ABSTRACT
Hedgehog (Hh) is an evolutionarily conserved signaling protein that has essential roles in animal development and homeostasis. We investigated Hh signaling in the region of the Drosophila wing imaginal disc that produces Hh and is near the tracheal air sac primordium (ASP) and myoblasts. Hh distributes in concentration gradients in the anterior compartment of the wing disc, ASP and myoblasts, and activates genes in each tissue. Some targets of Hh signal transduction are common to the disc, ASP and myoblasts, whereas others are tissue-specific. Signaling in the three tissues is cytoneme-mediated and cytoneme-dependent. Some ASP cells project cytonemes that receive both Hh and Branchless (Bnl), and some targets regulated by Hh signaling in the ASP are also dependent on Bnl signal transduction. We conclude that the single source of Hh in the wing disc regulates cell type-specific responses in three discreet target tissues.
KEY WORDS: Drosophila, Hedgehog, Myoblasts, Trachea, Wing disc
Summary: Hedgehog produced by the wing imaginal disc regulates cell type-specific responses in three discreet target tissues: wing disc, myoblast and tracheal cells.
INTRODUCTION
Morphogens are evolutionarily conserved signaling proteins that pattern tissues during development and regulate cell differentiation in stem cell niches. They spread across the tissues they target, generating distributions that elicit concentration-dependent responses. The Hedgehog (Hh) morphogen was discovered because of its roles in embryonic and imaginal disc development (Mohler, 1988; Nüsslein-Volhard and Wieschaus, 1980), and in humans, defects in Hh signaling are associated with congenital diseases and are implicated in malignancies such as basal cell carcinoma, medulloblastoma and pancreatic cancer (Hui and Angers, 2011; Roberts et al., 2017). Elucidating the function and mechanisms of Hh signaling is important to both developmental biology and medicine.
Studies of Hh signaling in the wing pouch primordium of the Drosophila wing imaginal disc have elucidated many fundamental features of Hh signaling. The wing disc is a flattened sac with two closely juxtaposed and connected single-cell layered sheets. The layer with columnar epithelial cells contains most of the disc cells and includes the progenitors of both the adult wing and notum. In the wing pouch primordium, hh is expressed exclusively by the posterior compartment cells, and Hh protein disperses to form a concentration gradient that extends over a distance of approximately 10 anterior compartment cells. In a concentration-dependent manner, the Hh gradient induces expression of patched (ptc), decapentaplegic (dpp, a Drosophila BMP), knot (kn) and engrailed (en), and regulates the intracellular distribution of cubitus interruptus (ci) and smoothened (smo) (Torroja et al., 2005). In contrast to the extensive studies of Hh signaling in the wing primordium, little is known about Hh signaling elsewhere in the disc. Hh signaling has not been characterized in the notum primordium or in two other tissues that associate physically and functionally with the notum primordium: the myoblast progenitors of the adult flight muscles and the progenitors of the tracheal tubes that transport air. The wing disc-associated tracheal progenitors include the air sac primordium (ASP) that will give rise to the adult dorsal air sacs that deliver air to the thoracic flight muscles (Huang and Kornberg, 2015; Sato and Kornberg, 2002).
Both the myoblasts and ASP are situated inside the basement membrane that envelops the disc and are adjacent to the epithelium cells of the notum primordium. In this region of the disc, Hh, Dpp, Branchless (Bnl, a Drosophila FGF), and Wingless (Wg, a Wnt homolog) are produced in different, partially overlapping groups of cells (Fig. 1A). Dpp and Bnl signal directly to the ASP, and Wg signals to myoblasts that respond by activating Notch signaling in the ASP (Du et al., 2018; Huang and Kornberg, 2015; Roy et al., 2014). Several targets of Hh signaling are expressed in the notum, but the roles and mechanisms of Hh signaling in the notum and its adjacent cells are unknown.
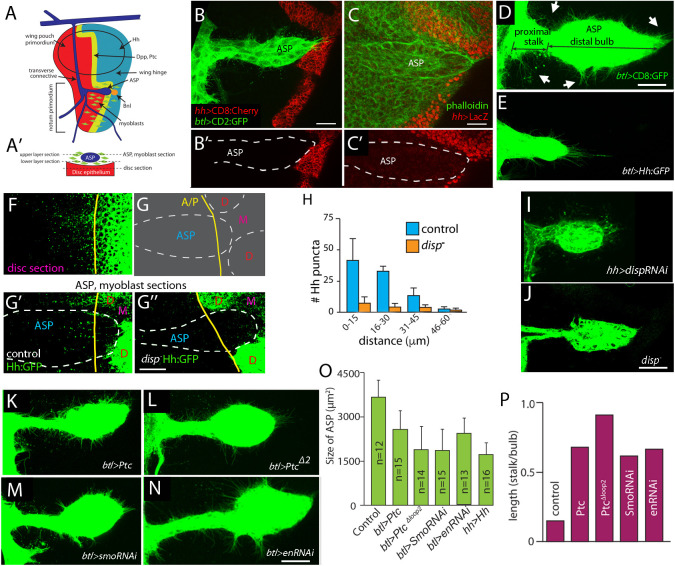
Fig. 1.
Hh signaling in ASP. (A) Schematic indicating wing disc, ASP, myoblasts and dpp-, ptc-, bnl- and hh-expressing cells. (A′) Cross-section showing ASP, myoblasts and disc epithelium; dashed lines, optical sections transecting ASP and disc. (B-C′) ASP outlined by white dashed lines (B′,C′), marked by green fluorescence (btl-lexA>CD2:GFP) (B), Phalloidin (C); hh expression marked by red fluorescence (B,B′, hh-Gal4>CD8:Cherry; (C,C′, hh-lacZ). (D) ASP marked with green fluorescence (btl-Gal4>CD8:GFP); distal bulb, proximal stalk and cytonemes (arrows) indicated. (E) Morphologically abnormal ASP that expressed Hh ectopically (btl-Gal4>Hh:GFP, mCD8:GFP). (F) Hh:GFP expression in notum where ASP overlies. Yellow line, compartment boundary. (G) Schematic of tissues in (G′,G″). D, disc; M, myoblasts; A/P, compartment boundary. (G′,G″) Hh:GFP (green) expressed in disc present also in control (G′) but not disp mutant (G″) ASP (white dashed line). Images: ASP, myoblast section. (H) Plot of fluorescent Hh:GFP puncta as a function of proximal/distal position in control and disp mutants; n=4 each genotype. (I,J) Morphologically abnormal ASP (I) that expressed disp RNAi in posterior compartment (hh-Gal4>dispRNAi) and disp mutant (J). (K-N) Abnormal ASP in genotypes expressing Ptc (K), PtcΔloop2 (L), smo RNAi (M) and en RNAi (N) driven by (btl-Gal4). (O,P) Quantification of size (O) and shape (P; ratio between average stalk and bulb length; n=5-7 each genotype). All differences between control and mutant conditions statistically significant (unpaired two-tailed Student's t-test; P<0.05). Scale bars: 20 μm.
Previous studies show that movement of Dpp and Bnl from the disc to the ASP is mediated by cytonemes. Cytonemes are specialized signaling filopodia that transport morphogens and facilitate cell-to-cell transfer of signaling proteins at synapses that link producing and target cells. These synaptic contacts share features with chemical synapses of neurons, including components that function in pre-synaptic compartments such as the voltage-gated calcium channel, synaptotagmin and synaptobrevin (Huang et al., 2019). Cells that do not make functional synapses do not signal (Huang et al., 2019; Roy et al., 2014). Cytonemes have been observed in many developmental contexts, including Drosophila epithelial, myoblast and germline cells, between cells in mouse and zebrafish embryos, and vertebrate cells in culture (González-Méndez et al., 2019; Taberner et al., 2020; Zhang and Scholpp, 2019). In the wing disc, both posterior and anterior compartment cells of the wing pouch project cytonemes that are required for Hh signaling. In the ASP, cytonemes project from the tip and medial regions and take up Bnl and Dpp, respectively. Myoblasts project cytonemes that take up Wg produced in the wing disc and project cytonemes that activate Notch signaling in the ASP.
Here, we characterize Hh signaling in the notum primordium and report that Hh from the disc posterior compartment activates Hh signaling in three target tissues: anterior compartment cells of the disc, the ASP and the myoblasts. The key findings are that Hh signaling in the ASP is cytoneme-mediated and gene targets of Hh signaling differ in the disc, ASP and myoblasts.
RESULTS
Growth and morphogenesis of the ASP relies on Hh signaling
The ASP is a single cell layered epithelial tube that extends posteriorly across the notum primordium. It develops de novo during the third larval instar (L3) from the transverse connective tracheal branch, and its distal tip extends to the disc posterior compartment (Fig. 1A-D). Surrogate measures of hh gene expression (hh-lacZ and hh-Gal4; UAS-mCherry) give no evidence for hh transcription in the ASP (Fig. 1B-C′), but ectopic expression of Hh:GFP in the ASP suppressed its growth and morphogenesis (Fig. 1E). This response indicates that the ASP is sensitive to Hh; the closest and most likely source is the disc.
