Abstract
Objective:
Hydrogel scaffolds hold promise for a myriad of tissue engineering applications, however, often lack tissue-mimetic architecture. Therefore, in this work, we sought to develop a new technology for the incorporation of aligned tubular architecture within hydrogel scaffolds engineered from the bottom-up.
Approach:
We report a platform fabrication technology -- magnetic templating-- distinct from other approaches in that it uses dissolvable magnetic alginate microparticles (MAMs) to form aligned columnar structures under an applied magnetic field. Removal of the MAMs yields scaffolds with aligned tubular microarchitecture that can promote cell remodeling for a variety of applications. This approach affords control of microstructure diameter and biological modification for advanced applications. Here, we sought to replicate the microarchitecture of the native nerve basal lamina using magnetic templating of hydrogels composed of glycidyl methacrylate hyaluronic acid and collagen I.
Main results:
Magnetically templated hydrogels were characterized for particle alignment and micro-porosity. Overall MAM removal efficacy was verified by 96.8% removal of iron oxide nanoparticles. Compressive mechanical properties were well-matched to peripheral nerve tissue at 0.93 kPa and 1.29 kPa, respectively. In vitro, templated hydrogels exhibited approximately 36% faster degradation over 12 hours, and were found to guide axon extension from dorsal root ganglia. Finally, in a pilot in vivo study utilizing a 10-mm rat sciatic nerve defect model, magnetically templated hydrogels demonstrated promising results with qualitatively increased remodeling and axon regeneration compared to non-templated controls.
Significance:
This simple and scalable technology has the flexibility to control tubular microstructure over long length scales, and thus the potential to meet the need for engineered scaffolds for tissue regeneration, including nerve guidance scaffolds.
1. Introduction
Porous architecture is a key attribute of advanced tissue engineering scaffolds. Specifically, porous architecture is influential in aiding cellular infiltration and diffusion of key nutrients and waste products [1]. A major engineering challenge in development of biomimetic architecture is achieving controlled tailored features under aqueous processing conditions. To this end we sought to develop a flexible platform fabrication technology to form tunable, aligned columnar porous architecture within hydrogel scaffolds capable of scale-up to long lengths as well as chemical and biological modification for advanced applications. We chose to utilize this technology to develop biomimetic peripheral nerve repair scaffolds, replicating the microarchitecture of the native nerve basal lamina with aligned porous tubules with diameters on the order of ~10 μm.
In the US alone, approximately 560,000 peripheral nerve injury (PNI) repair procedures are performed annually [2]. The socioeconomic impact of peripheral nerve injury is significant, resulting in over 8 million restricted activity days and over 5 million disability days yearly [3]. According to a recent independent study, the US transected peripheral nerve injury repair market is estimated at $1.32-$1.93 billion [2]. Autologous nerve grafts (autografts) are the current gold standard for repair of peripheral nerve defects greater than 3 cm in length. Autologous nerve is harvested from the patient’s less-critical cutaneous nerves, such as the sural nerve in the lower limb. However, autologous nerve grafts (autografts) require additional surgery sites, carry the risk of associated donor site morbidity, and have limited availability and size-ranges, which restrict their use for extensive lesions and large diameter nerve grafts, such as those commonly required in brachial plexus injuries [4]. Furthermore, autografts are the most expensive nerve repair method available in the clinic [2], and most importantly, recent studies indicate only 40-50% of patients regain motor function recovery after treatment with these primarily sensory nerve autografts [5].
Decellularized nerve allografts, such as the Avance® graft are a clinically available off-the-shelf alternative to autologous nerve grafts, for nerve gaps up to 7 cm [2, 6, 7]. However, processed allografts rely on a limited cadaveric tissue source and are more expensive than nerve guidance conduit alternatives [2], in part because of the time and labor-intensive procedure to clear the harvested nerve of cells. Furthermore, as with all allograft tissue, there remains a risk of disease transmission, however remote. Multiple artificial nerve guidance conduits (NGCs) are currently approved by the FDA, however their use has been limited to direct nerve apposition and small peripheral nerve gaps less than 3 cm [8]. No clinically available NGCs utilize microscale 3D luminal guidance mimicking the peripheral nerve extracellular matrix for repair of gaps greater than 3 cm. Given the limitations associated with autologous nerve grafts, processed allografts, and NGCs, there is a strong motivation to develop new peripheral nerve regeneration scaffolds [9, 10].
We believe the success of processed nerve allografts in repairing nerve gaps up to 7 cm can be attributed to the maintenance of the native extracellular matrix (ECM) with ~10 μm diameter basal lamina tubules providing 3D topographical guidance to direct axonal growth and nerve repair [9, 11, 12]. Further, we believe that inferior regeneration using processed allograft compared to autograft in 7-12 cm peripheral nerve gaps suggests a limitation of 3D topographical guidance. This limitation may be overcome by chemical and biological factors present in native nerve but removed from allografts during processing. Unfortunately, post-modification of processed nerve allografts to incorporate chemical and biological cues or cells remains challenging, especially for long nerve gaps. Therefore, we hypothesize that the development of biocompatible, off-the-shelf regeneration scaffolds containing an aligned tubular microarchitecture mimicking natural nerve and compatible with incorporation of chemical and biological factors may overcome these limitations and enhance regeneration across long peripheral nerve gaps.
Physical guidance, such as through 3D topography, is a critical aspect of biomaterial scaffolds. In all tissue engineering applications, physical architecture and porosity are critical for nutrient and waste diffusion and to guide cellular infiltration and remodeling [1]. In nerve tissue engineering, the lack of physical guidance within hollow nerve guidance conduits currently used in nerve repair is believed to be a primary factor in their limited capability for regeneration of nerve gaps greater than 3 cm in humans [8]. Further, aligned micro- and nano-topography has been shown to promote cellular migration and axonal alignment, even when physical cues are in direct competition with biochemical factors such as nerve growth factor (NGF) and laminin [13]. Multiple methods have been developed to incorporate topography in devices for peripheral nerve repair, such as surface-patterned scaffolds [14, 15], micro- and nanofiber conduits [16, 17], and creation of 3D channels [18, 19]. The primary limitation of surface patterning is that it only provides a 2D microenvironment to direct growth and does not provide adequate guidance for directed growth and migration across long nerve gaps in vivo. Micro- and nanofibrous mats have been developed for use on the luminal wall of NGCs, however the fibers are not completely straight and aligned over macro-scale distances and the voids between fibers do not replicate the tubular channel architecture found in native nerve tissue. 3D scaffold alignment has been achieved using magnetically aligned fibrillar collagen hydrogels, however this method has limitations such as fibrillar relaxation after removal of the magnetic field. Biomaterial scaffolds incorporating 3D channels have been obtained using foaming [20], freeze-drying [21], and dissolution of aligned fibers [22]. However, none of these methods is currently capable of forming highly-aligned tubular structures in the ~10 μm diameter range, relevant for peripheral nerve repair, while allowing for scalable processing of large, biocompatible scaffolds. Additionally, these approaches can be incompatible with incorporation of chemical and biological cues. The limitations inherent to current methods stress the importance of developing new, scalable methods for producing biocompatible repair scaffolds containing both topographical and biochemical cues for repair of long nerve gaps.
Additionally, iron oxide nanoparticles have been studied in the context of peripheral nerve regeneration with conjugated neurotrophic factors [23, 24], and recently, for development of anisotropic structures within hydrogel scaffolds [25]. However, our approach is unique in that it is the first to utilize magnetic nanoparticles to create sacrificial anisotropic structures to develop 3D porous geometry within hydrogel scaffolds.
Naturally derived extracellular matrix (ECM) components were used for the biomaterial scaffolds in this study. Scaffolds produced from ECM components found naturally in peripheral nerve have lower risk of immunogenicity because of the ECM’s biocompatibility and inherent roles in physiologic wound healing. Furthermore, these natural materials can be remodeled by the body during regeneration. We believe these features afford natural scaffolds a greater potential for nearterm clinical success. Our hydrogel scaffolds are composed of two components: hyaluronic acid and collagen I, both of which are present in native peripheral nerve tissues [26, 27]. Specifically, we utilize a chemically modified form of hyaluronan, glycidyl-methacrylate hyaluronic acid (GMHA), to create robust, UV-crosslinkable, and mechanically tunable hydrogels. The biocompatibility of our GMHA hydrogels has been verified in vivo by our lab [28]. However, hyaluronan is non-cell adhesive. Addition of an interpenetrating, physically crosslinked collagen I network provides the scaffolds with cell-adhesive properties and incorporates this most abundant component of the natural peripheral nerve ECM [27]. Furthermore, we have previously demonstrated successful neuronal cell growth in 3D culture using GMHA-collagen hydrogels in vitro [29, 30]. In this study, hydrogels composed of 20 mg/mL GMHA and 1.5 mg/mL collagen I were used exclusively. A GMHA concentration of 20 mg/mL was chosen as this concentration was recently studied by our lab to support 3D templating and introduction of porous architecture [31]. A collagen I concentration of 1.5 mg/mL was chosen as this concentration is commonly used for tissue engineering applications in literature [32-35], and was the highest concentration that could uniformly and reliably be added to the templated hydrogel solutions.
