Abstract
Background
Chronic rhinosinusitis (CRS) is a chronic inflammatory disease associated with a substantial personal and socioeconomic burden. Monitoring of patient‐reported outcomes by mobile technology offers the possibility to better understand real‐life burden of CRS.
Methods
This study reports on the cross‐sectional evaluation of data of 626 users of mySinusitisCoach (mSC), a mobile application for CRS patients. Patient characteristics of mSC users were analysed as well as the level of disease control based on VAS global rhinosinusitis symptom score and adapted EPOS criteria.
Results
The mSC cohort represents a heterogeneous group of CRS patients with a diverse pattern of major symptoms. Approximately half of patients reported nasal polyps. 47.3% of all CRS patients were uncontrolled based on evaluation of VAS global rhinosinusitis symptom score compared to 40.9% based on adapted EPOS criteria. The impact of CRS on sleep quality and daily life activities was significantly higher in uncontrolled versus well‐controlled patients. Half of patients had a history of FESS (functional endoscopic sinus surgery) and reported lower symptom severity compared to patients without a history of FESS, except for patients with a history of more than 3 procedures. Patients with a history of FESS reported higher VAS levels for impaired smell.
Conclusion
Real‐life data confirm the high disease burden in uncontrolled CRS patients, clearly impacting quality of life. Sinus surgery improves patient‐reported outcomes, but not in patients with a history of more than 3 procedures. Mobile technology opens a new era of real‐life monitoring, supporting the evolution of care towards precision medicine.
Keywords: Mobile health technology, nasal polyp, patient‐reported outcome measure, real‐world evidence, visual analogue scale
This study reports on the cross‐sectional evaluation of patient‐reported outcome measures in 626 chronic rhinosinusitis patients of which half reported nasal polyp disease. Over 40% of patients were uncontrolled, which clearly impacted their quality of life. Sinus surgery improved patient‐reported outcomes, but not in patients with a history of more than 3 procedures. Abbreviations: CRSw/sNP, chronic rhinosinusitis with/without nasal polyps; FESS, functional endoscopic sinus surgery; VAS, visual analogue scale.

Abbreviations
- CRSw/sNP
chronic rhinosinusitis with/without nasal polyps
- FESS
functional endoscopic sinus surgery
- VAS
visual analogue scale
1. INTRODUCTION
Chronic rhinosinusitis (CRS) is defined as a chronic inflammatory disorder of the nose and paranasal sinuses. 1 , 2 The clinical presentation consists of an impaired sense of smell, facial pain or pressure, postnasal drip, rhinorrhoea and/or nasal congestion for a consecutive period of at least 12 weeks. 1 CRS affects 10.9% of the European citizens according to population‐bases studies and is associated with a significant socioeconomic burden. 3 , 4 CRS can be divided into two major subtypes: with (CRSwNP) or without (CRSsNP) nasal polyps. 5 Sinonasal type 2 inflammation is found in the majority of European CRSwNP patients. 6 Although CRS patients frequently report co‐morbidities such as asthma and allergic rhinitis, it is unclear if a causal relationship between atopy and CRS truly exists. 7 , 8 In a study of almost 600 patients, more severe asthma was found to be associated with a more severe CRS with allergic sensitization and/or nasal polyps. 9
The cornerstone of CRS treatment includes saline rinsing and anti‐inflammatory treatment with prolonged topical or short‐course oral corticosteroids. 1 Functional endoscopic sinus surgery (FESS) is recommended in case of failure of maximal pharmaceutical treatment. Nonetheless, a substantial percentage of CRS patients still experiences bothersome symptoms interfering with daily life despite standard pharmaceutical and surgical therapy. 10 According to EPOS guidelines, uncontrolled disease is defined by the presence of three or more of the following features: nasal blockage, anterior or posterior nasal secretions, facial pain, impaired sense of smell and sleep disturbance or fatigue; in addition to signs of diseased sinonasal mucosa and/or need of long‐term antibiotics or systemic corticosteroids in the past 1‐3 months. 1 Forty per cent of patients were found to be uncontrolled despite pharmaceutical and surgical treatment in a tertiary referral centre. 11
Mobile health (mHealth) applications are emerging as novel tools for self‐management in chronic respiratory diseases (CRDs). 12 Apps aim to reinforce patient empowerment by monitoring disease activity, education, personalised messaging and feedback, and facilitating interaction between the patient and the healthcare provider. This engagement may improve medication adherence, health outcomes as well as quality of life. Moreover, the mHealth applications offer the possibility of repeated and remote monitoring of the patients’ disease status, simultaneously collecting large sets of real‐life data on patient‐reported outcome measures. 13
Recently, a mobile application (app) that enables self‐monitoring and patient education, called mySinusitisCoach (mSC), was launched by a consortium of medical experts dealing with CRDs, united by the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA). 14 Similar to the MACVIA Allergy Diary app for patients with allergic rhinitis, 13 a visual analogue scale (VAS) is used to assess the level of disease control. The use of VAS on smartphone screens was validated to assess allergic rhinitis symptoms and disease control. 15
In this study, we report on the cross‐sectional evaluation of baseline data of 626 users of mSC. We recorded the profile of patients using mSC as well as real‐life information on disease control, phenotype and treatment.