To investigate whether Hh is present in the ASP despite the lack of expression, we monitored ASP preparations using an Hh:GFP bacterial artificial chromosome (BAC) transgene that produces fluorescent Hh and is haplosufficient (Chen et al., 2017). In normal (control) larvae, we detected fluorescent puncta in the ASP and observed that Hh:GFP in the ASP is graded, with greater amounts in the cells closest to the Hh-producing disc cells (Fig. 1F-G′,H). We also examined ASPs isolated from larvae that are deficient for dispatched (disp). Disp is required for Hh release from Hh-producing cells, and because maternal Disp is provided to the embryo, disp mutants have sufficient residual function to develop to the L3 stage, albeit with small discs (Burke et al., 1999). Fluorescence of Hh:GFP is reduced in disp mutant ASPs (Fig. 1G″,H). In addition, growth of the ASP is reduced and ASP morphogenesis is abnormal under conditions of disp RNAi expression and in disp mutants (Fig. 1I,J). These results are consistent with the idea that the ASP takes up Hh from the wing disc and that ASP growth and morphogenesis depends on Hh signaling.
To analyze Hh signaling in the ASP, we genetically modified ptc, smo and en, three genes that are Hh targets in other contexts (Fig. 1K-N). Ectopic overexpression of either the normal Ptc protein or a dominant negative mutant (PtcΔloop2) decreases the size of the ASP by 47% and 78%, respectively (Fig. 1K,L,O). The normal ASP has a short narrow stalk proximal to the transverse connective and a bulbous distal end (Fig. 1A,B,D). Ectopic expression of Ptc or PtcΔloop2 increases the relative length of the stalk by factors of 4.5 and 6, respectively (Fig. 1K,L,P). These results are consistent with the idea that Hh signaling is necessary for normal ASP growth and morphogenesis.
Smo is essential for Hh signaling, and expression of smo RNAi decreases Hh signaling in the cells that normally respond to Hh in the wing disc (Casso et al., 2008). To assess the role of Smo in the ASP, we expressed smo RNAi in the ASP and observed both that the size of the ASP decreased by 70% and that the relative length of the stalk increased 4.1-fold (Fig. 1M,O,P). The third known Hh target we monitored is en, which encodes a homeodomain-containing transcriptional repressor. Although en is a ‘selector gene’ for the posterior compartment (Bejarano and Milán, 2009; García-Bellido, 1975), in which it is a positive regulator of hh (Tabata et al., 1992), en is also activated by Hh signaling in the wing primordium (Blair, 1992; de Celis and Ruiz-Gómez, 1995; Strigini and Cohen, 1997). We expressed en RNAi in the ASP and observed that the size of the ASP decreased by 24% and the relative length of the stalk increased 4.4-fold (Fig. 1N-P). These phenotypes are consistent with the idea that Smo and en are targets of Hh signaling in the ASP and that smo and en are necessary for ASP growth and development.
To better understand how Hh signaling might affect the size of the ASP, we compared the numbers of cells, dividing cells and dying cells in control ASPs and in ASPs that express Ptc, PtcΔloop2 or smo RNAi. Cell numbers were determined by direct counts of DAPI-stained ASP nuclei, dividing cells were identified by α-pH3 antibody staining, and dying cells were identified by α-caspase Dcp1 antibody staining. Expression of the transgenes reduced both the number of cells and number of dividing cells, but did not increase the frequency of cell death (Fig. S1). These results suggest that Hh signaling affects ASP size by controlling the rate of cell division. The contrast between the ∼42% reduction in cell number and >87% reduction in the number of dividing cells may reflect the fact that, whereas dividing cells are responsible for the growth of the upper layer of the ASP, the lower layer has no dividing cells and is populated with cells that migrate from the transverse connective and upper layer (Lin, 2009). Our results suggest that reduced Hh signaling may not affect cell migration, which is consistent with the observation that smo mutant clones do not affect cell migration in the ASP (Cabernard and Affolter, 2005).
The ASP is the progenitor of the thoracic dorsal air sacs (Sato and Kornberg, 2002), and by examining the scutellum of wild-type (WT) adult flies, we could detect two almond-shaped dorsal air sac lobes (Fig. S2A). In contrast, the dorsal air sacs in flies in which Hh signaling was suppressed by either overexpression of Ptc or expression of smo RNAi were smaller, misshapen and unequal in size (Fig. S2B,C). We conclude that the effects of Hh misregulation are consistent with the idea that Hh signaling is essential for normal ASP and dorsal air sac morphogenesis.
Graded output of Hh targets in the ASP
We next investigated how genes that are targets of Hh signaling in other tissues are expressed in the ASP. We first examined Ptc, Smo, Ci (the transcription factor that mediates transcriptional outputs of Hh signal transduction), En and the Hh co-receptor Interference hedgehog (Ihog) (Lum et al., 2003). In other contexts, Hh signaling elevates Ptc, Smo and En amounts, and suppresses Ihog and levels of nuclear Ci.
We monitored Ptc and Smo proteins by fluorescence of BAC-encoded Ptc:Cherry and Smo:GFP, respectively (Chen et al., 2017), Ci protein by fluorescence of Cherry:Ci (a CRISPR-generated knock-in, see Fig. S3A and Materials and Methods), and En protein by α-En antibody staining. These assays are quantitative and reveal that Ptc, Smo and En are elevated in cells at the distal tip of the ASP that are closest to Hh-expressing disc cells, and that nuclear Ci is elevated in proximal cells (Fig. 2A-D). These expression patterns indicate that Hh signaling is highest at the distal tip and lower proximally, and that Hh signaling upregulates levels of Ptc, Smo and En, and downregulates levels of nuclear Ci. In contrast, α-Ihog antibody staining indicated that Ihog is constant across the ASP proximal-distal axis (Fig. S4), suggesting that Ihog is regulated independently of Hh.
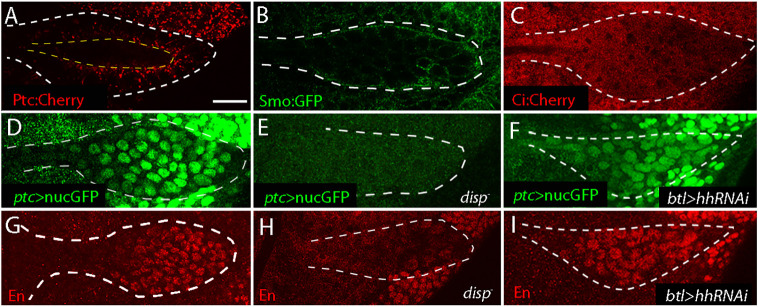
Fig. 2.
Graded output of Hh targets in ASP. (A-C) ASP distributions of Ptc:Cherry (A), Smo:GFP (B) and Ci:Cherry (C). Ptc:Cherry (A) localizes apically (dashed yellow lines). (D-F) ASP (white dashed line) ptc>nucGFP fluorescence of control (D), disp− (E) and genotype expressing hh RNAi in ASP (F, btl-Gal4 >hhRNAi). (G-I) ASP (white dashed line) α-En fluorescence of control (G), disp− (H) and genotype expressing hh RNAi in ASP (I, btl-Gal4 >hhRNAi). Scale bar: 20 μm.
To determine whether the Hh targets change in response to different levels of Hh signaling, we monitored disp mutants for En by α-En antibody staining and ptc expression by fluorescence of nuclear GFP expressed under the control of a segment of the ptc regulatory region (ptc>nucGFP). We also expressed hh RNAi in tracheal cells with the breathless (btl) pan-tracheal driver to determine whether hh expression in the ASP contributes to the expression of these Hh targets. Expression of En and ptc was not detected in ASPs of disp mutants and expression of hh RNAi was without effect (Fig. 2D-I).
To further characterize the dependence of ptc and En expression on Hh signaling in the ASP, we monitored their expression in genotypes that enhance or reduce Hh signaling. As shown in Fig. 3A-H, expression of Ptc or smo RNAi in the ASP reduced fluorescence of ptc>nucGFP and α-En staining, whereas expression of Hh:CD2, a membrane-tethered Hh form (Strigini and Cohen, 1997) increased both ptc and En. En and Ptc were also reduced in somatic clones expressing Ptc and smo RNAi (Fig. S5). These results are consistent with the idea that Hh signaling determines the patterns of ptc and En expression in the ASP.
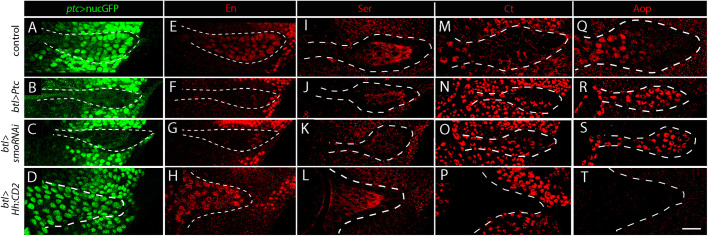
Fig. 3.
ASP expression of novel Hh target genes. (A-D) Compared with control (A), fluorescence of ptc>nucGFP (green) is decreased by expression of Ptc (B, btl-Gal4>Ptc), smo RNAi (C, btl-Gal4>smoRNAi), and increased by expression of Hh:CD2 (D, btl-Gal4 >Hh:CD2). ASP is outlined by white dashed lines. (E-H) Compared with control (E), α-En antibody staining is decreased by expression of Ptc (F, btl-Gal4>Ptc), smo RNAi (G, btl-Gal4>smoRNAi), and increased by expression of Hh:CD2 (H, btl-Gal4 >Hh:CD2). (I-L) Compared with control (I), α-Ser antibody staining is decreased in the ASP by Ptc expression (J, btl-Gal4>Ptc) and smo RNAi (K, btl-Gal4>smoRNAi), and is increased by expression of Hh:CD2 (L, btl-Gal4>Hh:CD2). (M-P) Compared with control (M), α-Ct antibody staining is increased in the ASP by Ptc expression (N, btl-Gal4>Ptc) and smo RNAi (O, btl-Gal4>SmoRNAi) and is decreased by expression of Hh:CD2 (P, btl-Gal4>Hh:CD2). (Q-T) Compared with control (Q), α-Aop antibody staining is increased in the ASP by Ptc expression (R, btl-Gal4>Ptc) and smo RNAi (S, btl-Gal4>smoRNAi) and is decreased by expression of Hh:CD2 (T, btl-Gal4>Hh:CD2). Scale bar: 20 μm.
