Abstract
Introduction
Establishing efficacy of and molecular pathways for statins has the potential to impact incidence of Alzheimer's and age‐related neurodegenerative diseases (NDD).
Methods
This retrospective cohort study surveyed US‐based Humana claims, which includes prescription and patient records from private‐payer and Medicare insurance. Claims from 288,515 patients, aged 45 years and older, without prior history of NDD or neurological surgery, were surveyed for a diagnosis of NDD starting 1 year following statin exposure. Patients were required to be enrolled with claims data for at least 6 months prior to first statin prescription and at least 3 years thereafter. Computational system biology analysis was conducted to determine unique target engagement for each statin.
Results
Of the 288,515 participants included in the study, 144,214 patients (mean [standard deviation (SD)] age, 67.22 [3.8] years) exposed to statin therapies, and 144,301 patients (65.97 [3.2] years) were not treated with statins. The mean (SD) follow‐up time was 5.1 (2.3) years. Exposure to statins was associated with a lower incidence of Alzheimer's disease (1.10% vs 2.37%; relative risk [RR], 0.4643; 95% confidence interval [CI], 0.44–0.49; P < .001), dementia 3.03% vs 5.39%; RR, 0.56; 95% CI, 0.54–0.58; P < .001), multiple sclerosis (0.08% vs 0.15%; RR, 0.52; 95% CI, 0.41–0.66; P < .001), Parkinson's disease (0.48% vs 0.92%; RR, 0.53; 95% CI, 0.48–0.58; P < .001), and amyotrophic lateral sclerosis (0.02% vs 0.05%; RR, 0.46; 95% CI, 0.30–0.69; P < .001). All NDD incidence for all statins, except for fluvastatin (RR, 0.91; 95% CI, 0.65‐1.30; P = 0.71), was reduced with variances in individual risk profiles. Pathway analysis indicated unique and common profiles associated with risk reduction efficacy.
Discussion
Benefits and risks of statins relative to neurological outcomes should be considered when prescribed for at‐risk NDD populations. Common statin activated pathways indicate overarching systems required for risk reduction whereas unique targets could advance a precision medicine approach to prevent neurodegenerative diseases.
Keywords: age, Alzheimer's disease, amyotrophic lateral sclerosis, bioinformatics, biology pathway analysis, cholesterol, multiple sclerosis, neurodegenerative diseases, Parkinson's disease, statins
1. INTRODUCTION
It is estimated that nearly 100 million Americans are afflicted by at least one neurological disease, costing 800 million dollars per year in the United States. 1 As the elderly segment of the population grows, the number of patients and cost will increase. 1 The prevalence of Alzheimer's disease (AD) in the United States is currently 5.8 million patients, 2 with an additional 2.2 million suffering from other forms of dementia 1 that combined account for 2.4% of the general population. 1 Multiple sclerosis (MS) and Parkinson's disease (PD) each affect approximately 1 million persons in the United States 3 , 4 and amyotrophic lateral sclerosis (ALS) affects 18,000 people. 1
In the face of the increasing incidence of age‐related neurodegenerative diseases (NDD), 1 , 2 , 5 we conducted this study to identify currently prescribed therapeutics that may alter NDD risk and their biological pathways. Specifically, several studies have suggested an association between hypercholesterolemia and dementia 6 , 7 , 8 , 9 and AD. 6 , 7 , 10 , 11 , 12 , 13
The drug class of 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase (HMGCR) inhibitors, commonly known as statins, are the primary pharmacological treatment for prevention and lowering cholesterol levels in blood. 6 , 7 , 13 , 14 , 15 As such, statins are the first‐line treatment for hyperlipidemia and prevention of coronary heart disease. 10 , 14 , 15 , 16 , 17 , 18 Statins are potent inhibitors of cholesterol biosynthesis via their primary mechanism of inhibiting HMGCR; 15 however, it is well known that statin therapies have pleiotropic effects in multiple biological pathways. 7 , 10 , 19 , 20 , 21 , 22 There are currently three generations of HMGCR inhibitors on the market: the first generation of statins includes lovastatin, pravastatin, and fluvastatin; the second generation includes simvastatin and atorvastatin; and rosuvastatin and pitavastatin constitute the third‐generation statins. 23
Worldwide, statins are the most prescribed cholesterol‐lowering medication and recent data suggest that 27.9% of adults aged 40 years old or older are on statin therapy. 14 In 2012–2013, 39.2 million individuals—accounting for 221 million prescriptions—were using statins, generating $16.9 billion in U.S. sales. 24 Multiple studies report an association between statin use and AD, 10 , 11 , 12 , 13 , 20 dementia, 7 , 21 , 25 , 26 MS, 27 , 28 PD, 29 , 30 and ALS. 31 , 32 Current meta‐analyses for AD, 21 , 33 dementia, 21 MS, 34 PD, 35 and ALS 36 suggest that statin use is associated with NDD risk decrements, except for ALS—as the evidence is insufficient to draw any conclusion—and all call for the addition of further studies to add to the existent literature.
Analyses reported herein were designed to determine potential associations between statin therapies and NDD risk. Our study was conducted using a United States–based population insurance claims records data set and a large patient population to survey a variety of NDD outcomes and their association with statin exposure. Furthermore, we report the association of individual statin types with each NDD outcome and we describe common and divergent biological networks in an attempt to understand the mechanism of risk reduction.
