The mesolimbic dopamine system is at the center of information processing and sends projections throughout the brain that have been linked to reward, motivation, salience, novelty, and the regulation of affective states (1). This system has been a major focus of psychiatric disease research given the critical role it plays in mood disorders - including anxiety disorders - where its dysregulation has been linked to the expression of negative affective states in human and animal models (2,3). Therefore, a significant effort has focused on understanding how the dopamine system encodes information, and how dopamine projections balance competing information to drive the expression of adaptive and maladaptive behaviors.
The rapid advancement of circuit-based techniques in the neuroscience field has allowed us to begin parsing the complex information encoded within specific projection populations. With this unprecedented resolution, we can now define how dopamine release influences microcircuits in downstream brain regions to drive behavior. While a large amount of effort has focused on ventral tegmental area (VTA) projections to regions like the prefrontal cortex, ventral striatum, hippocampus, and amygdala, less well understood is the effect of dopamine released into neighboring regions in the midbrain – like the interpeduncular nucleus (IPN). The IPN is ventral to the VTA, and dopaminergic processes originating from VTA neurons can be visualized in this region (4). While VTA projections to reward-related brain regions such as the ventral striatum have been associated with valence processing and motivation, the IPN has been linked to opposing actions such as reduced motivation, aversion, and negative affective states (5,6). The manuscript by DeGroot et al. (2020) combines a wide range of tools to elegantly parse how dopamine released into the IPN from neighboring VTA neurons affects IPN physiology to drive anxiety-like behavior.
This manuscript establishes the precise receptor-based mechanisms that mediate dopaminergic regulation of the IPN to control the expression of anxiety-like behavior (4). Using the fluorescent dopamine sensor GRABDA2m to visualize spatially defined dopamine release with optical imaging, DeGroot et al. (2020) showed that dopamine levels are elevated in the IPN after a pharmacological challenge with the dopamine agonist amphetamine, indicating that the processes from VTA neurons that extend into the IPN do in fact release dopamine in this area. Then using in vivo optogenetics to evoke and inhibit dopamine release from these VTA→IPN processes, they showed that excitation increased - while inhibition reduced - anxiety-like behavior, leaving motor behavior unaffected. Thus, dopamine release in the IPN from VTA dopamine neurons is a potent regulator of the expression of anxiety-like behavior.
The next critical question hinged on defining how dopamine influences neuronal activity in the IPN to drive behavior. Using ex vivo electrophysiology in the ventral IPN (vIPN) they identified two distinct neuronal populations: one that was excited by exogenous dopamine application and one where activity was reduced – termed Type A and Type B neurons, respectively. Both effects were mediated by alterations in excitatory pre-synaptic release probability and were abolished by the application of a D1, but not a D2, dopamine receptor antagonist. The divergent response of two different neuronal populations, both controlled by D1 receptors, suggested an upstream cellular population could be involved in coordinating these disparate downstream responses.
The vIPN receives innervation from GABAergic projection populations originating in the caudal (cIPN), thus they posited that dopamine could be influencing cIPN neurons that in turn regulate downstream Type A and Type B neurons. First, they identified a GABAergic D1 receptor expressing population of neurons in the cIPN (Type C) that, when optically activated, recapitulated the effects of bath applied dopamine in the vIPN, suggesting that dopamine is acting via this cell population. Using pharmacological approaches, they confirmed that these D1 receptor-expressing Type C cIPN neurons synapse in the vIPN, are activated by dopamine through D1 receptors, and modulate excitatory presynaptic inputs into Type A and Type B neurons via GABA release. Previous work has shown that GABAB receptors can promote excitatory neurotransmitter release from presynaptic terminals in some circuits (7); thus, in this model GABAB receptors on Type A inputs enhance glutamate release and while GABA receptors on Type B inputs reduce release. They then specifically linked this D1-receptor-based mechanism to the expression of anxiety-like behavior, showing that a D1 receptor agonist was anxiogenic and D1 antagonist was anxiolytic when infused into the IPN.
