Abstract
Background:
Evidence-based outpatient treatment for opioid use disorder (OUD) consists of medications that treat OUD (MOUD) and psychosocial treatments (e.g., psychotherapy or counseling, case management). Prior studies have not examined the use of these components of care in a commercially insured population.
Methods:
We analyzed claims data from a large national commercial insurer of enrollees age 17–64 identified with OUD (2008–2016, N=87,877 persons and 122,708 person-years). Multinomial logistic regression models identified factors associated with receiving in a given year: 1) both MOUD and psychosocial visits, 2) MOUD without psychosocial visits, 3) psychosocial visits without MOUD, or 4) neither. We estimated predicted probabilities for key variables of interest.
Results:
Identification of OUD nearly tripled during the observation period (0.17% in 2008, 0.45% in 2016). Among person-years identified as having OUD, 36.3% included MOUD (8.1% both MOUD and psychosocial visits and 28.2% MOUD without psychosocial visits). In adjusted analyses, women had a lower probability of receiving either treatment alone or in combination (e.g., MOUD plus psychosocial visits: women=6.7%[6.5%–6.9%] vs. men= 9.2%[9.0%–9.4%]). Moderate/severe vs. mild OUD was associated with a higher probability of receiving MOUD (e.g., MOUD plus psychosocial visits: 8.7%[8.6%–8.9%] vs. .9%[0.7%–1.0%]). In contrast, an OUD overdose was associated with a greater probability of receiving neither treatment (78.2%[77.4%–79.0%] vs. 55.5%[55.2%–55.8%]). Over time, the probability of receiving each MOUD and psychosocial treatment category increased relative to 2008, but reached a peak and then plateaued or declined, by the end of the study period.
Conclusions:
A significant treatment gap exists among individuals identified with OUD in this commercially insured population, with greater risks of receiving no treatment for women and for individuals with mild versus moderate or severe OUD. Overdose is associated with receiving neither MOUD nor psychosocial treatment. While treated prevalence initially increased relative to 2008, rates of treatment subsequently plateaued. Additional study and monitoring to elucidate barriers to OUD treatment in commercially insured populations are warranted.
1. Introduction
Recent prevalence rates of opioid use disorder (OUD) indicate that approximately 2 million adults in the U.S. (about .8%) have OUD (SAMHSA, 2018b). OUD prevalence, overdose, and deaths have increased substantially in the U.S. during the past decade (CDC, 2019; Han et al., 2017; Han, Compton, Jones, & Cai, 2015; Rudd, Aleshire, Zibbell, & Gladden, 2016; Saha et al., 2016; SAMHSA, 2019a). Evidence-based OUD care requires ongoing care and management in the outpatient setting in both acute and maintenance phases, to improve recovery outcomes (Blodgett, Maisel, Fuh, Wilbourne, & Finney, 2014; HHS, 2016; McLellan, Lewis, O’Brien, & Kleber, 2000; Tai & Volkow, 2013).
Methadone, buprenorphine, and naltrexone are medications that treat OUD (often abbreviated as MOUD). MOUD improve treatment retention, and reduce opioid use and mortality (Sordo et al., 2017; Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016). Additionally, psychosocial treatments (which include psychotherapy or counseling, case management, community behavioral supportive services, and psychosocial rehabilitation) are a common component of evidence-based OUD care, and can be effective in improving OUD outcomes in certain patient populations (Amato, Minozzi, Davoli, & Vecchhi, 2011; Gossop, Stewart, & Marsden, 2005; Weiss & Rao, 2017). While not all patients require the same array or intensity of services (Carroll & Weiss, 2017; Day & Mitcheson, 2017; Dutra et al., 2008), and more research is needed to better delineate characteristics of patient populations and specific psychosocial treatments that are associated with improved patient outcomes (Blanco & Volkow, 2019), there is evidence that patients who have access to a full array of psychosocial services along with MOUD have better outcomes independent of whether they used each of these services (McLellan, Arndt, Metzger, Woody, & O’Brien, 1993).
As is the case for substance use disorders more broadly, most individuals with OUD in the U.S. do not receive any treatment (SAMHSA, 2019b). Among those who do, many receive only inpatient care, with no outpatient follow up or medication (Naeger, Mutter, Ali, Mark, & Hughey, 2016). Stigmatizing societal beliefs about substance use disorders (including among healthcare workers) that may influence treatment-seeking behaviors, patient insight regarding the need for or skepticism about treatment, logistical challenges, and financial challenges have all been identified as barriers to care (Bearnot, Fine, Rigotti, & Baggett, 2019; Kennedy-Hendricks et al., 2017; SAMHSA, 2019a, 2019b; Volkow, 2020). Additional barriers to receiving MOUD in particular include low adoption of MOUD in substance use disorder treatment programs, insurance coverage restrictions such as prior authorization requirements for MOUD, shortages of MOUD prescribers, and patient preferences or beliefs about using medications as part of OUD treatment (Abraham, Knudson, Rieckmann, & Roman, 2013; Huskamp, Riedel, Barry, & Busch, 2018; Jones, Campopiano, Baldwin, & McCance-Katz, 2015; Mojtabai & Crum, 2016; Priester et al., 2016; Reif, Creedon, Horgan, Stewart, & Garnick, 2017; Reif et al., 2016; Roman, Abraham, & Knudsen, 2011; Uebelacker, Bailey, Herman, Anderson, & Stein, 2016).
Prior research also finds that women face additional challenges in accessing substance use disorder treatment, relative to men. They are less likely to enter treatment (Back, Payne, Simpson, & Brady, 2010), and when they do, they are less likely to receive treatment that takes into account specific needs that may disproportionately affect them (e.g., higher rates of co-occurring psychiatric disorders; need for childcare, transportation, and other supportive services) (Back et al., 2010; Greenfield, Back, Lawson, & Brady, 2010; Herbeck, Jeter, Cousins, Abdelmaksoud, & Crèvecoeur-MacPhail, 2016). However, prior research has not described whether women with OUD are less likely to receive OUD treatments in general, or only specific treatments (e.g., MOUD or psychosocial visits).
Further, more research is needed regarding patient clinical characteristics that may influence whether patients use MOUD or psychosocial treatments, either alone or in combination, as part of their OUD care. For example, clinical severity of OUD or the presence of co-occurring substance use disorders or mental health conditions may influence patients’ or providers’ care decisions. Another clinically important patient population are those who have experienced an opioid overdose. Among Medicaid enrolled individuals, several single-state studies found low rates of MOUD following opioid overdose (Koyawala, Landis, Barry, Stein, & Saloner, 2019; Larochelle et al., 2018), or small increases in rates of MOUD following overdose (Frazier et al., 2017; Koyawala et al., 2019). In one Medicaid program, use of counseling visits post–opioid overdose was lower than prior to the overdose event (Koyawala et al., 2019). However, research shows wide variation in OUD treatment utilization across states, related at least in part to their Medicaid coverage policies for OUD care (Grogan et al., 2016; Mark, Lubran, McCance-Katz, Chalk, & Richardson, 2015; Meinhofer & Witman, 2018). There is no prior research that examines OUD treatment among commercially insured individuals who have an OUD overdose in a given year.
Most research examining OUD treatment focuses on the use of MOUD. There is scarce prior research, particularly in the commercially insured population, that examines predictors of receiving both MOUD and psychosocial treatments, MOUD without psychosocial treatment, psychosocial treatment without MOUD, or neither of these treatments. Existing studies among the commercially insured and in national health plans (Morgan, Schackman, Leff, Linas, & Walley, 2018; Thomas et al., 2018) do not include information about this fuller array of OUD treatment. In this study, we examine claims data from a large, national commercial insurer to (1) investigate patient characteristics associated with OUD treatment (i.e., MOUD, psychosocial visits, both, or neither), and (2) consider how utilization patterns have changed over time during the evolution of the OUD crisis in the U.S. We focus on outpatient care because of its critical importance in providing sustained treatment and relapse prevention for patients with OUD.
2. Materials and methods
The Harvard Medical School Internal Review Board reviewed and approved this study.
2.1. Study population
We use claims data from a national health insurance company covering 2008–2016 to develop a person-year cohort. The study cohort included enrollees between ages 17 and 64 who were continuously enrolled at least 10 months of a given year with medical, behavioral health, and pharmacy benefits managed by the insurer, and who during the year were identified as having an OUD. We defined OUD in the claims as having at least one claim with an OUD diagnosis in any diagnostic field (ICD-9 codes 304.0, 304.7, 305.5; ICD-10 code F11) or with a claim for fatal or nonfatal OUD overdose (ICD-9 codes 965.0, E850.0–E850.2; ICD-10 codes T40.0–T40.4, or T40.6) (2013).