2. METHODS
2.1. Data source
The Humana database as described in Branigan et al. 37 was used for this study. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study was approved by the University of Arizona Institutional Review Board. Requirements for informed consent were waived because the data were deidentified.
2.2. Study design and variables
A subset of 1,959,483 patients with non‐melanoma skin cancer was selected from the Humana dataset. The statin exposure group is defined as patients with a medication charge for any of the HMGCR inhibitors (Table S1 in supporting information). The non‐statin exposure group are the patients without any medication charges for the above drugs. The outcome variable was defined as the occurrence of the first NDD diagnosis for each outcome of interest based on International Classification of Diseases, Ninth Revision (ICD‐9), Clinical Modification and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Procedure Coding System codes in the patient's medical claims data. NDD includes AD, dementia, MS, PD, and ALS (Table S3 in supporting information). Age in the statin exposure group is defined by the age at diagnosis of first statin exposure. Following the analysis in Branigan et al., 37 an analysis of comorbidities known to be associated with NDD outcomes was conducted (Table S3).
2.3. Statistical analysis
Statistical analyses were conducted between February 6 and May 9, 2020. Patient demographic statistics (Table 1) and incidence statistics were analyzed using unpaired two‐tailed t‐tests or χ2 tests, as appropriate, to test the significance of the differences between continuous and categorical variables. In all analyses, a two‐sided P < .05 was considered statistically significant.
TABLE 1.
Baseline characteristics for propensity score–matched patients with or without statin exposure
| Propensity score matched * | |||||
|---|---|---|---|---|---|
| Without statin exposure | With statin exposure | ||||
| n | % | n | % | P‐Value | |
| Number of patients | 144,301 | 144,214 | |||
| Age | |||||
| 45 to 49 | 11,764 | 8.15% | 6157 | 4.27% | 0.99 |
| 50 to 54 | 10,991 | 7.62% | 7523 | 5.22% | |
| 55 to 59 | 11,293 | 7.83% | 9844 | 6.83% | |
| 60 to 64 | 9175 | 6.36% | 10,623 | 7.37% | |
| 65 to 69 | 35,097 | 24.32% | 37,460 | 25.98% | |
| 70 to 74 | 27,748 | 19.23% | 33,170 | 23.00% | |
| 75 to 79 | 18,073 | 12.52% | 21,233 | 14.72% | |
| 80 to 84 | 11,300 | 7.83% | 11,169 | 7.74% | |
| 85 to 89 | 2343 | 1.62% | 2717 | 1.88% | |
| 90 and over | 6517 | 4.52% | 4318 | 2.99% | |
| Sex | |||||
| Female | 82,584 | 57.23% | 75,773 | 52.54% | 0.99 |
| Male | 61,717 | 42.77% | 68,441 | 47.46% | |
| Ethnicity | |||||
| White | 105,254 | 72.94% | 115,135 | 79.84% | 0.99 |
| Black | 3269 | 2.27% | 5561 | 3.86% | |
| Asian | 258 | 0.18% | 494 | 0.34% | |
| Hispanic | 774 | 0.54% | 1593 | 1.10% | |
| Other | 887 | 0.61% | 1316 | 0.91% | |
| Unknown | 33,859 | 23.46% | 20,115 | 13.95% | |
| Region | |||||
| Midwest | 33,456 | 23.18% | 28,916 | 20.05% | 0.99 |
| Northeast | 3053 | 2.12% | 2663 | 1.85% | |
| South | 91,930 | 63.71% | 98,772 | 68.49% | |
| West | 15,862 | 10.99% | 13,863 | 9.61% | |
| Comorbidities | |||||
| T2DM | 3817 | 2.65% | 9536 | 6.61% | 0.003 |
| HTN | 12,102 | 8.39% | 13,550 | 9.40% | |
| CVD | 2469 | 1.71% | 8901 | 6.17% | |
| STROKE | 2361 | 1.64% | 7274 | 5.04% | |
| CKD | 3500 | 2.43% | 9256 | 6.42% | |
| COPD | 2192 | 1.52% | 4741 | 3.29% | |
| CCI | |||||
| 0–4 | 117,275 | 81.27% | 138,329 | 95.92% | 0.99 |
| 5–10 | 24,743 | 17.15% | 5500 | 3.81% | |
| 11+ | 2283 | 1.58% | 385 | 0.27% | |
Abbreviations: CCI, Charlson Comorbidity Index.; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HTN, hypertension; T2DM, type‐2 diabetes mellitus.
Adjusted for age, sex, region, comorbidities and CCI.
To estimate the association between statin and NDD, a propensity score–matched population was generated as previously described 37 to account for confounding variables between treatment/control group assignment and NDD outcomes. Logistic regression was first used to estimate the probability for each subject to receive statin therapy given their age, sex, race, region, comorbidities of interest, and Charlson Comorbidity Index (CCI) score. The propensity score algorithm incorporated demographic variables and the comorbidities that were statistically significant in the regression model: age, sex, race, region, type 2 diabetes (T2DM), hypertension (HTN), cardiovascular disease (CVD), cerebrovascular disease and related conditions (STROKE), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD). In this study, only the propensity score–matched population was included as the study group (Figure 1) for the purpose of statistical reporting and analysis. Cumulative hazard ratios were created using the propensity score–matched population in the Bellwether‐PearlDiver interface. Median adherence rates for each statin group were calculated as previously described. 37
FIGURE 1.
