Abstract
Objective
The objective was to assess the 2‐year clinical performance of three drug‐eluting stents in all‐comer patients with severely calcified coronary lesions.
Background
Severe lesion calcification increases cardiovascular event risk after coronary stenting, but there is a lack of data on the clinical outcome of all‐comers with severely calcified lesions who were treated with more recently introduced drug‐eluting stents.
Methods
The BIO‐RESORT trial (clinicaltrials.gov: NCT01674803) randomly assigned 3,514 all‐comer patients to biodegradable polymer Synergy everolimus‐eluting stents (EES) or Orsiro sirolimus‐eluting stents (SES), versus durable polymer Resolute Integrity zotarolimus‐eluting stents (ZES). In a post hoc analysis, we assessed 783 patients (22.3%) with at least one severely calcified target lesion.
Results
At 2‐year follow‐up (available in 99% of patients), the main composite endpoint target vessel failure occurred in 19/252 (7.6%) of the EES and in 33/265 (12.6%) of the ZES‐treated patients (p = .07). Target vessel failure occurred in 24/266 (9.1%) of the SES‐treated patients (vs. ZES: p = .21). There was a difference in target vessel revascularization, which was required in EES in 6/252 (2.4%) patients and in ZES in 20/265 (7.7%) patients (p = .01); the target vessel revascularization rate in SES was 9/266 (3.4%, vs. ZES: p = .04). Multivariate analysis showed that implantation of EES, but not SES, was independently associated with lower target vessel revascularization rates than in ZES.
Conclusions
In BIO‐RESORT participants with severely calcified target lesions, treatment with EES was associated with a lower 2‐year target vessel revascularization rate than treatment with ZES.
Keywords: calcified stenosis, clinical trial, coronary stents, percutaneous coronary intervention
1. INTRODUCTION
Severe coronary artery calcification is associated with an increased risk of adverse cardiovascular events following percutaneous coronary intervention (PCI). 1 Risk factors contributing to the development of coronary calcification include advanced age, diabetes, male sex and renal dysfunction. Coronary artery calcification, which can be found in 20–30% of all patients who undergo PCI, 2 , 3 , 4 , 5 , 6 may result in a reduced vascular compliance and impair myocardial perfusion. 1
In severely calcified coronary lesions the radiopacity of calcium hampers the x‐ray visibility of PCI devices, 1 which may result in technical challenges and problems that include impaired stent deliverability, under‐expansion or incomplete apposition of stents, and sometimes geographical miss of the target lesion. In addition, when being advanced through calcified coronary vessels, the polymer‐coating of drug‐eluting stents (DES) can be damaged, which may reduce the efficacy of preventing lesion recurrence. 7 , 8 All these factors contribute to the increased risk of cardiovascular events (e.g., lesion recurrence and repeated revascularization) that have been reported for calcified target lesions. 6 , 9 , 10 Although coronary calcification increases the rate of target lesion recurrence in DES, 11 early generation DES improved clinical outcome as compared to bare‐metal stents. 12 , 13 Newer DES include devices with biodegradable polymers and thinner struts 14 that have shown excellent results in randomized clinical trials that assessed broad patient populations. 15 , 16 , 17 , 18 , 19 Calcified lesions may represent a true challenge for the radial force of stents with particularly thin struts, but data on the outcome of PCI with these devices in severely calcified lesions are scarce.
BIO‐RESORT is a large‐scale, randomized clinical trial in all‐comer patients that compares two contemporary very‐thin strut biodegradable polymer DES versus a thin‐strut durable polymer DES. Clinical outcome after PCI with all three stents was shown to be favorable. 20 , 21 In the current post hoc analysis, we assessed the clinical outcome of PCI with the three DES in the challenging population of all‐comer patients who were all treated for severely calcified lesions.
2. METHODS
2.1. Study design and participants
The present study was performed in participants of the BIO‐RESORT trial. Details of the randomized BIO‐RESORT trial and 2‐year follow‐up data have been published. 20 , 21 In brief, the 3‐arm, multicenter, investigator‐initiated BIO‐RESORT trial (NCT01674803) randomized and assessed 3,514 all‐comer patients undergoing PCI with DES implantation in 4 Dutch centers for coronary intervention, between December 2012 and August 2015. Randomization was done in a 1:1:1 fashion with biodegradable polymer everolimus‐eluting stents (EES; Synergy, Boston Scientific, Marlborough, MA) or sirolimus‐eluting stents (SES; Orsiro, Biotronik, Bülach, Switzerland) versus durable polymer zotarolimus‐eluting stents (ZES; Resolute Integrity, Medtronic, Santa Rosa, CA). Web‐based randomization was performed with the use of a custom‐designed computer program in random block sizes of 6 and 3, stratified according to the presence of diabetes mellitus. All coronary syndromes, de novo and restenotic lesions, and lesions in native vessels or bypass grafts were permitted. There was no limit for lesion length, reference vessel size, and number of lesions or vessels to be treated.
