ABSTRACT
FGF8 signaling plays diverse roles in inner ear development, acting at multiple stages from otic placode induction to cellular differentiation in the organ of Corti. As a secreted morphogen with diverse functions, Fgf8 expression is likely to be spatially restricted and temporally dynamic throughout inner ear development. We evaluated these characteristics using genetic labeling mediated by Fgf8mcm gene-targeted mice and determined that Fgf8 expression is a specific and early marker of Type-I vestibular hair cell identity. Fgf8mcm expression initiates at E11.5 in the future striolar region of the utricle, labeling hair cells following EdU birthdating, and demonstrates that sub-type identity is determined shortly after terminal mitosis. This early fate specification is not apparent using markers or morphological criteria that are not present before birth in the mouse. Although analyses of Fgf8 conditional knockout mice did not reveal developmental phenotypes, the restricted pattern of Fgf8 expression suggests that functionally redundant FGF ligands may contribute to vestibular hair cell differentiation and supports a developmental model in which Type-I and Type-II hair cells develop in parallel rather than from an intermediate precursor.
KEY WORDS: FGF8, Hair cell, Vestibular, Inner ear, Cre recombinase, Mouse
Summary: The two classes of vestibular hair cell are established during embryogenesis and are readily distinguished from each other by Fgf8 expression shortly after terminal mitosis.
INTRODUCTION
The vestibular sensory organs of the vertebrate inner ear contain mechanosensory receptors called hair cells that enable the detection of linear and rotational accelerations of the head, and gravity. Vestibular hair cells can be broadly divided into two classes based upon morphological and synaptic criteria. Type-I hair cells have a characteristic ampoule-like morphology and an afferent nerve terminal which forms a large calyx surrounding the hair cell. In contrast, Type-II hair cells have a columnar morphology and afferent nerve terminals that resemble synaptic boutons. Type-I hair cells are also physiologically unique, with low-voltage activated K+ currents that enable faster receptor potentials with smaller voltage responses than Type-II hair cells (Rennie et al., 1996; Rusch et al., 1998; Eatock and Songer, 2011).
In the mouse, the terminal mitosis and differentiation of vestibular hair cells begins at embryonic day (E) 11.5 and continues through postnatal development, with over half of the hair cells emerging after birth (Burns et al., 2012; Raft et al., 2007; Ruben, 1967). It has been proposed that hair cells appear sequentially, with the majority of Type-I hair cells developing at embryonic stages and Type-II hair cells emerging postnatally (McInturff et al., 2018). A noteworthy difference between these cell types is the requirement for postnatal expression of Sox2 in Type-II hair cells, as revealed by the increased number of Type-I hair cells that appear at the expense of the Type-II population after postnatal deletion of Sox2 (Lu et al., 2019). This has led to the alternative hypothesis that cell identity is determined by Sox2 and is not established until postnatal development, concurrent with Sox2 downregulation in the Type-I cells (Warchol et al., 2019; Hume et al., 2007; Oesterle et al., 2008). The commonalities and differences between these alternative etiologies have been difficult to resolve because the molecular, morphological and synaptic characteristics that distinguish hair cell subtypes are also not available until later postnatal stages [postnatal day (P) 2-12] (Simmons et al., 2010; Perry et al., 2003; McInturff et al., 2018).
Fibroblast growth factor 8 (FGF8) belongs to a family of secreted morphogens that signal locally to neighboring cells to promote differentiation. For example, in the organ of Corti, FGF8 released from inner hair cells induces neighboring pillar cell differentiation. Fewer inner pillar cells differentiate in Fgf8 knockouts, whereas overexpression leads to ectopic pillar cell formation (Jacques et al., 2007). FGF8 binds with varying specificity and affinity to FGF receptor tyrosine kinases (FGFR1-3) activating intracellular signaling cascades leading to transcription factor activation (Ornitz et al., 1996; Pirvola et al., 2000; Roehl and Nusslein-Volhard, 2001; Firnberg and Neubuser, 2002). Outside of the ear, Fgf8 is expressed in multiple important signaling centers during embryogenesis and the complete loss of FGF8 function causes early embryonic lethality by disrupting movements of nascent mesoderm in the primitive streak (Crossley and Martin, 1995; Meyers et al., 1998; Sun et al., 1999; Moon and Capecchi, 2000). Studies using hypomorphic Fgf8 alleles and Cre/Lox-mediated conditional mutagenesis have elucidated crucial roles for FGF8 in left-right axis determination, formation and patterning of the forebrain, olfactory bulb and the cerebellum/midbrain-hindbrain junction, pharyngeal gland development, cardiovascular and craniofacial development, renal development, and limb outgrowth and patterning (Frank et al., 2002; Boulet et al., 2004; Macatee et al., 2003; Reifers et al., 1998; Wright and Mansour, 2003; Lee et al., 1997; Crespo-Enriquez et al., 2012; Griffin et al., 2013; Gao et al., 2018; Lewandoski et al., 2000; Moon and Capecchi, 2000). In the developing neocortex a mutually opposing balance between FGF8 and Emx2 and related transcription factors contributes to functional domain patterning in a process called arealization (Fukuchi-Shimogori and Grove, 2001; O'Leary and Nakagawa, 2002; Muzio and Mallamaci, 2003). Thus, FGF8 can regulate tissue patterning by signaling between neighboring domains in addition to neighboring cells and is generally necessary for multiple differentiation and patterning events throughout embryonic development.
The diverse roles of FGF8 likely mean that Fgf8 expression is spatially restricted at different stages of otic development and also highly dynamic as development progresses from one stage to the next. To evaluate this, we examined the spatial and temporal dynamics of Fgf8 expression during inner ear development using Cre-mediated genetic labeling and in situ hybridization (ISH). These analyses were directed towards the vestibular maculae because domains of Emx2 expression in the utricle and saccule determine hair cell stereociliary bundle orientation (Holley et al., 2010; Jiang et al., 2017) and the reciprocal relationship between FGF8 and Emx2 determines a boundary of Emx2 expression in the neocortex (Fukuchi-Shimogori and Grove, 2003; Fukuchi-Shimogori and Grove, 2001). We found transient Fgf8 expression in auditory and vestibular neurons, and sustained specific expression in Type-I hair cells beginning shortly after terminal mitosis at E11.5 and continuing through postnatal development. Genetically labeled vestibular hair cells were distributed throughout the sensory epithelia, exhibited ampoule-like morphology, calyceal afferents, and expressed the molecular marker osteopontin (OPN, also known as Spp1), all of which are consistent with the Type-I hair cell identity. Despite this unique pattern of expression, Fgf8 conditional knockout mice showed no changes in hair cell types, stereociliary bundle polarity and organization, or formation of the calyx-synapse, suggesting that, as observed in other developmental contexts, there may be functional redundancy with other FGF family members during hair cell development. Although the contribution of FGF signaling to vestibular development remains unknown, genetic labeling experiments demonstrate that hair cell sub-type specification occurs earlier than anticipated, happening shortly after terminal mitosis, and establish Fgf8 expression as the earliest marker distinguishing vestibular hair cell types.
RESULTS
Generation of the Fgf8mcm allele
Genetic labeling enables heterochronic gene expression studies in which the cells or progeny of cells expressing a gene of interest during a defined period of development can be permanently labeled for evaluation at later stages. To generate a mouse line in which patterns of Fgf8 gene expression could be evaluated using this approach, cDNA encoding a fusion protein of Cre recombinase flanked by mutated hormone-binding domains of the murine estrogen receptor (mER:Cre:mER) (Verrou et al., 1999) was inserted into the Fgf8 locus. Commonly called CreER, these modified recombinases localize to the cytoplasm and remain inactive until binding the estrogen analog tamoxifen. Once activated, CreER translocates to the nucleus and catalyzes genetic recombination and excision of DNA located between tandem pairs of 32 base pair LoxP sequences (Zhang et al., 1996). To obtain CreER production in Fgf8-expressing tissues without disrupting Fgf8 function, a cassette containing an IRES sequence (Kaminski et al., 1990; Jang and Wimmer, 1990) followed by the mER:Cre:mER cDNA (Verrou et al., 1999) was inserted into the 3′ untranslated region (UTR) of the Fgf8 gene (Fig. 1A). This cassette and strategy have been successfully applied at other loci, and Flp-recombinase/Frt-mediated excision of the NeoR negative selection gene was completed to avoid hypomorphic effects of NeoR on expression from Fgf8 or other loci (Frank et al., 2002). To determine whether this novel Fgf8mcm allele retained intact Fgf8 expression, phenotypes were examined and Fgf8 mRNA expression was evaluated in animals heterozygous or homozygous for Fgf8mcm. Whole-mount ISH for Fgf8 mRNA revealed no detectable difference in hybridization patterns or intensity between wild-type and Fgf8mcm/mcm homozygous embryos (Fig. 1B,C). Furthermore, these embryos develop normally without phenotypes observed in Fgf8 null, hypomorphic or conditional mutant embryos. Fgf8mcm/mcm homozygotes survive to adulthood, are indistinguishable from wild-type animals, and reproduce normally. As even a modest decrease in Fgf8 function has phenotypic consequences in many systems, these results indicate that the presence of the IRES:mER:Cre:mER insertion into the 3′UTR of Fgf8 does not significantly affect gene function.
Fig. 1.