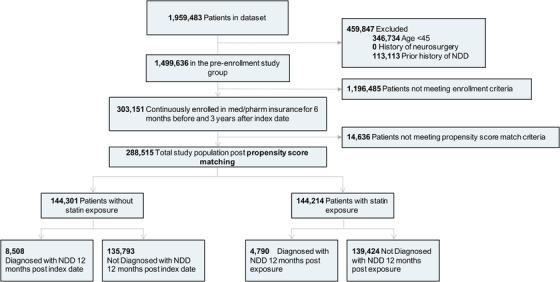
Study design and patient breakdown. Abbreviation: NDD, neurodegenerative disease
RESEARCH IN CONTEXT
Systematic review: To contextualize research reported herein, the authors mined existing resources including PubMed, Google Scholar, Gene Ontology, STRING, and DrugBank. Although previous reports indicate that statins are associated with reduced risk of Alzheimer's disease, a mechanistic understanding of off‐target neurological pathways and consideration of different classes of statins and their impact on age‐related neurodegenerative diseases remain to be considered. Unique and common mechanisms across different statin therapies could account for differences in efficacy to reduce risk.
Interpretation: Evidence provided herein indicates that statins significantly mitigate neurodegenerative disease risk. Differences in risk reduction profiles for each statin are reported. Computational system biology pathway analysis indicated unique and common mechanisms associated with risk reduction efficacy.
Future directions: Clinical and preclinical research to elucidate unique neurological pathways activated by statins could advance a precision medicine approach to prevent neurodegenerative diseases of aging, especially in at‐risk populations.
HIGHLIGHTS
Statins are associated with decreased incidence of Alzheimer's disease, dementia, Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Each statin lowered the incidence of neurodegenerative diseases (NDD) with the exception of fluvastatin.
Pitavastatin and atorvastatin exerted greatest reduction of NDD diagnosis.
Unique and common pathways of statins were associated with risk reduction profile.
Unique statin targets could advance a precision medicine approach to prevent NDD.
2.4. Pathway analysis
Biological pathway analysis was conducted using network‐based approach. First, for each statin identified, the related gene targets were extracted using DrugBank database. 38 Next, the gene targets were subsequently used to seed a protein‐protein interaction (PPI) network, which extract protein interactors of the target gene and obtain a comprehensive overview of the statin actions. In this step, the STRING database was used to extract PPI; 39 only high‐confidence PPIs were retrieved, ie, PPIs derived from only experimental and database evidence and with a STRING score cut‐off of 700. 40 , 41 Finally, for each drug, enrichment analysis of the related targets and their first protein interactors was performed to identify significant (P‐value < .05) gene ontology biological processes (GO‐BP) characteristic of each statin. GO‐BP enrichment was further analyzed to exclude redundant and similar GO‐BP terms. To this goal, we computed the semantic similarity between each GO‐BP term resulting in the enrichment using Python package GOATOOLS 42 and filtered for redundant GO‐BPs following the reported similarity threshold process. 43 Results were finally compared across the different type of statins to identify specific and common pathways and mechanisms of action.
3. RESULTS
Of 1,959,483 patients in the dataset, 288,515 patients met the inclusion, exclusion, enrollment, and propensity score–matching criteria (Figure 1). All analyses in the control group matched those defined in the statin exposure group (including time‐based analysis) based on patient demographics and predefined NDD‐relevant comorbidities. 44 , 45 , 46 , 47 , 48
The index dates were selected as the first date of a statin prescription for the treatment group and 6 months after the first patient claim record in the database for the control group. The 6 months prior to the index date was used to calculate the baseline comorbidities for both groups. A date of 1 year after the index date was assigned as the study start date to survey records for NDD diagnosis.
Of the 288,602 patients enrolled in the study, 144,214 (mean [standard deviation (SD)] age, 67.22 [3.8] years) received statins and 144,301 (mean [SD] age, 65.97 [3.2] years) were not treated with statins (Figure 1). The number of patients in each individual statin therapy group and the median adherence rate for each drug are described in Table S1. The statin exposure group includes generic and name brand drug codes for every individual statin, reported in Table S2 in supporting information.
Patients in the study ranged between 45 and 90 years of age or older with a median range from 65 to 80 years old and were predominantly identified as White with the greatest number of records coming from the southern United States, as the Humana dataset was constructed based on insurance network coverage (Table 1). There were no statistical differences between the statin exposure group and the non‐statin exposure group in terms of age, sex, region, or CCI. The mean (SD) follow‐up time was 5.1 (2.3) years.