The trial complied with the Declaration of Helsinki and the CONSORT 2010 Statement and was approved by the Medical Ethics Committee Twente and the institutional review boards of all participating centers. All patients provided written informed consent. For the current analysis, patients treated in at least one severely calcified target lesion (based on qualitative coronary angiographic analysis) were included.
2.2. Definition of target lesion calcification
Experienced angiographic analysts of Thoraxcentrum Twente performed qualitative and quantitative coronary angiographic analyses of all cases according to current standards, using the software QAngio XA (Version 7.3, Medis, Leiden, The Netherlands). Target lesion calcification was prospectively classified, in analogy with previous studies. 5 , 6 Severe target lesion calcification was defined as readily apparent radiopaque densities noted prior to contrast injection without cardiac motion and generally involved both sides of the arterial wall. 5 , 6
2.3. Procedures and follow‐up
Coronary interventions were performed according to standard techniques, current medical guidelines and the operator's judgment. Clinical follow‐up data was obtained at visits to outpatient clinics, or if not feasible, by telephone follow‐up or a medical questionnaire. Research staff was blinded to the assigned treatment.
The stent platform of the Synergy EES is made from platinum–chromium struts with a varying strut thickness (74 μm for stent diameters ≤2.5 mm, 79 μm for 3.0–3.5 mm stents, and 81 μm for 4.0 mm stents). The struts have an abluminal biodegradable 4 μm poly(lactic‐co‐glycolic acid) coating that is resorbed within 4 months and elutes everolimus within 3 months. The Orsiro SES has a circumferential biodegradable coating that is resorbed within 24 months and is thicker on the abluminal side (7.4 μm) than on the luminal side (3.5 μm) that elutes sirolimus within 4 months. The stent platform is made from cobalt–chromium struts of 60 μm (for stents ≤3.0 mm) or 80 μm (for >3.0 mm stents) and is covered with a thin passive coating of amorphous silicon carbide. The durable polymer coating of the Resolute Integrity ZES is a 6 μm thick blend of three polymers and the stent platform is made from 91 μm round cobalt–chromium struts. 20
2.4. Clinical endpoints, monitoring and event adjudication
Clinical endpoints were prespecified according to definitions of the Academic Research Consortium. 22 , 23 The composite endpoint target vessel failure comprised cardiac death, target vessel‐related myocardial infarction or clinically indicated target vessel revascularization (TVR). Secondary endpoints included target lesion failure (a composite of cardiac death, target vessel myocardial infarction or clinically indicated target lesion revascularization), and stent thrombosis. Data monitoring and independent clinical event adjudication were performed by an external research organization (Diagram, Zwolle, The Netherlands). The clinical event committee was blinded for the assigned stent type at all times.
2.5. Statistical analysis
Continuous variables were compared between groups with the Student's t test or Wilcoxon Rank Sum test, as appropriate, while categorical variables were assessed with chi‐square test. The Kaplan–Meier method was used to calculate the time to clinical endpoint and the log‐rank test was applied for between‐group comparisons. Hazard ratios were computed with Cox proportional hazards regressions analysis. Potential confounders were identified if in univariate analysis a p value <.15 was found. The first multivariate Cox regression model included all potential confounders (i.e., arterial hypertension, previous myocardial infarction, previous coronary bypass surgery, and total stent length per patient), and with stepwise backwards selection only true confounding factors (i.e., arterial hypertension) were kept in the model. We performed an additional sensitivity analysis in patients who required single vessel treatment. All confidence intervals (CI) and p values are two‐sided, p values <.05 were considered significant. Statistical analyses were performed with SPSS, Version 24 (IBM Corp., Armonk, NY).
3. RESULTS
3.1. Patient characteristics
Of all 3,514 BIO‐RESORT trial participants, 783 (22.3%) all‐comer patients were treated in severely calcified lesions (Figure 1). These patients were 67.0 ± 10.0 years old, and there was no difference between stent groups in clinical characteristics except for history of myocardial infarction, which was less prevalent in EES versus ZES (p = .02), and previous coronary bypass surgery, which was less prevalent in SES versus ZES (p = .04). Baseline patient characteristics and procedural details are presented in Table 1.
