Highlights
-
•
Chronic neurological disease and acute abnormalities are present in COVID-19 brain autopsies.
-
•
Acute hypoxic-injury, hemorrhage, and minimal inflammation are frequently observed.
-
•
Low levels of viral SARS-CoV-2 RNA are present; cellular source remains unknown.
Keywords: COVID-19, SARS-CoV-2, Brain autopsies, Neuropathology, Neuropathogenesis, Immunohistochemistry, Reverse transcriptase polymerase chain reaction
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19) for which there have been over 50 million confirmed cases and 1.2 million deaths globally. While many SARS-CoV-2 infected individuals are asymptomatic or experience respiratory symptoms, extrapulmonary manifestations, including neurological symptoms and conditions, are increasingly recognized. There remains no clear understanding of the mechanisms that underlie neurological symptoms in COVID-19 and whether SARS-CoV-2 has the potential for neuroinvasion in humans. In this minireview, we discuss what is known from human autopsies in fatal COVID-19, including highlighting studies that investigate for the presence of SARS-CoV-2 in brain and olfactory tissue, and summarize the neuropathological consequences of infection. Incorporating microscopic and molecular findings from brain tissue into what we know about clinical disease will inform best practice management guidance and direct research priorities as it relates to neurological morbidity from COVID-19.
1. Manuscript
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped, single-stranded positive-sense RNA betacoronavirus, is the causative agent of coronavirus disease 2019 (COVID-19), for which there have been over 50 million confirmed cases and 1.2 millions deaths worldwide as of November 8, 2020 [1,2]. Morbidity and mortality are more common in older individuals and those with comorbidities, including cardiovascular disease, hypertension, obesity, and diabetes, although young people with no comorbidities are also at risk for critical illness [[3], [4], [5]]. While many SARS-CoV-2 infected individuals are asymptomatic or experience predominantly respiratory symptoms, extrapulmonary manifestations, including neurological symptoms and conditions, are increasingly recognized [[6], [7], [8]]. The majority of current studies on neurological manifestations are case reports or retrospective series focused on hospitalized patients through the extraction of medical record data, which have described disorders of consciousness, delirium, and neuromuscular and cerebrovascular complications [[7], [8], [9], [10]]. Smell and taste disturbances in the absence of nasal obstruction are particularly characteristic of COVID-19, leading to speculation regarding the olfactory nerve as a possible route of central nervous system entry [11,12]. Other neurological findings include headache, myalgia, rhabdomyolysis, Guillain-Barre syndrome, encephalopathy, and myelopathy with rare cases of encephalitis based on imaging or cerebrospinal fluid [8,[13], [14], [15], [16], [17], [18]]. SARS-CoV-2 has not been detected in cerebrospinal fluid in the majority of patients tested [8,19], highlighting the need for studies of autopsy brain tissue to understand COVID-19 neuropathogenesis and develop neurocognitive preserving treatment strategies.
Autopsies provide a wealth of information about the decedents, regardless of whether a likely cause of death was identified pre-mortem [20,21]. Due to initial uncertainties regarding the infectious properties of SARS-CoV-2 and limitations in personnel and personal protective equipment availability, autopsies for COVID-19 patients have been limited, although an increasing number of studies are now being published (reviewed in [[22], [23], [24]]). Reports of detailed neuropathological examinations have lagged behind general autopsy series, in part due to the initial focus on lung pathology combined with the longer (2–3 weeks) formalin fixation time preferred by most neuropathologists before cutting brains. Additional factors include the reluctance of some institutions to perform brain removal in COVID-19 cases due to concerns over electric bone saw generated aerosols, which can be effectively contained through the use of vacuum filters or hand saws [25,26]. Included in this review are peer-reviewed studies of autopsy findings published in English between January 1, 2020, and November 5, 2020. Two different databases (PubMed, Google Scholar) were searched for key terms, including COVID-19, nCoV-2019, and SARS-CoV-2, crossed with autopsy, histology, histopathology, neuropathology, and post-mortem. This search was complemented with three review articles [[22], [23], [24]], text word searching and examining references in identified articles. A total of 24 studies were identified that included 149 individuals (range 1–43 subjects per series). Reported gross and microscopic findings and results of SARS-CoV-2 targeted studies are summarized in Table 1 . Representative gross, microscopic, and ultrastructural findings are illustrated in Fig. 1 .
Table 1.