Although the CCI was not statistically significantly different, the NDD‐relevant comorbidity profile was significantly different between patients who received statins and those who did not (diabetes, 9536 of 144,214 [6.61%] vs 3817 of 144,301 [2.65%]; hypertension, 13,550 of 144,214 [9.40%] vs 12,102 of 144,301 [8.39%]; cardiovascular disease, 8901 of 144,214 [6.17%] vs 2469 of 144,301 [1.71%]; stroke, 7274 of 144,214 [5.04%] vs 2361 of 144,301 [1.64%]; chronic kidney disease, 9256 of 144,214 [6.42%] vs 3500 of 144,301 [2.43%]; chronic obstructive pulmonary disease, 4741 of 144,214 [3.29%] vs 2192 of 144,301 [1.52%]; Table 1).
Analysis of the propensity score–matched population data showed that statin exposure compared to control was associated with a significant decrease in the incidence of AD (1463 of 132,990 [1.10%] vs 3151 of 132,990 [2.37%]; relative risk [RR], 0.4643; 95% confidence interval [CI], 0.44–0.49; P < .001), dementia (4029 of 132,990 [3.03%] vs 7164 of 132,990 [5.39%]; relative risk [RR], 0.56; 95% CI, 0.54–0.58; P < .001), MS (106 of 132,990 [0.08%] vs 203 of 132,990 [0.15%]; RR, 0.52; 95% CI, 0.41–0.66; P < .001), PD (645 of 132,990 [0.48%] vs 1221 of 132,990 [0.92%]; RR, 0.53; 95% CI, 0.48–0.58; P < .0001), ALS (33 of 132,990 [0.02%] vs 72 of 132,990 [0.05%]; RR, 0.46; 95% CI, 0.30–0.69; P < .001; Table 2). These data are represented in Figure 2A for each of the individual NDD, as well as the combined NDD group.
TABLE 2.
Relative risk of propensity score matched patients developing NDDs after receiving statins
| All NDD combined | AD | Dementia | MS | PD | ALS | |
|---|---|---|---|---|---|---|
| Without statin exposure | ||||||
| # Patients | 8508 | 3151 | 7164 | 203 | 1221 | 72 |
| % | 6.40% | 2.37% | 5.39% | 0.15% | 0.92% | 0.05% |
| With statin exposure | ||||||
| # Patients | 4790 | 1463 | 4029 | 106 | 645 | 33 |
| % | 3.60% | 1.10% | 3.03% | 0.08% | 0.48% | 0.02% |
| Relative risk | 0.56 | 0.46 | 0.56 | 0.52 | 0.53 | 0.46 |
| 95%CI | 0.54 to 0.58 | 0.44 to 0.49 | 0.54 to 0.58 | 0.41 to 0.66 | 0.48 to 0.58 | 0.30 to 0.69 |
| NNT | 35.77 | 78.79 | 42.42 | 1371 | 230.9 | 3410 |
| P‐value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; CI, confidence interval; MS, multiple sclerosis; NDD, neurodegenerative diseases; NNT, number needed to treat; PD, Parkinson's disease.
FIGURE 2.
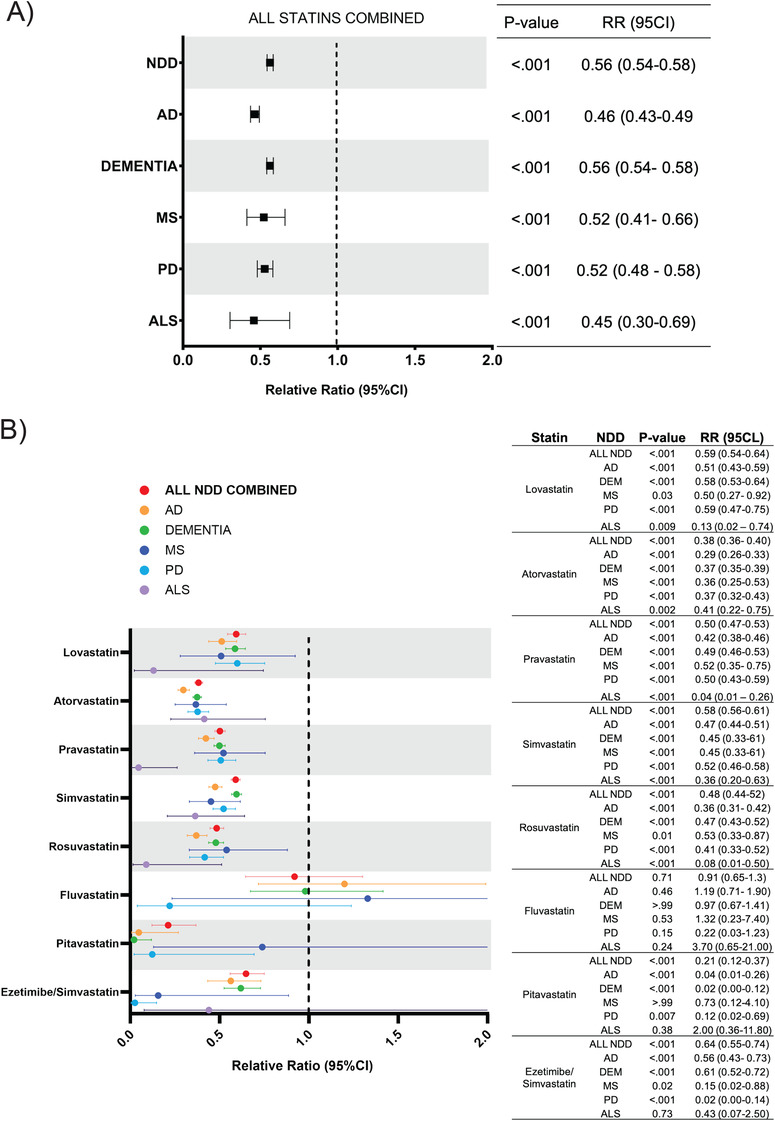
Relative risk of developing NDDs for patients receiving any statin versus individual statins. A, Risk ratio for each NDD based on exposure to any statin. B, Risk ratio for composite NDD group and every NDD for individual statin therapies. Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis; NDD, neurodegenerative diseases; PD, Parkinson's disease; RR, relative risk
To identify the effect of each individual statin, analysis of the incidence of each NDD with respect to an individual statin (eg, simvastatin) was conducted. The statin exposure group was subdivided into eight drugs: lovastatin (n = 14,185), atorvastatin (n = 53,554), pravastatin (n = 40,225), simvastatin (n = 71,140), rosuvastatin (n = 20,675), fluvastatin (n = 493), pitavastatin (n = 887), and ezetimibe/simvastatin (n = 4200).