2.2. Primary outcome
Our primary outcome of interest was a four-category, mutually exclusive service use variable defined as: 1) both MOUD and at least one outpatient psychosocial visit; 2) MOUD without any outpatient psychosocial visits; 3) at least one outpatient psychosocial visit without any MOUD; and 4), neither any MOUD nor outpatient psychosocial visits.
2.2.1. MOUD
We defined MOUD as the three medications that the Food and Drug Administration has approved for the treatment of OUD (buprenorphine, naltrexone, and methadone). We used pharmacy claims to identify prescription fills for buprenorphine (including buprenorphine/naloxone preparations) and naltrexone. We also included Health care Procedure Coding System (HCPCS) codes for methadone services (H0020, J1230, S0109); intramuscular (IM) naltrexone injection (J2315); and buprenorphine oral, injection, and implant administration (J0570-J0575, J0592)(Huskamp, Busch, et al., 2018).
2.2.2. Psychosocial visits
We identified outpatient psychosocial visits using Common Procedure Terminology (CPT) Evaluation and Management or behavioral health-specific CPT codes or Healthcare Common Procedure Coding System (HCPCS) codes for psychotherapy/counseling, case management, psychosocial rehabilitation, and supportive behavioral health services (Huskamp, Busch, et al., 2018) (See Appendix Table A1 for details). To count as an OUD visit, all outpatient visits were required to have OUD as the primary or secondary diagnosis on the claim (denoting that OUD was a prominent focus of the visit). We excluded from the outpatient visit definition any visit or provider types that we did not expect to involve OUD care specifically (e.g., radiologic or laboratory services; visits with providers of medical specialties such as oncology, cardiology, surgery).
2.3. Explanatory variables
Enrollee demographic variables included sex, U.S. geographic region (Northeast, South, Midwest, West), employee status (versus dependent), rural residence (defined using the Rural Urban Commuting Area (RUCA) codes), and age (17–24, 25–34, 35–44, 45–54, 55–64). We created several enrollee clinical variables, including the presence of any claims during the calendar year for OUD overdose, higher severity OUD (defined by the use of ICD-9 code 304.0 [dependence] versus 305.6 [abuse] and ICD-10 code F11.2x [moderate/severe] versus F11.1x [mild]), comorbid mental health condition (i.e., whether the enrollee had at least one claim in any diagnostic field with a diagnosis of a mental health condition [ICD-9 codes: 295–302, 306–314; ICD-10 codes F20–F69, F84, F90–F99]), and comorbid non-OUD substance use disorder (including alcohol) (i.e., at least one claim with a diagnosis in any diagnostic field of one of the following: ICD-9 codes: 291, 292, 303, 304.1–304.9, 305.0, 305.2–305.4, 305.6, 305.7, 305.9; ICD-10 codes: F10, F12–F16, F18, F19) during the person-year. Finally, we created dichotomous variables for each study year. For descriptive purposes, we defined a variable for OUD hospitalization in a given person-year as a hospitalization in which an OUD was in any diagnostic field.
To account for provider practice patterns, we used provider tax identification numbers (TINs) to assign each patient to a practice. A TIN practice may consist of a single provider, multiple providers in a single- or multi-site practice, or a hospital/healthcare organization. We assigned patients to the practice where they received the most days of OUD services in that year. Similar to an intent-to-treat analysis, we assigned enrollees who were associated with more than one primary TIN (i.e., enrollees who were in multiple years of the data and in different years had a different primary TIN), to the first TINs observed during our study period. We excluded emergency room (ER) visits as counting toward TIN assignment, because during the data years of our study ERs were not a level of care typically engaged in initiating or maintaining medications for chronic/maintenance OUD treatment.
2.4. Statistical analysis
We calculated the proportion of enrollees each year diagnosed with OUD, and among them, the proportion falling into each of the treatment categories.
We estimated a multinomial logistic regression model for receipt of each of the four mutually exclusive treatment categories (Agresti, 2002). The explanatory variables in the model included the demographic and clinical variables describe, and year. We used a generalized estimating equation approach to account for repeated measures per study enrollee as well as for within-TIN correlation among enrollee treatment outcomes.
We computed odds ratios and 95% confidence intervals and for several explanatory variables (calendar year, gender, comorbid mental health condition, comorbid substance use disorder, moderate or severe [vs. mild] OUD, and OUD overdose), we estimated the probability of treatment outcomes for enrollees with these characteristics. To obtain the estimated probabilities and corresponding 95% confidence intervals, we adopted a bootstrapping procedure. We drew 1000 random samples from the study cohort with replacement, estimated the multinomial model in each sample, and obtained the predicted probabilities for each treatment outcome category for each enrollee. We then averaged the probabilities of each treatment outcome over enrollees with the specific characteristic over the 1000 samples. For instance, we estimated the probabilities of the treatment outcomes among males and among females. We constructed ninety-five percent confidence intervals by ordering the estimated probabilities and identifying the 0.025th and 0.975th values.
We did not adjust for the multiplicity of statistical testing and thus do not report p-values. We did not adjust the confidence intervals.
3. Results
Between 2008 and 2016 there were more than 17 million unique enrollees in the health plan; the proportion who were diagnosed with OUD each year, while small overall, nearly tripled from 0.17% in 2008 to 0.45% in 2016 (Figure 1). Throughout the 9-year period, there were 122,708 person-years and 87,877 persons diagnosed with OUD. Three-quarters (75.3%) of the individuals identified with OUD were in the cohort for only one year, and these individuals represented half (50.0%) of the OUD person-years (data not shown). The mean and median number of patients with OUD per TIN was 1 each year, the maximum ranged from N=75–205 patients per TIN each year, and at least 75% of the patients were associated with only 1 TIN each year (data not shown).
Figure 1:
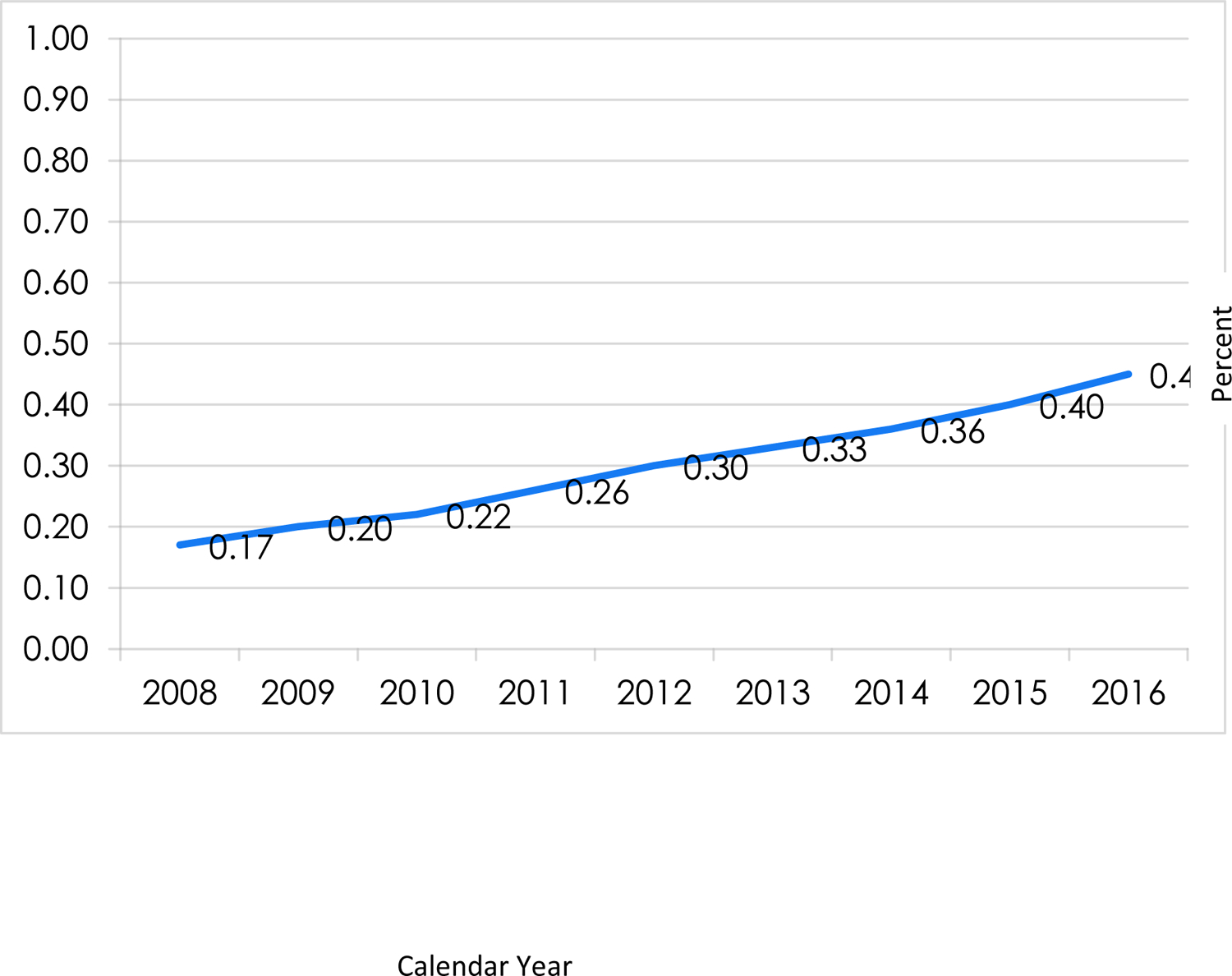
Unadjusted prevalence of OUD identification in a commercial health plan population, 2008–2016 (N=41,874,503 person-years in health plan, N=122,708 person-years with OUD identified).
