Abstract
The Ministry of AYUSH recommended the use of a decoction of the mixture of Ocimum tenuiflorum, Cinnamomum verum, Piper nigrum, Zingiber officinale, and Vitis vinifera as a preventive measure by boosting the immunity against the severity of infection caused by a novel coronavirus (COVID-19). The present study aimed to identify the probable modulated pathways by the combined action of AYUSH recommended herbal tea and golden milk formulation as an immune booster against COVID-19. Reported phytoconstituents of all the medicinal plants were retrieved from the ChEBI database, and their targets were predicted using DIGEP-Pred. STRING database and Cytoscape were used to predict the protein–protein interaction and construct the network, respectively. Likewise, MolSoft and admet SAR2.0 were used to predict the druglikeness score and ADMET profile of phytoconstituents. The study identified the modulation of HIF-1, p53, PI3K-Akt, MAPK, cAMP, Ras, Wnt, NF-kappa B, IL-17, TNF, and cGMP-PKG signaling pathways to boost the immune system. Further, multiple pathways were also identified which are involved in the regulation of pathogenesis of the multiple infections and non-infectious diseases due to the lower immune system. Results indicated that the recommended herbal formulation not only modulated the pathways involved in boosting the immunity but also modulated the multiple pathways that are contributing to the progression of multiple disease pathogenesis which would add the beneficial effect in the co-morbid patients of hypertension and diabetes. The study provides the scientific documentation of the role of the Ayurvedic formulation to combat COVID-19.
Keywords: AYUSH, COVID-19, Herbal tea, Immunity promotion
1. Introduction
The novel coronavirus disease (COVID-19) has spread over the globe infecting more than 2 million populations leading to more than 100 thousand deaths. Subjects suffering from infectious and non-infectious diseases including diabetes, hypertension, kidney disorder are considered at more risk from this viral infection due to lower immunity [1]. Hence, enhancing the immunity (natural body system) may possess the major contribution as a prophylactic measure against multiple pathogenic conditions as well as maintaining optimum health [2].
Ayurveda utilizes the concept of “Dinacharya” and “Ritucharya” to maintain a healthy life that utilizes the gifts of nature (herbal medicines) as daily/seasonal regimes to maintain healthy life [3]. Ayurveda; an ancient science of life suggests simplifying the lifestyle, and also promotes awareness in uplifting and maintaining one’s immunity via the utilization of many plants/herbs [3] which are easily available in the kitchen garden of a majority of the society.
Based on the Ayurvedic and scientific literature, the Ministry of AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy), India issued an advisory where it recommended the use of Kadha (herbal tea/decoction) composed of Ocimum tenuiflorum (Tulsi), Cinnamomum verum (Dalchini), Piper nigrum (Kalimirch), Zingiber officinale (Shunthi) and Vitis vinifera (Munakka) for self-care which will develop immunity against severe infection caused by COVID-19. Further, it also recommended the consumption of golden milk; a half teaspoon of Curcuma longa (turmeric) powder in 150 ml hot milk once or twice a day [3]. However, the scientific evidence of all these herbal combinations for boosting immunity has not been proposed yet. Hence, in the present study, we proposed to elucidate the probable interaction of the phytoconstituents from individual ingredients of the AYUSH recommended formulation to boost the immunity by gene-set enrichment and network pharmacology approach to support with scientific shreds of evidence.
2. Materials and methods
2.1. Mining of phytoconstituents and their targets
The phytoconstituents of 6 medicinal plants i.e. C. verum, C. longa, O. tenuiflorum, P. nigrum, Vitis Vinifera, Z. officinale were retrieved from ChEBI (https://www.ebi.ac.uk/chebi/) database. The targets of each phytoconstituents were identified using DIGEP-Pred [4] for proteins at the pharmacological activity (Pa) > 0.7.
2.2. Enrichment and network analysis
The list of probable targets was queried in the STRING [5] database, and enrichment analysis of protein–protein interaction was performed for biological process, molecular function, and cellular components. Further, the probably modulated pathways were also identified concerning the KEGG pathway database. Cytoscape [6] was used to construct the network among the plants, their phytoconstituents, modulated proteins, and regulated pathways. The network was treated as directed, node size as “low values to small sizes” and map node color from “low values to bright colors” and the whole network was analyzed based on edge count.