FIGURE 1.
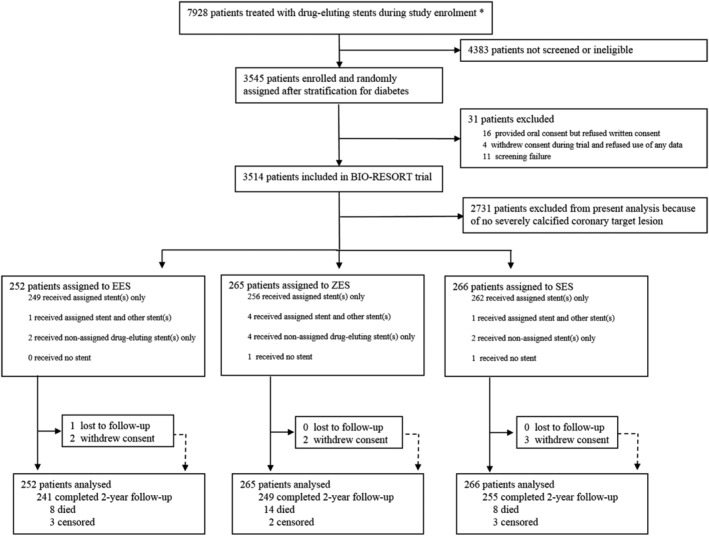
Study flow diagram. *Total number of patients treated with drug‐eluting stents during enrolment period, irrespective of study eligibility. EES, everolimus‐eluting stents; SES, sirolimus‐eluting stents; ZES, zotarolimus‐eluting stents
TABLE 1.
Baseline characteristics for patients of the three stent groups
| EES n = 252 | ZES n = 265 | SES n = 266 | |
|---|---|---|---|
| Demographics and medical history | |||
| Age, years | 67.3 ± 10.0 | 67.0 ± 9.8 | 66.8 ± 10.2 |
| Female sex | 71 (28.2) | 81 (30.6) | 71 (26.7) |
| Body mass index, kg/m2 | 27.5 ± 4.1 | 27.1 ± 3.8 | 27.4 ± 4.2 |
| Current smoker | 60/243 (24.7) | 65/261 (24.9) | 74/259 (28.6) |
| Diabetes, medically treated | 53 (21.0) | 55 (20.8) | 54 (20.3) |
| Hypertension | 124 (49.2) | 150 (56.6) | 131 (49.2) |
| Hypercholesterolemia | 111 (44.0) | 119 (44.9) | 106 (39.8) |
| Previous myocardial infarction | 37 (14.7) a | 60 (22.6) | 50 (18.8) |
| Previous percutaneous coronary intervention | 52 (20.6) | 49 (18.5) | 53 (19.9) |
| Previous coronary artery bypass grafting | 25 (9.9) | 34 (12.8) | 20 (7.5) b |
| Left ventricular ejection fraction <30% | 7 (2.8) | 5 (1.9) | 7 (2.6) |
| Renal insufficiency (severe) c | 10 (4.0) | 13 (4.9) | 18 (6.8) |
| Clinical presentation | |||
| ST‐elevation myocardial infarction | 69 (27.4) | 60 (22.6) | 53 (19.9) |
| Non‐ST‐elevation myocardial infarction | 42 (16.7) | 53 (20.0) | 43 (16.2) |
| Unstable angina | 45 (17.9) | 48 (18.1) | 61 (22.9) |
| Stable angina | 96 (38.1) | 104 (39.2) | 109 (41.0) |
| Details of target lesions and procedures | |||
| Multivessel treatment | 59 (23.4) | 71 (26.8) | 62 (23.3) |
| Severely calcified target vessel d | |||
| Right coronary artery | 125 (49.6) | 120 (45.3) | 118 (44.4) |
| Left anterior descending artery | 125 (49.6) | 138 (52.1) | 152 (57.1) |
| Left circumflex artery | 42 (16.7) | 61 (23.0) | 50 (18.8) |
| Chronic total occlusion | 14 (5.6) | 15 (5.7) | 15 (5.6) |
| Rotablator | 11 (4.4) | 17 (6.4) | 17 (6.4) |
| Cutting balloon | 14 (5.6) | 10 (3.8) | 19 (7.1) |
| Maximum implantation pressure, atm | 16.0 ± 2.8 | 15.9 ± 2.8 | 15.7 ± 2.8 |
| Postdilation | 223 (88.5) | 226 (85.3) | 229 (86.1) |
| Maximum postdilation pressure, atm | 22.3 ± 4.7 | 22.2 ± 5.0 | 22.6 ± 4.6 |
| Total stent length per patient, mm | 38 (24–62) | 44 (28–67) | 40 (24–62) |
Note: Values are mean ± SD, n (%) or median (interquartile range).