Summary of Published COVID-19 Reports with Autopsy Brain Findings.
| Reference | No. Cases Included; autopsy type | Macroscopic Evaluation | Microscopic Evaluation | SARS-CoV-2 Protein | SARS-CoV-2 RNA |
|---|---|---|---|---|---|
| Puelles et al. 2020 [41] Wichmann et al. 2020 [49] Matschke et al. 2020 [35] |
43; subset full autopsy with brain findings | Edema (n = 23), fresh territorial infarct (n = 6) | Fresh ischemic infarct (n = 6), astrocytosis, microgliosis, perivascular, parenchymal, and leptomeningeal T cells (n = 43) | Viral spike or nucleocapsid IHC positive in 16/40 cases (rare cells in medulla; 2 cases with vagus or glossopharyngeal nerves) | qRT-PCR positive (13/27; median 4700 viral E gene copies/cell; range <1000 to 162,000) in frontal lobe and/or medulla |
| Solomon et al. 2020 [37] | 18; brain-only findings | No specific findings | Mild to moderate acute hypoxic injury (n = 18); rare foci of perivascular and leptomeningeal inflammation (n = 3) | Viral nucleocapsid IHC negative in all cases | qRT-PCR positive (n = 5; 5.0–59.4 N1/N2 copies/μL) |
| Remmelink et al. 2020 [32] | 11; full autopsy with brain findings | Recently drained subdural hematoma (n = 1); cerebral hemorrhage (n = 1) | Cerebral hemorrhage or hemorrhagic suffusion (n = 8), focal ischemic necrosis (n = 3), edema and/or vascular congestions (n = 5), diffuse or focal spongiosis (n = 10) | N.A. | qRT-PCR positive (n = 9; viral E gene; Ct: 28.67–35.11) |
| Schurink et al. 2020 [38] | 11; full autopsy with brain findings | No specific findings | Hypoxic changes, activation/clusting of microglia, astrogliosis, perivascular cuffing of T cells most prominent in olfactory bulbs and medulla (n = 11); neutrophilic plugs (n = 3) | Viral nucleocapsid IHC negative in 11 cases | N.A. |
| Fabbri et al. 2020 [31] | 10; full autopsy with brain findings | Edema and meningeal congestion (n = 10), cerebral infarction (n = 3), uncal herniation (n = 2), purulent leptomeninges (n = 1), subarachnoid hemorrhage (n = 1) | Global hypoxic-ischemic injury (n = 10), acute hypoxic injury (all), intravascular microthrombi (n = 10), macro and/or microinfarcts (n = 10); perivascular microhemorrhage (n = 10), microglial activation (n = 5), perivascular/leptomeningeal lymphocytic inflammation (n = 1) | N.A. | qRT-PCR positive in olfactory nerve and brain tissue in (n = 1; RdRp, E, and N genes) |
| Schaller et al. 2020 [50] | 10; full autopsy with brain findings | No specific findings | No specific findings | N.A. | N.A. |
| Hanley et al. 2020 [34] | 9; full autopsy with brain findings | Hemorrhagic conversion of middle cerebral artery stroke (n = 1) | Moderate to intense microglial activation; mild T- cell infiltrate around blood vessels and capillaries, and ischemic changes of variable extent in the neurons of the cortex and the white matter (n = 5) | N.A. | qRT-PCR positive (n = 4; 101 to 104 viral E gene copies per μg total RNA); Subgenomic viral RNA positive (n = 1; Ct ∼32) |
| Deigendesch et al. 2020 [36] Menter et al. 2020 [26] * |
7; full autopsy with brain findings | Moderate global brain edema without cerebral mass displacement (n = 1) | Microglial activation in pons, medulla, and olfactory bulb; sparse perivascular and leptomeningeal infiltrates of lymphocytes; mild acute hypoxic-ischemic encephalopathy (n = 3) | N.A. | qRT-PCR positive in olfactory bulb (n = 4), optic nerve (n = 2); not detected in brainstem or cerebellum (ORFab1, S, and N genes) |
| von Weyham et al. 2020 [27] | 6; full autopsy with brain findings | Massive hemorrhage and herniation (n = 2); petechial bleedings (n = 4) | Hypoxic alterations (n = 6); lymphocytic meningitis and encephalitis (n = 6); brainstem neuronal cell loss in (n = 4), axon degeneration (n = 3) | N.A. | N.A. |