2.3. Druglikeness and ADMET profile
Druglikeness score of each phytoconstituent was calculated based on Lipinski’s rule of five [7] using MolSoft (https://molsoft.com/mprop/). Likewise, the probability for human intestinal absorption, caco-2, and blood–brain barrier permeability, human oral bioavailability, P-glycoprotein, CYP3A4, CYP2C9, CYP2D6, and CYP1A2 inhibition, eye irritation, Ames mutagenesis, human ether-a-go-go inhibition, and hepatotoxicity was predicted using admetSAR2.0 [8].
3. Results
3.1. Phytoconstituents and their targets
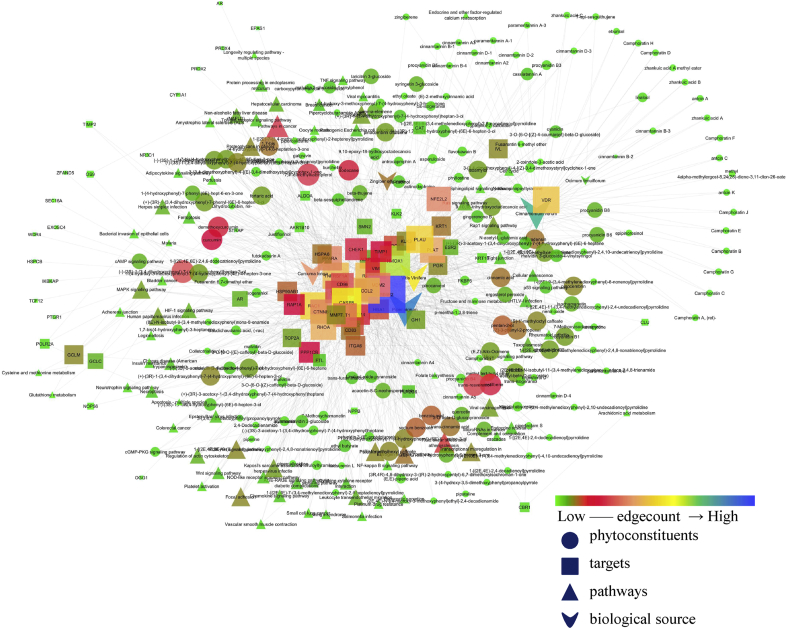
Total 221 phytoconstituents were identified in which 40 were from C. verum, 27 from C. longa, 3 from O. tenuiflorum, 91 from P. nigrum, 40 from V. vinifera, and 20 from Z. officinale in which 173 compounds were identified to modulate the proteins at Pa>0.7. Among them, (−)-(3S)-1-(3,4-hydroxyphenyl)-7-(4-hydroxyphenyl)heptan-3-ol was predicted to have the highest interaction with multiple proteins i.e. 18. Likewise, MMP2 was predicted to be a majorly targeted protein by 58 phytoconstituents. The list of phytoconstituents and the source of each compound are summarized in supplementary S1. By these 173 phytoconstituents, a total of 524 interactions were made with multiple proteins. The complete interaction of the multiple phytoconstituents with their targets and regulated pathways is represented in Fig. 1.
Fig. 1.
Interaction of bioactives from medicinal plants with their proteins and regulated pathways. In the figure higher the node interaction, the higher is its size and vice versa. Similarly, the node with lower interaction is indicated by green color and gradually increases to blue. Medicinal plants, their phytoconstituents, modulated targets, and regulated pathways are represented by ν, ●, ■, and ▲ respectively.
3.2. Gene set enrichment analysis
Gene set enrichment analysis identified eighty different pathways to be modulated by the bioactives from the herbal tea and golden tea in which pathways in cancer was majorly regulated with the lowest false discovery rate (5.37E-10) by modulating 17 genes i.e. AR, CASP8, CDK4, CTNNB1, ESR2, HMOX1, HSP90AB1, ITGA6, KLK3, MDM2, MMP2, NFE2L2, NOS2, NQO1, RAC1, RARA, and RHOA. Phytoconstituents were also identified to regulate the proteins which were involved in multiple pathogeneses (infectious and non-infectious diseases) including immunology (supplementary file 2; Table S1). Likewise, GO analysis identified the 29 proteins to be modulated from extracellular region i.e. ADIPOQ, AKR1B10, ALDOA, CAT, CCL2, CD14, CD86, CLU, ESR2, FLT1, FTL, GH1, HBA1, HMOX1, HSP90AB1, HSPA6, ITGA6, KLK2, KLK3, KRT1, MMP2, MMP7, NPPB, PLA2G6, PLAT, PLAU, TIMP1, TIMP2, and TNFRSF1A with the lowest false discovery rate (6.21E-07). Further, protein binding and response to the organic substance were identified as the primary regulated molecular function and biological processes respectively. Similarly, the protein–protein interaction of each modulated protein and hit GO analysis are represented in Fig. S1 and Fig. S2 of supplementary file 2 respectively. The detail of the GO analysis of regulated proteins is provided in supplementary file 3.
