temperamental fear and parents’ combined AD severity were significantly positively related to infant HR during both tasks (Novel stimuli task: AD severity score: B = 0.42, SE = 0.14, β = .22, p = .003; Habituation task: couples’ temperamental fear: B = 4.09, SE = 2.03, β = .16, p = .047; AD severity score: B = 0.55, SE = 0.16, β = .27, p = .001); significance was at trend level for parents’ temperamental fear in the novel stimuli task (B = 2.91, SE = 1.66, β = .13, p = .083). Parents’ combined AD status was significantly related to infant HR during the habituation task, F(2,123) = 4.11, p = .019. Higher HR during the habituation task was found in infants of couples with both parents diagnosed with lifetime AD (B = 7.53, SE = 2.88, β = .51, p = .010), but not in couples with only one parent with a lifetime AD (B = 1.63, SE = 2.83, β = .11, p = .564). The associations between parental anxiety measures and HRV or the habituation index were nonsignificant. Adjustment of the analyses for the covariates SES, child gender, child age and gestational resulted in similar outcomes: all significant results remained statistically significant.
Adjustment of the analyses for parents’ depression (disorder and severity) resulted in similar outcomes, with the majority of significant findings remaining statistically significant (five out of eight = 63%), one becoming significant at trend level and two becoming nonsignificant. Moreover, parents’ MD was not a significant predictor in any of the models, highlighting that parental anxiety was the strongest predictor for infant hyperarousal. More specifically, when adjusting for parents’ MD status the following predictors were still significant: Novel stimuli task: AD severity score: p = .021; Habituation task: AD in both parents: p = .038; and AD severity score: p = .003. When adjusting for MD severity, the following significant results remained statistically significant: Novel stimuli task: AD severity score: p = .026 and Habituation task: AD severity score: p = .010, and the following result became significant at trend level: Habituation task: AD in both parents: p = .061. Couples temperamental fear was no longer a significant predictor in the habituation task: adjustment for parents’ MD: p = .113 and adjustment for parents’ MD severity: p = .163.
Infant autonomic arousal predicting fearful temperament
A significant time*baseline HR interaction was found for the novel stimuli task (F(2,230.9) = 3.08, p = .048), indicating that the association between baseline HR and fearful temperament was dependent on age. Therefore, stratified analyses (linear regression analyses at the different child ages) were conducted. Higher baseline HR during the novel stimuli task predicted a more fearful temperament at 2.5 years (B = 0.007, SE = 0.001, β = .21, p = .030). Stronger HR reactivity and less HR recovery during the novel stimuli task at 4 months predicted a more fearful temperament (B = 0.007, SE = 0.003, β = .20, p = .016; B = 0.007, SE = 0.002, β = .22, p = .002, respectively; see also Table S4). The associations of HRV measures with child fearful temperament were nonsignificant, as was the relation of the habituation index with fearful temperament. Adjustment of the analyses for the covariates SES, child gender, child age and gestational resulted in similar outcomes. All significant results remained statistically significant.
Discussion
Autonomic hyperarousal was examined as a dispositional risk factor for ADs by relating parental anxiety to physiological arousal in their 4‐month‐old offspring, and by relating infants’ physiological arousal to their own fearful temperament. A more fearful temperament in parents (self‐report), presence of ADs in both parents (clinician report) and a higher combined severity of ADs in parents (clinician report) were related to higher HR in their 4‐month‐old infant, supporting tonic hyperarousal as a dispositional risk factor for ADs. Autonomic hyperarousal seemed to be linked specifically to parents’ anxiety, since the majority of significant findings remained significant after adjustment for parents’ MD or parents’ MD severity, and parents’ depression did not uniquely predict infant hyperarousal in addition to parents’ anxiety. This provides further support for the tripartite model of anxiety and depression (Clark & Watson, 1991). For parents’ fearful temperament, relations were less strong, which may be explained by the modest, though, acceptable internal consistency of the questionnaire scale used to assess parents’ fearful temperament. Regarding child fearful temperament, more HR reactivity and less HR recovery at 4 months (novel stimuli task), in other words, phasic hyperarousal, predicted a more fearful temperament during infancy and toddlerhood. In addition, higher tonic HR at 4 months prospectively predicted a more fearful temperament at 2.5 years only.