| Bradley et al. 2020 [28] | 5; full autopsy with brain findings | Scattered punctate subarachnoid hemorrhages (n = 1) | Rare microhemorrhages in the brainstem (n = 1) | N.A. | N.A. |
| Kantonen et al. 2020 [30] | 4; full autopsy with brain findings | Mild brain swelling, discoloration of watershed areas, lacunar infarcts, and microhemorrhages in cerebral and cerebellar white matter, deep gray matter, and brain stem (n = 1) | High density acute microhemorrhages, severe hypoxic-ischemic injury, scattered T lymphocytes, and axonal spheroids (n = 1); mild to moderate hypoxic-ischemic injury (n = 3) | Viral spike IHC negative in brain, olfactory mucosa, and carotid body | qRT-PCR negative in brain and olfactory mucosa (RdRp, N. and E genes) |
| Bussani et al. 2020 [51] | 3; fill autopsy with brain findings | N.A. | Gliosis, neuronal loss, vascular rarefaction | N.A. | N.A. |
| Barton et al. 2020 [52] | 2; full autopsy with brain findings | No gross abnormalities | N.A. | N.A. | N.A. |
| Jaunmuktane et al. 2020 [29] | 2; brain-only findings | Large acute and subacute infarcts (n = 1); white matter microhemorrhages and microinfarcts (n = 1) | Hemorrhages and infarcts (n = 2); mild leptomeningeal inflammation (n = 1) | N.A. | N.A. |
| Kirschenbaum et al. 2020 [39] | 2; brain-only findings | N.A. | Perivascular leukocytic infiltrates in basal ganglia and intravascular microthrombi (n = 2); prominent leukocytic infiltrates in olfactory epithelium (n = 2) | N.A. | N.A. |
| Al-Dalahmah et al. 2020 [33] | 1; full autopsy with brain findings | Cerebellar hemorrhage, acute infarcts in the dorsal pons and medulla, tonsillar herniation | Global hypoxia; numerous microglial nodules and neuronophagia in the inferior olives and cerebellar dentate nuclei; mild perivascular and sparse parenchymal and leptomeningeal lymphocytes; perivascular hemorrhages; chronic active inflammation in olfactory epithelium; red neurons in olfactory bulb and normal tract | Viral nucleocapsid IHC negative | qRT-PCR positive in nasal epithelium (Mean Ct 31.75, 278 copies/μL RNA), olfactory bulb (Ct 36.70, 11 copies/μL); Cerebellar clot (Ct 33.0, 559 copies/μL), and cerebellum (Ct 37.17, 8 copies/μL); Viral ISH negative |
| Craver et al. 2020 [53] | 1; full autopsy with brain findings | No CNS lesions identified | No CNS lesions identified | N.A. | N.A. |
| Dolhnikoff et al. 2020 [54] | 1; full autopsy with brain findings | N.A. | Microglial reactivity | N.A. | N.A. |
| Lax et al. 2020 [55] | 1: full autopsy with brain findings | No acute alterations | No acute alterations | N.A. | N.A. |
| Paniz-Mondolfi et al 2020 [12] | 1; brain-only findings | N.A. | N.A. | TEM showed viral like particles in frontal lobe sections | qRT-PCR positive (four different assays targeting ORF1/a and E-gene, N1, N2, N3, N2 and E-gene, and ORF1ab and S genes) |
| Reichard et al 2020 [14] | 1; brain-only findings | Mild brain swelling and hemorrhagic white matter lesions | Focal hemorrhage, ADEM-like lesions, microinfarcts, damaged axons, hypoxic-ischemic injury | N.A. | N.A. |
Abbreviations: ADEM, acute disseminated encephalomyelitis; Ct, cycle threshold; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; E gene, SARS-CoV-2 envelope gene; ORF1ab, open reading frame 1ab; IHC, immunohistochemistry; ISH, in-situ hybridization; RdRp, RNA-dependent RNA polymerase gene; N.A., not available or evaluated; TEM, transmission electron microscopy.
Provided data on angiotensin converting enzyme – 2 (ACE2) IHC in brain tissue and olfactory bulb.