Note: denominator = enrollees ages 17–64 with at least 10 months of continuous medical, behavioral health and pharmacy benefits in the health plan in a given year (independent of whether or not have OUD diagnosis in claims). Denominators by year: 2008 N=5,308,713; 2009 N=5,390,291; 2010 N=4,865,956; 2011 N=4,512,867; 2012 N= 4,390,082; 2013 N=4,306,309; 2014 N=4,315,893; 2015 N=4,342,254; 2016 N=4,442,138. Total N of unique individuals in health plan 2008–2016=17,440,108 individuals.
Nearly half of the person-year study sample were employees (48.9%), 42.3% were female, and most were located in urban areas (92.4%) (Table 1 and Appendix Table A2). We identified the majority of the OUD person-year sample (92.6%) as having more severe OUD; 6.1% included an opioid overdose claim during the year. Comorbid mental health and substance use disorders were prevalent (63.3% and 48.0% person-years, respectively).
Table 1:
Unadjusted person-year characteristics of commercial health plan enrollees diagnosed with OUD (2008–2016), and stratified by OUD treatment received (N= 87,877 persons and 122,708 person-years).
| Total | ||
|---|---|---|
| N | % | |
| Age group | ||
| 17–25 | 26,988 | 22.0 |
| 26–35 | 27,355 | 22.3 |
| 36–45 | 24,589 | 20.0 |
| 46–55 | 25,794 | 21.0 |
| 56–64 | 17,982 | 14.7 |
| Employee | 59,959 | 48.9 |
| Female | 51,922 | 42.3 |
| US Region | ||
| Northeast | 39,037 | 31.8 |
| Midwest | 12,747 | 10.4 |
| West | 25,083 | 20.4 |
| South | 45,841 | 37.4 |
| Urban | 113,373 | 92.4 |
| Comorbid mental health condition | 77,721 | 63.3 |
| Comorbid substance use disorder | 58,877 | 48.0 |
| OUD overdose claim | 7,450 | 6.1 |
| OUD moderate/severe | 113,647 | 92.6 |
| Inpatient | 18,479 | 15.1 |
| Year | ||
| 2008 | 9,161 | 7.5 |
| 2009 | 11,035 | 9.0 |
| 2010 | 10,756 | 8.8 |
| 2011 | 11,558 | 9.4 |
| 2012 | 13,042 | 10.6 |
| 2013 | 14,101 | 11.5 |
| 2014 | 15,600 | 12.7 |
| 2015 | 17,256 | 14.1 |
| 2016 | 20,199 | 16.5 |
| OUD treatment received | ||
| MOUD & psychosocial visits | 9,959 | 8.1 |
| MOUD but no psychosocial visits | 34,543 | 28.2 |
| Psychosocial visits but no MOUD | 8,853 | 7.2 |
| Neither MOUD nor psychosocial visits | 69,353 | 56.5 |
More than a third (36.3%) of the person-years included any MOUD (8.1% included both MOUD and psychosocial visits, 28.2% included MOUD but no psychosocial visits), 7.2% included psychosocial visits but no MOUD and more than half (56.5%) of the person-years included neither. Of the 36.3% that included any MOUD (independent of whether they included a psychosocial visit), 4.7% used methadone, 28.7% buprenorphine, and 4.5% naltrexone (data not shown).
Multinomial model point estimates, standard errors, odds ratios, and 95% confidence intervals can be found in the appendix (Appendix Tables A3 and A4). Table 2 provides the estimated mean probabilities for key enrollee variables of interest. Women, compared to men, were associated with lower probabilities of receiving either of the treatments, alone or in combination (e.g., both MOUD and psychosocial visits (Percent [95% CI]= 6.7%[6.5%–6.9%] for women vs. 9.2%[9.0%–9.4%] for men); MOUD but no psychosocial visits=24.1%[23.8%–24.5%] for women vs. 30.2%[29.8%–30.5%] for men).
Table 2:
Estimated percentages of receiving OUD medication and psychosocial visits, OUD medication but no psychosocial visits, psychosocial visits but no OUD medication, and neither MOUD nor psychosocial visits among commercial health plan enrollees with OUD, 2008–2016 (N= 87,877 persons and 122,708 person-years).
| MOUD & psychosocial visits | MOUD but no psychosocial visits | Psychosocial visits but no MOUD | Neither MOUD nor psychosocial visits | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (%) |
95%CI | Mean (%) |
95%CI | Mean (%) |
95%CI | Mean (%) |
95%CI | |
| Age | ||||||||
| 17–24 | 11.3 | 11.0–11.6 | 24.0 | 23.5–24.4 | 13.0 | 12.7–13.3 | 51.7 | 51.2–52.2 |
| 25–34 | 11.3 | 10.9–11.6 | 34.8 | 34.3–35.3 | 8.6 | 8.3–8.9 | 45.3 | 44.8–45.9 |
| 35–44 | 7.6 | 7.3–8.0 | 31.7 | 31.2–32.3 | 5.7 | 5.4–5.9 | 55.0 | 54.4–55.6 |
| 45–54 | 5.2 | 5.0–5.5 | 25.4 | 24.9–25.9 | 4.5 | 4.3–4.7 | 64.8 | 64.3–65.4 |
| 55–64 | 3.8 | 3.5–4.1 | 19.4 | 18.8–20.0 | 3.8 | 3.5–4.0 | 73.0 | 72.4–73.7 |
| Gender | ||||||||
| male | 9.2 | 9.0–9.4 | 30.2 | 29.8–30.5 | 8.0 | 7.8–8.2 | 52.7 | 52.3–53.0 |
| female | 6.7 | 6.4–6.9 | 24.1 | 23.8–24.5 | 6.4 | 6.2–6.5 | 62.9 | 62.5–63.2 |
| Comorbid substance use disorder | ||||||||
| yes | 9.5 | 9.3–9.7 | 26.5 | 26.2–26.8 | 10.3 | 10.1–10.5 | 53.7 | 53.4–54.1 |
| no | 6.8 | 6.6–7.0 | 28.9 | 28.5–29.2 | 4.4 | 4.3–4.6 | 59.9 | 59.5–60.2 |
| Comorbid mental health condition | ||||||||
| yes | 8.4 | 8.3–8.6 | 27.6 | 27.3–27.9 | 8.1 | 7.9–8.2 | 55.9 | 55.6–56.2 |
| no | 7.6 | 7.4–78.8 | 27.8 | 27.3–28.1 | 6.0 | 5.8–6.2 | 58.6 | 58.1–59.0 |
| Moderate/severe OUD | ||||||||
| yes | 8.8 | 8.6–8.9 | 28.5 | 28.2–28.7 | 7.5 | 7.3–7.6 | 55.3 | 55.0–55.6 |
| no | .9 | .7–1.0 | 16.8 | 16.2–17.5 | 5.4 | 5.0–5.8 | 76.9 | 76.2–77.6 |
| OUD overdose claim | ||||||||
| yes | 4.2 | 3.9–4.6 | 13.4 | 12.8–14.0 | 4.2 | 3.8–4.5 | 78.2 | 77.4–79.0 |
| no | 8.4 | 8.2–8.5 | 28.6 | 28.3–28.8 | 7.6 | 7.4–7.7 | 55.5 | 55.2–55.8 |
Note: There has been no adjustment for multiplicity. Multinomial regression models adjusted for age, gender, employee status, U.S. region, urban region, comorbid substance use disorders, comorbid mental health conditions, moderate/severe vs. mild OUD, nonfatal overdose, and calendar year.
Having a comorbid substance use disorder was associated with higher probabilities of receiving psychosocial visits (with or without MOUD) in a given year ( e.g., both MOUD and psychosocial visits 9.5%[9.3%–9. 7%] vs. 6.8%[6.6%–7.0%]), as was a comorbid mental health condition (e.g., both MOUD and psychosocial visits: 8.4%[8.3%–8.6%] vs. 7.6%[7.4%–7.9%]). Enrollees diagnosed with moderate or severe OUD (vs. mild) were considerably more likely to receive MOUD (e.g., both MOUD and psychosocial visits: moderate/severe OUD 8.7%[8.6%–8.9%] vs. .9%[.7%–1.0%]). The opposite was true regarding overdose claims, which were associated with greater probability of receiving neither MOUD nor psychosocial visits (78.2%[77.4%–79.0%] vs. 55.5% vs. 55.2%–55.8%]).