We extended these studies to Serrate (Ser), Cut (Ct) and Anterior open (Aop), and obtained evidence that they are also Hh targets in the ASP (Fig. 3I-T). Ser is expressed in the ASP bulb, but Ser decreases with overexpression of Ptc (btl>Ptc) and downregulation of Smo (btl>smoRNAi) and increases with expression of Hh:CD2 (btl>Hh:CD2). Ct and Aop are elevated in the more proximal cells relative to distal ASP cells, and the amounts in the more distal ASP cells increase in ASPs with decreased Hh signaling caused by Ptc overexpression (btl>Ptc) and downregulation of Smo (btl>smoRNAi). Neither Ct nor Aop expression was detected in ASPs that express Hh:CD2, consistent with the idea the Hh signaling represses both genes. Kn and Dpp are also Hh targets in the wing pouch, but we did not detect α-Kn antibody staining (Fig. S4B), and dpp is not expressed in the ASP (Roy et al., 2014). We conclude that Hh functions as a paracrine signal that generates graded distributions of Ptc, Smo, Ci, En, Ser, Ct and Aop in the ASP.
Hh produced in the wing disc induces signal transduction in myoblasts
During L3, myoblasts proliferate and spread across the wing disc to cover approximately 2/3 of the notum primordium (Fig. 1A) (Gunage et al., 2014; Huang and Kornberg, 2015). Approximately 125 myoblasts lie directly over Hh-expressing posterior compartment disc cells near the wing hinge primordium (Fig. 4A). This region is also near the distal tip of the ASP. We investigated whether these myoblasts either express Hh or respond to Hh made by the wing disc, using two surrogate sensors for hh expression, hh-lacZ and hh-Gal4. We did not detect activity of either sensor (Fig. 4B-C″), but did find evidence for Hh signaling. We detected disp-dependent fluorescence of Hh:GFP and Smo:GFP in myoblasts in animals that express these proteins from BAC transgenes (Chen et al., 2017). The myoblasts with Hh:GFP and Smo:GFP fluorescence include those that overlie the disc posterior compartment, in which Hh is expressed, as well as myoblasts near the posterior compartment that overlie the anterior compartment (Fig. 4D-E′). Antibodies directed against Ptc and En detected these targets of Hh signaling in the same cells (Fig. 4F,G). The amounts of Hh, Smo, Ptc and En in the myoblasts vary with position: they are greatest in myoblasts closest to the Hh-expressing cells of the wing disc posterior compartment and are lower in myoblasts that are more distant and overlie the nearby anterior compartment. The amounts of Hh, Smo, Ptc and En in the myoblasts were reduced in disp mutants (Fig. 4D′,E′,F′,G′). The amount of Ci also varies with position, with lower apparent levels in cells with greater amounts of Hh and higher nuclear levels in cells with no apparent Hh (Fig. 4H). The levels of nuclear Ci correlate negatively with Ptc. These findings suggest that myoblasts in the immediate vicinity of the posterior compartment take up Hh and activate Hh signal transduction. We also tested for expression of Ct, Aop, Ser, Dpp and Kn. Expression of Ct and Aop is uniform and independent of disp (Fig. S6A-B′). We did not detect expression of either Kn, Ser or Dpp (Fig. S6D-F). These findings suggest that Ct, Aop, Ser, Dpp and Kn are not Hh targets in the disc-associated myoblasts.
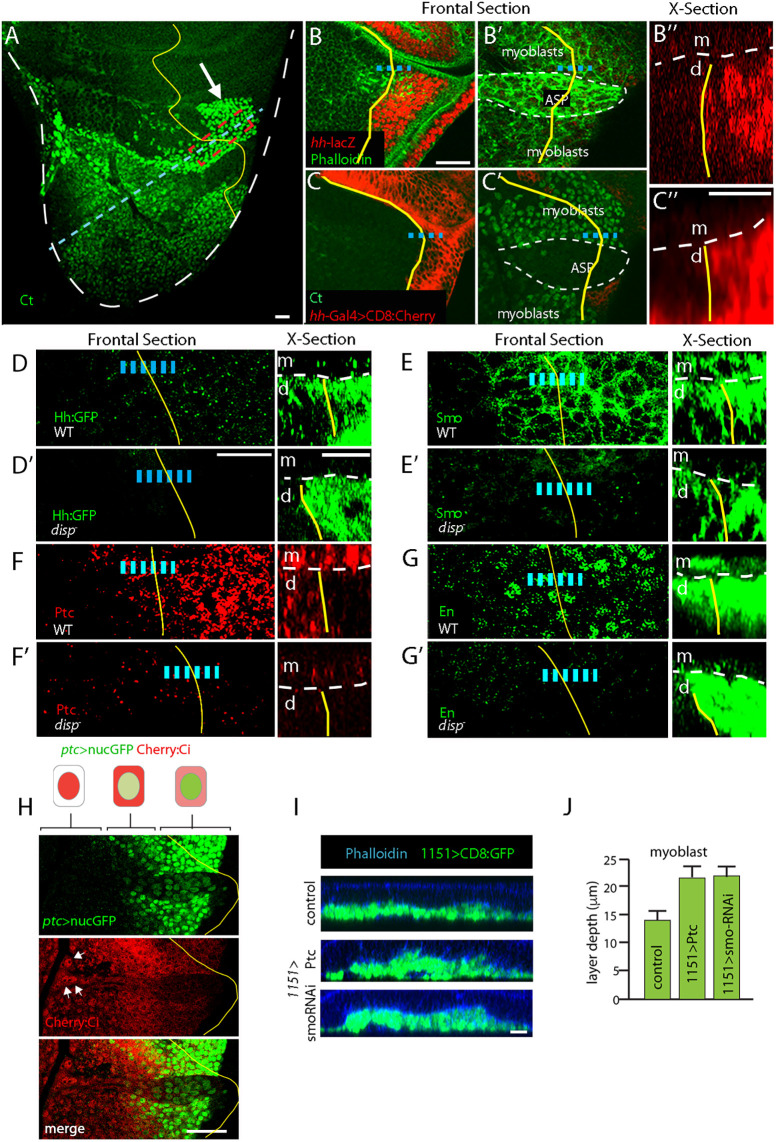
Fig. 4.
Wing disc-derived Hh activates signal transduction in myoblasts. (A) Myoblast α-Ct staining (green) populates notum. White arrow, myoblasts juxtaposed to disc cells expressing hh. (B-C″) hh-lacZ (B, red) and hh-Gal4 (C, red) expression in notum but not in myoblasts or ASP (B′,C′). (D-G′) Hh target gene expression in myoblasts. Hh:GFP fluorescence (D), Smo:GFP fluorescence (E), α-Ptc staining (F) and α-En staining (G) in WT (D,E,F,G) and disp mutants (D′,E′,F′,G′). (H) Images of ASP and myoblast that express ptc>nucGFP (green) and Cherry:Ci (red). Arrows indicate regions of the ASP with nuclear Cherry:Ci localization. Schematics represent cells in regions with different ptc>nucGFP and Cherry:Ci subcellular distributions. Dashed lines (B″,C″,D-G′ X-sections) show boundary between myoblasts (m) and disc cells (d). Yellow line (A-H) indicates A/P compartment border; blue dashed line shows the region imaged in cross section. In D-G′, Hh targets analyzed in red-boxed region shown in A. Cells along dashed blue line imaged in I. (I) Orthogonal sections of control (1151-Gal4>+), Ptc overexpression (1151-Gal4, UAS-Ptc) and smo RNAi expression (1151-Gal4, UAS-smoRNAi). Disc cells visible by Phalloidin staining (blue); layers of myoblasts visible by fluorescence of CD8:GFP (1151-Gal4, UAS-CD8:GFP). (J) Depth of the myoblast layers for genotypes shown in I. Difference between control, Ptc overexpression and smo RNAi expression, statistically significant (unpaired two-tailed Student's t-test, P<0.05). n=5 for each genotype. Scale bars: 20 μm (A-C′,D′,E-I); 10 μm (C″,D′, X-section).
To investigate whether Hh signaling has a role in myoblast development, we analyzed the myoblasts in genetic conditions that disrupt Hh signaling. Whereas the myoblasts constitute several cell layers that form a stack of ∼14 µm in WT discs at late L3 (Gunage et al., 2014), the depth of these myoblast layers increased under conditions of myoblast expression of Ptc or smo RNAi that reduce Hh signaling (Fig. 4I,J).
