Abstract
Deep chlorophyll maxima (DCM) are common in stratified lakes and oceans, and phytoplankton growth within DCM often contributes significantly to total system production. Theory suggests that properties of DCM should be predictable by trophic state, with DCM becoming deeper, broader, and less productive with greater oligotrophy. However, rigorous tests of these expectations are lacking in freshwater systems. We use data generated by the U.S. EPA from 1996 to 2017, including in situ profile data for temperature, photosynthetically active radiation (PAR), chlorophyll, beam attenuation (c p), and dissolved oxygen (DO), to investigate patterns in DCM across lakes and over time. We consider trophic state, 1% PAR depth (z 1%), thermal structure, and degree of photoacclimation as potential drivers of DCM characteristics. DCM depth and thickness generally increased while DCM chlorophyll concentration decreased with decreasing trophic state index (greater oligotrophy). The z 1% was a stronger predictor of DCM depth than thermal structure. DCM in meso‐oligotrophic waters were closely aligned with maxima in c p and DO saturation, suggesting they are autotrophically productive. However, the depths of these maxima diverged in ultra‐oligotrophic waters, with DCM occurring deepest. This is likely a consequence of photoacclimation in high‐transparency waters, where c p can be a better proxy for phytoplankton biomass than chlorophyll. Our results are generally consistent with expectations from DCM theory, but they also identify specific gaps in our understanding of DCM in lakes, including the causes of multiple DCM, the importance of nutriclines, and the processes forming DCM at higher light levels than expected.
Deep chlorophyll maxima (DCM) are common features in mesotrophic to oligotrophic water bodies with positively stratified water columns, but the mechanisms driving their magnitude and depth can vary widely across systems due to differences in physical and biological factors. Processes contributing to DCM formation may include phytoplankton growth at depth, phytoplankton accumulation along the pycnocline, high zooplankton grazing on epilimnetic phytoplankton, photoinhibition of phytoplankton growth near the surface, and phytoplankton photoacclimation to different light environments (Cullen 1982; Camacho 2006). While all of these processes can affect chlorophyll distributions, production at depth and photoacclimation are often indicated as primary causes of DCM formation and thus are of particular relevance when considering DCM across a range of environments. These mechanisms are influenced by factors such as water clarity, nutrient availability, and lake productivity, which are generally correlated with the trophic state of a water body.
In water bodies with stable stratification and nutrient depletion within the mixed layer, there is relatively low primary production in surface waters due to nutrient limitation. Low surface production generally correlates with high water clarity, which allows for phytoplankton growth at or below the thermocline, where there is greater nutrient availability (Cullen 2015). Thus, maximum phytoplankton production often occurs near the top of a nutricline in oligotrophic waters, causing the development of a deep biomass maximum (DBM) of phytoplankton and an associated DCM (Jamart et al. 1977; Banse 1987; Beckmann and Hense 2007). In some cases, production within DCM contributes over 60% of the total areal primary production (Moll et al. 1984; Weston et al. 2005; Giling et al. 2017), making DCM production important for estimates of system productivity (Hemsley et al. 2015).
However, DCM may also be caused by the physiological adaptation of phytoplankton to their light environment (photoacclimation) rather than high phytoplankton biomass. Although chlorophyll a (herein referred to as chlorophyll) is a commonly‐used proxy for phytoplankton abundance, it may not be an accurate measure of biomass across depths due to the variable nature of phytoplankton chlorophyll content. Variation in phytoplankton particulate organic carbon to chlorophyll ratios occurs because of inherent differences among taxa (Geider 1993) and due to photoacclimation, whereby algal cells increase their chlorophyll content when exposed to low light or decrease their chlorophyll content in high light environments. In ultra‐oligotrophic systems with high water clarity, DCM can be largely attributed to differences in photoacclimation across depth strata (Steele 1964; Fennel and Boss 2003; White and Matsumoto 2012), rather than high phytoplankton biomass at depth. In such cases, DCM are not necessarily associated with elevated phytoplankton biomass.
DCM have been studied extensively in offshore marine systems with stable stratification and long‐term nutrient depletion within the mixed layer (Herbland and Voituriez 1979; Yentsch 1980; Cullen 1982). Of course, other marine and freshwater systems that maintain stable stratification during summer periods can also develop a nutricline and exhibit seasonal DCM, which may contribute significantly to summer production (Coon et al. 1987; Estrada et al. 1993; Barbiero and Tuchman 2001a). Cullen (2015), in a review of DCM formation based largely on marine studies, summarized common features of systems with DCM and demonstrated that the depth of the DCM is typically inversely related to chlorophyll concentration at the DCM, water column integrated chlorophyll, and integrated primary productivity (Yentsch 1974, 1980; Herbland and Voituriez 1979; Beckmann and Hense 2007). It can thus be inferred that some characteristics of DCM should be predictable from the trophic state of a water body, defined herein as a function of chlorophyll and total phosphorus during spring (isothermal conditions) (Table 1, see “Methods” section for details). For example, with decreasing trophic state (oligotrophication), the DCM should likely form deeper in the water column, decrease in magnitude, and become less productive. Previous observations from freshwater systems generally agree with these expectations (Moll and Stoermer 1982; Barbiero et al. 2001; Camacho 2006), although extensive studies of DCM dynamics for lakes are fewer than for offshore marine environments.
Table 1.
Water quality variables indicative of lake trophic state, including Secchi depth, extracted chlorophyll concentration, and total phosphorus (TP) during spring and summer surveys. N is the number of samples for spring chlorophyll measurements (note sample size for Secchi depth is lower because it is only taken during daytime). Values given are the mean ± one standard deviation. All available years of quality‐approved data were used for each variable. Data source EPA‐GLNPO Great Lakes environmental database (GLENDA) (https://cdx.epa.gov/).
| n | Secchi depth (m) 1996–2015 | Chlorophyll (μg L−1) 2002–2016 | Total phosphorus (μg L−1) 1996–2014 | Trophic state index | Trophic state | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring 20‐m | Summer | Spring 20‐m | Summer | ||||||
| Epilimnion | DCM | Epilimnion | DCM | ||||||||
| Superior | 533 | 15.0 ± 4.6 | 12.1 ± 3.5 | 0.70 ± 0.35 | 1.05 ± 0.69 | 1.57 ± 0.71 | 1.60 ± 0.81 | 1.39 ± 0.92 | 1.47 ± 0.74 | 17.9 ± 3.17 | Ultra‐oligotrophic |
| Huron | 363 | 13.3 ± 7.6 | 15.0 ± 4.8 | 0.76 ± 0.29 | 0.59 ± 0.38 | 1.57 ± 1.08 | 1.46 ± 0.67 | 1.30 ± 0.76 | 1.42 ± 0.72 | 18.3 ± 3.29 | Ultra‐oligotrophic |
| Michigan | 326 | 13.2 ± 8.1 | 11.9 ± 6.3 | 1.01 ± 0.45 | 0.97 ± 0.54 | 2.11 ± 1.33 | 2.06 ± 0.99 | 1.51 ± 0.72 | 1.65 ± 0.80 | 21.9 ± 2.80 | Ultra‐oligotrophic to Oligotrophic |
| Northern | 146 | 13.7 ± 6.6 | 10.8 ± 4.8 | 0.96 ± 0.30 | 1.18 ± 0.60 | 2.47 ± 1.50 | 2.16 ± 0.97 | 1.55 ± 0.73 | 1.52 ± 0.65 | 22.2 ± 2.80 | |
| Southern | 180 | 12.6 ± 8.7 | 13.0 ± 6.7 | 1.01 ± 0.54 | 0.81 ± 0.40 | 1.82 ± 1.03 | 2.00 ± 1.01 | 1.49 ± 0.80 | 1.76 ± 0.85 | 21.7 ± 2.80 | |
| Ontario | 231 | 13.7 ± 6.8 | 6.4 ± 2.3 | 1.55 ± 0.91 | 2.87 ± 1.67 | 3.51 ± 2.12 | 2.97 ± 1.14 | 2.52 ± 0.99 | 2.56 ± 1.09 | 26.6 ± 3.09 | Oligotrophic |
| Western | 116 | 13.2 ± 5.9 | 6.8 ± 2.2 | 1.63 ± 0.97 | 2.92 ± 1.40 | 3.77 ± 2.30 | 2.90 ± 1.13 | 2.38 ± 1.24 | 2.54 ± 1.29 | 26.8 ± 2.54 | |
| Central | 115 | 14.2 ± 8.0 | 6.1 ± 2.7 | 1.48 ± 0.86 | 2.82 ± 1.34 | 3.20 ± 1.76 | 3.05 ± 1.13 | 2.63 ± 0.80 | 2.58 ± 0.88 | 26.5 ± 3.50 | |
| Erie | 369 | 3.4 ± 3.8 | 5.3 ± 2.9 | 4.54 ± 3.94 | 4.01 ± 3.55 | 2.59 ± 5.46 | 6.39 ± 4.42 | 3.34 ± 2.11 | 3.05 ± 1.07 | 35.6 ± 5.23 | Eutrophic to Oligotrophic |
| Western | 97 | 1.2 ± 1.0 | 2.1 ± 1.5 | 3.42 ± 2.36 | 6.80 ± 4.43 | — | 7.96 ± 6.55 | 4.59 ± 7.44 | — | 35.2 ± 7.45 | Eutrophic |
| Central | 189 | 2.7 ± 2.1 | 6.2 ± 2.1 | 6.24 ± 4.74 | 3.04 ± 6.19 | 2.97 ± 7.00 | 5.18 ± 9.14 | 2.83 ± 1.37 | 3.18 ± 1.05 | 36.5 ± 4.97 | Meso‐eutrophic |
| Eastern | 83 | 8.3 ± 5.5 | 6.8 ± 2.3 | 2.09 ± 1.69 | 2.31 ± 1.00 | 2.05 ± 1.41 | 7.03 ± 2.51 | 2.73 ± 2.05 | 2.91 ± 0.92 | 33.6 ± 3.85 | Meso‐oligotrophic |
General hypotheses about DCM in lakes along a trophic state gradient were proposed by Moll and Stoermer (1982), the fundamentals of which are consistent with the principles presented by Cullen (2015). Using data from lakes Michigan and Superior, Moll and Stoermer (1982) emphasized that DCM in large oligotrophic lakes typically form deep and are broader than those in more productive lakes (Fee 1976), and the authors noted that DCM chlorophyll concentrations were lower in the more oligotrophic Lake Superior than in Lake Michigan. In addition, multiple studies have shown that water clarity is an important factor determining DCM depth across lakes of varying types (Hamilton et al. 2010; Leach et al. 2018), while lake size (surface area and maximum depth) may be important for determining DCM thickness (Leach et al. 2018). The depth range at which phytoplankton growth can occur increases with lake transparency, which may lead to deeper and broader DCM in more oligotrophic waters with high water clarity (Beckmann and Hense 2007). There has been less attention to studying the relative distributions of production, biomass, and chlorophyll within the water column, and assessing how these distributions change across a trophic state gradient is an important step toward improving our ability to assess the ecological importance of DCM.
The Laurentian Great Lakes are particularly interesting systems in which to study DCM because they are among the largest lakes in the world, bridging the size gap between marine systems and smaller lakes. In addition, these lakes exhibit a trophic state gradient, ranging from ultra‐oligotrophic (Lake Superior) to eutrophic (western Lake Erie) (Table 1). Several of the Great Lakes have undergone oligotrophication over the past decades due to decreased nutrient loads; the rapid spread of non‐native dreissenid mussels has also affected nutrient cycling while increasing water clarity (Madenjian et al. 2002; Mills et al. 2003; Dove and Chapra 2015). As a result, the trophic state of lakes Michigan and Huron have converged toward that of Lake Superior (Barbiero et al. 2012), and Lake Ontario has also become more oligotrophic (Dove 2009; Rudstam et al. 2017). In addition to changes in spring total phosphorus and water clarity, summer phytoplankton (Reavie et al. 2014) and zooplankton communities (Barbiero et al. 2019) have shifted toward dominance by more oligotrophic species in lakes Huron, Michigan and Ontario. These changes suggest bottom‐up impacts on the food web (Bunnell et al. 2014) and have driven increased interest in studying DCM dynamics across the Great Lakes (Pothoven and Fahnenstiel 2013; Oliver et al. 2014; Koops et al. 2015; Watkins et al. 2015; Scofield et al. 2017).
Previous research on DCM in the Great Lakes system includes multiple studies of lakes Superior, Michigan, and Ontario, as well as a single cross‐lake comparison based on summer data from 1998 (Barbiero and Tuchman 2001b). Photoacclimation and phytoplankton settling appear to be important drivers of DCM depth and magnitude in Lake Superior (Barbiero and Tuchman 2004; White and Matsumoto 2012), while the DCM in Lake Michigan has historically contributed up to 30–60% of summertime production (Brooks and Torke 1977; Moll et al. 1984; Fahnenstiel and Scavia 1987). However, chlorophyll concentrations of DCM in Lake Michigan have decreased significantly since the 1970s as oligotrophication has occurred (Fahnenstiel et al. 2010b), again suggesting convergence toward Lake Superior. There are fewer studies from Lake Ontario prior to the 2000s, but the available data suggest that in previous decades, maximum primary production occurred in the epilimnion and there was net respiration below the thermocline—consistent with light limitation rather than nutrient limitation (Stadelmann et al. 1974; Boyd 1980). When DCM did form, they occurred at relatively shallow depths near the top of the thermocline and had chlorophyll concentrations only slightly higher than those found in the epilimnion. In recent years, however, DCM have become nearly ubiquitous during summer stratification, are associated with DBM, and are productive (Twiss et al. 2012; Watkins et al. 2015; Scofield et al. 2017). Although these studies suggest that the Great Lakes DCM are consistent with patterns observed in marine systems, a comprehensive cross‐lake comparison using standardized methods will provide more robust insights about DCM formation in the Great Lakes and allow us to compare theory of DCM dynamics with observations in large freshwater systems.
This study tests whether hypotheses derived from established theory about DCM (reviewed in Cullen 2015) apply to the Great Lakes, especially with respect to changing DCM dynamics across a trophic state gradient. We use data collected through the US Environmental Protection Agency's Great Lakes National Program Office (GLNPO) monitoring program from 1996 to 2017 to ask whether differences in DCM across lakes agree with expectations based on previous research, and to clarify gaps in DCM theory for large lakes. We specifically address the following questions: (1) Are differences in DCM depth, thickness, and chlorophyll concentration consistent with expectations based on lake trophic state? We hypothesize that DCM will be deeper, broader, and have lower chlorophyll concentrations in more oligotrophic lakes. (2) Are indicators of DCM‐forming mechanisms consistent with expectation based on lake trophic state? We examine potential mechanisms using beam attenuation coefficient (proportion of light attenuated m−1 due to particles) and dissolved oxygen saturation profiles as proxies for phytoplankton biomass and productivity distributions, respectively; we also use the ratio of particulate organic carbon (POC) to chlorophyll (POC : Chl) available from a subset of sites to investigate the potential importance of photoacclimation. We hypothesize that in relatively productive systems (such as lakes Erie and Ontario), the DCM will co‐occur with a particle biomass peak and a peak in oxygen saturation. With oligotrophication, biomass and oxygen saturation peaks will occur less frequently, and photoacclimation will be a more important driver of DCM formation. (3) What abiotic factors are correlated with differences in DCM characteristics, both across lakes and over time? We consider water clarity, thermal structure, and nutrient concentrations as potential drivers of trends in the DCM. Taken together, these research questions allow us to investigate whether a general framework for understanding DCM mechanisms across a trophic state gradient, developed primarily from marine systems, can be applied to large lakes.
