Highlights
-
•
High utilization of clinical preventive services reduces mortality during outbreaks.
-
•
The prevention of comorbidities can reduce the COVID-19 death toll.
-
•
Prevention of hypertension bears the highest potential to decrease COVID-19 deaths.
Keywords: Covid-19, Public health, Preventive medicine
Abstract
The recent COVID-19 pandemic has highlighted inadequacies in both national and international preparedness. The outbreak has resulted in an overburdening and incapacitation of health systems worldwide, as well as numerous deaths of individuals with comorbidities.
We have performed a simulation study to examine the effect of comorbidities and their prevention on the clinical outcome and mortality of patients during the COVID-19 pandemic. The data from past and present outbreaks indicate that individuals with comorbidities are significantly more susceptible to infections and yield poorer clinical outcomes. Our simulation study revealed that the prevention of morbidities like hypertension, diabetes, and cardiovascular disease bears an enormous potential to decrease the COVID-19 death toll. The accumulating evidence emphasizes our ability to reduce both the susceptibility of uninfected individuals to pathogenic factors, as well as the mortality of infected individuals during pandemics, by adopting a more comprehensive approach to disease prevention. Higher utilization of clinical preventive services is critical to reduce pandemic deaths and increase our preparedness for future outbreaks.
1. Introduction
Pandemics are outbreaks of infectious diseases that result in increased morbidity and mortality over a wide geographic area that cause significant economic, social, and political disruption. Unfortunately, recent events, most notably the COVID-19 outbreak, have highlighted inadequacies in both national and international preparedness and response. Not only the government and state leaders face difficulties, but the problem also affects every citizen. Numerous civilians are forced to deal with panic, insecurity, misinformation, and doubt. To further advance our preparedness and ability to respond to infectious disease outbreaks, it is crucial to identify the most significant challenges facing policymakers, based on past evidence, existing data, and future predictions. In this paper, we present data showing health status as a potentially significant factor in pandemic preparedness.
2. Outbreaks on the rise
Pandemics have occurred throughout history and appear to be increasing in frequency. Leading experts have been declaring for years that another pandemic whose rate and severity will match those of the Spanish flu is a matter not of if but of when (Gates, 2015). Evidence points out that the risk of outbreaks has grown over the past century (Morse, 1995, Jones et al., 2008). Numerous factors, such as population growth, urbanization, increased travel and interconnectedness, a higher requirement for animal protein, habitat loss, environmental changes, and growing interactions at the human-animal interface affect the risk of a pandemic event by increasing the likelihood of a spark event or the potential spread of a pathogen (Tilman and Clark, 2014, Zell, 2004). Probabilistic modeling and analytical tools such as exceedance probability have shown that in any given year, the probability of influenza pandemic is about 1% (Group, 2018). Data published by the Institute for Disease Modeling shows that if a highly contagious and lethal airborne pathogen, like the 1918 influenza, appeared today, over 30 million people globally would die in just six months (Gates, 2018).
Less than two decades into the century, the world has already witnessed numerous outbreaks of varying degrees of contagiousness and lethality (Table 1). Currently, the world is struggling with the COVID-19 outbreak. In less than four months after the onset, the number of confirmed cases has reached almost two million and continues to rise. With the global population predicted to reach close to 10 billion by 2050, and with a steady increase in travel and trade, public health systems will have less time to identify and contain an infection before it spreads.
Table 1.
Notable epidemics and pandemics of the 21st century, as of April 2020.
