ABSTRACT
Here, we advocate a highly favourable opportunity for the treatment of COVID-19 disease by repurposing a long-serving medical agent with an excellent history of clinical use, namely heparin. Heparin is best known as an anticoagulant, but it also exhibits direct antiviral activity against many enveloped viruses and has anti-inflammatory activity. The high incidence of thromboembolic events in COVID-19 patients suggests that coagulopathy plays an important role in the SARS-CoV-2 pathogenesis. This already makes heparin a unique, potentially curative agent that can be used immediately to help resolve the ongoing crisis associated with SARS-CoV-2 infection and COVID-19 disease. We demonstrate here in vitro that heparin does indeed inhibit SARS-CoV-2 infection. The three concurrent modes of activity of heparin (antiviral, anticoagulant and anti-inflammatory) against SARS-CoV-2/COVID-19 form a unique therapeutic combination. Thus, repurposing of heparin to fight SARS-CoV-2 and COVID-19 appears to be a powerful, readily available measure to address the current pandemic.
KEYWORDS: COVID-19, heparin, SARS-CoV-2, Inhalation, pulmonary coagulation
Introduction
COVID-19 is an emerging infectious disease with a high case fatality rate. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is transmitted between humans by respiratory droplets, aerosols or rarely via contaminated surfaces.1,2 SARS-CoV-2 is a betacoronavirus which was described in Wuhan, China in December 2019 for the first time.2 It is genetically 79.5% identical to SARS-CoV which caused the SARS outbreak in 2002 and similar to the MERS-CoV (Middle East respiratory syndrome-related coronavirus) discovered in 2012.3
The virus primarily infects the respiratory tract, causing no or mild symptoms in the majority of cases, while a smaller proportion develops severe disease with pneumonia and, upon spread to other body compartments, multi-organ failure. Currently, patients with COVID-19 are mostly treated with supportive care. Accordingly, literature survey reveals that the highest success registered to date lies not in the realm of antiviral drugs to address the infectivity of SARS-CoV-2, but is instead due to this symptomatic and supportive treatment of COVID-19 itself.3 Widely available vaccination strategies are not expected before the year 2021.6 As the development of novel drugs usually takes years, the fastest route to establish a new treatment option is to repurpose an existing drug.4,5 Indeed, a number of drug preparations developed in another context are currently being evaluated in clinical trials for COVID-19. Of these, the direct antiviral agent remdesivir has recently received an emergency use authorisation from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).7 However, according to the most recent data, remdesivir only shortens hospitalisation and weakens disease course without reducing overall mortality.3 The first repurposed drug was dexamethasone, which reduced 28-day mortality among patients receiving invasive mechanical ventilation, as recently communicated by the RECOVERY consortium and published as a preprint manuscript.8 Conversely, several promising repurposed formulations have failed.9,10 Active viral replication and shedding seems to take place in the upper respiratory tract during the early phase of COVID-19,5 followed by either immune-mediated viral clearance or transmission to the lower respiratory tract.11 In its later stages, COVID-19 presents with progressive respiratory failure, possibly caused by viral replication in the lungs and cytokine-induced hyperinflammation.4 Hence, one strategy to prevent clinical deterioration and respiratory failure might be to impede locoregional viral replication in the early disease phase, eg by inhaled drug formulations.12–14
Thromboembolic events during COVID-19
Besides obvious respiratory symptoms, the broad tropism of SARS-CoV-2 appears responsible for a variety of atypical manifestations of COVID-19.15 Accordingly, a series of manuscripts reported the importance of anticoagulation to prevent and/or treat thromboembolic complications in COVID-19 patients.16–19 Along these lines, a recent autopsy study, among others, confirmed thromboembolic events as a major cause of death in critically ill COVID-19 patients, all pointing toward an obviously relevant prothrombotic condition caused by SARS-CoV-2.17,19 Thus, systemic anticoagulation becomes one of the major columns in lowering death rates in critically ill COVID-19 patients. A recent study conducted a propensity score matching to illuminate the thromboembolic event rate in non-COVID-19 ARDS (acute respiratory distress syndrome) and COVID-19 ARDS patients. Although both groups comprised similar numbers of patients receiving either prophylactic or therapeutic dosing of heparin, thromboembolic events occurred significantly more frequently in COVID-19 ARDS than in non-COVID-19 ARDS patients (18% vs 6%, respectively).16 Accordingly, anticoagulation even appears to reduce mortality in severe COVID-19 patients fulfilling sepsis-induced coagulopathy (SIC) criteria or having markedly elevated D-dimers.20 Therefore, the latter criteria identify a high-risk population particularly likely to benefit from systemic anticoagulation.20 Thus, even more aggressive anticoagulation therapy like the salvage use of tissue plasminogen activator (tPA) may improve recovery of COVID-19 ARDS patients;21 however, such measures require further prospective evaluation.
