Abstract
The current technological advancements in emerging 3D printing technologies are indeed propitious. To date, ground-breaking 3D printing technologies are used in automobile, aerospace, clothing, pharma, and biomedical industries by creating pre-requisite engineered and tailored end-user products reaching standard sets. 3D printing is also becoming a crucial technology in support of enhanced health care and general emergency response since the beginning of the COVID-19 pandemic. As the world is facing a significant lack of medicinal supplies, manufactures are struggling to fulfill demands due to the ongoing COVID-19 pandemic. The decline in the diagnostic testing kits supply chained to increased interest in 3D printed Nasopharyngeal (NP) swabs. This article has reviewed and studied the sensitivity of the NP swabs and various NP swab designs. The process of different 3D printing technologies that are employed to address the swab shortages during COVID-19 is explained in detail. The paper ends with the conclusions drawn from the literature review.
Keywords: COVID-19, SARS-CoV-2, NP swabs, RT-PCR, 3D printing, 3D printed NP swabs
1. Introduction
In December 2019, an infectious disease named COVID-19 emerged due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China [1], [2]. Admittedly, its ultimate origin is unclear, while numerous experts have referred to their statements that suggest few animals, in particular snakes and bats [3]. This localised Wuhan crisis has steadily spread through all continents [4]. Flu symptoms such as dry cough, fever, trouble breathing, weakness, etc. are experienced by the patients [5], [6]. Droplet transmission through sneezing, coughing or simply speaking are the important ways of virus diffusion, resulting in infecting others who inhale these droplets [7]. COVID-19 affects the human respiratory system [8]. Researchers strive to understand the virus entirely and continuously monitor the evolution of new rapid diagnostics [9] and vaccines [10]. It’s important to focus on increasing the number of tests conducted for the early detection of the virus because they conclude that this may be a crucial way to combat the COVID-19 pandemic. NP swabs are used as a golden standard for testing, they play a vital role to guide the individuals in preventing more person-to-person COVID-19 transfers by detecting infected people to minimise and mitigate the COVID-19 pandemic by isolating them and also to trace their contacts. Nevertheless, the value of broad testing relies on the quality of the test and the impact of treatment on the results of the test. The existing medical devices cannot accommodate patient numbers every day if the number of reported cases surge rapidly [11]. Then the supply chain for the medical device is interrupted. When demand grows unexpectedly, manufacturers cannot prepare for the required devices, where creativity and new production methods are highly required. Global supply of the personal protective equipment (PPE), ventilators, including nasopharyngeal swabs is lacking, due to the rising and potential demands of testing for COVID-19. 3D printing can be an appropriate alternative technology to address these issues. 3D printing technology eliminates machine adjustment and setup times and makes it possible to build complex and customized parts by enhancing cost efficiency and productivity [12]. The feasibility of sharing developed open-source designs is possible using this technology. Then the designs can be prototyped around the world using the open-sourced designs. In this review, the sensitivity of NP swabs is discussed along with the design of NP swabs and their development using different 3D printing techniques in detail.
2. Nasopharyngeal swabs for COVID-19 diagnosis
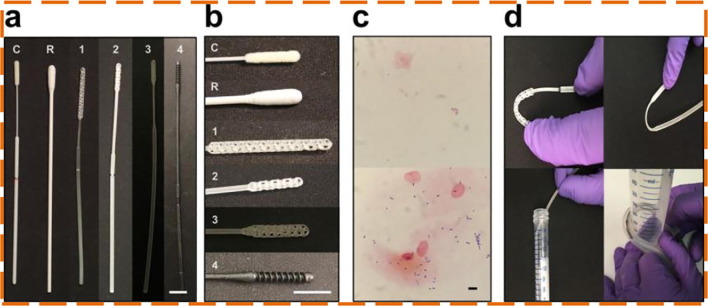
Laboratory tests used for the COVID-19 detection, typically detect the viral Ribonucleic acid (RNA) employing a nucleic acid amplification with the aid of a polymerase chain reaction (PCR) for which the majority of the respiratory samples are obtained using nasopharyngeal swabs [13]. Using NP swabs for COVID-19 testing is considered to be a gold standard [14]. It is evident from the literature that Flocked NP Swabs are the potential alternatives to the traditional rayon swabs [15], [16], [17]. As a protocol for the diagnosis of active COVID-19 infection, the Centers for Disease Control and Prevention (CDC) also recommends NP swabs for sample collections [18]. The standard procedure is followed for collecting the NP swab sample [19]. Additionally, several researchers also reported the sample collection technique using NP swabs intending to explain the procedure properly that can result in performing better [20], [21]. Few studies reported that saliva [22] and combined NP and oropharyngeal (OP) swabs [23], [24] samples are yielding quality results than NP swabs. On the contrary, researchers also reported that NP swabs hold higher sensitivity than saliva [25], OP swabs [26], [27], [28], Nasal swabs [29], and Mid-Turbinate Nasal Swabs [30]. During an international attempt to tackle the pandemic, COVID-19 diagnostic testing grew significantly, which resulted in the medical supply chain disruption of Flocked NP swabs [31]. 3D printing is a strong production choice that fulfills the requirement for NP swabs [32]. The 3D printed swabs come with intricate tip structures to improve the efficiency of sampling. Callahan, Cody J., et al. [33] assessed 160 swab designs and 48 materials by 24 firms, laboratories, and employees. Four manufacturers (Resolution Medical, EnvisionTec, Origin.io, HP, In) prototypes were focused as they found to have the potential to replace the Flocked NP swabs (Fig. 1 a). Same as control swabs, all the 4 prototyped NP swabs were also 15–16 cm in length with a nose tip diameter of 2 to 3 mm, a 2 to 4 mm diameter thick shaft, a thin neck with 1 to 2 mm in diameter, and 4 to 7 cm long and a breakpoint from the head tip of 7 to 8 mm is designed to capture posterior nasopharynx secretions, is a Food and Drug Administration (FDA) class I exempt medical device (Fig. 1a and b). The swab is placed into the nasopharynx and rotated to absorb material several times before being dropped into the container containing a few milliliters of the medium. The lying method along with a protective mask during nasopharyngeal swab collection can substantially increase patient satisfaction, co-operation, and minimise pain, sneezing, coughing, and nausea compared to the commonly used sitting procedure [34]. A shaft breakpoint allows the release of the head into the vial, then sealed and sent for inspection. Multiple branch points on a tapered strut, a central connected with a polygonal matrix with some sort of spiral are commonly featured designs (Fig. 1b). Surface texture, stiffness, and collection of the sample (Fig. 1c) were balanced by manufacturers. Changes of the central longitudinal strut permitted different degrees of impact cushioning, stability, and versatility (Fig. 1d). Every prototype demonstrated excellent agreement with the control swab in reverse transcription-polymerase chain reaction (RT-PCR) tests (K = 0.85 to 0.89).
Fig. 1.
(a) Conventional (C,R) and 3D printed (1,2,3,4) swabs (b) Swab heads close-ups (c) Gram stains of cheek swabs with 3D printed (bottom) and Conventional (top) swabs (d) Material testing of the swabs [33].