The incidence for all NDD for every individual statin was reduced in the statin exposed group compared to control patients (Figure 2B). Pitavastatin showed the strongest reduction in NDD incidence (RR, 0.21; 95% CI, 0.12–0.37; P < .001), followed by atorvastatin (RR, 0.38; 95% CI, 0.36–0.40; P < .001), rosuvastatin (RR, 0.48; 95% CI, 0.44–0.52; P < .001), pravastatin (RR, 0.50; 95% CI, 0.47–0.53; P < .001), simvastatin (RR, 0.58; 95% CI, 0.56–0.61; P < .001), lovastatin (RR, 0.59; 95% CI, 0.54–0.64; P < .001), and ezetimibe/simvastatin (RR, 0.64; 95% CI, 0.55–0.74; P < .001). Fluvastatin showed no significant change in the associated risk of developing NDD (RR, 0.91; 95% CI, 0.65–1.30; P = 0.71).
The cumulative hazard ratios with 95% CI were generated from the propensity score–matched population for development of all NDD combined, AD, dementia, and PD to evaluate the rate of disease conversion for statin versus non‐statin exposed groups (Figure 3). Differences in the rate of disease conversion in the hazard ratios corroborate the results seen in the Chi‐square analysis.
FIGURE 3.
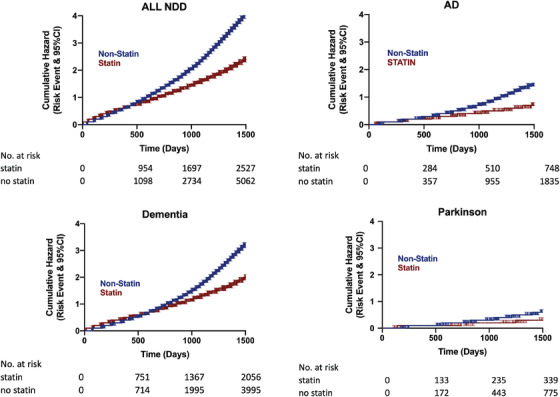
Hazard ratios for propensity score–matched patients for developing NDD, AD, dementia, PD. Abbreviations: AD, Alzheimer's disease; NDD, neurodegenerative diseases; PD, Parkinson's disease
To better understand the differences in the risk reduction efficacy profile for each statin, we used a systems biology approach to identify protein/gene pathways associated with each statin therapy (Figure 4). This analysis determined common versus unique biological mechanisms of action for each statin therapy. Consistent with their primary mechanism of action, each statin targeted HMGCR (Figure 4A). Based on non‐canonical statin targets four clusters emerged with each cluster associated with a unique set of first interactors. The first cluster included pitavastatin and rosuvastatin, which targeted integrin subunit alpha L (ITGAL), whose first interactors are predominantly intercellular adhesion molecules. The second cluster included fluvastatin and pravastatin that targeted histone deacetylase 2 (HDAC2), whose first interactors are involved in epigenetic regulation. The third cluster, comprising lovastatin and simvastatin, targeted both HDAC2 and ITGAL. Finally, atorvastatin was unique and represented the fourth cluster, which included the targets nuclear receptor subfamily 1 group I member 3 (NR1I3), aryl hydrocarbon receptor (AHR), dipeptidyl peptidase 4 (DPP4), in addition to HDAC2 (same as the second and third cluster), whose first interactors are associated with downstream signaling pathways.
FIGURE 4.
