ABSTRACT
Adult Paget's disease of bone is the second commonest metabolic bone condition after osteoporosis. The condition is characterised by increased bone cell activity, with bone-resorbing osteoclasts often larger and containing more nuclei than normal and osteoblasts producing increased amounts of disorganised bone. This leads to expanded bone of poor quality possessing both sclerotic and lytic areas. Paget's disease of bone has a strong genetic element, with a family history being noted in 10–20% of cases. A number of genetic defects have been found to be associated with the condition. The most common disease-associated variants identified affect the SQSTM1 gene, providing insights into disease aetiology, with the clinical value of knowledge of SQSTM1 mutation status currently under active investigation. The diagnosis may be suggested by an isolated raised total alkaline phosphatase (ALP) without other identifiable causes. This can be confirmed on plain X-ray and the extent determined by isotope bone scan. The mainstay of treatment are the bisphosphonates, especially intravenous zoledronate which results in long-term suppression of bone turnover. ALP is the usual means of monitoring the condition, although more specific bone turnover markers can be helpful, especially in coincident liver disease. Patients should be followed up to monitor for biochemical relapse or development of complications, which may require medical or surgical intervention.
KEYWORDS: Paget's disease of bone, pathogenesis, management, complications
Key points
Paget's disease of bone is the second commonest metabolic bone condition after osteoporosis.
Unregulated bone turnover in affected bones results in enlargement leading to deformity, fragility and compression phenomena and, very rarely, malignant transformation.
Long-term biochemical remission can be achieved with bisphosphonate therapy, especially with intravenous zoledronate.
Diagnosis suggested by isolated raised alkaline phosphatase or characteristic appearances on X-ray.
It is important to refer new patients for specialist care.
Introduction
Adult Paget's disease of bone (PDB) was first described by Sir James Paget in 1877, a condition he referred to as osteitis deformans.1 It is characterised by increased bone cell activity that results in expanded bone possessing both sclerotic and lytic areas. It is initiated by enhanced resorption by abnormal multinucleated osteoclasts followed by disorganised bone formation by osteoblasts.2 There is a high rate of bone remodeling at affected sites in the skeleton; the bone is highly metabolically active and has a high blood flow which can make the overlying skin feel warm to the touch.3 PDB is the second most common metabolic bone disorder after osteoporosis. Despite this, it is both under-diagnosed and under-treated, with many patients never coming to medical attention.4
Pathogenesis
PDB is more common in males than females.4 It is unusual under the age of 40 years. It can affect single bones (monostotic) or many bones (polyostotic).2 The UK and other populations of British descent have the highest prevalence of PDB, with up to 2% in those over 55 years estimated to be affected, as reported in 2008.5 The condition is often asymmetric and does not spread from bone to bone; it is unusual for new bones to become involved after diagnosis.6 Interestingly, the incidence has declined rapidly in recent years.7
In this condition, there is loss of the normal regulation of bone resorption and formation with the process biased towards one or the other depending on the phase of the disorder.8 The resultant bone has an altered and often abnormal architecture. In the early phase of PDB, there are increased numbers of enlarged osteoclasts with more nuclei than normal and increased bone resorption. Subsequently there is increased formation of new bone. The result is a highly vascular bone with areas of lysis and sclerosis within the same bone. In the final sclerotic phase, formation predominates and collagen fibres are deposited in a haphazard way, the bone is disorganised and is weaker. Of note, there is a modest increase in incidence of osteosarcoma.2
Genetic factors play an important role in PDB, with 10–20% of patients having a family history.9–11 Defects in the SQSTM1 gene are the most well characterised; this gene encodes the SQSTM1/p62 protein (sequestosome-1) and is found in up to 50% of familial cases of PDB.9,10 The SQSTM1/p62 protein is an important regulator of osteoclast RANK-mediated NF-kappa B signaling with mutation-associated activation of this pathway likely linked to disease aetiology.11 The declining incidence coupled with the fact that not everyone with gene defects develops Paget's suggests that environmental factors must also play a role.12,13 To date, what these triggers are remains uncertain.
Clinical features, complications and presentation
The clinical presentation and complications of PDB are a result of the increased bone cell activity in affected bone, bone expansion, deformity and poor bone quality (Table 1). In order of frequency, PDB affects the femur, spine, skull, sternum and pelvis, but can be found in any bone in the body.14 Many patients are asymptomatic and the diagnosis is often incidental as a result of an isolated raised alkaline phosphatase (ALP) or imaging undertaken for other reasons. Subsequent X-ray and isotope bone scan may then confirm the diagnosis and extent of the disease.