Flexural, torsion, and tensile tests are done on the 4 different 3D printed swabs (Formlabs NP Swab, Abiogenix FAST Spiral NP Swab, EnvisionTEC NP Swab, and Resolution Medical Lattice Swab) and compared to 1 conventional nylon flocked swab (Fosun Pharma NP Swab) [35]. Flexural tests suggest that lower flexibility is observed in EnvisionTEC, Formlabs NP, Abiogenix FAST Spiral NP swabs when compared with the conventional nylon flocked swabs. Similar flexibility as that of conventional nylon flocked swab is shown by Resolution Medical Lattice swabs. Tensile tests demonstrated better or comparable performance by Resolution Medical Lattice swabs, EnvisionTEC NP, Formlabs NP, Abiogenix FAST Spiral NP swabs when compared with conventional nylon flocked swabs. For all the sterilisation methods tested, the EnvisionTEC NP and Abiogenix FAST Spiral NP swabs performance are comparable at both pre and post-sterilization. No performance loss is recorded for Resolution Medical Lattice swabs, EnvisionTEC NP, and Abiogenix FAST Spiral NP swabs after autoclaving for 5–7 days. For the two autoclave protocols tested, the NP Swabs of Formlabs showed a major performance degradation immediately after autoclaving, later the performance is enhanced after 7 days of autoclaving. Even after this shift in efficiency, Formlabs NP swabs still performed better in comparison to the conventional nylon flocked swabs. Torsion tests indicated that all 3D printed swabs resisted less rotation when compared to conventional nylon flocked swabs. Indeed, greater torques are resisted by the 3D printed swabs when compared to conventional nylon flocked swabs. It is still unknown if this performance disparity will result in negative performance in clinical testing.
3. Stereolithography printed nasopharyngeal swabs
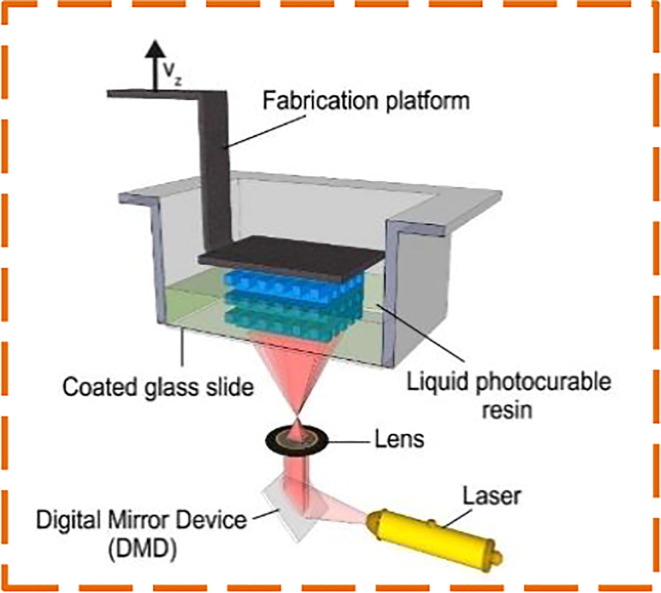
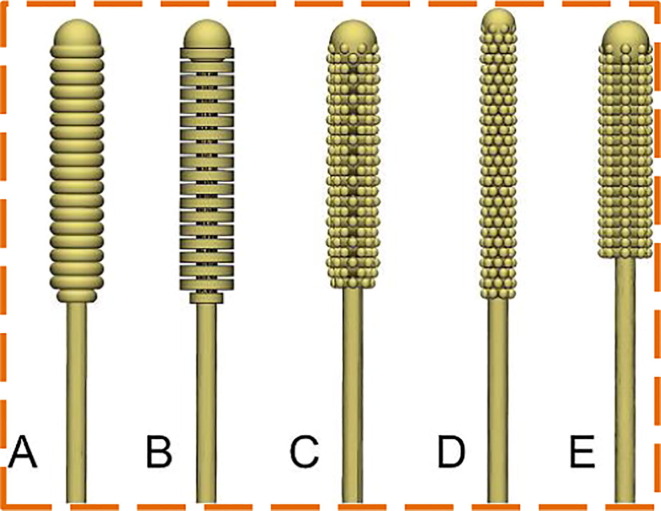
A vital component of the COVID-19 testing package is the nasopharyngeal swab. The pandemic remains a significant influence on the supply chain. To address the swab shortages, Jonathan Ford et al. [36] investigated to substitute the conventional flocked NP swab with the 3D printed swab. Stereolithography (SLA) based Formlabs Form 3B and Form 2 printers are employed to print swabs since they are easily accessible with biocompatible and comparatively inexpensive materials for local deployment, along with FDA cleared software. In SLA, an ultraviolet (UV) laser beam is used for curing the polymer resin layer-by-layer to create an object as shown in Fig. 2 . Fusion 360 (United States) or 3-matic (Belgium), Solidworks (France) are utilized to prototype the Computer-Aided Design (CAD) models. More than twenty tip designs are prototyped, few instances can be found in Fig. 3 . The authors collaborated with a radiologist and experts at USF Health Infectious Disease, Otolaryngology to concretize the swab designs which would be able to obtain an appropriate COVID test sample while retaining patient protection and comfort in mind. A design consisting of approximately 70 mm breakpoint and a 150 mm in total length was finalised after numerous design modifications. The rounded nose tip is designed in view of patient comfort with a 3.87 mm diameter and 15 mm length. The shaft and neck diameters are 2.45 mm and 1.5 mm respectively. With a diameter of 5 mm and 1.75 mm long the base is designed. Adding possible waste and post-processing materials, 0.76 ml of resin is used to print each swab which results in the approximate printing cost of US$ 0.25 for each swab. An interim patent is received by the USF Health for the principle and design of the 3D printed NP swab.
Fig. 2.
Schematic illustration of SLA setup [45].
Fig. 3.
Different 3D swab designs [36].
The final swab designs were tested in the bench lab to ensure that adequate geometries were obtained to allow viral trials. Surgical Guide resin Version 1 that is validated in medical application [37], is used to print the swabs, with the base in direct contact with the platform without any support or rafts at a depth of 100 μm. 3D printed swab's STL was then arrayed using the Preform program for 3D printing. To prevent possible complications, 324–380 swabs were found to be printed safely in batches with the balancing of most of the swabs and the maximum reduced contact points. To remove the uncured resin left from the printing process Form Wash is used to rinse the 3D printed swabs in 99% isopropyl alcohol for 20 min. Swabs remain to be on the build plate during this washing process. Manual washing can also be performed using the Finishing Kit, in which the swabs need to be detached from the build plate. The prints are air-dried for at least 30 min after the complete wash. To organize the swabs, a loose rubber band is placed around them before gently removing from the building platform. Then the swabs are shifted onto the curing rack and put in the Form Cure from the base with the pointing tip down for 30 min at 60 °C (Form 2) or for 30 min at 70 °C (Form 3B). Bent shafts or swabs with neck issues are eliminated with the inverted NP swabs suspension during curing by the rack. Overcrowding the curing rack with the swabs can be avoided to maintain adequate airflow and UV exposure for each swab. The 3D printed swabs are placed in sterilisation pouches for steam after cure and are prepared for sterilization. A pre-vacuum steam sterilization cycle set at 132 °C with 4 min of sterilizing time and a dry period of 30 min is a suitable sterilisation cycle. After sterilisation, the swabs are packed individually, autoclaved, and then packaged to complete the COVID-19 test kit with the test tube and Viral Transport Medium (VTM). Synthetic swabs are compared with the 3D printed swabs by testing each individual with both the swabs in alternative nostrils. Testing results of the Northwell Health and USF Health/TGH indicate a 94% confirmation with the 3D printed swab compared to the synthetic swab and threshold period (Ct) values do not vary statistically, concluding that 3D printed swabs are a feasible substitute for the synthetic swab.