Abbreviations: EES, everolimus‐eluting stents; SES, sirolimus‐eluting stents; ZES, zotarolimus‐eluting stents.
Previous myocardial infarction was less prevalent in EES versus ZES, p = .02.
Previous coronary artery bypass grafting was less prevalent in SES versus ZES, p = .04.
Defined as an estimated glomerular filtration rate of <30 ml/min/1.73 m2 or the need for dialysis.
Only severely calcified target vessels are presented. Patients were allowed to be treated in multiple severely calcified vessels, therefore, the percentages add up to more than 100%.
3.2. Two‐year clinical outcomes
Two‐year follow‐up was available in 775/783 patients (99.0%; one lost to follow‐up, seven withdrew consent; all censored at dropout). The main composite endpoint target vessel failure occurred in 19/252 (7.6%) patients treated with EES, 33/265 (12.6%) patients treated with ZES (EES vs. ZES: HR 0.59, 95% CI 0.34–1.04, p‐logrank = .07) and 24/266 (9.1%) patients treated with SES (SES vs. ZES: HR 0.72, 95% CI 0.42–1.21, p‐logrank = .21) (see Figure 2). There was also no statistically significant between‐stent difference in the rates of the individual safety endpoints cardiac death, target vessel myocardial infarction, and stent thrombosis.
FIGURE 2.
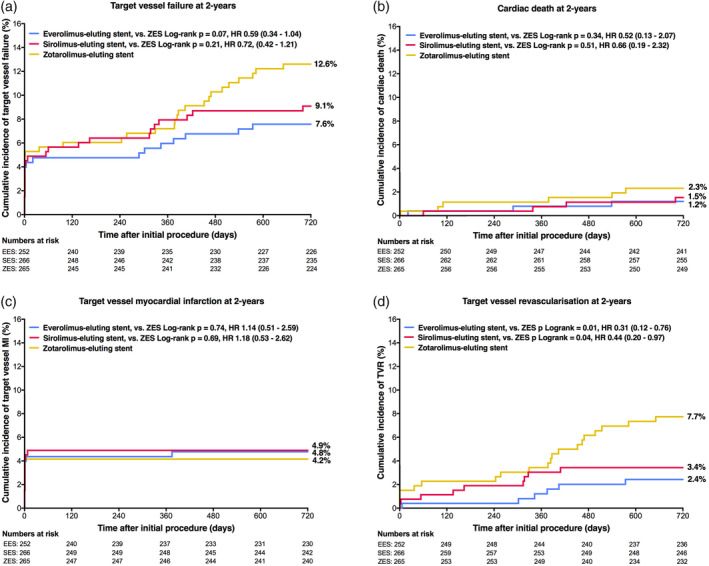
Kaplan–Meier cumulative event curves for target vessel failure and its individual components at 2‐year follow‐up. Target vessel failure (a), a composite of cardiac death (b), target vessel‐related myocardial infarction (c), or clinically indicated target vessel revascularization (d). HR, hazard ratio; MI, myocardial infarction; ZES, zotarolimus‐eluting stents
But there was a significant between‐DES difference in the efficacy endpoint TVR, which occurred in 6/252 (2.4%) patients treated with EES, 20/265 (7.7%) patients treated with ZES (EES vs. ZES: HR 0.31, 95% CI 0.12–0.76, p‐logrank = .01), and 9/266 (3.4%) patients treated with SES (SES vs. ZES: HR 0.44, 95% CI 0.20–0.97, p‐logrank = .04). A sensitivity analysis in 591 patients with single‐vessel treatment confirmed these findings: TVR rates were in EES, ZES, and SES 2.6, 8.0, and 3.5%, respectively (EES vs. ZES: HR 0.32, 95% CI 0.12–0.89, p‐logrank = .02; SES vs. ZES: HR 0.43, 95% CI 0.18–1.06, p‐logrank = .06). Clinical outcome of patients without severely calcified target lesions is presented in Table S1.
