Dear Editor,
The subject of hematological malignancies treatment in the era of COVID-19 is of great importance as many teams are working hard to define the right balance: controlling the disease while not exposing their patients to the high risk of COVID-19 [1].
Ibrutinib is a Bruton tyrosine kinase (BTK) inhibitor, widely used in the treatment of some lymphoproliferative diseases like mantle cell lymphoma and Waldenström’s macroglobulinemia [2]. Treon et al. suggested a protective effect of ibrutinib on pulmonary injury in COVID-19 disease patients. They reported these protective effects on pulmonary injury of continuing or even increasing ibrutinib therapy at a maximal dose of 420 mg/day. All six patients were on ibrutinib treatment for Waldenström’s macroglobulinemia. The only patient on a reduced dose of 140 mg of ibrutinib was also the only patient to develop respiratory insufficiency, which improved upon re-initiating ibrutinib, and subsequently quickly resolved upon increasing the dose to 420 mg/day [3]. They suggest that ibrutinib as a BTK inhibitor is able to inhibit and decrease the inflammatory cytokines that are activated through the toll-like receptor (TLR) pathway in epithelial ATII cells and alveolar macrophages. These cytokines are deemed responsible for the majority of pulmonary injury in COVID-19 disease.
Herein we report on two patients treated at our center with ibrutinib, both with underlying mantle cell lymphoma. They had COVID-19 and developed respiratory illness. Both patients discontinued ibrutinib, and both fully recovered. We describe these two cases and discuss further, the possible mechanisms of action of ibrutinib in the context of COVID-19.
Cases presentation
Case 1
The first patient is a 71-year old male. He was contacted by his hematologist for a routine check-up. The patient had already significant dyspnea and unproductive cough. The patient refused to be admitted to hospital. One week later, the patient’s status had worsen, and the emergency services were contacted. He was then admitted to hospital presenting with symptoms suggestive of COVID-19, including dyspnea with oxygen-dependence at 5 L/min, diarrhea and the hematologist immediately discontinued ibrutinib.
This patient had a biological inflammatory syndrome with leukocytosis, neutrophilia at 10 000/μl, and C-reactive protein (CRP) at 204 mg/l. He also had a newly diagnosed lymphopenia of 570/μl.
PCR result of SARS-CoV-2 were negative; however, a CT angiogram of the chest, performed on the second day of hospitalization, was very suggestive of COVID-19 pulmonary disease with bilateral ground glass opacities and nodular condensations, as shown in Figs. 1 and 2 .
Fig. 1.
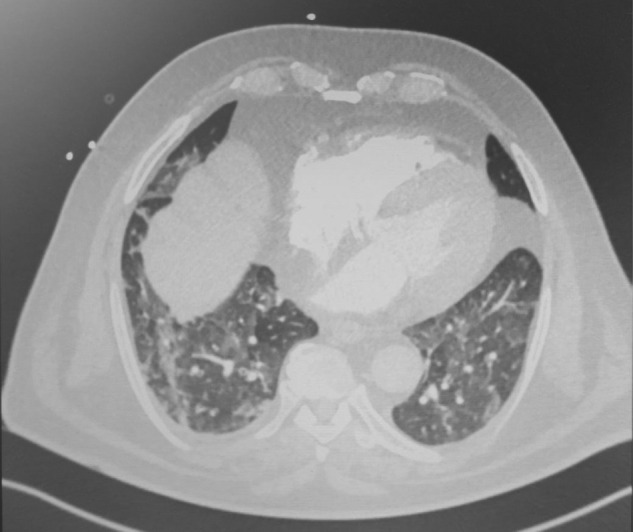
Case 1: chest angiogram at admission showing ground glass opacities and nodular condensations.
Fig. 2.
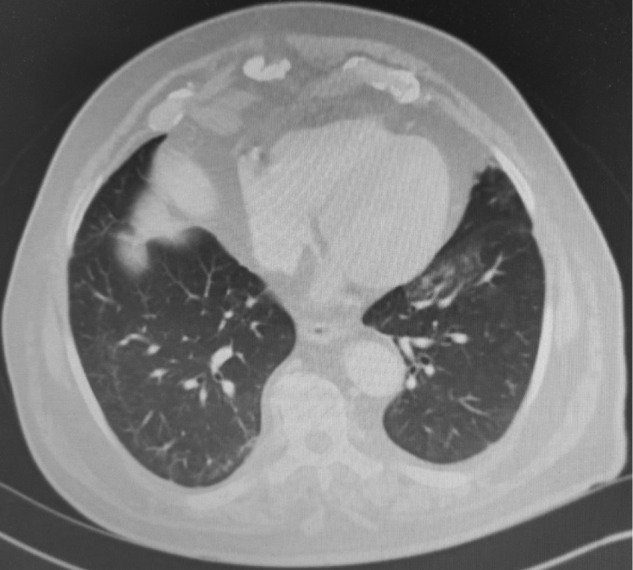
Case 1: chest angiogram at 4 weeks after hospital admission showing remarkable regression of ground glass opacities and nodular condensations.