Our results provide further support for autonomic hyperarousal as a dispositional risk factor for ADs. The relations we found between parental anxiety measures and infant tonic hyperarousal supplement the results of Turner et al. (2005), who reported tonic autonomic hyperarousal in 7‐ to 12‐year‐old offspring of parents with ADs. We also found evidence for phasic autonomic hyperarousal as a dispositional risk factor for ADs, as infants’ phasic autonomic hyperarousal was associated with infant fearful temperament. Our results strengthen previous findings in older children reported by Grillon et al. (1997) and Merikangas et al. (1999).
We found no evidence for impaired physiological adaptation as a dispositional risk factor for ADs. These results are in line with Grillon et al. (1997) and Merikangas et al. (1999), who also found no deviant habituation in older offspring of parents with ADs, but not with Turner et al. (2005). The differences may be due to our use of HR rather than the potentially more sensitive electrodermal activity used by Turner et al. (2005). Furthermore, Grillon et al. (1997) and Merikangas et al. (1999) used a conditioning paradigm rather than a habituation paradigm, which may explain why their findings differed from Turner et al.’s (2005). In addition, Grillon et al. (1997), Merikangas et al. (1999) and we used repeated noises as a stimulus, which may be less fear relevant than the snake stimulus used by Turner et al. (2005). Alternatively, absence of significant relations between habituation and a fearful temperament that we found may supplement the results of Moehler et al. (2006) in very young infants. Together these studies suggest that impaired physiological adaptation from repeated noises is only visible at a very young age, as Moehler et al. (2006) found this in infants of 2 weeks and not in infants of 6 weeks old, and we not found at the age of 4 months. In sum, results regarding habituation are inconclusive and additional studies are needed using various stimuli at different ages before firm conclusions can be drawn.
We found no indications for deviant parasympathetic activity as a dispositional risk factor for developing ADs as we found no deviations in HRV during infancy. Our HR and HRV results, together with those of Grillon et al. (1997), Merikangas et al. (1999) and Turner et al. (2005) in older children, suggest that hyperarousal associated with the risk for ADs is sympathetically rather than parasympathetically driven.
Taken together, since parents’ anxiety was associated with infant tonic hyperarousal, which in turn predicted infant fearful temperament at the age of 2.5 years, our results provide strongest support for tonic hyperarousal as a mechanism explaining the intergenerational transmission of ADs. The fact that we did not find an association of tonic hyperarousal with fearful temperament at the ages of 4 months and 1 year may be explained by developmental processes in temperament and in the autonomic system; it is known that the association of temperament with autonomic functioning is not yet very stable during infancy (Snidman, Kagan, Riordan, & Shannon, 1995). With regard to phasic arousal, we cannot rule out its role in the intergenerational transmission of ADs, despite the fact that we found no evidence for an association of parents’ anxiety with infants phasic arousal. Again, developmental processes in autonomic functioning may have affected the association at the age of 4 months as well as the extent to which parents’ anxiety indices assessed either fear or anxiety.
This study has several strengths. We used a high‐risk‐design to study the early premorbid characteristics of children at risk for developing ADs, thus showing that hyperarousal is present before an actual AD. Furthermore, we used a multimethod approach to measure mothers’ and fathers’ anxiety, we measured infant autonomic hyperarousal in two different contexts and child fearful temperament using a large battery of behavioural measures at three different ages during infancy and toddlerhood. Especially the finding of elevated HR at 4 months in offspring of parents with ADs was consistent across tasks, across methods and after correction for potential confounders, highlighting its robustness.