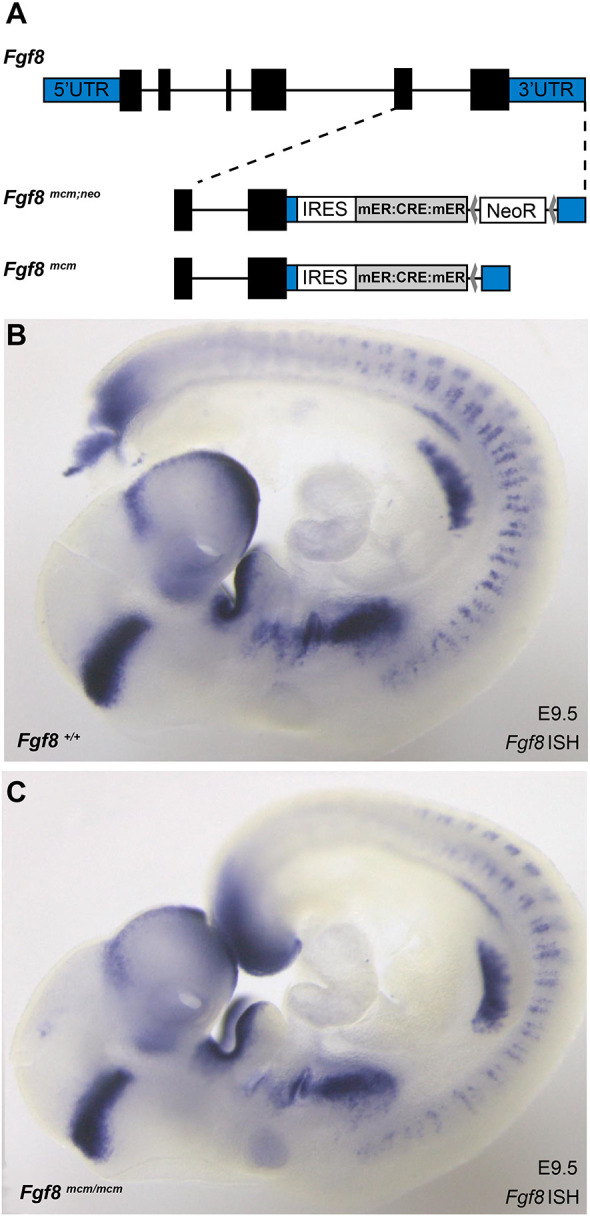
Fgf8mcm engineering strategy does not impact gene expression or function. (A) The gene targeting strategy used to generate Fgf8mcm mice inserts an IRES mER:CRE:mER expression cassette into the 3′UTR of the Fgf8 gene. 5′ and 3′ UTRs are marked in blue shading and the Fgf8 coding sequence exons are black. An Frt-flanked Neomycin resistance gene (NeoR) used for selection of targeted embryonic stem cell clones was removed using FLP recombinase to produce the final Fgf8mcm allele. (B,C) Whole-mount ISH for Fgf8 reveals no detectable differences in the staining pattern or intensity between wild-type (B) and Fgf8mcm/mcm homozygous (C) embryos at E9.5.
Embryonic Fgf8mcm genetic labeling labels a subset of vestibular hair cells
To evaluate Fgf8 expression in the developing vestibular system, Fgf8mcm/+ males were crossed with ROSAai9tom/ai9tom female mice allowing for tamoxifen-dependent Cre-mediated labeling of Fgf8- expressing cells by the red fluorescent protein tdTomato. Single injections of tamoxifen at E17.5 resulted in robust tdTomato expression in a subset of vestibular hair cells identified by βII-spectrin labeling (Fig. 2A-E). Following injections, labeled hair cells were present at E18.5 throughout the utricle (Fig. 2A,B), saccule (Fig. 2C,D) and within the semi-circular canal cristae (Fig. 2E). For each sensory organ, Fgf8mcm activation only labeled a subset of the total vestibular hair cells. This readout of Fgf8 expression is accurate and is unlikely the result of incomplete recombination because it resembles the distribution of Fgf8 mRNA visualized by whole-mount ISH at P0. Fgf8 mRNA also appears in a salt-and-pepper pattern within subsets of cells in the utricle (Fig. 2F), saccule (Fig. 2G) and cristae (Fig. 2H). In the cochlea, Fgf8 mRNA is also present in a subset of hair cells, the single row of inner hair cells, as previously described (Fig. 2I; Jacques et al., 2007).
Fig. 2.
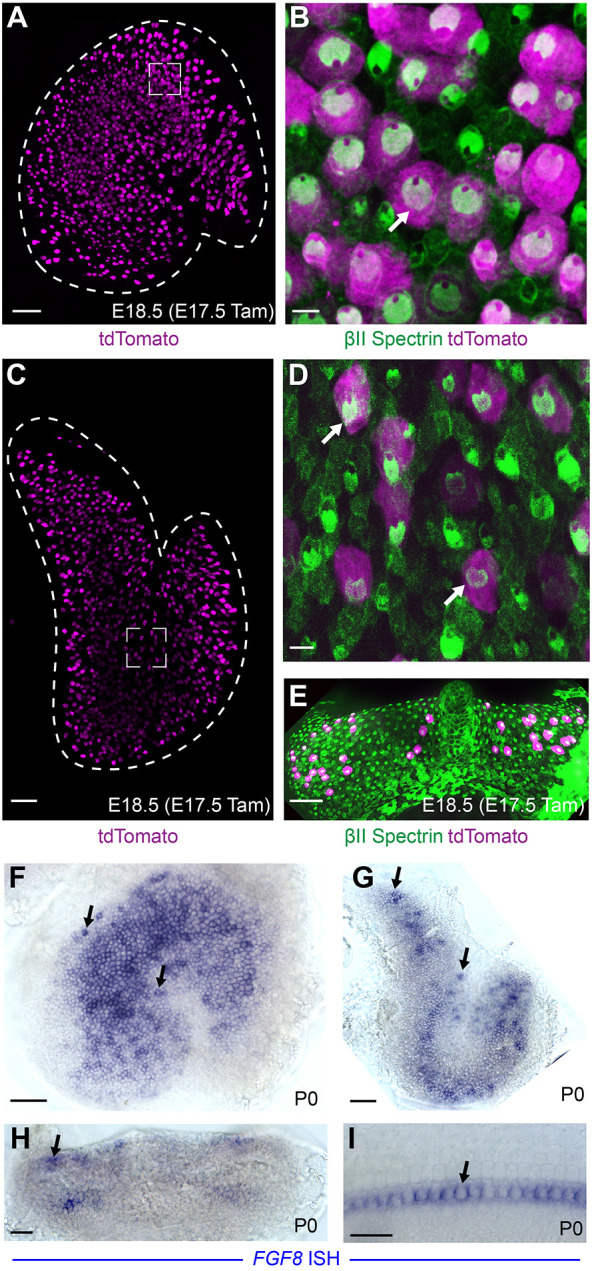
Fgf8mcm genetic labeling labels subsets of vestibular hair cells. (A) tdTomato fluorescence is present throughout the sensory epithelia in an Fgf8mcm/+; ROSAtdTom(Ai9)/WT utricle at E18.5 following tamoxifen induction at E17.5. (B) Higher magnification image from the framed region in A shows tdTomato colocalization with βII spectrin (example marked by arrow). (C,E) tdTomato is also induced by Fgf8mcm in vestibular hair cells throughout the saccule (C) and cristae (E). (D) Higher magnification image from the framed region in C shows tdTomato colocalization with βII spectrin (examples marked by arrows). (F-I) Whole-mount ISH for endogenous Fgf8 in the utricle (F), saccule (G), cristae (H) and cochlea (I). Arrows highlight examples of mRNA expression in individual hair cells. Scale bars: 50 µm in A,C,E,F,G,H; 20 µm in B,D,I.
Interestingly, early injections of tamoxifen at E11.5 similarly labeled a subset of vestibular hair cells when evaluated in the E18.5 utricular maculae (Fig. 3A,B). Previous birthdating experiments using tritiated thymidine or 5-ethynyl-2-deoxyuridine (EdU) incorporation demonstrate that the earliest-born vestibular hair cells undergo terminal mitosis at E11.5 (Ruben, 1967; Jiang et al., 2017) and these cells are enriched in the striolar region of the mature utricle. Similarly, Fgf8mcm genetic labeling at E11.5 included striolar hair cells based upon their oncomodulin expression when imaged at E18.5 (Fig. 3A,B) (Simmons et al., 2010; Hoffman et al., 2018). In contrast, induction at later stages (E15.5-E17.5) labeled a broader regional population that included extrastriolar hair cells in the lateral and medial regions (Fig. 2A; Fig. S1). Furthermore, newly-born hair cells labeled by EdU at E12.5 are also genetically labeled by Fgf8mcm when EdU and tamoxifen are co-administered (Fig. 3C-E′) and these cells populate the striolar region at E18.5 (Fig. 3C). As EdU is only retained in cells that have undergone their last mitosis, and CreER activation by tamoxifen is transient, colocalization indicates Fgf8 promoter activation shortly after cell division. Together, these regional and temporal patterns of Fgf8mcm activity demonstrate that Fgf8 expression is initiated in newly-born hair cells, after their specification and during the initial stages of their differentiation, and that expression is maintained throughout embryonic development.