2. METHODS
2.1. Users
The app could be freely downloaded from the 2 most common digital distribution platforms (Apple App Store and Google Play Store) and was available in 3 countries (Belgium, the Netherlands and United Kingdom). The app was promoted via various national scientific meetings of ENT doctors as well as flyers and posters in the waiting rooms of the outpatient clinic of both academic and non‐academic centres. Patients were considered for use of the mSC application when they filled out to have 2 or more sinonasal symptoms and/or to have a doctor‐based diagnosis of CRS. The mobile application was recommended to be used for better follow‐up and increased patient empowerment of CRS patients. All mSC users (n = 626) who completed the RhinoSinusitis Diary 14 between 10 November 2017 and 2 April 2019 were included in the study.
2.2. Ethical aspects
The Terms of Use and Privacy Statement, available in the local language, allow the use of the results for research purposes. An institutional review board (IRB) approval was not required.
2.3. Patient profile
The following patients' characteristics were collected in the patient profile of mSC: demographic characteristics (year of birth, gender, country, language), smoking status, presence of co‐morbidities (allergic rhinitis or AR, asthma, chronic obstructive pulmonary disease or COPD) and disease‐related factors (presence of nasal polyps and history of functional endoscopic sinus surgery or FESS). Country‐specific medication for nose, eyes and lungs was recorded.
2.4. Patient outcomes
Information on general and specific CRS symptoms (impaired smell, facial pain or pressure, postnasal drip, nasal secretions and nasal blockage) was obtained using a VAS score (0‐100 mm), 14 which has been previously validated for use on smartphone screens. 15 The major symptom was defined as the CRS symptom with the highest VAS score. In addition, three additional questions addressed the impact of CRS on sleep quality, lower airway symptoms and daily life activities as described earlier. 14 A usability test was performed with a limited number of patients to evaluate the ease to complete the Health Diary as part of the registration process of the app as medical device class I. Patients were able to complete the health diary multiple times per day. The last recorded values for the respective day were used for analysis.
2.5. Assessment of disease control
The level of disease control assessed by the patient in the mSC app was based on the VAS global sinusitis symptom score, which correlates with SinoNasal Outcome Test‐22 (SNOT‐22) scores. 16 The following cut‐off levels were applied: controlled (VAS ≤ 20 mm), partly controlled (VAS >20 mm and ≤50 mm) and uncontrolled (VAS >50 mm and ≤100 mm). Alternatively, disease control was defined based on the adapted EPOS criteria for comparison in Figure 1 only. Five characteristics (nasal blockage, rhinorrhoea/postnasal drip, facial pain, impaired sense of smell and sleep disturbance or fatigue) are deemed critical to determine the level of disease control according to EPOS guidelines: controlled (no symptoms present), partly controlled (presence of at least one symptom) or uncontrolled (presence of 3 or more symptoms). Clinical signs of inflammation and medication history were not taken into account. For the current study, a VAS level higher than 50 mm was used to define the presence of one of the respective symptoms.
Figure 1.
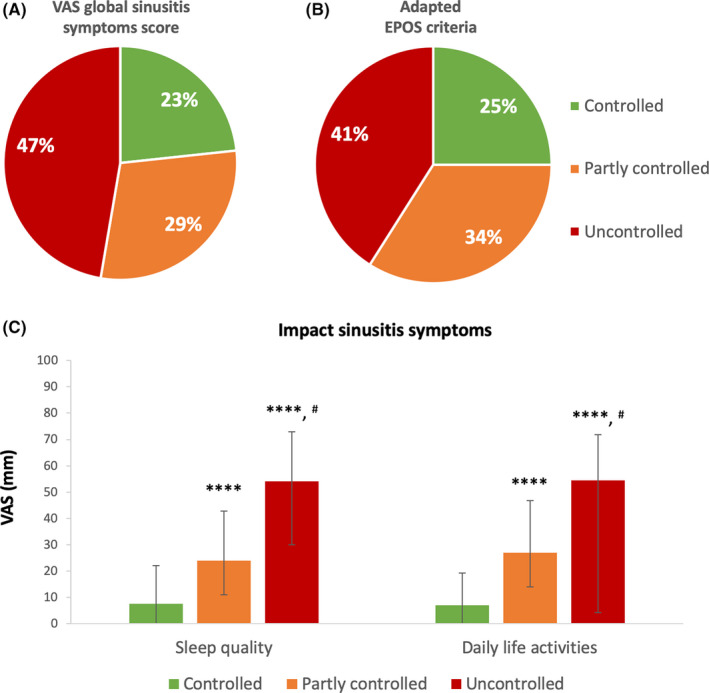
Evaluation of disease control and impact of sinusitis symptoms on sleep quality and daily life activities. A. The level of disease control was defined by VAS global sinusitis symptom score: controlled (VAS ≤ 20 mm), partly controlled (VAS > 20 mm and ≤ 50 mm) and uncontrolled (VAS > 50 mm and ≤ 100 mm). B. Disease control was assessed by evaluation of 5 characteristics (nasal blockage, rhinorrhoea/postnasal drip, facial pain or pressure, impaired sense of smell and sleep disturbance or fatigue) that are deemed critical to determine the level of disease control according to EPOS guidelines: controlled (no symptoms present), partly controlled (presence of one symptom) or uncontrolled (presence of 3 or more symptoms). A VAS level higher than 50 mm defined the presence of one of the respective symptoms. C. The impact of the patients’ rhinosinusitis symptoms on sleep quality and daily life activities were evaluated by VAS: “How much are your sinusitis symptoms affecting your sleep quality?” and “How much are your sinusitis symptoms affecting your work and daily activities today?” Data are presented as median with interquartile range. Three groups of CRS patients stratified by disease control were compared by use of Kruskal‐Wallis test and Dunn's multiple comparison test (as post hoc test). ****: P < .0001 compared to controlled, #: P < .0001 compared to partly controlled
2.6. Evaluation of app use
The number of times the app has been used was defined as the sum of days that the user completed the VAS for global rhinosinusitis symptoms. The time span over which the app was used was defined as the number of days between the first and the last time of use. The patients were advised to use the app on a weekly base. Reminders to complete the health diary were fixed on “weekly” by default to ensure continued data input. Longitudinal patient‐reported data were not included in the current report.