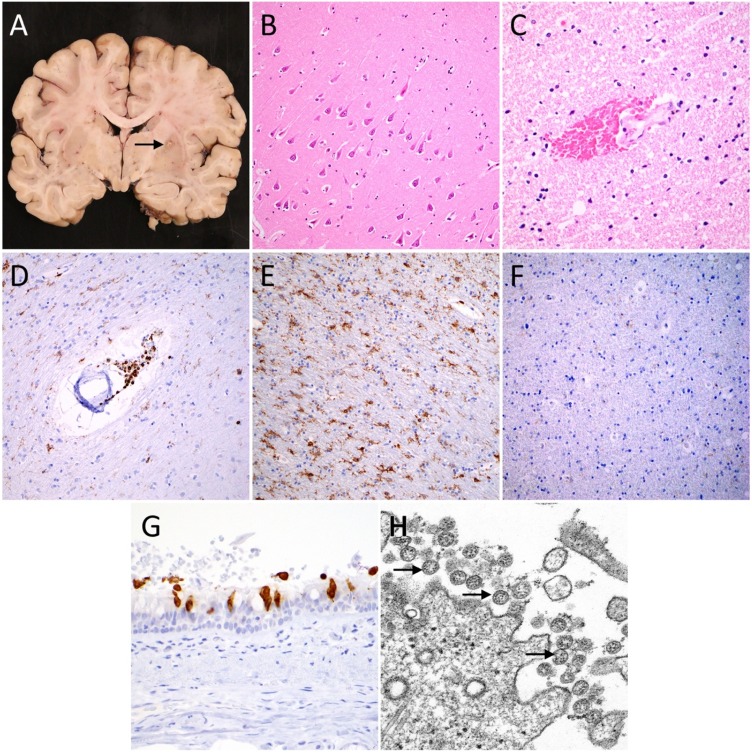
Fig. 1.
Neuropathological findings of COVID-19. (A) Coronal brain slice from a 55 year old man who died from COVID-19 contains a calcified nodule (arrow) in the right globus pallidus, but is otherwise unremarkable. (B) Hematoxylin and eosin stained section of hippocampus shows scattered hypereosinophilic neurons indicative of acute hypoxic injury. (C) Hematoxylin and eosin stained section shows extravasated red blood cells suggestive of microhemorrhage (deep pink). (D) CD45 immunostaining (brown) highlights a small collection of perivascular immune cells. (E) CD45 immunostaining (brown) also highlights numerous resident immune cells of the brain parenchyma (microglia). (F) In comparison to panel E, a patient without COVID-19 shows minimal CD45 immunostaining (brown). (G) SARS-CoV-2 nucleocapsid immunohistochemistry (brown) shows a cytoplasmic staining pattern in respiratory epithelial cells of the trachea. (H) Transmission electron micrograph of SARS-CoV-2 from cultured cells shows spherical extracellular viral particles (arrows). Images B-F taken at 200x magnification, G at 400x magnification, and are each from a different patient. Image H is from the Centers for Disease Control and Prevents Public Health Image Library, courtesy of Courtesy Cynthia S. Goldsmith and A. Tamin.
Gross brain autopsy findings were reported individually or in aggregate for 142 subjects. In keeping with the high prevalence of comorbidities in this patient population, evidence of prior brain disease was frequently identified, including neurodegeneration, prior strokes, tumor resection, demyelinating disease, and atherosclerosis. Acute gross abnormalities were much more limited, and a direct causal relationship with SARS-CoV-2 infection was not always straightforward to identify. A total of 92 (65 %) of the gross brain examinations reported either no significant findings or no acute abnormalities. Of the remaining 50 cases, multiple findings were often described in individual brains. Hemorrhage was the most common abnormality reported, ranging from petechial bleedings and punctate subarachnoid hemorrhages (n = 9) [14,[27], [28], [29], [30], [31]], to large cerebral/cerebellar hemorrhages (n = 4) [27,32,33], hemorrhagic conversion of middle cerebral artery stroke (n = 1) [34], and a recently drained subdural hematoma (n = 1) [32]. Large acute and/or subacute infarcts (n = 11) [29,31,33,35] as well as lacunar infarcts/microinfarcts and watershed infarcts (n = 2) [29,30] were identified in several cases. Severe edema resulting in herniation (n = 5) [27,31,33] as well as mild to moderate edema without herniation (n = 34) [14,30,31,35,36] were also present.