Methods
Study system
The Laurentian Great Lakes are a chain of deep glacial freshwater lakes that contain one of the largest surface freshwater resources on Earth, representing approximately 20% of the world's supply. Water generally flows through the Great Lakes system from west to east, draining from Lake Superior and Lake Michigan to Lake Huron, through the St. Clair River, fluvial Lake St. Clair, and Detroit River to Lake Erie, and over Niagara Falls to Lake Ontario (Fig. 1); the Great Lakes watershed eventually drains to the Atlantic Ocean through the St. Lawrence River (NOAA 2016). Lake Superior is the largest of the Great Lakes in terms of surface area (82,097 km2), and it also has the greatest volume due to its depth (mean 149 m, max 406 m). Lake Ontario is the second deepest (mean 86 m, max 244 m), although it has the smallest surface area (19,009 km2), followed by Lake Michigan (mean 85 m, max 281 m, surface area 57,753 km2) and Lake Huron (mean 59 m, max 229 m, surface area 59,565 km2). Lake Erie is the shallowest (mean depth 19 m, max 64 m, surface area 25,655 km2), although its three basins vary greatly in depth. The western basin of Lake Erie is shallowest (mean 7.4 m), followed by the central basin (mean 22 m) and eastern basin (mean 45 m) (NOAA 2016).
Figure 1.
Map of the Laurentian Great Lakes showing station locations for the Great Lakes National Program Office (GLNPO) long‐term monitoring program, including standard, master, benthos, and fish stations. Only standard and master stations were used for long‐term trend analysis. The gray line denotes the boundary between the United States and Canada, and dashed lines denote basin divisions for Lakes Michigan, Ontario, and Erie.
The Great Lakes represent a trophic state gradient, ranging from ultra‐oligotrophic in lakes Superior and Huron to eutrophic in western Lake Erie, based on both spring and summer metrics for trophic state (Table 1). In recent decades, average springtime Secchi depth (referred to herein as Secchi) measurements ranged from 15.0 m in Lake Superior to 1.2 m in western Erie; during summer, Secchi measurements were comparable to the spring, with the deepest values in Lake Huron (15.0 m) rather than Lake Superior (12.1 m) and the shallowest in western Lake Erie (2.1 m). Spring integrated (top 20 m) chlorophyll (Chl) and total phosphorus (TP), which are generally good indicators of trophic state, were lowest in lakes Huron and Superior, which are similar (0.76 and 0.70 μg L−1 chl; 1.56 and 1.60 μg L−1 TP, respectively), followed by Lake Michigan (1.01 μg L−1 chl and 2.06 μg L−1 TP) and Lake Ontario (1.55 μg L−1 chl and 2.97 μg L−1 TP). Lake Erie was most eutrophic (4.54 μg L−1 chl and 6.39 μg L−1 TP), but the three basins are different enough to be considered separately, with increasing trophic state from east to west, especially during summer (Table 1). Data are reported separately for the northern and southern basins of Lake Michigan and the western and central basins of Lake Ontario, due to higher chlorophyll concentrations in northern Lake Michigan during summer (Table 1) and differences in thermocline depth between basins in Lake Ontario during August (Table 2). During summer, DCM occur in the offshore regions of all five of the lakes, although they are uncommon in central Lake Erie and generally do not form in western Lake Erie, which is polymictic during summer.
Table 2.
Summer physical variables for the Great Lakes based on data collected during summer surveys from 1996 to 2017, including average site depth for all profiles analyzed, thermal structure (epilimnion, thermocline, and hypolimnion), maximum relative thermal resistance to mixing (RTRM), maximum thermal gradient, and the 1% PAR depth (daytime profiles only). Values shown are the mean ± one standard deviation.
| n profiles | Thermocline present (%) | Site depth (m) | Epilimnion depth (m) | Thermocline depth (m) | Hypolimnion depth (m) | RTRM | Thermal gradient (°C m−1) | 1% PAR depth (m) | |
|---|---|---|---|---|---|---|---|---|---|
| Superior | 473 | 99 | 169 ± 54 | 12.5 ± 5.3 | 17.2 ± 8.6 | 33.7 ± 13.2 | 22.9 ± 21.8 | −1.7 ± 1.1 | 35.6 ± 8.9 |
| Huron | 359 | 100 | 82 ± 27 | 11.9 ± 3.7 | 16.7 ± 5.7 | 35.1 ± 9.3 | 49.8 ± 33.2 | −2.6 ± 1.2 | 39.8 ± 10.0 |
| Michigan | 388 | 100 | 109 ± 58 | 11.9 ± 4.5 | 16.5 ± 5.5 | 34.8 ± 9.2 | 54.7 ± 51.1 | −2.6 ± 1.2 | 31.9 ± 8.2 |
| Northern | 226 | 100 | 116 ± 70 | 11.8 ± 4.8 | 16.8 ± 6.4 | 34.5 ± 10.3 | 47.4 ± 49.8 | −2.3 ± 1.0 | 29.9 ± 6.4 |
| Southern | 162 | 100 | 99 ± 34 | 12.0 ± 4.1 | 16.0 ± 4.0 | 35.2 ± 7.7 | 60.9 ± 51.6 | −3.0 ± 1.2 | 33.9 ± 9.3 |
| Ontario | 229 | 99 | 99 ± 47 | 11.5 ± 4.7 | 14.7 ± 5.5 | 33.1 ± 10.5 | 49.8 ± 33.2 | −2.7 ± 1.3 | 20.8 ± 5.5 |
| Western | 112 | 100 | 102 ± 33 | 9.5 ± 3.7 | 11.9 ± 4.5 | 28.5 ± 7.2 | 56.4 ± 37.3 | −2.9 ± 1.4 | 21.8 ± 6.1 |
| Central | 117 | 97 | 95 ± 57 | 12.9 ± 4.9 | 17.5 ± 4.9 | 38.1 ± 11.2 | 44.4 ± 28.4 | −2.6 ± 1.3 | 20.0 ± 4.9 |
| Erie | 459 | 81 | 24 ± 13 | 12.7 ± 4.2 | 15.6 ± 4.7 | 20.7 ± 7.2 | 76.6 ± 62.9 | −4.0 ± 3.0 | 16.1 ± 6.9 |
| Western | 135 | 35 | 10 ± 1 | 5.5 ± 1.7 | 5.9 ± 1.7 | 5.7 ± 1.1 | 17.0 ± 35.3 | 0.3 ± 0.5 | 7.5 ± 3.4 |
| Central | 217 | 100 | 23 ± 1 | 13.2 ± 3.1 | 16.0 ± 2.3 | 17.7 ± 2.1 | 110.0 ± 54.3 | −6.2 ± 2.0 | 18.2 ± 3.8 |
| Eastern | 107 | 100 | 45 ± 9 | 14.4 ± 4.2 | 18.9 ± 3.6 | 29.0 ± 4.9 | 72.6 ± 51.4 | −4.3 ± 1.9 | 22.1 ± 5.6 |
Long‐term monitoring summary
The US Environmental Protection Agency's GLNPO began collecting in situ profile data in 1996 as part of their long‐term monitoring program for the Laurentian Great Lakes. The GLNPO surveys sampled consistent stations across each of the Great Lakes during April and August each year (1996–2017). Monitoring stations include four categories: standard, master, benthos (every year) and fish (every other year) (Fig. 1); all surveys were conducted from the US EPA R/V Lake Guardian. At each site, a rosette assembly equipped with 12 Niskin bottles and the following instrumentation was deployed: Seabird CTD, Seapoint Fluorometer (Seapoint Sensors, Exeter, New Hampshire; calibrated annually by manufacturer), Biospherical/Licor sensor for photosynthetically active radiation (PAR); transmissometer (WETlab C‐Star) measuring beam attenuation due to particles (660 nm wavelengths) across a 25 cm pathlength, and dissolved oxygen (DO) probe (SBE 43). The rosette was deployed at a constant speed of 0.5 m s−1 during down‐casts, and discrete‐depth water samples for chemical analyses were collected on the up‐casts. Water samples were collected at standard, master, and fish stations, but not at benthos stations. Sample depths ranged from the surface to two meters above the bottom (typically six depths at standard and fish stations and 12 depths at master stations, depending on the station depth). The discrete depths for sample collection, including the depth of the DCM, were determined by the EPA Chief Scientist based on real‐time plots of the downcast profile data. During isothermal conditions (spring survey), an integrated water sample was collected by equally mixing water from 2, 5, 10, and 20 m depth. When the water column was stratified, an integrated epilimnetic sample was mixed using equal amounts of water from the top, middle, and bottom of the epilimnion.
At all sites where water samples were collected, all depths sampled were analyzed for extracted chlorophyll and total phosphorus (TP), which is typically the limiting nutrient in the Great Lakes (Guildford and Hecky 2000). At master stations, the integrated epilimnion sample and a discrete‐depth DCM sample (when present) were also analyzed for particulate organic carbon (POC). All water for chlorophyll analysis was filtered and processed using EPA Standard Operating Procedure (SOP) LG404 (Revision 8, 2017) and frozen. Samples were analyzed for chlorophyll by the non‐acidification method (Welschmeyer 1994) according to the EPA SOP LG405 (Revision 10, 2017) using a calibrated Turner Designs 10‐AU bench‐top fluorometer. Because there are quality control concerns for the extracted chlorophyll data collected prior to 2002, we do not report extracted chlorophyll values for early years even though we did use the profile data in our analysis of the depths at which various peak features occurred. TP and particulates data were available for 2000–2015 and were determined using EPA SOPs LG204 and LG206/LG 207, respectively. All SOPs are available via the EPA GLNPO portal website (https://login.glnpo.net). Nutrient and extracted chlorophyll data presented herein were downloaded from the Great Lakes Environmental Database (GLENDA) via the U.S. EPA central data exchange website (https://cdx.epa.gov/, accessed 7.19.18). POC : Chl in g : g were calculated using the POC concentrations and extracted chlorophyll concentrations for the integrated epilimnion water and DCM water at master stations.
Spring nutrient data were used to calculate a trophic state index for each site (excluding benthos sites, where water samples were not collected, see Fig. 1). The trophic state index (TSI) used herein was calculated by taking the mean value of the TSI based on spring chlorophyll and spring TP according to Carlson (1977), as follows:
| (1) |
| (2) |
Profile processing
The raw profile data for temperature (°C), photosynthetically active radiation (PAR, μE (m2s)−1), chlorophyll fluorescence (F chl, μg L−1), beam attenuation coefficient due to particles (c p, m−1), and dissolved oxygen (DO, mg L−1) were binned to 0.5‐m depth resolution using Seabird's SBE processing software, and only down‐cast data were used for further analysis. The c p data generated from transmissometer measurements offer a good proxy for POC concentrations in the water column (Bishop 1999; Gardner et al. 2000; Fennel and Boss 2003). Oxygen saturation percentages were calculated from DO concentrations (mg L−1) and temperature data (Baca and Arnett 1976). DO saturation data provide indications of positive net ecosystem production (NEP); positive heterograde dissolved oxygen curves often co‐occur with DCM in lakes (Parker et al. 1991; Matthews and Deluna 2008; Scofield et al. 2017), which indicate positive NEP at depth (although this is not always the case, Wilkinson et al. 2015, see “Discussion” section).
The 1% PAR depth (z 1%) was defined as the depth at which 1% surface PAR occurred and was calculated using the light attenuation coefficient (K PAR) for the site as follows: z 1% = −ln(0.01)/K PAR, where K PAR was determined by the linear slope of the natural log‐transformed PAR profiles. We estimated K PAR using a simple linear regression fit to natural log‐transformed PAR data from the surface to the approximate 1% PAR depth (estimated as the depth at which the measured PAR value was equal to 1% of the maximum value observed within the profile). As a quality control measure, we used an adjusted R 2 threshold value of 0.90 to define high quality profiles. If the R 2 was lower than this threshold, we considered the fit poor, and we instead used a light extinction coefficient estimated from Secchi data. Of available daytime profiles with PAR data, 13% of the profiles had poor quality. Secchi is well‐established as a reliable index of water clarity, and Secchi data are generally well‐correlated with light extinction coefficients in a variety of water bodies (Tyler 1968; Wetzel 2001). We estimated the light extinction coefficient based on Secchi using a relationship optimized to our dataset using paired light attenuation coefficients from high quality PAR profiles and Secchi data taken concurrently: K PAR = 1.07/(Secchi) + 0.05 (Fig. S1). Although this relationship does introduce some bias for sites with high transparency (can underestimate the 1% PAR depth), this may have affected only a small number of our samples, as only 6 of 55 total sites with z 1% > 40 m were based on Secchi data.
Several processes can affect in situ chlorophyll fluorescence and bias the shape of the chlorophyll fluorescence profiles. Non‐photochemical quenching commonly causes in situ chlorophyll fluorescence measurements to be biased low at high light levels. Irradiance and temperature can affect the function of photosystem 2 in algae and plants (Yamamoto 2016), altering chlorophyll a fluorescence yield (Kornyeyev et al. 2004; Suggett et al. 2010). Although previous research and other recent studies have used different methods to correct profiles for quenching (Mignot et al. 2011; Thomalla et al. 2018; Xing et al. 2018), our dataset includes discrete depth extracted chlorophyll measurements from water samples collected with every profile; thus, we used an approach that takes advantage of these concurrent measurements to ground‐truth and calibrate our in situ chlorophyll fluorescence data. In addition to concerns related to non‐photochemical quenching, factory calibrations do not fully account for variability in the relationship between in situ fluorescence and extracted chlorophyll values. To address these issues, we used an approach that combines a field‐calibration of the in situ fluorometer data and a correction for non‐photochemical quenching; we optimized this calibration using data for in situ chlorophyll fluorescence (F chl), extracted chlorophyll (Chl) data from discrete‐depth water samples, measured PAR values at the sample depth, and measured temperature at the sample depth. Scofield et al. (2017) derived an equation for correcting the F chl data for the effects of non‐photochemical quenching on fluorescence response in Lake Ontario, which we optimized to the present dataset:
| (3) |
where F chl is the estimated chlorophyll concentration based on in situ fluorescence and the factory‐provided calibration coefficient, Chl is extracted chlorophyll in μg L−1, Temp is temperature in °C, PAR is photosynthetic active radiation in μE (m2s)−1, and a, b, and c are fitted constants. Note that the 25 μE (m2s)−1 cutoff value for a PAR effect on fluorescence is modified slightly from Scofield et al. 2017, to better fit the across‐lakes dataset. We used data from all lakes and the years 2002–2017 to obtain the values for the parameters a, b, and c that minimized the difference between corrected chlorophyll data and extracted chlorophyll data (see Table S1). The coefficients from the model fit to data collected after 2002, years for which we have confidence in the quality of the extracted chlorophyll data, were also applied to data from 1996 to 2001. Data collected in 2011 were fitted separately, as there was an apparent instrument calibration issue such that in situ fluorescence measurements were biased high. Applying these corrections resolved the observed bias in data with high PAR values, as well as corrected the fluorescence to chlorophyll correlation for instrument calibration issues (Table S1, Fig. S2).
Water column profile data were then processed using algorithms developed to detect thermal structure (lower epilimnion and upper hypolimnion boundaries, and the thermocline), as well as the top, peak, and bottom depths of maxima (Xu et al. 2019). Thermal structure was determined based on piecewise linear representation using the bottom‐up approach (Keogh et al. 2004), and the thermocline depth was defined as the mid‐point of the linear segment with the strongest thermal gradient (Xu et al. 2019). The metalimnion boundaries were determined using a temperature gradient cut‐off value of 0.1°C m−1 (algorithm optimized using the same dataset presented herein, see Xu et al. 2019); the epilimnion was defined as the depth range above the shallowest piecewise regression segment with the stated minimum gradient, and the hypolimnion was defined as the depth below which the minimum gradient was not observed for any segment. If this minimum gradient was not observed in the piecewise regressions, the profile was considered unstratified (see Xu et al. 2019 for details). We also calculated relative thermal resistance to mixing (RTRM) for each 0.5‐m depth interval, defined as the difference in water density for the given interval relative to the difference in water density at 4°C and 5°C (Vallentyne 1957); the maximum observed RTRM within each profile was used as a metric of water column stability.