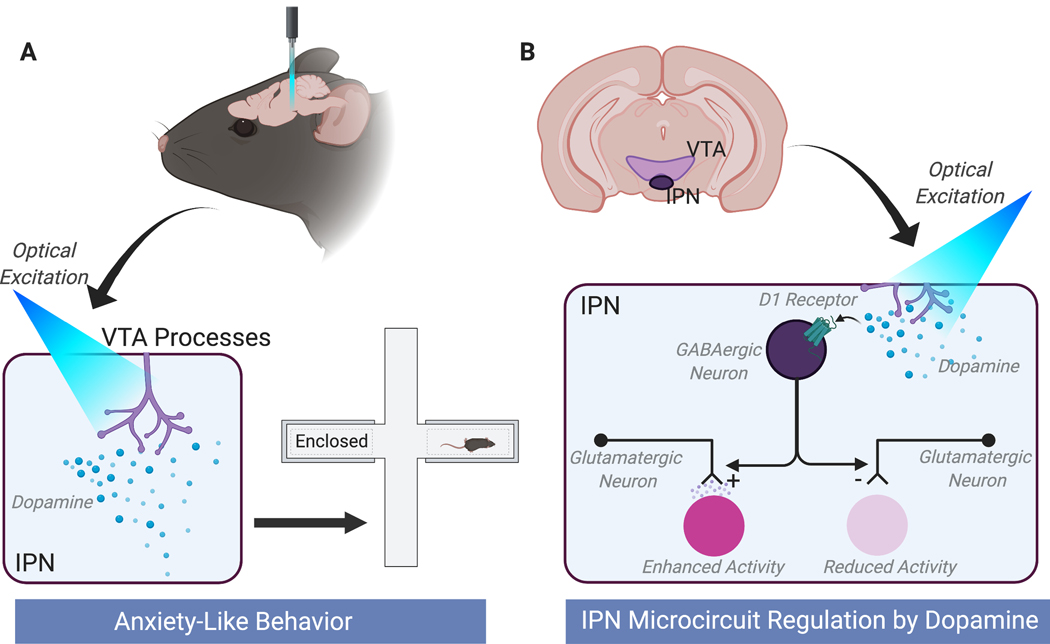
Together, these studies outline a circuit whereby VTA processes in the IPN release dopamine which activates dopamine D1 receptors to modulate the activity of a GABAergic neuronal population in the cIPN (Type C). Activation of these neurons then alters the activity of the vIPN through differentially regulating pre-synaptic release from excitatory inputs onto downstream neurons – increasing the activity of Type A neurons and decreasing the activity of Type B. The production of this circuit patten in the IPN ultimately drives the expression of anxiety-like behavior (Figure 1).
Figure 1. An interpeduncular nucleus (IPN) microcircuit is regulated by dopamine to drive anxiety-like behavior.
DeGroot et al. (2020) present an elegant set of studies showing that increasing dopamine in the IPN regulates microcircuit activity to drive the expression of anxiety-like behavior. (A) Optically activating ventral tegmental area (VTA) processes in the IPN increased anxiety-like behavior in rodents. Inhibition of these processes had an anxiolytic effect (not shown). (B) Proposed circuit mechanism of dopaminergic regulation of the IPN. Dopamine released in the caudal IPN (cIPN) activates D1 receptors on GABAergic neurons (Type C neurons) that project to the ventral (vIPN) to regulate presynaptic release probability of excitatory inputs. Depending on the input this either increases (left) or decreases (right) release probability to increase (Type A neurons) or decrease (Type B neurons) cellular activity in two morphologically distinct cellular populations downstream. The authors suggest that this occurs through a monosynaptic mechanism where GABAB receptors on Type A inputs enhance glutamate release probability – an effect that has been observed in other circuits (7) -, while GABA receptors on Type B inputs reduce release probability. Created with BioRender.com
These data clearly establish a potent role of dopamine in the IPN in regulating IPN circuit activity. Another major question moving forward is whether this represents a defined projection (VTA→IPN) or whether dopamine released into the IPN occurs through other non-canonical dopamine release mechanisms. In addition to classical vesicular release from axons, dopamine neurons in the VTA exhibit dopamine release from their cell bodies and dendrites – termed somatodendritic release (8). This dopamine release is fundamentally different from axonal release. It is regulated by different release machinery and is differentially sensitive to typical release regulators like calcium (8). Previous work, has shown that in the substantia nigra, dendritically released dopamine acts through a similar mechanism to the work outlined here, where dopamine acts at D1 receptors on the terminals of striatonigral axons to enhance GABA release and amplify inhibition of the principle cells of the substantia nigra (9). Thus, it will be important to understand whether this is a defined projection population whereby the VTA sends axons to the IPN or whether somatodendritic release into the IPN – which is directly adjacent to the VTA - acts as a feedback mechanism following the activation of neurons that send axons to other regions.