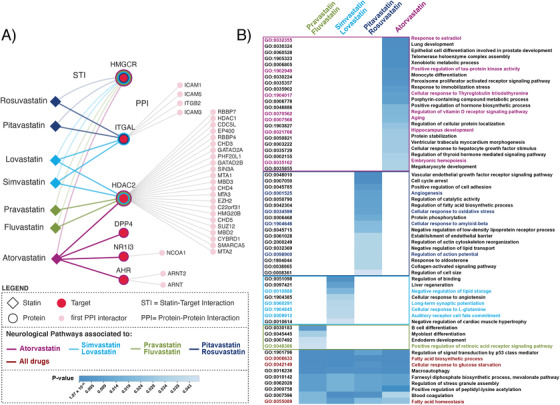
Gene ontology biological processes analysis: unique versus common. A, Protein‐protein interactors shown are of the highest confidence (STRING score >800). Extended interactome shown in Figure S1 in supporting information. B, Unique and common gene ontology terms of interest for statin clusters. Extended version in Figure S1
Pathway analysis using GO‐BP enrichment identified unique and common gene networks for each cluster (Figure 4B). For the purposes of this report, we focused on neurological pathways. Pitavastatin and rosuvastatin, the first cluster, included angiogenesis, cellular response to oxidative stress, cellular response to amyloid beta, and regulation of action potential. Fluvastatin and pravastatin, the second cluster, included positive regulation of retinoic acid receptor signaling as a relevant neurological pathway. Lovastatin and simvastatin, the third cluster, involved negative regulation of lipid storage, long‐term synaptic potentiation, cellular response to L‐glutamine, and auditory receptor cell fate commitment pathways. Last, atorvastatin, the fourth cluster, comprised response to estradiol, positive regulation of tau‐protein kinase activity, cellular response to thyroglobulin triiodothyronine, regulation of vitamin D receptor signaling, aging, hippocampus development, and embryonic hemopoiesis.
The common GO‐BP pathways for the four clusters are mainly involved in metabolic processes, such as fatty acid biosynthetic process and cellular response to glucose starvation (Figure 4B). Additionally, those statins that target ITGAL (first and third clusters) are involved in immunological pathways as regulation of immune response, inflammatory response, and antigen processing of exogenous peptide antigen via major histocompatibility complex class I (Figure S1 in supporting information). Statins that target HDAC2 gene (second, third, and fourth clusters) share pathways including Notch signaling; oligodendrocyte differentiation; positive regulation of gluconeogenesis; cellular response to hydrogen peroxide; transforming growth factor beta receptor signaling; and neurological pathways including layer formation in cerebral cortex, neuron maturation, neuron apoptotic process, cerebral cortex neuron differentiation, and smoothened signaling pathway involved in spinal cord motor neuron cell fate specification (Figure S1).
3.1. Limitations
As this study is a retrospective analysis of a claims database, there are several limitations. Importantly, patients included may have obtained services beyond those included in this dataset, such as lifestyle modifications, which are recommended as first‐line treatment in addition to the cholesterol‐lowering therapies. 18 This study used a claims dataset, which relies on the physician's diagnosis and the ICD code assigned to each patient presentation. Because the diagnosis is clinical, there may be overlap between AD and dementia codes given similar presentations despite different underlying pathophysiologies. Furthermore, there could be biases in the prescribing trends for statins that cannot be controlled in the model. Additionally, two statins (pitavastatin and fluvastatin) had fewer than 1000 claims; and select patient demographics (eg, socioeconomic status) are not commonly included in insurance claims and thus were not assessed. Finally, there could be factors, known and unknown, that may not be adequately addressed in this analysis despite propensity‐score matching.
4. DISCUSSION
This study investigated the association between statin therapies and their non‐canonical neurologically relevant mechanisms of action and their efficacy of risk reduction across multiple age‐related NDD. Results reported herein are consistent with previously published outcomes for statin therapy and a single NDD 7 , 10 , 11 , 12 , 13 , 14 , 20 , 21 , 25 , 27 , 28 , 31 , 32 , 33 , 34 , 35 , 36 , 49 thereby validating our findings.
Based on this foundation, we extended our analyses to include multiple statins and multiple NDDs along with their biological pathways to develop overall neurologic risk profiles. Further, our analysis sought to identify and describe differences in efficacy between each statin therapeutic for NDD risk reduction. The comparative analysis of statin efficacy indicated that all statins reduce the incidence risk of NDD, except for fluvastatin, which showed no statistically significant differences. Atorvastatin and pitavastatin showed the greatest risk reduction for NDD in the study. Of interest is the fact that pitavastatin is dosed on a different scale than the other statins (10, 20, 40, 80 mg)23 where lower doses (1, 2, and 4 mg) yield comparable cholesterol‐reduction profiles. 50 There is limited literature regarding blood–brain barrier (BBB) permeability for pitavastatin. Although the study suggests that pitavastatin may be beneficial for reducing risk for AD and dementia, the number of patients in the subgroup was small and requires further validation.
Further, we conducted a system biology analysis to determine common and unique mechanisms of NDD risk reduction. This approach enabled the identification of trends based on unique and common biological targets and enriched gene ontology pathways. Validating this approach, each statin targeted the HMGCR pathway. The GO‐BP enrichment analysis identified unique pathway profiles that categorized each statin into four different clusters. The assignment of statins into clusters was defined by shared gene ontology networks. Pathway profiles were consistent with comparable risk reduction profiles (overlapping 95% CI). For example, the cluster including lovastatin and simvastatin, which targets both ITGAL and HDAC2 genes, showed no significant differences for NDD relative risk reduction. This trend of similar cluster‐based risk reduction profiles was evident across multiple NDDs. In contrast, the group containing pravastatin and fluvastatin, which act on HDAC2 and share a common pathway profile, showed statistically different relative risk ratios, where fluvastatin was the only statin to not significantly reduce the risk of NDD. Of note, fluvastatin is known to have low level of permeability at the BBB, 20 which may explain, in part, the lack of significant effect.