Hh signaling in the notum primordium
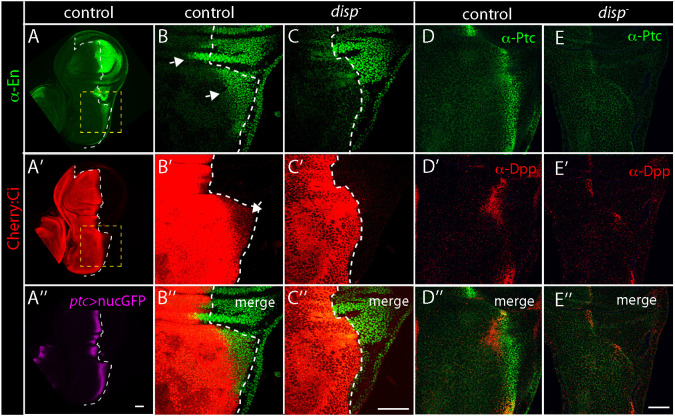
Hh signaling in the notum primordium has not been previously investigated. We monitored the expression of genes that are Hh targets in other tissues. We stained L3 wing discs with α-En antibody and observed that En is present in the posterior compartment as well as in ∼175 cells on the anterior side of the compartment boundary near the distal tip of the ASP (Fig. 5A,B). To test whether En expression in the anterior compartment is Hh-dependent, we examined disp mutants and detected significantly lower amounts of En than in controls (Fig. 4B,C). Additional evidence for Hh signaling in this region was obtained by monitoring Ci. Fluorescence of Cherry:Ci in anterior compartment cells that express En is reduced relative to more anterior portions of the disc (Fig. 5A′). This reduction of Ci amounts is disp-dependent (Fig. 5B′-C″), consistent with the idea that Hh signaling regulates both En and Ci in these cells. Ptc expression in the notum (Fig. 5A″) is disp-dependent (Fig. 5D,E). We also analyzed expression of Dpp in the notum primordium and tested whether it is under the control of Hh. Dpp is expressed in a stripe of cells, with the majority overlapping with or alongside cells that express Ptc. Expression of Dpp is significantly lower in disp mutants, consistent with the idea that Dpp expression is dependent on Hh signaling (Fig. 5D-E″). Ser, Ct, Kn and Aop are not expressed in the notum primordium (Fig. S7), suggesting that they are not targets of Hh signaling in the notum.
Fig. 5.
Targets of Hh signaling in notum. (A-A″) α-En staining (green), Cherry:Ci fluorescence (red), ptc nuclear GFP reporter fluorescence (purple) in wing disc; A/P compartment border marked by white dashed line. (B-C″) α-En (green) and Cherry:Ci fluorescence (red) in notum region outlined by dashed yellow dashed box in A. Arrows indicate anterior compartment α-En staining (B,B′) and Cherry:Ci fluorescence that change in disp. (D-E″) α-Ptc staining (green), α-Dpp staining (red) in WT control and disp. Scale bars: 50 μm.
Hh signaling in the ASP is mediated by cytonemes
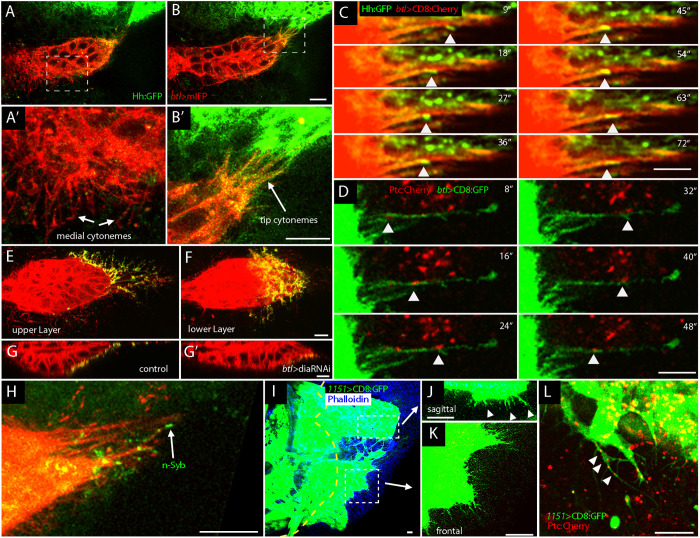
To investigate whether Hh in ASP cells is taken up by cytonemes, we examined preparations from animals with cytonemes marked by membrane-tethered fluorescent proteins. Panels A, B, and B′ in Fig. 6 show that cytonemes containing CD4:mIFP (Yu et al., 2015) extend from the ASP tip and take up Hh:GFP from the wing disc. Cytonemes also extend from the medial region of the ASP, but these cytonemes do not extend toward Hh-expressing disc cells and do not take up Hh:GFP (Fig. 6A,A′). Time-lapse imaging shows that cytonemes that extend from the ASP tip contain motile puncta that have Hh:GFP (Fig. 6C) and Ptc:Cherry (Fig. 6D). These results are consistent with the conclusion that ASP cytonemes transport Hh from the disc to the ASP.
Fig. 6.
Cytonemes mediate Hh signaling between the ASP, myoblast and notum. (A-B′) ASP expressing mIFP (B, red, btl>mIFP), disc expressing Hh:GFP from BAC (A, green). Optical sections with medial (A) and tip cytonemes (B). A′ and B′ show high magnification images of boxed regions in A and B, respectively; note Hh:GFP in tip cytonemes but not in the medial cytonemes. (C,D) Time-lapse video images show Hh:GFP puncta (arrowheads) (C, red, btl>CD8:Cherry) and Ptc:Cherry puncta (D, arrowheads) moving along ASP cytonemes (green, btl>CD8:GFP). (E,F) GRASP fluorescence (green) indicates contact in upper and lower layers of ASP between ASP cytonemes (red) and Hh-producing disc cells. Genotype: btl>Cherry:CAAX, CD4:GFP1-10, hh>CD4:GFP11. (G,G′) ASP sagittal views; GRASP-marked contacts (green) decreased dia RNAi expression (G′) compared with control (G). Genotypes: G, btl>Cherry:CAAX, CD4:GFP1-10, hh>CD4:GFP11; G′, btl>Cherry:CAAX, CD4:GFP1-10, diaRNAi, hh>GFP11. (H) n-syb GRASP (green) indicates that cytonemes synapse with hh-producing disc cells. Genotype: btl>Cherry:CAAX, CD4:GFP1-10, hh>n-syb:GFP11. (I-K) Myoblasts (green, 1151-Gal4>mCD8:GFP) populate notum (blue, Phalloidin). Yellow dashed line, A/P compartment boundary. Myoblast cytonemes project toward notum P compartment in magnified sagittal sections (J, top boxed region) and (K, bottom boxed region). (L) Ptc:Cherry puncta in myoblast cytonemes (arrowheads). Genotype: 1151-Gal4>mCD8:GFP, Ptc:Cherry BAC. Scale bars: 10 μm.
GRASP (GFP reconstitution across synaptic partners) is a technique that generates fluorescence at contacts made by cells that express membrane-tethered extracellular fragments of GFP (transmembrane domain of mouse CD4 fused to GFP1-10 and to GFP11) that together can reconstitute fluorescent GFP if they are stably and closely juxtaposed (Feinberg et al., 2008). GRASP fluorescence marks contacts between disc cells that express Dpp and disc cells that take up Dpp (Roy et al., 2014), between disc cells that express Hh and disc cells that take up Hh (Chen et al., 2017; González-Méndez et al., 2017), and between disc cells that make Bnl and ASP cells that take up Bnl (Roy et al., 2014). To test whether ASP cytonemes contact disc cells that produce Hh, we expressed CD4:GFP1-10 in the ASP and CD4:GFP11 in Hh-expressing disc cells, and marked ASP cytonemes with membrane-tethered Cherry (Cherry:CAAX). In optical section images of the ASP between the ‘upper’ and ‘lower’ layer of the ASP (Fig. 1A′), we observed GFP fluorescence along ASP cytonemes (Fig. 6E). In optical sections at the lower layer of the ASP or in sagittal sections, GRASP fluorescence is present in the region between the ASP and wing disc (Fig. 6F,G). We also monitored the GRASP-marked contacts in genetic conditions that compromise cytonemes. Pulsed expression of RNAi directed against diaphanous (dia), a formin-encoding gene, decreases the number of cytonemes without affecting cell viability or cell polarity (Bischoff et al., 2013; Chen et al., 2017; González-Méndez et al., 2017; Roy et al., 2014); and it reduces fluorescence of CD4:GFP11–CD4:GFP1-10 GRASP (Fig. 6G,G′). Further evidence for the nature of the contacts that ASP cytonemes make with Hh-producing disc cells was obtained by using a variant GRASP system that substitutes the cytoplasmic and transmembrane domains of n-Synaptobrevin (Syb), a constituent of synaptic vesicles, for the CD4 portion of CD4:GFP1-10. Syb GRASP identifies active synapses in neuronal tissue (Macpherson et al., 2015), and marks contacts between Hh-producing and Hh-receiving cells in the wing disc (Chen et al., 2017). As shown in Fig. 6H, expression of Syb:GFP1-10 in the wing disc posterior compartment and expression of CD4:GFP11 in the ASP generates fluorescence at contacts between ASP cytonemes and Hh-expressing disc cells. In sum, these experiments show that cytonemes at the ASP tip synapse with Hh-expressing disc cells and are consistent with the idea that Hh moves from the disc to the ASP at these contacts.
Myoblasts also extend cytonemes to the disc (Fig. 6I-L). Some orient directly toward underlying disc cells and are visible in sagittal sections (Fig. 6J); others extend across the disc and are visible in frontal sections (Fig. 6K). Punctal Cherry fluorescence is visible in myoblast cytonemes in animals that have the Ptc:Cherry-encoding BAC (Fig. 6L).