2. Materials and Methods
2.1. GMHA Synthesis
GMHA was synthesized as previously published [28]. Briefly, hyaluronic acid sodium salt (~1.5-1.8 MDa, Sigma-Aldrich, St. Louis, MO, 53747) was dissolved in 50% acetone and 50% water, incubated at room temperature with 6.7% triethylamine (Sigma-Aldrich, T0886) for 30 min, then 6.3% glycidyl methacrylate (Sigma-Aldrich, 779342) for 20-24 hours. The resulting methacrylated HA was precipitated twice in 20X volume of 100% acetone (ThermoFisher, Waltham, MA, A1820) for purification, sterile filtered (Corning, Corning, NY, 430767), and lyophilized. GMHA was stored under desiccant at −20°C prior to use.
2.2. Magnetic Templating
Magnetic alginate microparticles (MAMs) were obtained by water-in-oil emulsion crosslinking of sodium alginate and iron oxide nanoparticle (IONP) mixtures using calcium chloride, as described previously [36]. In this study, polydisperse MAMs with diameters between 100’s of nm to 20 μm were utilized. MAMs were lyophilized and stored under desiccant at −20°C.
Before hydrogel templating, MAMs were weighed and hydrated in a known volume of double distilled water (ddH2O) for accurate gravimetric-based concentrations. MAMs were added to the hydrogel precursor mixture with final concentrations of 20 mg/mL GMHA, 0.3% Irgacure 2959 photoinitiator (BASF, Ludwigshafen, Germany, 55047962), and 1.5 mg/mL collagen I (Corning, 354249). MAMs were uniformly dispersed by mixing on an asymmetrical mixer at 2500 RPM for 1.5 min (Flacktek SpeedMixer DAC 150.1 FVZ). The complete hydrogel precursor solution was then loaded into a syringe and injected into 32 x 3 x 1.7 mm silicone molds (Grace bio-labs, Bend, OR, 622203). A custom magnet array was fabricated and composed of 12, 5.1 cm outer-radius x cm inner-radius x 2.5 tall, 30° arc segment magnets (K&J Magnetics, Pipersville, PA, AY0X030-T) inside a 3D-printed magnet holder (Figure 1B). Hydrogels were placed in the magnetic array for 30 min at 4°C, protected from light. Finally, the molds were removed from the magnet and immediately placed under a 365 nm UV light with intensity of 10-16 mW/cm2 at 17 cm from the bulb for 10 min of UV GMHA crosslinking, followed by incubation at 37°C for at least 35 min for collagen thermal gelation. The high viscosity of the precursor hydrogel solution helps to prevent MAM settling prior to hydrogel crosslinking.
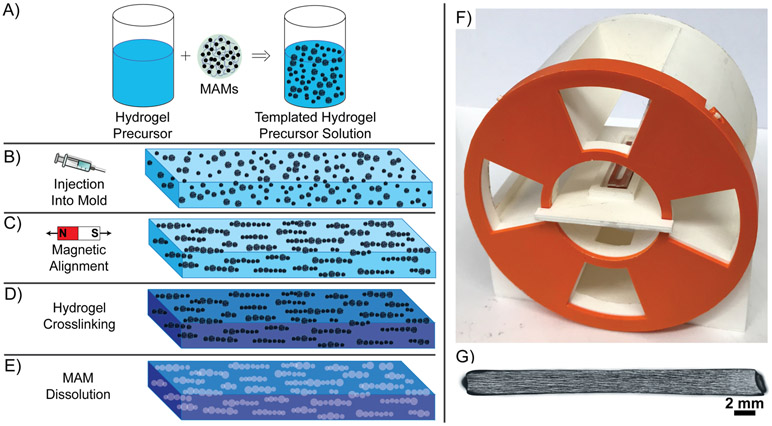
Figure 1. Overview of Magnetic Templating.
A) MAMs are added to a concentrated hydrogel precursor solution and asymmetrically mixed to create a uniform templated hydrogel precursor solution. B) The templated hydrogel precursor solution is injected into a mold of desired geometry between glass slides. C) The hydrogel precursor is aligned within a uniform magnetic field for linear assembly of MAMs. D) The hydrogel is crosslinked around the aligned MAMs. E) After MAM dissolution processing, hydrogels with linear 3D porous microarchitecture are generated. F) Complete magnetic array in 3D-Printed magnet-holder, features 4 radially aligned windows for UV exposure. G) Example 3 cm long magnetically templated hydrogel with complete magnetic particle alignment.
After hydrogel crosslinking, MAM degradation processing was conducted. Specifically, hydrogels were submerged in sterile-filtered EDTA (0.1M, ThermoFisher, SS412) for 14 days, with the EDTA being replaced every 3 days.
2.3. Visualization and Semi-Quantification of Alignment
Comparison of hydrogels templated at varying MAM concentrations from 2-5 mg/mL was conducted after templating GMHA-col hydrogels with the respective MAM concentrations. Three hydrogels per group were fabricated as described above and imaged at 10X magnification via transmitted light microscopy and a z-stack of ~200 μm into the hydrogel on a Zeiss Axioimager Z2 microscope immediately after crosslinking. Fast Fourier Transform (FFT) image analysis processing was conducted on all longitudinally aligned hydrogels for semi-quantification of the effect of MAM concentration on magnetic alignment, identically to published protocols [37]. An example FFT processing overview is shown in Figure S1 in the Supporting Information. All aligned samples were imaged with alignment in the horizontal direction, therefore each image was rotated 45 degrees to minimize superposition of horizontal edge effects in FFT processing on the data from the direction of alignment. The final image was flattened and saved as a TIFF file. Subsequent image processing was conducted using ImageJ software [38]. Each image was identically processed by enhancing contrast (50% saturated pixels, normalized and equalized histogram), and the background was saturated (using sliding paraboloid settings, smoothing disabled, and a rolling ball radius of 100 pixels). The images were then converted to binary and FFT transformed. Finally, using the oval profile plugin [39], the radial sums were measured for each image using a 450-pixel diameter circle, with 360 points to generate radial sums across each degree of alignment around the center-point. Resulting data were compiled in Microsoft Excel for subsequent analysis. The ratio to the mean orthogonal angle was calculated as a semi-quantitative measure of morphological alignment of each image as described by Taylor et al. [37].
For hydrogel visualization before and after MAM-dissolution, approximately 2 mm thick longitudinally aligned hydrogels and unaligned controls were imaged (Zeiss Axioimager Z2) and maximum projections of ~200 μm z-stacks were taken to visualize particle alignment. After MAM dissolution, hydrogels were identically imaged using light microscopy.
2.4. Dextran-FITC Imaging of Porosity and Confocal Microscopy
Porosity was visualized by soaking magnetically templated hydrogels with dextran fluorescein isothiocyanate conjugate (dextran-FITC). After MAM dissolution, hydrogels were equilibrated in 1 mg/mL 500 kDa dextran-FITC (Sigma-Aldrich, 46947) in PBS for 24 hours, then rinsed in 1X PBS for 2-3 hours to allow contrast between the bulk hydrogel and porous geometry prior to imaging on a Zeiss LSM710 laser-scanning confocal microscope.
2.5. Iron Quantification
Removal of iron oxide nanoparticles after MAM-dissolution processing was quantified through the o-phenanthroline UV-vis assay for iron content. Uniformly sized hydrogels were collected before and after MAM dissolution and were degraded in 50 units/mL hyaluronidase solution in PBS. For each sample, 10 μl aliquots were taken in triplicate and digested in 1 ml of 70% nitric acid (ThermoFisher, A-467-2) at 101°C for 12 hours to further degrade excess polymer and expose the iron oxide nanoparticles. After digestion, 10 μl aliquots were taken from each sample, placed into 1.5 mL glass vials, and heated to 115°C for 1 hour to evaporate all liquid. Aliquots of 46 μl of deionized water and 30 μl of 8.06 M hydroxylamine (Sigma-Aldrich, 431362-50G) were added to each vial to reduce iron samples from Fe+3 to Fe+2. After 1 hour, 49 μl of 1.22 M sodium acetate (Sigma-Aldrich, S8750-500G) and 75 μl of 13 mM 1,10-phenanthroline (Sigma-Aldrich, 77500-25G-F) were added to each vial, allowing for the formation of iron (II)-o-phenanthroline complexes. For each sample, 100 μl was loaded into a 96-well quartz plate and the absorbance values were read at 508 nm using a SpectraMax® M5 Multimode plate reader. Using a calibration curve prepared from a dilution series of Fe standard (Sigma-Aldrich, 56209-100ML) as the stock, the concentration of the iron was related to the absorbance value, allowing the iron concentrations to be quantified. The results were adjusted for the dilution of the hydrogel samples in hyaluronidase for final results in μg iron per μL of hydrogel.