Microscopic findings were reported for 146 of the cases in these studies. Similar to the gross examinations, histopathology identified correlates of pre-existing disease, including neurodegeneration, chronic/subacute strokes, hepatic encephalopathy, and arteriolosclerosis. No specific findings were reported for 25 (17 %) of the cases. Mild to moderate acute hypoxic injury was the most common abnormality (n = 58) [14,27,30,31,33,34,[36], [37], [38]], while severe hypoxic-ischemic injury (n = 1) [30] and infarcts/focal ischemic necrosis (n = 22) [14,29,31,32,35] were identified in several cases. Focal microhemorrhage or hemorrhagic suffusion was also frequently reported (n = 23) [14,[28], [29], [30], [31], [32], [33]], although intravascular microthrombi (n = 12) [31,39] or neutrophilic plugs (n = 3) [38] were less common. Mild focal perivascular, parenchymal, and leptomeningeal T-cell predominant lymphocytic infiltrates were identified in a large number of cases without clear evidence of vasculitis or meningoencephalitis (n = 81) [27,[29], [30], [31],[33], [34], [35], [36], [37], [38], [39]]. Moderate to intense microglial activation was noted, particularly in the brainstem (n = 73), although similar results were also reported in COVID-19-negative individuals with systemic inflammatory/septic clinical courses [31,[33], [34], [35], [36],38]. Axonal damage was identified in a few cases (n = 5) [14,27,30]. Acute disseminated encephalomyelitis (ADEM)-like lesions were reported in a single case [14]. The olfactory system was examined to varying degrees, identifying prominent acute and chronic inflammation in the olfactory epithelium (n = 14) [33,38,39], microglial activation (n = 18) [36] and red neurons (n = 1) [33] in the olfactory bulb, and only unremarkable age-related corpora amylacea in olfactory tracts.
Researchers across the globe have employed multiple strategies to directly assess for the presence of SARS-CoV-2 in brain tissue, including immunohistochemistry, in situ hybridization (ISH), targeted quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), and transmission electron microscopy. At this time, immunohistochemistry, using antibodies that recognize the viral nucleocapsid (N) or spike (S) proteins, have been negative in most attempted human cases (n = 58) [30,33,35,37,38], with the exception of a recent case series that reported positive staining in vagus and glossopharyngeal nerves and scattered cells in the medulla in a total of 16 cases [35]; in situ hybridization for viral RNA has been negative (n = 1) [33]. Viral spike protein has been reported to be present in the olfactory epithelium in 5/6 patients; however, brain findings from these cases were not discussed [40]. A number of qRT-PCR assays have been employed targeting the N, S, envelope (E), open reading frame (ORF) 1/a, ORF1ab, or RNA-dependent reverse transcriptase (RdRp) genes, identifying low levels of virus in frozen or formalin-fixed paraffin-embedded brain tissue (34/84; 41 %) [12,[30], [31], [32], [33], [34], [35], [36], [37],41] and olfactory bulb/tract (n = 9/36; 25 %) [31,33,36,37]. Viral subgenomic RNA, a marker of actively replicating virus, was positive in a single case (n = 1/5; 20 %) [34]. Transmission electron microscopy (TEM) without immunolabeling reported virus-like particles in the frontal lobe (n = 1) [12].
While additional COVID-19 autopsy series continue to be published, the overall picture of acute hypoxic-injury, hemorrhage, and mild to moderate non-specific inflammation is unlikely to change significantly. Evidence of direct viral involvement in the brain or olfactory nerve is limited to the detection of low levels of viral RNA and rare viral antigen in cranial nerves and scattered brainstem cells. Diagnosis of coronavirus particles by electron microscopy is challenging due to similar appearing normal cellular structures, which has created significant controversy in the literature [42,43]. Due to the inherent bias of autopsy studies for severe, fatal disease, and additional institutional restrictions for which cases include brain evaluation, the frequency and extent of neuropathological findings are likely to be overestimated relative to the average COVID-19 patient. At the time of this review, pediatric autopsies, including individuals with multisystem inflammatory syndrome in children (MIS-C), remain extremely limited. While the number of pediatric COVID-19 cases accounts for <2 % of all cases [44], data obtained from brain tissue in this age-group can help address the unique pathophysiology of SARS-CoV-2 infection, including age-dependent immune-responses, hypercoagulability, and degree of hypoxic-ischemic injury.
Additional remaining areas of interest include characterizing the effects of remdesivir and other potential antiviral therapeutics, immunomodulatory medications including dexamethasone, anti-IL-6 or other monoclonal antibodies, and anticoagulants on brain tissue. Given that the therapeutic response to COVID-19 vastly differs between institutions, it remains a challenge to understand how therapeutic choices during acute hospitalization are responsible for the variability in observed neurological manifestations and neuropathological findings. Also, while not surprisingly this early in the pandemic, long-term neuropathological sequelae in COVID-19 survivors remain unstudied. There is evidence that neurological symptoms, including fatigue and headaches, linger for weeks to months in a subset of affected patients [45,46] and studies determining mechanisms for persistent neurological symptoms are needed.