2.7. Statistics
Statistical analyses were performed with GraphPad Prism VI for Macintosh (GraphPad Software Inc, San Diego, USA) by use of Kruskal‐Wallis, Dunn's multiple comparison test (as post‐test) and Mann‐Whitney U test when appropriate. Chi‐square test was used to compare proportions between groups. Normality was analysed prior to between‐group analysis by Shapiro‐Wilk test. t test was performed if data were normally distributed. A difference was considered to be significant when P < .05.
3. RESULTS
3.1. Patient profile
Table 1 summarises the patient characteristics of the mSC users. An equal proportion of men (308; 49.2%) and women (318; 50.8%) used the app, with a mean age of 43.9 ± 13.4 years. A detailed age distribution histogram is shown in Figure S1. The app was used on average 8, 9 and 16 times by, respectively, controlled, partly controlled and uncontrolled patients over a mean period of 9 weeks (data not shown).
Table 1.
Patient characteristics of mySinusitisCoach users
| Total | Controlled | Partly controlled | Uncontrolled | P value* | |
|---|---|---|---|---|---|
| Number of patients, N (%) | 626 | 146 (23.3%) | 184 (29.4%) | 296(47.3%) | |
| Age, years (mean ± SD) | 43.9 ± 13.4 | 43.8 ± 14.1 | 44.2 ± 14.4 | 43.8 ± 12.5 | .95 |
| Male/female | 308/318 | 73/76 | 88/95 | 147/147 | .92 |
| Chronic rhinosinusitis | |||||
| sNP | 48.2% | 44.9% | 48.4% | 49.6% | .68 |
| wNP a | 51.8% | 55.1% | 51.6% | 50.4% | .68 |
| Pharmaceutical treatment | |||||
| Nasal corticosteroids | 45.2% | 39.9% | 44.8% | 43.5% | .54 |
| Oral corticosteroids | 4.2% | 4.4% | 2.7% | 4.4% | .46 |
| Antibiotics | 3.4% | 3.8% | 3.8% | 2.3% | .40 |
| Inhaled corticosteroids | 15.5% | 17.4% | 16.9% | 13.6% | .47 |
| Sinus surgery | |||||
| Primary | 31.9% | 34.9% | 29.5% | 32.0% | .58 |
| Revision | 20.3% | 20.8% | 19.1% | 20.7% | .90 |
| Allergic rhinitis b | 48.2% | 54.6% | 44.8% | 47.1% | .20 |
| Asthma c | |||||
| Childhood onset | 8.5% | 9.8% | 8.8% | 7.3% | .76 |
| Adulthood onset | 23.7% | 24.1% | 21.3% | 23.9% | .71 |
| COPD d | 5.9% | 9.0% | 6.0% | 4.3% | .17 |
| Smoking status | |||||
| Current smoker | 11.8% | 11.4 | 9.8% | 13.3% | .52 |
| Ex‐smoker | 18.6% | 18.8% | 20.8% | 17.0% | .59 |
| Never smoker | 69.6% | 69.8% | 69.4% | 69.7% | 1.00 |
Unknown in 82 patients.
Unknown in 37 patients.
Unknown in 86 patients.
Unknown in 67 patients.
Comparison between controlled, partly controlled and uncontrolled groups.
Approximately half of the CRS population, 282 patients (51.8%), reported physician‐diagnosed nasal polyps (unknown in 82 subjects). Three hundred twenty‐six patients (52.1%) reported to currently take pharmaceutical treatment (nasal corticosteroids (INS), oral corticosteroids (OCS), antibiotics or inhaled corticosteroids (ICS), anti‐histamine or leukotriene receptor antagonist). In total, 327 patients (52.2%) reported a history of FESS (1 FESS: 200 patients, 2 FESS: 69 patients, 3 FESS: 34 patients, >3 FESS: 24 patients). Co‐morbidities such as allergic rhinitis, asthma and COPD were reported in, respectively, 284 (48.2%), 174 (32.2%) and 33 (5.9%) of patients.