In adjusted analyses, MOUD plus psychosocial treatment use increased among those diagnosed with OUD from 2008 through 2013 (2008 percent [95%CI]=3.5%[3.2%–3.8%], 2013 12.6%[12.2%–13.0%]), but began to decrease thereafter [Figure 2 and Appendix Table A5]). MOUD use without psychosocial treatment also increased initially relative to 2008 but plateaued in 2010 and 2011 and then decreased thereafter through 2016 (2008: 23.9%[23.3%–24.6%], 2011: 39.0%[38.3%–39.6%], 2016: 20.3%[19.9%–20.7%]). The estimated probability of psychosocial visits without MOUD use declined from 2009 to 2012, relative to 2008, increased in 2013 and remained elevated relative to 2008, but declined somewhat from its peak after 2014 (2008: 5.7%[5.3–6.0], 2014: 10.7%[10.3%–11.0%], 2016: 9.0%[8.7%–9.3%]).
Figure 2:
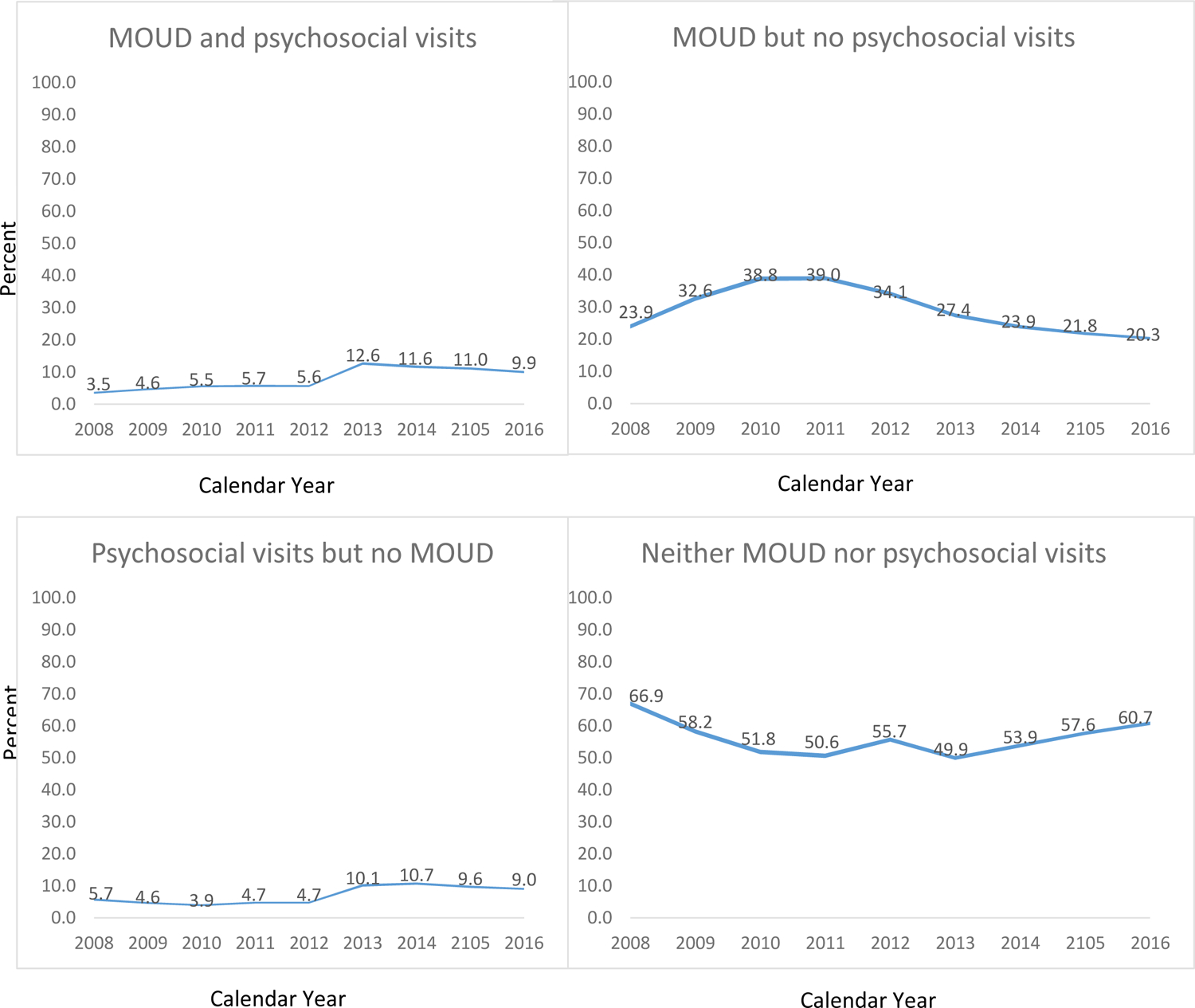
Adjusted mean percentages of receiving opioid use disorder (OUD) medication and psychosocial visits, OUD medication but no psychosocial visits, psychosocial visits but no OUD medication, and neither OUD medication nor psychosocial visits among commercial health plan enrollees with OUD, 2008–2016 (N= 87,877 persons and 122,708 person-years).
Note: Point estimates noted in graphs; 95% CIs in lines. There has been no adjustment for multiplicity. Multinomial regression models adjusted for age, gender, employee status, U.S. region, urban region, comorbid substance use disorders, comorbid mental health conditions, moderate/severe vs. mild OUD, nonfatal poisoning, and calendar year.
4. Discussion
We found, consistent with population-level estimates of OUD treatment rates (SAMHSA, 2019b), that the majority of individuals identified with OUD in this commercially insured population did not receive treatment. Our estimate of MOUD among individuals diagnosed with OUD (36.3%) was slightly higher than, but similar to, that observed in a recent study examining a different national, commercially insured population (25.0% in 2014 and 29.7% in 2015) (Thomas et al., 2018). Several prior studies investigated MOUD in Medicaid programs (McCarty, Gu, McLlveen, & Lind, 2019; Meinhofer & Witman, 2018; Pro, Utter, Haberstroh, & Baldwin, 2020; Sharp et al., 2018); however, only one used a definition of MOUD use similar to ours, and found rates of 50% in 2013 and 45% in 2014 (McCarty et al., 2019). Wide variation in state Medicaid coverage of OUD treatment (Grogan et al., 2016; Mark et al., 2015; Meinhofer & Witman, 2018) makes it unclear how widely generalizable these findings are to other Medicaid programs.
A low proportion of individuals with OUD are receiving MOUD (National Academies of Sciences; Volkow & Wargo, 2018). Since 2016, several federal, state, and national consortium efforts were undertaken to increase access to buprenorphine prescribing (“Comprehensive Addiction and Recovery Act of 2016,” 2016; “Medication Assisted Treatment for Opioid Use Disorders; Final Rule,” 2016; “Providers Clinical Support System”; “State Targeted Response Technical Assistance (STR-TA) Consortium”). Another potential reason for low rates of MOUD is that medication utilization management policies such as prior authorization or step therapy, historically, have made it more difficult for some to access MOUD (American Society of Addiction Medicine, 2013; Barnett, 2018; Huskamp, Riedel, et al., 2018; Legal Action Center, 2015). In 2017, after the end of our study period a number of large national insurers, including the plan we studied, discontinued most or all prior authorization requirements for MOUD in their commercial products (Mattina, 2017). Future research should continue to monitor MOUD coverage restrictions among health plans, as well as examine the effect of the removal of prior authorization requirements on MOUD use.
We also found that having lower-severity OUD was associated with a lower probability of receiving MOUD. Individuals with milder symptoms may be less likely to accept medications. Alternatively, doctors may have been less likely to prescribe thinking it is not clinically indicated given that randomized controlled trials and meta-analyses of MOUD predominately have been conducted among individuals with more severe OUD (i.e., opioid dependence, or “physiologic dependence” in more recent clinical trials since DSM-5 was published)(American Psychiatric Association). However, even individuals with milder severity OUD are at risk for overdose, and current recommendations about the importance of MOUD in improving patient outcomes do not distinguish between mild or more severe OUD (Blanco & Volkow, 2019).
Our findings of an initial increase but then subsequent decrease in MOUD use (with or without psychosocial treatment) in OUD outpatient care indicates that use of the most effective OUD treatment (i.e., treatment that includes MOUD) has been, unfortunately, decreasing in this population in recent years. Several explanations for this decrease are possible, such as changes in the composition of individuals identified with OUD over the observation years, or a decrease in access to MOUD prescribers during years when “demand” (i.e., increasing rates of individuals identified as having OUD) was increasing. Additionally, we found that the probability of receiving neither of these treatments increased during the latter years of our study (beginning in 2013), raising the concern that there may have been a broader shortage of available clinicians who could deliver OUD care. These findings warrant further study and monitoring.