An identical study is reported by Decker, Summer J., et al. [38] where similar design parameters and methodology is used to 3D print NP swab with “cattail” tip design which is modeled in Materialise (3-matic). No major differences are observed in the comparison of Flocked NP and 3D printed swab results with three separate test methods (Roche Cobas, NeuMoDx, and CDC research) indicating that 3D printed swabs can be used safely across platforms. Substantial variations are not reported between swab values (p = 0.152 and p = 0.092) in the cycle threshold (Ct) of the gene targets and RNase P genes in the CDC assay, where RNase P targets performed slightly better than 3D printed swabs (p > 0.001). In the NeuMoDx test (p = 0.401 and p = 0.484), the Ct values were not substantially different for the swabs, as well as for the Roche Cobas assay (p = 0.05 and p = 0.05). For both the swabs, the general clinical correlation between the diagnosis of COVID-19 is found to be Kappa = 0.901 (95.88%) and that is a strong agreement [39], where coefficients of Kappa are correlation tests between categorical variables often used as coefficients of reliability or validity [40].
The greater the NP swab surface area, the more intuitively the amount of cell-mucus matrix gets accumulated and discharged, resulting in increased test sensitivity. With this purpose in view, Tay, Joshua K., et al. [41] designed the NP python swab with a square-shaped helix design with the reservoir concept by knowing the nasal passage diameter through which the NP swab is to be moved from beginning to end and the helix makes 1.5 turns. This can help to maintain adequate structural integrity by resisting the forces of push and turning by enabling surface handling (weaving, flexing, sculpting). The helix blades gaps and internal offset of the blades allow the swab to capillarily collect and inject the cellular mucus matrix into the middle reservoir. When the tip of the NP swab is redirected and agitated into the VTM, the cellular-mucus matrix is trapped inside the middle reservoir, and adhering to the swab tip is released. Rhino 6 for Windows is used to develop the helix design of the NP swab and is then prototyped with the Formlabs Form 3 printer using Surgical Guide Resin in a similar methodology. Later, the 3D printed swabs undergo a flexural and tensile test in compliance with international requirements (ISO 178:2010 Plastics – Determination of Flexural Properties and ISO 527-1:2012 Plastics – Determination of Tensile Properties) and also were tested for fluid absorption and release rate, from which it is concluded that the 3D printed swab has potential to test SARS-CoV-2. The 3D printed swab showed excellent correspondence in pair clinical trials for COVID-19 patients with the values of the RT- PCR cycle threshold (Ct) at r = 0.943 and 0.918 for each Sarbecovirus E-gene and SARS-CoV-2 ORF 1/a respectively, compared to Copan FLOQSwab.
Complete negative and positive percentage agreements on a dual-assay RT-PCR platform are 100% and 90% respectively. Although high cycle thresholds resulted in discordant samples, various factors including the swabbing technique, the patient's viral lower nasopharyngeal, and the laboratory examination influences the precision of COVID-19 detection using NP swabs. This is evident in the recent study, that 89% sensitivity is observed in the pooled RT-PCR testing but with a large range of susceptibility between studies ranging from 50 to 100 % [42]. Even the Python swab had slightly higher Ct values (37.48–38.12), implying that it captured less than the FLOQSwab obtained, but the overall agreement was excellent for both swabs. The minor variations in Ct values probably have no big implications for the accuracy of the highly sensitive testing of the RT-PCR, very small amounts of material are highly sensitive. La Scola, Bernard, et al. [43] study revealed that for patients with ≥34 E-gene, Ct values failed in isolating the virus in the cell culture, which suggests a lack of infectiveness and recommends to release the non-hospitalized patients from hospital treatment or strict containment. Another research showed that Ct values <24 and <8 days of illness were only contagious with cell culture [44]. Authors state that the Python swab is secure, patient-acceptable, and is a feasible alternative to conventional swabbing and also helps to relieve swab shortages during the rising COVID-19 pandemic.
When compared to adult NP swabs, pediatric NP swabs are more flexible, smaller, and thinner, which are in even more shortage due to the COVID-19 pandemic. Since adult swabs are too large and inflexible to test children, particularly those under the age of 3 years. At first, 3D printed swabs replicating the size of the commercial pediatric swab (COPAN Flock Innovations, Puritan Diagnostics) were assessed at Texas Children’s Hospital but the 3D printed swab was not versatile enough and could damage the child's nasal passages. It is not advisable to simply cut down the diameter for the flexible shaft, because the swab would bend in both directions and the thinner shaft would threaten to break in situ. For this reason, Starosolski, Z., et al. [46] developed and manufactured new 3D printed swabs for young children and infants. To endorse the pediatric NP swabs design, a random selection of maxillofacial CT datasets was made from 5 patients database, 11 to 34 months old and unremarkable for maxillofacial pathologies. 3D Slicer is used to create an STL file from the images to proceed for printing. Formlabs Elastic Resin and Surgical Guide Resin are utilized to 3D print NP passages and NP swabs respectively. Fusion 360 was used to design the NP swabs.
Five swab designs are tested by the authors, 2 mini commercial swabs, PURITAN Diagnostics (Flocked swab), COPAN Flock Technologies (Flocked swab); and 3 3D printed swabs, that are having approximately 50 mm shaft length, 78 mm handle, and 14 mm transition zone with various cross-sectional flexible shaft: 1.2 mm for Design I, 1.2 mm × 0.8 mmø for Design E and 1.2 mm × 0.8 mmø for Design ES along with the brush having slanted posterior edge. Each swab was navigated by one person between the external nares and the posterior nasopharynx. Testing orders were randomised for each swab and anatomical model. Each test is repeated 3 times. An observer with a handheld stopwatch monitored the time for full navigation and graded the strength upon insertion with a 3 level resistance score (3, hard: that requires extra strength for resistance; 2, medium: needs low strength; and 1, simple insertion: no resistance). Matlab R19a is used to perform all the statistical calculations. After testing all 5 swabs, COPAN Flock Technologies (Flocked swab) is unable to get inserted in anatomical model 1. Design ES is selected for clinical studies as the optimal design, based on the lowest resistance and shorter navigation time. In terms of swab tips, commercial swab designs vary with the 3D printed swabs designs, as 3D printed swabs have the hemispheric nubs with solid tips while flocked fibrous tips are found in commercial swabs. In vitro as well as in vivo studies, however, they were shown to be almost similar in the transfer of samples for RT-PCR viral assays in different laboratories [47], [48]. Researchers also state that Design ES 3D printed pediatric swabs are used for 1000 patients with no problems reported.