3.2. Burden of uncontrolled disease
Upon patient assessment of the level of disease control by the VAS global rhinosinusitis symptom score, 47.3% of patients were identified with uncontrolled disease versus 23.3% and 29.4% of patients with partly controlled and well‐controlled disease, respectively (Figure 1A). No major differences were observed in patient profile characteristics among the three groups (Table 1). CRS patients with uncontrolled disease show higher median VAS for the impact of CRS symptoms on sleep quality as well as daily life activities compared to the partly controlled and controlled patients (Figure 1C and Supplementary Table S1). All specific symptoms were significantly higher in partly controlled (P < .0001) and uncontrolled (P < .0001) patients compared to controlled patients (Supplementary Table S2).
Alternatively, when the adapted EPOS criteria for disease control were applied, 40.9% of patients were defined as uncontrolled compared to 33.9% and 25.2% of patients with partly controlled and well‐controlled disease, respectively (Figure 1B).
3.3. CRSwNP versus CRSsNP
CRS patients with nasal polyps (CRSwNP) reported higher VAS scores for impaired smell than CRS patients without nasal polyps (CRSsNP) (P < .0001), whereas CRSsNP showed higher VAS scores for facial pain compared to CRSwNP (P < .0001) (Figure 2, Supplementary Table S3). Median VAS for major symptom was significantly higher in patients with CRSwNP compared to CRSsNP. Impaired sense of smell was the most bothersome symptom reported by both CRSwNP and CRSsNP (Figure 3). Almost half of CRSwNP patients reported impaired sense of smell as most bothersome symptom (Figure 3).
Figure 2.
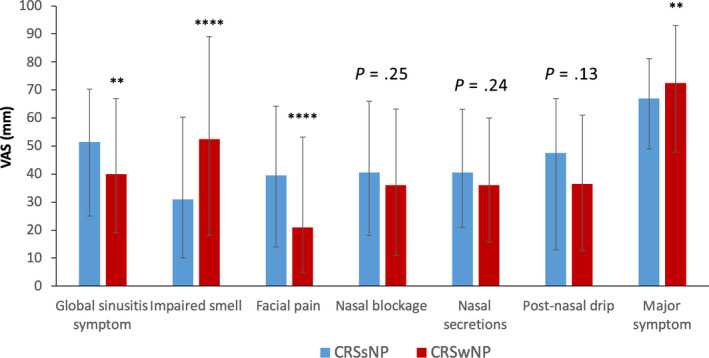
Comparison of global and specific rhinosinusitis symptoms between CRSsNP and CRSwNP phenotypes. Global rhinosinusitis symptoms were assessed by VAS: “How much are your global sinusitis symptoms bothering you today?”. Major symptom was defined as the most bothersome (highest VAS score) specific CRS symptom. Data are presented as median with interquartile range (missing information on NP status in 82 patients). Mann‐Whitney U test was used for in‐between group comparison. **: P < .01, ****: P < .0001
Figure 3.
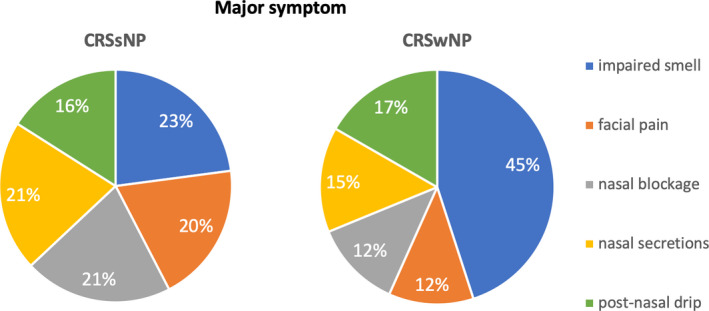
Analysis of the major symptom between CRSsNP and CRSwNP phenotypes. The proportion of patients with a particular major symptom was compared between CRSsNP and CRSwNP. Major symptom was defined as the most bothersome (highest VAS score) specific CRS symptom. Missing information on NP status in 82 patients
3.4. Asthma co‐morbidity
Self‐reported asthma was significantly higher in CRSwNP (42.2%) compared to CRSsNP (16.5%; P < .0001; Figure 4A). Median VAS bronchial symptoms (data from 176 patients) were significantly higher in uncontrolled compared to partly controlled (P < .05) and controlled (P < .0001) CRS patients (Figure 4B). No significant differences in global and specific CRS symptoms were observed between CRS patients with or without asthma (data not shown).
Figure 4.