Fig. 3.
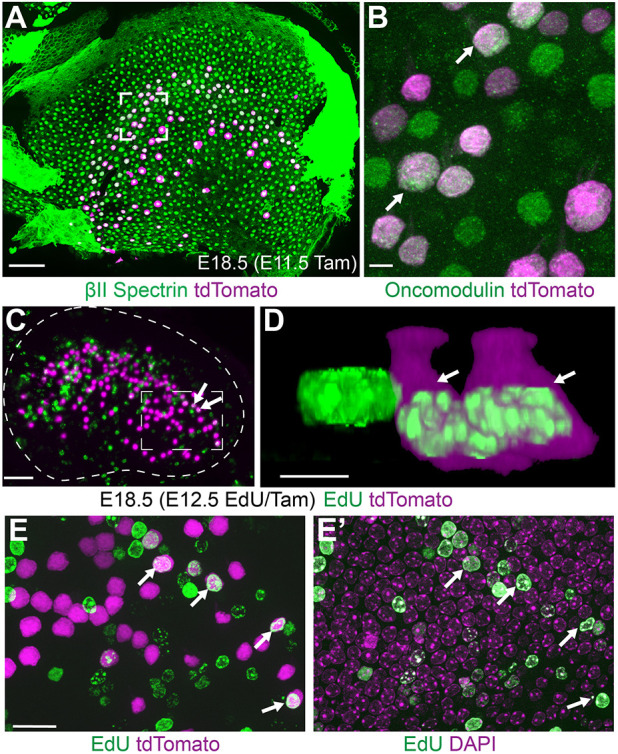
Fgf8mcm genetic labeling occurs shortly after hair cell terminal mitosis. (A) Tamoxifen induction at E11.5 induces tdTomato expression in hair cells throughout the central region of the utricle in which the striolar region lies at E18.5. (B) Higher magnification image from the framed region in A shows tdTomato colocalization with oncomodulin-positive hair cells (examples marked by arrows). (C) Newly divided hair cells labeled by EdU incorporation at E12.5 are genetically labeled by the tdTomato reporter with concurrent tamoxifen induction (examples marked by arrowheads). Dashed line indicates the boundary of the utricular sensory epithelium. (D) Higher magnification 3D-reconstruction of EdU-labeled hair cells. Arrowheads correspond to the two tdTomato-positive cells indicated in C. (E,E′) Higher magnification image corresponding to the framed region in C similarly reveals vestibular hair cells containing EdU and labeled by tdTomato (E) and DAPI (E′). Scale bars: 50 µm in A,C; 5 µm in B,D; 10 µm in E,E′.
Genetic labeling reveals transient Fgf8 expression in auditory and vestibular neurons
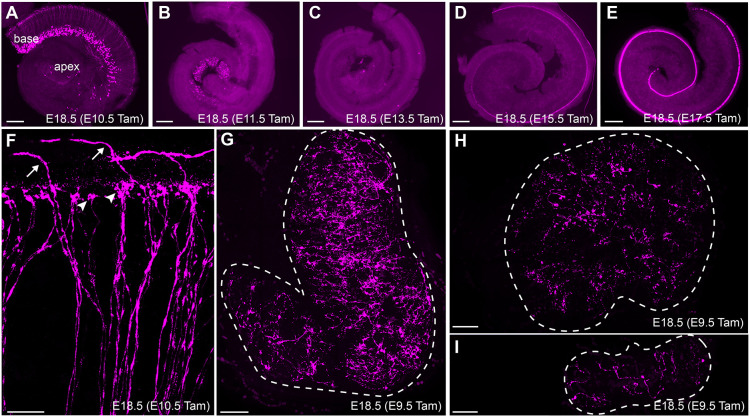
Although tamoxifen injections before E10.5 failed to label hair cells, transient Fgf8 expression was evident in the spiral ganglion neurons (SGNs) during these early stages of cochlear development. This transient expression follows a development gradient, with tamoxifen injections from E10.5 to E13.5 revealing progressive Fgf8 activity in SGNs from the cochlear base to the apex (Fig. 4A-C), similar to the expression pattern of pro-neural genes such as neurogenin 1 (Koundakjian et al., 2007). Close examination of the peripheral processes of labeled SGNs show that Fgf8 is expressed by both Type-I and Type-II SGNs based upon the morphology of tdTomato-labeled peripheral axons innervating the organ of Corti (Fig. 4F). Consistent with previous reports (Jacques et al., 2007), Fgf8mcm genetic labeling also robustly marks inner hair cells, with the onset of tdTomato expression also appearing in a developmental gradient initiating in the cochlear base and progressing towards the cochlear apex (Fig. 4D,E). tdTomato-positive neuronal processes were also observed throughout the utricle, saccule and cristae following tamoxifen delivery at E9.5 (Fig. 4G-I) indicating early expression in vestibular neurons as well. More importantly, tamoxifen induction at later stages (E11.5, E17.5) predominantly labels hair cells and not vestibular neurons demonstrating that, as in the SGNs, Fgf8 expression is transient within vestibular neurons and restricted to early stages of neuronal development.
Fig. 4.
Fgf8 expression in auditory and vestibular neurons is transient. (A-E) Fgf8mcm genetic labeling in the developing cochlea selectively labels SGNs following tamoxifen induction at E10.5 (A) and E11.5 (B). Little to no labeling is seen with tamoxifen induction at E13.5 (C), whereas inner hair cells are labeled following tamoxifen induction at E15.5 (D) and E17.5 (E). (F) Higher magnification image of tdTomato labeling in peripheral axons of auditory neurons following tamoxifen induction at E10.5 reveals Type-I SGNs (arrowheads) which innervate inner hair cells, and Type-II SGNs (arrows) that extend further, turn towards the cochlear base and innervate outer hair cells. (G-I) Tamoxifen induction at E9.5 labels the peripheral processes of vestibular neurons innervating the saccule (G), utricle (H) and cristae (I). Dashed line indicates the boundary of the vestibular sensory organ. Scale bars: 200 µm in A-E,G-I; 20 µm in F.
Embryonic Fgf8mcm genetic labeling selectively labels Type-I vestibular hair cells
The two different types of vestibular hair cells in the mouse can be distinguished from each other based upon morphologic and synaptic criteria which begin to emerge at about the time of birth (19 days post-copulation). These features were evaluated in cryosections collected from E18.5 Fgf8mcm/+; tdTomato-positive embryos following tamoxifen induction at E17.5. At this early stage, many tdTomato-positive hair cells already exhibited an ampoule morphology characteristic of Type-I vestibular hair cells (Fig. 5A). Often, these putative Type-I hair cells were also contacted by a neuronal process that was immunoreactive for neurofilament (NFH) that appeared to be nascent calyceal termini, although formation of these synaptic structures is not complete until P14 (Fig. 5A′). Other tdTomato-positive hair cells had simpler or possibly immature synaptic contacts and hair cell morphologies that could not be distinguished from Type-II hair cells. Cells with these latter characteristics were more abundant in the periphery of the sensory epithelia.
Fig. 5.
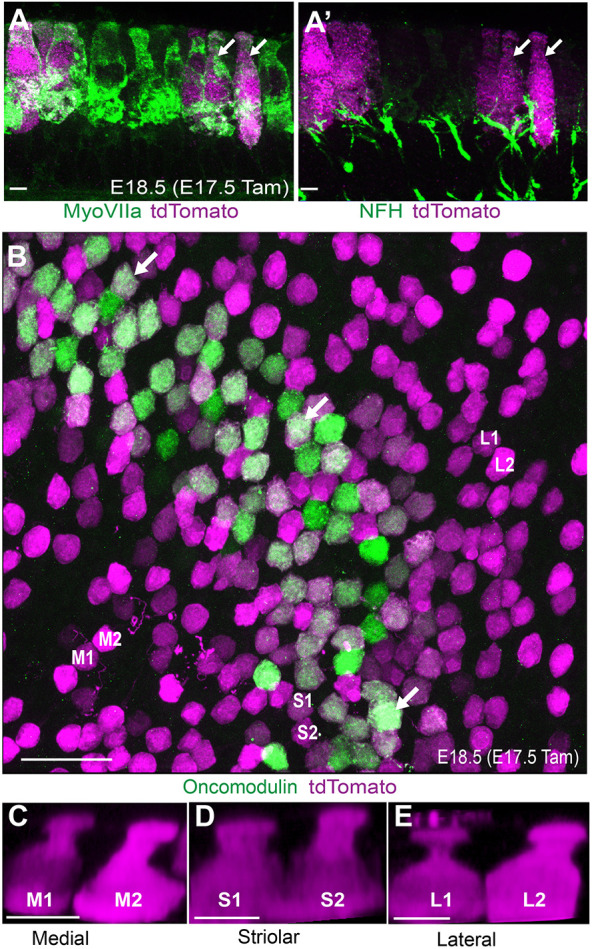
Fgf8mcm genetic labeling labels Type-I hair cells at embryonic stages. (A) Cross-section through the embryonic utricular sensory epithelia following tamoxifen induction at E17.5 showing tdTomato expression in hair cells with Type-I morphology characterized by a restriction at the apical neck (arrows). (A′) tdTomato-labeled hair cells are contacted by presumptive developing calyceal synapses (arrows). (B) Whole-mount immunolabeling of Fgf8mcm utricle reveals colocation of tdTomato reporter and the striolar Type-I hair cell marker oncomodulin (examples illustrated by arrows). (C-E) 3D-reconstructions of tdTomato-positive oncomodulin-negative hair cells in medial extrastriolar (M1, M2; C), striolar (S1, S2; D), and lateral extrastriolar (L1, L2; E) regions also reveals ampoule-like Type-I hair cell morphology. Scale bars: 5 µm in A,C-E; 20 µm in B.