Furthermore, to cut the print time of SLA, a two-part design is employed by Gallup, Nicole, et al. [49]. OpenSCAD is utilized to design the two components (swab head with breakpoint and handle). The swab heads rear and front are tapered for easy access of the swab insert and remove. An offset sequence is chosen for knob positions. For ensuring the collection of specimens and printability, the gap between knobs is chosen. The length of the swab is reduced and fit into a handle. SLA based open source Prusa SL-1 3D printer is used to print the swab tip, while FDM based LulzbotTaz 6 3D printer is used to print the handle. As the number of SLA-based printers are much fewer when compared to the total number of Fused Deposition Modelling (FDM) based printers. A UV LED box is developed, manufactured, and tested to cure the resin print head. These swabs are fabricated at a cost of US $0.0603/swab including swab head and handle. Although clinical testing is needed for these swabs before medical practices.
4. Fused deposition modelling printed nasopharyngeal swabs
As SLA printers for resin nasopharyngeal swabs are validated previously, further, FDM printers are used to 3D print NP swabs using polyester and PLA as raw materials [50]. In FDM, the filament is melted only beyond its glass transition temperature and then extruded to or over previous extrusions in a line, generating the product layer after layer [51]. The FDM setup schematic illustration is shown in Fig. 4 . Compared to SLA printers, the FDM printers are less costly and are easily obtained to manufacture NP swabs using PLA. Irrespective of the wide availability of the material, PLA is also bio-degradable and non-toxic as it comes from sustainable resources including tapioca and corn. The chemical and physical properties of PLA along with their biocompatibility are well defined [52], [53] but PLA is not yet determined to fit viral specimens for diagnosis based on PCR [54]. Conversely, certain restrictions are imposed on using FDM [55]. The lattice pattern for the high-resolution tip of the SLA printer is difficult to reproduce by the FDM because of its inherent properties. Alternatively, researchers tried to establish a procedure to quickly and reliably add a synthetic absorbent material like polyester to the tip of the swab. The viral viability of polyester in transport media is also proved to be non-toxic and is widely utilized by a range of commercial manufacturers of the swab [56]. Dremel™ 104 3D4 and Prusa™ I3 MK2S 3D printers are used to print NP swabs for a batch of 60 swabs per printer and the printing time estimated is 3 h. To achieve a swab with desired material characteristics, a 180o bending test is used to demonstrate that the PLA swabs retain their tensile strength as they are maneuvered through the nasal cavity for the balance between rigidity and flexibility. At 60 °C PLA reaches its glass transition temperature, where the material gets soften by losing its rigidity. Accordingly, low-temperature sterilisation with hydrogen peroxide plasma is used to sterilize and package the swabs at 45 °C with evaporated hydrogen peroxide.
Fig. 4.
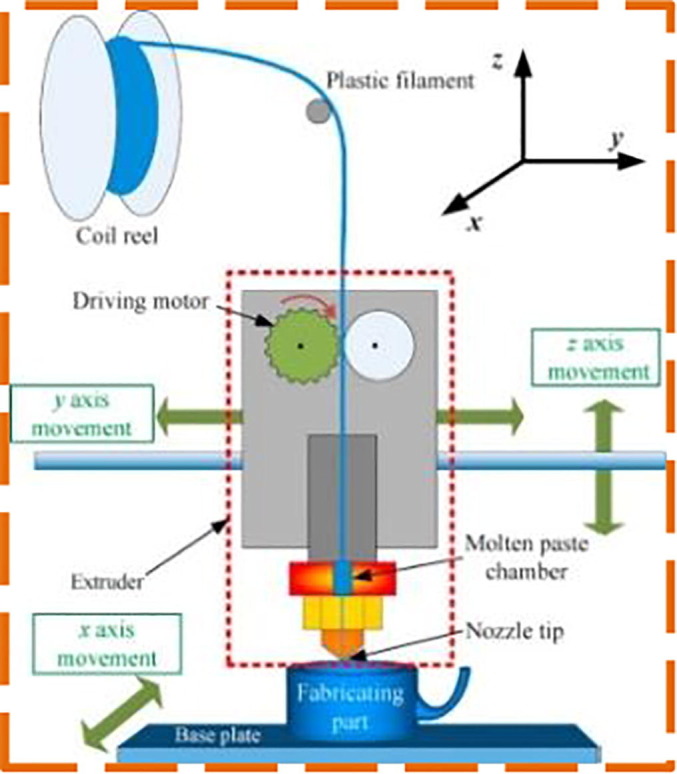
Schematic illustration of FDM setup [60].
The total length of the 3D printed NP swab is 150 mm along with the tapered breakpoint at 90 mm from the swab tip. To avoid using adhesive substrates, 6 small “barbs” are placed at the end of the shaft to allow the polyester fibers to retain the swab. Then it is rotated in a clockwise direction to complete the rolling of the polyester. A swab applicator is developed and 3D printed to maintain consistency in the polyester diameter (4 mm) which is mounted on the swab tips. The dimensional consistency of the swabs is cross-checked after printing the first 50 swabs. The initial 10 3D printed swabs successfully cleared the 180° bend test. Additionally, no bacteria growth is observed in any of the randomly selected 10 swabs in the sterility test. Almost 80.8% agreement is found between the control swab and the 3D printed swab. The prototype swab is more sensitive when all positive outcomes are considered as true positives. Cotton tipped swabs are investigated in 44 adults and found that they are equivalent to rayon tipped swabs in capturing the NP specimens. The sensitivity of the cotton-tipped swabs for detecting SARS-CoV-2 is evaluated by Freire-Paspuel et al. [57]. In the collection of the specimens needed for the detection of SARS-CoV-2 in 44 adults, authors found that cotton-tipped swabs are equivalent to rayon tipped swabs. Besides that, in capturing the human DNA from salivary samples that polyester swabs have greater extraction and absorption efficiency than cotton or rayon swabs [56]. In parallel, a similar study is reported with the polyester tip diameter of 3.5 mm [58]. Authors compared the 3D printed swab with the commercial swab and 91% positive concurrence (Cohen’s kappa = 0.81) is demonstrated.
Besides that, Cox, Jesse L., and Scott A. Koepsell [59] also fabricated and studied the NP swabs using FDM. Rhinoceros 5.5.5 software is used to draft the swab design of length 15 cm, 6.5 cm × 2 mm handle along with a thin 1 mm diameter long shaft. A “brush” consists of 0.50 mm thick disks in series that are spaced every 1 mm apart and are positioned over a distal 1 cm shaft. A “score”, 5 cm up the handle shaft is also integrated to quickly split the swab into conical lower tubes with VTM. PrusaSlicer 2.3.0 software is loaded with the STL file and processed for printing on the Prusa MK3S printer. Swabs are printed using a relatively cheap and widely accessible food-grade plastic, polyethylene terephthalate glycol (PETG) filament. PETG is a chemically inert, long-lasting, and structurally suitable thermoplastic polyester. Nozzle temperature of 250 °C, a layer height of 0.15 mm along with the 90 °C bed temperature is selected for printing. Around 3 h 40 min of a total print time is observed for printing 50 swabs simultaneously while one swab's print time is about 5 min. Utilizing the STERRAD process the swabs are sterilized, where the surface areas are coated with 58% hydrogen peroxide with a vacuum at 54°–63 °C. The hydrogen peroxide is extracted out of the chamber after exposure, and it takes about 50 min to complete the process. No plastic deterioration was registered, and one day after the submission to the sterile core facility, the swab for clinical use was available. Later, the 3D printed swabs are compared with the commercial swabs by testing 2 individuals, where the results are correlated.