Potential advantage of inhaled heparin
There are various therapeutic options to achieve systemic anticoagulation. Among these, unfractionated but also fractionated heparins have a long clinical history of being well tolerated, with limited and manageable side effects.22 Thus, heparin is currently included in the WHO recommendation for COVID-19 treatment via subcutaneous systemic administration.23 In addition, heparin administration via inhalation has also been proven to be safe in patients.24 Specifically, inhaled dosages of up to 150,000 IU/d heparin marked a threshold dose to cause systemic effects detected by activated partial thromboplastin time (aPTT) prolongation while causing no relevant side effects in patients with distinct chronic lung disease.13,20 Accordingly, in 2006 an orphan designation to improve mucosal clearance in cystic fibrosis patients with inhaled heparin has been provided by the EMA. Further to being the most prominent approved anticoagulant, heparin is also known to have antiviral activity against diverse viruses, including SARS-CoV-2 (Fig 1).25–27 Thus, the overall effect of heparin to treat SARS-CoV-2/COVID-19 is unique, and, unlike directly acting antivirals, covers both the pathogen and the ensuing disease. Specifically, it has three modes of action: direct antiviral,25,26,28 anti-inflammatory,29 and anticoagulant,22 as outlined below.
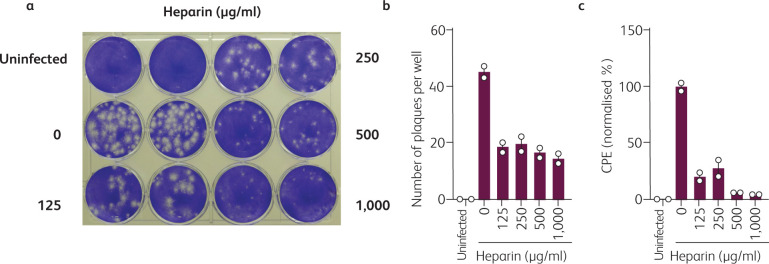
Fig 1.
Heparin inhibits SARS-CoV-2 plaque formation. a) 800,000 Vero E6 cells were seeded in a 12-well plate the day before infection to result in a 100% confluent cell monolayer. The next day, medium was removed, cells were washed once with PBS and medium containing heparin (Sigma-Aldrich) was added. Cells were then inoculated with SARS-CoV-2 isolate BetaCoV/Netherlands/01/NL/2020 (#010V-03903; European virus archive – global) in 500 μl and incubated for 2 h at 37°C with shaking every 15 to 30 min. Next, cells were overlaid with 1.5 ml of 0.8% Avicel RC-581 (FMC Corporation) in medium containing heparin and incubated for 3 days. Cells were fixed, stained with crystal violet and imaged. b) Virus-induced plaques shown in Fig 1a were counted. c) Cytopathic effect (CPE) was quantified using a custom ImageJ macro as the percentage of non-stained area within one well. Raw images were converted to greyscale, regions of interest (= one well) defined, automatic thresholding (Minimum algorithm) applied and total/white pixel areas calculated. Values represent means of two technical replicates ± sd.