A subset of Type-I hair cells produce oncomodulin, which is a calcium-binding protein found in Type-I hair cells populating the striolar region of the utricle, and are contacted by afferent neurons that form a complex calyx encompassing multiple hair cells. tdTomato and endogenous oncomodulin colocalize in the striolar region following tamoxifen induction at E17.5, consistent with Fgf8 expression in Type-I hair cells (Fig. 5B). The striolar and extrastriolar regions also contain Type-I hair cells that are contacted by a simple calyx and do not express oncomodulin, but still develop the characteristic ampoule-like morphology of Type-I hair cells. 3D reconstruction of individual tdTomato-positive oncomodulin-negative cells from the striolar or medial and lateral extrastriolar regions revealed this stereotypic Type-I hair cell morphology (Fig. 5C-E). Altogether the morphology and overall distribution of genetically labeled cells is consistent with selective Fgf8mcm activity in Type-I hair cells. tdTomato-positive cells with similar ampoule-like morphology were also seen in the saccule and semi-circular cristae, suggesting that Fgf8 expression is a general marker of vestibular hair cell identity. The defining characteristic of Type-I hair cells is the calyceal synapse, which can be readily seen using neuronal markers such as βIII-tubulin at P14. To definitively establish whether embryonic hair cells expressing Fgf8 differentiated into Type-I hair cells, tamoxifen was administered at E11.5 and tdTomato-positive hair cells were evaluated for the presence of a synaptic calyx at P14 (Fig. 6A). The majority of these cells were surrounded by a ring of βIII-tubulin, indicating the presence of a calyx and confirming Type-I hair cell identity.
Fig. 6.
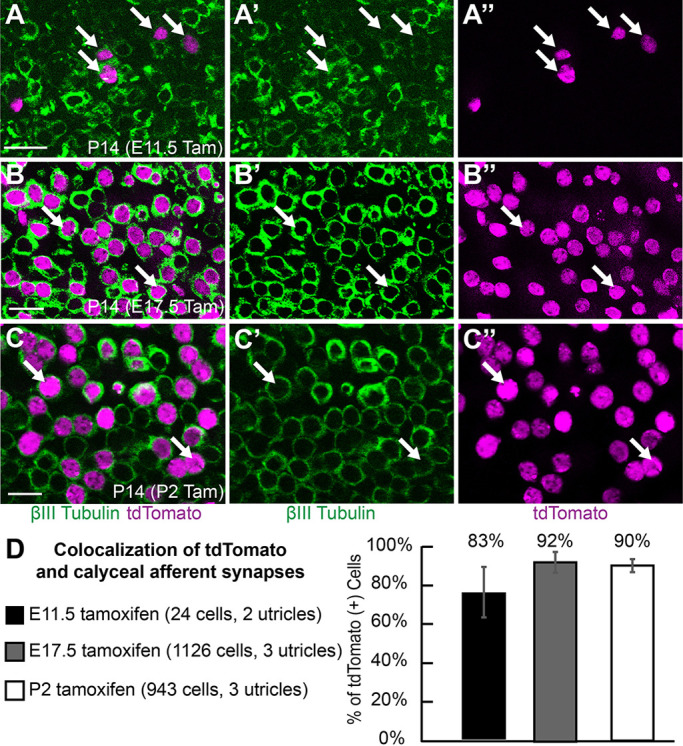
Fgf8mcm genetic labeling selectively labels Type-I hair cells contacted by calyxes. (A) Following tamoxifen induction at E11.5, tdTomato-positive hair cells are surrounded by βIII-tubulin rich calyces (arrows) when evaluated at P14, consistent with Type-I hair cell identity. (B) Following tamoxifen induction at E17.5, tdTomato-positive hair cells are surrounded by βIII-tubulin rich calyces (arrows) when evaluated at P14. (C) Following tamoxifen induction at P2, tdTomato-positive hair cells are similarly contacted by calyces (arrows) at P14. A′, B′, C′ show βIII-tubulin channel; A″, B″, C″ show tdTomato channel. (D) The frequency of calyx formation about tdTomato-positive hair cells following tamoxifen induction at different developmental stages. Data are mean±s.d. Scale bars: 10 µm.
Postnatal Fgf8 genetic labeling is restricted to Type-I hair cells
Recently it was proposed that hair cell sub-types differentiate sequentially, with the Type-II cells predominantly appearing at postnatal stages and, conversely, Type-I hair cells differentiating in the embryo (McInturff et al., 2018). In this scenario, genes expressed in hair cells at embryonic stages may appear to preferentially label Type-I hair cells in genetic labeling experiments, when in fact they may not be cell type-specific. To determine whether Fgf8mcm sequentially labeled Type-I and then Type-II hair cells through the course of utricle development, tamoxifen was delivered at E11.5, E17.5, or P2. For each injection paradigm, tdTomato-positive cells were evaluated at P14 when cell type-specific molecular markers are available, such as the Type-I hair marker osteopontin, the Type-II markers Anxa4 and Calb2 (McInturff et al., 2018; Sayyid et al., 2019; Perry et al., 2003), or the presence of a calyx. Regardless of the time of tamoxifen induction, tdTomato genetic labeling predominantly labeled Type-I hair cells, as evidenced by the presence of calyceal synapses (Fig. 6A-C) and, at all stages, tamoxifen administration resulted in a comparable frequency of tdTomato-positive cells contacted by a calyx (Fig. 6D, Table S1).
The postnatal delivery of tamoxifen at P2 also failed to label cells expressing markers associated with Type-II hair cell identity. When evaluated at P14, very few tdTomato-positive hair cells expressed Type-II markers Anxa4 and Calb2 (Fig. 7B-D, Table S2). It is not unexpected that, on occasion, tdTomato was found in hair cells expressing Anxa4 or Calb2, as this marker is not absolutely restricted to Type-II hair cells and can be detected in a small proportion of cells expressing other Type-I markers (McInturff et al., 2018). In contrast, following tamoxifen delivery at P2, the majority of labeled hair cells colocalized with the Type-I marker osteopontin at P14 (Fig. 7A,D, Table S2). Finally, unlike the transient Fgf8mcm activity observed in neurons, expression in hair cells appeared to be sustained across the range of developmental time points that tamoxifen was administered (E11.5 through P2).
Fig. 7.
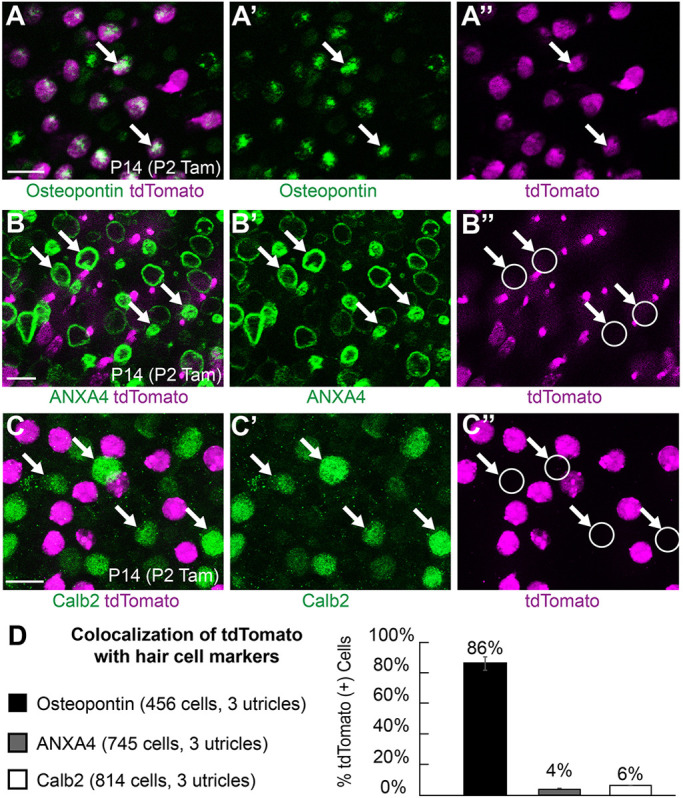
Fgf8mcm genetic labeling selectively labels Type-I but not Type-II hair cells at postnatal stages. (A-A″) tdTomato-positive hair cells labeled by tamoxifen induction at P2 colocalize with the Type-I hair cell marker osteopontin (examples marked by arrows). (B-B″) Hair cells labeled by tamoxifen induction at P2 (arrows) do not colocalize with the Type-II hair cell marker Anxa4. (C-C″) Hair cells labeled by tamoxifen induction at P2 do not colocalize with Type-II hair cell marker Calb2 (examples marked by arrows). (D) The frequency of tdTomato colocalization with cell type-specific markers following tamoxifen induction at P2. Data are mean±s.d. Scale bars: 10 µm.