5. Selective laser sintering printed nasopharyngeal swabs
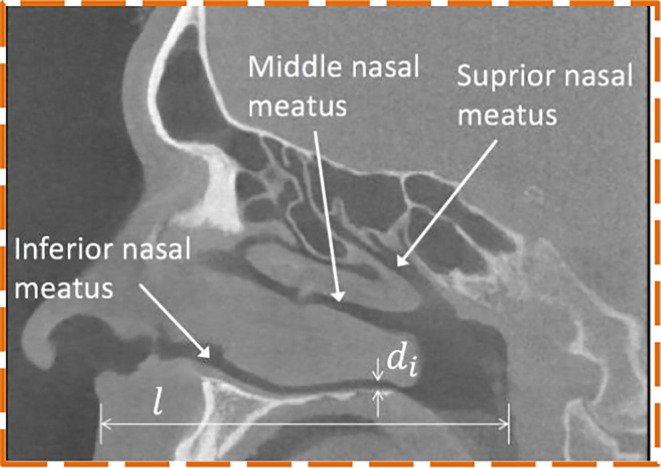
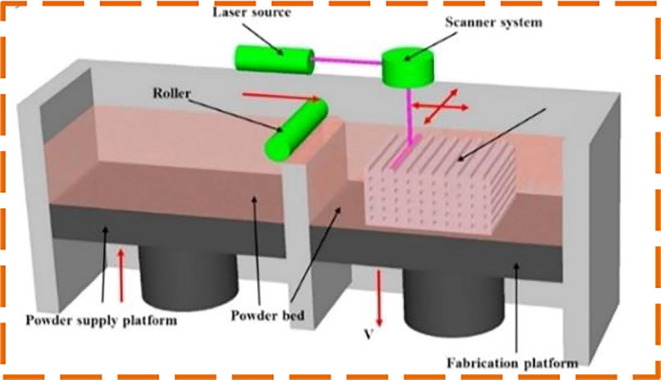
Meanwhile, the availability of test swabs for hospitals is becoming increasingly scarce due to the exponential growth of infection instances and transportation limitations across countries [61]. Despite that, most of the commercial swabs available in the market are of only one standard size that has not taken account of patients' various nostrils’ sizes and may cause discomfort to certain patients during testing. Patient-specific NP swab is also highly desirable from this perspective. To address these limitations, Sun, Y. et al. [62] worked on the patient-specific NP swab by developing a unique design platform in Matlab that can be 3D printed easily to fill the shortage of the swabs. One significant aspect of the unique patient design is that the swab's scale is extracted from the patient's nasal passage scale. The computed tomography scan reveals the patient's nasal cavity in a sagittal plane, which specifically investigates the three nasal meatuses (passages) as shown in Fig. 5 . To automatically measure the size of the nasal passages, the patient’s CT data is processed into a single Matlab function. Based on boundary representations (B-rep) surface models are employed to model the swab geometries. This allows direct 3D printing of the realized swab by eliminating the transfer of data between various types of geometry files, making the design process considerably easier by preventing data loss in the conversion process [63]. A comprehensive overview of B-rep-based modeling geometry is available in [64].To minimise the patient's discomfort, the length and the diameter of the head are equal to 0.25 l and di accordingly to capture adequate respiratory samples. Neck length and diameter are respectively chosen as 1 mm and 0.5 l for versatility in the neck along with a 0.5 mm diameter for a thin breaking point. The holder’s diameter is di, so it does not get stuck at the nostril opening. Selective Laser Sintering (SLS) technique is adopted to fabricate the NP swab using polyamide (PA2200). SLS uses a laser, which serves as a power source to sinter pulverized material and ends up in creating the complex 3D objects (as shown in Fig. 6 ). SLS is a proven technology to produce polymerized materials for bioimplants [65]. But, the cost of the SLS printer and it’s post-processing equipment makes it more expensive even for desktop models when compared to FDM and SLA printers. The mechanical performance of the 3D printed NP swab design is also evaluated using FEA. On the tip of the swab, with an angle of 45° to the Z-axis the load is fixed to imitate the nasopharynx resistance during the collection of the sample. Flexible neck holds the most stresses and deformations, where no major deformations are found at the breaking point of the swab.
Fig. 5.
Nasal cavity computed tomography scan [62].
Fig. 6.
SLS process schematic illustration [67].
Consequently, Williams, Eloise, et al. [66] also adopted SLS technology to fabricate the 3D printed NP using PA2200. Feedback from the engineering and clinical researchers is also considered to iterate the design process. The iterations include maximizing the cell collection by optimizing the tip geometry, enhancing the geometry of the swab (ease to use, the position of the breakpoint, and flexibility), and ensuring the consistency of patient comfort with the overall design parameters. The Design G has an approximate total length of 147 mm with a breakpoint of 72 mm along with a tip length of 20 mm. The diameter of the shaft and neck is 2.50 mm and 1 mm respectively. The Design G 3D printed NP swabs are compared with the Flocked Kang Jian swabs and Flocked Copan ESwabs. In this evaluation, a quality agreement is found between the flocked swabs and 3D printed swabs for the detection of SARS-CoV-2.
6. Other 3D printing technologies printed nasopharyngeal swabs
Arnold, Forest W., et al. [68] compared the efficiency of the 3D printed swab with the commercial swab. A commercial swab is taken into reference while designing the 3D printed swab. To facilitate the swab breakage into VTM, a notch from the tip between 80 and 100 mm is included. An open lattice configuration is chosen with a domed tip. Digital Light Processing (DLP) based NewPro3D 3D printer is employed to print the swab with Envisiontec E1 guide soft material. DLP is a similar technology to SLA, the only notable variation is the light source used to curate the resin. In SLA UV laser is used to cure the resin, wherein DLP the UV laser is replaced with the digital projector screen [70]. After printing, swabs are washed twice for 10 min in 99% alcohol and 99% isopropyl alcohol. Then the swabs are dried at 38 °C for 30 min and after that curing is done for 10 min at the UV curing unit. Compared to the commercial swab, the 3D printed swab performance is reasonable.
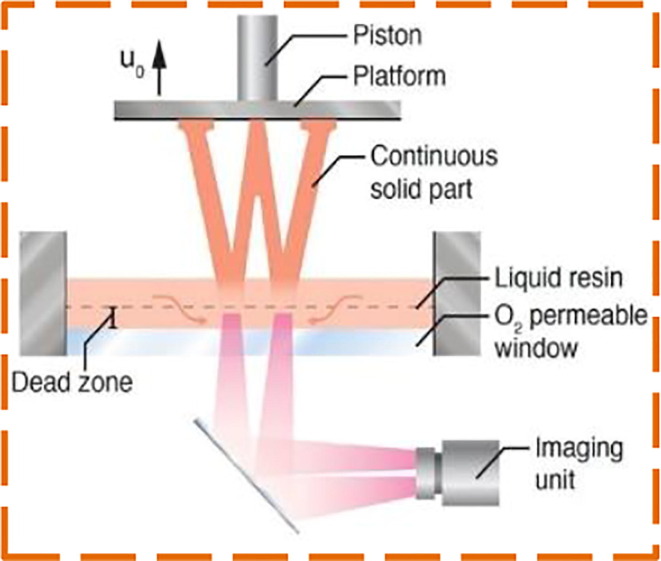
Moreover, Digital Light Synthesis (DLS) technology is also adopted to 3D print the NP swabs by Bennett, Ian, et al. [71]. By its oxygen-permeable window, DLS differs from the essentially similar DLP principles of additive manufacturing, which prevents polymerization triggered by UV at the window surface so that it does not bind to the window. Light is projected into the UV-curable resin reservoir via an oxygen-permeable window. The build platform rises when the part solidifies as a series of UV images are projected as shown in Fig. 7 . With the dimensional reference of commercial swabs, the authors designed the 3D printed swab. A breakpoint is included from the distal end to a handle of 70 mm. The design concept of a lattice bulb is used at the distal end using software developed at Carbon, Inc. Keeping the patient comfort in mind, inside the bulb tip, a helical core that is tapered fully is included along with an open unit cell design. These improvements enhanced bulb flexibility and durability while preserving the collection volume of the sample. Keystone® Industries’ KeySplint Soft® Clear material is used to 3D printed swabs. In a human clinical sample of suspected patients of COVID-19, these latticed 3D printed NP swabs have shown non-inferiority.