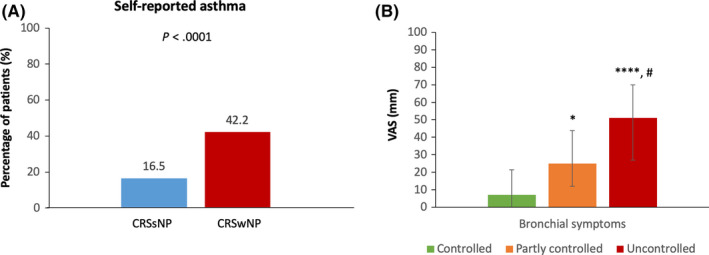
Prevalence of self‐reported asthma (A) and burden of bronchial symptoms (B) in mSC users. A. Information on self‐reported asthma was extracted from the health profile of mSC users. B. Bronchial symptoms were assessed by VAS: “How much is shortness of breath or wheezing bothering you today?”. Data are presented as median with interquartile range. Three groups of CRS patients stratified by disease control were compared by use of Kruskal‐Wallis test and Dunn's multiple comparison test (as post hoc test). *: P < .05 compared to controlled, ****: P < .0001 compared to controlled, #: P < .01 compared to partly controlled
3.5. Impact of treatment on CRS symptoms
3.5.1. Surgical treatment
Figure 5 shows the VAS score for global and specific CRS symptoms in patients stratified by the number of FESS procedures. Global rhinosinusitis symptoms as well as facial pain, nasal blockage and nasal secretions were lower in patient with a history of FESS (1, 2, 3 procedures) compared to patients without a history of FESS. In contrast, patients with a history of FESS reported higher levels of impaired sense of smell compared to patients without history of FESS (P = .003). Interestingly, patients with a history of more than 3 FESS procedures showed the highest level of symptoms, exceeding levels of poor control seen in patients without a history of FESS. The symptom that contributed most to uncontrolled disease in these patients was again impaired sense of smell (Supplementary Figure S2E). The proportion of patients with a history of FESS was significantly higher in CRSwNP (66.0%) compared to CRSsNP (20.6%) patients (P < .0001; Supplementary Table S4). Analysis of the impact of FESS on global and specific CRS symptoms in CRSwNP patients showed similar tendencies as in the full CRS patient cohort (Supplementary Figure S3A‐B).
Figure 5.

Evaluation of global and specific rhinosinusitis symptoms in CRS patients stratified by the number of functional endoscopic sinus surgery (FESS) procedures. Information on the number of FESS procedures was extracted from the health profile of mSC users. Five groups of CRS patients stratified by the number of FESS procedures were compared by use of Kruskal‐Wallis test and Dunn's multiple comparison test (as post hoc test). **: P < .01 compared to 0 FESS procedures
3.5.2. Pharmaceutical treatment
More than half of the CRS patients reported taking pharmaceutical treatment, that is INS (45.2%), OCS (4.2%), antibiotics (3.4%) and/or ICS (15.5%) (Table 1). Global rhinosinusitis symptoms were not significantly lower in patients who reported taking INS (P25‐median‐P75: 20 ‐ 48 ‐ 68) compared to those who did not report taking INS (P25‐median‐P75: 29‐ 52 ‐ 70). The subgroups of patients taking OCS, antibiotics or ICS were too small to perform an adequate analysis.
4. DISCUSSION
This study is the first of its kind providing real‐life data of CRS patients using mHealth technology. Here we report on the cross‐sectional analysis of data from 626 users of mSC in Belgium, The Netherlands and United Kingdom. Forty‐seven per cent of CRS patients were classified as uncontrolled based on self‐evaluation of VAS for global sinusitis symptoms. Uncontrolled patients reported a significantly higher impact of CRS symptoms on sleep quality and daily life activities compared to patients with well‐controlled disease underlining the impact of CRS on different aspects of patient's quality of life.
Achieving or maintaining optimal disease control is key in the decision‐making process of CRS management. EPOS guideline defines disease control by the presence of 5 cardinal symptoms with or without endoscopic signs of infection and the need for systemic therapies. 1 However, so far, no validated tools or questionnaires are however available to assess CRS disease control in real life. SNOT‐22 is the best‐established validated CRS questionnaire to assess the impact of CRS on quality of life. Recently, an association between SNOT‐22 and VAS global rhinosinusitis symptoms score was demonstrated, 16 pointing towards the usefulness of a simple VAS based score to monitor patients with CRS. The use of VAS has previously been validated to assess disease control in patients with allergic rhinitis using the MACVIA Allergy Diary app. 15
The proportion of patients who were identified with uncontrolled disease in the current analysis is in line with previous real‐life reports. We here demonstrated that 296 patients (47.3%) are uncontrolled by use of VAS global rhinosinusitis symptoms compared to 256 patients (40.9%) by use of adapted EPOS criteria. EPOS definition of disease control could however not be entirely assessed given the lack of information on signs of infection and need for systemic therapies. A previous study using the EPOS criteria to assess disease control showed that 40% of CRS patients were uncontrolled despite pharmaceutical and surgical treatment in a tertiary centre. 11 One might however argue that the use of mHealth technology to assess disease control is likely to be biased towards those patients with uncontrolled disease. In that respect, we showed that uncontrolled patients are using mSC over a longer period of time than well‐controlled patients. In addition, patients with uncontrolled disease may be overrepresented in the current cohort because of preferential advertisement of mSC in tertiary centres. Nevertheless, our cross‐sectional data on disease control seem to mirror previous real‐life data.
Furthermore, the patients were advised to use the app on a weekly base. Our results showed that patients with controlled or partly controlled CRS followed this advice. Patients with uncontrolled disease doubled the frequency of completing the health diary, which likely reflects the presence of increased symptoms.