Hair cell development and innervation appears normal in Fgf8 CKOs
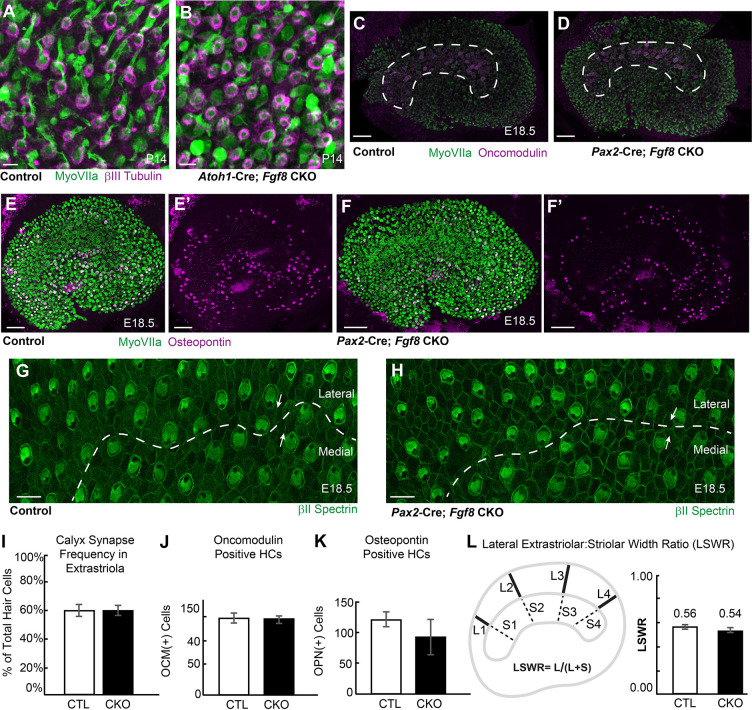
As FGFs are known secreted signaling molecules, restricted expression of Fgf8 in Type-I hair cells suggests that FGF8 acts through autocrine and/or paracrine mechanisms during vestibule development. Consistent with a paracrine function in the cochlea, paracrine FGF8 signaling from inner hair cells promotes the differentiation of neighboring pillar cells (Jacques et al., 2007). Despite this, no phenotypic differences in hair cells or cellular organization within the vestibular maculae were identified in Fgf8 CKOs compared with littermate controls (Fig. 8). CKOs were generated using two Cre lines frequently used for inner ear research, the hair cell-restricted Atoh1-Cre (Matei et al., 2005) and Pax2-Cre, which is active in the otic placode and also portions of the CNS and kidney (Ohyama and Groves, 2004). Atoh1-Cre-mediated gene deletion was incomplete as revealed by ISH, with some Fgf8 mRNA persisting in the striolar region of the utricle at P0 (Fig. S2). Pax2-Cre on the other hand results in the complete loss of Fgf8 mRNA throughout the utricle (Fig. S2); however, Pax2-Cre, Fgf8 CKO mice die at birth. Based upon CKO phenotypes, Fgf8 is not required for formation of the calyceal synapses that surround Type-I hair cells because these structures remain intact in extrastriolar regions of the utricle lacking Fgf8 mRNA in Atoh1-Cre; Fgf8 CKOs at P14 (Fig. 8A,B,I, Table S3). The number and distribution of Type-I hair cells as determined using oncomodulin to label Type-I hair cells in the striolar region (Fig. 8C,D,J, Table S3) and osteopontin to label Type-I hair cells in the extrastriolar regions (Fig. 8E,F,K) is also not changed in Fgf8 CKOs. Finally, in the developing neocortex, Fgf8 acts in opposition to the transcription factor Emx2 to regulate cortical patterning (Fukuchi-Shimogori and Grove, 2003; Muzio and Mallamaci, 2003; O'Leary and Nakagawa, 2002; O'Leary and Sahara, 2008). Although Emx2 also functions in the utricle to regulate stereociliary bundle orientation and formation of the line of polarity reversal (Jiang et al., 2017), these features are not disrupted by the loss of Fgf8 as there are no changes in hair cell polarity (Fig. 8G,H) or the relative position of the striolar region in Pax2-Cre; Fgf8 CKOs (Fig. 8L).
Fig. 8.
Fgf8 conditional knockout utricles are phenotypically normal. (A,B) Whole-mount immunolabeling using βIII-tubulin shows no changes in the average frequency of calyceal synapses in extrastriolar regions of Atoh1-Cre; Fgf8 CKOs (B) and littermate control utricles (A) when evaluated at P14. (C,D) There are no differences in the distribution of oncomodulin-positive Type-I hair cells in the striolar region of Pax2-Cre; Fgf8 CKO (D) compared with littermate control utricles (C) at E18.5. Dashed lines indicate the boundaries of the striolar region. (E-F′) There are no differences in the distribution of osteopontin-positive Type-I hair cells in the extrastriolar regions of Pax2-Cre; Fgf8 CKO (F) compared with littermate control utricles (E) at E18.5. E′, F′ show osteopontin channel only. (G,H) A line of polarity reversal (dashed line) forms normally and stereociliary bundle orientation (arrows) is undisturbed in Pax2-Cre; Fgf8 CKOs (H) similar to littermate controls (G). (I-K) The frequency of calyceal synapses (I) and number of Type-I hair cells expressing oncomodulin (J) and osteopontin (K) at E18.5 is not changed between littermate control (CTL) and Fgf8 CKO utricles. (L) The position of the striolar region relative to the lateral border of the utricle is normal in the Pax2-Cre; Fgf8 CKO utricle based upon measures of the lateral extrastriolar:striolar width ratio (LSWR). Data are mean±s.d. Scale bars: 10 µm in A,B,G,H; 50 µm C-F.
DISCUSSION
Using CreER-mediated genetic labeling, we demonstrate temporally regulated, transient expression of Fgf8 in auditory and vestibular neurons before E11.5 and sustained expression in Type-I vestibular hair cells at all subsequent developmental stages investigated (E11.5 to P2). We anticipate that the engineered Fgf8mcm allele will be a valuable experimental resource that provides genetic access to this unique population of hair cells. This should enable further studies of vestibular development and regeneration, and create new approaches for understanding Type-I hair cell function through the application of genetically encoded tools for manipulating their function. Fgf8mcm may also be applied for studies of cochlear inner hair cell function, in which selective Fgf8 expression has been well documented (Jacques et al., 2007). The Fgf8mcm allelic design also provides distinct advantages over alternative strategies for placing Cre recombinase at the Fgf8 locus. First, insertion of the IRES:mER-Cre-mER expression cassette into the 3′UTR of Fgf8 does not perturb its expression. This is significant because FGF8 signaling is dose dependent, as evidenced by severe developmental defects and lethality of hypomorphs (Frank et al., 2002; Meyers et al., 1998). Second, the tamoxifen-dependent CreER activity in the Fgf8mcm line allows recombination to be restricted to hair cells and avoids recombination in those neurons that transiently express Fgf8. Finally, for developmental studies, the early onset of Fgf8 expression, and hence CreER activity, will allow many target genes to be deleted before their transcription is initiated in hair cells.
Cre recombinase activity from the engineered Fgf8mcm allele is detected in the utricular maculae at the earliest stages of hair cell differentiation, beginning shortly after terminal mitosis, and indicates that cell-type specification occurs earlier than has been documented using other criteria. Given the permanence of genetic labeling, it appears to be unlikely that Fgf8 is expressed in Type-II hair cells or their precursors during this time. Consistent with this, tdTomato-positive hair cells at P14 express the Type-I marker osteopontin, rarely colocalize with the Type-II biased marker Anxa4, and are always contacted by calyceal synaptic endings. Perhaps more striking than this cell specificity is the observation that Fgf8mcm can be activated in newly born hair cells at E11.5, a time when the initial cohort of hair cells is completing mitosis and beginning to differentiate. Together these observations suggest that vestibular hair cell identity is defined at the earliest stages of their development and refutes alternative models in which Type-I and Type-II hair cells are derived postnatally from a shared hair cell precursor.
It is more difficult to reconcile the early Type-I restricted expression of Fgf8 with the potential role that Sox2 may have in defining vestibular hair cell identity after birth (Lu et al., 2019). The vestibular sensory epithelia are unique because expression of the progenitor cell marker Sox2 is maintained throughout postnatal development in vestibular supporting cells and Type-II hair cells. Although Sox2 is transiently expressed in Type-I hair cells, it is not downregulated until later stages, when these two cell types are readily distinguished using morphological and synaptic criteria. Based upon this correlation, Sox2 downregulation after birth in a subset of cells has been suggested to be the mechanism by which the two hair cell types are established. Consistent with this potential mechanism, the postnatal deletion of Sox2 results in an increase in the ratio of Type-I to Type-II hair cells (Lu et al., 2019). A parsimonious synthesis of the findings from these studies would be that Fgf8 expression distinguishes between committed and non-committed states of cell fate divergence, and that only Fgf8-expressing cells are restricted to the Type-I identity. In contrast, Fgf8-negative cells may retain the potential to differentiate as either cell type, possibly doing so during postnatal development through a switching mechanism that is Sox2-dependent. It remains to be shown whether Fgf8 expression is activated following Sox2 gene deletion at postnatal stages or whether the two cohorts of Type-I hair cells that would be generated through these parallel paths might differ from each other.