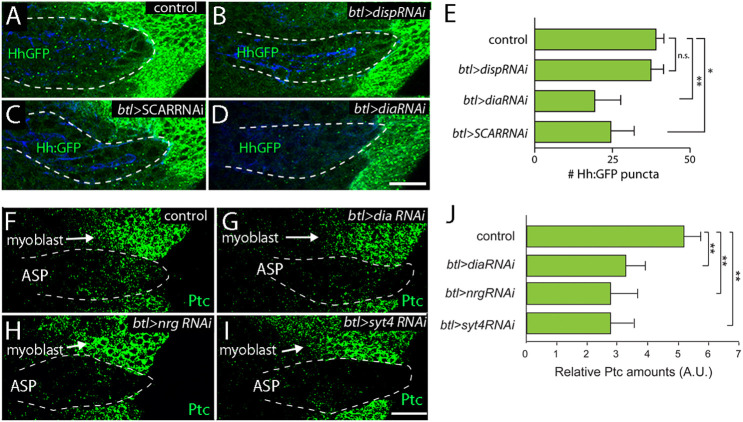
To obtain evidence for cytoneme-mediated Hh uptake, we expressed RNAi transgenes that decrease disp, dia, SCAR, neuroglian (nrg) and synaptotagmin 4 (syt4) expression, and monitored both the presence of Hh and Hh signaling in the ASP. Previous studies established that these conditions of dia RNAi, SCAR RNAi, nrg RNAi or syt4 RNAi expression decrease cytoneme-mediated signaling without disrupting cell polarity, morphology or viability (Bischoff et al., 2013; Chen et al., 2017; González-Méndez et al., 2017; Roy et al., 2014). In controls and in animals that express disp RNAi in the ASP, Hh:GFP fluorescence is present in similar amounts (Fig. 7A,B). In contrast, Hh:GFP fluorescence in the ASP is reduced in disp mutants (Fig. 1G). Knockdown of dia or SCAR in the ASP decreases Hh:GFP fluorescence by ∼50% (Fig. 7C-E), consistent with the idea that cytoneme ablation decreases transport of Hh to the ASP. We monitored Hh signal transduction by staining for Ptc, and observed that knockdown of dia, nrg and syt4 in the ASP decreases amounts of Ptc in the ASP by ∼50% (Fig. 7F-J). These results are consistent with the idea that cytonemes are required for Hh transport to the ASP and for Hh pathway activation in the ASP.
Fig. 7.
Cytonemes required for Hh transport and signaling in ASP. (A-E) Hh:GFP from disc distributes at similar levels in control (A, btl>+; Gal80ts) and disp knockdown (B, btl>dispRNAi; Gal80ts) ASPs, but at reduced amounts in SCAR (C, btl>ScarRNAi; Gal80ts) and dia (D, btl>diaRNAi; Gal80ts) knockdown ASPs. E shows Quantification of Hh:GFP puncta in control and knockdown ASPs. n=5-7. (F-J) α-Ptc staining (green) in control (F, btl>+; Gal80ts), dia (G, btl>diaRNAi; Gal80ts), nrg (H, btl>nrgRNAi; Gal80ts) and syt4 (I, btl>syt4RNAi; Gal80ts) ASPs. n=12. *P<0.05, **P<0.005 (unpaired two-tailed Student's t-test). n.s. not significant. Scale bars: 25 μm.
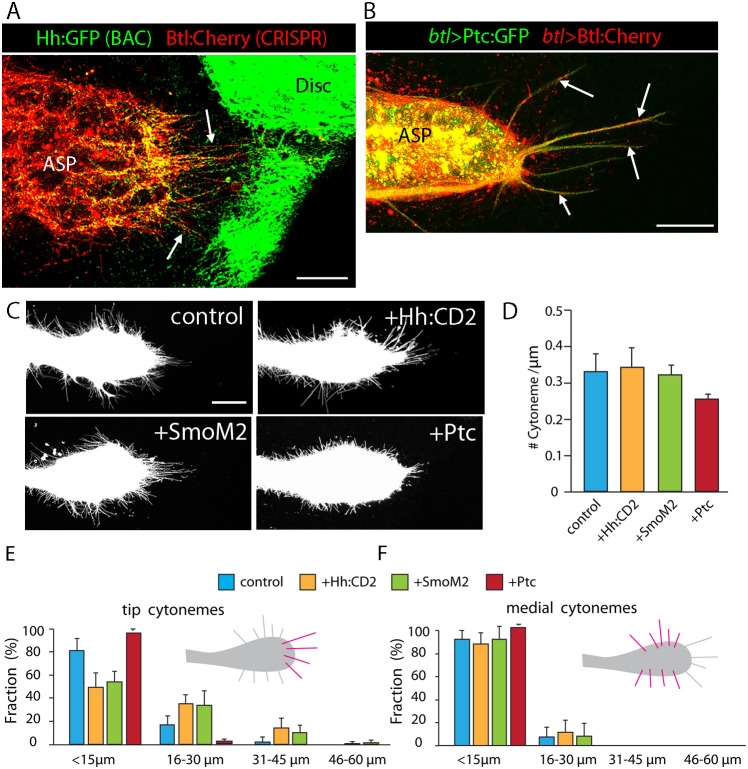
ASP cytonemes that take up Hh from the disc extend from the distal tip (Fig. 6). Because previous studies have shown that Bnl is also taken up from the disc by tip cytonemes, in contrast to the cytonemes that extend from medial regions and take up Dpp (Roy et al., 2014), we investigated cytoneme-mediated uptake of Hh in more detail. We first examined preparations from flies that expressed BAC-encoded Hh:GFP (Chen et al., 2017) and Btl:Cherry generated by CRISPR-generated knock-in (Du et al., 2018). Both Bnl-expressing cells and Hh-expressing cells are distal to the ASP tip, and ASP tip cytonemes in these preparations contain both Hh:GFP and Btl:Cherry (Fig. 8A). This indicates that tip cytonemes might take up both Hh and Bnl from the disc and might have receptors for Hh and Bnl. We examined preparations from animals with btl-Gal-driven expression of Ptc:GFP and Btl:Cherry, and detected both Ptc:GFP and Btl:Cherry in tip cytonemes (Fig. 8B). These results are consistent with the idea that tip cytonemes might take up either or both signaling proteins.
Fig. 8.
Cytonemes respond to Hh signaling. (A) BAC-encoded Hh:GFP (green) and CRISPR-generated Btl:Cherry knock-in (red) in ASP and disc. Tip cytonemes marked by Btl:Cherry and Hh:GFP (arrows). Genotype: Hh:GFP/+; Btl:Cherry/+. (B) Arrows show tip cytonemes containing Ptc:GFP and Btl:Cherry in ASP that expresses Ptc:GFP (green) and Btl:Cherry (red). Genotype: btl-Gal4, UAS-Btl:Cherry; UAS-Ptc:GFP/+. (C) ASPs with btl-Gal4 UAS-mCD8:GFP only (control) or with Hh:CD2, SmoM2 and Ptc. Genotypes: control (btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/+); +Hh:CD2 (btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/UAS-Hh:CD2), +SmoM2 (btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/UAS-SmoM2) and +Ptc (btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/UAS-Ptc). (D-F) Graphs showing density of ASP cytonemes measured as # cytonemes/µm of ASP perimeter (D), and fraction of cytonemes of indicated lengths at ASP tip (E) and medial region (F). The statistically significant differences between control and experimental genotypes (unpaired two-tailed Student's t-test, P<0.05) are cytoneme lengths for UAS-Ptc and number of tip cytonemes for UAS-Hh:CD2 and UAS-SmoM2 in D. For each genotype (n=10), except UAS-Ptc (n=4). Scale bars: 25 μm.
We next analyzed cytonemes extending from ASPs that ectopically express Hh:CD2, an engineered Hh that substitutes the transmembrane domain of the mouse CD2 protein for the C-terminal cholesterol of mature Hh (Strigini and Cohen, 1997), SmoM2, a constitutively active mutant (Xie et al., 1998), or Ptc. Hh:CD2 is a membrane-tethered chimeric protein that has signaling activity, but unlike the normal protein does not move efficiently beyond the cells that express it. Because the ASP does not grow normally in the presence of constitutive expression of Hh:GFP (Fig. 1E), Hh:CD2 (Fig. S8), SmoM2 (Fig. S8) or Ptc (Fig. 1K), we expressed Hh:CD2, SmoM2 and Ptc for a limited time during L3 by conditional inactivation of Gal80ts. Under this regimen, the ASP grows to normal size and morphology to late L3 at permissive temperature (18°C), and cytonemes are analyzed after 24 h of transgene expression at non-permissive temperature (29°C). We found that neither ectopic expression of Hh:CD2 nor SmoM2 changed either the total number of ASP cytonemes or the lengths of medial cytonemes relative to controls. However, the average length of tip cytonemes increased. Ectopic expression of Ptc reduced both the total number and lengths of cytonemes (Fig. 8C-F). Because Hh:CD2, SmoM2 and Ptc were expressed only in tracheal cells and the disc was genetically unaltered in this experiment, the significant cytoneme phenotype is evidence that Hh signaling and uptake in the ASP cells contributes to cytoneme production or behavior. This effect is consistent with the conclusion that the tip cytonemes mediate Hh uptake from the disc and with the positive feedback model of Du et al. (2018) that links cytoneme production and stability to signaling.
Hh signals to disc, ASP and myoblast cells
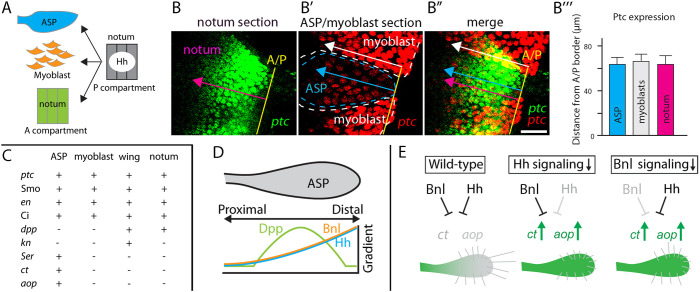
Our findings show that Hh produced in the disc signals to the ASP (Fig. 1), myoblasts (Fig. 4) and anterior compartment cells of the notum (Fig. 5), and that the three types of responding cells are near the wing hinge region of the disc in close proximity to disc posterior compartment cells of the notum (Fig. 1A). These cells present a setting in which a single source of Hh activates signal transduction in three different types of cells (Fig. 9A). The confocal images in the three panels of Fig. 9B-B″ show the Hh-responding cells in two optical sections, one that focuses on the disc epithelium (Fig. 9B) and another that focuses on the plane that includes ASP and myoblast cells (Fig. 9B′). The anterior extent of Hh signaling in the disc is not constant in this region, but the anterior extents of signaling are similar in the disc and ASP where they are juxtaposed, and are similar in the disc and myoblasts where they are juxtaposed (Fig. 9B‴). Myoblasts that overlie posterior compartment disc cells also activate Hh signal transduction (Fig. 4D-G), although posterior compartment disc cells block autocrine Hh signaling (Ramírez-Weber et al., 2000).