Heparin exerts direct antiviral activity
Heparin is a broadly active antiviral agent that inhibits different enveloped viruses including coronaviruses.25–27 Applying heparin per inhalationem could imply that concentrations of heparin would be highest in tissues that are most affected by SARS-CoV-2, namely the upper and lower respiratory tract. Of note, inhaled heparin has a proven broad distribution in the respiratory tract including the alveolar space.7,30–32 Specifically, the antiviral activity of heparin is based on affinity of viral glycoproteins to negatively charged glycosaminoglycans such as sulfated heparan, which are ubiquitously expressed on the surface of mammalian cells. It has recently been demonstrated in a published and a preprint article that soluble heparin interacts with the SARS-CoV-2 spike protein28,33 and inhibits SARS-CoV-2 spike pseudovirus entry.34 In line with these findings, another preprint article showed that porcine heparin prevents wildtype SARS-CoV-2 infection of Vero E6 cells.26
Thus, we here set out to determine the antiviral activity of heparin against SARS-CoV-2. We conducted a viral plaque reduction assay in Vero E6 cells inoculated with a Dutch SARS-CoV-2 isolate in the absence or presence of various concentrations of heparin. Heparin not only resulted in a decrease in the number of plaques (Fig 1a,b) but in particular also resulted in a decrease in the size of the plaques (Fig 1a). In order to quantify the plaque sizes, we calculated the total virus-induced cytopathic effect per well using a custom ImageJ macro as the percentage of non-stained area within one well (Fig 1c). This evaluation shows that in the presence of 500–1,000 μg/ml heparin, viral replication is almost completely inhibited, and at 125–250 μg/ml suppressed by more than 60%. Thus, heparin prevents SARS-CoV-2 infection and subsequent replication with a half maximal inhibitory concentration (IC50) below 125 μg/ml.
Anti-inflammatory and anticoagulant effects of heparin in the lung
Apart from these direct antiviral properties, heparin is known to have anti-inflammatory activity via, among other mechanisms, its ability to neutralise a variety of cationic immune mediators including IL-6 and IL-8 and to inhibit chemotaxis.35,36 In mammals, heparin is found in mast cells, suggesting its physiological usefulness in mucosal and connective tissue. Inhaled heparin has been used in patients with cystic fibrosis for its proposed mucolytic activity and a reduction of serum levels of IL-6 and IL-8 were observed.29,37 A third proposed mechanism of action of inhaled heparin in COVID-19 patients is its anticoagulative property unfolding locally in the lung.38 ARDS, one of the severe and potentially lethal complications of SARS-CoV-2, is marked, among other features, by a deposition of fibrin in the alveolar space and microcirculation, leading to hyaline membranes and ultimately to thrombosis, a process called pulmonary coagulopathy (PC). The anticoagulative and fibrinolytic properties of heparin could prevent this through per inhalationem application by preventing alveolar collapse, lung oedema and impaired gas exchange.7 Thus, PC might be more relevant during COVID-19-associated ARDS than during classical ARDS, as a significantly perturbed gas exchange, eg from fibrin deposition, may lead to perturbed surfactant function and thus alveolar collapse. Unusually high D-dimers in critically ill COVID-19 patients might therefore serve as an early indicator of PC subsequently leading to alveolar damage.39 Of note, systemically elevated D-dimers are currently considered to be strong predictors of severe COVID-19 disease.39 In this light, the anti-inflammatory and thrombolytic activity of heparin in injured lungs40 could serve as effective measures against COVID-19.
Finally, inhalation is strongly suggested herein as a route of administration, as the lungs are most affected in COVID-19, and this strategy has proven to be highly effective for a range of other drugs.41 A randomised, placebo-controlled study currently aims to determine if nebulised heparin may reduce the severity of lung injury caused by COVID-19.13 Similarly, the COVID-19 HOPE (nebulised Heparin-N-acetylcysteine in COVID-19 Patients by Evaluation of pulmonary function) trial tests the combination treatment of nebulized heparin with N-acetylcysteine (NAC), which may have the potential to improve pulmonary function and reduce or eliminate mechanical ventilation in patients with a coronavirus infection.42 Of note, substantial increase of PC has also been reported for influenza pneumonia-associated ARDS.20
Conclusion
The three concurrent modes of activity of heparin against SARS-CoV-2/COVID-19 form a unique therapeutic combination. Repurposing of heparin to fight SARS-CoV-2 and COVID-19 appears to be a powerful, readily available measure to address the current pandemic. The first clinical evidence to validate this hypothesis is already available,43 and stronger efforts are now needed to fully realise this potential. Thus, clinical trials designed in a prospective randomised controlled manner are urgently warranted to rapidly accelerate this unique strategy toward clinical application.
Acknowledgments
CC is part of the International Graduate School in Molecular Medicine, Ulm. This work was supported by grants from the MWK Baden-Württemberg to JM and AK, and the Horizon 2020 project Fight-nCoV to JM and ANZ.