3.3. Multivariate analysis
Multivariate analysis revealed that the implantation of EES was independently associated with a lower risk of TVR (adjusted HR 0.32, 95% CI 0.13–0.80, p = .02). The lower rate of TVR after the implantation of SES lost statistical significance after adjustment for confounders, with an adjusted HR 0.46, 95% CI 0.12–1.02 p = .06. Further clinical outcomes at 2‐year are presented in Table 2.
TABLE 2.
Clinical events during 2‐year follow‐up
| All patients N = 783 | |||||||
|---|---|---|---|---|---|---|---|
| EES Synergy n = 252 | ZES Resolute Integrity n = 265 | SES Orsiro n = 266 | Hazard ratio [95% CI] EES vs. ZES | p‐logrank EES vs. ZES | Hazard ratio [95% CI] SES vs. ZES | p‐logrank SES vs. ZES | |
| Target vessel failure | 19 (7.6) | 33 (12.6) | 24 (9.1) | 0.59 [0.34–1.04] | .07 | 0.72 [0.42–1.21] | .21 |
| Cardiac death | 3 (1.2) | 6 (2.3) | 4 (1.5) | 0.52 [0.13–2.07] | .34 | 0.66 [0.19–2.32] | .51 |
| Target vessel myocardial infarction | 12 (4.8) | 11 (4.2) | 13 (4.9) | 1.14 [0.51–2.59] | .74 | 1.18 [0.53–2.62] | .69 |
| Target vessel revascularisation | 6 (2.4) | 20 (7.7) | 9 (3.4) | 0.31 [0.12–0.76] | .007 | 0.44 [0.20–0.97] | .04 |
| Target lesion failure | 19 (7.6) | 30 (11.5) | 22 (8.3) | 0.65 [0.37–1.16] | .14 | 0.72 [0.42–1.25] | .24 |
| Target lesion revascularisation | 6 (2.4) | 16 (6.2) | 7 (2.7) | 0.38 [0.15–0.98] | .04 | 0.43 [0.18–1.04] | .05 |
| Definite‐or‐probable stent thrombosis | 2 (0.8) | 3 (1.2) | 2 (0.8) | 0.70 [0.12–4.16] | .69 | 0.66 [0.11–3.96] | .65 |
| Definite stent thrombosis | 2 (0.8) | 2 (0.8) | 2 (0.8) | 1.04 [0.15–7.40] | .97 | 0.99 [0.14–7.05] | .99 |
Note: Event rates are expressed as n (%) and were calculated with the use of the Kaplan–Meier method. All target vessel revascularizations were clinically indicated.
Abbreviations: CI, confidence interval; EES, everolimus‐eluting stents; SES, sirolimus‐eluting stents; ZES, zotarolimus‐eluting stents.
4. DISCUSSION
4.1. Main findings
Among all‐comer patients with at least one severely calcified target lesion, there were no statistically significant differences in the 2‐year rate of the main composite endpoint target vessel failure between patients treated with biodegradable polymer Synergy EES or Orsiro SES versus durable polymer Resolute Integrity ZES.
Yet, the efficacy endpoint TVR was significantly less often reached after treatment with Synergy EES and Orsiro SES versus Resolute Integrity ZES. For Synergy EES, this statistically significant finding was confirmed in a sensitivity analysis in patients with single‐vessel treatment. Furthermore, multivariate analysis showed that during 2‐year follow‐up the implantation of Synergy EES was independently associated with a lower risk of TVR. After treatment with Orsiro SES the TVR rate was low, but multivariate analysis did not show a statistically significant difference versus Resolute Integrity ZES.
There was no between‐stent difference in various safety endpoints, such as cardiac death, target vessel myocardial infarction, and stent thrombosis. Overall, clinical adverse event rates were low as compared to previous studies that assessed patients treated in severely calcified coronary lesions, 2 , 5 , 10 , 12 which may be partly related to the high rate of stent postdilation and the high‐balloon pressures applied.
4.2. Previous studies
There is lack of data on the performance of new‐generation biodegradable polymer DES in severely calcified coronary lesions. Some previous studies assessed other DES in calcified coronary target lesions, but to the best of our knowledge, no other randomized study assessed these two biodegradable polymer study stents in patients with severely calcified target lesions. In patient‐level pooled analyses from previous TWENTE trials, 5 , 6 patients with severely calcified target lesions were also treated with Resolute Integrity ZES. Two‐year target vessel failure and TVR rates in patients with stable angina (16.4 and 7.6%, respectively) and in patients with acute coronary syndromes (12.4 and 6.8%) were quite similar to the corresponding event rates in the BIO‐RESORT all‐comer patients treated with Resolute Integrity ZES in the current study (12.6 and 7.7%).