We anticipated that patients with a younger age profile would be overrepresented within the mSC cohort. However, we found age ranges that equal those that have been reported previously. 6 , 17 This indicates the willingness of older CRS patients to use mHealth technology to monitor their symptoms and also suggests that the mSC app is easy to use for patients across different age groups.
A considerable amount of literature has been published demonstrating an association between asthma and CRS, especially with the CRSwNP phenotype. 7 , 8 , 18 We show here that 32.2% patients reported a physician‐based diagnosis of asthma. This proportion of patients corresponds with previous studies evaluating the prevalence of asthma in CRS patients in tertiary centres, 8 but is higher than a recent report showing concomitant asthma in 16.3% of CRS patients in a population‐based study. 18 The higher proportion of patients with late‐onset compared to early‐onset asthma in our cohort confirms previous studies. 7 In our study, allergic rhinitis was reported as a co‐morbidity in 48.2% of CRS patients, which is in line with other studies demonstrating skin prick test positivity in up to half of CRS patients. 8
A low level of adherence to pharmaceutical treatment was observed without differences depending on the level of disease control of the patients. This could be due to patients who experienced side effects of corticosteroids, corticofobia or underreporting in the app by the patient.
FESS is a treatment option for CRS patients with uncontrolled disease despite pharmaceutical treatment. 19 Managing nasal polyps in CRSwNP patients remains a therapeutic challenge with recurrence rates at 1 year after FESS of 38% and at 12 years after surgery in up to 78.9% of patients. 20 , 21 In the latter study, revision surgery took place in 36.8% of patients over the 12‐year period. 20 Our study showed that 20.3% of CRS patients had a history of revision surgery. With every consecutive FESS procedure, VAS for both global and the majority of specific CRS symptoms declined. However, for a subgroup of patients with a history of more than three surgeries (n = 16), VAS for global and specific CRS symptoms was equally high or even higher compared to patients without a history of sinus surgery. In this subgroup, additional surgery will likely not further improve disease control, underlining the need for alternative treatment options. On a short‐term, however, evidence suggests that there is a temporarily beneficial effect of revision surgery but that the interval between successive operations becomes shorter. 22
On the other hand, impaired sense of smell seemed to be unresponsive to FESS treatment, possibly indicating the fact that sinus surgery typically spares the olfactory cleft. The lack of beneficial effect of revision FESS could also be explained by several other reasons. Firstly, patients with more severe CRSwNP and hence more rapid recurrence of nasal polyps might not benefit very much from endoscopic sinus surgery. The research group of Peter Hellings already reported in Allergy on uncontrolled CRSwNP after sinus surgery in 80% of aspirin‐intolerant CRSwNP patients. 11 Secondly, FESS in the olfactory area may be associated with scar formation, preventing smell improvement to occur even after nasal polyp removal. It is generally accepted by the sinus surgery community that scarification and/or direct injury to the olfactory nerve might occur after or during sinus surgery, respectively. Thirdly, the subgroup of patients with a higher number of FESS procedures are likely to be enriched by more severe CRS patients who are characterized by increased severity of smell impairment. Other studies have shown short‐term relief of both objective and subjective olfactory measurements. 23 However, cross‐sectional analysis of hyposmia and anosmia prevalence showed rates ranging from 60% to 70%. 24 In line with this, impaired sense of smell was the most bothersome symptom reported by mSC users. This points at the significance of routine olfactory testing in patients with CRS in order to characterize olfactory loss in a more detailed way. 25
For the refractory subgroup of CRSwNP patients, biologic treatment that is currently being investigated for patients with nasal polyps may be an alternative treatment approach. 26 , 27 , 28 , 29 Based on the favourable clinical outcomes of phase II‐III clinical trials as well as the high unmet need among CRSwNP patients, criteria for biologic therapy have been developed by a multidisciplinary expert team united by EUFOREA aiming to provide a framework for implementation of biologics in clinical practice for CRSwNP patients. 30
Collecting real‐life data through mobile technology has several advantages over existing disease registries. 31 , 32 , 33 It allows longitudinal collection of patient‐reported outcome measures that are usually not present in electronic health records, thereby being complementary to other real‐life registries. Additionally, well‐adopted mHealth applications allow longitudinal data collection in a wide variety of centres as well as at different levels of healthcare delivery. When analysing real‐life data from mHealth applications, one should, however, be aware of a potential bias towards specific profiles of patients that are more keen on or familiar with using mHealth technology as well as bias because of the self‐reported nature of the data. Also, the fact that patients did not complete the health diary on fixed days may have introduced a certain degree of bias but is inherent to the real‐life nature of the implementation of such technology for at‐home patient follow up. However, this study only reports on patients with a full record of the patient‐reported outcome measures and does not include any longitudinal data. Lastly, we acknowledge that saline irrigations were not included as a treatment option in the mSC application.
In order to optimally meet the needs of patients and healthcare providers, mSC has been updated and upgraded with a series of additional functionalities in combination with a physician dashboard to be used in the outpatient clinic, now called Galenus Health. Such improvements will further support the adoption of e‐health support of patients with asthma, rhinitis and chronic rhinosinusitis.