The GO‐BP analysis identified potential pathways that underlie the protective profile of statins and the differences in risk reduction efficacy between statins. We hypothesize that the non‐canonical neurological targets represent pathways underlying the impact of each statin on NDD risk profile. Further, the unique pathways for each cluster may explain the differences across statins in relative risk reduction. Differences in the risk reduction profile within the same cluster may be explained, in part, through BBB permeability. Because lipid dysregulation is a common feature of NDD, the fact that all statins have a common target, HMGCR, corroborates common gene ontology pathways that may be responsible for the overall protective effect of statins on NDD.
This study aimed to establish the risk profile of statin therapeutics on the incidence of NDD. In addition, a unique biology pathways analysis was conducted to elucidate potential mechanisms underlying the differences between each statin profile. Each statin varied in its efficacy to reduce NDD incidence, except for fluvastatin, and interestingly these results paralleled the neurological pathways targeted by these drugs.
Results reported herein are based on a large clinical population from across the United States (Humana insurance claims), which allows for clinical translation relevant to NDD in at‐risk populations. Future clinical and pre‐clinical research is needed to elucidate biological mechanisms underlying statin therapies and development of NDD. With this foundation, a precision medication approach will be possible in which prescription guidelines of cholesterol‐lowering medication can be adapted for each population with respect to their neurological health profile. Common pathways indicate overarching systems required for risk reduction, whereas unique targets could advance a precision medicine approach to prevent and treat neurodegenerative diseases in genetic at‐risk and aging populations.
CONFLICTS OF INTEREST
Dr Brinton reported receiving grants from the Women's Alzheimer's Movement and the National Institute on Aging during the conduct of the study. No other disclosures were reported.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We would like to thank Patrick Ronaldson, Ph.D., for his insights regarding statin therapies and the blood–brain barrier.
This work was supported by the National Institute on Aging (grants P01AG026572 [Perimenopause in Brain Aging and Alzheimer's Disease], T32AG061897 [Translational Research in Alzheimer's Disease and Related Dementias (TRADD)], and R37AG053589 [Aging and Estrogenic Control of the Bioenergetic System in Brain]) and the Women's Alzheimer's Movement to Dr Brinton.
Torrandell‐Haro G, Branigan GL, Vitali F, Geifman N, Zissimopoulos JM, Brinton RD. Statin therapy and risk of Alzheimer's and age‐related neurodegenerative diseases. Alzheimer's Dement. 2020;6:e12108 10.1002/trc2.12108
REFERENCES
- 1. Gooch C, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479‐484. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2020 Alzheimer's disease facts and figures. Alzheimer's Dement. 2020:391‐460. [Google Scholar]
- 3. National Multiple Sclerosis Society. https://www.nationalmssociety.org/. Accessed November 6, 2020.
- 4. Parkinson's Foundation . https://www.parkinson.org/. Accessed November 6, 2020.
- 5. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time‐current evidence. Nat Rev Neurol. 2017;13(6):327‐339. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Tian Q, Liu D, et al. Causal association of circulating cholesterol levels with dementia: a mendelian randomization meta‐analysis. Transl Psychiatry. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loera‐Valencia R, Goikolea J, Parrado‐Fernandez C, Merino‐Serrais P, Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019;190:104‐114. [DOI] [PubMed] [Google Scholar]
- 8. Mcfarlane O, Kędziora‐Kornatowska K. Cholesterol and Dementia: A Long and Complicated Relationship. Curr Aging Sci. 2020;13(1):42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appleton JP, Scutt P, Sprigg N, Bath PM, Hypercholesterolaemia and vascular dementia. Clin Sci (Lond). 2017;131(14):1561‐1578. [DOI] [PubMed] [Google Scholar]
- 10. Daneschvar HL, Aronson MD, Smetana GW. Do statins prevent Alzheimer's disease? A narrative review. Eur J Intern Med. 2015;26(9):666‐669. [DOI] [PubMed] [Google Scholar]
- 11. Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer's disease. Alzheimer's Res Ther. 2017;9(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geifman N, Kennedy RE, Schneider LS, Buchan I, Brinton RD. Data‐driven identification of endophenotypes of Alzheimer ’ s disease progression : implications for clinical trials and therapeutic interventions. Alzheimers Res Ther. 2018;10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zissimopoulos JulieM, Barthold Douglas, Roberta Diaz Brinton GJ. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2017;3331(2):225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu Q, Paulose‐Ram R, Burt VL, Kit BK. Prescription cholesterol‐lowering medication use in adults aged 40 and over : United States, 2003 – 2012. NCHS Data Brief. 2014;(177):1‐8. [PubMed] [Google Scholar]
- 15. Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3‐11. [DOI] [PubMed] [Google Scholar]
- 16. Chou R, Dana T, Blazina I, et al. Evidence synthesis number 139 statin use for the prevention of cardiovascular disease in adults : a systematic review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US; ); 2016 Nov. (Evidence Syntheses, No. 139.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK396415/ [PubMed] [Google Scholar]
- 17. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263‐1282. [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285‐e350. [DOI] [PubMed] [Google Scholar]