The peak‐detection algorithm developed by Xu et al. (2019) was applied to corrected F chl profiles to detect the DCM, c p profiles to detect the DBM, and DO saturation profiles to detect the deep oxygen maximum (DOmax). For each profile, all peaks in the data were identified by noting points where the slope changed from positive to negative (with increasing depth). Individual peaks were then categorized as significant or nonsignificant based on threshold values for the peak magnitude (the maximum value of the peak) and the peak range (the difference between maximum and minimum values within a peak feature). The minimum thresholds for magnitude (x min) and range (r min) were calculated relative to the total range observed in the data, as follows:
| (4) |
| (5) |
where X is the set of values for the full profile of the variable (F chl, c p, or DO saturation). A peak was determined significant if both its magnitude and height exceeded the given thresholds (for further details on algorithm parameterization and performance, see Xu et al. 2019). Where more than one significant peak was detected within a profile, we used only the highest magnitude peak for the current analysis; the DCM, DBM, or DOmax was then defined by the depth at which the maximum value occurred within the largest significant peak of that variable. Two half Gaussian curves with the mean being the depth of the DCM, DBM, or DOmax and separate standard deviations (σ 1 and σ 2) were fit to the data above and below each maximum point. The top and bottom boundaries of the peak were defined as the maximum depth −2.5σ 1 and the maximum depth +2.5σ 2, respectively; we refer to the differences between the top and bottom boundaries as the thickness (m). Algorithm outputs were checked against the raw profile data for all variables, and manual corrections were applied where the algorithm detection was problematic, based on expert opinion and algorithm performance on other profiles.
Data analysis
All available profiles were used in our analysis of DCM characteristics, but only standard and master stations were used for the time series because water samples were collected at these sites every year. All data analysis was completed using the program R software packages (R Core Team 2018), and plots were generated using base R or the package “ggplot2” (Wickham 2016). Calculations for integrated chlorophyll were completed using the “trapz” function within the R package “pracma” (Borchers 2018). Integrated chlorophyll concentrations by depth layer (epilimnion, metalimnion, and hypolimnion) were calculated for all sites where a thermocline was detected for the purpose of comparison across lake trophic state, regardless of whether a DCM was present. Differences in POC : Chl were tested using non‐parametric methods because the assumption of homogeneity of variance did not hold; pairwise differences by depth layer and lake were tested using the “kruskalmc” function within the R package “pgirmess” (Giraudoux 2018). All statistical tests were performed at the level α = 0.05.
Results
Profile processing
In total, we used 1908 summer profiles from the years 1996–2017 in our analysis of lakes Superior (473), Huron (359), Michigan (388), Ontario (229), and Erie (459) (Table 2). Sites were mostly sampled in August, but some years included the last days of July (for Lake Michigan, the first lake sampled) or first days of September (for Lake Superior, the last lake sampled) due to differences in the ship's calendar and weather delays. All included stations were greater than 20 m in depth, as algorithms were optimized to perform at offshore sites. Thermal structure (Table 2), as well as the depths of the DCM, DBM, and DOmax at stratified sites (Table 3), were consistently detected (see Fig. 2 for examples of algorithm performance). Some minor manual corrections were made based on visual examination of profiles and algorithm output. Issues with peak detection were primarily associated with the presence of multiple significant peaks or especially noisy profiles, as there were some inconsistencies regarding where the top and bottom of maximum features were detected in such cases. We therefore made manual adjustments to the depth range of the DCM to encompass the appropriate extent of the DCM feature when this occurred, based on the algorithm performance on typical profiles. All profiles from western Lake Erie (135 profiles) were excluded from further DCM analysis, as the western basin is eutrophic, shallow (mean depth 7.4 m), and often is well‐mixed rather than stratified. The algorithms used were not optimized for such shallow eutrophic sites, and thus they did not perform well for DCM detection and would often characterize noise as a DCM. Visual inspection of profiles from western Lake Erie confirmed that DCM were not present. Data on thermal structure for western Lake Erie were still used, and integrated chlorophyll values by depth stratum were calculated when stratification was present.
Table 3.
Summary of deep chlorophyll maxima (DCM), deep biomass maxima (DBM), and deep oxygen maxima (DOmax) characteristics in the Laurentian Great Lakes, based on summer survey profiles from 1996 to 2017, organized by lake and basin (see Fig. 1). N = the number of profiles where a thermocline was detected. For the DCM, DBM, and DOmax, each of the following are given: the percentage of stratified profiles with the feature, the depth of the maximum value, and the magnitude of the maximum value. In addition, the for the DCM: multiple peaks = the proportion of profiles with a DCM that had two or more peaks present. DCM thickness = the depth range of the highest magnitude DCM feature (see text for details). All values shown are the mean ± one standard deviation.
| n profiles | DCM present (%) | Multiple peaks (%) | DCM depth (m) | DCM thickness (m) | DCM chlorophyll (μg L−1) | DBM present (%) | DBM depth (m) | DBM c p (m−1) | DOmax present (%) | DOmax depth (m) | DOmax saturation (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superior | 469 | 89 | 20 | 27.8 ± 7.7 | 38.5 ± 11.9 | 2.14 ± 1.03 | 80 | 25.0 ± 8.3 | 1.13 ± 1.98 | 91 | 22.7 ± 13.8 | 110 ± 8 |
| Huron | 359 | 91 | 27 | 34.8 ± 10.2 | 26.4 ± 11.9 | 2.20 ± 1.26 | 78 | 31.3 ± 8.8 | 1.01 ± 0.87 | 97 | 22.5 ± 6.1 | 122 ± 43 |
| Michigan | 388 | 81 | 34 | 27.8 ± 8.6 | 26.0 ± 12.7 | 2.73 ± 3.27 | 77 | 25.4 ± 6.7 | 1.30 ± 1.36 | 95 | 20.5 ± 6.0 | 119 ± 9 |
| Northern | 226 | 75 | 29 | 26.2 ± 8.7 | 27.4 ± 13.8 | 2.74 ± 1.81 | 70 | 25.0 ± 7.2 | 1.36 ± 1.62 | 92 | 20.6 ± 7.2 | 116 ± 9 |
| Southern | 162 | 90 | 40 | 29.7 ± 8.2 | 24.3 ± 11.2 | 2.72 ± 4.43 | 86 | 25.8 ± 6.1 | 1.22 ± 0.96 | 99 | 20.4 ± 3.8 | 123 ± 8 |
| Ontario | 226 | 65 | 17 | 17.5 ± 4.7 | 17.5 ± 6.9 | 5.63 ± 3.15 | 60 | 18.9 ± 5.1 | 1.90 ± 1.33 | 74 | 17.9 ± 7.9 | 116 ± 13 |
| Western | 112 | 82 | 11 | 17.0 ± 4.3 | 17.8 ± 6.2 | 6.13 ± 2.98 | 77 | 18.4 ± 4.7 | 2.00 ± 1.32 | 87 | 16.5 ± 9.2 | 121 ± 13 |
| Central | 114 | 47 | 28 | 18.5 ± 5.1 | 17.1 ± 7.9 | 4.92 ± 3.28 | 44 | 19.9 ± 5.6 | 1.71 ± 1.33 | 62 | 20.0 ± 5.1 | 110 ± 11 |
| Erie | 371 | 30 | 5 | 12.2 ± 6.1 | 5.2 ± 4.6 | 5.56 ± 3.29 | 26 | 13.6 ± 7.4 | 2.73 ± 2.95 | 11 | 13.0 ± 8.1 | 97 ± 19 |
| Central | 217 | 28 | 2 | 14.5 ± 2.7 | 5.3 ± 2.4 | 4.99 ± 2.70 | 20 | 15.0 ± 2.7 | 2.03 ± 1.16 | 2 | 14.0 ± 1.1 | 104 ± 10 |
| Eastern | 107 | 31 | 12 | 19.5 ± 3.7 | 10.7 ± 5.8 | 4.50 ± 2.50 | 49 | 21.1 ± 4.3 | 1.41 ± 0.84 | 33 | 21.0 ± 3.8 | 98 ± 9 |
Figure 2.
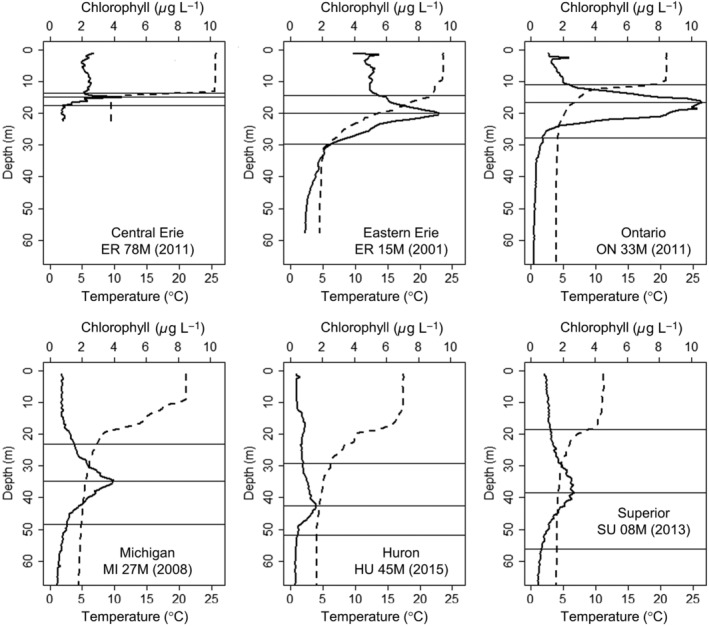
Examples of common deep chlorophyll maximum (DCM) shapes observed in the Laurentian Great Lakes. The dashed vertical line shows temperatures, and the solid line shows quenching‐corrected chlorophyll concentrations. Horizontal lines indicate the algorithm detection for the start, peak, and end depths of the DCM.
DCM characteristics across lakes
DCM were most common in lakes Superior and Huron (89% and 91% of all stratified profiles, respectively), frequent in lakes Michigan and Ontario (81% and 65%, respectively), and less common in eastern and central Lake Erie (31% and 28%, respectively) (Table 3). DCM were more common in southern Lake Michigan (90%) than in the northern basin (75%), and they were more common in western Lake Ontario (82%) than in the central basin (47%). In general, the lack of a DCM was associated with unstratified conditions, high epilimnetic chlorophyll concentrations, or deep thermoclines that were below the z 1%, such as in central Lake Ontario. At sites with a DCM, the occurrence of multiple significant peaks was most common in lakes Michigan (34%) and Huron (27%), followed by lakes Superior (20%), Ontario (17%), and Erie (5%). In part due to the high occurrence of multiple chlorophyll peaks, the shape of chlorophyll profiles was generally more variable in lakes Michigan and Huron than in lakes Superior, Ontario, and Erie (Fig. 3). The depths at which DCM occurred were greatest in Lake Huron (34.8 ± 10.1 m, 1 SD), followed by lakes Superior and Michigan (28.1 ± 7.3 and 27.8 ± 8.7 m, respectively). DCM were shallower in Lake Ontario (17.4 ± 4.5 m) and eastern Lake Erie (19.3 ± 3.7 m), while central Lake Erie had the shallowest DCM (14.5 ± 2.6 m). Generally, DCM thickness decreased with increasing lake trophic state, as DCM were broadest in Lake Superior (39.2 ± 11.8 m) and narrowest in Lake Erie (7.2 ± 4.7 m). When plotted against TSI, both the depth and thickness of DCM were greater at sites with low TSI values, with ultra‐oligotrophic sites typically having the deepest and broadest DCM (Fig. 4).
Figure 3.
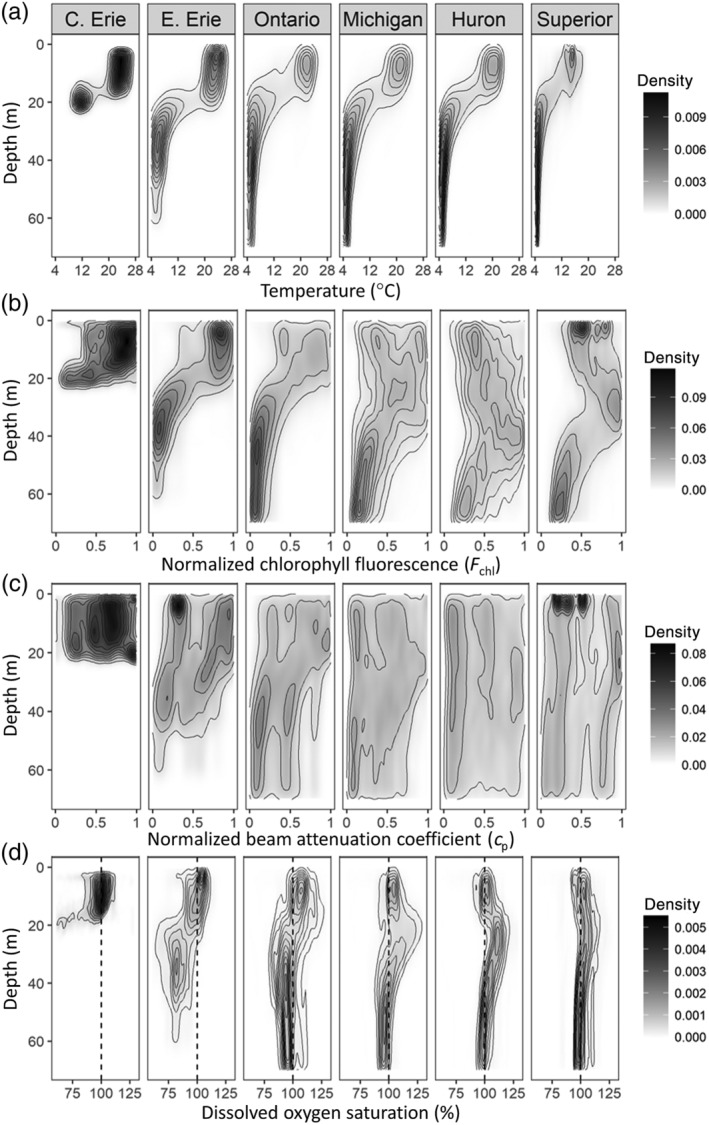
Density plots for all profile data, binned to 0.5‐m intervals. High density regions indicate where profiles are similar for that depth, while diffuse regions suggest greater variability in the data. (a) Temperature (°C), (b) normalized chlorophyll (maximum of 1), (c) normalized beam attenuation coefficient (maximum of 1), and (d) dissolved oxygen saturation percent, with 100% saturation indicated by the dotted line. Data shown include all profiles generated at all standard sampling sites from 1996 to 2017.
Figure 4.
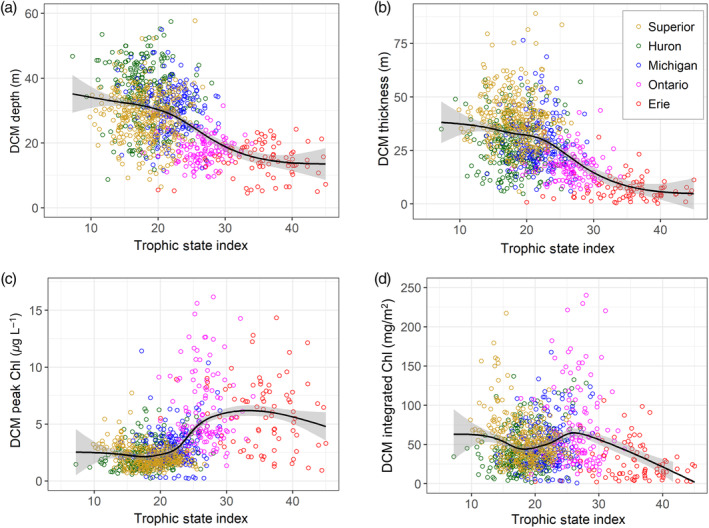
Characteristics of deep chlorophyll maxima (DCM) in the Great Lakes vs. trophic state index (TSI) calculated from mean of TSI from chlorophyll and total phosphorus concentrations the prior spring season (Carlson 1977). (a) Depth of the DCM (m); (b) thickness of the DCM (m); (c) chlorophyll concentration (μg L−1) at the DCM peak; (d) integrated chlorophyll for the full depth range of the DCM feature (mg m−2). All dependent variables were calculated from corrected chlorophyll profiles based on data from 1996 to 2017. The smoothed line represents a generalized additive model with confidence intervals, generated within the R package ggplot2 (Wickham 2016).