After performing blood cultures and following local guidelines, antibiotic treatment was started with ceftriaxone, which was subsequently switched to piperacillin/tazobactam 16 g/24 h the same day. Azithromycin was added, the same day, at a dose of 500 mg on the first day followed by 250 mg for the next four days.
Because of the high clinical suspicion of COVID-19 alongside the suggestive radiological features, and despite the initial negative PCR result, the patient started on an anti-IL-6R antibody that blocks the IL-6 pathway. The patient’s status was ameliorated remarkably and progressively. He returned home on day 7 after admission.
The patient tested serologically negative for COVID-19 antibodies more than 2 months after symptom initiation. Ibrutinib was reintroduced 6 weeks after the initial admission date, according to department guidelines, equal to 4 weeks of full recovery of COVID-19.
The patient was treated for mantle cell lymphoma with ibrutinib at a dose of 560 mg, up from January 2019. Initial diagnosis was in April 2016, with first line treatment by R-CHOP followed by rituximab maintenance. At the end of maintenance treatment, disease relapse was noted in the cervical area, at which time ibrutinib treatment was started. Treatment was intensified and followed by autologous stem cell transplant. It is worth noting that the patient had some important comorbidities such as uncontrolled diabetes mellitus type 2, with HbA1c at 10.4 %, and macro-angiopathic complications, including ischemic coronaropathy, carotid stenosis and peripheral obstructive disease.
Case 2
The second patient, also male, was treated for mantle cell lymphoma with ibrutinib at a dose of 560 mg per day. A lymphoma - related periodic PET scan first alerted doctors to the possibility of COVID-19 infection by the demonstration of hypermetabolic interstitial images at both pulmonary bases, suggestive of an infection. Due to his intact clinical status the patient was not hospitalized and stayed at home for the remainder of his infection period. Ibrutinib was promptly discontinued during presentation of mild fever and respiratory symptoms. The clinical situation evolved favorably, and the patient remained unhospitalized. Serological tests for SARS-CoV-2 were conducted one month after this period with positive results suggesting a history of COVID-19 infection.
This patient was diagnosed with mantle cell lymphoma stage IV in 1999. After treatment with R-CHOP/R-DHAP followed by high-dose chemotherapy and autologous transplant, he remained in remission until 2018. Upon disease relapse, treatment with ibrutinib and rituximab was initiated with achievement of a good disease response. He also received tenofovir for a chronic hepatitis B infection.
The patient had resumed ibrutinib treatment almost 4 weeks after full recovery.
No adverse effects were registered on mantle cell lymphoma control for either of the two patients.
Discussion
Patients with COVID-19 experience mild to moderate disease in 80 % of cases, the rest may present with severe symptoms leading to critical conditions for which there is massive concern due to the rapid viral dissemination and limited medical resources, even in developed countries [4,5]. Patients with hematological malignancies and COVID-19 have an increased risk of death compared to the general population, depending on the age of the patient, time since diagnosis, disease status or type of hematological malignancy [[6], [7], [8]]. Lamure et al. reported a 30-day overall survival à 71 % in patients with lymphoma hospitalized for COVID-19 [9]. However, the impact of ibrutinib on patients’ outcomes was not assessed.
Treon et al. have recently reported that Waldenström’s disease patients on ibrutinib may benefit from continuation of their therapy despite the diagnosis of COVID-19.
These results were supported by laboratory findings suggesting the importance of inflammatory cytokines in lung injury by SARS-CoV-2 and the capacity of BTK-inhibitors and HCK to reduce them [3].
While severe manifestations of COVID-19 may be the result of over released cytokines and chemoattractants such as IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10/CXCL-10, MCP-1/CCL2, MIP-1a/CCL3, and TNF-alpha, (blood) interferon (INF)-γ concentration usually increases during viral infections [10,11].
Ren et al. found that ibrutinib could not block TNFα production in monocytes primed with IFNγ, or in co-cultures of monocytes and NK cells. Thus, they expect that it is likely that FcγR-activated monocytes can still produce not only IL-12, but also other chemokines/cytokines in mixed cellular environments. These cytokines could serve to activate neighboring cells [12].
When taken collectively, these data may suggest that although ibrutinib can effectively inhibit proinflammatory responses in isolated cells, its ability to do so within the whole organism may be limited, especially when the secretion of IFNy is increased. This may explain why early clinical results showed an enhanced effect when adding rituximab or ofatumumab to ibrutinib in the treatment for chronic lymphocytic leukemia [13].
Although ibrutinib is considered by some to be a potential promising treatment for pulmonary consequences of chronic graft-versus-host disease (cGVHD) [8], serious adverse events are usually the reason behind treatment discontinuation in almost 30%–40 % of cases. These adverse events may include asthenia, pneumonitis, fungal and bacterial infections, while diarrhea of grade ≥3 occurs in more than two-thirds of patients [14,15].
This may also explain the relatively good results for these two patients when discontinuing ibrutinib after being infected with SARS-CoV-2. No ICU hospitalization was needed, and no accompanying severe co-infections were reported.