| Type | Outbreak | Starting year | Localization (no. of countries) | Morbidity | Mortality | Lethality | Reference |
|---|---|---|---|---|---|---|---|
| pandemic | COVID-19 | 2019 | 199 | 1 844 863 | 117 021 | 6,34% | (World Health Organization, 2019) |
| Zika virus | 2015 | 87 | 3 589 confirmed cases in both Americas India: 290 Thailand:1,698 | – | – | (WHO, 2019) | |
| Swine flu influenza | 2009 | Global | 753 500 000–1 233 000 000 | 151,700–575,400 | 0,05% | (Dawood et al., 2012, Kelly et al., 2011) | |
| SARS | 2003 | 37 | 8 098 | 744 | 9,19% | (Wang and Jolly, 2004) | |
| epidemic | West Africa Ebola virus disease | 2013 | 10 | 28 616 | 11 310 | 39,52% | (World Health Organization, 2016) |
| MERS | 2012 | 27 | 2 519 | 866 | 34,3% | (World Health Organization, 2019) |
3. Pandemic preparedness and response
The growing concerns about the outbreak threat led individual countries as well as the World Health Organization to devise plans and strategies for increasing pandemic preparedness (Group, 2018, Achonu et al., 2005, World Health Organization, 2005)
On 15th September 2020, WHO published a Global Burden of Disease Study, highlighting the urgent need for all public health drivers to improve preventive care services. The current syndemic occurrence of chronic diseases, social inequalities, and COVID-19 can be only a harbinger of future communicable disease pandemics. However, global healthy life expectancy is continuously rising (between 1990 and 2019), the overall life expectancy recorded a lower increase. Collected data indicates that we are living more and more years in poor health.
The three risks associated with the highest number of deaths are:
-
1.
High systolic blood pressure (10.8 million deaths)
-
2.
Tobacco (8.71 million deaths)
-
3.
Dietary risks (e.g., low fruit, high salt) (7.94 million deaths)
It is crucial to notice the value of preventive measures and lifestyle modifications in limiting these statistics.
Oppenheim et al. have developed an Epidemic Preparedness Index, which includes health capacities as well as non-health system factors to measure a nation's capacity to detect and respond to outbreaks (Oppenheim et al., 2019). The index takes into consideration several critical capacities, such as:
-
•
Government health system capable of identifying, tracking, managing, and treating patients;
-
•
Adequate infrastructure to disseminate information and allocate resources;
-
•
Fundamental bureaucratic and public administration capacities;
-
•
Ability to mobilize financial reserves to pay for disease response and weather the economic shock of the crisis;
-
•
Ability to initiate adequate risk communications.
Top-ranked countries have efficient state institutions, strong economies, and satisfactory investment in the health sector. They have established specific competencies crucial to identifying and handling disease outbreaks, in particular surveillance, mass vaccination, and risk communications. Poorly ranked countries are likely to suffer from political uncertainty, inefficient public administration, scarce resources for public health, and gaps in fundamental outbreak detection and response systems.
However, the risk of pathogen spread is not only determined by the level of preparedness of a nation. The initiation and progression of an outbreak are influenced by pathogen-specific factors, in particular genetic adaptation and mode of transmission, as well as human-population factors, such as the density of the population and the susceptibility to infection (Sands et al., 2016). It is well-established that factors that affect an individual’s immune system, such as comorbid diseases and obesity, amplify transmission rates, and increase morbidity and mortality (Toole, 1990, Brundage and Shanks, 2008, Murray et al., 2006). The susceptibility of uninfected individuals to the pathogenic factor is primarily determined by their health status. Thus, a good overall level of health of the population emerges as a critical way to prevent mass casualties and to avoid overburdening and incapacitating a health care system, which may lead to a twofold increase in all-cause mortality during outbreaks (Simonsen et al., 2013). We propose that the health status of a population is a factor that has not yet been incorporated into pandemic preparedness considerations.
4. Susceptibility to infections
The data from past and present outbreaks indicate that individuals with comorbidities are more susceptible to infections and yield poorer clinical outcomes. For instance, the 2019-nCoV infection is more likely to affect individuals with comorbidities and may result in severe and even fatal respiratory diseases such as acute respiratory distress syndrome (Chen et al., 2020). A recent study from the COVID-19 outbreak in China reported that patients with underlying diseases, such as hypertension, diabetes, and cardiovascular disease, were at higher risk of developing severe disease and receiving intensive care unit (ICU) care (Wang et al., 2020). Moreover, patients with preexisting comorbid conditions had a significantly elevated case-fatality rate- 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer (Wu and McGoogan, 2020).