Table 1.
Clinical presentations of Paget's disease
| System | Clinical findings |
|---|---|
| Musculoskeletal These are all common features of Paget's disease of bone |
Acetabular protrusion Bone deformity Bone pain Fractures Spinal stenosis Osteoarthritis of neighbouring joints |
| Neurological Deafness, spinal stenosis/vascular steal are all common |
Basilar invagination Cerebellar dysfunction Obstructive hydrocephalus Cranial nerve palsies Spinal stenosis/cauda equina syndrome Deafness Tinnitus Vascular steal syndrome Para- or quadriplegia |
| Cardiovascular All uncommon |
Increased cardiac output Congestive cardiac failure Aortic stenosis Endocardial calcification Atherosclerosis |
| Metabolic All are rare |
Hypercalcaemia Hyperuricaemia Immobilisation hypercalciuria Nephrolithiasis |
| Neoplasia Sarcomas are very rare but are important not to miss due to their serious nature and are potentially lethal |
Sarcoma (osteosarcoma, chondrosarcoma or fibrosarcoma) Giant cell tumour |
Bone pain occurs in about 50% of cases presenting clinically.15 The pain may arise from the affected bone itself, altered biomechanics of limb deformity (eg bowed tibia) and secondary osteoarthritis.2,15,16 Approximately, 10–30% of PDB patients sustain fractures.2 These may initially be incomplete traversing of the cortex especially the outer borders of bowed bones (fissure fractures). Fissure fractures predominantly affect weight-bearing bones, which are at high risk of complete fracture. Bone healing seems to take place normally.2 The development of osteosarcomas within the affected bone, or more rarely chondro- or fibrosarcomas, can also cause pain, a soft tissue mass and also a rise in ALP level.2,4
Neurological complications occur as a result of bony overgrowth leading to compression of which the commonest is hearing loss. Compression at the base of the skull may lead to basilar invagination, cerebellar dysfunction and even obstructive hydrocephalus. Thoracic and lumbar spine involvement may cause nerve entrapment and even spinal canal stenosis, but sometimes this is the result of the increased vascularity causing vascular steal phenomena. The increased bone vascularity could potentially cause increased cardiac output and high-output cardiac failure should the disease be extensive and uncontrolled. There is an association between PDB and aortic stenosis, arteriosclerosis and intracardiac calcification.14
Diagnosis
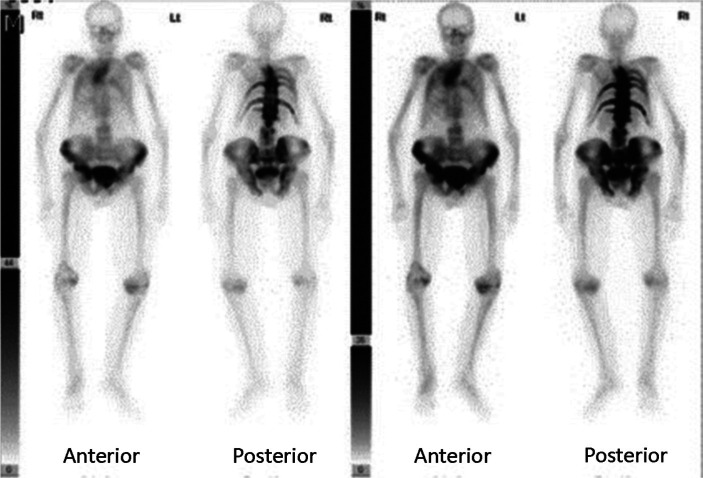
The possibility of PDB is often first raised by an isolated raised ALP, in which isoenzymes reveal to be largely of bone origin. It also causes an elevation of other bone turnover markers (BTMs). Other causes of this need to be excluded, including vitamin D deficiency, hyperparathyroidism, hyperthyroidism, renal osteodystrophy and malignancy. Bone metastases, including those arising from prostate cancer and myeloma, can usually be differentiated by blood tests, plain X-ray and other imaging. Very occasionally, it is necessary to resort to bone biopsy to be certain. X-ray of affected bone will usually be diagnostic and show the characteristic features of the illness (Fig 1). The changes can also be detected with computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) CT which can be useful in distinguishing from other potential causes and to assess complications. Isotope bone scans (Fig 2), in addition to targeted X-ray, are recommended as a means of fully and accurately defining the extent of metabolically active PDB.17
Fig 1.