Fig. 9.
Hh signals to multiple targets. (A) Hh from disc signals to notum cells, myoblasts and ASP. (B-B″) ptc-expressing cells in disc, ASP and myoblast, detected by the fluorescence of ptc-nuclear GFP in two optical sections. GFP fluorescence is green in notum section, red in ASP/myoblast section. Yellow line, A/P compartment border. Extent of ptc expression in the anterior compartment, ASP and myoblast marked by magenta, blue and white arrows, respectively. (B‴) Ptc expression extent from the A/P border in the ASP, myoblasts and notum. n=7. (C) Transcriptional targets of Hh signaling in myoblast, ASP, wing and notum. (D) Distributions of Dpp, Bnl and Hh taken up in the ASP. (E) Cartoon depicting roles of Bnl and Hh regulating expression of ct and aop (green) in ASP of wild-type and mutant genotypes that decrease Hh or Bnl signaling. Cytoneme lengths decrease with reduction in either Hh or Bnl signaling. Scale bar: 25 μm.
Another difference in the action of Hh on the three cell types is the suite of target genes that Hh signal transduction regulates in each (Fig. 9C). ptc, en, Smo and Ci are targets of Hh signaling in all three tissues, but only disc cells express dpp and only ASP cells regulate kn, Ser, ct and aop in response to Hh (Figs 1–4). Expression of ct and aop in the ASP is noteworthy because both genes are also regulated by Bnl signaling (Du et al., 2018). Bnl is taken up from disc cells, forming a concentration gradient in the ASP that is highest distally and lower proximally (Fig. 9D). The contours of this gradient are indistinguishable from the Hh gradient (Fig. 1J), and both Hh and Bnl signal transduction are required to regulate ct and aop (Fig. 9E).
DISCUSSION
These findings show that Hh produced in the notum region of the wing disc activates signaling in the tracheal ASP, wing disc-associated myoblasts, as well as anterior compartment cells of the notum. During L3, as the ASP extends across the basal surface of the disc from the transverse connective to the posterior compartment in which Hh is expressed, its growth and morphology are dependent on Hh taken up from the disc. During this period, myoblasts also proliferate and migrate over the disc basal surface, and they spread around and under the ASP. Myoblasts that are in the region where Hh moves from the disc to the ASP depend on Hh they take up to regulate their growth. And anterior compartment wing disc cells depend on Hh signaling to express Dpp. The Hh-producing disc cells are the source of Hh for these three tissues, and because the wing disc and its associated trachea and myoblasts are physically separate from other tissues inside the larva, it is likely that the disc is the only source. The role of cytonemes supports this idea. Hh produced in the disc is released basally from the polarized columnar epithelial cells (Callejo et al., 2011), and cytonemes mediate Hh uptake by the ASP and myoblasts (Fig. 6). Although most Drosophila organs rely on Hh signaling, and available evidence indicates that cytoneme-mediated transport is the universal mechanism of Hh conveyance, this study revealed novel aspects of Hh signaling in this particular context of disc, trachea and myoblasts.
Whereas the number of different morphogen proteins is small, the number of tissues that they control and the number of different morphologies they mold is many times larger. The apparent versatility of this system of growth and morphogenesis control is presumably a function of the context dependence of signal transduction, and the different suites of gene targets that are activated in different tissues. In the ASP, myoblasts, notum and wing pouch, several genes are activated by Hh in only one target (e.g. kn in the wing pouch; Ser, ct and aop in the ASP). dpp is activated in two targets (e.g. wing pouch and notum), and Ptc, Smo, Ci and en are regulated by Hh in all four (Fig. 9C). Although finding that Hh signaling activates different genes in different target cells is not novel, this is the first context in which a morphogen signaling protein produced by a single source has been found to elicit distinct responses in different target tissues. This finding has interesting implications for mechanisms of signal dissemination.
There is almost complete spatial overlap between cells that are activated by Hh in the notum anterior compartment, the ASP and myoblasts (Fig. 9B). All disc anterior compartment cells, ASP cells and myoblasts respond if they are within ∼60 µm of the nearest Hh-expressing cell (Fig. 9B). This pattern of Hh signaling suggests that the process that disseminates Hh is similar for the three tissues. There is, however, a difference in the region of the disc posterior compartment in which the disc cells express Hh but do not activate Hh signal transduction. Whereas wing disc posterior compartment cells inhibit autocrine Hh signaling (Ramírez-Weber et al., 2000), the myoblasts and ASP cells that overlie these Hh-expressing posterior compartment cells do take up and transduce the disc-produced Hh (Fig. 4).
Hh signaling is cytoneme-mediated (Bischoff et al., 2013; Chen et al., 2017; González-Méndez et al., 2019; Huang et al., 2019), and several studies have shown that cytoneme distributions across tissues parallel signaling. In the wing disc and abdominal histoblasts, the lengths and numbers of cytonemes correlates with graded responses to Hh signaling (Bischoff et al., 2013; Chen et al., 2017; González-Méndez et al., 2017), and cytoneme distributions and Bnl signaling are similarly correlated in the ASP (Du et al., 2018). The model proposed by Du et al. (2018) accounts for the matching gradients of cytonemes, morphogen protein amounts and signaling with evidence that ‘morphogens self-generate precise tissue-specific gradient contours through feedback regulation of cytoneme-mediated dispersion’. This model invokes an essential role of signaling in cytoneme propagation and/or stability, a functionality that involves positive feedback to signal transduction. This idea is supported by our observation that activation of Hh signaling by ectopic expression of Hh:CD2 or SmoM2 in the ASP increases the lengths of ASP cytonemes (Fig. 8E).
Although multiple signaling pathways have been shown to be important in tissue regeneration and immune responses (Houtz et al., 2017; Jiang et al., 2009), and in Drosophila leg and eye development (Estella et al., 2008; Newcomb et al., 2018; Tomlinson and Struhl, 2001), we have little understanding how multiple signaling inputs might be integrated for a coordinated output. The ASP receives Dpp, Bnl and Hh from the wing disc, and all distribute spatially in the ASP in concentration gradients. Whereas highest levels of the Dpp gradient are in the medial region of the ASP (Roy et al., 2011), the Bnl and Hh gradients both extend along the proximo-distal axis, with highest levels at the tip of the ASP (Du et al., 2018; Fig. 9D). The similarly graded distributions of Bnl and Hh may be a consequence of a shared process of uptake from sources in the wing disc. We found that tip cytonemes take up both Bnl and Hh and contain both the Btl and Ptc receptors (Fig. 8), suggesting that a single cytoneme might convey both signaling proteins. This contrasts with Dpp uptake, which a previous study has shown is localized to the medial ASP cells and medial cytonemes, and not to tip cells or tip cytonemes (Roy et al., 2014). The presence of Tkv in medial but not tip cytonemes and the presence of Btl in tip cytonemes but not medial cytonemes suggested that cytonemes might be specific for a particular signaling pathway. The findings reported here that tip cytonemes convey both Bnl and Hh may indicate either that cytoneme selectivity may not be general to all signaling pathways, or that our experiments which analyze steady state distributions are biased to detect the most abundant components. Nevertheless, the conclusion which is supported by this work is that a cytoneme can mediate signaling for more than one signaling pathway.
This attribute has interesting ramifications for the relationships between signaling pathways. We found that the combined distributions of Bnl and Hh are crucial to target gene regulation – ct and aop are targets of both Bnl and Hh signal transduction and have similar patterns of expression along the proximo-distal axis. Both ct and aop are normally expressed in the proximal stalk region and not in the more distal bulb, but in ASPs with reduced Bnl or Hh signaling, Ct and Aop expression are elevated in the bulb. This reveals that suppression of Ct and Aop expression in the bulb is dependent on both Bnl and Hh signaling and that neither Bnl nor Hh signaling is sufficient (Fig. 9E). In addition, cytonemes are also dependent on both Bnl and Hh signaling – reduction of either Bnl or Hh signaling reduces both cytoneme number and length (Fig. 8C-F; Du et al., 2018). These effects on cytonemes were observed upon downregulation of Hh signaling despite the normal state for components of Bnl signaling, and were observed upon downregulation of Bnl signaling despite the normal state for components of Hh signaling. We consider this finding to support the positive feedback model of Du et al. (2018) and suggest that coordination of different signaling pathways may involve the processes of signal transduction that control signaling protein uptake.