Loss-of-function experiments using Fgf8 CKOs generated with Pax2-Cre or Atoh1-Cre fail to demonstrate a requirement for FGF8 function during vestibular hair cell development. This is likely owing to genetic redundancy with other FGFs that preserve signaling in the absence of FGF8 and, consistent with this possibility, Fgf3 and Fgf10 are expressed during vestibular development in the mouse (Hossain and Morest, 2000; Pirvola et al., 2000; Ohuchi et al., 1997; Pauley et al., 2003). Furthermore, genetic redundancy in FGF signaling has been well described in cochlear development (Wright and Mansour, 2003; Mansour et al., 2013). One event where FGF8 functions independently is during pillar cell differentiation: the absence of Fgf8 expression results in fewer pillar cells. FGF8 signals through FGFR3 to regulate pillar cell development in this context (Jacques et al., 2007). However, FGF8 must signal through different receptors in the utricle because FGFR3 is absent from the developing vestibule based upon genetic labeling experiments using Fgfr3-iCre mice (Fig. S3 and Anttonen et al., 2012). In contrast, Fgfr1 and Fgfr2 are expressed in vestibular hair cells of mice and rats, and these receptors are activated by FGF8 (Saffer et al., 1996; Burns et al., 2015; Pirvola et al., 2000). Moreover, the number of vestibular hair cells is reduced in Fgfr1 CKO mice (Ono et al., 2014), and targeted deletion of Fgfr2b results in severe dysgenesis of the cochleovestibular labyrinth (Pirvola et al., 2000), demonstrating their necessity for vestibular morphogenesis and development. Together, these findings suggest that multiple FGFs are released by Type-I vestibular hair cells and may be received by FGFR1 and/or FGFR2 rather than FGFR3, as observed for FGF8-dependent pillar cell development in the cochlea.
As a secreted signaling molecule, FGF8 is most likely to function through a paracrine pathway to promote local differentiation or through an autologous mechanism that reinforces Type-I differentiation. Based upon FGF8 function in the cochlea, one role for FGF-signaling in vestibular sensory development may be to promote supporting cell differentiation. Unfortunately, the lack of molecular markers and evidence for supporting cell diversity in the utricle makes it difficult to test this hypothesis (Shim et al., 2005; Jacques et al., 2007). An exciting alternative is that FGF signaling directs afferent neuron differentiation and formation of the calyceal synapse that is unique to the Type-1 hair cells. The existence of a hair cell signal that directs post-synaptic specialization is suggested by the morphology of dimorphic afferent neurons, which contact both Type-I and Type-II hair cells but only form calyceal synaptic structures around the Type-I cell. Notably, FGF7 and FGF22 have been shown to promote the differentiation of excitatory and inhibitory synapses as presynaptic organizers (Terauchi et al., 2010), consistent with a potential role for FGF signaling in vestibular synaptic development. Finally, in the developing neocortex Fgf8 regulates cortical patterning through a process called arealization, in which FGF8 expression in an anterior domain opposes the posterior expression of the transcription factor Emx2. We originally hypothesized that a similar process regulated Emx2 expression in the utricle and thus determined the position of the line of polarity reversal that divides hair cells with opposite stereociliary bundle orientations (Deans, 2013; Jiang et al., 2017). However, the observed Fgf8 expression throughout the utricle, including the Emx2-expressing domain in the lateral extrastriolar region, is inconsistent with this model. Moreover, the position of the line of polarity reversal is not altered in Fgf8 mutants.
A remarkable characteristic of utricular hair cells in the mouse is their potential to regenerate at perinatal stages of development. Curiously, the majority of vestibular hair cells regenerated in the mouse are of the Type-II identity (Golub, 2012; Bucks, 2017). Understanding and directing this process to occur at later stages, and generating the correct proportion of hair cell types, is essential if regeneration strategies are to successfully replace lost hair cells. Although the contribution of FGF8 towards identity specification is not known, Fgf8 expression in Type-I hair cells demonstrates that identity is established earlier than expected and shortly after terminal mitosis. This expression pattern further suggests that FGF or other morphogenic signaling pathways might be manipulated during the course of regeneration to direct cell fate. Thus, the Fgf8mcm line may catalyze directed regeneration strategies, while the distinct cell-specific expression makes this line a valuable resource for studying hair cell development and function.
MATERIALS AND METHODS
Design and construction of the Fgf8mcm allele
Fgf8mcm mice were generated by inserting an IRES sequence (Jang and Wimmer, 1990; Kaminski et al., 1990) upstream of the mER:Cre:mER cDNA encoding a fusion protein with two copies of the mouse estrogen receptor (mER) flanking Cre recombinase, and a Neomycin-resistance (NeoR) selection cassette flanked by Frt recombination sites (Verrou et al., 1999). This assembly was inserted into an Eag1 site in the Fgf8 gene located 36 bp 3′ of the Fgf8 stop codon and 5′ to the endogenous polyadenylation sequence. The modified genomic fragment was shuttled into a plasmid backbone flanked by thymidine kinase genes. The linearized targeting vector was electroporated into mouse embryonic stem cells and subjected to selection with G418 and gancyclovir. Positive clones were identified by digesting genomic DNA, Southern blotting, and hybridizing with a 3′ flanking probe, and targeted clones were confirmed to be correct by additional analyses using internal genomic probes and probes within the targeting vector. Following successful gene targeting the NeoR selection cassette was removed by Flp-mediated recombination of the flanking Frt sites. Genotyping of the wild-type and targeted alleles was performed by PCR using the following primers: Fgf8 exon 5 sense 5′ GAGGTGCACTTCATGAAGCGC; Fgf8 antisense, 5′ ATTTCAGGAGAACAGACCAGAG; sense for Frt site in mER:Cre:mER, 5′ ATACTATCTAGAGAATAGGAACTTCG.
Mouse strains and husbandry
For tamoxifen-inducible genetic labeling, Fgf8mcm/+ males were crossed with ROSAtdTom(Ai9)/tdTom(Ai9) reporter mice (Madisen et al., 2010), and CreER was activated with a single intraperitoneal (IP) injection of tamoxifen (37.5-75 mg/kg, Sigma-Aldrich, #T5648) solubilized in corn oil and delivered to the timed-pregnant dam at embryonic stages ranging from E9.5 to E17.5 as indicated. When embryonic inductions were followed by postnatal collections, β-estradiol (75 μg/kg, Sigma-Aldrich, #E8875) was co-injected to help alleviate the anti-estrogen effects of tamoxifen. In some cases, where a natural birth did not occur on the 19th day of gestation, pups were delivered by Cesarean-section and cross fostered. Postnatal activation was carried out using a single IP injection of tamoxifen (75 mg/kg) at P2. For Fgf8 mutant analysis, Fgf8 CKOs were generated by crossing Fgf8LoxP/LoxP female mice with Pax2-Cre-positive or Atoh1-Cre-positive Fgf8+/− males. Cre-positive progeny were analyzed, with Fgf8LoxP/− mice serving as CKOs and Fgf8LoxP/wt as littermate controls. For these studies, Pax2-Cre mice (Ohyama and Groves, 2004) were provided by A. Groves (Baylor College of Medicine, Houston, TX, USA), Atoh1-Cre mice (Matei et al., 2005) and tdTom(Ai9) reporter mice were purchased from The Jackson Laboratory (Strains #011104 and #007909). Fgf8 KO and Fgf8Loxp/Loxp lines have been previously described (Park et al., 2006). For timed breeding and tissue staging, noon on the day in which a vaginal plug was detected was considered E0.5 and the day of birth was considered P0. Mice of either sex were used interchangeably for all experimental analyses. Mouse colonies were maintained at the University of Utah under Institutional Animal Care and Use Committee approved guidelines, and genotyped using allele-specific PCR reactions.