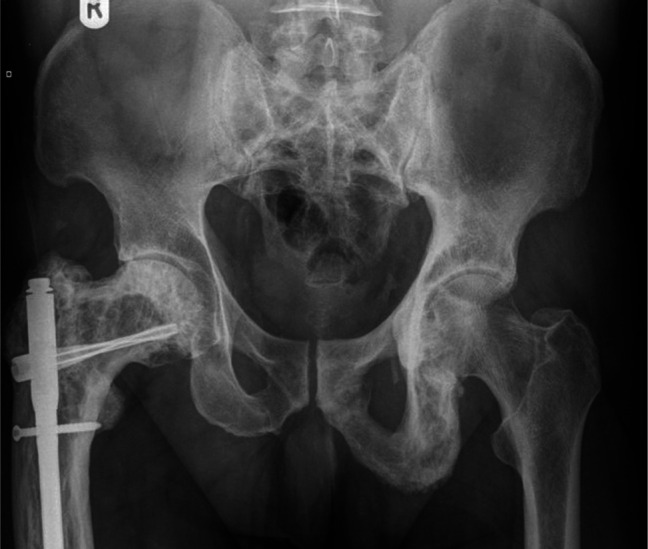
X-ray confirming the presence of Paget's disease of bone affecting the right femur and pelvis. This was performed after the patient presented with a right hip fracture due to low trauma.
Fig 2.
Isotope bone scan of a patient with extensive Paget's disease of bone affecting multiple bones.
Treatment
The pharmacological treatment of PDB is targeted at reducing osteoclast function and at relieving symptoms and complications. Pain from PDB itself will improve with treatment, but often arises from complications (eg secondary osteoarthritis). It is important to ensure that patients are vitamin D replete (≥50 nmol/L), have good calcium intake and normal calcium levels prior to treatment, otherwise hypocalcaemia can be induced.
The current first-line treatment for active disease or those at risk of complications is intravenous 5 mg zoledronate because of its potency and prolonged duration of action. Zoledronate brings about rapid normalisation of BTMs and relieves pain from active PDB.17,18 Oral bisphosphonates and intravenous pamidronate also work, although their duration of response is much shorter.14,17,18 In the UK, risedronate is licensed for PDB at 30 mg daily for 2 months. Salmon calcitonin is now rarely used in PDB; it requires daily subcutaneous injections and relapse is rapid after cessation.14,17,18
Management of complications
Bisphosphonates are excellent at inducing biochemical remission. However, patients can be biochemically well controlled yet still have pain or problems from complications (eg secondary osteoarthritis). Practical difficulties resulting from deformity and abnormal biomechanics can be helped by physiotherapy, orthotics and occupational therapy. Orthopaedic surgery may be required for correction of deformity, joint replacement, prophylactic nailing to prevent imminent fracture for fissure fractures and nerve compression. Pagetic bone activity should be brought under control with medical therapy prior to surgery in order to reduce vascularity and minimise the risk of excessive blood loss.18 Total hip or knee replacements are recommended for patients with PDB who develop osteoarthritis in whom medical treatment is inadequate.17 In general, hearing loss does not seem to respond to treatment of the PDB.17,18 Neurosurgical intervention is sometimes necessary for problems arising from compressive phenomena (eg spinal stenosis). Sometimes, the cause is a vascular steal from active pagetic bone in vertebrae, which may well be reversed by the intravenous bisphosphonate.17,18 If medical management should fail, then surgical opinions should be sought urgently. High-output cardiac failure may respond to treatment of PDB. Malignant transformation to an osteosarcoma has a poor prognosis and should be referred to a specialist centre for sarcomas.
Monitoring
The measurement of BTMs provides an objective means of assessing disease activity, response to treatment and biochemical relapse. Serum total ALP correlates well with the amount of skeletal involvement on X-ray and activity on isotope bone scans.2,18 Total ALP is recommended as the most commonly used marker to monitor PDB. However, it is unreliable in the presence of liver or biliary tract disease and can be within the normal range at presentation, particularly in monostotic disease when more specific BTMs would be advantageous.13,17,18
Bone specific markers include formation markers (bone specific alkaline phosphatase (BSAP) and procollagen type 1 amino terminal propeptide (P1NP)) and resorption markers (such as serum β C-terminal telopeptide (sβCTX)). These can all be used to monitor response to therapy and biochemical relapse, and are especially useful if total ALP is normal or in the presence of liver disease. BSAP and P1NP are recommended, especially if ALP values are normal and clinical suspicion of metabolically active PDB is high.17,18
Potent bisphosphonates result in long-term suppression of bone turnover, and this can last at least 6 years with zoledronate, which also gives better quality of life measurements than risedronate.19 The initial assessment of biochemical and clinical response to treatment should take place between 3–6 months after treatment. Further annual follow-up will be needed to asses biochemical relapse and for potential complications.18
Conclusion
Adult PDB is a very common metabolic bone disorder, most frequently affecting men and women over 40 years of age. Although, often asymptomatic, it can cause pain and result in considerable morbidity. The diagnosis is commonly missed and, even when identified, it is poorly understood and inadequately treated despite the efficacy of bisphosphonates. Identified patients should be referred for specialist care.