Based on extracted chlorophyll data at all standard sites, the DCM chlorophyll concentrations were lowest in lakes Superior and Huron, which were not significantly different from each other (mean 1.6 μg L−1), and highest in Lake Ontario (mean 3.5 μg L−1, max 8.7 μg L−1). Note, though, that there was a greater range of values in Lake Huron (max 4.1 μg L−1) than in Lake Superior (max 3.0 μg L−1). When plotted against the station TSI, DCM peak chlorophyll was consistently low at sites with TSI below approximately 20, while the highest DCM chlorophyll concentrations were observed at sites with TSI between 25 and 40 (Fig. 4c). Profile data were used to calculate integrated DCM chlorophyll, which was also highest for mid‐range TSI values, although some sites with a low TSI had high integrated DCM chlorophyll due to the broad thickness of the DCM (such as sites in Lake Superior, Fig. 4d). When split by depth stratum, the relative contribution of the hypolimnetic chlorophyll decreased with increasing TSI (note that very oligotrophic sites are also typically very deep), while the importance of epilimnetic chlorophyll increased; the amount of chlorophyll within the metalimnion was highest at mid‐range TSI sites (Fig. 5).
Figure 5.
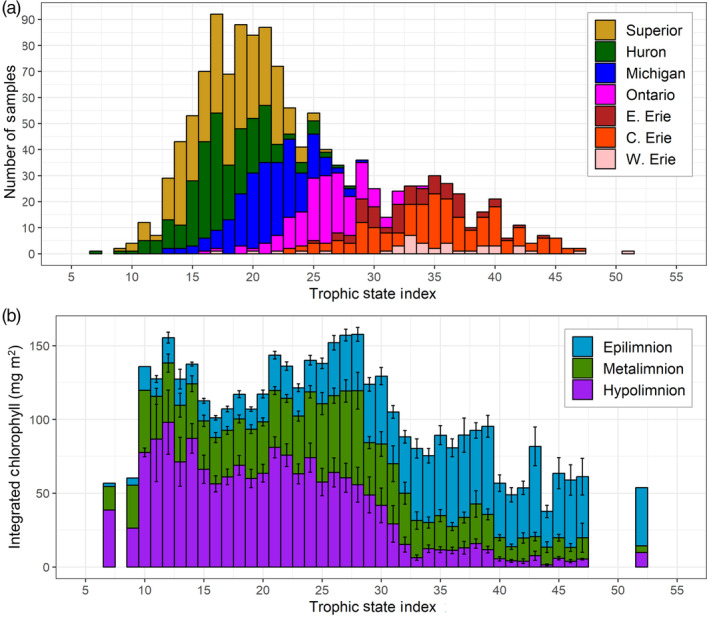
(a) Number of profiles by trophic state index (rounded to the nearest integer) for all sites where a thermocline was detected, colored by lake. (b) Integrated water column chlorophyll (mg m−2) within the epilimnion, metalimnion, and hypolimnion. Error bars show one standard error of the mean value for each depth layer. Trophic state index (TSI) shown is the mean of TSI values calculated from chlorophyll and total phosphorus concentrations the prior spring season (Carlson 1977, see text).
Both DBM and DOmax were also common for all lakes except Lake Erie (Table 3), although DOmax were observed more frequently than DBM. The depths at which DBM occurred were more variable than DCM depths, but the distributions of DO saturation data were more consistent (Fig. 3). In lakes Superior, Huron, and Michigan, DO supersaturation often extended well into the hypolimnion (Fig. 3d). DO saturation peaks were commonly within the metalimnion in lakes Ontario and Erie, although DO saturation minimum features were also frequently observed in these lakes, as DO depletion sometimes occurred near the metalimnion‐hypolimnion boundary.
While significant peaks in all three variables were often observed, the depths at which these peaks occurred did not always coincide. In the upper more oligotrophic lakes (Superior, Huron, and Michigan), there were differences in the distributions of the three peaks (Figs. 6, 7). The distributions of DOmax were shallowest, followed by DBM, and DCM exhibited the deepest distribution. When DCM were relatively shallow, the three peaks were more closely aligned. In lakes Ontario and Erie, the overall distributions of observed DCM, DBM, and DOmax were similar. In general, the differences between the DCM and DBM or DOmax increased with DCM depth (Fig. 7).
Figure 6.
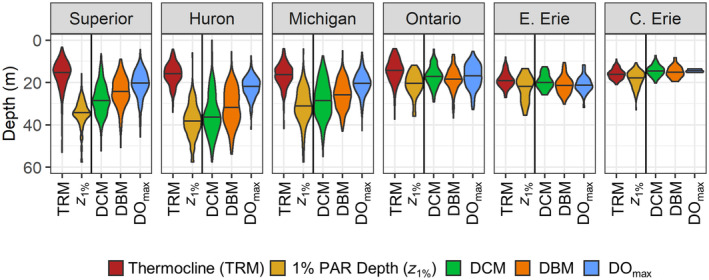
Violin plots showing the distribution of data for the depths of: (left panels) thermocline (TRM) and 1% PAR depth (z 1%); (right panels) deep chlorophyll maximum (DCM), deep biomass maximum (DBM), and deep oxygen maximum (DOmax). Data shown are for all sites sampled from 1996 – 2017. The line within each violin indicates the median. Plots are ordered from most oligotrophic (Superior and Huron) to most eutrophic (Central Erie).
Figure 7.
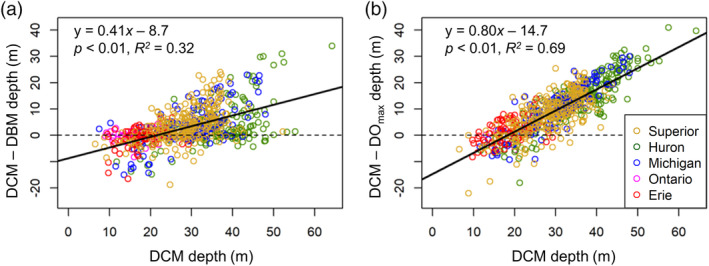
The differences between the deep chlorophyll maximum (DCM) depth and (a) the deep biomass maximum (DBM) depth, (b) deep oxygen maximum (DOmax) depth plotted against DCM depth. The dotted line marks the zero line, where the DCM and DBM/DOmax occur at the same depth, and the solid line is the linear regression.
Thermal structure and 1% PAR depth
The distributions of DCM depths were similar to those of the z 1% depths in lakes Huron and Michigan, whereas the median DCM depth was between those of the z 1% and thermocline depths in Lake Superior (Fig. 6). In lakes Ontario and Erie, the DCM, z 1%, and thermocline depths had similar distributions. Thermocline depths were similar across the five lakes (Fig. 6), but there were differences in the stratification strength, likely driven by differing summer surface temperatures across latitudes (Table 2). On average, stratification was strongest in central Lake Erie (RTRM 76.6, SD 62.9, maximum thermal gradient −6.2, SD 2.0°C m−1) and weakest in Lake Superior (RTRM 22.9, SD 21.8, maximum thermal gradient −1.7, SD 1.1°C m−1). Although the DCM was closely associated with the thermocline in lakes Ontario and Erie, there was not a strong correlation of DCM depth with thermocline depth across all five lakes because the DCM typically occurred near the bottom of the metalimnion or in the hypolimnion in lakes Michigan, Huron, and Superior. Rather, the DCM depth was significantly correlated with the z 1% depth overall (p < 0.01, R 2 = 0.34, n = 608, Fig. 8a). Although the DCM often occurred shallower than the z 1%; these anomalies were most common for relatively deep DCM at oligotrophic sites (especially in lakes Huron and Superior, Fig. 8c,d). Furthermore, DCM chlorophyll concentration (for 2002–2017) exhibited a significant negative nonlinear response to z 1% (p < 0.01, n = 608, Fig. 8b), such that higher water clarity was associated with lower magnitude DCM.
Figure 8.
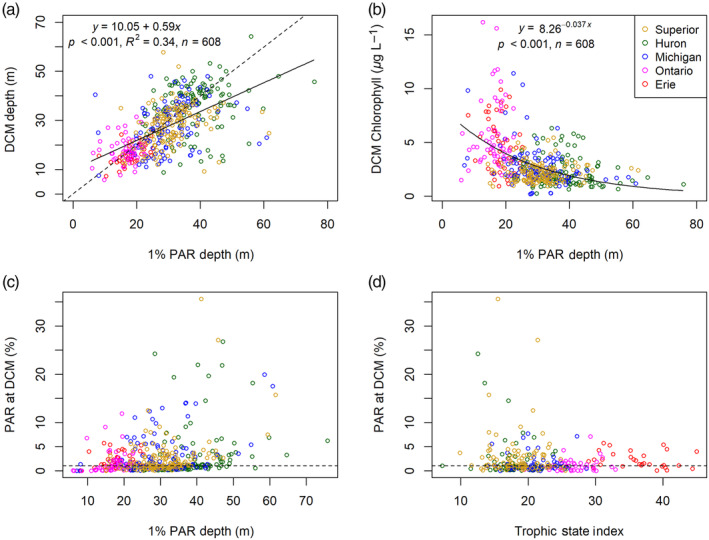
Relationships showing the importance of water clarity for determining deep chlorophyll maximum (DCM) characteristics. (a) DCM depth vs. 1% PAR depth (z 1%), including the 1 : 1 line (dotted line) and linear regression (solid line); (b) DCM chlorophyll (μg L−1) vs. z 1% with a nonlinear fit (c) Percent surface PAR at the DCM vs. z 1%; (d) Percent surface PAR at the DCM vs. trophic state index.
Data for POC from master stations indicate that photoacclimation is important when water clarity is high, such as in lakes Superior, Huron, and Michigan. Based on paired epilimnetic and DCM samples (sites where water samples for both depths were collected concurrently), there were larger differences in POC : Chl between the epilimnion and DCM in lakes Huron (mean diff = −97.0 g : g, p < 0.05, n = 31), Michigan (mean diff = −79.0 g : g, p < 0.05, n = 33), and Superior (mean diff = −72.8 g : g, p < 0.05, n = 33) than in Lake Ontario (mean diff = −24.1 g : g, p = 0.05, n = 21) (Fig. 9). There was no significant difference in POC : Chl between depth layers in Lake Erie, which had a small sample size due to infrequent formation of a DCM at the master stations (n = 3). While the POC : Chl within the DCM was not significantly different across lakes, there were differences in the epilimnetic POC : Chl among the lakes. The epilimnetic POC : Chl was not measurably different for lakes Michigan, Huron, and Superior. However, the POC : Chl was significantly higher in lakes Michigan (n = 35) and Huron (n = 36) than in Lake Ontario (n = 22, all p < 0.05); the POC : Chl ratio in the epilimnion of eastern Lake Erie (n = 12) was significantly lower than that of Lake Huron (p < 0.05) but not measurably different from the other lakes. Sample sizes differ between these comparisons because epilimnion samples were always collected, while DCM samples were only collected at sites where the Chief Scientist determined one was present based on real‐time profile data.
Figure 9.
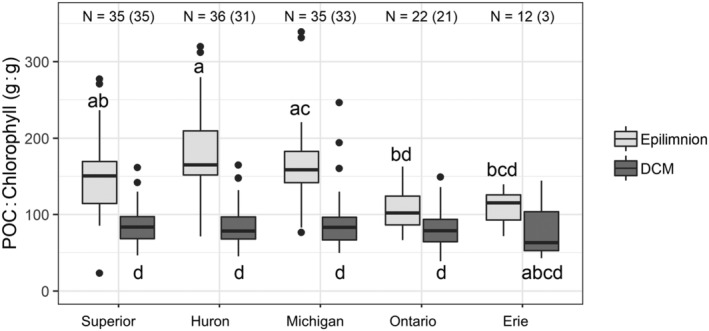
Particular organic carbon (POC) to chlorophyll ratios for the epilimnion and deep chlorophyll maximum (DCM) by lake, based on POC and extracted chlorophyll concentrations at master stations (Fig. 1) from 2000 to 2014. Boxes show the first and third quartiles with the median (line), whiskers extend to most extreme data point within 1.5 times the interquartile range from the box, and dots show outliers. Sample size is noted for samples from the epilimnion and DCM (in parentheses). Letters indicate significantly different groups based on pairwise comparisons after a significant Kruskal–Wallis test (Giraudoux 2018).
Time series
From 1996 to 2017, some lakes had significant trends in TSI, z 1%, DCM, and DBM characteristics (Fig. 10, Fig. 11), while changes in stratification, summertime TP, and DOmax depths were generally absent or inconsistent across lakes. TSI increased significantly in Lake Superior (p < 0.01, slope = 0.22 TSI year−1, R 2 = 0.14, n = 234) and decreased in both lakes Huron (p < 0.01, slope = −0.24 TSI year−1, R 2 = 0.17, n = 166) and Michigan (p < 0.01, slope = 0.14 TSI year−1, R 2 = 0.07, n = 146) (Fig. 10a). The z 1% deepened in lakes Huron (p < 0.01, slope = 0.45 m year−1, R 2 = 0.10, n = 113) and Michigan (p < 0.01, slope = 0.63 m year−1, R 2 = 0.18, n = 105) (Fig. 10b). In contrast, RTRM did not change significantly over time, and thermal structure (e.g., thermocline depth) was fairly consistent over the study period.
Figure 10.
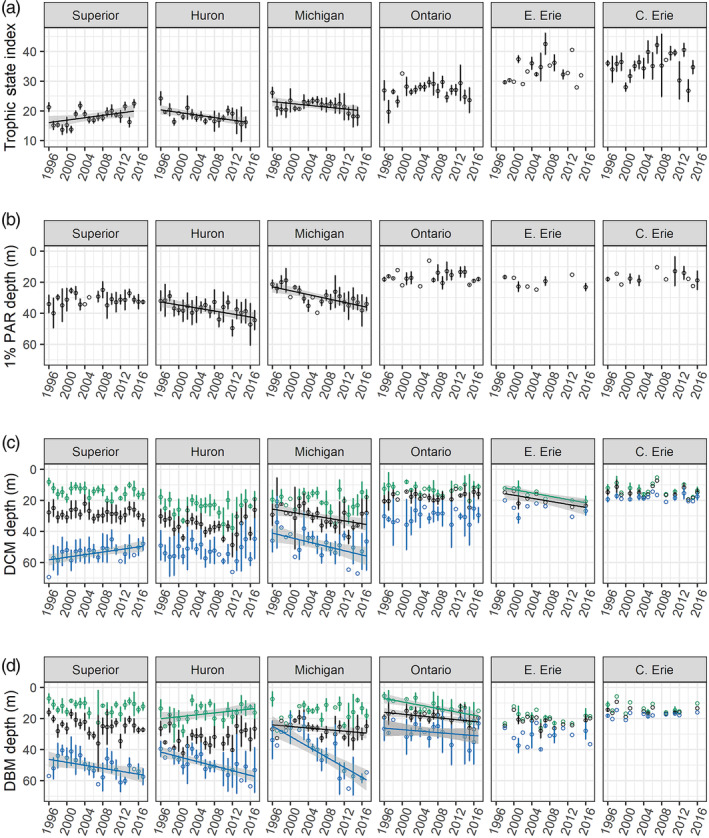
August time series plots (mean depth ± two standard errors) for data collected at all standard sampling stations: a) trophic state index, b) 1% PAR depth (z 1%), c) deep chlorophyll maximum (DCM), d) deep biomass maximum (DBM). For (c) and (d), colors indicate the top (green), peak (black), and bottom (blue) of the given feature. Lines show significant linear trends.
Figure 11.