Fig. 7.
Schematic diagram of DLS printing process [69].
7. Conclusion
To conclude, this paper presents a detailed review of various 3D printed NP swab designs, their manufacturing using 3D printing technologies and their promising potential to overcome the dearth of testing kits. The scarcity of diagnostic testing kits and the transformed development by allowing health care personals to produce NP swabs in the battle against COVID-19 can be assisted by 3D printing. In addition to conventional NP swabs and viral transport media kits, 3D printing has proved to be a reliable, cost-effective, and fast solution for COVID-19 testing and with the ability to mitigate substantial supply chain obstacles with growing testing requirements. SLA printed NP swabs have performed remarkably well with conventional NP swabs when compared to other 3D printed NP swabs. However, more research is to be done on other raw materials, besides Surgical Guide resin. Although, PLA has been used in FDM NP swabs, which is biodegradable and non-toxic, it is yet to be tested to see if it’s viable for viral specimens for PCR based diagnosis. FDM printers though less expensive than SLA printers, have some inherent limitations. Further research may be one of the potential ways to address the same. Although the literature suggests that 3D printing swabs can be replaced in place of conventional NP swabs, but few reported designs need to undergo large-scale clinical trials to know their true potential. Patient’s comfort is also to be kept in mind while performing large scale trials of these designs. Other methods like DLP, DLS and SLS have shown promising results, however, more trials are to be made to be able to reach a conclusion so far as their effectiveness is concerned. Moreover, optimized design studies with suitable 3D printing technologies are furthermore needed to enhance the test quality and also to meet the supply chain demands.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to “Elsevier (License numbers: 4918060982968, 4918061299271, 4918070098840, 4918070238276), American Society for Microbiology, Springer Nature, Infinite Science Publishing” for granting the copyright permission to various figures in this paper.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M. Brida, M. Chessa, H. Gu, M.A. Gatzoulis, The globe on the spotlight: Coronavirus disease 2019 (COVID-19), Int. J. Cardiol., 310 (2020) 170–172. [DOI] [PMC free article] [PubMed]
- 5.Kobayashi T., Jung S., Linton N.M., Kinoshita R., Hayashi K., Miyama T., Anzai A., Yang Y., Yuan B., Akhmetzhanov A.R., Suzuki A., Nishiura H. Communicating the risk of death from novel coronavirus disease(COVID-19) JCM. 2020;9(2):580. doi: 10.3390/jcm9020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linton N., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A., Jung S., Yuan B., Kinoshita R., Nishiura H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. JCM. 2020;9(2):538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohanski M.A., Lo L.J., Waring M.S. Review of indoor aerosol generation, transport, and control in the context of COVID-19. Int. Forum. Allergy Rhinol. 2020 doi: 10.1002/alr.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020;10 doi: 10.1038/d41587-020-00010-2. [DOI] [PubMed] [Google Scholar]
- 10.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Meltzer M.I., Wortley P.M. Flusurge—a tool to estimate demand for hospital services during the next pandemic influenza. Med. Decis. Making. 2006;26(6):617–623. doi: 10.1177/0272989X06295359. [DOI] [PubMed] [Google Scholar]
- 12.Durfee W.K., Iaizzo P.A. Medical applications of 3D printing. Eng. Med. 2019:527–543. [Google Scholar]
- 13.R. Patel, E. Babady, E.S. Theel, G.A. Storch, B.A. Pinsky, K. St. George, T.C. Smith, S. Bertuzzi, Report From The American Society For Microbiology Covid-19 International Summit, 23 March 2020: value of diagnostic testing for SARS–COV-2/COVID-19, mBio, 11 (2) (2020) mBio.00722-20. [DOI] [PMC free article] [PubMed]
- 14.Péré H., Podglajen I., Wack M., Flamarion E., Mirault T., Goudot G., Hauw-Berlemont C., Le L., Caudron E., Carrabin S., Rodary J., Ribeyre T., Bélec L., Veyer D. Nasal swab sampling for SARS-COV-2: a convenient alternative in times of nasopharyngeal swab shortage, McAdam AJ, ed. J. Clin. Microbiol. 2020;58(6):e00721–e820. doi: 10.1128/JCM.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Diab A., Azzeh M., Ghneim R., Ghneim R., Zoughbi M., Turkuman S., Rishmawi N. Issa A-E-R, Siriani I, Dauodi R, Kattan R, Hindiyeh MY, Comparison between pernasal flocked swabs and nasopharyngeal aspirates for detection of common respiratory viruses in samples from children. J. Clin. Microbiol. 2008;46(7):2414–2417. doi: 10.1128/JCM.00369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan K.H., Peiris J.S.M., Lim W., Nicholls J.M., Chiu S.S. Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J. Clin. Virol. 2008;42(1):65–69. doi: 10.1016/j.jcv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Daley P., Castriciano S., Chernesky M., Smieja M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J. Clin. Microbiol. 2006;44(6):2265–2267. doi: 10.1128/JCM.02055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention, Interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for coronavirus disease 2019 (COVID-19), COVID-19, Mar 9 (2020).