During the H1N1 influenza pandemic, reports on risk factors showed that obesity and hypertension not only increased the risk of death (odds ratio (OR) 2.74 and 1.49, respectively) but also were significantly associated with the requirement for hospitalization, ICU care, and ventilator support (Mertz et al., 2013). Moreover, the risk of severe outcomes after hospitalization was highest among patients with diabetes (relative risk (RR) 2.2) and with preexisting heart disease (RR 2.1) (Campbell et al., 2010). Also, it was found that smokers were at a significantly higher risk of mortality (OR 5.97) (Al et al., 2014). Similarly, during the outbreak of severe acute respiratory syndrome (SARS), the presence of comorbidities increased the mortality risk to RR 9.0, with heart disease (RR 9.2), and diabetes (RR 4.7) being the most critical comorbidities (Chan et al., 2003). The presence of comorbidities in patients with Middle East respiratory syndrome (MERS) was associated with the development of severe disease. MERS patients with underlying diseases, such as obesity, diabetes, and cardiac disease, had around four times higher risk of mortality (RR 3.74) (Yang et al., 2017).
It is evident that the population's health status is a crucial determinant of mortality during outbreaks of infectious diseases.
5. The effect of comorbidities on mortality of patients with COVID-19 – A simulation study
To date, there is no published data on the effect of comorbidities on patients with COVID-19 in developed countries. To gain an insight into the effect of comorbidities on mortality of patients suffering from COVID-19 in developed countries, we conducted a simulation study based on results reported by Wu et al. applied to the adult population of the United States (Wu and McGoogan, 2020). We propose the following set of assumptions for the simulation:
-
1.
We assume that the probability of SARS-CoV-2 infection is equal for both patients with comorbidities and patients without comorbidities. We consider this assumption realistic because current preventive measures in the United States, including social distancing and quarantine, apply to both groups. We have decided to study the case of the United States in this simulation because of its large population, a high percentage of patients with comorbidities, and availability of reliable data for analysis.
-
2.
We assume that case-fatality rates for patients with hypertension (6%), diabetes (7.3%), and cardiovascular disease (10.5%) reported by Wu et al. can be a reasonable starting point for simulation for the adult population of the United States (Wu and McGoogan, 2020).
-
3.
We assume that the current case-fatality rate for the United States reflects the actual susceptibility of adult Americans to COVID-19. At the time of this analysis, the CDC reports 24,582 deaths and 605,390 cases of COVID-19 as of April 15th, 2020 (the study was performed in March and April 2020 in Wroclaw, Poland), which yields a case-fatality rate of COVID-19 of 4.06%. This figure is significantly higher than CFR 2.3% reported by Wu et al (Wu and McGoogan, 2020).
Demographic characteristics of COVID-19 cases as of April 14th, 2020, yields 7,001 cases among persons under the age of 18 out of 398,852 in total, which is 1.76%. No information on the age structure of deaths is available to the authors. Nevertheless, exclusion of persons under the age of 18 from the CFR calculation would not change it significantly, and so the figure of 4.06% will be used in this simulation as CFR of COVID-19 for the adult population of the United States.
In order to simulate the effect of comorbidities on mortality of patients with COVID-19, we require information on the percentage of the adult population suffering from each of the analyzed comorbidities:
-
1.
108 million, or 45% of adults in the United States have hypertension defined as a systolic blood pressure ≥ 130 mm Hg or a diastolic blood pressure ≥ 80 mm Hg or are taking medication for hypertension (Centers for Disease Control and Prevention, 2019);
-
2.
million, or 13% of all US adults had diagnosed or undiagnosed diabetes (Report, 2020);
-
3.
Excluding high blood pressure, the prevalence of the cardiovascular disease among US adults is 9% overall (Benjamin et al., 2019).
Based on the assumptions above, we calculate the case-fatality rate for patients without each of the analyzed comorbidities and implied risk ratios:
-
1.
Patients without hypertension need to have a case-fatality rate of 2.47% in order to obtain CFR of 4.06% for the entire adult population of the United States, given the fact that 45% of US adults have hypertension and assumed CFR for persons with hypertension is 6% (assumption 2). This yields an implied risk ratio of 2.43.
-
2.
Patients without diabetes need to have a case-fatality rate of 3.58% in order to obtain CFR of 4.06% for the entire adult population of the United States, given the fact that 13% of US adults have diabetes and assumed CFR for persons with diabetes is 7.3% (assumption 2). This yields an implied risk ratio of 2.04.