Although serological tests were negative for the first case and a PCR-confirmed COVID-19 couldn’t be documented, highly suggestive radiological signs alongside the typical clinical manifestations (general, respiratory and digestive) in addition to high CRP level on admission [4] were indicative of COVID-19 infection.
The delay between the first symptom and the PCR test was more than 11 days, the estimated median time needed for a positive-to-negative PCR test result conversion of upper respiratory tract samples [10].
With respect to the first case’s laboratory tests, serological tests (IgM and IgG) could have rendered false-negative results in 10–30% of cases, according to a recent Cochrane systematic review. Moreover, no robust data to date are available to interpret test sensitivity for results obtained beyond the 35th day of symptom initiation, as it was the case for our first patient [16].
The patient was treated by a recombinant human IL-6Rα antagonist. Could this treatment have affected antibody productivity in response to SARS-CoV-2 by blocking an important inflammatory pathway? In our opinion, this question is worth thorough discussion [17].
Finally, rapid clinical amelioration and an unaffected lymphoma control were observed in both cases suggesting a short temporary discontinuation policy success in our mantle cell lymphoma patients during the acute phase of COVID-19 infection.
Conclusion
Although the protective effect of anti-proinflammatory cytokines may be rational when talking about BTKs, this effect may be limited to circumstances of viral infection due to predisposed high concentrations of interferon gamma and to the probability of concomitant infections.
Continuation and discontinuation policies should rely more widely on outcome experience; meanwhile case-by-case decisions should also be considered.
Informed consents
Informed consents were obtained from the patients for the publication of their cases.
Declaration of Competing Interest
FM reports lecture honoraria from Therakos/Mallinckrodt, Biocodex, Janssen, Keocyt, Sanofi, JAZZ pharmaceutical and Astellas, all outside the submitted work.
RD reports lecture honoraria from Keocyt, Sanofi and Novartis, all outside the submitted work.
TA reports honoraria from BIOTEST, outside the submitted work.
Acknowledgements
Authors would like to thank the patients for their participation. Authors would like also to thank medical and paramedical staff of hematology and cell therapy department of Saint-Antoine hospital, AP-HP, Paris for their dedication to patients’ service.
References
- 1.Malard F., Mohamad Mohty. Management of patients with multiple myeloma during the COVID-19 pandemic. Lancet Haematol. 2020;7(6):e435–e437. doi: 10.1016/S2352-3026(20)30124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parmar Sapna, Patel Khilna, Pinilla-Ibarz Javier. Ibrutinib (imbruvica): a novel targeted therapy for chronic lymphocytic leukemia. P & T. 2014;39(7):483–519. [PMC free article] [PubMed] [Google Scholar]
- 3.Treon S., Castillo J., Skarbnik A., Soumerai J., Ghobrial I., Guerrera M.L. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19–infected patients. Blood. 2020;135(21):1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsuliman T., Alasadi L., Alkharat B., Srour M., Alrstom A. A review of potential treatments to date in COVID-19 patients according to the stage of the disease. Curr Res Transl Med. 2020;68(3):93–104. doi: 10.1016/j.retram.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Chris Bates Bates C., Morton C.E. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(August (7821)):430–436. doi: 10.1038/s41586-020-2521-4.TPP. Horsforth, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malard F., Genthon A., Brissot E., Van De Wyngaert Z., Marjanovic Z., Ikhlef S. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passamonti F., Cattaneo C., Arcaini L., Arcaini L., Bruna R., Cavo M. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;(August (13)) doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamure S., Duléry R., Di Blasi R., Chauchet A., Laureana C., Deau-Fisher B. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsuliman T., Sulaiman R., Ismail S., Srour M., Alrstom A. COVID-19 paraclinical diagnostic tools: updates and future trends. Curr Res Transl Med. 2020;68(3):83–91. doi: 10.1016/j.retram.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. ISSN 0009-8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren L., Campbell A., Fang H., Gautam S., Elavazhagan S., Fatehchand K. Analysis of the effects of the Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib on monocyte Fcγ receptor (FcγR) function. J Biol Chem. 2016;291:3043–3052. doi: 10.1074/jbc.M115.687251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilhan O., Seval G.C., Uzay A., Kaya E., Ozturk Z.N., Deveci B. Ibrutinib as a promising treatment for pulmonary complications due to refractory chronic graft versus host disease. Biol Blood Marrow Transplant. 2020;26(Suppl. (3)):S191. doi: 10.1016/j.bbmt.2019.12.757. ISSN 1083-8791. [DOI] [Google Scholar]
- 14.Varughese T., Taur Y., Cohen N., Palomba M.L., Seo S., Hohl T. Serious infections in patients receiving ibrutinib for treatment of lymphoid Cancer. Clin Infect Dis. 2018;67(5):687–692. doi: 10.1093/cid/ciy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galimberti S., Baldini C., Baratè C., Ricci F., Balducci S., Grassi S. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S. Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;(July) doi: 10.1136/annrheumdis-2020-218122. Published Online First: 03. [DOI] [PMC free article] [PubMed] [Google Scholar]