- 19.World Health Organization . World Health Organization; 2006. Collecting, preserving and shipping specimens for the diagnosis of avian influenza A (H5N1) virus infection: guide for field operations. [Google Scholar]
- 20.Marty F.M., Chen K., Verrill K.A. How to obtain a nasopharyngeal swab specimen, Ingelfinger JR, ed. N. Engl. J. Med. 2020;382(22) doi: 10.1056/NEJMvcm2010260. [DOI] [PubMed] [Google Scholar]
- 21.Karligkiotis A., Arosio A., Castelnuovo P. How to obtain a nasopharyngeal swab specimen. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2015949. [DOI] [PubMed] [Google Scholar]
- 22.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., Warren J.L., Geng B., Muenker M.C., Moore A.J., Vogels C.B.F., Petrone M.E., Ott I.M., Lu P., Venkataraman A., Lu-Culligan A., Klein J., Earnest R., Simonov M., Datta R., Handoko R., Naushad N., Sewanan L.R., Valdez J., White E.B., Lapidus S., Kalinich C.C., Jiang X., Kim D.J., Kudo E., Linehan M., Mao T., Moriyama M., Oh J.E., Park A., Silva J., Song E., Takahashi T., Taura M., Weizman O.-E., Wong P., Yang Y., Bermejo S., Odio C.D., Omer S.B., Dela Cruz C.S., Farhadian S., Martinello R.A., Iwasaki A., Grubaugh N.D., Ko A.I. Saliva or nasopharyngeal swab specimens for detection of SARS-COV-2. N. Engl. J. Med. 2020;383(13):1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBlanc J.J., Heinstein C., MacDonald J., Pettipas J., Hatchette T.F., Patriquin G. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ek P., Böttiger B., Dahlman D., Hansen K.B., Nyman M., Nilsson A.C. A combination of naso- and oropharyngeal swabs improves the diagnostic yield of respiratory viruses in adult emergency department patients. Infectious Diseases. 2019;51(4):241–248. doi: 10.1080/23744235.2018.1546055. [DOI] [PubMed] [Google Scholar]
- 25.Jamal AJ, Mozafarihashjin M, Coomes E, Powis J, Li AX, Paterson A, Anceva-Sami S, Barati S, Crowl G, Faheem A, Farooqi L, Khan S, Prost K, Poutanen S, Taylor M, Yip L, Zhong XZ, McGeer AJ, Mubareka S, Toronto Invasive Bacterial Diseases Network COVID-19 Investigators, Coleman BL, Chen D, Farshait N, Gold W, Kandel CE, Katz K, Kozak R, Mazzulli T, Muller M, Opavsky A, Ostrowski M, Plevneshi A, Rau N, Ricciuto D, Richardson D, Rose D, Sales V, Walmsley S, Sensitivity of nasopharyngeal swabs and saliva for the detection of Severe Acute Respiratory Syndrome Coronavirus 2, Clinical Infectious Diseases,(2020) ciaa848. [DOI] [PMC free article] [PubMed]
- 26.Wang X., Tan L., Wang X., Liu W., Lu Y., Cheng L., Sun Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infectious Diseases. 2020;94:107–109. doi: 10.1016/j.ijid.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M.R. Patel, D. Carroll, E. Ussery, H. Whitham, C.A. Elkins, J. Noble-Wang, J.K. Rasheed, X. Lu, S. Lindstrom, V. Bowen, J. Waller, G. Armstrong, S. Gerber, J.T. Brooks, Performance of oropharyngeal swab testing compared to nasopharyngeal swab testing for diagnosis of COVID-19—United States, January-February 2020, Clinical Infectious Diseases, (2020) ciaa759. [DOI] [PMC free article] [PubMed]
- 28.Wang H., Liu Q., Hu J., Zhou M., Yu M., Li K., Xu D., Xiao Y., Yang J., Lu Y., Wang F., Yin P., Xu S. Nasopharyngeal swabs are more sensitive than oropharyngeal swabs for covid-19 diagnosis and monitoring the SARS-COV-2 load. Front. Med. 2020;7:334. doi: 10.3389/fmed.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irving S.A., Vandermause M.F., Shay D.K., Belongia E.A. Comparison of nasal and nasopharyngeal swabs for influenza detection in adults. Clinical Med. Res. 2012;10(4):215–218. doi: 10.3121/cmr.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinninti S., Trieu C., Pati S.K., Latting M., Cooper J., Seleme M.C., Boppana S., Arora N., Britt W.J. BoppanaSBComparing Nasopharyngeal and Mid-Turbinate Nasal Swab Testing for the Identification of SARS-CoV-2. Clin. Infect. Dis. 2020:ciaa882. doi: 10.1093/cid/ciaa882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeiren C., Marchand-Senécal X., Sheldrake E., Bulir D., Smieja M., Chong S., Forbes J.D., Katz K. Comparison of Copan Eswab and FLOQswab for COVID-19 PCR diagnosis: working around a supply shortage. J. Clin. Microbiol. 2020;58(6):e00669–e720. doi: 10.1128/JCM.00669-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tino R., Moore R., Antoline S., Ravi P., Wake N., Ionita C.N., Morris J.M., Decker S.J., Sheikh A., Rybicki F.J., Chepelev L.L. COVID-19 and the role of 3D printing in medicine. 3D Print. Med. 2020;6(1):11. doi: 10.1186/s41205-020-00064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan C.J., Lee R., Zulauf K.E., Tamburello L., Smith K.P., Previtera J., Cheng A., Green A., Abdul Azim A., Yano A., Doraiswami N., Kirby J.E., Arnaout R.A. Open Development and Clinical Validation Of Multiple 3D-Printed Nasopharyngeal Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck. J. Clin. Microbiol. 2020;58(8):e00876–e920. doi: 10.1128/JCM.00876-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.M. Siyuan, L. Yimei, H. Tianyu, Y. Zaichun, S. Jiangu,o Y. Shiyong, Y. Zhiqiang, P. Yizhi, L. Gaoxing,X. Zhi, Clinical application effect of modified nasopharyngeal swab sampling for 2019 novel coronavirus nucleic acid detection, Chinese J. Burns 36 (2020) E009 (In Chinese) [DOI] [PubMed]
- 35.Spadaccini C., Duoss E., Shusteff M., Tooker A., Haque R. Livermore; CA (United States): 2020. Swab Testing Results, Lawrence Livermore National Lab (LLNL) [Google Scholar]
- 36.J. Ford, T. Goldstein, S. Trahan, A. Neuwirth, K. Tatoris, S. Decker, A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing, 3D Print. Med. 6 (1) (2020) 21. [DOI] [PMC free article] [PubMed]
- 37.Bencharit S., Staffen A., Yeung M., Whitley D., Laskin D.M., Deeb G.R. In vivo tooth-supported implant surgical guides fabricated with desktop stereolithographic printers: fully guided surgery is more accurate than partially guided surgery. J. Oral Maxillofac. Surg. 2018;76(7):1431–1439. doi: 10.1016/j.joms.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Decker S.J., Goldstein T.A., Ford J.M., Teng M.N., Pugliese R.S., Berry G.J., Pettengill M., Silbert S., Hazelton T.R., Wilson J.W., Shine K., Wang Z.-X., Hutchinson M., Castagnaro J., Bloom O.E., Breining D.A., Goldsmith B.M., Sinnott J.T., O’Donnell D.G., Crawford J.M., Lockwood C.J. Kim K,3D Printed Alternative to the Standard Synthetic Flocked Nasopharyngeal Swabs Used for COVID-19 testing. Clin. Infect. Dis. 2020:ciaa1366. doi: 10.1093/cid/ciaa1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chmura Kraemer H., Periyakoil V.S., Noda A. Kappa coefficients in medical research. Statist. Med. 2002;21(14):2109–2129. doi: 10.1002/sim.1180. [DOI] [PubMed] [Google Scholar]