In fact, there were a number of experiments included within this study that suggest that dopaminergic projections into the IPN originate from neurons that also project to the nucleus accumbens. Using a retrograde traveling adeno-associated virus (AAV), cell bodies in the VTA were labeled based on their projection targets. Injections into the nucleus accumbens that resulted in the labeling of VTA neurons in close proximity to the IPN allowed for tracing of processes from these cells into the IPN. However, when less proximal populations were labeled (following injections into the striatum), VTA→IPN processes were not visible. These data suggest that IPN processes originate from accumbal projecting VTA neurons with cell bodies proximal to the IPN. Thus, it is possible that dopamine release in the IPN serves as a negative regulatory mechanism to balance information gated through accumbal projections.
From a behavioral perspective, it will be important to define exactly what other environmental factors influence IPN dopamine release to drive behavior. There is evidence that – in addition to promoting anxiety - IPN activation influences novelty-based learning (6). Therefore, to understand what dopamine release in the IPN ultimately encodes, it will be necessary to understand how activation and inhibition of dopamine release in the IPN influence behavior across a wide range of stimuli that span valence, novelty, and behavioral control. These factors are often co-occurring in behavioral tasks, so it will be particularly important to use behavioral tasks designed to dissociate these factors from one another to gain a comprehensive understanding of exactly how dopamine in the IPN responds to stimuli in the environment to ultimately drive behavior (10). Even within the realm of aversive processing it will be important to outline how dopamine release in the IPN maps onto defined behavioral responses in specific contexts, and whether release drives behavior or is purely associated with the generation of negative affective states.
Lastly, this work outlines a precise mechanism by which dopamine regulation of the IPN results in anxiety-like behavior. Thus, this novel and well-defined circuit provides an optimal framework for assessing how differences in D1 receptor expression, physiological activity of Type A, B, or C neurons, or dopamine release in the IPN can confer vulnerability to the development of anxiety-disorders. Similarly, plasticity induced by repeated stress exposure in any part of this pathway could serve as a key mechanism that ultimately leads to the development of anxiety. Also, given that preventing dopamine release in the IPN had an anxiolytic effect, this region could underlie both susceptibility and resilience to anxiety.
Together, DeGroot et al. (2020) outline how bi-directional modulation of VTA processes in the IPN controls the expression of anxiety-like behavior in rodents. They then outline the precise circuit-based mechanisms by which dopamine exerts these effects. This well-executed series of studies expands our understanding of how distinct dopamine projections underlie different behavioral processes depending on their projection target. This also will serve as a foundation for better understanding the neural dysfunction that underlies the expression and development of anxiety-disorders.
Acknowledgements:
This work was supported by NIH grants DA042111, DA048931, funds from the Brain and Behavior Research Foundation, the Whitehall Foundation and the Edward Mallinckrodt, Jr. Foundation.
Footnotes
Financial Disclosures: Dr. Calipari reported no biomedical financial interests or potential conflicts of interest
References:
- 1.Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010): Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TMK, et al. (2011): Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety [no. 5]. Nature Neuroscience 14: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang G-J, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. (1994): Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16: 255–262. [DOI] [PubMed] [Google Scholar]
- 4.DeGroot SR, Zhao-Shea R, Chung L, Klenowski PM, Sun F, Molas S, et al. (2020): Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, et al. (2018): Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat Commun 9 10.1038/s41467-018-04654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molas S, Zhao-Shea R, Liu L, DeGroot SR, Gardner PD, Tapper AR (2017): A circuit-based mechanism underlying familiarity signaling and the preference for novelty. Nat Neurosci 20: 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, et al. (2016): Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell 166: 716–728. [DOI] [PubMed] [Google Scholar]
- 8.Rice ME, Patel JC (2015): Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc Lond B Biol Sci 370 10.1098/rstb.2014.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radnikow G, Misgeld U (1998): Dopamine D1 Receptors Facilitate GABAASynaptic Currents in the Rat Substantia Nigra Pars Reticulata. J Neurosci 18: 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutlu MG, Zachry JE, Brady LJ, Melugin PR, Kelly SJ, Sanders C, et al. (2020): A novel multidimensional reinforcement task in mice elucidates sex-specific behavioral strategies. Neuropsychopharmacology 45: 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]