2.6. Indentation Compressive Testing
Indentation compressive testing experiments were conducted to analyze alterations in compressive modulus with varying MAM concentration and to analyze compressive mechanical properties of bulk hydrogels in comparison with peripheral nerve tissue. Indentation testing was conducted on approximately 2 mm tall x 3 mm wide hydrogels cut to approximately 5 mm in length. All templated hydrogels were templated with tubular architecture in parallel with the hydrogel length. Indentation testing was conducted using a Bruker BioSoft In Situ indenter using a 3 mm diameter spherical indentation tip. Indentation was performed at a strain rate of 20 μm/second, up to ~8.33% total strain. The sample was then allowed to relax at constant strain for 90 seconds to reach quasistatic state. All indentation methods and data processing were conducted as described by Stewart et al. [40]. Briefly, relaxation data was fit to rearrangement of the Hertz contact model to calculate the effective modulus with a parabolic contact area, then the effective modulus was fit to a standard linear solid (SLS) model to estimate the strain-rate independent steady-state modulus as t → ∞.
2.7. In Vitro Hyaluronidase Degradation Testing
In vitro degradation profiles were obtained by gravimetric recording of hydrogels exposed to 50 Unit/mL hyaluronidase (Sigma-Aldrich, H3506) in PBS for a total of 12 hours. All hydrogels were equilibrated in 1X PBS for 24 hours then weighed prior to testing. Hydrogels were then incubated in 2 mL of hyaluronidase at 37°C and weighed hourly. After each weight was recorded, hydrogels were placed in 2 mL of fresh hyaluronidase.
2.8. In Vitro Testing: Dorsal Root Ganglia Outgrowth
Whole rat dorsal root ganglia were dissected from the vertebral column of post-natal day 1-3 Sprague Dawley-Tg(UBC-EGFP)2BalRrrc rats. The complete DRG culture medium used in this study was composed of neurobasal culture medium (ThermoFisher, 21103049) supplemented with 10% fetal bovine serum, 10 ng/mL nerve growth factor (R&D Systems, Minneapolis, MN, 556-NG), penicillin-streptomycin-amphotericin B antibiotic/antimycotic (MP Biomedicals, Solon, OH, 091674049), Glutamax supplement (ThermoFisher, 35050079), and 1% B-27 (ThermoFisher, 17504044). Magnetically templated hydrogels were equilibrated in complete DRG culture medium for 1-3 days prior to culture. DRGs were placed on the top surface of the pre-formed hydrogels. Half was culture medium was replaced in each sample every 2-3 days of culture. After 7 days, hydrogels were submerged in 4% paraformaldehyde (ThermoFisher, AC416780250) for fixation then washed in 1X PBS. Hydrogels were stained with mouse anti-βIII tubulin (Abcam, Cambridge, MA, 7751), rabbit anti-S100 (Sigma-Aldrich, S2644), and 4′,6-diamidino-2-phenylindole (DAPI) (ThermoFisher, D1306) at concentrations of 1:1000, 1:500, and 1:1000, respectively. Secondary immunostaining was conducted with goat anti-mouse Alexa Fluor 568 (Abcam, 175473) and goat anti-rabbit Alexa Fluor 647 (Abcam, 150079) at concentrations of 1:500 each. After staining, hydrogels were imaged using a Zeiss LSM710 laser-scanning confocal microscope.
2.9. In Vivo Testing: Rat Sciatic Nerve Injury
Magnetically templated hydrogels were tested against non-templated hydrogels of identical composition, and a positive control of rat nerve isograft, with a total n of 3 per group (1 used for longitudinal analysis and 2 used for cross-sectional analysis) designated for the 4 week endpoint and an n of 1 per group (used for longitudinal analysis) at the 2 week endpoint. Implantable hydrogel-filled conduits were prepared by cutting pre-formed hydrogels to 10 mm long segments with ~1.2 mm square cross-sectional dimensions. Hydrogel segments were individually wrapped in 12 mm long by 7 mm wide decellularized small intestinal submucosa (SIS) membranes (Cook Biotech, West Lafayette, IN) formed into conduits with a ~1 mm circumferential overlap and 1 mm overhangs at each end. Hydrogel-filled implants were equilibrated in DMEM/F-12 culture medium (Corning, 10-090-CV) to remove EDTA and neutralize pH levels and stored at 37° C for 7 days prior to implantation. Sciatic implantation was conducted on 10-week-old Lewis rats (Charles River).
All in vivo experiments were conducted in accordance with the University of Florida Institute for Animal Care and Use Committee guidelines and approval (Protocol #201508854). Rats were anesthetized with isofluorane and core body temperature was carefully monitored and maintained using a rectal temperature probe and a far-infrared warming pad as part of the RightTemp monitoring system (Kent Scientific Corporation, Torrington, CT). The sciatic nerve was isolated by carefully separating the muscles of the upper hindlimb. For isograft positive controls, 10 mm of sciatic nerve was harvested from a littermate donor rat for implantation into the experimental animal. The proximal transection was made 4 mm distal to the ligaments of the greater sciatic foramen and the nerve stump was sutured into the 1 mm SIS-overhang of the hydrogel-filled conduit or to the isograft control sample. Then, a transection was made for removal of exactly 8 mm of the distal sciatic nerve and the distal nerve stump was sutured into the hydrogel-filled conduit or to the isograft control sample for tension-free repair with a 10 mm final gap.
After 2 or 4 weeks of recovery following implantation, the animals were anesthetized with isofluorane for harvest of the regenerated sciatic nerves, including > 2 mm of proximal and distal nerve stumps, prior to pentobarbital/phenytoin chemical euthanasia.
2.10. Histology, Immunofluorescence, and Image Analysis
The regenerated nerves were immediately placed in 4% paraformaldehyde for 24 hours, washed daily in 1X PBS for 3 days, and stored in a solution of 30% sucrose (ThermoFisher, S5500) and 0.5% sodium azide (Sigma-Aldrich, S2002) in 1X PBS for at least 24 hours prior to embedding and cryosectioning. All washes were conducted at 4° C. All nerves were embedded in optimal cutting temperature compound (Electron Microscopy Sciences, Hatfield, PA, 6255001) for 24 hours, frozen at −80° C, and cryosectioned (Leica, CM1950). Longitudinal sections (20 μm thickness) and cross-sections (10 μm thickness) were immediately deposited onto gelatin-coated glass slides, dried for 2 hours on a slide warmer at 37° C, then stored at −80° C prior to staining. Two and four-week longitudinal sections (n = 1 animal per group) were stained with hematoxylin and eosin (H&E) and imaged using a Zeiss Axio Observer Z1 microscope.
For cross-sectional analysis after 4 weeks of implantation, slides were blocked for 1 hour in blocking buffer composed of PBS with 0.03%, Triton X-100 (ThermoFisher, BP151100), and 0.3% Goat Serum (Bio-Techne, Minneapolis, MN, NBP2-23475) then incubated in a mouse monoclonal anti-RT97 heavy chain antibody (RT97 Deposited to the DSHB Hybridoma Bank by Wood, J., University of Iowa) and a polyclonal rabbit anti-myelin basic protein (MBP) antibody (Sigma-Aldrich, M3821) primary antibodies at concentrations of 1:400 and 1:100 respectively in blocking buffer for 24 hours at 4° C. Slides were washed 3X for 1 hour each in PBS at room temperature, followed by 1 hour incubation at 4° C in goat anti-mouse Alexa Fluor 568 (Abcam, 175473) and goat anti-rabbit Alexa Fluor 647 (Abcam, 150079) secondary antibodies at concentrations of 1:500 each in blocking buffer. Slides were washed 3X for 1 hour each in PBS at room temperature, then treated with 1:1000 DAPI in ddH2O for 10 min at room temperature. Slides were then coverslipped with Fluoromount-g mounting medium (Southern Biotech, Birmingham, AL, 0100-01). Fluorescence imaging was conducted on a Zeiss Axioimager Z2. Four to five consecutive sections were imaged for each sample (at 2, 5, and 12 mm graft locations for the proximal implant, midgraft, and distal nerve locations) for each animal with n = 2 animals per group. RT97 fluorescence staining was quantified for axonal density using Zen software at each location, and MBP fluorescence intensity sum was quantified at the midpoint of each group.