-
3.
Patients without cardiovascular disease need to have a case-fatality rate of 3.42% in order to obtain a CFR of 4.06% for the entire adult population of the United States given the fact that 9% of US adults have cardiovascular disease and assumed CFR for persons with cardiovascular disease is 10.5% (assumption 2). This yields an implied risk ratio of 3.07.
Given the implied risk ratios for hypertension, diabetes, and cardiovascular disease, we can simulate the percentage of prevented COVID-19 deaths due to reduced comorbidity. The results of the simulation are presented in Table 2. A limitation of the simulation study is that it does not take into consideration the occurrence of more than one comorbidity, nor the subtype (e.g. diabetes type 1 or type 2) and the clinical stage of the disease.
Table 2.
Percentage of prevented COVID-19 deaths due to reduced comorbidity.
| Hypertension | Diabetes | Cardiovascular disease | ||
|---|---|---|---|---|
| Reduction of prevalence of comorbid disease | 5% | 2,0% | 0,6% | 0,8% |
| 10% | 3,9% | 1,2% | 1,6% | |
| 15% | 5,9% | 1,8% | 2,4% | |
| 20% | 7,8% | 2,4% | 3,1% | |
| 25% | 9,8% | 3,0% | 3,9% | |
| 30% | 11,7% | 3,6% | 4,7% |
Based on our simulation, prevention of hypertension bears the highest potential to decrease the COVID-19 death toll – up to almost 12% if the population with hypertension is reduced by 30%. On the other hand, prevention of diabetes has the lowest potential to limit COVID-19 deaths of the three studied comorbidities – due both to a relatively low-risk ratio and prevalence of diabetes.
It is essential to mention that the results presented in this simulation also do not depend on the percentage of the population infected by the coronavirus. Of course, the higher the infected population, the higher the total mortality, and the higher the number of prevented deaths given the reduction of the prevalence of the particular comorbid disease.
At the time of this analysis, the actual risk ratios for COVID-19 for patients with hypertension, diabetes, or cardiovascular disease in the United States remain unknown. In order to study the sensitivity of presented simulation results to changes of implied risk ratios, we calculated the percentage of prevented COVID-19 deaths due to reduced prevalence of comorbid disease as a function of implied risk ratios based on two assumptions:
-
1.
We assume that the probability of becoming infected by COVID-19 is equal for both patients with comorbidities and patients without comorbidities.
-
2.
We assume that higher utilization of clinical preventive services will reduce the population suffering from analyzed comorbidities by at least one-fourth (25%) (Adler et al., 2015, Borsky et al., 2018)
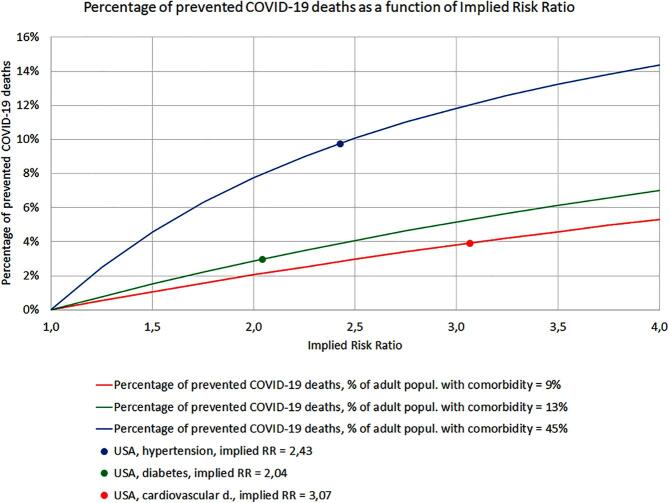
The results are shown in Fig. 1. Each curve represents the percentage of prevented COVID-19 deaths as a function of the implied risk ratio connected to each of the three studied comorbid diseases. Curves for cardiovascular disease (9% prevalence) and diabetes (13% prevalence) are relatively flat and seem almost linear– the potential for preventing COVID-19 deaths remains below 8%, even for risk ratios as high as 4. The third curve corresponds to hypertension with a 45% prevalence in US adults and has a high potential of preventing COVID-19 deaths – above 5% even for risk ratios as low as 1.6.