Immunofluorescent labeling and imaging
Inner ear tissues were fixed using a 4% paraformaldehyde (PFA) solution prepared in 67 mM Sorensons’ phosphate buffer (pH 7.4) for 2 h on ice. For whole-mount immunofluorescent labeling, inner ears were micro-dissected to isolate the cochlea or utricular maculae and detergent-permeabilized using blocking solution [5% normal donkey serum, 1% bovine serum albumin (BSA) in PBS] supplemented with 0.5% Triton X-100. Primary antibodies and Phalloidin Alexa Fluor 488 (Invitrogen, A12379, 1:1500) were diluted in blocking solution supplemented with 0.1% Tween-20 and incubated overnight at 4°C. For osteopontin and Anxa4 immunolabeling, tissue was fixed in 4% PFA prepared in PBS for 1 h at room temperature, and 10% donkey serum in PBS was used as a blocking solution as previously described (McInturff et al., 2018). Tissue was washed thoroughly using PBS with 0.05% Tween-20 and incubated with blocking solution supplemented with 0.1% Tween-20 and containing species-specific Alexa-conjugated secondary antibodies (Invitrogen: A11122, A21202, A31571; The Jackson Laboratory: 715-605-150, 705-165-147, 711-605-152; all diluted 1:1500). Tissue was washed thoroughly with PBS/0.05% Tween-20 and mounted for fluorescence microscopy using Prolong Gold Antifade Mountant (Thermo Fisher Scientific, #P10144). For immunolabeling of cryosections, tissue was fixed as described above using 4% PFA, cryoprotected by passing through a post-fixation sucrose gradient, and frozen in Neg50 (Richard Allan Scientific) before sectioning at 20 μm using a Leica CM3050 cryostat. Immunolabeling of tissue sections on SuperFrost Plus microscopy slides (Thermo Fisher Scientific, #12-550-15) was completed as described for whole-mount tissues except that Tween-20 was excluded from blocking and wash solutions. Fluorescent images were acquired using structured illumination microscopy using a Zeiss Axio Imager M.2 with ApoTome.2 attachment and an Axiocam 506 monochrome camera. Images were processed using Zeiss Zen software followed by figure preparation with Adobe Photoshop and Adobe Illustrator. 3D reconstructions of hair cells were completed using Fluorender software provided by University of Utah Scientific Computing and Imaging Institute. Quantification of hair cells or calyces, and area measurements, were facilitated by FIJI (ImageJ2). For measurements of the lateral extrastriolar:striolar width ratio the striolar region was defined by the presence of oncomodulin-positive hair cells and region boundaries were fit using the curvature tool (Adobe Illustrator) to demarcate the smallest area containing all of these cells. The boundaries of the utricular sensory epithelia were similarly demarcated based upon myosin VIIa labeling. Commercial antibodies used in this study were: goat anti-Anxa4 (R&D Systems, AF4146, 1:200), rabbit anti-βIII-tubulin (Covance, PRB-435P, 1:500), rabbit anti-dsRed to detect tdTomato (Clontech, 632496, 1:5000), mouse anti-myosin VIIa (Developmental Studies Hybridoma Bank, 138-1, deposited by D. J. Orten, 1:100), rabbit anti-myosin VIIa (Proteus Biosciences, 25-6790, 1:1000), rabbit anti-NF200 (Millipore, AB1989, 1:1500), goat anti-oncomodulin (Santa Cruz Biotechnology, sc-7466, 1:250), goat anti-osteopontin (R&D Systems, AF808, 1:200) and rat anti-tdTomato (Kerafast, EST203, 1:2000).
EdU birthdating
For EdU labeling of dividing cells, Fgf8mcm/+ males were crossed with ROSAtdTom(Ai9)/tdTom(Ai9) reporter mice as previously described and pregnant dams received three IP injections of EdU (10 μg/kg) at 2 h apart on E12.5. Stock solutions of EdU (1 μg/μl) were prepared in PBS before injection and stored at −20°C. At E12.5, the pregnant dam was subsequently injected with 75 mg/kg tamoxifen to induce Cre-mediated genetic labeling as previously described. All embryos were collected at E18.5 and fixed in 4% PFA solution prepared in 67 mM Sorensons’ phosphate buffer (pH 7.4) for 2 h on ice. Following dissection, tissue was permeabilized in PBS containing 0.5% Triton X-100 for 20 min, washed with three exchanges of PBS containing 3% BSA, and EdU was detected using the Click-iT cross-linking reaction and Alexa Fluor azide 488 dye as per the manufacturer's specifications (Thermo Fisher Scientific, C10337), with volumes adapted for a 96-well plate. Tissue was washed twice with two rapid exchanges of PBS containing 3% BSA and processed for whole-mount immunofluorescent labeling as previously described.
In situ hybridization
For whole-mount ISH, tissue was fixed using 4% PFA prepared in PBS (pH 7.4) overnight at 4°C and stored in 100% MeOH at −20°C. For whole-mount hybridization of inner ear tissues, ear capsules were dissected to expose sensory epithelia, which was subsequently rehydrated through a decreasing MeOH gradient [75%, 50%, 25% and then PBS with 0.05% Tween-20 (PBSt)]. Tissue was washed with PBSt, digested with 0.75 mg/ml Proteinase K (Ambion, #Am2546) for 25 min at room temperature, rinsed with PBSt, and subsequently fixed in 4% PFA/0.25% glutaraldehyde for 25 min at room temperature. Tissue was pre-hybridized for 3 h in 5× SSC hybridization buffer at 65°C and then hybridized with 150 ng/ml digoxigenin-labeled anti-sense probes for 18 h at 65°C with constant rocking. Tissue received three 50-min washes in 50% formamide, 2× SSC, 1% SDS solution, followed by 1 h in ISH blocking solution [1× MABT, 10% Blocking Reagent (Roche, #11-096-176-001), 2% and heat inactivated sheep serum]. Alkaline phosphatase-conjugated anti-digoxigenin antibodies (Roche, #11-093-274-910) were diluted 1:2500 in ISH blocking solution and rocked overnight at 4°C. After antibody labeling, tissue was washed six times for 1 h in Tris buffered saline with 0.05% Tween-20 (TBST) solution followed by alkaline phosphatase detection using BM Purple substrate (Roche, 11-442-074-001). After detection, tissues were post-fixed in 4% PFA/0.25% glutaraldehyde, cleared through an increasing MeOH gradient and mounted onto slides using Sorensons’ phosphate buffer (pH 7.4), 31% glycerol, and 6.25% polyvinyl alcohol for imaging using the Zeiss Axio Imager M.2 and Axiocam 105 color camera. Fgf8 mRNA distribution in wild-type mice and CKO mice was detected using an anti-sense Fgf8 probe specific to Fgf8 exon 5 which is deleted in CKOs, and was provided by S. Mansour (University of Utah).
Supplementary Material
Acknowledgements
We thank Suzanne Mansour and Lisa Urness for facilitating the exchange of mouse lines and ISH probe templates, and Orvelin Roman Jr and Sarah Siddoway for animal care and genotyping.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.M.R., M.R.D.; Methodology: E.M.R.; Formal analysis: E.M.R.; Investigation: E.M.R., M.R.D.; Resources: A.M.M.; Writing - original draft: E.M.R.; Writing - review & editing: A.M.M., M.R.D.; Visualization: E.M.R.; Supervision: M.R.D.; Project administration: M.R.D.; Funding acquisition: M.R.D.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (NIH) (R01DC013066 to M.R.D.), and Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH (T32HD007491 to E.M.R.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.192849.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.192849.reviewer-comments.pdf
References
- Anttonen T., Kirjavainen A., Belevich I., Laos M., Richardson W. D., Jokitalo E., Brakebusch C. and Pirvola U. (2012). Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci. Rep. 2, 978 10.1038/srep00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet A. M., Moon A. M., Arenkiel B. R. and Capecchi M. R. (2004). The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev. Biol. 273, 361-372. 10.1016/j.ydbio.2004.06.012 [DOI] [PubMed] [Google Scholar]
- Burns J. C., On D., Baker W., Collado M. S. and Corwin J. T. (2012). Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. J Assoc. Res. Otolaryngol. 13, 609-627. 10.1007/s10162-012-0337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Kelly M. C., Hoa M., Morell R. J. and Kelley M. W. (2015). Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat. Commun. 6, 8557 10.1038/ncomms9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks S. A., Cox B. C., Vlosich B. A., Manning J. P., Nguyen T. B. and Stone J. S. (2017). Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. eLife6, e18128. 10.7554/eLife.18128 [DOI] [PMC free article] [PubMed]
- Crespo-Enriquez I., Partanen J., Martinez S. and Echevarria D. (2012). Fgf8-related secondary organizers exert different polarizing planar instructions along the mouse anterior neural tube. PLoS ONE 7, e39977 10.1371/journal.pone.0039977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley P. H. and Martin G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439-451. [DOI] [PubMed] [Google Scholar]
- Deans M. R. (2013). A balance of form and function: planar polarity and development of the vestibular maculae. Semin. Cell Dev. Biol. 24, 490-498. 10.1016/j.semcdb.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock R. A. and Songer J. E. (2011). Vestibular hair cells and afferents: two channels for head motion signals. Annu. Rev. Neurosci. 34, 501-534. 10.1146/annurev-neuro-061010-113710 [DOI] [PubMed] [Google Scholar]
- Firnberg N. and Neubüser A. (2002). FGF signaling regulates expression of Tbx2, Erm, Pea3, and Pax3 in the early nasal region. Dev. Biol. 247, 237-250. 10.1006/dbio.2002.0696 [DOI] [PubMed] [Google Scholar]
- Frank D. U., Fotheringham L. K., Brewer J. A., Muglia L. J., Tristani-Firouzi M., Capecchi M. R. and Moon A. M. (2002). An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 129, 4591-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T. and Grove E. A. (2001). Neocortex patterning by the secreted signaling molecule FGF8. Science 294, 1071-1074. 10.1126/science.1064252 [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T. and Grove E. A. (2003). Emx2 patterns the neocortex by regulating FGF positional signaling. Nat. Neurosci. 6, 825-831. 10.1038/nn1093 [DOI] [PubMed] [Google Scholar]