2.11. Statistical Methods
All statistical analyses were conducted in GraphPad Prism 6 statistical software. Paired Student’s t-tests were conducted using 95% confidence intervals. For FFT analysis and indentation compressive analyses, a one-way ANOVA with Tukey’s HSD post-hoc test was used to account for multiple comparisons for statistical significance within each comparison for an overall confidence interval of at least 95%. All error bars represent standard deviation (SD).
3. Results
3.1. Development of Magnetically Templated Hydrogels
In this study we report a facile and scalable process for the preparation of naturally derived hydrogels with aligned 3D tubular microarchitecture that mimics the natural nerve basolaminal extracellular matrix. We refer to these scaffolds as “magnetically templated hydrogels”. Magnetically templated hydrogels composed of glycidyl-methacrylated hyaluronic acid and collagen I (GMHA-Col) were generated as illustrated in Figure 1A-E. Our goal for this technology was to use MAMs as sacrificial templating agents to create aligned, tubular, porous architecture with micron-scale diameters and millimeter-scale lengths within natural ECM hydrogels. To accomplish this goal, we developed a custom-made magnetic array (Figure 1F), as a scalable method for alignment of MAMs within magnetically templated hydrogels up to 3 cm in length (Figure 1G). The magnetic array was found to have an average magnetic flux density of 76.15 ± 3.70 mT in the central 3 cm of the array, as measured using a F. W. Bell series 9950 gaussmeter/teslameter. This magnetic array also allows for UV exposure and thus, hydrogel crosslinking within the magnetic field.
3.2. Hydrogel Alignment Increases with MAM Concentration
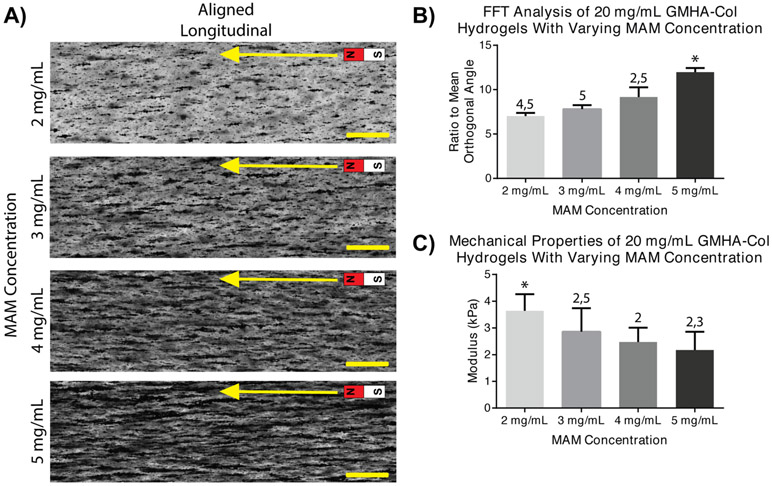
GMHA-Col scaffolds were developed with magnetic alginate microparticle (MAM) concentrations of 2-5 mg/mL in the hydrogel precursor solution for analysis of the effects of MAM concentration on particle alignment and hydrogel mechanical properties. A cutoff of 5 mg/mL MAMs was used as higher concentrations were found to demonstrate poor crosslinking with incomplete UV crosslinking through the thickness of the 2 mm-thick hydrogel molds. Qualitatively, an increase in overall opacity can be noted with increasing particle concentration (Figure 2A). Additionally, increasing MAM concentration appears to generate increased particle alignment, with longer linear aggregates of particle chains.
Figure 2. Effect of MAM Concentration on Hydrogel Alignment and Mechanical Properties.
A) Magnetically templated GMHA-Col hydrogels with varying MAM concentrations from 2 to 5 mg/mL. Light micrographs of longitudinal sections of freshly crosslinked aligned hydrogels, containing dark, opaque MAMs. Scale bars = 500 μm, arrow indicates the direction of magnetic alignment. B) Ratio to mean orthogonal angle derived from FFT analysis. Increasing ratio to mean orthogonal angle indicates increased morphological alignment, with 5 mg/mL MAM concentrations resulting in the highest alignment. C) Indentation compressive mechanical properties of freshly crosslinked hydrogels at varying MAM concentrations. Decreased mechanical properties noted at progressively higher MAM concentrations. For all graphs: * indicates statistically significant differences from all other groups and numbers 2-5 indicate statistically significant differences to 2-5 mg/mL MAM groups respectively. Error bars represent standard deviation.
A semi-quantification of magnetic particle alignment was conducted via FFT image analysis on three samples per group. In this analysis, the morphological alignment of structures in images of each group was quantified via calculation of the ratio of the mean orthogonal angle, a metric of alignment ranging from 0 (no-alignment) to a theoretical maximum of 1 (complete morphological alignment). The ratio of the mean orthogonal angle is shown for 20 mg/mL GMHA-Col hydrogels (Figure 2B). The ratio to the mean orthogonal angle was found to linearly increase with increasing MAM concentrations, to a maximum of 11.97 +/− 0.47 % at 5 mg/mL MAM concentration, significantly higher than all other MAM concentrations. Indentation testing (Figure 2C) was performed on identical hydrogels at varying MAM concentrations to study the effect on compressive mechanical properties. Results demonstrate statistically significant differences and decreasing steady-state moduli with increasing MAM concentration.
A concentration of 5 mg/mL of MAMs in the hydrogel solution was chosen for all subsequent experiments as this concentration was proven as the upper-limit allowing for optimal particle-chain length (as demonstrated by FFT analysis) on the millimeter scale. A concentration of 5 mg/mL also retained uniformity of UV-crosslinking throughout the thickness of the hydrogel, as evidenced by no visible deformation of the hydrogel geometry throughout the cross section. Higher MAM concentrations were found to result in a highly opaque hydrogel precursor solution, which resulted in incomplete crosslinking of 2 mm thick hydrogels upon UV irradiation.
3.3. Porous Architecture is Generated After MAM Dissolution
Macroscopic images of hydrogels before and after MAM dissolution processing are shown in Figure 3A and 3B, respectively. These images demonstrate particle removal through MAM dissolution processing as indicated by a decrease in both color and opacity of the bulk hydrogel. Longitudinally aligned templated hydrogels (Figure 3D) and unaligned controls (Figure 3C) were imaged under transmitted light microscopy demonstrating the overall effect of magnetic alignment of MAMs prior to hydrogel crosslinking.
Figure 3. MAM Dissolution Processing Successfully Removes MAMs and Generates Porous Microarchitecture.
A) Macroscopic view of crosslinked hydrogel after magnetic templating, and B) Macroscopic view of magnetically templated hydrogel after MAM dissolution. C) Crosslinked hydrogel after MAM templating without magnetic alignment (unaligned), projection of ~200 μm light microscopic z-stack. D) Crosslinked, aligned magnetically templated hydrogel, projection of ~200 μm light microscopic z-stack. E) Magnetically templated hydrogel after MAM dissolution, projection of ~200 μm light microscopic z-stack. F) Templated hydrogel soaked in dextran-FITC to visualize pores after MAM dissolution, maximum projection of a ~250 μm thick confocal micrograph.
After MAM dissolution processing, removal of MAMs was verified by light microscopy with a substantial reduction of the presence of dark, opaque MAMs (Figure 3E). After particle removal, hydrogels were soaked in dextran-FITC then rinsed for ~3 hours in PBS to allow for visualization of the pores remaining inside the hydrogels. Confocal micrographs demonstrate tubular porous architecture remains intact after MAM dissolution, with millimeter scale lengths (Figure 3F). Iron concentrations before and after MAM dissolution indicate removal of most of the initial iron from templated hydrogels (i.e., average residual iron concentration less than 133 μg iron/ mL of hydrogel, the limit of detection of the iron quantification assay performed), Figure S2 in the Supporting Information.
3.4. Templated Hydrogels are Mechanically Matched to Nerve
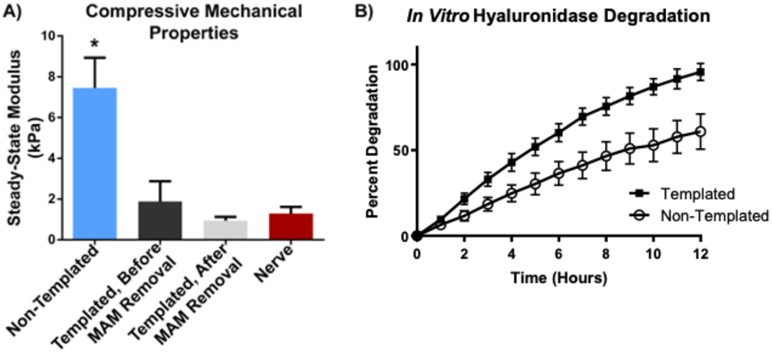
Indentation compressive testing (Figure 4A) results demonstrate a statistically significant decrease in mechanical properties with magnetic templating alone, in the absence of MAM dissolution processing, with non-templated hydrogels having a steady-state modulus of 7.45 +/− 1.49 kPa (n = 8), compared to 1.88 +/− 1.00 kPa for magnetically templated hydrogels (n = 12). After MAM dissolution processing, magnetically templated hydrogels demonstrated a trend toward decreased mechanical properties with a steady-state modulus of 0.93 +/− 0.20 kPa (n = 12), however, this difference relative to the hydrogel prior to MAM dissolution was not statistically significant. Finally, rat sciatic nerve tissue (n = 4) demonstrated a steady-state compressive modulus of 1.29 +/− 0.33 kPa. This result was very similar to magnetically templated hydrogels after MAM dissolution processing (difference was not statistically significant). Therefore, magnetically templated hydrogels were found to be well-matched to the mechanical properties of native peripheral nerve tissue.