In conclusion, this real‐life study confirms the high burden of uncontrolled disease in CRS patients, which clearly impacts daily functioning of CRS patients. Sinus surgery improves patient‐reported outcomes, but not in patients with a history of more than 3 procedures. Mobile technology opens a new era of real‐life studies, which supports the evolution towards preventive and predictive medicine. 34 , 35
CONFLICT OF INTEREST
Dr Alobid reports personal fees from Roche, personal fees from Menarini, personal fees from Mylan, personal fees from Novartis, personal fees from MSD, outside the submitted work. Dr Bachert reports personal fees from Sanofi, GSK, AstraZeneca and Novartis and grants and personal fees from Mylan, outside the submitted work. Dr Bernal‐Sprekelsen has nothing to disclose. Dr Bjermer is member of the Board of EUFOREA that owns the mySinusitisCoach application. Dr Callebaut has nothing to disclose. Dr Cardell reports grants from Sanofi, outside the submitted work. Dr Carrie has nothing to disclose. Dr Castelnuovo has nothing to disclose. Dr Cathcart has nothing to disclose. Dr Clement has nothing to disclose. Dr Constantinidis has nothing to disclose. Ms Cools has nothing to disclose. Dr Cornet has nothing to disclose. Dr Cox has nothing to disclose. Dr Correia‐de‐Sousa reports other from Boheringer Ingelheim, grants, personal fees and other from GSK, grants and other from AstraZeneca, personal fees from Mundipharma, outside the submitted work. Dr De Bont has nothing to disclose. Ms Deneyer is employed by Change Accelerator in Respiratory Diseases. Dr Delsupehe has nothing to disclose. Dr Devos has nothing to disclose. Dr Doulaptsi has nothing to disclose. Dr Fokkens reports that the Amsterdam University Medical Centres, location AMC receives grants and Stichting AERO receives personal fees from Sanofi and Novartis, grants from Gsk, from Meda, from ALK, from Allergy therapeutics, outside the submitted work. Dr Gane has nothing to disclose. Dr Gevaert has nothing to disclose. Dr Hellings reports personal fees from Sanofi, personal fees from Allergopharma, personal fees from Stallergenes, grants and personal fees from Mylan, outside the submitted work. He is also member of the Board of EUFOREA that owns the mySinusitisCoach application. Dr Hopkins has nothing to disclose. Dr Hox reports personal fees from Consultant work for ALK, outside the submitted work. Dr Hummel reports grants from Sony, Stuttgart, Germany, grants from Smell and Taste Lab, Geneva, Switzerland, grants from Takasago, Paris, France, grants from aspuraclip, Berlin, Germany, outside the submitted work. Dr Hosemann has nothing to disclose. Dr Jacobs has nothing to disclose. Dr Jorissen has nothing to disclose. Dr Kjeldsen reports other from Astra Zeneca, outside the submitted work. Dr Landis has nothing to disclose. Dr Lemmens has nothing to disclose. Dr Leunig has nothing to disclose. Dr Lund has nothing to disclose. Mr Mariën is employed by Change Accelerator in Respiratory Diseases and advisor to EUFOREA. Dr Mullol reports personal fees and other from SANOFI‐GENZYME & REGENERON, NOVARTIS and ALLAKOS, grants and personal fees from MYLAN Pharma and URIACH Group, personal fees from Mitsubishi‐Tanabe, Menarini, UCB, AstraZeneca, GSK and MSD, outside the submitted work. Dr Onerci has nothing to disclose. Mrs Palkonen has nothing to disclose. Mrs Proano has nothing to disclose. Dr Prokopakis has nothing to disclose. Dr Pugin has nothing to disclose. Dr Ryan reports personal fees and non‐financial support from Mylan, personal fees from Chiesi, personal fees from GSK, personal fees from Novartis, personal fees from Regeneron, personal fees from BI, outside the submitted work. Dr Riechelmann has nothing to disclose. Dr Sahlstrand Johnson reports grants and personal fees from Medtronic, outside the submitted work. Dr Toppila‐Salmi reports personal fees from ERT, Roche products, Sanofi Pharma and Novartis, grants from GSK, outside the submitted work. Dr Segboer has nothing to disclose. Dr Seys is employed by Change Accelerator in Respiratory Diseases. Dr Speleman has nothing to disclose. Dr Steinsvik has nothing to disclose. Dr Surda has nothing to disclose. Dr Tomazic has nothing to disclose. Dr Vanderveken has nothing to disclose. Dr Van Gerven has nothing to disclose. Dr Van Zele has nothing to disclose. Dr Verfaillie has nothing to disclose. Dr Verhaeghe has nothing to disclose. Dr Vierstraete has nothing to disclose. Dr Vlaminck has nothing to disclose. Dr Wagenmann reports personal fees from ALK‐Abelló, Allergopharma, AstraZeneca, Bencard Allergie, Genzyme, HAL Allergie, Infectopharm, LETI Pharma, MEDA Pharma, Novartis, Sanofi Aventis, Stallergenes and Teva, outside the submitted work.
Supporting information
Fig S1
Fig S2
Fig S3A
Fig S3B
Table S1‐S4
Seys SF, De Bont S, Fokkens WJ, et al. Real‐life assessment of chronic rhinosinusitis patients using mobile technology: The mySinusitisCoach project by EUFOREA. Allergy. 2020;75:2867–2878. 10.1111/all.14408
Sven F. Seys and Shana De Bont are shared first authorship.