This study also has several limitations. First, we conducted multiple tests per hypothesis, which is associated with an increased risk of making a type I error. Results adjusted for multiple testing using the Benjamini–Hochberg procedure (Benjamini & Hochberg, 1995) are presented in Tables S3 and S4. Second, our sample consisted primarily of relatively highly educated, intact (i.e. biological parents, married/cohabiting at the start of the study) Caucasian families, thereby limiting generalizability of our findings to other populations. In addition, our sample came from the general population, which may to some extent explain why we found low associations between parents’ anxiety indices and child fearful temperament, and why we found low rank order stability for fearful temperament from infancy to toddlerhood. Our sample may not have included extremely anxious (i.e. treatment seeking parents) and fearless parents and, consequently, infants with extremely low and high fearful temperament. This potential restriction of range in parents’ anxiety and child fearful temperament suggests that associations between parents’ anxiety and infant autonomic hyperarousal and between infant autonomic hyperarousal and child fearful temperament may be even stronger in a sample with a larger variation in parents’ anxiety and child temperament. Third, although we measured autonomic hyperarousal early in infancy, parental anxiety may also have influenced the infants’ autonomic arousal through parent–partner dynamics and parent–child interactions. Therefore, autonomic arousal at the age of 4 months may not be seen as solely genetically determined. Fourth, we related autonomic hyperarousal to a fearful temperament rather than to presence of an AD in the children themselves. Therefore, our evidence for autonomic hyperarousal as a premorbid characteristic of ADs is indirect.
Several directions for future research are indicated. First, actual mediation of autonomic hyperarousal indices in the association between parents’ anxiety and child fearful temperament should be tested. Second, to further assess aetiological pathways of ADs, studies may prospectively relate premorbid autonomic hyperarousal to presence of an AD during late childhood and adolescence, the periods in which ADs typically develop. Third, studies may explore autonomic hyperarousal as an endophenotype of ADs by addressing other endophenotype criteria (Gottesman & Gould, 2003) by conducting genetic linkage studies and studying hyperarousal in relatives of individuals with an AD.
If our findings are replicated and early autonomic hyperarousal becomes an established premorbid indicator of ADs, early detection of children at risk for developing ADs may be improved. In addition, preventive strategies that affect autonomic hyperarousal such as relaxation techniques or meditation (e.g. Varvogli & Darviri, 2011) may be further developed and tested in children and parents.
In conclusion, parental prenatal anxiety (disorders) predicted infants’ autonomic arousal, which in turn predicted later fearful temperament in children. Hence, we found support for autonomic hyperarousal as a predisposing risk factor for ADs. Future research may focus at aetiological mechanisms linking autonomic hyperarousal to actual development of ADs.
Supporting information
Table S1. Correlation coefficients (with p‐values below) of the main study measures.
Table S2. Correlation coefficients (with p‐values below) of the main study measures and covariates.
Table S3. Regression coefficients of main effects of infant physiological activity regressed on parents’ temperamental fear, AD status, and AD severity.
Table S4. Regression coefficients of main effects of child fearful temperament regressed on infant physiological activity.
Acknowledgements
The authors would like to thank Sarah de Schutter, M.Sc., Annefleur Visscher, M.Sc., Jasmijn Rahder, M.Sc. and Hester Verwey, M.Sc. for their work in the recruitment and data collection phase. The authors have declared that they have no competing or potential conflicts of interest.
Key points.
Autonomic hyperarousal has been proposed as a predisposition to anxiety disorders; however, studies on autonomic hyperarousal in children at risk for anxiety disorders are scarce.
This study showed that parents’ anxiety predicts their infants’ autonomic hyperarousal, which in turn predicts child fearful temperament.
Our findings suggest that autonomic hyperarousal is a genetic predisposition to anxiety disorders.
Anxiety treatments may focus more on reducing autonomic hyperarousal.
Conflict of interest statement: No conflicts declared.