- 40.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. [PubMed] [Google Scholar]
- 41.J.K. Tay, G.B. Cross, C.K. Lee, B. Yan, J. Loh, Z.Y. Lim, N. Ngiam, J. Chee, S.W. Gan, A. Saraf, W.T.E. Chow, H.L. Goh, C.H. Siow, D.W. Lian, W.S. Loh, K.S. Loh, V.T. Chow, D.Y. Wang, J.Y. Fuh, C.-C. Yen, J.E. Wong, D.M. Allen, Design and Clinical Validation of a 3D-Printed Nasopharyngeal Swab for COVID-19 Testing,medRxiv [Preprint], (2020). DOI: https://doi.org/10.1101/2020.06.18.20134791
- 42.Kim H., Hong H., Yoon S.H. Diagnostic performance of Ct and Reverse Transcriptase Polymerase Chain Reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020;296(3):E145–E155. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Scola B., Le Bideau M., Andreani J., Hoang V.T., Grimaldier C., Colson P., Gautret P., Raoult D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1059. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., Doan K., Bastien N., Li Y., Van Caeseele P.G., Poliquin G. Predicting infectious Severe Acute Respiratory Syndrome Coronavirus 2 from diagnostic samples. Clin. Infect. Dis. 2020:ciaa638. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 46.Starosolski Z., Admane P., Dunn J., Kaziny B., Huisman T.A., Annapragada A. Design of 3D-printed nasopharyngeal swabs for children is enabled by radiologic imaging. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullis K.B., Faloona F.A. Specific synthesis of DNA in vitro via a Polymerase-Catalyzed Chain Reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 49.Gallup N., Pringle A.M., Oberloier S., Tanikella N.G., Pearce J.M. Parametric nasopharyngeal swab for sampling COVID-19 and other respiratory viruses: Open source design, SLA 3-D printing and UV curing system. HardwareX. 2020;8 doi: 10.1016/j.ohx.2020.e00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.M. Alghounaim, S. Almazeedi, S. Al Youha, J. Papenburg, O. Alowaish, G. AbdulHussain, R. Al-Shemali, A. Albuloushi, S. Alzabin, K. Al-Wogayan, Y. Al-Mutawa, S. Al-Sabah, Low-cost polyester-tipped 3-Dimensionally-printed nasopharyngeal swab for the diagnosis of severe acute respiratory syndrome-related coronavirus 2(SARS-CoV-2), J. Clin. Microbiol. (2020) JCM.01668-20. [DOI] [PMC free article] [PubMed]
- 51.Masood S.H. Advances in fused deposition modeling. Comprehensive Mater. Process. 2014:69–91. [Google Scholar]
- 52.Singhvi M.S., Zinjarde S.S., Gokhale D.V. Polylactic acid: synthesis and biomedical applications. J. ApplMicrobiol. 2019;127(6):1612–1626. doi: 10.1111/jam.14290. [DOI] [PubMed] [Google Scholar]
- 53.Gupta B., Revagade N., Hilborn J. Poly(Lactic acid) fiber: an overview. Prog. Polym. Sci. 2007;32(4):455–482. [Google Scholar]
- 54.Zuniga J.M., Cortes A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med. Devices. 2020;17(6):477–481. doi: 10.1080/17434440.2020.1756771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015;9(1):4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruijns B.B., Tiggelaar R.M., Gardeniers H. The extraction and recovery efficiency of pure DNA for different types of swabs. J. Forensic Sci. 2018;63(5):1492–1499. doi: 10.1111/1556-4029.13837. [DOI] [PubMed] [Google Scholar]
- 57.B. Freire-Paspuel, P.A. Vega-Marino, A. Velez, P. Castillo, I. Gomez-Santos, M. Cruz, M.A. Garcia-Bereguiain, Cotton Tipped Plastic Swabs for SARS-CoV2 RT-QPCR Diagnosis to Prevent Supplies Shortage,medRxiv [Preprint], (2020). DOI: https://doi.org/10.1101/2020.04.28.20079947 [DOI] [PMC free article] [PubMed]
- 58.S. Alyouha, S. AlMazeedi, M. Alghounaim, Y. Al-Mutawa, S. AlSabah, Polyester tipped 3-Dimensionally printed swab that costs less than US$0.05 and can easily and rapidly be mass produced, BMJ Innov. (2020) bmjinnov-2020-000483.
- 59.Cox J.L., Koepsell S.A. 3D-printing to address COVID-19 testing supply shortages. Laboratory Medicine. 2020;51(4):e45–e46. doi: 10.1093/labmed/lmaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin Y., Li H., He Y., Fu J. Quantitative analysis of surface profile in fused deposition modelling. Addit. Manuf. 2015;8:142–148. [Google Scholar]
- 61.S. Pfeiffer, Despite early warnings, US took months to expand swab production for COVID-19 test, (2020).
- 62.Sun Y., Mercader A., Lueth T.C. Design of 3D-printable nasopharyngeal swabs in Matlab for COVID-19 testing. Trans. Additive Manuf. Meets Med. 2020;2(1) [Google Scholar]
- 63.Y. Sun, L. Xu, J. Yang, T.C. Lueth, Automatic design in MATLAB using PDE toolbox for shape and topology optimization, Advanced Materials: Design, Processing, Characterization, and Applications. American Society of Mechanical Engineers, 12 (2019) V012T10A002.
- 64.Sun Y., Liu Y., Xu L., Zou Y., Faragasso A., Lueth T.C. Automatic design of compliant surgical forceps with adaptive grasping functions. IEEE Rob. Autom. Lett. 2020;5(2):1095–1102. [Google Scholar]
- 65.K.S. Munir, Y. Li, C. Wen, Metallic scaffolds manufactured by selective laser melting for biomedical applications, Metallic Foam Bone (2017) 1–23.
- 66.Williams E., Bond K., Isles N., Chong B., Johnson D., Druce J., Hoang T., Ballard S.A., Hall V., Muhi S., Buising K.L., Lim S., Strugnell D., Catton M., Irving L.B., Howden B.P., Bert E., Williamson D.A. Pandemic printing: a novel 3D-printed swab for detecting SARS-CoV-2. Med. J. Aust. 2020;213(6):276–279. doi: 10.5694/mja2.50726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.A. Muzaffar, M.B. Ahamed, K. Deshmukh, T. Kovářík, T. Křenek, S.K.K. Pasha, 3D and 4D printing of pH-responsive and functional polymers and their composites, 3D and 4D Printing of Polymer Nanocomposite Materials, (2020) 85–117.
- 68.Arnold F.W., Grant G., Bressoud P.F., Furmanek S.P., Chung D., Sbaih N., Karmali D., Cahill M., Pantalos G. A Comparison Efficacy Study of Commercial Nasopharyngeal Swabs versus a Novel 3D Printed Swab for the Detection of SARS-CoV-2. University of Louisville J. Respiratory Infections. 2020;4(1):41. [Google Scholar]
- 69.Redmann A., Oehlmann P., Scheffler T., Kagermeier L., Osswald T.A. Thermal curing kinetics optimization of epoxy resin in Digital Light Synthesis. Addit. Manuf. 2020;32 [Google Scholar]
- 70.Kadry H., Wadnap S., Xu C., Ahsan F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019;135:60–67. doi: 10.1016/j.ejps.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Bennett I., Bulterys P.L., Chang M., DeSimone J.M., Fralick J., Herring M., Kabaria H., Kong C., Larson B., Lu O., Maxner A., Meyer E., Patterson S., Pollack S., Rolland J., Schmidt S., Seshadri S., Swarup K., Thomas C., Van Wert R. The Rapid Deployment of a 3D Printed Latticed Nasopharyngeal Swab for COVID-19 Testing Made Using Digital Light Synthesis. medRxiv [Preprint] 2020 doi: 10.1101/2020.05.25.20112201. [DOI] [Google Scholar]