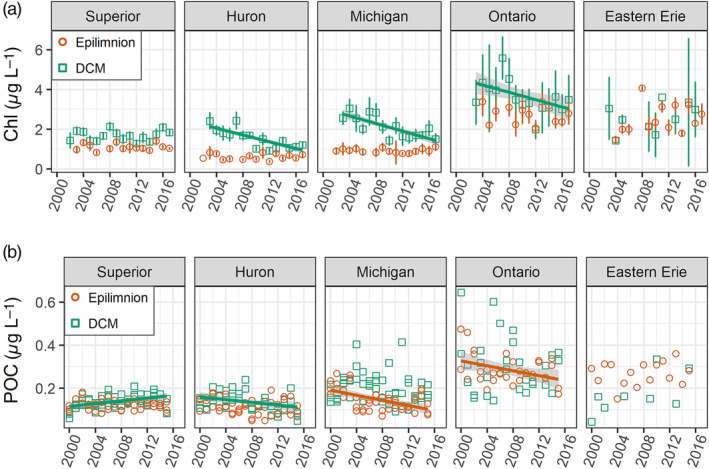
August time series for data for the epilimnion and the deep chlorophyll maximum (DCM): (a) chlorophyll concentration (mean ± two standard errors), and (b) particulate organic carbon (POC, all data for master stations only). Lines show significant linear trends. Data source EPA‐GLNPO GLENDA data base (https://cdx.epa.gov/).
DCM characteristics had significant temporal trends in lakes Superior, Michigan, and eastern Erie, while DBM changed in all lakes except Lake Erie over the study period. Lake Superior had contrasting trends in the bottom boundaries of DCM, which became shallower (p = 0.01, slope = −0.30 m year−1 R 2 = 0.02, n = 266) and DBM, which became deeper over the time period (p < 0.01, slope = 0.45 m year−1, R 2 = 0.06, n = 240). In Lake Huron, there were no significant trends in DCM features, but the DBM became broader as the top boundary became slightly shallower (p = 0.01, slope = −0.23 m year−1, R 2 = 0.04, n = 161) and the bottom boundary of the DBM deepened (p < 0.01, slope = 0.84 m year−1, R 2 = 0.16, n = 161), leading to an increasing DCM thickness over time. In Lake Michigan, both the DCM and the DBM deepened, in terms of the peaks depths (DCM: p = 0.01, slope = 0.28 m year−1, R 2 = 0.04, n = 143; DBM: p < 0.01, slope = 0.29 m year−1, R 2 = 0.09, n = 136) and the bottom boundaries (DCM: p < 0.01, slope = 0.58 m year−1, R 2 = 0.08, n = 143; DBM: p < 0.01, slope = 2.04 m year−1, R 2 = 0.55, n = 136). In contrast, Lake Ontario had no significant changes in the DCM, but the DBM deepened significantly (top: p < 0.01, slope = 0.45 m year−1, R 2 = 0.22; peak: p < 0.01, slope = 0.25 m year−1, R 2 = 0.10; bottom: p = 0.01, slope = 0.43 m year−1, R 2 = 0.09, all n = 76). Surprisingly, DCM deepened significantly in eastern Lake Erie: depth increased for both the top (p < 0.01, slope = 0.43 m year−1, R 2 = 0.61, n = 15) and the peak (p = 0.01, slope = 0.30 m year−1, R 2 = 0.34, n = 15) of DCM, although the sample size was low. The DBM, however, did not exhibit any significant changes in depth for Lake Erie.
The chlorophyll concentrations at the DCM significantly decreased in lakes Huron (p < 0.01, slope = −0.11 (μg L−1) year−1, R 2 = 0.29, n = 164), Michigan (p < 0.01, slope = −0.12 (μg L−1) year−1, R 2 = 0.20, n = 170), and Ontario (p = 0.02, slope = −0.12 (μg L−1) year−1, R 2 = 0.06, n = 107), while there were no significant changes to epilimnetic chlorophyll concentrations in any of the lakes over the study period (extracted chlorophyll data for 2002–2014) (Fig. 11). Based on particulates data at master stations (data for 2000–2015), POC in the DCM increased in Lake Superior (p < 0.01, slope = 3.18 (μg L−1) year−1, R 2 = 0.20, n = 47) and decreased only in Lake Huron over the study period (p = 0.02, slope = −3.21 (μg L−1) year−1, R 2 = 0.11, n = 43). Epilimnetic POC decreased significantly in lakes Michigan (p < 0.01, slope = −5.92 (μg L−1) year−1, R 2 = 0.28, n = 48) and Ontario (p = 0.03, slope = −5.70 (μg L−1) year−1, R 2 = 0.13, n = 32) (Fig. 11). The POC : Chl ratio at the DCM increased significantly in Lake Superior over the study period (p = 0.02, slope = 2.81 g : g year−1, R 2 = 0.12, n = 47); in Lake Michigan, there was also an increase in POC : Chl, but it was not significant at the 0.05 level (p = 0.08, slope = 3.76 g : g year−1, R 2 = 0.07, n = 48).
Discussion
Variation in DCM characteristics
There were clear patterns in the frequency of occurrence, depth, thickness, and chlorophyll concentration of DCM along the trophic state gradient from lakes Superior, Huron, and Michigan to Lake Ontario and Erie. Generally, the patterns of DCM characteristics we observed in the Great Lakes agreed with expectations based on stratified marine systems as described by Cullen (2015), previous observations across the Great Lakes (Moll and Stoermer 1982), and those in smaller lakes (Fee 1976; Hamilton et al. 2010; Leach et al. 2018). DCM formation was more common with increasing oligotrophy, with frequency of occurrence ranging from 30% in Lake Erie (excluding western basin) to 89% and 91% in lakes Superior and Huron, respectively. Furthermore, DCM generally increased in depth, became broader, and decreased in chlorophyll concentrations with increasing oligotrophy (decreasing TSI, Fig. 4). These patterns are consistent with expectations based on observations from other systems that span a trophic state gradient; for example, stable DCM are common in offshore oligotrophic oceans (Herbland and Voituriez 1979; Yentsch 1980; Cullen 1982; Mignot et al. 2014), while more variable DCM tend to occur in shallower and more productive embayments or lakes (Onitsuka et al. 2018). However, relatively few studies have investigated DCM across water bodies with a range of trophic states using consistent methods. Many studies on individual water bodies, as well multilake and review papers (Cullen 2015; Leach et al. 2018), suggest that water clarity is a key factor driving the depth at which DCM occur. Given that water clarity and trophic state are highly correlated, it is not surprising that trophic state may explain much of the observed variation in DCM.
While many of these patterns were consistent on the lake‐wide scale, differences in DCM between basins within lakes Michigan, Ontario, and Erie warrant further discussion. In lakes Michigan and Ontario, the major differences between basins were in DCM frequency. In Lake Michigan, DCM occurred more frequently in the southern basin than the northern basin (90% and 75% with DCM, respectively). The northern basin of Lake Michigan often has weaker stratification (Table 2) and may be somewhat more productive than the southern basin during summer (note summer chlorophyll data, Table 1), both of which may affect DCM formation. However, there were not significant differences between basins in the depth, thickness, or chlorophyll concentrations of DCM when present. Similarly, in Lake Ontario, there were differences in frequency of formation between basins (71% of profiles in the western basin and 38% in the central), but not in characteristics of DCM when present. The likely cause of the observed difference in DCM frequency for Lake Ontario is thermocline depth relative to the z 1%. In Lake Ontario, the thermocline was significantly deeper in the central basin than in the western basin (mean depth of 17.5 and 11.9 m, respectively, p < 0.01), such that the z 1% was sometimes shallower than the thermocline depth (and thus presumably shallower than the nutricline) in the central basin. In such cases, DCM were generally absent. However, the timing of the summer surveys for the Great Lakes (August) is late in the stratified season, especially for lakes Erie and Ontario, which are at lower latitudes; the thermocline generally deepens considerably in the central basin of Lake Ontario over the summer due to prevailing wind and circulation patterns (Beletsky et al. 1999). Thus, the absence of a DCM in August is not necessarily indicative of a lack of DCM formation in central Lake Ontario, and previous studies have shown a DCM to be almost ubiquitous in offshore Lake Ontario during June through July and less common by September (Twiss et al. 2012; Watkins et al. 2015; Scofield et al. 2017).
Differences in DCM among basins in Lake Erie were expected due to variation in the bottom depth and trophic state of different regions of the lake (Table 1). The western basin of Lake Erie generally does not have DCM because it is both eutrophic and shallow (sample sites 9–12 m); given that stable stratification is uncommon (due to wind‐driven mixing events) and epilimnetic production is high, conditions for DCM formation typically do not occur. The central basin of Lake Erie is mesotrophic and does form DCM (28% of observed profiles, Table 3), but they were typically much shallower than those in eastern Erie and the rest of the Great Lakes. Bottom depth may affect differences in DCM thickness across lakes (Leach et al. 2018), in part because depth affects stratification patterns and limits the maximum depth of a DCM, but also because lake depth is negatively correlated with lake trophic state (Carpenter 1983). The DCM occurring in Lake Erie's central basin were generally very shallow and narrow, occurring near to the top of the thermocline (which is also the approximate depth of the z 1%).
Mechanisms of DCM formation
Our results suggest that the processes contributing to DCM vary across the trophic state gradient in the Great Lakes and provide additional support for previous observations that peaks in production, phytoplankton biomass, and chlorophyll often occur at different depths in oligotrophic to ultra‐oligotrophic waters (Sterner 2010). We base this assertion on the occurrence and distributions of DBM and DOmax as related to the DCM. Overall, the DOmax tended to occur shallowest, within the metalimnion, while DBM were somewhat deeper, and DCM occurred below both the other peaks (Fig. 7). This was common in lakes Superior, Huron, and Michigan (Figs. 6, 7); overall, the depth differences between the DCM and both the DBM and DOmax increased with increasing DCM depth (Fig. 7). The observed discrepancies in depths among DCM, DBM, and DOmax suggest that the processes regulating these variables may interact in different ways as trophic state changes, such that the depths at which maxima occur for phytoplankton production, biomass, and chlorophyll diverge in more oligotrophic systems. Similar patterns have been observed in some marine and freshwater systems (including Lake Superior, see Sterner 2010), but this is not always the case. For example, some data from Lake Tahoe (an ultra‐oligotrophic lake) suggest the DCM and DBM typically coincide, even when the DCM occur very deep in the water column (Abbott et al. 1984; Coon et al. 1987). In our dataset, peaks in production and phytoplankton biomass appear to be shallower than the DCM, suggesting that the location of the DCM is at least in part driven by photoacclimation in these lakes. Photoacclimation has previously been established as an important process contributing to DCM formation, especially in oligotrophic waters (Fennel and Boss 2003; Kiefer et al. 1975; Steele 1964; Taylor et al. 1997), and our results support this hypothesis.
Data for POC : Chl also indicate that differences between the epilimnion and DCM chlorophyll concentrations are strongly affected by photoacclimation in lakes Michigan, Huron, and Superior; this agrees with other recent assessments of DCM in these lakes (Barbiero and Tuchman 2004; White and Matsumoto 2012). It is important to account for photoacclimation in systems with high water clarity, as POC : Chl observed within the epilimnia of these lakes were significantly higher than those in the DCM (Fig. 9); thus, chlorophyll data alone will underestimate phytoplankton biomass within the epilimnion and/or overestimate DCM biomass. Even so, the concentration of POC within the DCM was significantly higher than that of the epilimnion in lakes Superior, Huron, and Michigan, suggesting that variable photoacclimation with depth is not the sole cause of the observed DCM. However, we note that POC is not only phytoplankton carbon, as it also includes detritus, heterotrophic flagellates, etc. Thus, differences in the abundance of non‐phytoplankton POC could affect our interpretation of POC : Chl data.
In contrast to the upper lakes, DCM were closely aligned in depth with the DBM and DOmax in lakes Ontario and Erie (Fig. 6), indicating that the DCM are closely associated with biomass peaks and are likely productive in these lakes; this is consistent with other recent observations of DCM in Lake Ontario (Twiss et al. 2012; Scofield et al. 2017), as well as with observations in shallow lakes and more meso‐eutrophic water bodies forming DCM (Camacho 2006). While DBM frequently co‐occurred with DCM in both the central and eastern basins of Lake Erie, DOmax were much less common. Rather, DO minima were often observed within the metalimnion. Oxygen depletion is common in the central basin of Lake Erie (Scavia et al. 2014; Rucinski et al. 2016), and high bacterial decomposition rates below the thermocline could cause the oxygen minima in these more productive basins of Lake Erie (Fig. 3). However, some biomass within the DCM of these mesotrophic lakes may be due a combination of bacterial, protozoan, and mesozooplankton, rather than phytoplankton alone (Adrian and Schipolowski 2003).
The shallower depths observed for the DOmax relative to the DCM is consistent with the fact that both higher light intensity and warmer temperatures (within the range observed for Great Lakes) will increase photosynthetic rates even under nutrient stressed conditions. However, DOmax were sometimes present even when peaks were not detected in the other variables. Physical processes may also contribute to the observed metalimnetic oxygen peaks, as has been observed in small lakes (Wilkinson et al. 2015). Solar warming of metalimnetic water cut‐off from atmospheric exchange can lead to supersaturation of DO below the thermocline, even in the absence of positive NEP. In our dataset, however, supersaturation of oxygen did occur over a broad depth range below the thermocline in lakes Michigan, Huron, and Superior, rather than just near the thermocline, suggesting that there is positive NEP occurring over a greater depth range in these lakes (Fig. 3). In lakes Ontario and Erie, the DOmax depth typically coincided with that of a DBM; high phytoplankton biomass at the same depth of a DOmax suggests that the layer is productive and the DO saturation peak is not due only to physical processes. Other examples of highly productive DCM have been observed in a range of systems, including the Great Lakes (Fahnenstiel and Scavia 1987).
Central Erie is likely near the threshold trophic state for productive DCM formation, given that DCM often do not form when the z 1% zone is shallower than the thermocline (as is common in Lake Erie); in such cases, maximum phytoplankton biomass typically occurs in the epilimnion (Hamilton et al. 2010) and growth is limited primarily by light rather than nutrients. Our data suggest that in cases that are below this eutrophic extreme (indicated by a z 1% that reaches the metalimnion), shallow and narrow DCM form in the metalimnion of central Lake Erie. Growth in these shallow DCM are likely co‐limited by light and nutrient availability, and phytoplankton that can maintain their position at this optimum depth probably dominate this layer (Klausmeier and Litchman 2001). Such DCM could be formed by in situ growth within the layer, as well as by more transient processes such as vertical migration by buoyant or mobile species (Cullen 1985; Oliver 1994; White et al. 2006), in which case time of sampling could influence the frequency of DCM observation. Passive accumulation of phytoplankton cells due to physical processes could also form a DCM along the pycnocline (Steinbuck et al. 2010; Durham and Stocker 2012). A combination of these processes can lead to the formation of multiple biomass peaks in the water column, which was also observed in our study (Table 3).
Of course, several of these additional processes, as well as in situ growth and photoacclimation, may influence DCM dynamics across lakes. For example, phytoplankton migration for nutrient acquisition (Heaney and Eppley 1981; Ralston et al. 2007; Baek et al. 2009; Ryan et al. 2010), variable phytoplankton sinking rates (Steele and Yentsch 1960), and physical processes (even in the absence of stable stratification, Lewis et al. 2017) can be important factors affecting DCM formation. Vertical migrations of mobile plankton follow a variety of patterns that could accentuate DCM or cause additional peaks (Lande et al. 1989; Cullen and MacIntyre 1998; Prairie et al. 2011), and these features can vary with time of day. In addition, nutrient excretion by migrating zooplankton and/or grazing pressure by zooplankton can impact DCM/DBM dynamics (Pilati and Wurtsbaugh 2003; Sawatzky et al. 2006; Oliver et al. 2014). There may also be phytoplankton community differences with depth related to fine‐scale niche partitioning (Sommer 1982; Cullen and MacIntyre 1998; Latasa et al. 2017), as previously observed in Lake Ontario (Twiss et al. 2012). In ultra‐oligotrophic systems with high water clarity, light is attenuated over a greater depth range; thus, there may be more vertical distance over which phytoplankton may adapt to grow at different combinations of isolumes and nutrient levels, leading to niche partitioning and distinct phytoplankton communities at the DBM and DCM.