References
- Aktar, E. , Colonnesi, C. , de Vente, W. , Majdandžić, M. , & Bögels, S.M. (2017). How do parents' depression and anxiety, and infants' negative temperament relate to parent–infant face‐to‐face interactions? Development and Psychopathology, 29, 697–710. [DOI] [PubMed] [Google Scholar]
- Ask, H. , Torgersen, S. , Seglem, K.B. , & Waaktaar, T. (2014). Genetic and environmental causes of variation in adolescent anxiety symptoms: A multiple‐rater twin study. Journal of Anxiety Disorders, 28, 363–371. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. [Google Scholar]
- Berntson, G.G. , Quickley, K.S. , & Lozano, D. (2007). Cardiovascular psychophysiology In Cacioppo J.T., Tassinary L.G. & Berntson G.G. (Eds.), Handbook of psychophysiology (3rd edn, pp. 182–210). New York: Cambridge University Press. [Google Scholar]
- Bolton, D. , Eley, T.C. , O'connor, T.G. , Perrin, S. , Rabe‐hesketh, S. , Rijsdijk, F. , & Smith, P. (2006). Prevalence and genetic and environmental influences on anxiety disorders in 6‐year‐old twins. Psychological Medicine, 36, 335–344. [DOI] [PubMed] [Google Scholar]
- Bradley, M.M. , & Lang, P.J. (2007). Emotion and motivation In Cacioppo J.T., Tassinary L.G. & Berntson G.G. (Eds.), Handbook of psychophysiology (3rd edn, pp. 581–607). New York: Cambridge University Press. [Google Scholar]
- Brown, T.A. , Barlow, D.H. , & Di Nardo, P.A. (1994). Anxiety disorders interview schedule adult version: Client interview schedule. Oxford, UK: Oxford University Press. [Google Scholar]
- Buss, K.A. (2011). Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology, 47, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins, S.D. , Fox, N.A. , & Marshall, T.R. (1996). Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development, 67, 523–540. [PubMed] [Google Scholar]
- Cannon, W.B. (1914). The emergency function of the adrenal medulla in pain and the major emotions. American Journal of Physiology, 33, 356–372. [Google Scholar]
- Clark, L.A. , & Watson, D. (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Clauss, J.A. , & Blackford, J.U. (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta‐analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, M.E. , Schell, A.M. , & Filion, D.L. (2007). The electrodermal system In Cacioppo J.T., Tassinary L.G. & Berntson G.G. (Eds.), Handbook of psychophysiology (3rd edn, pp. 159–181). New York: Cambridge University Press. [Google Scholar]
- de Vente, W. , Majdandžić, M. , Colonnesi, C. , & Bögels, S.M. (2011). Intergenerational transmission of social anxiety: The role of paternal and maternal fear of negative child evaluation and parenting behaviour. Journal of Experimental Psychopathology, 2, 509–530. [Google Scholar]
- Durbin, C.E. , Klein, D.N. , Hayden, E.P. , Buckley, M.E. , & Moerk, K.C. (2005). Temperament emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114, 28–37. [DOI] [PubMed] [Google Scholar]
- Evans, D.E. , & Rothbart, M.K. (2007). Developing a model for adult temperament. Journal of Research in Personality, 41, 868–888. [Google Scholar]
- Fox, N.A. , Henderson, H.A. , Marshall, P.J. , Nichols, K.E. , & Ghera, M.M. (2005). Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology, 56, 235–262. [DOI] [PubMed] [Google Scholar]
- Friedman, B.H. (2007). An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74, 185–199. [DOI] [PubMed] [Google Scholar]
- Goldsmith, H.H. , & Rothbart, M.K. (1996). The Laboratory Temperament Assessment Battery, locomotor version (manual), Technical Manual, Department of Psychology, University of Wisconsin, Madison, WI, USA. [Google Scholar]
- Gottesman, I.I. , & Gould, T.D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry, 160, 636–645. [DOI] [PubMed] [Google Scholar]
- Grillon, C. , Dierker, L. , & Merikangas, K.R. (1997). Startle modulation in children at risk for anxiety disorders and/or alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 925–932. [DOI] [PubMed] [Google Scholar]
- Gustavsson, A. , Svensson, M. , Jacobi, F. , Allgulander, C. , Alonso, J. , Beghi, E. , … & Gannon, B. (2011). Cost of disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21, 718–779. [DOI] [PubMed] [Google Scholar]
- Hettema, J.M. , Neale, M.C. , & Kendler, K.S. (2001). A review and meta‐analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry, 158, 1568–1578. [DOI] [PubMed] [Google Scholar]
- Kagan, J. , Reznick, J.S. , & Snidman, N. (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473. [PubMed] [Google Scholar]