References
- 1.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science 2020; 368:356–60. [DOI] [PubMed] [Google Scholar]
- 2.Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. N Engl J Med 2020;382:2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - Preliminary Report. N Engl J Med 2020, in press ( 10.1056/NEJMoa2007764). [DOI] [PubMed] [Google Scholar]
- 4.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discovery 2019;18:41–58. [DOI] [PubMed] [Google Scholar]
- 5.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020;19:149–50. [DOI] [PubMed] [Google Scholar]
- 6.Graham BS. Rapid COVID-19 vaccine development. Science 2020;368:945–6. [DOI] [PubMed] [Google Scholar]
- 7.Dixon B, Santamaria JD, Campbell DJ. A phase 1 trial of nebulised heparin in acute lung injury. Crit Care 2008;12:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020: 2020.2006.2022.20137273. [Google Scholar]
- 9.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;382:2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465–9. [DOI] [PubMed] [Google Scholar]
- 12.Dixon B, Schultz MJ, Smith R, et al. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care 2010;14:R180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smoot P. Nebulized heparin for the treatment of COVID-19 induced lung injury. https://clinicaltrials.gov/ct2/show/NCT04397510.
- 14.Gilead Sciences Inc Working to supply remdesivir for COVID-19. www.gilead.com/purpose/advancing-global-health/covid-19/working-to-supply-remdesivir-for-covid-19.
- 15.Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020;383:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020, in press ( 10.1007/s00134-020-06062-x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J 2020;56:2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoneham SM, Milne KM, Nuttal E, et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med 2020;20:e76–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020, in press ( 10.7326/M20-2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol 2016;13:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury R, Barrett CD, Moore HB, et al. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg 2020;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oduah EI, Linhardt RJ, Sharfstein ST. Heparin: past, present, and future. Pharmaceuticals (Basel) 2016;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med 2003;31:S329–36. [DOI] [PubMed] [Google Scholar]
- 24.Yip LY, Lim YF, Chan HN. Safety and potential anticoagulant effects of nebulised heparin in burns patients with inhalational injury at Singapore General Hospital Burns Centre. Burns 2011;37:1154–60. [DOI] [PubMed] [Google Scholar]
- 25.Lang J, Yang N, Deng J, et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLOS ONE 2011;6:e23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mycroft-West C, Su D, Elli S, et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. bioRxiv 2020: 2020.2002.2029.971093. [Google Scholar]
- 27.Vicenzi E, Canducci F, Pinna D, et al. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg Infect Dis 2004;10:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mycroft-West CJ, Su D, Pagani I, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv 2020: 2020.2004.2028.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young E. The anti-inflammatory effects of heparin and related compounds. Thrombosis Research 2008;122:743–52. [DOI] [PubMed] [Google Scholar]
- 30.Bendstrup KE, Jensen JI. Inhaled heparin is effective in exacerbations of asthma. Respir Med 2000;94:174–5. [DOI] [PubMed] [Google Scholar]
- 31.Markart P, Nass R, Ruppert C, et al. Safety and tolerability of inhaled heparin in idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv 2010;23:161–72. [DOI] [PubMed] [Google Scholar]
- 32.Monagle K, Ryan A, Hepponstall M, et al. Inhalational use of antithrombotics in humans: Review of the literature. Thromb Res 2015;136:1059–66. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Jin W, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res 2020;181:104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tandon R, Sharp JS, Zhang F, et al. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. bioRxiv 2020; 2020.06.08.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lever R, Page CP. Non-anticoagulant effects of heparin: an overview. Handb Exp Pharmacol 2012:281–305. [DOI] [PubMed] [Google Scholar]
- 36.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992;12:17–46. [PubMed] [Google Scholar]
- 37.Ledson M, Gallagher M, Hart CA, Walshaw M. Nebulized heparin in Burkholderia cepacia colonized adult cystic fibrosis patients. Eur Respir J 2001;17:36–8. [DOI] [PubMed] [Google Scholar]
- 38.Juschten J, Tuinman PR, Juffermans NP, et al. Nebulized anticoagulants in lung injury in critically ill patients-an updated systematic review of preclinical and clinical studies. Ann Transl Med 2017;5:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit Care Med 2014;42:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman SP. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv Drug Deliv Rev 2018;133:5–18. [DOI] [PubMed] [Google Scholar]
- 42.Quay S. The COVID-19 HOPE clinical trial. https://drquay.com/covid-19-doctor/.
- 43.Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. medRxiv 2020; 2020.2003.2028.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]