Fig. 1.
The percentage of prevented COVID-19 deaths as a function of Implied Risk Ratio. Each curve represents the percentage of prevented COVID-19 deaths as a function of the implied risk ratio connected to each of the three studied comorbid diseases. Curves for cardiovascular disease (9% prevalence) and diabetes (13% prevalence) are relatively flat and seem almost linear– the potential for preventing COVID-19 deaths remains below 8% even for risk ratios as high as 4. The third curve corresponds to hypertension with a 45% prevalence in US adults and has a high potential of preventing COVID-19 deaths – above 5% even for risk ratios as low as 1.6.
It is worth pointing out that as long as assumption 1 holds (the probability of becoming infected by COVID-19 is equal for both patients with comorbidities and patients without comorbidities), the results presented above do not depend on the percentage of the population infected by the coronavirus, nor do they depend on the actual case-fatality rates for patients with and without comorbidities. This means that the prevention of diseases like hypertension, with a very high prevalence in the population, will yield a significant reduction of mortality during future outbreaks of diseases like COVID-19, even if the actual risk ratios turn out to be moderately low.
Economic evaluations of preventive services show favorable cost-effectiveness of the majority of interventions intended to prevent or control morbidities at an early stage, in particular conditions that are responsible for a large share of the world’s burden of diseases, such as diabetes, hypertension, and cardiovascular disease (Van Gils et al., 2011, Li et al., 2010, Vos et al., 2010, Cohen et al., 2008). In the face of damaging consequences of an outbreak on the economy and increasingly constrained resources, preventive activities emerge as a realistic way of achieving better health results.
6. Population health and preventive health services
It is estimated that 60% of adult Americans suffer from at least one chronic disease or condition, and over 40% have multiple morbidities (Buttorff and Ruder, 2017). By 2030, more than 80 million people alone in the United States will have at least three chronic diseases (Waters, 2018).
Chronic conditions, including cardiac disease, cancer, chronic lung disease, stroke, diabetes, are the leading causes of poor health, long-term disability, and death. Approximately one-third of all deaths are attributable to heart disease or stroke, and every year, nearly 2 million people are diagnosed with cancer (Center for Disease Control and Prevention, 2020).
Health care systems worldwide do not currently leverage available resources to support prevention strategies; on the contrary, we are observing disproportionate emphasis on sick care rather than helping people stay well (World Health Organization, 2008). A bulk of health care expenses in most developed countries can be attributed to the diagnosis and treatment of chronic diseases and conditions which can be effectively prevented (Center for Disease Control and Prevention, 2020).
Clinical preventive services are available for multiple chronic morbidities. These services include interventions before the occurrence of the disease (primary prevention), detection and treatment of the disease at an early stage (secondary prevention), and management of the disease to slow or stop its progression (tertiary prevention). These strategies, coupled with lifestyle modifications, can considerably decrease the incidence of chronic diseases, as well as disability and death associated with chronic disease (Centers for Disease Control and Prevention UD, 2009). Nonetheless, clinical preventive services are massively underutilized despite the escalating burden of chronic diseases, the availability of evidence-based instruments to prevent them, and the well-established effectiveness of prevention strategies (Centers for Disease Control and Prevention UD, 2009, US Department of Health and Human Services, 2018, Adepoju et al., 2015). For instance, only 8% of Americans receive all recommended, high-priority, appropriate clinical preventive services, and nearly 5% received none (Borsky et al., 2018). A 2019 study found that clinical preventive services are mostly underutilized due to an implementation gap, which is caused by disproportionate financial incentives for healthcare providers that prioritizes treatment over prevention (Levine et al., 2019). A critical improvement in the quality of health care could be achieved by increasing the number of people who receive proven preventive services that are demonstrated to reduce mortality and increase the quality of life.