We found low use of both MOUD and psychosocial visits in the same year. At its highest during the study period (in 2013), the probability of receiving both of these treatments was 12.6% (12.2%–13.0%). While not all patients at all stages of OUD care need psychosocial treatment, and further research is needed to clarify for which patients, whether, how best, or when in the course of treatment patients should receive both MOUD and psychotherapy (Blanco & Volkow, 2019), these rates seem low, given that the defining symptoms of OUD (similar to substance use disorders in general) include significant impairments in social and role functioning in one’s work or school life and home life (American Psychiatric Association, 2013).
Women, compared to men, had lower probabilities of receiving each of the three “active” OUD treatment categories (both treatments, MOUD without psychosocial treatment, and psychosocial treatment without MOUD). This observation is consistent with prior research findings that women are less likely to receive treatment for their OUD (Back et al., 2010). Overall, women are also less likely to enter substance use disorder treatment, and when they do enter treatment, they are less likely to receive treatment that takes into account specific needs that may disproportionately affect them (e.g., higher rates of co-occurring psychiatric disorders; need for childcare, transportation, and other supportive services) (Back et al., 2010; Greenfield et al., 2010; Herbeck et al., 2016). It is notable that most programs lack gender-specific components of treatment that can address these special needs (SAMHSA, 2018a). This finding extends our understanding of the disparity in OUD care for women compared to men—it elucidates that not only are women less likely to receive treatment in general but are also less likely to receive specifically MOUD (with or without psychosocial treatments).
Unfortunately, we also found that individuals with an overdose claim in a given year were markedly less likely to use MOUD or psychosocial treatment in that year—about a 50% lower predicted probability of each of the three active OUD treatment outcomes. Whether this is related to low rates of MOUD use following opioid overdose, as has been found in other research (Larochelle et al., 2018), or that individuals at risk for opioid overdose (before or after the overdose occurred) are perhaps also less likely to engage in OUD treatment, or both, is unknown. Still, it underscores that also among the commercially insured, individuals with overdose histories are a vulnerable population that receives disproportionately inadequate OUD care. We did not link the claims data to vital statistics/deaths data; therefore, our identification of opioid overdose is likely underestimated. Still, our strong association between opioid overdose in a given year and OUD treatment highlights the important public health need to develop policies and programs that better identify and engage patients in OUD care; for example, initiating buprenorphine treatment for OUD in emergency departments and making linkages for subsequent follow-up in primary care (D’Onofrio et al., 2017).
Several mental health and substance use disorder clinical considerations were also associated with different OUD treatment. For example, comorbid mental health and substance use disorders were associated with higher probability of psychosocial treatment (with and without MOUD). More pronounced were the differences among individuals with moderate or severe (vs. mild) OUD; in particular more severe OUD was associated with much greater probability of receiving MOUD (with and without psychosocial treatment). Thus, comorbid mental health or substance use disorders were associated with higher probability of psychosocial treatment in general, while more severe OUD was associated with higher probability of MOUD in general.
Interestingly, three-quarters of individuals identified as having an OUD (75%) were only in the OUD cohort for a single person-year over the period 2008–2016. Prior research documents that continuous enrollment of at least 12 months among commercial health plan enrollees, on average and not specific to individuals with OUD, ranges from 62% to 72% (Chung et al., 2019; 2015). No doubt, some of the OUD enrollees in our cohort discontinued enrollment due to the typical enrollment/disenrollment patterns of commercial insurance. However, this finding also likely represents continuously enrolled individuals with OUD who are identified in claims data as having OUD in some years, but not others. Future research is needed to better understand the relationship among enrollment, disenrollment, OUD identification, and OUD treatment among the commercially insured.
There are several limitations to this study. First, individuals identified as having an OUD and receiving OUD treatment in commercially insured populations may be undercounted. For example, due to patient concerns of stigma, some patients may not disclose opioid use, providers may not code OUD diagnoses in the claims, and some patients may choose to self-pay rather than bill their insurance for OUD care. Also, some enrollees may receive treatment in their employee assistance program or at public, state-funded substance use disorder programs that did not bill insurance, including some opioid treatment programs that offer methadone. Last, approximately half (50.3%) of the person-years among individuals identified with OUD were excluded from the study because they did not include at least 10 months of continuous enrollment in pharmacy benefit that the insurer managed. It is unclear whether, or to what extent, these limitations might influence the study findings. Additionally, our study represents one large national health insurer, thus our findings may not be generalizable to OUD treatment utilization in other commercial insurers.
4. Conclusions
In this study of a large commercially insured population that included 9 years of claims data, we found, consistent with population health estimates, that a minority of the person-years of care among individuals with OUD included MOUD. Our findings support the need for further monitoring of OUD care and the specific components of that care, given that in this commercially insured population, more recent estimates indicate a decline in the use of MOUD in particular, as well as OUD care more generally. Further, the low use of OUD care that included both medications and psychosocial visits suggests psychosocial visits are possibly underutilized as important adjuncts to care with MOUD. Our finding that women were less likely than men to receive MOUD, with and without psychosocial visits, is particularly concerning, given the evidence of better OUD outcomes for individuals treated with these medications. Finally, individuals who may be at greatest risk for death from an OUD—those who have had treatment for an opioid overdose—were markedly less likely to receive MOUD or psychosocial treatment, which highlights an important focus for those wishing to improve OUD care. This study underscores important features of treatment provision for patients with OUD and significant gaps in care. This information can help to guide clinician, health plan, and policymaker efforts to improve OUD treatment in the future.
Highlights.
A minority of commercially insured adults with opioid use disorder (OUD) use OUD medication.
Women are less likely to use OUD treatments—medications and/or psychosocial treatment.
Individuals with a claim for OUD overdose have lower probability of using OUD care.
OUD medication use initially increased relative to 2008, but then decreased relative to its peak.
Acknowledgements:
Funding for this research was provided by the Brandeis/Harvard NIDA Center to Improve System Performance of Substance Use Disorder Treatment (P30 DA035772). Hocine Azeni, M.A. provided expert statistical programming. NIDA had no role in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Funding: The National Institute of Drug Abuse (P30 DA035772, Brandeis/Harvard NIDA Center to Improve System Performance of Substance Use Disorder Treatment) supported this work. Hocine Azeni, M.A. provided expert statistical programming. NIDA had no role in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix
Table A1:
Health care Procedure Coding System (HCPCS) and Common Procedure Terminology (CPT) codes used to define SUD outpatient visits
Outpatient visits were defined as CPT or HCPCS codes for the following types of care. “X” denotes whether category/codes are used to define a particular type of visit (e.g., outpatient psychosocial visit, psychotherapy/counseling, non-psychotherapy psychosocial visit, or E&M visit). All HCPCS codes are without modifier unless specified otherwise.
| Visit Category | Revenue Codes | Outpatient psychosocial visit | Psychotherapy/counseling visit | Non-psychotherapy psychosocial visit | Assessment or evaluation & management visit |
|---|---|---|---|---|---|
| Crisis psychotherapy |
CPT: 90839, 98040 |
X | x | ||
| Individual/group/family therapy or counseling |
CPT: 90804- 90815, 90832- 90838, 90842- 90844, 90846- 90849, 90853, 90855, 90857, 90875, 90876, 99412. HCPCS: G0071- G0082, H0004 (without modifier or with modifiers HD, HF, HG, HQ, HR, TF), H005 (without modifier or with modifiers HD, HQ), H0046 HE, H2019 (with modifiers HQ and HR), H2033, H5010, H5020, H5025, S9454, T1006 (without modifier or with modifier SA), T1012. |
X |
x |
||
| On-site behavioral health services5 |
HCPCS: H0022, H0023, H0038, H2019 (without modifier or with modifier HM, HN, HO), H2020- H2022, H202, H5030, T1011 HE, T1026. |
X |
x |
||
| Community supportive behavioral health services |
HCPCS: H0036, H0037, H2013, H2015 (without modifier and modifiers HE, HN, HQ), H2016 |
X | x | ||
| Case management services |
HCPCS:
G0351, H0006 (without modifier and with modifier HD), T1016 HB, T1017 (without modifier and with modifiers HE, HK, TL), T022, T023 |
X | x | ||
| Psychosocial rehabilitation/ occupational therapy |
CPT: 97003, 97004. HCPCS: H2001, H2014, H2017, H2018, H2023- H2026, H5220, H5230, H5240, H5299, Z0002 |
X | x | ||
| Methadone services | H0020 (methadone administration and/or service) | x | x | x | x |
| Evaluation and management/assessment services |
CPT: 90792, 90801, 90802, 90820, 90862, 99201- 99205, 99211- 99215, 99241- 99245. HCPCS: G0463, G0466, G0467, G0469, G0470, H0001 (without modifier or with modifiers U1, HN, HO, TS), H0016, H2000 (with modifier HP only), H2010 (without modifier or with modifiers HE, HF, HP), M0064, T1015 (except not if modifiers HA or HF), T2011. |
x |
On site behavioral health services: are codes for the following HCPCS services: “Alcohol and/or drug intervention service (planned facilitation)”, “Behavioral health outreach service (planned approach to reach a targeted population)”, “self-help/Peer services, per 15min”, “therapeutic on-site services”, “therapeutic behavioral services”, “psychoeducational service”, “Other services by social worker, psychiatric nurse, etc, per hour”
Table A2:
Unadjusted person-year characteristics of commercial health plan enrollees diagnosed with OUD (2008–2016), stratified by OUD treatment received (N= 87,877 persons and 122,708 person-years).