- Gao B., Ajima R., Yang W., Li C., Song H., Anderson M. J., Liu R. R., Lewandoski M. B., Yamaguchi T. P. and Yang Y. (2018). Coordinated directional outgrowth and pattern formation by integration of Wnt5a and Fgf signaling in planar cell polarity. Development 145, dev163824 10.1242/dev.163824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. N., Compagnucci C., Hu D., Fish J., Klein O., Marcucio R. and Depew M. J. (2013). Fgf8 dosage determines midfacial integration and polarity within the nasal and optic capsules. Dev. Biol. 374, 185-197. 10.1016/j.ydbio.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub J. S., Tong L., Ngyuen T. B., Hume C. R., Palmiter R. D., Rubel E. W. and Stone J. S. (2012). Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci.32, 15093-15105. 10.1523/JNEUROSCI.1709-12.2012 [DOI] [PMC free article] [PubMed]
- Hoffman L. F., Choy K. R., Sultemeier D. R. and Simmons D. D. (2018). Oncomodulin expression reveals new insights into the cellular organization of the murine utricle striola. J. Assoc. Res. Otolaryngol. 19, 33-51. 10.1007/s10162-017-0652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley M., Rhodes C., Kneebone A., Herde M. K., Fleming M. and Steel K. P. (2010). Emx2 and early hair cell development in the mouse inner ear. Dev. Biol. 340, 547-556. 10.1016/j.ydbio.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain W. A. and Morest D. K. (2000). Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. J. Neurosci. Res. 62, 40-55. [DOI] [PubMed] [Google Scholar]
- Hume C. R., Bratt D. L. and Oesterle E. C. (2007). Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr. Patterns 7, 798-807. 10.1016/j.modgep.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques B. E., Montcouquiol M. E., Layman E. M., Lewandoski M. and Kelley M. W. (2007). Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134, 3021-3029. 10.1242/dev.02874 [DOI] [PubMed] [Google Scholar]
- Jang S. K. and Wimmer E. (1990). Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 4, 1560-1572. 10.1101/gad.4.9.1560 [DOI] [PubMed] [Google Scholar]
- Jiang T., Kindt K. and Wu D. K. (2017). Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. eLife 6, e23661 10.7554/eLife.23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A., Howell M. T. and Jackson R. J. (1990). Initiation of encephalomyocarditis virus RNA translation: the authentic initiation site is not selected by a scanning mechanism. EMBO J. 9, 3753-3759. 10.1002/j.1460-2075.1990.tb07588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian E. J., Appler J. L. and Goodrich L. V. (2007). Auditory neurons make stereotyped wiring decisions before maturation of their targets. J. Neurosci. 27, 14078-14088. 10.1523/JNEUROSCI.3765-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Danielian P. S., Fritzsch B. and Mcmahon A. P. (1997). Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 124, 959-969. [DOI] [PubMed] [Google Scholar]
- Lewandoski M., Sun X. and Martin G. R. (2000). Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 26, 460-463. 10.1038/82609 [DOI] [PubMed] [Google Scholar]
- Lu J., Hu L., Ye B., Hu H., Tao Y., Shu Y., Chiang H., Borse V., Xiang M., Wu H. et al. (2019). Increased Type I and decreased Type II hair cells after deletion of Sox2 in the developing mouse utricle. Neuroscience 422, 146-160. 10.1016/j.neuroscience.2019.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee T. L., Hammond B. P., Arenkiel B. R., Francis L., Frank D. U. and Moon A. M. (2003). Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development 130, 6361-6374. 10.1242/dev.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci.13, 133-140 10.1038/nn.2467 [DOI] [PMC free article] [PubMed]
- Mansour S. L., Li C. and Urness L. D. (2013). Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 27, 2320-2331. 10.1101/gad.228957.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V., Pauley S., Kaing S., Rowitch D., Beisel K. W., Morris K., Feng F., Jones K., Lee J. and Fritzsch B. (2005). Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 234, 633-650. 10.1002/dvdy.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcinturff S., Burns J. C. and Kelley M. W. (2018). Characterization of spatial and temporal development of type I and type II hair cells in the mouse utricle using new cell-type-specific markers. Biol. Open 7, bio038083 10.1242/bio.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M. and Martin G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136-141. 10.1038/ng0298-136 [DOI] [PubMed] [Google Scholar]
- Moon A. M. and Capecchi M. R. (2000). Fgf8 is required for outgrowth and patterning of the limbs. Nat. Genet. 26, 455-459. 10.1038/82601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio L. and Mallamaci A. (2003). Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb. Cortex. 13, 641-647. 10.1093/cercor/13.6.641 [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Campbell S., Taylor R. R., Forge A. and Hume C. R. (2008). Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol. 9, 65-89. 10.1007/s10162-007-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H., Nakagawa T., Yamamoto A., Araga A., Ohata T., Ishimaru Y., Yoshioka H., Kuwana T., Nohno T., Yamasaki M. et al. (1997). The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 124, 2235-2244. [DOI] [PubMed] [Google Scholar]
- Ohyama T. and Groves A. K. (2004). Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38, 195-199. 10.1002/gene.20017 [DOI] [PubMed] [Google Scholar]
- O'Leary D. D. M. and Nakagawa Y. (2002). Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 12, 14-25. 10.1016/S0959-4388(02)00285-4 [DOI] [PubMed] [Google Scholar]
- O'Leary D. D. M. and Sahara S. (2008). Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 18, 90-100. 10.1016/j.conb.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Kita T., Sato S., O'Neill P., Mak S.-S., Paschaki M., Ito M., Gotoh N., Kawakami K., Sasai Y. et al. (2014). FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 10, e1004118 10.1371/journal.pgen.1004118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Xu J., Colvin J. S., Mcewen D. G., Macarthur C. A., Coulier F., Gao G. and Goldfarb M. (1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297. 10.1074/jbc.271.25.15292 [DOI] [PubMed] [Google Scholar]
- Park E. J., Ogden L. A., Talbot A., Evans S., Cai C.-L., Black B. L., Frank D. U. and Moon A. M. (2006). Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133, 2419-2433. 10.1242/dev.02367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S., Wright T. J., Pirvola U., Ornitz D., Beisel K. and Fritzsch B. (2003). Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. 227, 203-215. 10.1002/dvdy.10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B., Jensen-Smith H. C., Ludueña R. F. and Hallworth R. (2003). Selective expression of β tubulin isotypes in gerbil vestibular sensory epithelia and neurons. J. Assoc. Res. Otolaryngol. 4, 329-338. 10.1007/s10162-002-2048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Spencer-Dene B., Xing-Qun L., Kettunen P., Thesleff I., Fritzsch B., Dickson C. and Ylikoski J. (2000). FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 20, 6125-6134. 10.1523/JNEUROSCI.20-16-06125.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S., Koundakjian E. J., Quinones H., Jayasena C. S., Goodrich L. V., Johnson J. E., Segil N. and Groves A. K. (2007). Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134, 4405-4415. 10.1242/dev.009118 [DOI] [PubMed] [Google Scholar]
- Reifers F., Bohli H., Walsh E. C., Crossley P. H., Stainier D. Y. and Brand M. (1998). Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125, 2381-2395. [DOI] [PubMed] [Google Scholar]
- Rennie K. J., Ricci A. J. and Correia M. J. (1996). Electrical filtering in gerbil isolated type I semicircular canal hair cells. J. Neurophysiol. 75, 2117-2123. 10.1152/jn.1996.75.5.2117 [DOI] [PubMed] [Google Scholar]
- Roehl H. and Nüsslein-Volhard C. (2001). Zebrafish pea3 and erm are general targets of FGF8 signaling. Curr. Biol. 11, 503-507. 10.1016/S0960-9822(01)00143-9 [DOI] [PubMed] [Google Scholar]
- Ruben R. J. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 220 Suppl., 1-44. [PubMed] [Google Scholar]
- Rüsch A., Lysakowski A. and Eatock R. A. (1998). Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J. Neurosci. 18, 7487-7501. 10.1523/JNEUROSCI.18-18-07487.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer L. D., Gu R. and Corwin J. T. (1996). An RT-PCR analysis of mRNA for growth factor receptors in damaged and control sensory epithelia of rat utricles. Hear. Res. 94, 14-23. 10.1016/0378-5955(95)00228-6 [DOI] [PubMed] [Google Scholar]
- Sayyid Z. N., Wang T., Chen L., Jones S. M. and Cheng A. G. (2019). Atoh1 directs regeneration and functional recovery of the mature mouse vestibular system. Cell Rep. 28, 312-324.e4. 10.1016/j.celrep.2019.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K., Minowada G., Coling D. E. and Martin G. R. (2005). Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell 8, 553-564. 10.1016/j.devcel.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Simmons D. D., Tong B., Schrader A. D. and Hornak A. J. (2010). Oncomodulin identifies different hair cell types in the mammalian inner ear. J. Comp. Neurol. 518, 3785-3802. 10.1002/cne.22424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Meyers E. N., Lewandoski M. and Martin G. R. (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834-1846. 10.1101/gad.13.14.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi A., Johnson-Venkatesh E. M., Toth A. B., Javed D., Sutton M. A. and Umemori H. (2010). Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 465, 783-787. 10.1038/nature09041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrou C., Zhang Y., Zürn C., Schamel W. W. A. and Reth M. (1999). Comparison of the tamoxifen regulated chimeric Cre recombinases MerCreMer and CreMer. Biol. Chem. 380, 1435-1438. 10.1515/BC.1999.184 [DOI] [PubMed] [Google Scholar]
- Warchol M. E., Massoodnia R., Pujol R., Cox B. C. and Stone J. S. (2019). Development of hair cell phenotype and calyx nerve terminals in the neonatal mouse utricle. J. Comp. Neurol. 527, 1913-1928. 10.1002/cne.24658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. J. and Mansour S. L. (2003). Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130, 3379-3390. 10.1242/dev.00555 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Riesterer C., Ayrall A.-M., Sablitzky F., Littlewood T. D. and Reth M. (1996). Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 24, 543-548. 10.1093/nar/24.4.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.