The occurrence of multiple chlorophyll peaks at different depths in the water column, which may be associated with several of the above processes, can complicate analysis and interpretation of DCM. In our dataset, chlorophyll profiles in lakes Michigan and Huron were characterized by greater variability than the other lakes, and around a quarter to a third of the profiles had multiple significant peaks (Table 3). Similar patterns were seen in the c p profiles, which also exhibited double peaks. These multiple peaks caused greater variability in the distributions of normalized chlorophyll and c p (Fig. 3), as well as confounded the relationship between the DCM and DBM. For example, there were some sites at which there were multiple peaks for both chlorophyll and c p profiles, but the deeper peaks had higher chlorophyll concentrations and the shallower peak had a higher c p values. This suggests that the processes contributing to chlorophyll peaks are variable with depth, not just across sites. Shallower peaks within one profile may be associated with high biomass and/or production (Coon et al. 1987; Sterner 2010) and dominated by species that thrive under higher light but are nutrient‐limited, while deeper DCM may be dominated by species adapted to grow at colder, lower light environments (Edwards et al. 2016). However, these dynamics are not well‐documented in the Great Lakes system, and more detailed investigations of profiles with multiple peaks will improve our understanding of DCM dynamics in these large lakes.
Previous work on phytoplankton community structure in the Great Lakes suggests that the DCM is often a unique phytoplankton community from that of the epilimnion, with larger‐bodied forms of siliceous algae (diatoms and chrysophytes) in the DCM compared to the epilimnion (Twiss et al. 2012; Bramburger and Reavie 2016; Scofield et al. 2017). However, these comparisons have largely been based on discrete depth DCM samples compared to integrated epilimnetic samples; additional studies examining a greater number of depths (such as at the DBM indicated by c p) or using profiling instruments with multiple wavelengths for algal class determination, such as the FluoroProbe (bbe Moldaenke), would provide phytoplankton community structure data at greater depth resolution. Such studies will be important for understanding phytoplankton community changes with depth, the formation of multiple phytoplankton peaks, and the bottom‐up impacts of DCM dynamics on zooplankton grazers (Twiss et al. 2012).
Thermal structure and 1% PAR depth
Water clarity is clearly an important variable affecting the DCM depth and thickness, DCM chlorophyll concentration, and the degree of photoacclimation of phytoplankton. Previous research has identified z 1% as the most important factor regulating the location of DCM in various lakes (Leach et al. 2018), and the DCM often occurs around the 1–2% light level in other systems (Williamson et al. 1996; Pérez et al. 2007; Hamilton et al. 2010). The z 1% appears to be a stronger determinant of DCM characteristics than thermal structure in our dataset as well. Thermal structure did not vary greatly across the lakes and was not generally associated with differences in DCM depth: the DCM was well within the hypolimnion in some cases (e.g., Lake Superior) and at the top of the thermocline in others (e.g., central Lake Erie). However, we note that DCM are typically absent in cases where the thermocline depth is deeper than z 1%, presumably because there is not enough light reaching the thermocline (and presumed nutricline) for net phytoplankton growth at depth; in such cases, phytoplankton growth is likely higher in the epilimnion, and strong nutriclines may not be present. Thus, the thermocline depth relative to z 1% is an important variable determining DCM formation. In addition to the importance of water clarity for determining DCM depth, the chlorophyll concentration at the DCM declined with deeper z 1%, indicating that DCM tend to decrease in magnitude with increasing water clarity. The importance of water clarity observed in this study agrees with a broad range of previous work in both freshwater and marine systems (Cullen 2015; Leach et al. 2018).
However, one key difference between our study and much previous research is that we frequently observed DCM that occurred at higher light levels than expected (often up to 5% surface PAR, at times > 10% surface PAR) (Fig. 8). This contrasts with some previous observations in marine waters, where the DCM is typically associated with the z 1% and occurs at relatively stable isolumes (Letelier et al. 2004; Mignot et al. 2014); such discrepancies have previously been observed in ultra‐oligotrophic lakes (Abbott et al. 1984). A variety of factors could explain this deviation from expectation, such as higher upward nutrient flux in systems with weak stratification, or differences in phytoplankton community structure. Work in Lake Tahoe has shown that DCM depth can vary over relatively short time scales (days) and these changes are largely driven by changes to nutrient flux and diffusion; in systems with relatively weak stratification or particularly strong storm events, wind events can increase nutrient flux upward—strengthening the DCM and/or allowing it to form shallower (Abbott et al. 1984; Coon et al. 1987). Thus, our hypothesis for this observation is that nutriclines may be less consistent in lakes Superior and Huron, which have cooler surface temperatures and weaker stratification (Table 2), allowing for DBM and/or DCM formation at shallower depths than expected.
One significant limitation of our study is a lack of high‐resolution nutrient data. Nutricline dynamics are clearly important for understanding DCM‐ and DBM‐forming mechanisms (Cermeño et al. 2008; Gong et al. 2017; Kiefer et al. 1975; Mignot et al. 2014; see Cullen 2015 for review). Because we only have nutrient data for a few depths, we were unable to characterize nutricline depths with high resolution and test hypotheses related to nutricline depth. Phosphorus is often the limiting nutrient in freshwater systems, and fast‐response probes for in situ measurements of dissolved phosphate are not as reliable or widely used as those for nitrate; furthermore, dissolved phosphorus concentrations in the Great Lakes are low enough that they are often at detection limits even for laboratory methods. Thus, our knowledge of nutricline dynamics in freshwaters lags that of marine systems. Additional research investigating nutrient dynamics with greater depth resolution in the Great Lakes may help elucidate possible mechanisms driving DCM at shallower depths than z 1%.
Long‐term change and impacts
Our time series data support previous observations that lakes Huron and Michigan are continuing to undergo oligotrophication, becoming increasingly similar to Lake Superior (Barbiero et al. 2012). Lake Huron and Lake Michigan both exhibited significant long‐term trends across a range of variables, including trophic state, water clarity, DCM and DBM characteristics. Both lakes Huron and Michigan have low TSI and increasing densities of dreissenid mussels in the offshore zone (Fahnenstiel et al. 2010b; Burlakova et al. 2018), which may interact to impact nutrient cycling and water clarity (Stańczykowska and Lewandowski 1993; Idrisi et al. 2001; Fahnenstiel et al. 2010a) and in turn DCM dynamics. One key point regarding long‐term trends across the lakes is that there were clear differences in trends for the DCM and DBM. This observation reemphasizes the importance of distinguishing between the two in analyses of phytoplankton vertical structure. As abiotic conditions change (e.g., nutrient concentrations, water clarity, water column stability), DCM and DBM may be affected differently due to interactions among the mechanisms driving their formation.
Differences in DCM and DBM characteristics are important to consider when evaluating the food web implications of variation in the vertical distribution of phytoplankton distribution within the water column. While chlorophyll data alone are not sufficient to predict the importance of subsurface phytoplankton to total water column biomass, improving our framework for how DCM characteristics change along a trophic state gradient informs our interpretation of chlorophyll data (for example, how DCM and DBM depths are related in different systems). Furthermore, the temperatures at which DBM occur affect bioenergetic trade‐offs for zooplankton grazers, with deeper (and thus typically colder) DBM favoring large‐bodied cold‐adapted zooplankton species, such as large calanoid copepods common in the Great Lakes (e.g., Senecella calanoides, Limnocalanus macrurus). Significant changes to zooplankton abundance and community structure have occurred in lakes Ontario, Michigan, and Huron over the past two decades: along with declines in total biomass, the relative importance of calanoid copepods has increased as species that prefer warmer surface waters have declined (Barbiero et al. 2019). This vertical redistribution of resources may favor native cold‐water fish species, such as coregonids, over non‐native alewife and has implications for both restoration efforts and the economics of the Great Lakes fisheries (Dettmers et al. 2012).
Conclusions
Our results extend previous work in the Laurentian Great Lakes by providing the first long‐term cross‐lake comparison of DCM using standardized methods, improving our understanding of DCM dynamics in large lakes. In summary, DCM were more common, deeper, broader, and lower in chlorophyll concentration with increasing oligotrophy. These general characteristics were strongly related to the trophic state index of individual sites, based on spring TP and chlorophyll data, suggesting that spring indices of trophic state may be useful for predicting DCM characteristics the following summer season in temperate lakes. Our study also reiterates that water clarity, measured by z 1% herein, is a helpful variable for predicting DCM characteristics. While z 1% is clearly useful for predicting DCM depth and concentration across a broad trophic state gradient, one key observation of our study that differs from previous work is the occurrence of DCM at higher light levels than expected in ultra‐oligotrophic waters with high water clarity (lakes Superior and Huron in particular. While further research is needed to assess drivers of this pattern, we suggest that stratification strength may play a role via regulation of nutrient flux, as lakes Superior and Huron typically have relatively weak stratification due to their high latitudes.
The overall patterns we observed in the distributions of proxies for maxima in biomass and production (DBM and DOmax, respectively) were consistent with expectations based on previous observations in the Great Lakes, other large lakes, and marine systems (Moll and Stoermer 1982; Coon et al. 1987; Cullen 2015). Our study reiterates that the relative importance of processes contributing to the DCM are variable, as has been established by a wide breadth of studies (Barbiero and Tuchman 2001b; Cullen 1982; Cullen 2015; Durham and Stocker 2012); however, we expand upon previous literature by suggesting that these differences are predictable across a trophic state gradient in large lakes. In ultra‐oligotrophic waters, DOmax, DBM, and DCM often occur at different depths, with DCM usually occurring deepest in the water column. DCM may be largely attributed to photoacclimation in these systems, and thus chlorophyll profiles must be interpreted with caution. Rather, the use of other proxy variables for phytoplankton biomass, such as beam attenuation (c p), may be more useful for evaluating phytoplankton biomass distributions in ultra‐oligotrophic waters, and increased sampling of DBM is warranted.
Lastly, the time series presented here provides further evidence that lakes Michigan and Huron have undergone continued oligotrophication, converging toward the conditions of Lake Superior. Trends in lakes Michigan and Huron were mostly consistent across various metrics, while long‐term patterns were not as clear in other lakes. Understanding how DCM and DBM may respond to changes in trophic state is important for predicting food web impacts of ongoing long‐term change in the Great Lakes. Although this study is a step toward bridging DCM theory across freshwater and marine systems, several areas of further research are needed to continue this effort, including standardized analysis of profiles across a wider range of environments (i.e., pooling datasets across systems), high‐resolution nutrient data for freshwater systems, and greater attention to processes driving the formation of multiple DCM peaks.
Conflict of interest
None declared.
Supporting information
Figure S1 Relationship of the light extinction coefficient (K) to the secchi disk depth (meters) for all sites with paired secchi disk measurements and profiles of photosynthetically active radiation (PAR). K values were calculated from the slope of the linear relationship between the natural log transformed PAR vs. depth.
Figure S2. Scatter plots of in situ chlorophyll fluorescence (F chl) measurements vs. extracted values (Chl) for discrete depth water samples taken throughout the water column (a) before quenching corrections were applied, and (b) after quenching corrections were applied. Black dots indicate points where the PAR measurement was < 25 μE (m2s)‑1, and red dots indicate points with > 25 μE (m2s)‑1 PAR measurements, which are biased low prior to the correction. Only data from 2002 onward (through 2017) were used for the calibration because of quality control issues for extracted chlorophyll in earlier years. The model was optimized separately for 2011, when in situ fluorometer values were biased upward relative to other years (likely an issue with the manufacturer‐supplied calibration coefficient used). All other years showed a similar relationship and thus were pooled to fit the quenching equation parameters.
Table S1. Parameter fits for the quenching correction model for data subsets: 2002–2017, and 2011 (fit separately as the calibration constant was entered incorrectly that year). The parameters fitted for data from 2002 to 2017 were also applied to data from 1996 to 2001, for which there are concerns about the quality of the extracted chlorophyll data. Corrected profiles were then run through the peak detection algorithm.
Acknowledgments
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This study was supported by the U.S. Environmental Protection Agency (U.S. EPA) Cooperative Agreement to Cornell University (GL 00E01184‐0). We are grateful to the captains and crews of the R/V Lake Guardian and the technicians and graduate students at Cornell University that contributed to the data presented in this paper. We also thank Wenzhao Xu and Paris Collingsworth for their assistance troubleshooting our use of their algorithms for profile analysis. Patrick Sullivan, Nelson Hairston, Brian Weidel, and Elizabeth Hinchey Malloy provided helpful comments to improve this manuscript. The research described in this article has not been subjected to U.S. EPA review. Any opinions expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. EPA.
Associate editor: John Downing
References
- Abbott, M. R. , Denman K. L., Powell T. M., Richerson P. J., Richards R. C., and Goldman C. R.. 1984. Mixing and the dynamics of the deep chlorophyll maximum in Lake Tahoe. Limnol. Oceanogr. 29: 862–878. doi: 10.4319/lo.1984.29.4.0862 [DOI] [Google Scholar]
- Adrian, R. , and Schipolowski T.. 2003. Bacterial and protozoan mass accumulation in the deep chlorophyll maximum of a mesotrophic lake. Arch. Hydrobiol. 157: 27–46. doi: 10.1127/0003-9136/2003/0157-0027 [DOI] [Google Scholar]
- Baca, R. G. , and Arnett R. C.. 1976. A limnological model for eutrophic lakes and impoundments. Richland, WA: Battelle, Inc. Pacific Northwest Laboratories. [Google Scholar]
- Baek, S. H. , Shimode S., Shin K., Han M. S., and Kikuchi T.. 2009. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of vertical migration and cell division. Harmful Algae 8: 843–856. doi: 10.1016/J.HAL.2009.04.001 [DOI] [Google Scholar]
- Banse, K. 1987. Clouds, deep chlorophyll maxima and the nutrient supply to the mixed layer of stratified water bodies. J. Plankton Res. 9: 1031–1036. doi: 10.1093/plankt/9.5.1031 [DOI] [Google Scholar]
- Barbiero, R. P. , Little R. E., and Tuchman M. L.. 2001. Results from the U.S. EPA's biological open water surveillance program of the Laurentian Great Lakes: III. Crustacean zooplankton. J. Great Lakes Res. 27: 167–184. doi: 10.1016/S0380-1330(01)70630-2 [DOI] [Google Scholar]
- Barbiero, R. P. , and Tuchman M. L.. 2001a. Results from the U.S. EPA's biological open water surveillance program of the Laurentian Great Lakes: I. Introduction and phytoplankton results. J. Great Lakes Res. 27: 134–154. doi: 10.1016/S0380-1330(01)70628-4 [DOI] [Google Scholar]
- Barbiero, R. P. , and Tuchman M. L.. 2001b. Results from the U.S. EPA's biological open water surveillance program of the Laurentian Great Lakes: II. Deep chlorophyll maxima. J. Great Lakes Res. 27: 155–166. doi: 10.1016/S0380-1330(01)70629-6 [DOI] [Google Scholar]
- Barbiero, R. P. , and Tuchman M. L.. 2004. The deep chlorophyll maximum in Lake Superior. J. Great Lakes Res. 30: 256–268. doi: 10.1016/S0380-1330(04)70390-1 [DOI] [Google Scholar]
- Barbiero, R. P. , Lesht B. M., and Warren G. J.. 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. J. Great Lakes Res. 38: 368–380. doi: 10.1016/j.jglr.2012.03.009 [DOI] [Google Scholar]
- Barbiero, R. P. , Rudstam L. G., Watkins J. M., and Lesht B. M.. 2019. A cross‐lake comparison of crustacean zooplankton communities in the Great Lakes, 1997‐2016. J. Great Lakes, Res. 45: 672–690. doi: 10.1016/j.jglr.2019.03.012 [DOI] [Google Scholar]
- Beckmann, A. , and Hense I.. 2007. Beneath the surface: Characteristics of oceanic ecosystems under weak mixing conditions – A theoretical investigation. Prog. Oceanogr. 75: 771–796. doi: 10.1016/J.POCEAN.2007.09.002 [DOI] [Google Scholar]
- Beletsky, D. , Saylor J. H., and Schwab D. J.. 1999. Mean circulation in the Great Lakes. J. Great Lakes Res. 25: 78–93. doi: 10.1016/S0380-1330(99)70718-5 [DOI] [Google Scholar]
- Bishop, J. K. B. 1999. Transmissometer measurement of POC. Deep Sea Res. Part I Oceanogr. Res. Pap. 46: 353–369. doi: 10.1016/S0967-0637(98)00069-7 [DOI] [Google Scholar]
- Borchers, H. W. 2018. pracma: Practical numerical math functions. R package version 2.2.2. Available from https://CRAN.R-project.org/package=pracma.