The prospective, multicenter ADAPT‐DES registry assessed 8,582 all‐comer patients who underwent successful PCI with DES implantation. 2 Moderate‐to‐severe coronary artery calcification was observed in 2,644 (30.8%) patients, and these patients had a 2‐year target vessel failure rate of 14.2%, which is somewhat higher than in our present study. Yet, the ADAPT‐DES registry assessed patients who were treated with a variety of early‐ and new‐generation durable polymer DES, and not with the two new‐generation biodegradable polymer DES that were examined in BIO‐RESORT. As DES differ in various characteristics (i.e., design and strut thickness of metallic stent; material, distribution, and degradation (if any) of polymer coating; and type and pharmacodynamics of drug), we can only hypothesize that the rather biocompatible coatings and the ‐on average‐ thinner stent struts may have accounted for the lower target vessel failure rates in our present study.
A large multiethnic registry retrospectively assessed patients, who had undergone stent implantation between 2009 and 2013, according to coronary calcification. 24 A total of 994 (8.0%) of these patients were treated with second‐generation durable polymer DES for severely calcified lesions, and the 1‐year incidence of the composite clinical endpoint of death, myocardial infarction, or TVR (17.8%) was much higher than the 2‐year incidence of target vessel failure in our current study. A possible explanation could be differences in patient population, with a higher prevalence of comorbidities in the registry.
In a large patient‐level pooled dataset of 26 randomized clinical trials, the coronary artery calcification status of 11,557 women was assessed. 4 The coronary calcification status was known in 6,371 participants, of whom 1,622 (25.5%) had moderate‐to‐severely calcified coronary arteries. After 3‐year of follow‐up, moderate‐to‐severely calcified coronaries led to higher rates of adverse clinical events, irrespective of the generation of DES used. 4 While this registry included patients treated with early thick‐strut biodegradable polymer DES, it did not assess any of the contemporary very‐thin‐strut biodegradable polymer DES.
Three‐year clinical outcomes according to coronary calcification status were assessed among 6,296 patients in a patient‐level pooled analysis of seven stent trials. 3 Patients with severe lesion calcification had a higher 3‐year mortality as compared to patients without severe coronary calcification. In that study, multivariate analysis showed that severe calcification was independently associated with mortality, myocardial infarction, and repeat revascularization. The study, which did not include patients treated with one of the biodegradable polymer DES assessed in BIO‐RESORT, underlines that severe coronary calcification is not only a marker of advanced atherosclerosis but also a predictor of worse prognosis. 3
4.3. Stent design and clinical outcome
The material and design of the metallic stent may have an impact on the radial force of a DES. Platinum–chromium alloy has a higher radiographic visibility and a somewhat higher radial force, which allowed to reduce strut thickness in the very thin‐strut Synergy EES as compared to the previous cobalt–chromium‐based device. Both, Orsiro SES and Resolute Integrity ZES use cobalt–chromium stent backbones. The newer devices (i.e., the EES and SES), assessed in BIO‐RESORT, have thinner uncoated metallic stent struts with a flexibility that is higher than in their predecessors, but it was unknown whether the radial force of these very‐thin strut devices is sufficient in the complex, severely calcified target lesions of an all‐comer patient population.
In the present study, we perceived no signal for a potential problem with radial force following the use of very thin‐strut Synergy EES and Orsiro SES, as clinical adverse event rates of both of these DES were lower than with Resolute Integrity ZES. These findings should be interpreted in light of the applied high maximum balloon pressures and the very high postdilation rates (>85%), which both may have been advantageous. In addition, a bench study with scanning electron microscopy has shown no more than mild effects of aggressive postdilation on the polymer coatings of DES. 25 Furthermore, to achieve the best possible stent apposition despite severe lesion calcification, a high rate of stent postdilation and high balloon pressures may be expected in this particular patient population. In fact, the rates and pressures of stent postdilation were similar to previous substudies of other TWENTE trials that assessed patients with severely calcified target lesions. 5 , 6
The “ideal” DES for patients with severely calcified target lesions has not yet been determined, and in such patients adverse clinical event rates are still increased. Based on the results of our current study, the combination of very‐thin struts with sufficient radial force and good radiographic visibility appears to be highly suitable to achieve and maintain good clinical results in patients with severely calcified target lesions.