3.3. Druglikeness and ADMET profile
Among 221 phytoconstituents, eighty compounds were predicted to possess a positive druglikeness score in which camphoratin D from C. verum scored the highest druglikeness score i.e. 1.18 (Fig. S3). Among the 20 best hits from the list of phytoconstituents, all the compounds were predicted to get absorbed from the human intestinal tract. Among them, camphoratin B was predicted to have the highest oral bioavailability and was also predicted for the least toxicity profile like eye irritation, Ames mutagenesis, human either-a-go-go inhibition, and hepatotoxicity compared to the rest of the compounds (Fig. S4).
4. Discussion
Recently, AYUSH has advised utilizing Kadha (herbal tea/decoction) composing basil, cinnamon, black pepper, dry ginger, and raisin and golden milk to boost the immunity as a prophylactic measure against COVID-19. Due to the complex composition of multiple phytoconstituents from all these medicinal plants, the mixture of this combination could modulate multiple proteins and would help to boost immunity which can be explained via network pharmacology and gene-set enrichment analysis. Network pharmacology and gene set enrichment analysis are well-accepted approaches to identify the disease target, lead hit molecules and modulated pathways via “multiple component-protein interactions” [[9], [10], [11]].
Boosting immunity involves the modulation of multiple proteins that are involved in the homeostatic regulation. In the present study, the modulation of multiple pathways that are related to infectious/non-infectious diseases and the immune system are identified. A subject suffering from infectious/non-infectious diseases with compromised immune response [12]; are at higher risk to be affected by COVID-19. Hence, the combined action of herbal tea and golden milk which has been recommended by the AYUSH is not limited over the boosting of the immunity but also may modulate other pathways which are involved in the pathogenesis of multiple diseases provides beneficial effect to the patients with diabetes and hypertension.
Enrichment analysis of modulated proteins identified the regulation of HIF-1, p53, PI3K-AKT, MAPK, cAMP, Ras, Wnt, NF-kB, IL-17, TNF, and cGMP-PKG signaling pathway which are directly involved to boost immunity. The task of HIF-1α has been reported to be dysregulated in viral infection which is involved to boost the immunity by regulating the task of macrophages, neutrophils, dendritic cells, and lymphocytes during the hypoxic condition [13]; primarily occurs in COVID-19 infection due to improper exchange of O2/CO2 in lungs [14]. In the present study, we identified five genes i.e. ALDOA, FLT1, HMOX1, NOS2, and TIMP1 to be modulated related to this pathway. Further, PI3K-AKT functions as a rheostat in orchestrating the differentiation of memory CD8 T cells [15] which further regulate the functioning of multiple chemokines and cytokines [16] from multiple pathways like NF-k B, IL-17, and TNF signaling pathway which were found to be modulated in the present study. Hence, the next approach to boost the immunity by suggested Kadha could occur via regulation of PI3K-AKT, NF-kβ, interleukin, and TNF mediated cytokine regulation. Further, in infectious diseases including viral infection MAPK pathway gets targeted by pathogens. Since this pathway is involved in the synthesis of immunomodulatory cytokines like interleukins and TNF-α via the activation of p38 MAPK pathways, modulation of this pathway could play important coordination with an immune response via the promotion of Th1 and Th2 against extracellular infectious agents [17]. In the present study, the modulation of six proteins i.e. CD14, FLT1, HSPA6, RAC1, RAP1A, TNFRSF1A indicates the regulation of the MAPK pathways. Further, in COVID-19 there is an increase in cell apoptosis and necrosis in lung tissue. As the phytoconstituents were also predicted to modulate the Ras signaling pathway, the suggested herbal tea could also function in multiple cellular tasks including the regulation of cell survival and proliferation [18]. Additionally, we also identified the multiple pathways (Table S2) which get modulated in the pathogenesis of multiple infectious (bacterial/viral) and non-infectious diseases (diabetes, obesity, hypertension, etc) in which the immune system is compromised. Hence, the intake of this tea would also add a beneficial effect to maintain daily lifestyle and also improve the immunity system. The main ingredient of golden milk i.e. C. longa constitutes curcuminoids as an active biomarker that has been reported to possess anti-inflammatory and anti-viral properties [19]. Similarly, tulsi (Ocimum sanctum) is also reported for its detoxifying property and protection against infections which would help to clear the body from dead tissue after viral infection. Additionally, it is also reported to possess the anti-viral, anti-inflammatory property which would also help in healing the infected tissues in the lungs [20]. Likewise, other ingredients of herbal tea i.e. C. verum [21], P. nigrum (composes the piperamides) [22], Z. officinale [23], and V. vinifera [24,25] are also reported for their potential antiviral and anti-inflammatory property. Since COVID-19 is associated with viral infection and inflammation of lung tissues, the anti-viral and anti-inflammatory properties of the above medicinal plants may add a beneficial effect against COVID-19.