Figure 4. Magnetically Templated Hydrogels are Mechanically Matched to Native Nerve and Degrade Faster Than Non-Templated Hydrogels.
A) Steady-state compressive modulus of magnetically templated vs. non-templated GMHA-col hydrogels and rat sciatic nerve. Magnetic templating was found to result in a statistically significant decrease in mechanical properties. (n = 13 for each templated group, n = 16 for non-templated group). Magnetically templated hydrogels with or without MAM dissolution processing not statistically different from rat sciatic nerve (n = 4). * indicates statistical significance from all other groups with p=0.001. B) In vitro hydrogel degradation profiles obtained via gravimetric analysis of magnetically templated GMHA-Col hydrogels (after MAM removal) and non-templated controls treated with hyaluronidase for 12 hours. Percent degradation represents weight lost from each hydrogel at each time point normalized to initial hydrogel weight. The two groups demonstrated statistically significant differences at each time point observed. Error bars represent standard deviation.
3.5. Templated Hydrogels Degrade Faster In Vitro
In vitro degradation results (Figure 4B) indicate increased degradation rate of magnetically templated hydrogels (95.6 +/− 5.0 %, n = 10) compared to non-templated GMHA-Col controls (60.8 +/− 10.3 %, n = 12) over 12 hours. Statistically significant differences in hydrogel degradation were found at each time point measured, with p = 0.002 at 1 hour, and p < 0.001 for hours 2-12. These results indicate the potential for faster in vivo degradation of magnetically templated hydrogels compared to non-templated hydrogels with identical hydrogel composition.
3.6. Templated Hydrogels Guide Dorsal Root Ganglia Growth In Vitro
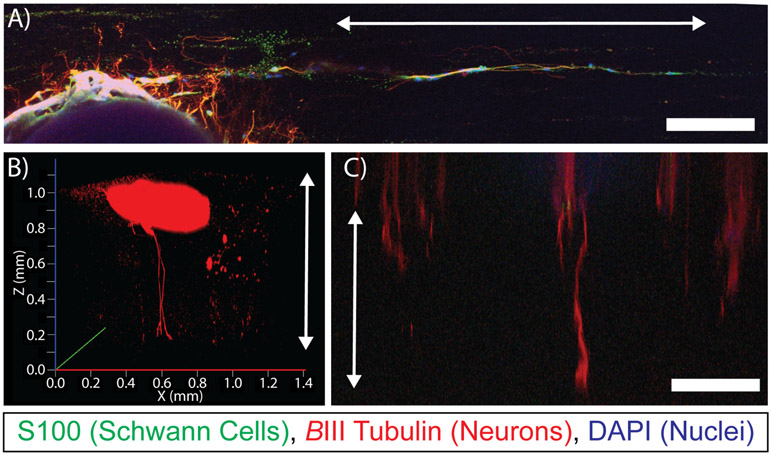
When dorsal root ganglia were cultured for 7 days on the surface of magnetically template hydrogels with aligned porosity parallel with the surface plane, outgrowth demonstrated linear regions of axonal elongation and Schwann cell migration directly in parallel with the direction of the aligned pores (Figure 5A). Furthermore, when DRGs were cultured for 7 days on the surface of a magnetically templated hydrogel with pores aligned perpendicular to the surface (Figure 5B,C), axons were found to penetrate into the hydrogel, and appeared to grow up to ~1 mm into the thickness of templated hydrogels.
Figure 5. Magnetically Templated Hydrogels Guide Dorsal Root Ganglia Growth In Vitro.
A) Confocal rendering of DRG outgrowth on surface of magnetically templated hydrogel, stained for S100 (Schwann cells, green), βIII Tubulin (neurons, red), and DAPI (nuclei, blue), arrow indicates direction of aligned porosity. B, C) Confocal renderings of axonal penetration throughout the thickness of approximately 1 mm thick hydrogels from each of 2 separate studies, arrows indicate the direction of aligned porosity. Scale bars = 250 μm.
3.7. Templated Hydrogels Support Better Regeneration In Vivo
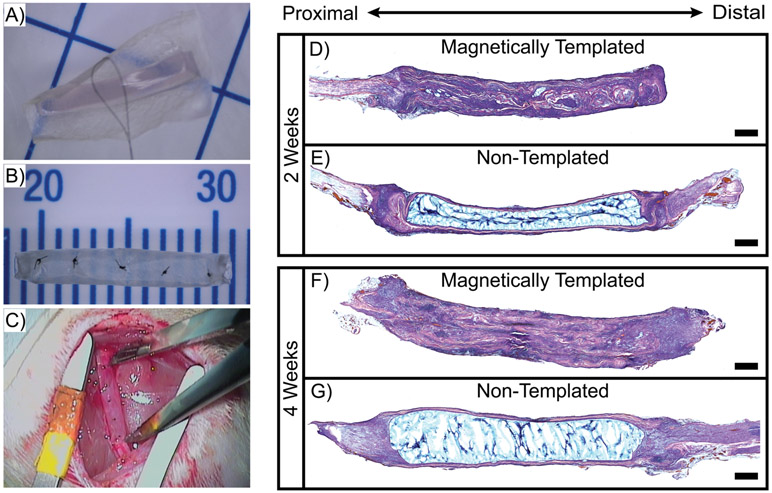
Magnetically templated hydrogels were studied in the context of short-term implantation in a pilot rat 10 mm sciatic nerve injury model. Representative images of the SIS-wrapping process and complete implantable hydrogel-filled conduits are shown in Figure 6A and 6B, respectively, and a magnetically templated hydrogel implant is shown immediately following implantation in the injury location in Figure 6C. After just 2 weeks of implantation, H&E stained longitudinal sections demonstrated stark differences in overall implant remodeling and cellular infiltration (Figure 6D,E). The magnetically templated hydrogel sample appeared largely remodeled after 2 weeks, with a great deal of cellular infiltration versus the non-templated hydrogel control, which appeared largely intact, with minimal remodeling or cellular infiltration.
Figure 6. Increased Cellular Remodeling of Magnetically Templated Hydrogels.
A) Representative pre-formed hydrogel being wrapped in SIS, grid = 1 cm, B) Representative hydrogel-filled conduit ready for implantation, C) Magnetically templated hydrogel-filled implant sutured in 10 mm-gap injury location, D-G) H&E stained 20 μm thick sections of longitudinally-sliced implants after 2 weeks (D,E) and 4 weeks (F,G) of implantation. Scale = 1 mm.