4.4. Limitations
The findings of this post hoc analysis are hypothesis generating and should, therefore, be interpreted with caution. Nevertheless, with a reasonable sample size of 783 patients and high 2‐year follow‐up rate (99%), the present subgroup analysis of a randomized all‐comer trial provides quite unique data about the treatment of severely calcified target lesions using three different DES. However, we cannot rule out potential effects of unmeasured confounders, and treatment details were classified on a patient‐level (rather than on a lesion‐level). In addition, the severity of coronary lesion calcification was determined by (blinded) angiographic analysts in a central core laboratory, based on coronary angiographic images and not on intravascular imaging. Routine intravascular ultrasound or optical coherence tomography assessment in severely calcified lesions could have further improved angiographic and clinical outcomes. Finally, the use of rotablator and cutting balloon was relatively low, to which many reasons may have contributed: (a) severely calcified plaques, seen on angiography, may be located on the outside of the vessel wall and no suitable target for these therapeutic devices; (b) a large proportion of patients was treated for an acute myocardial infarction in which rotablator use is controversial 26 ; (c) considering the increased procedural risk of rotablation, some centers follow a more restrictive policy of rotablator use; (d) data were obtained from an all‐comer stent trial rather than a dedicated rotablator‐ or calcified lesion study. The Resolute Integrity ZES is the predecessor of the present widely used Resolute Onyx ZES, which has struts that are slightly thinner and more visible. Future research may focus on clinical outcomes with this newest iteration of the ZES.
5. CONCLUSION
In patients with severely calcified target lesions, there was no significant between‐DES difference in the main composite endpoint target vessel failure and various safety endpoints. Nevertheless, the use of Synergy EES was independently associated with a lower 2‐year incidence of repeat target vessel revascularization as compared to the Resolute Integrity ZES. These findings are hypothesis generating and therefore further validation in randomized clinical trials and large‐scale prospective registries is required.
CONFLICT OF INTEREST
CvB reports that the research department of Thoraxcentrum Twente has received institutional research grants provided by Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. All other authors declared that they have no conflict of interest. The present substudy received no additional financial support. The investigator‐initiated BIO‐RESORT trial was equally funded by Biotronik, Boston Scientific, and Medtronic.
Supporting information
Appendix S1: Supporting Information.
Buiten RA, Ploumen EH, Zocca P, et al. Three contemporary thin‐strut drug‐eluting stents implanted in severely calcified coronary lesions of participants in a randomized all‐comers trial. Catheter Cardiovasc Interv. 2020;96:E508–E515. 10.1002/ccd.28886
REFERENCES
- 1. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703‐1714. [DOI] [PubMed] [Google Scholar]
- 2. Généreux P, Redfors B, Witzenbichler B, et al. Two‐year outcomes after percutaneous coronary intervention of calcified lesions with drug‐eluting stents. Int J Cardiol. 2017;231:61‐67. [DOI] [PubMed] [Google Scholar]
- 3. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient‐level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158‐1164. [DOI] [PubMed] [Google Scholar]
- 4. Giustino G, Mastoris I, Baber U, et al. Correlates and impact of coronary artery calcifications in women undergoing percutaneous coronary intervention with drug‐eluting stents. JACC: Cardiovasc Interv. 2016;9:1890‐1901. [DOI] [PubMed] [Google Scholar]
- 5. Huisman J, van der Heijden LC, Kok MM, et al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer‐coated drug‐eluting stents: a patient‐level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). Am Heart J. 2016;175:121‐129. [DOI] [PubMed] [Google Scholar]
- 6. Huisman J, van der Heijden LC, Kok MM, et al. Two‐year outcome after treatment of severely calcified lesions with newer‐generation drug‐eluting stents in acute coronary syndromes: a patient‐level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). J Cardiol. 2017;69:660‐665. [DOI] [PubMed] [Google Scholar]
- 7. Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75:905‐911. [DOI] [PubMed] [Google Scholar]