- Boyd, J. D. 1980. Metalimnetic oxygen minima in Lake Ontario, 1972. J. Great Lakes Res. 6: 95–100. doi: 10.1016/S0380-1330(80)72087-7 [DOI] [Google Scholar]
- Bramburger, A. J. , and Reavie E. D.. 2016. A comparison of phytoplankton communities of the deep chlorophyll layers and epilimnia of the Laurentian Great Lakes. J. Great Lakes Res. 42: 1016–1025. doi: 10.1016/J.JGLR.2016.07.004 [DOI] [Google Scholar]
- Brooks, A. S. , and Torke B. G.. 1977. Vertical and seasonal distribution of chlorophyll a in Lake Michigan. J. Fish. Res. Board Canada 34: 2280–2287. doi: 10.1139/f77-306 [DOI] [Google Scholar]
- Bunnell, D. B. , Barbiero R. P., Ludsin S. A., Madenjian C. P., Warren G. J., Dolan D. M., Brenden T. O., Briland R., Gorman O. T., He J. X., Johengen T. H., Lantry B. F., Lesht B. M., Nalepa T. F., Riley S. C., Riseng C. M., Treska T. J., Tsehaye I., Walsh M. G., Warner D. M., and Weidel B. C.. 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: Bottom‐up and top‐down regulation. Bioscience 64: 26–39. doi: 10.1093/biosci/bit001 [DOI] [Google Scholar]
- Burlakova, L. E. , Barbiero R. P., Karatayev A. Y., Daniel S. E., Hinchey E. K., and Warren G. J.. 2018. The benthic community of the Laurentian Great Lakes: Analysis of spatial gradients and temporal trends from 1998 to 2014. J. Great Lakes Res. 44: 600–617. doi: 10.1016/j.jglr.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho, A. 2006. On the occurrence and ecological features of deep chlorophyll maxima (DCM) in Spanish stratified lakes. Limnetica 25: 453–478. doi: 10.23818/limn.25.32 [DOI] [Google Scholar]
- Carlson, R. E. 1977. A trophic state index for lakes. Limnol. Oceanogr. 22: 361–369. doi: 10.4319/lo.1977.22.2.0361 [DOI] [Google Scholar]
- Carpenter, S. R. 1983. Lake geometry: Implications for production and sediment accretion rates. J. Theor. Biol. 105: 273–286. doi: 10.1016/S0022-5193(83)80008-3 [DOI] [Google Scholar]
- Cermeño, P. , Dutkiewicz S., Harris R. P., Follows M., Schofield O., and Falkowski P. G.. 2008. The role of nutricline depth in regulating the ocean carbon cycle. Proc. Natl. Acad. Sci. U. S. A. 105: 20344–20349. doi: 10.1073/pnas.0811302106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon, T. G. , Lopez M. M., Richerson P. J., Powell T. M., and Goldman C. R.. 1987. Summer dynamics of the deep chlorophyll maximum in Lake Tahoe. J. Plankton Res. 9: 327–344. doi: 10.1093/plankt/9.2.327 [DOI] [Google Scholar]
- Cullen, J. J. 1982. The deep chlorophyll maximum: Comparing vertical profiles of chlorophyll a. Can. J. Fish. Aquat. Sci. 39: 791–803. doi: 10.1139/f82-108 [DOI] [Google Scholar]
- Cullen, J. J. 1985. Diel vertical migration by dinoflagellates: Roles of carbohydrate metabolism and behavioral flexibility. Contrib. Mar. Sci. 27(Suppl.): 135–152. Retrieved from http://www.cmep.ca/jcullen/publications/1985/Cullen_1985_Migration.pdf [Google Scholar]
- Cullen, J. J. 2015. Subsurface chlorophyll maximum layers: Enduring enigma or mystery solved? Ann. Rev. Mar. Sci. 7: 207–239. doi: 10.1146/annurev-marine-010213-135111 [DOI] [PubMed] [Google Scholar]
- Cullen, J. J. , and MacIntyre J. G.. 1998. Behavior, physiology and the niche of depth‐regulating phytoplankton, p. 559–580. In Anderson D. M., Cembella A. D., and Hallegraeff G. M. [eds.], Physiological ecology of harmful algal blooms. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Dettmers, J. M. , Goddard C. I., and Smith K. D.. 2012. Management of Alewife using Pacific Salmon in the Great Lakes: Whether to manage for economics or the ecosystem? Fisheries 37: 495–501. doi: 10.1080/03632415.2012.731875 [DOI] [Google Scholar]
- Dove, A. 2009. Long‐term trends in major ions and nutrients in Lake Ontario. Aquat. Ecosyst. Health Manag. 12: 281–295. doi: 10.1080/14634980903136388 [DOI] [Google Scholar]
- Dove, A. , and Chapra S. C.. 2015. Long‐term trends of nutrients and trophic response variables for the Great Lakes. Limnol. Oceanogr. 60: 696–721. doi: 10.1002/lno.10055 [DOI] [Google Scholar]
- Durham, W. M. , and Stocker R.. 2012. Thin phytoplankton layers: Characteristics, mechanisms, and consequences. Ann. Rev. Mar. Sci. 4: 177–207. doi: 10.1146/annurev-marine-120710-100957 [DOI] [PubMed] [Google Scholar]
- Edwards, K. F. , Thomas M. K., Klausmeier C. A., and Litchman E.. 2016. Phytoplankton growth and the interaction of light and temperature: A synthesis at the species and community level. Limnol. Oceanogr. 61: 1232–1244. doi: 10.1002/lno.10282 [DOI] [Google Scholar]
- Estrada, M. , Marrasé C., Latasa M., Berdalet E., Delgado M., and Riera T.. 1993. Variability of deep chlorophyll maximum characteristics in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 92: 289–300. doi:10.3354/meps092289 [Google Scholar]
- Fahnenstiel, G. L. , and Scavia D.. 1987. Dynamics of Lake Michigan phytoplankton: The deep chlorophyll layer. J. Great Lakes Res. 13: 285–295. doi: 10.1016/S0380-1330(87)71652-9 [DOI] [Google Scholar]
- Fahnenstiel, G. , Nalepa T., Pothoven S., Carrick H., and Scavia D.. 2010a. Lake Michigan lower food web: Long‐term observations and Dreissena impact. J. Great Lakes Res. 36: 1–4. doi: 10.1016/j.jglr.2010.05.009 [DOI] [Google Scholar]
- Fahnenstiel, G. L. , Pothoven S., Vanderploeg H. A., Klarer D., Nalepa T., and Scavia D.. 2010b. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. J. Great Lakes Res. 36: 20–29. doi: 10.1016/j.jglr.2010.03.009 [DOI] [Google Scholar]
- Fee, E. J. 1976. The vertical and seasonal distribution of chlorophyll in lakes of the Experimental Lakes Area, northwestern Ontario: Implications for primary production estimates. Limnol. Oceanogr. 21: 767–783. doi: 10.4319/lo.1976.21.6.0767 [DOI] [Google Scholar]
- Fennel, K. , and Boss E.. 2003. Subsurface maxima of phytoplankton and chlorophyll: Steady‐state solutions from a simple model. Limnol. Oceanogr. 48: 1521–1534. doi: 10.4319/lo.2003.48.4.1521 [DOI] [Google Scholar]
- Gardner, W. D. , Richardson M. J., and Smith W. O.. 2000. Seasonal patterns of water column particulate organic carbon and fluxes in the Ross Sea, Antarctica. Deep Sea Res. II 47: 3423–3449. doi: 10.1016/S0967-0645(00)00074-6 [DOI] [Google Scholar]
- Geider, R. J. 1993. Quantitative phytoplankton physiology: Implications for primary production and phytoplankton growth. ICES Mar. Sci. Symp. 197: 52–62. [Google Scholar]
- Giling, D. P. , Staehr P. A., Grossart H. P., Andersen M. R., Boehrer B., Escot C., Evrendilek F., Gómez‐Gener L., Honti M., Jones I. D., Karakaya N., Laas A., Moreno‐Ostos E., Rinke K., Scharfenberger U., Schmidt S. R., Weber M., Woolway R. I., Zwart J. A., and Obrador B.. 2017. Delving deeper: Metabolic processes in the metalimnion of stratified lakes. Limnol. Oceanogr. 62: 1288–1306. doi: 10.1002/lno.10504 [DOI] [Google Scholar]
- Giraudoux, P. 2018. pgirmess: Spatial analysis and data mining for field ecologists. R package version 1.6.9. Available from https://CRAN.R-project.org/package=pgirmess.
- Gong, X. , Jiang W., Wang L., Gao H., Boss E., Yao X., Kao S., and Shi J.. 2017. Analytical solution of the nitracline with the evolution of subsurface chlorophyll maximum in stratified water columns. Biogeosciences 14: 2371–2386. doi: 10.5194/bg-14-2371-2017 [DOI] [Google Scholar]
- Guildford, S. J. , and Hecky R. E.. 2000. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 45: 1213–1223. doi: 10.4319/lo.2000.45.6.1213 [DOI] [Google Scholar]
- Hamilton, D. P. , O'Brien K. R., Burford M. A., Brookes J. D., and McBride C. G.. 2010. Vertical distributions of chlorophyll in deep, warm monomictic lakes. Aquat. Sci. 72: 295–307. doi: 10.1007/s00027-010-0131-1 [DOI] [Google Scholar]
- Heaney, S. I. , and Eppley R. W.. 1981. Light, temperature and nitrogen as interacting factors affecting diel vertical migrations of dinoflagellates in culture. J. Plankton Res. 3: 331–344. doi: 10.1093/plankt/3.2.331 [DOI] [Google Scholar]
- Hemsley, V. S. , Smyth T. J., Martin A. P., Frajka‐Williams E., Thompson A. F., Damerell G., and Painter S. C.. 2015. Estimating oceanic primary production using vertical irradiance and chlorophyll profiles from ocean gliders in the North Atlantic. Environ. Sci. Technol. 49: 11612–11621. doi: 10.1021/acs.est.5b00608 [DOI] [PubMed] [Google Scholar]
- Herbland, A. , and Voituriez B.. 1979. Hydrological structure analysis for estimating the primary production in the tropical Atlantic Ocean. J. Mar. Res. 37: 87–101. [Google Scholar]
- Idrisi, N. , Mills E. L., Rudstam L. G., and Stewart D. J.. 2001. Impact of zebra mussels (Dreissena polymorpha) on the pelagic lower trophic levels of Oneida Lake, New York. Can. J. Fish. Aquat. Sci. 58: 1430–1441. doi: 10.1139/f01-070 [DOI] [Google Scholar]
- Jamart, B. M. , Winter D. F., Banse K., Anderson G. C., and Lam R. K.. 1977. A theoretical study of phytoplankton growth and nutrient distribution in the Pacific Ocean off the northwestern U.S. coast. Deep Sea Res. 24: 753–773. doi: 10.1016/0146-6291(77)90498-2 [DOI] [Google Scholar]
- Keogh, E. , Chu S., Hart D., and Pazzani M.. 2004. Segmenting time series: A survey and novel approach, p. 1–21. In Last M., Kandel A., and Bunke H. [eds.], Data mining in time series databases. World Scientific. doi: 10.1142/9789812565402_0001 [DOI] [Google Scholar]
- Kiefer, D. A. , Olson R. J., and Holm‐Hansen O.. 1975. Another look at the nitrite and chlorophyll maxima in the central North Pacific. Deep‐Sea Res. Oceanogr. Abstr. 23: 1199–1208. doi: 10.1016/0011-7471(76)90895-0 [DOI] [Google Scholar]
- Klausmeier, C. A. , and Litchman E.. 2001. Algal games: The vertical distribution of phytoplankton in poorly mixed water columns. Limnol. Oceanogr. 46: 1998–2007. doi: 10.4319/lo.2001.46.8.1998 [DOI] [Google Scholar]
- Koops, M. A. , Munawar M., and Rudstam L. G.. 2015. The Lake Ontario ecosystem: An overview of current status and future directions. Aquat. Ecosyst. Health Manag. 18: 101–104. doi: 10.1080/14634988.2015.1004028 [DOI] [Google Scholar]
- Kornyeyev, D. , Holaday A. S., and Logan B. A.. 2004. Minimization of the photon energy absorbed by ‘closed’ reaction centers of photosystem 2 as a photoprotective strategy in higher plants. Photosynthetica 42: 377–386. doi: 10.1023/B:PHOT.0000046156.00556.b4 [DOI] [Google Scholar]
- Lande, R. , Li W. K. W., Horne E. P., and Wood A. M.. 1989. Phytoplankton growth rates estimated from depth profiles of cell concentration and turbulent diffusion. Deep‐Sea Res. 36: 1141–1159. doi: 10.1016/0198-0149(89)90097-6 [DOI] [Google Scholar]
- Latasa, M. , Cabello A. M., Morán X. A. G., Massana R., and Scharek R.. 2017. Distribution of phytoplankton groups within the deep chlorophyll maximum. Limnol. Oceanogr. 62: 665–685. doi: 10.1002/lno.10452 [DOI] [Google Scholar]
- Leach, T. H. , Beisner B. E., Carey C. C., Pernica P., Rose K. C., Huot Y., Brentrup J. A., Domaizon I., Grossart H. P., Ibelings B. W., Jacquet S., Kelly P. T., Rusak J. A., Stockwell J. D., Straile D., and Verburg P.. 2018. Patterns and drivers of deep chlorophyll maxima structure in 100 lakes: The relative importance of light and thermal stratification. Limnol. Oceanogr. 63: 628–646. doi: 10.1002/lno.10656 [DOI] [Google Scholar]
- Letelier, R. M. , Karl D. M., Abbott M. R., and Bidigare R. R.. 2004. Light driven seasonal patterns of chlorophyll and nitrate in the lower euphotic zone of the North Pacific Subtropical Gyre. Limnol. Oceanogr. 29: 508–519. doi: 10.4319/lo.2004.49.2.0508 [DOI] [Google Scholar]
- Lewis, D. M. , Brereton A., and Siddons J. T.. 2017. A large eddy simulation study of the formation of deep chlorophyll/biological maxima in un‐stratified mixed layers: The roles of turbulent mixing and predation pressure. Limnol. Oceanogr. 62: 2277–2307. doi: 10.1002/lno.10566 [DOI] [Google Scholar]
- Madenjian, C. P. , Fahnenstiel G. L., Johengen T. H., Nalepa T. F., Vanderploeg H. A., Fleischer G. W., Schneeberger P. J., Benjamin D. M., Smith E. B., Bence J. R., Rutherford E. S., Lavis D. S., Robertson D. M., Jude D. J., and Ebener M. P.. 2002. Dynamics of the Lake Michigan food web, 1970‐2000. Can. J. Fish. Aquat. Sci. 59: 736–753. doi: 10.1139/f02-044 [DOI] [Google Scholar]
- Matthews, R. , and Deluna E.. 2008. Metalimnetic oxygen and ammonium maxima in Lake Whatcom, Washington (USA). Northwest Sci. 82: 18–29. doi: 10.3955/0029-344X-82.1.18 [DOI] [Google Scholar]
- Mignot, A. , Claustre H., D'Ortenzio F., Xing X., Poteau A., and Ras J.. 2011. From the shape of the vertical profile of in vivo fluorescence to chlorophyll‐a concentration. Biogeosciences 8: 2391–2406. doi: 10.5194/bg-8-2391-2011 [DOI] [Google Scholar]
- Mignot, A. , Claustre H., Uitz J., Poteau A., D'Ortenzio F., and Xing X.. 2014. Understanding the seasonal dynamics of phytoplankton biomass and the deep chlorophyll maximum in oligotrophic environments: A Bio‐Argo float investigation. Global Biogeochem. Cycles 28: 856–876. doi: 10.1002/2013GB004781 [DOI] [Google Scholar]
- Mills, E. , Casselman J., Dermott R., Fitzsimons J., Gal G., Holeck K., Hoyle J., Johannsson O., Lantry B., Makarewicz J., Millard E., Munawar I., Munawar M., Owens R., Rudstam L. G., Schaner T., and Stewart T.. 2003. Lake Ontario: Food web dynamics in a changing ecosystem (1970–2000). Can. J. Fish. Aquat. Sci. 60: 471–490. doi: 10.1139/F03-033 [DOI] [Google Scholar]
- Moll, R. A. , and Stoermer E. F.. 1982. A hypothesis relating trophic status and subsurface chlorophyll maxima of lakes. Arch. Hydrobiol. 94: 425–440. [Google Scholar]
- Moll, R. A. , Brahce M. Z., and Peterson T. P.. 1984. Phytoplankton dynamics within the subsurface chlorophyll maximum of Lake Michigan. J. Plankton Res. 6: 751–766. doi: 10.1093/plankt/6.5.751 [DOI] [Google Scholar]
- NOAA Laboratory, G.L.E.R.L . 2016. About our Great Lakes: Lake by lake profiles [WWW Document]. [accessed 2018 July 27]. Available from https://www.glerl.noaa.gov/education/ourlakes/lakes.html.