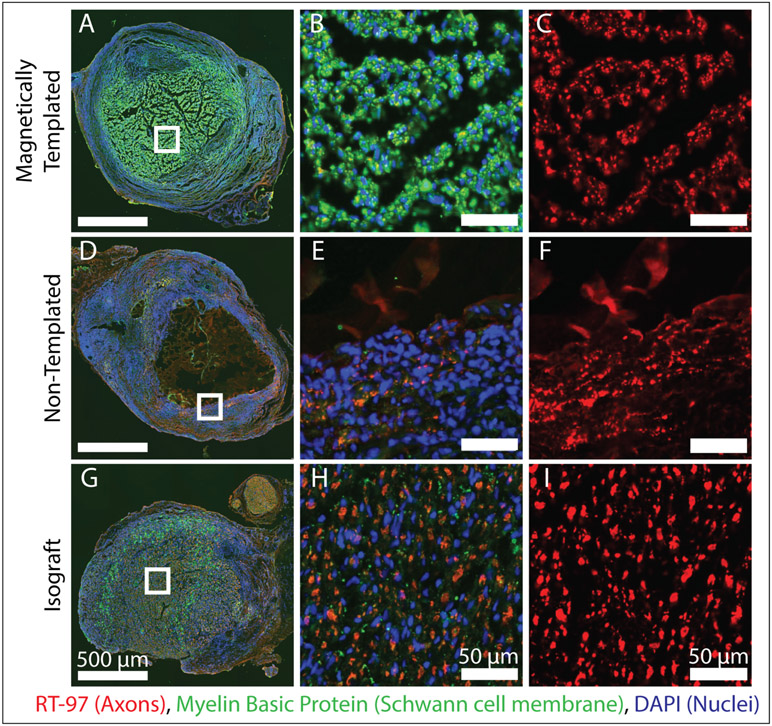
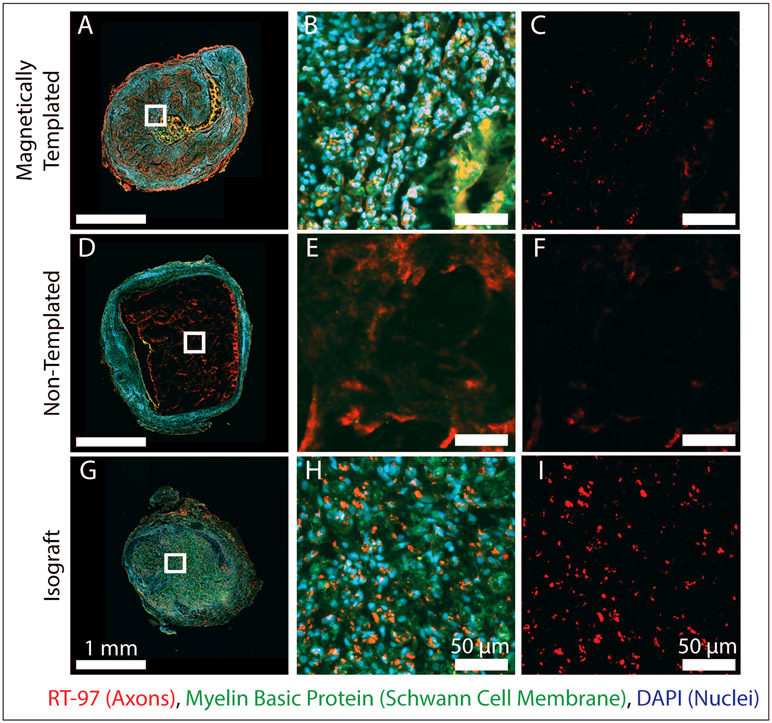
Representative cross sections from the proximal graft location after 4 weeks of implantation, are shown in Figure 7, alongside magnified views of the hydrogel lumen or the hydrogel-SIS interface for the non-templated hydrogel group, where the most abundant neurofilament staining was noted. Minimal to no axonal elongation occurred within the hydrogel lumen of the non-templated hydrogels and appeared to be completely localized to the hydrogel-SIS interface. Magnetically templated hydrogels demonstrated abundant myelin basic protein staining, often colocalized with neurofilament staining, suggesting these hydrogels promoted Schwann cell infiltration and proliferation within the hydrogel lumen. Non-templated hydrogels again demonstrated minimal cellular infiltration or remodeling throughout the length of the implant. Furthermore, Figure 8 contains representative cross sections at the midgraft location for all groups. These data demonstrate successful axonal growth to the midgraft location in magnetically templated hydrogels and isograft controls compared to no evident axons in the lumen of non-templated hydrogel controls.
Figure 7. Axonal Elongation and Schwann Cell Migration into the Proximal End of Experimental Implants After 4-Weeks of Implantation.
A-I) Fluorescence stained images of magnetically templated, non-templated, and isograft implants taken at the proximal implant location 2 mm into the device after 4-weeks in vivo. Left panel shows complete cross sections, with magnified views shown to the right as indicated by white boxes. Left panel shows RT97 staining for axons, and all other images are stained for RT97 for axons (red), Myelin basic protein (green), and DAPI (blue). (n = 2 / group). At the proximal graft location, isografts and magnetically templated hydrogels demonstrated axonal growth and Schwann cell infiltration in the luminal area, compared to minimal axonal growth localized to the hydrogel-SIS interface in non-templated samples.
Figure 8. Axonal Elongation and Schwann Cell Migration at the Mid-Graft Location of Experimental Implants After 4-Weeks of Implantation.
A-I) Fluorescence stained images of magnetically templated, non-templated, and isograft implants taken at the midgraft location of the device after 4-weeks in vivo. Left panel shows complete cross sections stained for RT97 for axons (red), Myelin basic protein (green), and DAPI (blue), with magnified views shown to the right as indicated by white boxes. Right column shows RT97 staining for axons. (n = 2 / group). At the midgraft location, isografts and magnetically templated hydrogels demonstrated axonal growth in the luminal area, compared to minimal to no axonal presence in non-templated samples.
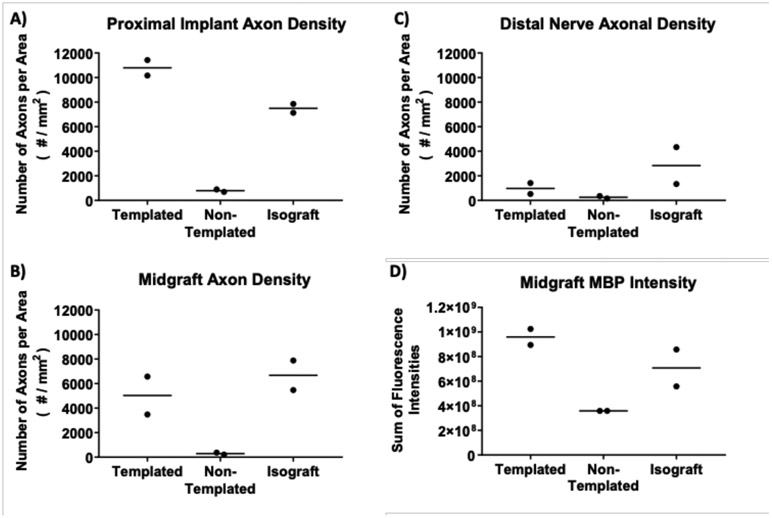
Representative centrally located areas of each nerve section were taken for neurofilament staining quantification to determine axonal density throughout the length of the implants (proximal implant location, midgraft location, distal nerve location, Figure 9 A, B, and C respectively). Results appear to indicate similar axonal density between the templated hydrogels and the fresh nerve isograft positive controls up until the mid-graft location at the early endpoint of 4 weeks. In both templated hydrogel and fresh nerve isograft groups, axonal density decreased at the distal graft location through to the distal nerve stump. This level of regeneration can be expected after 4 weeks of repair, with previous reports of axonal bridging of 10 mm defects beginning at 3 weeks postinjury using an empty conduit [3, 41]. Overall, axons were found to bridge the 10 mm defect when implanted with magnetically templated hydrogels for 4 weeks, and quantifiable axons were found in the distal nerve stump, albeit at lower density than for isograft controls.
Figure 9. Image Quantification of Cell Growth Into and Across Magnetically Templated Hydrogels and Controls After 4-Weeks Implantation.
Axonal densities obtained through RT97 quantification of cross sections throughout the implant after 4-weeks in vivo, (n = 2 / group). Axonal density quantification shown at the proximal implant location, ~2 mm into the regenerated implants (A), the midgraft location (B), and the distal nerve location (C). Myelin basic protein (MBP) staining intensity quantified as a measure of Schwann cells at the midgraft location (D), (n = 2 / group). Each point represents the mean quantified value from 4-5 consecutive sections from one individual animal, and the horizontal line represents the group mean. Overall, magnetically templated hydrogels appeared to have higher axon density than non-templated hydrogels at every location, and higher MBP intensity at the midgraft location.
Myelin basic protein staining was quantified for overall intensity within representative centrally-located areas of the implanted devices at the midgraft location (Figure 9D). At the midgraft location, myelin basic protein fluorescence intensity in templated hydrogel implants was on average higher than isograft implants, demonstrating potential Schwann cell infiltration into magnetically templated hydrogels similar to or higher than levels found within isograft positive controls. Minimal myelin basic protein intensity was found within non-templated scaffolds.
5. Discussion
We report a facile and scalable platform of magnetic templating for the preparation of naturally derived hydrogels with aligned 3D tubular microarchitecture. This approach allows for tremendous flexibility in controlling microstructural dimensions and for future biological modifications for advanced tissue engineering applications. Here, we used this novel fabrication method to create scaffolds with aligned tubular microstructures that mimic natural nerve tissue. In this study we sought to characterize magnetically templated hydrogels and demonstrate the powerful effects of 3D porous architecture alone on altering cell growth in vitro. Additionally, we conducted a pilot in vivo trial to demonstrate the ability of magnetic templating to aid in peripheral nerve regeneration.