| MOUD and psychosocial visits | MOUD but no psychosocial visits | Psychosocial visits but no MOUD | Neither MOUD nor psychosocial visits | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total | 9,959 | 26.4 | 34,543 | 28.2 | 8,853 | 7.2 | 69,353 | 56.5 |
| Age group | ||||||||
| 17–25 | 3,055 | 30.7 | 6,440 | 18.6 | 3,462 | 39.1 | 14,031 | 20.2 |
| 26–35 | 2,989 | 30.0 | 9,827 | 28.5 | 2,224 | 25.1 | 12,315 | 17.8 |
| 36–45 | 1,876 | 18.8 | 8,002 | 23.2 | 1,356 | 15.3 | 13,355 | 19.3 |
| 46–55 | 1,351 | 13.6 | 6,706 | 19.4 | 1,140 | 12.9 | 16,597 | 23.9 |
| 56–64 | 688 | 6.9 | 3,568 | 10.3 | 671 | 7.6 | 13,055 | 18.8 |
| Employee | 4,357 | 43.8 | 18,398 | 53.3 | 3,231 | 36.5 | 33,973 | 49.0 |
| Female | 3,523 | 35.4 | 12,799 | 37.1 | 3,318 | 37.5 | 32,282 | 46.6 |
| US Region | ||||||||
| Northeast | 4,540 | 45.6 | 11,246 | 32.6 | 3,868 | 43.7 | 19,383 | 28.0 |
| Midwest | 1,314 | 13.2 | 3,532 | 10.2 | 1,193 | 13.5 | 6,708 | 9.7 |
| West | 1,472 | 14.8 | 6,819 | 19.7 | 1,486 | 16.8 | 15,306 | 22.1 |
| South | 2,633 | 26.4 | 12,946 | 37.5 | 2,306 | 26.1 | 27,956 | 40.3 |
| Urban | 9,094 | 91.3 | 31,842 | 92.2 | 8,225 | 92.9 | 64,212 | 92.6 |
| Comorbid mental health condition | 6,666 | 66.9 | 21,635 | 62.6 | 6,324 | 71.4 | 43,096 | 62.1 |
| Comorbid substance use disorder | 5,694 | 57.2 | 15,504 | 44.9 | 6,134 | 69.3 | 31,545 | 45.5 |
| OUD overdose claim | 302 | 3.0 | 959 | 2.8 | 299 | 3.4 | 5,890 | 8.5 |
| OUD moderate/severe | 9,882 | 99.2 | 33,038 | 95.6 | 8,398 | 94.9 | 62,329 | 89.9 |
| Inpatient | 2,528 | 25.4 | 4,287 | 12.4 | 2,467 | 27.9 | 9,197 | 13.3 |
| Year | ||||||||
| 2008 | 323 | 3.5 | 2,163 | 23.6 | 511 | 5.6 | 6,164 | 67.3 |
| 2009 | 502 | 4.6 | 3,575 | 32.4 | 503 | 4.6 | 6,455 | 58.5 |
| 2010 | 585 | 5.4 | 4,143 | 38.5 | 415 | 3.9 | 5,613 | 52.2 |
| 2011 | 645 | 5.6 | 4,480 | 38.8 | 537 | 4.7 | 5,896 | 51.0 |
| 2012 | 711 | 5.5 | 4,432 | 34.0 | 598 | 4.6 | 7,301 | 56.0 |
| 2013 | 1,738 | 12.3 | 3,883 | 27.5 | 1,384 | 9.8 | 7,096 | 50.3 |
| 2014 | 1,746 | 11.2 | 3,766 | 24.1 | 1,606 | 10.3 | 8,482 | 54.4 |
| 2015 | 1,806 | 10.5 | 3,857 | 22.4 | 1,575 | 9.1 | 10,018 | 58.1 |
| 2016 | 1,903 | 9.4 | 4,244 | 21.0 | 1,724 | 8.5 | 12,328 | 61.0 |
Table A3:
Multinomial logistic regression results: person-year estimates of receiving MOUD and psychosocial visits, MOUD but no psychosocial visits, or psychosocial visits but no MOUD vs. receiving neither MOUD nor psychosocial visits among commercially insured health plan enrollees with OUD, 2008–2016 (N= 87,877 persons and 122,708 person-years).
| MOUD & psychosocial visits | MOUD but no psychosocial visits | Psychosocial visits but no MOUD | ||||
|---|---|---|---|---|---|---|
| Estimate | s.e. | Estimate | s.e. | Estimate | s.e. | |
| Intercept | −6.2880 | .0992 | −2.7014 | .0579 | −3.7596 | .0593 |
| Age (ref=55–64) | ||||||
| 17–24 | 1.1406 | .0487 | .5620 | .0361 | .7976 | .0340 |
| 25–34 | 1.2360 | .0462 | 1.0277 | .0326 | .5621 | .0334 |
| 35–44 | .8607 | .0482 | .7634 | .0336 | .3461 | .0345 |
| 45–54 | .3845 | .0492 | .3697 | .0330 | .1372 | .0338 |
| Male | .2430 | .0257 | .3735 | .0202 | .0675 | .0199 |
| Employee | −.0661 | .0280 | .0589 | .0215 | −.1723 | .0221 |
| Region (ref=South) | ||||||
| Northeast | .8232 | .0299 | .1763 | .0234 | .6493 | .0237 |
| Midwest | .6929 | .0412 | .1376 | .0345 | .5665 | .0331 |
| West | .1239 | .0360 | −.0685 | .0257 | .1649 | .0270 |
| Rural | −.0715 | .0587 | −.0124 | .0459 | −.0792 | .0470 |
| Comorbid SUD | .5629 | .0235 | −.1217 | .0179 | .8503 | .0203 |
| Comorbid MH | .9081 | .0268 | .1668* | .0180 | 1.2341 | .0236 |
| Mod/severe OUD | 1.7255 | .0663 | .9071 | .0375 | .0754 | .0321 |
| OUD overdose claim | −.9871 | .0504 | −1.2100 | .0428 | −.2048 | .0355 |
| Year (ref=2008) | ||||||
| 2009 | .3441 | .0595 | .4478 | .0318 | −.1116 | .0471 |
| 2010 | .7151 | .0603 | .7448 | .0336 | −.0953 | .0499 |
| 2011 | .6146 | .0613 | .7645 | .0340 | −.0235 | .0484 |
| 2012 | .4283 | .0606 | .4885 | .0338 | −.1785 | .0473 |
| 2013 | 1.8286 | .0557 | .3970 | .0361 | 1.2091 | .0422 |
| 2014 | 1.6415 | .0556 | .1889 | .0358 | 1.2021 | .0412 |
| 2015 | 1.5401 | .0553 | .0126 | .0354 | 1.0766 | .0407 |
| 2016 | 1.5034 | .0552 | −.0939 | .0349 | 1.1437 | .0401 |
Note: Reference category = neither OUD medications nor OUD psychosocial visits. There has been no adjustment for multiplicity.