- Kagan, J. , & Snidman, N. (1991). Infant predictors of inhibited and uninhibited profiles. Psychological Science, 2, 40–44. [Google Scholar]
- Kagan, J. , & Snidman, N. (1999). Early childhood predictors of adult anxiety disorders. Biological Psychiatry, 46, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Kaltsas, G.A. , & Crousos, G.P. (2007). The neuroendocrinology of stress In Cacioppo J.T., Tassinary L.G. & Berntson G.G. (Eds.), Handbook of psychophysiology (3rd edn, pp. 303–318). New York: Cambridge University Press. [Google Scholar]
- Majdandžić, M. , de Vente, W. , Colonnesi, C. , & Bögels, S.M. (2018). Fathers' challenging parenting behavior predicts less subsequent anxiety symptoms in early childhood. Behaviour Research and Therapy, 109, 18–28. [DOI] [PubMed] [Google Scholar]
- Merikangas, K.R. , Avenevoli, S. , Dierker, L. , & Grillon, C. (1999). Vulnerability factors among children at risk for anxiety disorders. Biological Psychiatry, 46, 1523–1535. [DOI] [PubMed] [Google Scholar]
- Moehler, E. , Kagan, J. , Parzer, P. , Wiebel, A. , Brunner, R. , & Resch, F. (2006). Relation of behavioral inhibition to neonatal and infant cardiac activity, reactivity and habituation. Personality and Individual Differences, 41, 1349–1358. [Google Scholar]
- Molenkamp, B. (2011). VSRRP98 Manual, Version 8.0. University of Amsterdam, The Netherlands. [Google Scholar]
- Olatunji, B.O. , Cisler, J.M. , & Tolin, D.F. (2007). Quality of life in the anxiety disorders: A meta‐analytic review. Clinical Psychology Review, 27, 572–581. [DOI] [PubMed] [Google Scholar]
- Olsson, A. , & Phelps, E.A. (2007). Social learning of fear. Nature Neuroscience, 10(9), 1095. [DOI] [PubMed] [Google Scholar]
- Pumprla, J. , Howorka, K. , Groves, D. , Chester, M. , & Nolan, J. (2002). Functional assessment of heart rate variability: physiological basis and practical applications. International Journal of Cardiology, 84, 1–14. [DOI] [PubMed] [Google Scholar]
- Rapee, R.M. (2012). Family factors in the development and management of anxiety disorders. Clinical Child and Family Psychology Review, 15, 69–80. [DOI] [PubMed] [Google Scholar]
- Rothbart, M.K. (1988). Temperament and the development of inhibited approach. Child Development, 59, 1241–1250. [PubMed] [Google Scholar]
- Schmitz, J. , Krämer, M. , Tuschen‐Caffier, B. , Heinrichs, N. , & Blechert, J. (2011). Restricted autonomic flexibility in children with social phobia. Journal of Child Psychology and Psychiatry, 52, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Schmitz, J. , Tuschen‐Caffier, B. , Wilhelm, F.H. , & Blechert, J. (2013). Taking a closer look: autonomic dysregulation in socially anxious children. European Child and Adolescent Psychiatry, 22, 631–640. [DOI] [PubMed] [Google Scholar]
- Snidman, N. , Kagan, J. , Riordan, L. , & Shannon, D.C. (1995). Cardiac function and behavioral reactivity during infancy. Psychophysiology, 32, 199–207. [DOI] [PubMed] [Google Scholar]
- Sylvers, P. , Lilienfeld, S.O. , & LaPrairie, J.L. (2011). Differences between trait fear and trait anxiety: Implications for psychopathology. Clinical Psychology Review, 31, 122–137. [DOI] [PubMed] [Google Scholar]
- Turner, S.M. , Beidel, D.C. , & Epstein, L.H. (1991). Vulnerability and risk for anxiety disorders. Journal of Anxiety Disorders, 5, 151–166. [Google Scholar]
- Turner, S.M. , Beidel, D.C. , & Roberson‐Nay, R. (2005). Offspring of anxious parents: reactivity, habituation, and anxiety‐proneness. Behaviour Research and Therapy, 43, 1263–1279. [DOI] [PubMed] [Google Scholar]
- Varvogli, L. , & Darviri, C. (2011). Stress management techniques: evidence‐based procedures that reduce stress and promote health. Health Science Journal, 5, 74. [Google Scholar]
- WHO Mental Health Survey Consortium (2004). Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Journal of the American Medical Association, 291, 2581–2590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation coefficients (with p‐values below) of the main study measures.
Table S2. Correlation coefficients (with p‐values below) of the main study measures and covariates.
Table S3. Regression coefficients of main effects of infant physiological activity regressed on parents’ temperamental fear, AD status, and AD severity.
Table S4. Regression coefficients of main effects of child fearful temperament regressed on infant physiological activity.