7. Conclusion
The recent COVID-19 pandemic has highlighted inadequacies in both national and international preparedness. Evidence points out that population health management emerges as a critical way to prevent mass casualties and to avoid overburdening and incapacitating a health care system during pandemics. Current models emphasize our ability to reduce the susceptibility of uninfected individuals to a pathogenic factor, as well as the mortality of infected individuals during pandemics by improving the population's health status. In this paper, we have shown that higher utilization of clinical preventive services and a more comprehensive approach to the prevention of morbidities like hypertension, diabetes, and cardiovascular diseases will yield a significant reduction of mortality during future outbreaks and substantially increase pandemic preparedness. Pandemic preparedness should take population health status and disease management into consideration.
8. Contributor ship statement
SA conceived the idea, designed the study, and wrote the manuscript. JG collected the data and wrote the manuscript. BB performed the simulation study, SM and GM reviewed and edited the manuscript.
Funding
Project number 2020/ABM/COVID19/0005, financed by the Medical Research Agency, Poland and Wroclaw Medical University, grant number STM.A210.20.118. The publication was prepared under the project financed from the funds granted by the Ministry of Science and Higher Education in the “Regional Initiative of Excellence” programme for the years 2019–2022, project number 016/RID/2018/19, the amount of funding 11 998 121.30 PLN.
Data sharing statement
The authors confirm that the data supporting the findings of this study are available within the article.
CRediT authorship contribution statement
Siddarth Agrawal: Conceptualization, Methodology. Justyna Gołębiowska: Writing - original draft. Bartłomiej Bartoszewicz: . Sebastian Makuch: Writing - review & editing. Grzegorz Mazur: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
N.A.
References
- Gates B. The next epidemic — lessons from Ebola. N. Engl. J. Med. 2015;372(15):1381–1384. doi: 10.1056/NEJMp1502918. [DOI] [PubMed] [Google Scholar]
- Morse S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995;1(1):7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D., Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515(7528):518–522. doi: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- Zell R. Global climate change and the emergence/re-emergence of infectious diseases. Int. J. Med. Microbiol. Suppl. 2004;293:16–26. doi: 10.1016/S1433-1128(04)80005-6. [DOI] [PubMed] [Google Scholar]
- Group, W.B., 2018. Disease Control Priorities Improving Health and Reducing Poverty. doi:10.1596/978-1-4648-0527-1_ch8. [PubMed]
- Gates B. Innovation for Pandemics. N. Engl. J. Med. 2018;378(22):2057–2060. doi: 10.1056/NEJMp1806283. [DOI] [PubMed] [Google Scholar]
- Achonu C., Laporte A., Gardam M.A. The financial impact of controlling a respiratory virus outbreak in a teaching hospital: lessons learned from SARS. Can. J. Public Health. 2005;96(1):52–54. doi: 10.1007/BF03404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Health Regulations, 2005. Second Edition. doi:10.1177/146642407109100301.
- Oppenheim B., Gallivan M., Madhav N.K., Brown N., Serhiyenko V., Wolfe N.D., Ayscue P. Assessing global preparedness for the next pandemic: development and application of an Epidemic Preparedness Index. BMJ Glob Health. 2019;4(1):e001157. doi: 10.1136/bmjgh-2018-001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands P., El Turabi A., Saynisch P.A., Dzau V.J. Assessment of economic vulnerability to infectious disease crises. Lancet. 2016;388(10058):2443–2448. doi: 10.1016/S0140-6736(16)30594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole M.J. Prevention of excess mortality in refugee and displaced populations in developing countries. JAMA. 1990;263(24):3296. doi: 10.1001/jama.1990.03440240086021. [DOI] [PubMed] [Google Scholar]
- Brundage J.F., Shanks G.D. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg Infect Dis. 2008 doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.JL., Lopez A.D., Chin B., Feehan D., Hill K.H. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368(9554):2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- Simonsen L., Spreeuwenberg P., Lustig R. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013 doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L.i. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Mertz D., Kim T.H., Johnstone J. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013 doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Rodin R., Kropp R., Mao Y., Hong Z., Vachon J., Spika J., Pelletier L. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. Can. Med. Assoc. J. 2010;182(4):349–355. doi: 10.1503/cmaj.091823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al S.H., Ibrahim A.S., Al M.M., Al-Khal A.L., Shaath S., Hamza N.A. Epidemiology, risk factors, clinical features, and outcome of adult patients with severe pandemic A/H1N1/2009 influenza in Qatar: a retrospective study. Infect Dis. Clin. Pract. 2014 doi: 10.1097/IPC.0000000000000148. [DOI] [Google Scholar]
- Chan J.W.M., Ng C.K., Chan Y.H. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003 doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.M., Hsu C.Y., Lai C.C. Impact of comorbidity on fatality rate of patients with middle east respiratory syndrome. Sci. Rep. 2017 doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2013–2. Atlanta, GA US Dep Heal Hum Serv. 2019.