The present study also attempted to identify the lead hit molecules with positive druglikeness scores which could be responsible to boost immunity. The druglikeness score was assessed using Lipinski’s Rule of Five which evaluates any synthetic or semi-synthetic organic molecule or any secondary metabolite from a medicinal plant to act like a drug for oral bioavailability based on its molecular weight, a number of hydrogen bond donor and acceptor, and lipophilicity [7]. The 20 lead hits i.e. cinnamtannin A2, B1, and D1, cassiatannin A, parameritannin A-1, procyanidin B1, and B2, zhankuic acid B, C, A methyl ester, camphoratin A, B, C, D, E, F, and J, antcin A and C, and methyl 4-α-methylergost-8,24 (28)-diene-3,11-dion-26-oate showing positive druglikeness score were also evaluated for their pharmacokinetic and toxicity probabilities in which cinnamtannin A2, B1, D1, and procyanidin B1 and B2 were predicted for high hepatotoxicity. Similarly, the above molecules were also predicted as hERG inhibitors and ames toxic. However, these predictions were based on the comparison of 20 best hits of positive druglikeness bioactives and further confirmations are to be made using suitable wet-lab experiments to confirm these findings.
Since the Ministry of AYUSH suggested the oral intake of the herbal tea and golden milk, we attempted to identify the probable lead hits to get absorbed from the human intestinal tract in which the majority of the compounds were predicted to be absorbed from the gastrointestinal tract. Based on the Rule of Five, we identified camphoratin D to possess the highest drug-likeness score with the molecular weight of 486.30, 6 hydrogen bond acceptors, 3 hydrogen bond donors, and 3.47 MolLogP; upregulated vitamin D3 receptor. Similarly, camphoratin B was predicted to have the highest human oral bioavailability. However, the single-molecule may not be as effective compared to the advised herbal tea as the amount required to boost the immunity may not be sufficient enough. Hence, intake of the multiple compounds in the form of herbal tea as suggested by AYUSH could be more beneficial rather than a single molecule for boosting the immune system as a prophylactic against COVID-19. Additionally, AYUSH has recommended utilizing the herbal tea in which only hydrophilic compounds may get solubilized. However, in the present study, we identified the combined interactions of all the hydrophilic and hydrophobic bioactives (Fig S3; MolLogP value) suggested in this process. Hence, the study suggests identifying if all the bioactives are extracted in the herbal tea or not via appropriate analytical techniques. However, this process is not required in golden milk as the whole powder is dissolved in the milk to drink. As the present study is a preliminary in-silico study, it is very difficult to predict the exact nature of phytoconstituents. Further, more elaborative biologically guided fractionation and identification of the major bioactives and validation by suitable in vivo studies are required to confirm the findings which are the future scope of the study. One of the major limitations of the present study is neither in-vitro nor in-vivo experimental data are provided to confirm immune-modulatory and anti-viral activities. Further, one caveat of this study could be that we did not measure the anti-covid potential of these formulations in experimental studies to validate the findings from above in-silico investigations.