MATERIALS AND METHODS
Fly lines
The following fly lines were used in this study: disp− (Burke et al., 1999); Gal4/LexA lines: btl-Gal4 (Sato and Kornberg, 2002), hh-Gal4 (Tanimoto et al., 2000), Ay-Gal4 [Bloomington Drosophila Stock Center (BDSC), #3953], btl-LHG (Roy et al., 2014), 1151-Gal4 (Roy and VijayRaghavan, 1997); UAS lines: UAS-SmoM2 (Zhu et al., 2003), UAS-mcd8:GFP (Roy et al., 2011), UAS-mcd8:Cherry (Roy et al., 2011), UAS-Ptc (Johnson et al., 1995), UAS-PtcΔloop2 (Briscoe et al., 2001), UAS-SmoRNAi [Vienna Drosophila Resource Center (VDRC), #9542], UAS–enRNAi (BDSC, #33715), UAS-Hh:GFP (Torroja et al., 2004), UAS-CD4:mIFP (Yu et al., 2015), UAS-CD4:GFP1-10 (from Kristen Scott, University of California, Berkeley, USA), UAS-SCAR-RNAi (VDRC, #21908), UAS-disp RNAi (BDSC, #27247), UAS-dia-RNAi (BDSC, #28541), UAS-hhRNAi (BDSC, #31475), UAS-Btl:Cherry (Roy et al., 2011), UAS-Ptc:GFP (Torroja et al., 2004), UAS-SmoM2 (BDSC, #57367); LexO lines: lexO-CD2:GFP (Torroja et al., 2004), lexO-CD4:GFP11 (Roy et al., 2014), lexO-n-syb-GFP11 (Macpherson et al., 2015), lexO-diaRNAi (Fereres et al., 2019), lexO-cherry:CAAX (from K. Basler, University of Zurich, Switzerland); BAC/Crispr/Fosmid knock-in lines: Ptc:Cherry BAC (Chen et al., 2017); Smo:GFP BAC (Chen et al., 2017); Ci:Cherry CRISPR (this study); Hh:GFP BAC (Chen et al., 2017), Btl:Cherry (Du et al., 2018); Enhancer traps: hh-lacZ (Lee et al., 1992), nuc>ptc:GFP (CB02030, FlyTrap).
All crosses were at 25°C except those involving temporal induction of gene expression using Gal80ts. For cytoneme ablation experiments, Gal80ts was carried out according to Roy et al. (2014). For overexpression of Hh:CD2, larvae were grown at 18°C to L3 and then incubated at 29°C for 24 h.
Cherry:Ci
Using CRISPR-Cas9 gene editing, mCherry together with a short linker was inserted at the N terminus of the ci coding sequence. The template vector contains a left homology arm, mCherry, and right homology arm that were amplified by PCR. The left homology arm contains an overlapping sequence with ∼1 kb sequence upstream of the transcriptional start site. The right homology arm fragment contains an overlapping sequence with ∼1 kb sequence from the transcriptional start site. The three fragments were joined and cloned into PBS-SK using Gibson Assembly (New England Biolabs). The resulting vector was designated as Cherry:Ci donor vector. gRNA was cloned into pCDF3 (Addgene plasmid #49410).
The following primers were used: L-arm-fwd, CGGTATCGATAAGCTTGATGTTTTGCGCTGTTTGTGGACGTTAGAGTG; L-arm-rev, GGACGAGCTGTACAAGTCATGGACTAACTTTAATGAAATGGACGCGTACGCG; Cherry fwd, CATTAAAGTTAGTCCATGACTTGTACAGCTCGTCCATGCCGC; Cherry rev, CGACGTCATTCTTGTTGATGGTGAGCAAGGGCGAGGAGGATAAC; R-arm-fwd, CTCGCCCTTGCTCACCATCAACAAGAATGACGTCGTTTTATAAATTC; R-arm-rev, CCGGGCTGCAGGAATTCGATGGCGTTGCCAATAACTTTTGCG. gRNA sequence: AAAATATGTAGGTAACGCGTAGG.
Function of Cherry:Ci was tested in CiD/Cherry:Ci heterozygotes and Cherry:Ci homozygotes and found to be indistinguishable from WT (Fig. S3B-E). Expression in wing disc is specific to anterior compartment (Fig. S3F).
Immunohistochemistry and fluorescent imaging
L3 larvae were dissected in PBS; wing discs together with the Tr2 trachea were fixed in 4% formaldehyde in PBS. Tissue was washed in PBS-Triton X-100 (0.3%) followed by Roche Blocking Solution. The following antibodies were used: α-Dpp-prodomain (1:200, from M. Gibson; Akiyama and Gibson, 2015), mouse α-GFP (Roche, 11814460001, 1:500), rabbit α-RFP (Rockland, 600-401-379, 1:500), mouse α-Ptc [Developmental Studies Hybridoma Bank (DSHB), Apa1, 1:500], mouse α-En (DSHB, 4D9, 1:25), DAPI (Thermo Fisher Scientific, 62248, 1:1000), Alexa633-conjugated-Phalloidin (Invitrogen, A22284, 1:500), mouse α-Cut (DSHB, 2B10, 1:200), mouse α-Aop (DSHB, 8B12H9, 1:25), rat α-Ser (1:500, from Kenneth Irvine; Papayannopoulos et al., 1998), rat α-Ihog (1:200, from X. Zheng; Yao et al., 2006), rat α-Ci-AbN (1:100; Aza-Blanc et al., 1997), rabbit α-PH3 Ser10 (Millipore, 06-570, 1:100), Rabbi α-Caspase (α-DCP-1, Cell Signaling Technologies, #9578, 1:500). Secondary antibodies from Invitrogen were used at 1:500 dilution: goat anti-mouse IgG, Alexa Fluor 488 (A-11001), goat anti-mouse IgG, Alexa Fluor 555 (A-21422), goat anti-rabbit IgG, Alexa Fluor 488 (A-11008), goat anti-rabbit IgG, Alexa Fluor 555 (A-21428), goat anti-rat IgG, Alexa Fluor 555 (A-21434), goat anti-rat IgG, Alexa Fluor 488 (A-11006). Samples were mounted in Vectashield Antifade Mounting Medium (Vector Laboratories, H-1000-10).
Unfixed wing discs and ASPs were observed using the hanging drop method (Huang and Kornberg, 2016). All images were taken using the FV3000 Olympus Confocal microscope with GaAsP PMT detectors. Images were analyzed and processed using ImageJ and Photoshop.
Analysis of the number of Hh:GFP puncta in the ASP
Maximum projections of 15 optical sections that span the upper and lower layers of the ASP were processed in ImageJ. For Fig. 1H, the position of each puncta was measured relative to the distance from the tip of the ASP. Histograms show the number of puncta in different bins at distances from the tip of the ASP. For Fig. 7, the total number of Hh:GFP puncta in the ASP was tabulated.
Analysis of cytoneme number and length
btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/+ (Control), btl-Gal4, UAS-mCD8:GFP/UAS-SmoM2; Gal80ts/+ (SmoM2 expression), btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/UAS-Hh:CD2 (Hh:CD2 expression), btl-Gal4, UAS-mCD8:GFP/+; Gal80ts/UAS-Ptc (Ptc expression) larvae were grown at 18°C until early L3 larvae and were incubated at 29°C for 24 h before dissection. ASP cytonemes were imaged using the hanging drop method (Huang and Kornberg, 2016). For the analysis of cytoneme density, the number of cytonemes in the ASP bulb was counted and the number is expressed relative to perimeter length. The length and location of the base was measured in ImageJ for each cytoneme. Cytonemes were categorized into tip (≤25 μm from tip) and medial (>25 μm from tip) based on the position of their base with respect to the tip of the ASP. The fraction of cytonemes in various length bins was measured for each sample. Ten samples were analyzed for each genotype except for UAS-Ptc (n=4).
Ptc intensity measurements in the ASP
Average projection of 15 optical sections was calculated in ImageJ for optical sections spanning the upper and lower layer of the ASP. α-Ptc staining intensity was measured inside the ASP in these images and normalized to α-Ptc staining intensity in the myoblast.
Analysis of the morphology of myoblast layers
In ImageJ, orthogonal sections were generated along the region of the notum shown in Fig. 4A; depths of the myoblast layers were measured from the basal side of the wing discs. Five samples were measured for each genotype.
Statistical analysis
All error bars show s.d. To analyze the statistical significance, Microsoft Excel was used to perform the unpaired two-tailed Student's t-test. P value below 0.05 was specified as being statistically significant.
Supplementary Material
Acknowledgements
We thank: K. Irvine, M. Gibson and X. Zheng for antibodies and the BDSC and VDRC for fly stocks. We thank Guilherme Oliveira Barbosa and Sol Fereres for contributing to the initial observation of hh signaling in the myoblast.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.H., T.B.K.; Methodology: R.H., T.B.K.; Validation: R.H.; Investigation: R.H.; Writing - original draft: R.H., T.B.K.; Writing - review & editing: R.H., T.B.K.; Visualization: T.B.K.; Supervision: T.B.K.; Funding acquisition: T.B.K.