- Report NDS. National Diabetes Statistics Report, 2020. Natl Diabetes Stat Rep. 2020.
- Benjamin E.J., Muntner P., Alonso A. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019 doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Adler A.J., Prabhakaran D., Bovet P. Reducing cardiovascular mortality through prevention and management of raised blood pressure: a world heart federation roadmap. Glob Heart. 2015 doi: 10.1016/j.gheart.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Borsky A., Zhan C., Miller T., Ngo-Metzger Q., Bierman A.S., Meyers D. Few Americans receive all high-priority, appropriate clinical preventive services. Health Aff. 2018 doi: 10.1377/hlthaff.2017.1248. [DOI] [PubMed] [Google Scholar]
- Van Gils P.F., Tariq L., Verschuuren M., Van Den Berg M. Cost-effectiveness research on preventive interventions: a survey of the publications in 2008. Eur. J. Public Health. 2011 doi: 10.1093/eurpub/ckq069. [DOI] [PubMed] [Google Scholar]
- Li R., Zhang P., Barker L.E., Chowdhury F.M., Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010 doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos Theo, Rob Carter J.B., Veerman Lennert, Magnus Anne, Linda Cobiac M.B. ACE-Prevention; 2010. Assessing Cost-Effectiveness in Prevention. [Google Scholar]
- Cohen J.T., Neumann P.J., Weinstein M.C. Does preventive care save money? Health economics and the presidential candidates. N. Engl. J. Med. 2008 doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- Buttorff, C., Ruder, T.B.M., 2017. Multiple Chronic Conditions in the United States. https://www.rand.org/pubs/tools/TL221.html.
- Waters, H.G.M., 2018. The Costs of Chronic Diseases in the US.
- Center for Disease Control and Prevention. Health and Economic Costs of Chronic Diseases. National Center for Chronic Disease Prevention and Health Promotion.
- World Health Organization. The World Health Report 2008. primary health Care – Now more than ever. World Heal Rep. 2008. doi:10.12927/hcpol.2013.22778.
- Centers for Disease Control and Prevention UD of, Services H and H. The Power of Prevention. Chronic Disease . . . the Public Health Challenge of the 21st Century.; 2009. https://www.cdc.gov/%0Dchronicdisease/pdf/2009-Power-of-Prevention.pdf.
- US Department of Health and Human Services. Clinical Preventive Services, 2018.
- Adepoju O.E., Preston M.A., Gonzales G. Health care disparities in the post-affordable care act era. Am. J. Public Health. 2015 doi: 10.2105/AJPH.2015.302611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Malone E., Lekiachvili A., Briss P. Health care industry insights: why the use of preventive services is still low. Prev. Chronic Dis. 2019;16:E30. doi: 10.5888/pcd16.180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The Coronavirus Disease 2019 (COVID-19): Situation report-36. Who. 2020. doi:10.3928/19382359-20200219-01.
- WHO. Zika Epidemiology Update. Who. 2019.
- Dawood F.S., Iuliano A.D., Reed C. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012 doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- Kelly H., Peck H.A., Laurie K.L., Wu P., Nishiura H., Cowling B.J. The age-specific cumulative incidence of infection with pandemic influenza H1N1 2009 was similar in various countries prior to vaccination. PLoS One. 2011 doi: 10.1371/journal.pone.0021828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.D., Jolly A.M. Changing virulence of the SARS virus: the epidemiological evidence. Bull. World Health Organ. 2004 doi: 10.1590/S0042-96862004000700013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Ebola Situation Report March 30, 2016. 15 April. 2015. doi:10.1007/s13398-014-0173-7.2.
- World Health Organization (WHO). MERS Update Situation. 2019. doi:10.1017/CBO9781107415324.004.