- Oliver, R. L. 1994. Floating and sinking in gas‐vacuolate cyanobacteria. J. Phycol. 30: 161–173. doi: 10.1111/j.0022-3646.1994.00161.x [DOI] [Google Scholar]
- Oliver, S. K. , Branstrator D. K., Hrabik T. R., Guildford S. J., and Hecky R. E.. 2014. Nutrient excretion by crustacean zooplankton in the deep chlorophyll layer of Lake Superior. Can. J. Fish. Aquat. Sci. 72: 390–399. doi: 10.1139/cjfas-2014-0209 [DOI] [Google Scholar]
- Onitsuka, G. , Yoshikawa Y., Shikata T., Yufu K., Abe K., Tokunaga T., Kimoto K., and Matsuno T.. 2018. Development of a thin diatom layer observed in a stratified embayment in Japan. J. Oceanogr. 74: 351–365. doi: 10.1007/s10872-018-0466-0 [DOI] [Google Scholar]
- Parker, B. C. , Wenkert L. J., and Parson M. J.. 1991. Cause of the metalimnetic oxygen maximum in Mountain Lake, Virginia. J. Freshw. Ecol. 63: 293–303. doi: 10.1080/02705060.1991.9665306 [DOI] [Google Scholar]
- Pérez, G. , Queimalinos C., Balseiro E., and Modenutti B.. 2007. Phytoplankton absorption spectra along the water column in deep North Patagonian Andean lakes (Argentina). Limnologica. 37: 3–16. doi: 10.1016/j.limno.2006.08.005 [DOI] [Google Scholar]
- Pilati, A. , and Wurtsbaugh W. A.. 2003. Importance of zooplankton for the persistence of a deep chlorophyll layer: A limnocorral experiment. Limnol. Oceanogr. 48: 249–260. doi: 10.4319/lo.2003.48.1.0249 [DOI] [Google Scholar]
- Pothoven, S. A. , and Fahnenstiel G. L.. 2013. Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. J. Great Lakes Res. 39: 287–294. doi: 10.1016/j.jglr.2013.02.005 [DOI] [Google Scholar]
- Prairie, J. C. , Franks P. J. S., Jaffe J. S., Doubell M. J., and Yamazaki H.. 2011. Physical and biological controls of vertical gradients in phytoplankton. Limnol. Oceanogr.: Fluids Environ. 1: 75–90. doi: 10.1215/21573698-1267403 [DOI] [Google Scholar]
- R Core Team . 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, https://www.R-project.org/ [Google Scholar]
- Ralston, D. K. , McGillicuddy D. J. Jr., and Townsend D. W.. 2007. Asynchronous vertical migration and bimodal distribution of motile phytoplankton. J. Plankton Res. 29: 803–821. doi: 10.1093/plankt/fbm061 [DOI] [Google Scholar]
- Reavie, E. D. , Barbiero R. P., Allinger L. E., and Warren G. J.. 2014. Phytoplankton trends in the Great Lakes, 2001‐2011. J. Great Lakes Res. 40: 618–639. doi: 10.1016/j.jglr.2014.04.013 [DOI] [Google Scholar]
- Rucinski, D. K. , DePinto J. V., Scavia D., and Beletsky D.. 2016. Modeling Lake Erie's hypoxia response to nutrient loads and physical variability. J. Great Lakes Res. 40: 1206–1211. doi: 10.1016/j.jglr.2014.02.003 [DOI] [Google Scholar]
- Rudstam, L. G. , Holeck K. T., Watkins J. M., Hotaling C., Lantry J. R., Bowen K. L., Munawar M., Weidel B. C., Barbiero R., Luckey F. J., Dove A., Johnson T. B., and Biesinger Z.. 2017. Nutrients, phytoplankton, zooplankton, and macrobenthos. In O'Gorman R. [ed.], The State of Lake Ontario in 2014, pp. 10–32. Great Lakes Fishery Commission Special Publication. Available from: http://www.glfc.org/pubs/SpecialPubs/Sp17_02.pdf [Google Scholar]
- Ryan, J. P. , McManus M. A., and Sullivan J. M.. 2010. Interacting physical, chemical and biological forcing of phytoplankton thin‐layer variability in Monterey Bay, California. Cont. Shelf Res. 30: 7–16. doi: 10.1016/j.csr.2009.10.017 [DOI] [Google Scholar]
- Sawatzky, C. L. , Wurtsbaugh W. A., and Luecke C.. 2006. The spatial and temporal dynamics of deep chlorophyll layers in high‐mountain lakes: Effects of nutrients, grazing and herbivore nutrient recycling as growth determinants. J. Plankton Res. 28: 65–86. doi: 10.1093/plankt/fbi101 [DOI] [Google Scholar]
- Scavia, D. , David Allan J., Arend K. K., Bartell S., Beletsky D., Bosch N. S., Brandt S. B., Briland R. D., Daloǧlu I., DePinto J. V., Dolan D. M., Evans M. A., Farmer T. M., Goto D., Han H., Höök T. O., Knight R., Ludsin S. A., Mason D., Michalak A. M., Peter Richards R., Roberts J. J., Rucinski D. K., Rutherford E., Schwab D. J., Sesterhenn T. M., Zhang H., and Zhou Y.. 2014. Assessing and addressing the re‐eutrophication of Lake Erie: Central basin hypoxia. J. Great Lakes Res. 40: 226–246. doi: 10.1016/j.jglr.2014.02.004 [DOI] [Google Scholar]
- Scofield, A. E. , Watkins J. M., Weidel B. C., Luckey F. J., and Rudstam L. G.. 2017. The deep chlorophyll layer in Lake Ontario: Extent, mechanisms of formation, and abiotic predictors. J. Great Lakes Res. 43: 782–794. doi: 10.1016/j.jglr.2017.04.003 [DOI] [Google Scholar]
- Sommer, U. 1982. Vertical niche separation between two closely related planktonic flagellate species (Rhodomonas lens and Rhodomonas minuta v. nannoplanctica). J. Plankton Res. 4: 137–142. doi: 10.1093/plankt/4.1.137 [DOI] [Google Scholar]
- Stadelmann, P. , Moore J. E., and Pickett E.. 1974. Primary production in relation to temperature structure, biomass concentration, and light conditions at an inshore and offshore station in Lake Ontario. J. Fish. Res. Board Canada 31: 1215–1232. doi: 10.1139/f74-145 [DOI] [Google Scholar]
- Stańczykowska, A. , and Lewandowski K.. 1993. Effect of filtering activity of Dreissena polymorpha (Pall.) on the nutrient budget of the littoral of Lake Mikołajskie Hydrobiol. 251: 73–79. doi: 10.1007/BF00007167 [DOI] [Google Scholar]
- Steele, J. 1964. A study of production in the Gulf of Mexico. J. Mar. Res. 3: 211–222. [Google Scholar]
- Steele, J. H. , and Yentsch C. S.. 1960. The vertical distribution of chlorophyll. J. Mar. Biol. Ass. U.K. 39: 217–226. doi: 10.1017/S0025315400013266 [DOI] [Google Scholar]
- Steinbuck, J. V. , Genin A., Monismith S. G., Koseff J. R., Holzman R., and Labiosa R. G.. 2010. Turbulent mixing in fine‐scale phytoplankton layers: Observations and inferences of layer dynamics. Cont. Shelf Res. 30: 442–455. doi: 10.1016/j.csr.2009.12.014 [DOI] [Google Scholar]
- Sterner, R. W. 2010. In situ‐measured primary production in Lake Superior. J. Great Lakes Res. 36: 139–149. doi: 10.1016/j.jglr.2009.12.007 [DOI] [Google Scholar]
- Suggett D. J., Prášil O., and Borowitzka M. A. [eds.]. 2010. Chlorophyll a fluorescence in aquatic sciences: Methods and applications. Dordrecht, The Netherlands: Springer. doi: 10.1007/978-90-481-9268-7 [DOI] [Google Scholar]
- Taylor, A. H. , Geider R. J., and Gilbert F. J. H.. 1997. Seasonal and latitudinal dependencies of phytoplankton carbon‐to‐chlorophyll a ratios: Results of a modelling study. Mar. Ecol. Prog. Ser. 152: 51–66. doi: 10.3354/meps152051 [DOI] [Google Scholar]
- Thomalla, S. J. , Moutier W., Ryan‐Keogh T. J., Gregor L., and Schutt J.. 2018. An optimized method for correcting fluorescence quenching using optical backscattering on autonomous platforms. Limnol. Oceanogr.: Methods 16: 132–144. doi: 10.1002/lom3.10234 [DOI] [Google Scholar]
- Twiss, M. R. , Ulrich C., Zastepa A., and Pick F. R.. 2012. On phytoplankton growth and loss rates to microzooplankton in the epilimnion and metalimnion of Lake Ontario in mid‐summer. J. Great Lakes Res. 38: 146–153. doi: 10.1016/j.jglr.2012.05.002 [DOI] [Google Scholar]
- Tyler, J. E. 1968. The secchi disk. Limnol. Oceanogr. 13: 1–6. [Google Scholar]
- Vallentyne, J. 1957. Principles of modern limnology. Am. Sci. 45: 218–244 Retrieved from www.jstor.org/stable/27826908 [Google Scholar]
- Watkins, J. M. , Weidel B. C., Rudstam L. G., and Holeck K. T.. 2015. Spatial extent and dissipation of the deep chlorophyll layer in Lake Ontario during the Lake Ontario lower foodweb assessment, 2003 and 2008. Aquat. Ecosyst. Health Manag. 18: 18–27. doi: 10.1080/14634988.2014.937316 [DOI] [Google Scholar]
- Welschmeyer, N. A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39: 1985–1992. doi: 10.4319/lo.1994.39.8.1985 [DOI] [Google Scholar]
- Weston, K. , Fernand L., Mills D. K., Delahunty R., and Brown J.. 2005. Primary production in the deep chlorophyll maximum of the Central North Sea. J. Plankton Res. 27: 909–922. doi: 10.1093/plankt/fbi064 [DOI] [Google Scholar]
- Wetzel, R. G. 2001. Limnology: Lake and river ecosystems. Academic Press. ISBN: 0080574394, 9780080574394 [Google Scholar]
- White, A. E. , Spitz Y. H., and Letelier R. M.. 2006. Modeling carbohydrate ballasting by Trichodesmium spp. Mar. Ecol. Prog. Ser. 323: 35–45. doi: 10.3354/meps323035 [DOI] [Google Scholar]
- White, B. , and Matsumoto K.. 2012. Causal mechanisms of the deep chlorophyll maximum in Lake Superior: A numerical modeling investigation. J. Great Lakes Res. 38: 504–513. doi: 10.1016/j.jglr.2012.05.001 [DOI] [Google Scholar]
- Wickham, H. 2016. ggplot2: Elegant graphics for data analysis. New York, NY: Springer‐Verlag; https://ggplot2.tidyverse.org [Google Scholar]
- Wilkinson, G. M. , Cole J. J., Pace M. L., Johnson R. A., and Kleinhans M. J.. 2015. Physical and biological contributions to metalimnetic oxygen maxima in lakes. Limnol. Oceanogr. 60: 242–251. doi: 10.1002/lno.10022 [DOI] [Google Scholar]
- Williamson, C. E. , Sanders R. W., Moeller R. E., and Stutzman P. L.. 1996. Utilization of subsurface food resources for zooplankton reproduction: Implications for diel vertical migration theory. Limnol. Oceanogr. 41: 224–233. doi: 10.4319/lo.1996.41.2.0224 [DOI] [Google Scholar]
- Xing, X. , Briggs N., Boss E., and Claustre H.. 2018. Improved correction for non‐photochemical quenching of in situ chlorophyll fluorescence based on a synchronous irradiance profile. Opt. Express 26: 24734–24751. doi: 10.1364/OE.26.024734 [DOI] [PubMed] [Google Scholar]
- Xu, W. , Collingsworth P. D., and Minsker B.. 2019. Algorithmic characterization of lake stratification and deep chlorophyll layers from depth profiling water quality data. Water Resour. Res. 55: 3815–3834. doi: 10.1029/2018WR023975 [DOI] [Google Scholar]
- Yamamoto, Y. 2016. Quality control of photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 7: 1136. doi: 10.3389/fpls.2016.01136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yentsch, C. S. 1974. The influence of geostrophy on primary production. Tethys 6: 111–118. [Google Scholar]
- Yentsch, C. S. 1980. Phytoplankton growth in the sea, p. 17–32. In Falkowski P. G. [ed.], Primary productivity in the sea. New York, NY: Plenum Press. doi: 10.1007/978-1-4684-3890-1_2 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Relationship of the light extinction coefficient (K) to the secchi disk depth (meters) for all sites with paired secchi disk measurements and profiles of photosynthetically active radiation (PAR). K values were calculated from the slope of the linear relationship between the natural log transformed PAR vs. depth.
Figure S2. Scatter plots of in situ chlorophyll fluorescence (F chl) measurements vs. extracted values (Chl) for discrete depth water samples taken throughout the water column (a) before quenching corrections were applied, and (b) after quenching corrections were applied. Black dots indicate points where the PAR measurement was < 25 μE (m2s)‑1, and red dots indicate points with > 25 μE (m2s)‑1 PAR measurements, which are biased low prior to the correction. Only data from 2002 onward (through 2017) were used for the calibration because of quality control issues for extracted chlorophyll in earlier years. The model was optimized separately for 2011, when in situ fluorometer values were biased upward relative to other years (likely an issue with the manufacturer‐supplied calibration coefficient used). All other years showed a similar relationship and thus were pooled to fit the quenching equation parameters.
Table S1. Parameter fits for the quenching correction model for data subsets: 2002–2017, and 2011 (fit separately as the calibration constant was entered incorrectly that year). The parameters fitted for data from 2002 to 2017 were also applied to data from 1996 to 2001, for which there are concerns about the quality of the extracted chlorophyll data. Corrected profiles were then run through the peak detection algorithm.