Development of tissue engineered scaffolds for peripheral nerve are a current focus of several research groups. Specifically, as described in our recent review article by Mobini et al. [8] multiple methods have been developed to develop scaffolds with hierarchical architecture and/or porosity to promote nerve repair; such as electrospinning [42-44], magnetically aligned collagen [45], solvent casting/template leaching [19], alginate capillary gels [46], 3D-printing [47], unidirectional freezing [48], and microdrilling [49]. The magnetic templating platform presented in this manuscript is unique in its ability to generate tubular porous geometry with mm-scale length and μm-scale diameters to mimic the peripheral nerve microarchitecture. Furthermore, these scaffolds have been fabricated using methods designed to be highly scalable and using aqueous processing conditions for future potential for incorporation of growth factors, adhesive moieties, drug release, and/or or cellular delivery, to help with repair of long-gap peripheral nerve injuries. In developing engineered scaffolds capable of repairing peripheral nerve injuries in humans, we hope to fulfill the unmet clinical need for alternatives to peripheral nerve autografts and processed allografts to eliminate additional surgical sites, high cost, reliance on human peripheral nerve sources and possibility of an adverse immune response. This novel process can also be used to develop hydrogels of varying composition and could be adapted for tissue engineering approaches targeting varying tissues with aligned architecture, including peripheral nerve, spinal cord, and potentially tendon, muscle, or skin repair.
Currently, magnetic alginate microparticle (MAM) alignment has been optimized to generate millimeter-scale non-continuous channels within GMHA-Col scaffolds. Utilizing naturally-based hydrogel scaffolds was believed to be critical in this approach, as in vivo degradation and remodeling of the natural hydrogel scaffolds through endogenous mechanisms for wound repair should allow for cellular migration and elongation through the bulk of the scaffold. Therefore, while porous channels were designed for optimization of directed, linear neural regeneration, porous continuity was not believed to be necessary in these scaffolds as regenerating cells should be able to remodel the hydrogel to grow from one porous channel into another. With proper design of the magnetic fields used to align the MAMs and further hydrogel optimization to minimize hydrogel precursor viscosity, longer chains should be obtainable, resulting in a more continuous porous architecture.
In this study, utilizing 20 mg/mL GMHA and 1.5 mg/mL collagen I, 5 mg/mL MAM concentrations were found to result in the optimal particle alignment as indicated by FFT analysis. Furthermore, these hydrogels were found to retain handling properties and complete crosslinking of 2 mm thick hydrogels.
Iron quantification results demonstrate substantial removal of iron oxide nanoparticles from the templated hydrogels through MAM dissolution processing. Residual iron concentrations of 11.5 μg/mL in processed magnetically templated hydrogels will have minimal to no effects on the biocompatibility of these scaffolds. Literature reports demonstrate iron toxicity above 223.4 μg/mL for rat alveolar epithelial cell toxicity [50], and 84 μg/mL for neurons [51].
Mechanical properties are a critical determinant of cell and tissue-level response to tissue-engineered scaffolds for virtually any application. Compressive analysis demonstrated a decrease in mechanical properties of magnetically templated hydrogels compared to non-templated hydrogels. This decrease can be caused by both incorporation of porous architecture or light absorption scattering during photopolymerization of the hydrogels caused by the dark, opaque MAMs. Therefore, we are unable to directly state what proportion of the decrease in mechanical properties (or other properties such as degradation rate) is attributable directly to porous architecture. However, we concluded that the mechanical properties of magnetically templated hydrogels were similar to peripheral nerve tissues and the magnetically templated hydrogels were therefore produced with bulk mechanical properties well matched to native nerve tissue.
Increased macro-scale porosity induced through magnetic templating was anticipated to increase the permeability of the hydrogel scaffolds and potentially influence properties such as degradation rate, important for promoting successful regeneration within these scaffolds. In vitro hyaluronidase degradation studies demonstrate significantly increased degradation of magnetically templated hydrogels compared to non-templated controls. These data correlate well with in vivo findings of increased hydrogel degradation and remodeling in magnetically templated hydrogels compared to non-templated controls; non-templated controls were still intact after 4 weeks of implantation as indicated by hematoxylin and eosin H&E stained longitudinal sections. In vitro hyaluronidase degradation assessment is commonly used to compare the degradation of varying hyaluronic acid based hydrogels [28, 52, 53]. While varying hydrogel and enzymatic degradation conditions make comparative assessment difficult across studies, our in vitro degradation assessment was performed identically to previous reports from our lab [31], and demonstrated similar results with non-templated hydrogels degrading ~60% over 12 hours. Additionally, our previous study [31] determined that the addition of macroscale porosity through salt templating increased degradation rate in a similar manner to magnetic templating.
Evidence of guided neuronal outgrowth in vitro suggests the 3D architecture in magnetically templated hydrogels has the capability to guide cellular migration. In vivo analysis of magnetically templated hydrogels suggests their ability to promote peripheral nerve regeneration. These findings are powerful given the limitations of human autologous nerve supply and donor site morbidities, especially in the context of extensive nerve lesions and where large diameter nerve grafts are required. In this study, we used non-templated hydrogels of identical composition to the magnetically templated hydrogels (20 mg/mL GMHA, 1.5 mg/mL col I) as a negative control. While this allowed a direct comparison of hydrogels with and without magnetic templating, the increased mechanical properties of the non-templated hydrogels determined in section 3.4 could have influenced their performance in vivo. Therefore, additional studies could be performed to compare in vivo regeneration in magnetically templated hydrogels to non-templated hydrogels with similar mechanical properties through the use of lower GMHA concentrations or decreased UV crosslinking duration. Further in vivo testing and functional recovery assessment will be necessary as the current pilot study lacks a sufficient sample size for statistical analysis.
This study demonstrates the novel method of magnetic templating. The magnetic templating technology used in this study has been developed with the flexibility for loading and/or immobilization of growth factors and/or biochemical factors, and even potentially cell delivery. The resulting combinatorial factors could further enhance regeneration by delivering numerous physical, chemical, and cellular cues in one device to meet the goal of an off-the-shelf peripheral nerve scaffold capable of repairing long nerve injuries in humans.
6. Conclusion
Here, we have presented a novel magnetic templating technology capable of inducing a highly aligned, 3D, tubular microarchitecture within naturally derived hydrogel scaffolds. These scaffolds have been tailored to mimic the microarchitecture, composition, and mechanical properties of native peripheral nerve tissue. Results demonstrate the ability for magnetically templated hydrogel to guide cellular migration and potentially aid in peripheral nerve regeneration after injury. These results suggest that magnetically templated hydrogels could facilitate nerve repair and meet the clinical need for off-the-shelf, cost effective repair of long-gap nerve injuries without relying on human nerve tissue.
Supplementary Material
Acknowledgments:
This work was funded by NIH NS093239. Provisional patent on the technology has been filed, PCT patent application WO2016/183162. The authors would like to thank Cook Biotech for their contribution of SIS for this study under materials transfer agreement #A14720.