Funding
This work was funded by the National Institutes of Health (T32HL007185 to R.H. and R35GM122548 to T.B.K.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.195974.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.195974.reviewer-comments.pdf
References
- Akiyama T. and Gibson M. C. (2015). Decapentaplegic and growth control in the developing Drosophila wing. Nature 527, 375-378. 10.1038/nature15730 [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P., Ramírez-Weber F.-A., Laget M.-P., Schwartz C. and Kornberg T. B. (1997). Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89, 1043-1053. 10.1016/S0092-8674(00)80292-5 [DOI] [PubMed] [Google Scholar]
- Bejarano F. and Milán M. (2009). Genetic and epigenetic mechanisms regulating hedgehog expression in the Drosophila wing. Dev. Biol. 327, 508-515. 10.1016/j.ydbio.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Bischoff M., Gradilla A.-C., Seijo I., Andrés G., Rodríguez-Navas C., González-Méndez L. and Guerrero I. (2013). Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat. Cell Biol. 15, 1269-1281. 10.1038/ncb2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. S. (1992). Engrailed expression in the anterior lineage compartment of the developing wing blade of Drosophila. Development 115, 21-33. [DOI] [PubMed] [Google Scholar]
- Briscoe J., Chen Y., Jessell T. M. and Struhl G. (2001). A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol. Cell 7, 1279-1291. 10.1016/S1097-2765(01)00271-4 [DOI] [PubMed] [Google Scholar]
- Burke R., Nellen D., Bellotto M., Hafen E., Senti K.-A., Dickson B. J. and Basler K. (1999). Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803-815. 10.1016/S0092-8674(00)81677-3 [DOI] [PubMed] [Google Scholar]
- Cabernard C. and Affolter M. (2005). Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell 9, 831-842. 10.1016/j.devcel.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Callejo A., Bilioni A., Mollica E., Gorfinkiel N., Andrés G., Ibáñez C., Torroja C., Doglio L., Sierra J. and Guerrero I. (2011). Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA 108, 12591-12598. 10.1073/pnas.1106881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casso D. J., Liu S., Iwaki D. D., Ogden S. K. and Kornberg T. B. (2008). A screen for modifiers of hedgehog signaling in Drosophila melanogaster identifies swm and mts. Genetics 178, 1399-1413. 10.1534/genetics.107.081638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Huang H., Hatori R. and Kornberg T. B. (2017). Essential basal cytonemes take up Hedgehog in the Drosophila wing imaginal disc. Development 144, 3134-3144. 10.1242/dev.149856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis J. F. and Ruiz-Gómez M. (1995). groucho and hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development 121, 3467-3476. [DOI] [PubMed] [Google Scholar]
- Du L., Sohr A., Yan G. and Roy S. (2018). Feedback regulation of cytoneme-mediated transport shapes a tissue-specific FGF morphogen gradient. eLife 7, e38137 10.7554/eLife.38137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C., McKay D. J. and Mann R. S. (2008). Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev. Cell 14, 86-96. 10.1016/j.devcel.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg E. H., Vanhoven M. K., Bendesky A., Wang G., Fetter R. D., Shen K. and Bargmann C. I. (2008). GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, 353-363. 10.1016/j.neuron.2007.11.030 [DOI] [PubMed] [Google Scholar]
- Fereres S., Hatori R., Hatori M. and Kornberg T. B. (2019). Cytoneme-mediated signaling essential for tumorigenesis. PLoS Genet. 15, e1008415 10.1371/journal.pgen.1008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A. (1975). Genetic control of wing disc development in Drosophila. In Cell Patterning (ed. R. Porter and J. Rivers), pp. 161-178. American Scientific Publishers. [DOI] [PubMed] [Google Scholar]
- González-Méndez L., Seijo-Barandiarán I. and Guerrero I. (2017). Cytoneme-mediated cell-cell contacts for Hedgehog reception. eLife 6, e24045 10.7554/eLife.24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Méndez L., Gradilla A.-C. and Guerrero I. (2019). The cytoneme connection: direct long-distance signal transfer during development. Development 146, dev174607 10.1242/dev.174607 [DOI] [PubMed] [Google Scholar]
- Gunage R. D., Reichert H. and VijayRaghavan K. (2014). Identification of a new stem cell population that generates Drosophila flight muscles. eLife 3, e03126 10.7554/eLife.03126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz P., Bonfini A., Liu X., Revah J., Guillou A., Poidevin M., Hens K., Huang H.-Y., Deplancke B., Tsai Y.-C. et al. (2017). Hippo, TGF-β, and Src-MAPK pathways regulate transcription of the upd3 cytokine in Drosophila enterocytes upon bacterial infection. PLoS Genet. 13, e1007091 10.1371/journal.pgen.1007091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. and Kornberg T. B. (2015). Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium. eLife 4, e06114 10.7554/eLife.06114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. and Kornberg T. B. (2016). Cells must express components of the planar cell polarity system and extracellular matrix to support cytonemes. eLife 5, e18979 10.7554/eLife.18979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Liu S. and Kornberg T. B. (2019). Glutamate signaling at cytoneme synapses. Science 363, 948-955. 10.1126/science.aat5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C.-C. and Angers S. (2011). Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 27, 513-537. 10.1146/annurev-cellbio-092910-154048 [DOI] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G. and Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343-1355. 10.1016/j.cell.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Grenier J. K. and Scott M. P. (1995). patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development 121, 4161-4170. [DOI] [PubMed] [Google Scholar]
- Lee J. J., von Kessler D. P., Parks S. and Beachy P. A. (1992). Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71, 33-50. 10.1016/0092-8674(92)90264-D [DOI] [PubMed] [Google Scholar]
- Lin L. (2009). Clonal Analysis of Growth Behaviors during Drosophila Larval Tracheal Development. Universit of Basel. [Google Scholar]
- Lum L., Yao S., Mozer B., Rovescalli A., Von Kessler D., Nirenberg M. and Beachy P. A. (2003). Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 299, 2039-2045. 10.1126/science.1081403 [DOI] [PubMed] [Google Scholar]
- Macpherson L. J., Zaharieva E. E., Kearney P. J., Alpert M. H., Lin T.-Y., Turan Z., Lee C.-H. and Gallio M. (2015). Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 6, 10024 10.1038/ncomms10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J. (1988). Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics 120, 1061-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb S., Voutev R., Jory A., Delker R. K., Slattery M. and Mann R. S. (2018). Cis-regulatory architecture of a short-range EGFR organizing center in the Drosophila melanogaster leg. PLoS Genet. 14, e1007568 10.1371/journal.pgen.1007568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. and Wieschaus E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801. 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V., Tomlinson A., Panin V. M., Rauskolb C. and Irvine K. D. (1998). Dorsal-ventral signaling in the Drosophila eye. Science 281, 2031-2034. 10.1126/science.281.5385.2031 [DOI] [PubMed] [Google Scholar]
- Ramírez-Weber F.-A., Casso D. J., Aza-Blanc P., Tabata T. and Kornberg T. B. (2000). Hedgehog signal transduction in the posterior compartment of the Drosophila wing imaginal disc. Mol. Cell 6, 479-485. 10.1016/S1097-2765(00)00046-0 [DOI] [PubMed] [Google Scholar]
- Roberts K. J., Kershner A. M. and Beachy P. A. (2017). The stromal Niche for epithelial stem cells: a template for regeneration and a brake on malignancy. Cancer Cell 32, 404-410. 10.1016/j.ccell.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. and VijayRaghavan K. (1997). Homeotic genes and the regulation of myoblast migration, fusion, and fibre-specific gene expression during adult myogenesis in Drosophila. Development 124, 3333-3341. [DOI] [PubMed] [Google Scholar]
- Roy S., Hsiung F. and Kornberg T. B. (2011). Specificity of Drosophila cytonemes for distinct signaling pathways. Science 332, 354-358. 10.1126/science.1198949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Huang H., Liu S. and Kornberg T. B. (2014). Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science 343, 1244624 10.1126/science.1244624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. and Kornberg T. B. (2002). FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev. Cell 3, 195-207. 10.1016/S1534-5807(02)00202-2 [DOI] [PubMed] [Google Scholar]
- Strigini M. and Cohen S. M. (1997). A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124, 4697-4705. [DOI] [PubMed] [Google Scholar]
- Tabata T., Eaton S. and Kornberg T. B. (1992). The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 6, 2635-2645. 10.1101/gad.6.12b.2635 [DOI] [PubMed] [Google Scholar]
- Taberner L., Bañón A. and Alsina B. (2020). Sensory neuroblast quiescence depends on vascular cytoneme contacts and sensory neuronal differentiation requires initiation of blood flow. Cell Rep. 32, 107903 10.1016/j.celrep.2020.107903 [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Itoh S., ten Dijke P. and Tabata T. (2000). Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell 5, 59-71. 10.1016/S1097-2765(00)80403-7 [DOI] [PubMed] [Google Scholar]
- Tomlinson A. and Struhl G. (2001). Delta/Notch and Boss/Sevenless signals act combinatorially to specify the Drosophila R7 photoreceptor. Mol. Cell 7, 487-495. 10.1016/S1097-2765(01)00196-4 [DOI] [PubMed] [Google Scholar]
- Torroja C., Gorfinkiel N. and Guerrero I. (2004). Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development 131, 2395-2408. 10.1242/dev.01102 [DOI] [PubMed] [Google Scholar]
- Torroja C., Gorfinkiel N. and Guerrero I. (2005). Mechanisms of Hedgehog gradient formation and interpretation. J. Neurobiol. 64, 334-356. 10.1002/neu.20168 [DOI] [PubMed] [Google Scholar]
- Xie J., Murone M., Luoh S.-M., Ryan A., Gu Q., Zhang C., Bonifas J. M., Lam C.-W., Hynes M., Goddard A. et al. (1998). Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90-92. 10.1038/34201 [DOI] [PubMed] [Google Scholar]
- Yao S., Lum L. and Beachy P. (2006). The Ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125, 343-357. 10.1016/j.cell.2006.02.040 [DOI] [PubMed] [Google Scholar]
- Yu D., Baird M. A., Allen J. R., Howe E. S., Klassen M. P., Reade A., Makhijani K., Song Y., Liu S., Murthy Z. et al. (2015). A naturally monomeric infrared fluorescent protein for protein labeling in vivo. Nat. Methods 12, 763-765. 10.1038/nmeth.3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. and Scholpp S. (2019). Cytonemes in development. Curr. Opin. Genet. Dev. 57, 25-30. 10.1016/j.gde.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A. J., Zheng L., Suyama K. and Scott M. P. (2003). Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17, 1240-1252. 10.1101/gad.1080803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.