Conflicts of interest
Dr Stephen P Tuck has received speaker fees from Ely Lilly, Servier, Internis Pharmaceuticals and Amgen.
References
- 1.Paget J. On a form of chronic inflammation of bones (osteitis deformans). Med Chir Trans 1877;60:37–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBoff MS. Metabolic bone disease. In: Kelly WN, Ruddy SR, Harris ED, Sledge C. (eds). Textbook of rheumatology: Vol 2, 5th edn. Philadelphia: WB Saunders, 1997:1574–80. [Google Scholar]
- 3.Al Nofal AA, Altayar O, Ben Khadra K, et al. Bone markers in Paget's disease of the bone: a systemic review and meta-analysis. Osteoporosis Int 2015;26:1875–91. [DOI] [PubMed] [Google Scholar]
- 4.Van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget's disease of bone in England and Wales. J Bone Mine Res 2002;17:465–71. [DOI] [PubMed] [Google Scholar]
- 5.Ethel SS, Roodman GD. Paget's disease of bone. In: Rosen CJ. (ed). Primer on the metabolic bone diseases and disorders of mineral metabolism, 7th edn. Washington: American Society for Bone and Mineral Research, 2008:335–42. [Google Scholar]
- 6.Takata S, Hashimoto J, Nakatsuka K, et al. Guidelines for diagnosis and management of Paget's disease of bone in Japan. J Bone Miner Metab 2006;24:359–67. [DOI] [PubMed] [Google Scholar]
- 7.Cundy T, Reid IR. Reprint: Paget's disease of bone. Clin Biochem 2012;45:970–5. [DOI] [PubMed] [Google Scholar]
- 8.Siris ES. Paget's disease of bones and joints. J Bone Miner Res 1998;13:1061–65. [DOI] [PubMed] [Google Scholar]
- 9.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget's disease of bone. Am J Hum Genet 2002;70:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hocking LJ, Lucas GJ, Darosjewska A, et al. Domaine-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum Mol Genet 2002;11:2735–9. [DOI] [PubMed] [Google Scholar]
- 11.Goode A, Long JE, Shaw B, et al. Paget disease of bone-associated UBA domain mutations of SQSTM1 exert distinct effects on protein structure and function. Biochim Biophys Acta 2014;1842:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralston SH, Afzal MA, Helfrich MH, et al. Multicenter blinded analysis of RT-PCR detection methods for paramyxoviruses in relation to Paget's disease of bone. J Bone Miner Res 2007;22:569–77. [DOI] [PubMed] [Google Scholar]
- 13.Layfield R. The molecular pathogenesis of Paget's disease of bone. Expert Rev Mol Med 2007;9:1–13. [DOI] [PubMed] [Google Scholar]
- 14.Tuck SP, Layfield R, Mikkayil B, Walker JA. Paget's disease of bone: a review. Rheumatology 2017;56:2050–9. [DOI] [PubMed] [Google Scholar]
- 15.Tan A, Ralston SH. Clinical presentation of Paget's disease: evaluation of a contemporary cohort and systematic review. Calcif Tissue Int 2014;95:385–92. [DOI] [PubMed] [Google Scholar]
- 16.Frank WA, Bress NM, Singer FR, Krane SM. Rheumatic manifestations of Paget's disease of bone. Am J Med 1974;56:592–603. [DOI] [PubMed] [Google Scholar]
- 17.Ralston SH, Corral-Gudino L, Cooper C, et al. Diagnosis and management of Paget's disease of Bone in adults: A clinical guideline. JBMR 2019;34:579–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer F, Bone HG, Hosking DJ, et al. Paget's disease of bone: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014;99:4408–22. [DOI] [PubMed] [Google Scholar]
- 19.Reid IR, Lyles K, Su G, et al. A single infusion of zoledronic acid produces sustained remissions in Paget disease: data to 6.5 years. J Bone Miner Res 2011;26:2261–70. [DOI] [PubMed] [Google Scholar]