- 8. Kitahara H, Kobayashi Y, Yamaguchi M, et al. Damage to polymer of undelivered sirolimus‐eluting stents. J Invasive Cardiol. 2008;20:130‐133. [PubMed] [Google Scholar]
- 9. Généreux P, Redfors B, Witzenbichler B, et al. Angiographic predictors of 2‐year stent thrombosis in patients receiving drug‐eluting stents: insights from the ADAPT‐DES study. Catheter Cardiovasc Interv. 2017;89:26‐35. [DOI] [PubMed] [Google Scholar]
- 10. Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes: pooled analysis from the HORIZONS‐AMI and ACUITY trials. J Am Coll Cardiol. 2014;63:1845‐1854. [DOI] [PubMed] [Google Scholar]
- 11. Theodoropoulos K, Mennuni MG, Dangas GD, et al. Resistant in‐stent restenosis in the drug eluting stent era. Catheter Cardiovasc Interv. 2016;88:777‐785. [DOI] [PubMed] [Google Scholar]
- 12. Zhang BC, Wang C, Li WH, Li DY. Clinical outcome of drug‐eluting versus bare‐metal stents in patients with calcified coronary lesions: a meta‐analysis. Intern Med J. 2015;45:203‐211. [DOI] [PubMed] [Google Scholar]
- 13. Bangalore S, Vlachos HA, Selzer F, et al. Percutaneous coronary intervention of moderate to severe calcified coronary lesions: insights from the National Heart, Lung, and Blood Institute dynamic registry. Catheter Cardiovasc Interv. 2011;77:22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byrne RA, Stone GW, Ormiston J, Kastrati A. Coronary balloon angioplasty, stents, and scaffolds. Lancet. 2017;390:781‐792. [DOI] [PubMed] [Google Scholar]
- 15. Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer‐coated, everolimus‐eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv. 2015;8:e002372. [DOI] [PubMed] [Google Scholar]
- 16. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable‐polymer sirolimus‐eluting stent versus durable‐polymer everolimus‐eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single‐blind, non‐inferiority trial. Lancet. 2014;384:2111‐2122. [DOI] [PubMed] [Google Scholar]
- 17. von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer‐coated (resolute onyx) versus ultrathin cobalt–chromium strut, bioresorbable polymer‐coated (Orsiro) drug‐eluting stents in allcomers with coronary artery disease (BIONYX): an international, single‐blind, randomised non‐inferiority trial. Lancet. 2018;392:1235‐1245. [DOI] [PubMed] [Google Scholar]
- 18. Kandzari DE, Mauri L, Koolen JJ, et al. Ultrathin, bioresorbable polymer sirolimus‐eluting stents versus thin, durable polymer everolimus‐eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843‐1852. [DOI] [PubMed] [Google Scholar]
- 19. Jensen LO, Thayssen P, Maeng M, et al. Randomized comparison of a biodegradable polymer ultrathin strut sirolimus‐eluting stent with a biodegradable polymer biolimus‐eluting stent in patients treated with percutaneous coronary intervention: the SORT OUT VII trial. Circ Cardiovasc Interv. 2016;9:e003610. [DOI] [PubMed] [Google Scholar]
- 20. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimuseluting and sirolimus‐eluting stents versus durable polymer zotarolimus‐eluting stents in allcomers with coronary artery disease (BIO‐RESORT): a three‐arm, randomised, non‐inferiority trial. Lancet. 2016;388:2607‐2617. [DOI] [PubMed] [Google Scholar]
- 21. Kok MM, Zocca P, Buiten RA, et al. Two‐year clinical outcome of all‐comers treated with three highly dissimilar contemporary coronary drug‐eluting stents in the randomised BIO‐RESORT trial. EuroIntervention. 2018;14:915‐923. [DOI] [PubMed] [Google Scholar]
- 22. Cutlip DE, Windecker S, Mehran R, et al. Academic research consortium clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344‐2351. [DOI] [PubMed] [Google Scholar]
- 23. Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871‐874. [DOI] [PubMed] [Google Scholar]
- 24. Copeland‐Halperin RS, Baber U, Aquino M, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer‐generation DES: findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859‐866. [DOI] [PubMed] [Google Scholar]
- 25. Basalus MWZ, Tandjung K, van Apeldoorn AA, Ankone MJK, von Birgelen C. Effect of oversized partial postdilatation on coatings of contemporary durable polymer‐based drug‐eluting stents: a scanning electron microscopy study. J Interv Cardiol. 2011;24:149‐161. [DOI] [PubMed] [Google Scholar]
- 26. Sakakura K, Ako J, Wada H, et al. Comparison of frequency of complications with on‐label versus off‐label use of rotational atherectomy. Am J Cardiol. 2012;110:498‐501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.