Table A4:
Multinomial logistic regression results: person-year adjusted odds ratios of receiving MOUD and psychosocial visits, MOUD but no psychosocial visits, or psychosocial visits but no MOUD vs. receiving neither MOUD nor psychosocial visits among commercially insured health plan enrollees with OUD, 2008–2016 (N= 87,877 persons and 122,708 person-years).
| MOUD & psychosocial visits | MOUD but no psychosocial visits | Psychosocial visits but no MOUD | ||||
|---|---|---|---|---|---|---|
| AOR | 95%CI | AOR | 95%CI | AOR | 95%CI | |
| Age (ref=55–64) | ||||||
| 17–24 | 3.90 | 3.46–4.40 | 1.65 | 1.54–1.76 | 3.67 | 3.30–4.08 |
| 25–34 | 4.34 | 3.87–4.87 | 2.65 | 2.50–2.82 | 3.10 | 2.79–3.44 |
| 35–44 | 2.68 | 2.37–3.02 | 2.06 | 1.93–2.19 | 1.92 | 1.71–2.14 |
| 45–54 | 1.60 | 1.41–1.81 | 1.41 | 1.33–1.50 | 1.34 | 1.20–1.50 |
| Male | 1.39 | 1.31–1.48 | 1.41 | 1.36–1.46 | 1.26 | 1.20–1.33 |
| Employee | .98 | .92–1.05 | 1.07 | 1.03–1.11 | .88 | .83–.93 |
| Region (ref=South) | ||||||
| Northeast | 2.27 | 2.12–2.43 | 1.18 | 1.13–1.23 | 2.14 | 2.01–2.28 |
| Midwest | 2.07 | 1.89–2.27 | 1.13 | 1.06–1.20 | 2.05 | 1.88–2.24 |
| West | 1.00 | .92–1.09 | .95 | .91–1.00 | 1.12 | 1.04–1.22 |
| Rural | 1.03 | .91–1.17 | .98 | .90–1.06 | 1.00 | .88–1.13 |
| Comorbid substance use disorder | 1.30 | 1.23–1.37 | .89 | .86–.92 | 2.05 | 1.94–2.17 |
| Comorbid mental health condition | 1.23 | 1.16–1.30 | 1.14 | 1.10–1.18 | 1.30 | 1.23–1.37 |
| Moderate/severe OUD | 16.16 | 12.66–20.63 | 2.60 | 2.43–2.78 | 2.33 | 2.09–2.60 |
| OUD overdose | .32 | .28–.36 | .30 | .28–.33 | .33 | .29–.37 |
| Year (ref=2008) | ||||||
| 2009 | 1.47 | 1.28–1.69 | 1.56 | 1.47–1.66 | .91 | .80–1.03 |
| 2010 | 2.04 | 1.78–2.35 | 2.11 | 1.98–2.25 | .87 | .76–.99 |
| 2011 | 1.97 | 1.71–2.27 | 2.11 | 1.98–2.25 | .97 | .85–1.10 |
| 2012 | 1.67 | 1.45–1.92 | 1.63 | 1.53–1.74 | .85 | .75–.96 |
| 2013 | 4.61 | 4.06–5.24 | 1.49 | 1.39–1.59 | 2.29 | 2.05–2.56 |
| 2014 | 4.04 | 3.55–4.58 | 1.19 | 1.12–1.27 | 2.32 | 2.08–2.59 |
| 2015 | 3.72 | 3.27–4.22 | 1.02 | .96–1.09 | 2.07 | 1.86–2.31 |
| 2016 | 3.53 | 3.11–4.01 | .93 | .87–.99 | 2.11 | 1.89–2.35 |
Note: Reference category = neither OUD medications nor OUD psychosocial visits. There has been no adjustment for multiplicity.
Table A5:
Multinomial logistic regression results: calendar year estimated percentages of receiving MOUD and psychosocial visits, MOUD but no psychosocial visits, or psychosocial visits but no MOUD vs. receiving neither MOUD nor psychosocial visits among commercially insured health plan enrollees with OUD, 2008–2016 (N= 87,877 persons and 122,708 person-years).
| MOUD & psychosocial visits | MOUD but no psychosocial visits | Psychosocial visits but no MOUD | Neither MOUD nor psychosocial visits | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (%) |
95%CI | Mean (%) |
95%CI | Mean (%) |
95%CI | Mean (%) |
95%CI | |
| Year | ||||||||
| 2008 | 3.5 | 3.2–3.8 | 23.9 | 23.3–24.6 | 5.7 | 5.3–6.0 | 66.9 | 66.2–67.6 |
| 2009 | 4.6 | 4.3–4.9 | 32.6 | 31.9–33.3 | 4.6 | 4.3–4.9 | 58.2 | 57.5–58.9 |
| 2010 | 5.5 | 5.2–5.8 | 38.8 | 38.1–39.5 | 3.9 | 3.6–4.2 | 51.8 | 51.1–52.5 |
| 2011 | 5.7 | 5.3–6.0 | 39.0 | 38.3–39.6 | 4.7 | 4.4–5.0 | 50.6 | 49.9–51.3 |
| 2012 | 5.6 | 5.3–5.9 | 34.1 | 33.5–34.8 | 4.7 | 4.4–5.0 | 55.7 | 55.0–56.3 |
| 2013 | 12.6 | 12.2–13.0 | 27.4 | 26.8–28.0 | 10.1 | 9.7–10.5 | 49.9 | 49.3–50.5 |
| 2014 | 11.6 | 11.2–12.0 | 23.9 | 23.3–24.4 | 10.7 | 10.3–11.0 | 53.9 | 53.2–54.4 |
| 2015 | 11.0 | 10.7–11.4 | 21.8 | 21.3–22.2 | 9.6 | 9.3–10.0 | 57.6 | 57.1–58.2 |
| 2016 | 9.9 | 9.6–10.3 | 20.3 | 19.9–20.7 | 9.0 | 8.7–9.3 | 60.7 | 60.2–61.3 |
Note: There has been no adjustment for multiplicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AJ, Knudson HK, Rieckmann T, & Roman PM (2013). Disparities in access to physicians and medications for the treatment of substance use disorders between publicly and privately funded treatment programs in the United States. J Stud Alcohol Drugs, 74, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An act to authorize the Attorney General and Secretary of Health and Human Services to award grants to address the prescription opioid abuse and heroin use crisis, and for other purposes., 130, Pub. L. No. 114–198, 695 Stat. (2016 July 22, 2016).
- Agresti A (2002). Chapter 7: Logit Model for Multinomial Responses Categorical Data Analysis: Second Edition: Wiley Series in Probability and Statistics. [Google Scholar]
- Amato L, Minozzi S, Davoli M, & Vecchhi S (2011). Psychosocial and pharmacological treatments versuspharmacological treatments for opioid detoxification (Review). Cochrane Database of Systematic Reviews(9), Art. No.: CD005031 10.1002/14651858.CD005031 [DOI] [PubMed]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association. [Google Scholar]
- American Society of Addiction Medicine. (2013). Advancing access to addiction medications: Implications for opioid addiction treatment. Retrieved from Rockville: https://www.asam.org/docs/default-source/advocacy/aaam_implications-for-opioid-addiction-treatment_final [Google Scholar]
- Back SE, Payne RL, Simpson AN, & Brady KT (2010). Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addict Behav, 35(1), 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B (2018). Insurers are making it harder for me to treat my opioid-addicted patients. Retrieved from https://www.washingtonpost.com/opinions/insurers-are-making-it-harder-for-me-to-treat-my-opioid-addicted-patients/2018/04/24/1ed674b0-2090-11e8-86f6-54bfff693d2b_story.html?noredirect=on&utm_term=.88e3cb2ac125
- Bearnot B, Fine DR, Rigotti NA, & Baggett TP (2019). Access to treatment for drug use disorders at US health centers: a national study. J Gen Int Med, 34(12), 2723–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, & Volkow ND (2019). Management of opioid use disorder in the USA: present status and future directions. Lancet, 393, 1760–1762. [DOI] [PubMed] [Google Scholar]
- Blodgett JC, Maisel NC, Fuh IL, Wilbourne PL, & Finney JW (2014). How effective is continuing care for substance use disorders? A meta-analytic review. Journal of Substance Abuse Treatment, 46(2), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, & Weiss RD (2017). The role of behavioral interventions in buprenorphine maintenance treatment: a review. American Journal of Psychiatry, 174(8), 738–747. 10.1176/appi.ajp.2016.16070792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2013). Prescription drug overdose data and statistics: Guide to ICD-9-CM and ICD-10 codes related to poisoning and pain; Version 1.3. Retrieved from Atlanta: https://www.cdc.gov/drugoverdose/pdf/pdo_guide_to_icd-9-cm_and_icd-10_codes-a.pdf [Google Scholar]
- CDC. (2019). 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Retrieved from https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillancereport.pdf.