There have been several efforts for sharing COVID-19 brain tissue, including the International Society of Neuropathology (ISN) Collaborative Efforts [47] and the COVID-19 Virtual Biobank at the University of Nebraska Medical Center [48]. To address many of the remaining unanswered questions regarding the neuropathological effects of COVID-19, large scale integrated studies from multiple institutions with relevant clinical metadata will be crucial. The ongoing collection of neurological tissue will be critical to inform best practice management guidance and to direct research priorities as it relates to neurological morbidity from COVID-19.
Funding sources
S.S.M. was supported by the National Institute of Mental Health at the National Institutes of Health [grant number K23MH115812], James S. McDonnell Foundation and the Harvard University Eleanor and Miles Shore Fellowship Program. I.H.S. was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [grant number R21NS119660].
Acknowledgements
We would like to acknowledge all front-line healthcare workers taking care of patients during the COVID-19 pandemic, and patients and their families who contribute research to help understand neurological disease.
References
- 1.University J.H. 2020. COVID-19 Map-Johns Hopkins Coronavirus Resource Center.https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O’Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. ISARIC4C investigators, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 7.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. https://jamanetwork.com/journals/jamaneurology/article-abstract/2766766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., Lantos J., Schenck E.J., Goyal P., Bruce S.S., Kahan J., Lansdale K.N., LeMoss N.M., Murthy S.B., Stieg P.E., Fink M.E., Iadecola C., Segal A.Z., Cusick M., Campion T.R., Jr., Diaz I., Zhang C., Navi B.B. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Hu Y., Li Y., Jin H., Hu B. 2020. Neurological Manifestations of Hospitalized Patients With COVID-19 in Wuhan, China: a Retrospective Case Series Study.https://papers.ssrn.com/abstract=3544840 [Google Scholar]
- 11.Cooper K.W., Brann D.H., Farruggia M.C., Bhutani S., Pellegrino R., Tsukahara T., Weinreb C., Joseph P.V., Larson E.D., Parma V., Albers M.W., Barlow L.A., Datta S.R., Di Pizio A. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107:219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paniz‐Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAbee G.N., Brosgol Y., Pavlakis S., Agha R., Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr. Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilotto A., Odolini S., Stefano Masciocchi S., Comelli A., Volonghi I., Gazzina S., Nocivelli S., Pezzini A., Focà E., Caruso A., Others Steroid-responsive encephalitis in Covid-19 disease. Ann. Neurol. 2020 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Rumeileh S., Abdelhak A., Foschi M., Tumani H., Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2020 doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roulson J., Benbow E.W., Hasleton P.S. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47:551–559. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 21.Shojania K.G., Burton E.C., McDonald K.M., Goldman L. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA. 2003;289:2849–2856. doi: 10.1001/jama.289.21.2849. [DOI] [PubMed] [Google Scholar]
- 22.Sessa F., Bertozzi G., Cipolloni L., Baldari B., Cantatore S., D’Errico S., Di Mizio G., Asmundo A., Castorina S., Salerno M., Pomara C. Clinical-forensic autopsy findings to defeat COVID-19 disease: a literature review. J. Clin. Med. Res. 2020;9 doi: 10.3390/jcm9072026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020 doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmukh V., Motwani R., Kumar A., Kumari C., Raza K. Histopathological observations in COVID-19: a systematic review. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 25.Hanley B., Lucas S.B., Youd E., Swift B., Osborn M. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 26.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., Frank S., Turek D., Willi N., Pargger H., Bassetti S., Leuppi J.D., Cathomas G., Tolnay M., Mertz K.D., Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395:e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T., Yarid N., Marshall D.A. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaunmuktane Z., Mahadeva U., Green A., Sekhawat V., Barrett N.A., Childs L., Shankar-Hari M., Thom M., Jäger H.R., Brandner S. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020;140:397–400. doi: 10.1007/s00401-020-02190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantonen J., Mahzabin S., Mäyränpää M.I., Tynninen O., Paetau A., Andersson N., Sajantila A., Vapalahti O., Carpén O., Kekäläinen E., Kantele A., Myllykangas L. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020 doi: 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabbri V.P., Foschini M.P., Lazzarotto T., Gabrielli L., Cenacchi G., Gallo C., Aspide R., Frascaroli G., Cortelli P., Riefolo M., Giannini C., D’Errico A. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 2020 doi: 10.1111/bpa.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remmelink M., De Mendonça R., D’Haene N., De Clercq S., Verocq C., Lebrun L., Lavis P., Racu M.-L., Trépant A.-L., Maris C., Rorive S., Goffard J.-C., De Witte O., Peluso L., Vincent J.-L., Decaestecker C., Taccone F.S., Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit. Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Dalahmah O., Thakur K.T., Nordvig A.S., Prust M.L., Roth W., Lignelli A., Uhlemann A.-C., Miller E.H., Kunnath-Velayudhan S., Del Portillo A., Liu Y., Hargus G., Teich A.F., Hickman R.A., Tanji K., Goldman J.E., Faust P.L., Canoll P. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol. Commun. 2020;8:147. doi: 10.1186/s40478-020-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., Thursz M., Manousou P., Corbett R., Goldin R., Al-Sarraj S., Abdolrasouli A., Swann O.C., Baillon L., Penn R., Barclay W.S., Viola P., Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deigendesch N., Sironi L., Kutza M., Wischnewski S., Fuchs V., Hench J., Frank A., Nienhold R., Mertz K.D., Cathomas G., Matter M.S., Siegemund M., Tolnay M., Schirmer L., Pröbstel A.-K., Tzankov A., Frank S. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Jr., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., de Bree G.J., Bulle E.B., Aronica E.M., Florquin S., Fronczek J., Heunks L.M.A., de Jong M.D., Guo L., du Long R., Lutter R., Molenaar P.C.G., Neefjes-Borst E.A., Niessen H.W.M., van Noesel C.J.M., Roelofs J.J.T.H., Snijder E.J., Soer E.C., Verheij J., Vlaar A.P.J., Vos W., van der Wel N.N., van der Wal A.C., van der Valk P., Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirschenbaum D., Imbach L.L., Ulrich S., Rushing E.J., Keller E., Reimann R.R., Frauenknecht K.B.M., Lichtblau M., Witt M., Hummel T., Steiger P., Aguzzi A., Frontzek K. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller S.E., Brealey J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98:231–232. doi: 10.1016/j.kint.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller S.E., Goldsmith C.S. Caution in identifying coronaviruses by Electron microscopy. J. Am. Soc. Nephrol. 2020;31:2223–2224. doi: 10.1681/ASN.2020050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parri N., Lenge M., Buonsenso D., Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group Children with Covid-19 in pediatric emergency departments in Italy. N. Engl. J. Med. 2020;383:187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group, persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., Gibbs K.W., Erickson H.L., Steingrub J.S., Smithline H.A., Gong M.N., Aboodi M.S., Exline M.C., Henning D.J., Wilson J.G., Khan A., Qadir N., Brown S.M., Peltan I.D., Rice T.W., Hager D.N., Ginde A.A., Stubblefield W.B., Patel M.M., Self W.H., Feldstein L.R., IVY Network Investigators, CDC COVID-19 Response Team, IVY Network Investigators Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, march-june 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVID-19, (n.d.). https://www.intsocneuropathol.com/2020/04/accessing-covid-19-brain-tissue/ (Accessed September 10, 2020).
- 48.COVID-19 Biorepository, (n.d.). https://covidbank.unmc.edu/ (Accessed September 10, 2020).
- 49.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B., Claus R. Postmortem examination of patients with COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bussani R., Schneider E., Zentilin L., Collesi C., Ali H., Braga L., Volpe M.C., Colliva A., Zanconati F., Berlot G., Silvestri F., Zacchigna S., Giacca M. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal eosinophilic myocarditis in a healthy 17-Year-Old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c) Fetal Pediatr. Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolhnikoff M., Ferreira Ferranti J., de Almeida Monteiro R.A., Duarte-Neto A.N., Soares Gomes-Gouvêa M., Viu Degaspare N., Figueiredo Delgado A., Montanari Fiorita C., Nunes Leal G., Rodrigues R.M., Taverna Chaim K., Rebello Pinho J.R., Carneiro-Sampaio M., Mauad T., Ferraz da Silva L.F., Brunow de Carvalho W., Saldiva P.H.N., Garcia Caldini E. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., Vander K., Bargfrieder U., Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]