- 19. Penkauskas T, Zentelyte A, Ganpule S, Valincius G, Preta G. BBA ‐ Biomembranes Pleiotropic effects of statins via interaction with the lipid bilayer : a combined approach. Biochim Biophys Acta Biomembr. 2020;1862(9):183306. [DOI] [PubMed] [Google Scholar]
- 20. Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68(11):1385‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, Wen J, Zhang Z. Statins use and risk of dementia: a dose–response meta analysis. Medicine (Baltimore). 2018;97(30):e11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao JK, Laufs U. Pleiotropic Effects of Statins. NIH Public Access. 2005;45:89‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapur NK, Musunuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc Health Risk Manag. 2008;4(2):341‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weintraub WS. Perspective on trends in statin use. JAMA Cardiol. 2017;2(1):11‐12. 10.1001/jamacardio.2016.4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegener. 2018;7(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith KB, Kang P, Sabbagh MN. The effect of statins on rate of cognitive decline in mild cognitive impairment. Alzheimer's Dement Transl Res Clin Interv. 2017;3(2):149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciurleo R, Bramanti P, Marino S. Role of statins in the treatment of multiple sclerosis. Pharmacol Res. 2014;87:133‐143. [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Xiao Y, Luo M, Hongye L. Statins for multiple sclerosis (Review). Cochrane Database Syst Rev. 2011;(12):CD008386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deck BL, Rick J, Morley JF, Chahine LM, Dahodwala N, Trojanowski JQ. Statins and cognition in Parkinson's disease. Journal of Parkinson's Disease. 2017;7(4):661‐667. 10.3233/JPD-171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu G, Sterling NW, Kong L, et al. Statins may facilitate Parkinson's disease: Insight gained from a large, national claims database. Mov Disord. 2017;32(6):913‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nefussy B, Hirsch J, Cudkowicz ME, Drory VE. Gender‐based effect of statins on functional decline in amyotrophic lateral sclerosis. J Neurol Sci. 2011;300(1‐2):23‐27. [DOI] [PubMed] [Google Scholar]
- 32. Drory VE, Bronipolsky T, Artamonov I, Nefussy B. Influence of statins treatment on survival in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2008;273(1‐2):81‐83. [DOI] [PubMed] [Google Scholar]
- 33. Williams DM, Finan C, Schmidt AF, Burgess S, Hingorani AD. Lipid lowering and Alzheimer disease risk: a mendelian randomization study. Ann Neurol. 2020;87(1):30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pihl‐Jensen G, Tsakiri GPA, Frederiksen JL. Statin treatment in multiple sclerosis : a systematic review and meta‐analysis. CNS Drugs. 2015;29(4):277‐291. [DOI] [PubMed] [Google Scholar]
- 35. Yan J, Qiao L, Tian J, et al. Effect of statins on Parkinson ’ s disease. Medicine (Baltimore). 2019;98(12):e14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng Z, Sheng L, Shang H. Statins and amyotrophic lateral sclerosis : a systematic review and meta‐analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(4):241‐245. [DOI] [PubMed] [Google Scholar]
- 37. Branigan GL, Soto M, Neumayer L, Rodgers K, Brinton RD. Association between hormone‐modulating breast cancer therapies and incidence of neurodegenerative outcomes for women with breast cancer. JAMA Netw open. 2020;3(3):e201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074‐D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein‐protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(D1):D447‐D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vitali F, Mulas F, Marini P, Bellazzi R. Network‐based target ranking for polypharmacological therapies. J Biomed Inform. 2013;46(5):876‐881. [DOI] [PubMed] [Google Scholar]
- 41. Vitali F, Cohen LD, Demartini A, et al. A network‐based data integration approach to support drug repurposing and multi‐target therapies in triple negative breast cancer. PLoS One. 2016;11(9):395‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klopfenstein DV, Zhang L, Pedersen BS, et al. OPEN GOATOOLS : a python library for gene ontology analyses. Sci Rep. 2018;18;8(1):10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bettembourg C, Diot C, Dameron O. Optimal threshold determination for interpreting semantic similarity and particularity : application to the comparison of gene sets and metabolic pathways using GO and ChEBI. PLoS One. 2015;. 10(7):e0133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and meta‐analysis. Alzheimer's Dement. 2018;14(11):1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta‐analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53(11):1149‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolters FJ, Segufa RA, Darweesh SKL, et al. Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta‐analysis. Alzheimer's Dement. 2018;14(11):1493‐1504. [DOI] [PubMed] [Google Scholar]
- 47. Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta‐analysis. BMC Med. 2016;14(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yohannes AM, Chen W, Moga AM, Leroi I, Connolly MJ. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta‐analysis of observational studies. J Am Med Dir Assoc. 2017;18(5):451. e1‐451.e11. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X‐J, Qin J‐J, Cheng X, et al. In‐Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID‐19. Cell Metab. 2020:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma N, Cui L. Comparative efficacy of pitavastatin and simvastatin in patients with hypercholesterolemia: a meta‐analysis of randomized controlled clinical trials. Drug Des Devel Ther. 2015;9:1859‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kazeen A, Anand R. Statins: practical considerations – a review. European Cardiology Review, 2017;9(2):71 10.15420/ecr.2014.9.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