- Chung H, Deshpande G, Zolotarjova J, Quimibo RA, Kern DM, Cochetti PT, & Willey VJ (2019). Health plan enrollment and disenrollment among individuals with and without established chronic disease in a U.S. commercially insured and Medicare Advantage population. J Manag Care Spec Pharm, 25(5), 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, Chawarski MC, O’Connor PG, Pantalon MV, Busch SH, Owens PH, … Fiellin DA (2017). Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. Journal of General Internal Medicine, 32(6), 660–666. 10.1007/s11606-017-3993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day E, & Mitcheson L (2017). Psychosocial interventions in opiate substitution treatment services: does the evidence provide a case for optimism or nihilism? Addiction, 112(8), 1329–1336. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165(2), 179–187. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Frazier W, Cochran G, Lo-Ciganic W-H, Gellad WF, Gordon AJ, Chang C-C, & Donohue JM (2017). Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA, 318(8), 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Stewart D, & Marsden J (2005). Effectiveness of drug and alcohol counselling during methadone treatment: content, frequency, and duration of counselling and association with substance use outcomes. Addiction, 101, 404–412. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, & Brady KT (2010). Substance abuse in women. Psychiatr Clin North Am, 33(2), 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan CM, Andrews C, Abraham A, Humphreys K, Pollack HA, Smith BT, & Friedmann PD (2016). Survey highlights dffrences in Medicaid coverage for subtance use treatment and opioid use disorder medications. Health Affairs, 12, 2289–2296. 10.1377/hlthaff.2016.0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, & Jones CM (2017). Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Annals of Int Med, 167(5), 293–302. [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, & Cai R (2015). Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA, 314(14), 1468–1478. [DOI] [PubMed] [Google Scholar]
- Herbeck DM, Jeter KE, Cousins SJ, Abdelmaksoud R, & Crèvecoeur-MacPhail D (2016). Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. Journal of Addictive Diseases, 35(4), 305–314. 10.1080/10550887.2016.1189659 [DOI] [PubMed] [Google Scholar]
- HHS. (2016). Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Retrieved from Washington, D.C.: https://addiction.surgeongeneral.gov/surgeon-generals-report.pdf [PubMed] [Google Scholar]
- Huskamp HA, Busch AB, Souza J, Uscher-Pines L, Rose S, Wilcock A, … Mehrotra A (2018). How is telemedicine being used in opioid and other substance use disorder treatment? Health Affairs, 37(12), 1940–1947. 10.1377/hlthaff.2018.05134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskamp HA, Riedel LE, Barry CL, & Busch AB (2018). Coverage of medications that treat opioid use disorder and opioids for pain management in marketplace plans. Medical Care, 56(6), 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E (2015). National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Pub Health, 105(8), e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, Barry CL, Gollust SE, Ensminger ME, Chisolm MS, & McGinty EE (2017). Social stigma toward persons with prescription opioid use disorder: Associations with public support for punitive and public-health oriented policies. Psychiatric Services, 68, 462–469. 10.1176/appi.ps.201600056 [DOI] [PubMed] [Google Scholar]
- Koyawala N, Landis R, Barry CL, Stein BD, & Saloner B (2019). Changes in outpatient services and medication use following a non-fatal opioid overdose in the West Virginia Medicaid program. Journal of General Internal Medicine, 34(6), 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, … Walley AY (2018). Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med, 169, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legal Action Center. (2015, March 15, 2015). Confronting an epidemic: The case for eliminating barriers to medication-assisted treatment of heroin and opioid addiction. Retrieved from http://lac.org/wp-content/uploads/2014/07/LAC-The-Case-for-Eliminating-Barriers-to-Medication-Assisted-Treatment.pdf
- Mark TL, Lubran R, McCance-Katz EF, Chalk M, & Richardson J (2015). Medicaid coverage of medications to treat alcohol and opioid dependence. Journal of Substance Abuse Treatment, 55, 1–5. doi:The role of health insurance on treatment for opioid use disorders:Evidence from the Affordable Care Act Medicaid expansion [DOI] [PubMed] [Google Scholar]
- Mattina C (2017). Aetna becomes latest insurer to end prior authorization for opioid treatment. American Journal of Managed Care Newsroom. Retrieved from http://www.ajmc.com/newsroom/aetna-becomes-latest-insurer-to-end-prior-authorization-for-opioid-treatment
- McCarty D, Gu Y, McLlveen JW, & Lind BK (2019). Medicaid expansion and treatment for opioid use disroders in Oregon: An interrupted time-series analysis. Addict Sci Clin Pract, 14(31). 10.1186/s13722-019-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GW, & O’Brien CP (1993). The effects of psychosocial services in substance abuse treatment. JAMA, 269, 1953–1959. [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, & Kleber HD (2000). Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA, 284(13), 1689–1695. [DOI] [PubMed] [Google Scholar]
- Medication Assisted Treatment for Opioid Use Disorders; Final Rule, 42 CFR 8 C.F.R. (2016). [PubMed] [Google Scholar]
- Meinhofer A, & Witman AE (2018). The role of health insurance on treatment for opioid use disorders: Evidence from the Affordable Care Act Medicaid expansion. Journal of Health Economics, 60, 177–197. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, & Crum RM (2016). Perceived unmet need for alcohol and drug use treatments and future use of sevices: Results from a longitudinal study. Drug and Alcohol Dependence, 127, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley AY (2018). Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment, 85, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeger S, Mutter R, Ali MM, Mark T, & Hughey L (2016). Post-discharge treatment engagement among patients with an opioid use disorder. Journal of Substance Abuse Treatment, 69, 64–17. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, E., and Medicine; Health and Medicine Division; Board on Health Sciences Policy. Medication-Assisted Treatment for Opioid Use Disorder: Proceedings of a Workshop—in Brief. Washington (DC): National Academies Press (US); 2018. November 30 [PubMed] [Google Scholar]
- Optum. (2015). Real world health care experiences from over 150 million unique indivividuals since 1993. Retrieved from https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf
- Priester MA, Browne T, Iachini A, Clone S, DeHart D, & Seay KD (2016). Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: An integrative literature review. Journal of Substance Abuse Treatment, 61, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro G, Utter J, Haberstroh S, & Baldwin J (2020). Dual mental health diagnoses predict the receipt of medication-assisted opioid treatment: Associations moderated by state Medicaid expansion status, race/ethnicity and gender, and year. Drug and Alcohol Dependence, 209 doi:https://www.sciencedirect.com/science/article/abs/pii/S0376871620301174?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- Providers Clinical Support System. Retrieved from https://pcssnow.org
- Reif S, Creedon TB, Horgan CM, Stewart MT, & Garnick DW (2017). Commercial health plan coverage of treatment for selected opioid use disorders from 2003 to 2014. Journal of Psychoactive Drugs, 49(2), 102–110. 10.1080/02791072.2017.1300360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Horgan CM, Hodgkin D, Matteucci A, Creedon T, & Stewart MT (2016). Access to addiction pharmacotherapy in private health plans. Journal of Substance Abuse Treatment, 66, 23–29. doi:doi:10.1016/j.jsat.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman PM, Abraham AJ, & Knudsen HK (2011). Using mediation-assisted treatment for substance use disorders: evidence of barriers and facilitators of implementation. Addictive Behaviors, 36, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Gladden RM (2016). Increases in drug and opioid overdose deaths - United States, 2000–2014. Morbidity and Mortality Weekly Report, 64, 1378–1382. [DOI] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou P, Zhang H, Jung J, … Grant BF (2016). Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. Journal of Clinical Psychiatry, 77(6), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. (2018a). National Survey of Substance Abuse Treatment Services (N-SSATS): 2017 Data on Substance Abuse Treatment Facilities. Retrieved from Rockville, MD: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/2017_NSSATS.pdf [Google Scholar]
- SAMHSA. (2018b). Results from the 2017 National Survey on Drug Use and Health: Prevalence Estimates, Standard Errors, P Values, and Sample Sizes. Retrieved from Rockville: [Google Scholar]
- SAMHSA. (2019a). Behavioral Health Barometer: United States, Volume 5: Indicators as measured through the 2017 National Survey on Drug Use and Health and the National Survey of Substance Abuse Treatment Services. HHS Publication No. SMA–19–Baro-17-US. Retrieved from Rockville, MD: [PubMed] [Google Scholar]
- SAMHSA. (2019b). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. (HS Publication No. PEP19–5068, NSDUH Series H-54.). Rockville, MD: Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Sharp A, Jones A, Sherwood J, Kutsa O, Honermann B, & Millett G (2018). Impact of Medicaid expansion on access to opioid analgesic medications and medication-assisted treatment. Am J Pub Health, 108(5), 642–648. 10.2105/AJPH.2018.304338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, … Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. British Medical Jounral, 357, j1550 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- State Targeted Response Technical Assistance (STR-TA) Consortium. Retrieved from https://www.getstr-ta.org/
- Tai B, & Volkow ND (2013). Treatment for substance use disorder: Opportunities and challenges under the Affordable Care Act. Soc Work Public Health, 28(3–4), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Ritter GA, Harris AHS, Garnick DW, Freedman KI, & Herbert B (2018). Applying American Society of Addiction Medicine performance measures in commercial health insurance and services data. J Addict Med, 12(4), 287–294. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication assisted treatment for opioid dependence: A systematic review. J Addict Dis, 35(1), 22–35. 10.1080/10550887.2016.1100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, & Stein M (2016). Patients’ beliefs about medications are associated with stated preferencefor methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. Journal of Substance Abuse Treatment, 66, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2020). Stigma and the Toll of Addiction. NEJM, 382, 1289–1290. [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Wargo EM (2018). Overdose prevention through medical treatment of opioid use disorders. Annals of Internal Medicine, 169(3), 190–192. [DOI] [PubMed] [Google Scholar]
- Weiss RD, & Rao V (2017). The Prescription Opioid Addiction Treatment Study: What have we learned. Drug and Alcohol Dependence, 173(Suppl 1), S48–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]