References:
- [1].O'Brien F, Biomaterials & Scaffolds for Tissue Engineering, Materials Today 14(3) (2011) 88–95. [Google Scholar]
- [2].Brattain K, Analysis of Peripheral Nerve injury Market in the United States, Magellan Medical Technology Consultants, Inc, 2013. [Google Scholar]
- [3].Belkas JS, Soichet MS, Midha R, Axonal guidance channels in peripheral nerve regeneration, Operative techniques in orthopaedics 14 (2004) 190–198. [Google Scholar]
- [4].Ray WZ, Mackinnon SE, Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy, Experimental Neurology 223(1) (2010) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee SK, Wolfe SW, Peripheral nerve injury and repair, J Am Acad Orthop Surg 8 (2000) 243–52. [DOI] [PubMed] [Google Scholar]
- [6].Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE, Wisotsky SM, Cho MS, Wilson C, Cooper EO, Ingari JV, Safa B, Parrett BM, Buncke GM, Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions, Microsurgery 32 (2012) 1–14. [DOI] [PubMed] [Google Scholar]
- [7].Zuniga JR, Sensory Outcomes After Reconstruction of Lingual and Inferior Alveolar Nerve Discontinuities Using Processed Nerve Allograft—A Case Series, Journal of Oral and Maxillofacial Surgery 73(4) (2015) 734–744. [DOI] [PubMed] [Google Scholar]
- [8].Mobini S, Spearman BS, Lacko CS, Schmidt CE, Recent advances in strategies for peripheral nerve tissue engineering, Current Opinion in Biomedical Engineering 4 (2017) 134–142. [Google Scholar]
- [9].Kehoe S, Zhang XF, Boyd D, FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy, Injury 43 (2012) 553–572. [DOI] [PubMed] [Google Scholar]
- [10].Subramanian A, Krishnan UM, Sethuraman S, Development of Biomaterial Scaffold for Nerve Tissue Engineering, Journal of Biomedical Science 16(1) (2015) 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE, Optimized acellular nerve graft is immunologically tolerated and supports regeneration, Tissue Engineering 10 (2004) 1641–1651. [DOI] [PubMed] [Google Scholar]
- [12].Spivey EC, Khaing ZZ, Shear JB, Schmidt CE, The fundamental role of subcellular topography in peripheral nerve repair therapies, Biomaterials 33 (2012) 4264–4276. [DOI] [PubMed] [Google Scholar]
- [13].Gomez, Chen, Schmidt CE, Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands, J. R. Soc Interface 4(13) (2007) 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun M, McGowan M, Kingham PJ, Terenghi G, Downes S, Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy, Journal of Materials Science: Materials in Medicine 21(10) (2010) 2765–2774. [DOI] [PubMed] [Google Scholar]
- [15].Hu C, Uchida T, Tercero C, Ikeda S, Ooe K, Fukuda T, Arai F, Negoro M, Kwon G, Development of biodegradable scaffolds based on magnetically guided assembly of Magnetic sugar particles, Journal of Biotechnology 159(1-2) (2012) 90–98. [DOI] [PubMed] [Google Scholar]
- [16].Murray-Dunning C, McArthur SL, Sun T, McKean R, Ryan AJ, Haycock JW, Three-Dimensional Alignment of Schwann Cells Using Hydrolysable Microfiber Scaffolds: Strategies for Peripheral Nerve Repair Methods in Molecular Biology 695 (2011) 155–166. [DOI] [PubMed] [Google Scholar]
- [17].Xie J, MacEwan MR, Liu W, jesuraj N, Li X, Hunter D, Xia Y, Nerve Guidance Conduits Based on Double-Layered Scaffolds of Electrospun Nanofibers for Repairing the Peripheral Nervous System, ACS Applied Material Interfaces 6(12) (2014) 9472–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biazar E, Design of Porous Alginate Conduit using a Freeze Drying Technique for Neural Engineering, Oriental Journal of Chemistry. 27(3) (2011) 1029–1032. [Google Scholar]
- [19].Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH, Templated Agarose Scaffolds Support Linear Axonal Regeneration, Tissue Engineering 12(10) (2006) 2777–2787. [DOI] [PubMed] [Google Scholar]
- [20].Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP, A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration, Tissue Engineering 6 (2000) 119–127. [DOI] [PubMed] [Google Scholar]
- [21].Stokols S, Tuszynski MH, Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury, Biomaterials 27 (2006) 443–451. [DOI] [PubMed] [Google Scholar]
- [22].Scott JB, Afshari M, Kotek R, Saul JM, The promotion of axon extension in vitro using polymer-templated fibrin scaffolds, Biomaterials 32 (2011) 4830–4839. [DOI] [PubMed] [Google Scholar]
- [23].Ziv-Polat O, Skaat H, Shahar A, Margel S, Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering, Int J Nanomedicine 2012, pp. 1259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ziv-Polat O, Shahar A, Levy I, Skaat H, Neuman S, Fregnan F, Geuna S, Grothe C, Haastert-Talini K, Margel S, The role of neurotrophic factors conjugated to iron oxide nanoparticles in peripheral nerve regeneration: in vitro studies, Biomed Res Int 2014 (2014) 267808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rose JC, Cámara-Torres M, Rahimi K, Köhler J, Möller M, Laporte LD, Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Atala A, Lanza R, Thomson J, Nerem R, Principles of Regenerative Medicine, 3 ed., Elsevier Science; 2018. [Google Scholar]
- [27].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, The Extracellular Matrix of Animals, Molecular Biology of the Cell, Garland Science, New York, 2002. [Google Scholar]
- [28].Leach JB, Bivens KA, P. CW Jr., Schmidt CE, Photocrosslinked Hyaluronic acid Hydrogels: Natural, Biodegradable Tissue Engineering Scaffolds, Biotechnology and bioengineering 825 (2003) 578–89. [DOI] [PubMed] [Google Scholar]
- [29].Suri S, Schmidt CE, Cell-laden hydrogel constructs of hyaluronic acid, collagen and laminin for neural tissue engineering, Tissue Engineering Part A 165 (2010). [DOI] [PubMed] [Google Scholar]
- [30].Suri S, Schmidt CE, Photopatterned Collagen-Hyaluronic acid interpenetrating polymer network hydrogels, Acta Biomaterialia 57 (2009) 2385–397. [DOI] [PubMed] [Google Scholar]
- [31].Thomas RC, Chung PE, Modi SP, Hardy JG, Schmidt CE, Sacrificial crystal templating of hyaluronic acid-based hydrogels, European Polymer Journal 87 (2017) 487–496. [Google Scholar]
- [32].Sieminski AL, Hebbel RP, Gooch KJ, The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro, Exp Cell Res 297(2) (2004) 574–84. [DOI] [PubMed] [Google Scholar]
- [33].Haessler U, Teo JC, Foretay D, Renaud P, Swartz MA, Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber, Integr Biol (Camb) 4(4) (2012) 401–9. [DOI] [PubMed] [Google Scholar]
- [34].Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S, Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices, Biomaterials 31(7) (2010) 1875–84. [DOI] [PubMed] [Google Scholar]
- [35].Zhou W, Blewitt M, Hobgood A, Willits RK, Comparison of neurite growth in three dimensional natural and synthetic hydrogels, J Biomater Sci Polym Ed 24(3) (2013) 301–14. [DOI] [PubMed] [Google Scholar]
- [36].Garcia A, Lacko C, Snyder C, Bohorquez A, Schmidt C, Rinaldi C, Processing-size correlations in the preparation of magnetic alginate microspheres through emulsification and ionic crosslinking, Colloids and Surfaces A: Physicochemical and Engineering Aspects 529 (2017) 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Taylor SE, Cao T, Talauliker PM, Lifshitz J, Objective Morphological Quantification of Microscopic Images Using a Fast Fourier Transform (FFT) Analysis, Curr Protoc Essent Lab Tech 95(Suppl 7) (2013) 9.5.1–9.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rasband WS, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. [Google Scholar]
- [39].O'Connell B, Oval Profile Plot ImageJ Plugin, 2002–2012. [Google Scholar]
- [40].Stewart DC, Rubiano A, Dyson K, Simmons CS, Mechanical characterization of human brain tumors from patients and comparison to potential surgical phantoms, PLoS One 12(6) (2017) e0177561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Williams LR, Longo FM, Powell HC, Lundborg G, Varon S, Spacial-Temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay, Journal of Comparative Neurology 218(4) (1983) 460–470. [DOI] [PubMed] [Google Scholar]
- [42].Panseri S, Cunha C, Lowery J, D el Carro U, Taraballi F, Amadio S, Vescovi A, Gelain F, Electrospun micro- and nano- fiber tubes for functional nervous regeneration in sciatic nerve transections, BMC Biotechnol 8 (2008) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xie J, Liu W, Macewan M, Bridgman P, Xia Y, Neurite outgrowth on electrospun nanofibers with uniaxial alignment: the effects of fiber density, surface coating, and supporting substrate, ACS Nano 8 (2014) 1878 – 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim Y, Haftel VK, Kumar S, Bellamkonda RV, The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps, Biomaterials 29(21) (2008) 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ceballos D, Navarro X, Dubey N, Wendelschafer-Crabb G, Kennedy WR, Tranquillo RT, Magnetically Aligned Collagen Gel Filling a Collagen Nerve Guide Improves Peripheral Nerve Regeneration, Experimental Neurology 158(2) (1999) 290–300. [DOI] [PubMed] [Google Scholar]
- [46].Treml H, Woelki S, Kohler H, Theory of capillary formation in alginate gels, Chem Phys 293 (2003) 341 – 353. [Google Scholar]
- [47].Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC, 3D Printed Anatomical Nerve Regeneration Pathways, Adv Funct Mater 25(39) (2015) 6205–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stokols S, Tuszynski M, The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury, Biomaterials 25 (2004) 5839 – 5846. [DOI] [PubMed] [Google Scholar]
- [49].Shahriari D, Shibayama M, Lynam DA, Wolf KJ, Kubota G, Koffler JY, Tuszynski MH, Campana WM, Sakamoto JS, Peripheral nerve growth within a hydrogel microchannel scaffold supported by a kink-resistant conduit, J Biomed Mater Res A 105(12) (2017) 3392–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Okeson CD, Riley MR, Riley-Saxton E, In vitro alveolar cytotoxicity of soluble components of airborne particulate matter: effects of serum on toxicity of transition metals, Toxicology in Vitro 18(5) (2004) 673–680. [DOI] [PubMed] [Google Scholar]
- [51].Pisanic II TR, Blackwell JD, Shubayev VI, Finones RR, Jin S, Nanotoxicity of iron oxide nanoparticle internalization in growing neurons, Biomaterials 28(16) (2007) 2572–2581. [DOI] [PubMed] [Google Scholar]
- [52].Burdick JA, Chung C, Jia X, Randolph MA, Langer R, Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks, Biomacromolecules 6(1) (2005) 386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vercruysse KP, Marecak DM, Marecak JF, Prestwich GD, Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid, Bioconjug Chem 8(5) (1997) 686–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.