REFERENCES
- 1. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:1‐298. [PubMed] [Google Scholar]
- 2. Bachert C, Pawankar R, Zhang L, et al. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA2LEN study. Allergy. 2011;66(9):1216‐1223. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population‐based sample. Allergy. 2017;72(2):274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Greve G, Hellings PW, Fokkens WJ, Pugin B, Steelant B, Seys SF. Endotype‐driven treatment in chronic upper airway diseases. Clin Transl Allergy. 2017;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449‐1456.e4. [DOI] [PubMed] [Google Scholar]
- 7. Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 8. Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin DC, Chandra RK, Tan BK, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):205‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prokopakis EP, Vlastos IM, Ferguson BJ, et al. SCUAD and chronic rhinosinusitis. Reinforcing hypothesis driven research in difficult cases. Rhinology. 2014;52(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 11. van der Veen J, Seys SF, Timmermans M, et al. Real‐life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72(2):282‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hellings PW, Borrelli D, Pietikainen S, et al. European Summit on the Prevention and Self‐Management of Chronic Respiratory Diseases: report of the European Union Parliament Summit (29 March 2017). Clin Transl Allergy. 2017;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bousquet J, Caimmi DP, Bedbrook A, et al. Pilot study of mobile phone technology in allergic rhinitis in European countries: the MASK‐rhinitis study. Allergy. 2017;72(6):857‐865. [DOI] [PubMed] [Google Scholar]
- 14. Seys S, Bousquet J, Bachert C, et al. mySinusitisCoach: patient empowerment in chronic rhinosinusitis using mobile technology. Rhinology. 2018;56(3):209‐215. [DOI] [PubMed] [Google Scholar]
- 15. Caimmi D, Baiz N, Tanno LK, et al. Validation of the MASK‐rhinitis visual analogue scale on smartphone screens to assess allergic rhinitis control. Clin Exp Allergy. 2017;47(12):1526‐1533. [DOI] [PubMed] [Google Scholar]
- 16. Doulaptsi M, Prokopakis E, Seys S, Pugin B, Steelant B, Hellings P. Visual analogue scale for sino‐nasal symptoms severity correlates with sino‐nasal outcome test 22: paving the way for a simple outcome tool of CRS burden. Clin Transl Allergy. 2018;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN rhinosinusitis cohort: a large European cross‐sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32‐42. [DOI] [PubMed] [Google Scholar]
- 18. Ostovar A, Fokkens WJ, Pordel S, et al. The prevalence of asthma in adult population of southwestern Iran and its association with chronic rhinosinusitis: a GA 2 LEN study. Clin Transl Allergy. 2019;9(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rudmik L, Soler ZM, Hopkins C, et al. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: a RAND/UCLA appropriateness study. Rhinology. 2016;54(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 20. Calus L, Van Bruaene N, Bosteels C, et al. Twelve‐year follow‐up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy. 2019;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith K, Orlandi R, Oakley G, Meeks H, Curtin K, Alt J. Long‐term revision rates for endoscopic sinus surgery. Int Forum Allergy Rhinol. 2019;9(4):402‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohli P, Naik AN, Farhood Z, et al. Olfactory outcomes after endoscopic sinus surgery for chronic rhinosinusitis: a meta‐analysis. Otolaryngol Neck Surg. 2016;155(6):936‐948. [DOI] [PubMed] [Google Scholar]
- 24. Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127(2):309‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hummel T, Whitcroft K, Andrews P, Altundag A, Cinghi C, Costanzo R. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1‐30. [DOI] [PubMed] [Google Scholar]
- 26. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110‐116.e1. [DOI] [PubMed] [Google Scholar]
- 27. Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989‐995.e8. [DOI] [PubMed] [Google Scholar]
- 28. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469‐479. [DOI] [PubMed] [Google Scholar]
- 29. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394(10209):1638‐1650. [DOI] [PubMed] [Google Scholar]
- 30. Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74(12):2312‐2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sleurs K, Seys S, Bousquet J, et al. Mobile health tools for the management of chronic respiratory diseases. Allergy. 2019;74(7):1292‐1306. [DOI] [PubMed] [Google Scholar]
- 32. Hellings PW, Akdis CA, Bachert C, et al. EUFOREA Rhinology Research Forum 2016: report of the brainstorming sessions on needs and priorities in rhinitis and rhinosinusitis. Rhinology. 2017;55(3):202‐210. [DOI] [PubMed] [Google Scholar]
- 33. Lund V. EUFOREA Rhinology Research Forum 2017: report of the brainstorming sessions on endotype‐driven treatment, patient empowerment and digital future in airways care. Rhinol Online. 2018;1(1):11‐19. [Google Scholar]
- 34. Hellings PW, Fokkens WJ, Bachert C, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis ‐ A EUFOREA‐ARIA‐EPOS‐AIRWAYS ICP statement. Allergy. 2017;72(9):1297‐1305. [DOI] [PubMed] [Google Scholar]
- 35. Muraro A, Lemanske RF, Hellings PW, et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis‐PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347‐1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3A
Fig S3B
Table S1‐S4