5. Conclusion
The present study evaluated the immunomodulatory effect of the AYUSH recommended Kadha (herbal tea) and golden tea simultaneously which could act as a prophylactic against COVID-19. Further, the study also identified the regulation of multiple signaling pathways like HIF-1, Estrogen, Rap1, p53, PI3K-Akt, Toll-like receptor, MAPK, cAMP, Ras, Wnt, Adipocytokine, NOD-like receptor, Chemokine, NF-kappa B, IL-17, TNF, Sphingolipid, and cGMP-PKG which could be beneficial to the subjects with immune system compromised pathogenesis.
Sources of funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2020.11.004.
Contributor Information
B.M. Patil, Email: drbmpatil@klepharm.edu, bmpatil59@hotmail.com.
Yadu Nandan Dey, Email: yadunandan132@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Opitz B., van Laak V., Eitel J., Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181(12):1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson L.B. The immune system. Essays Biochem. 2016;60(3):275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of AYUSH . 2020. Ayurveda’s immunity boosting measures for self care during COVID 19 crisis.https://www.ayush.gov.in/docs/123.pdf Available at: [Google Scholar]
- 4.Lagunin A., Ivanov S., Rudik A., Filimonov D., Poroikov V. DIGEP-Pred: web service for in silico prediction of drug-induced gene expression profiles based on structural formula. Bioinformatics. 2013;29(16):2062–2063. doi: 10.1093/bioinformatics/btt322. [DOI] [PubMed] [Google Scholar]
- 5.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipinski C.A. Lead- and drug-like compounds: the rule-of five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Yang H., Lou C., Sun L., Li J., Cai Y., Wang Z., et al. admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–1069. doi: 10.1093/bioinformatics/bty707. [DOI] [PubMed] [Google Scholar]
- 9.Khanal P., Patil B.M., Chand J., Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat Prod Bioprospect. 2020;10(5):325–335. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanal P., Patil B.M. Gene set enrichment analysis of alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed. 2019;9(6):263–270. doi: 10.4103/2221-1691.260399. [DOI] [Google Scholar]
- 11.Khanal P., Patil B.M. Integration of network and experimental pharmacology to decipher the antidiabetic action of Duranta repens L. J Integr Med. 2020 doi: 10.1016/j.joim.2020.10.003. S2095-4964(20)30109-16. [DOI] [PubMed] [Google Scholar]
- 12.NCBI (US) National Center for Biotechnology Information (US); Bethesda (MD): 1998. Genes and disease.https://www.ncbi.nlm.nih.gov/books/NBK22243/ [Internet] 1998-. Diseases of the Immune System. Available from: [Google Scholar]
- 13.Palazon A., Goldrath A.W., Nizet V., Johnson R.S. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E.H., Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front Immunol. 2013;4:20. doi: 10.3389/fimmu.2013.00020. Published 2013 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenway-Lynch C.S., Das A., Lackner A.A., Pahar B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2014;88(16):9442–9457. doi: 10.1128/JVI.00774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares-Silva M., Diniz F.F., Gomes G.N., Bahia D. The mitogen-activated protein kinase (MAPK) pathway: role in immune evasion by trypanosomatids. Front Microbiol. 2016;7:183. doi: 10.3389/fmicb.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson D.S., Chen Y.H. Ras family of small GTPases in immunity and inflammation. Curr Opin Pharmacol. 2012;12(4):458–463. doi: 10.1016/j.coph.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen M.M. Tulsi - Ocimum sanctum: a herb for all reasons. J Ayurveda Integr Med. 2014;5(4):251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Y., Jia L.N., Honma N., Hosono T., Ariga T., Seki T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects - a review. J Tradit Complement Med. 2012;2(1):27–32. doi: 10.1016/s2225-4110(16)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mair C.E., Liu R., Atanasov A.G., Schmidtke M., Dirsch V.M., Rollinger J.M. Antiviral and anti-proliferative in vitro activities of piperamides from black pepper. Planta Med. 2016;82(S 01):S1–S381. doi: 10.1055/s-0036-1596830. [DOI] [Google Scholar]
- 23.Kaushik S., Jangra G., Kundu V., Yadav J.P., Kaushik S. Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. Virusdisease. 2020;31(3):1–7. doi: 10.1007/s13337-020-00584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X., D’Souza D.H. Grape seed extract for control of human enteric viruses. Appl Environ Microbiol. 2011;77(12):3982–3987. doi: 10.1128/AEM.00193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo F., Di Lorenzo C., Regazzoni L., Fumagalli M., Sangiovanni E., Peres de Sousa L., et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019;10(4):1797–1807. doi: 10.1039/c8fo02175a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.