Supplemental digital content is available in the text.
KEY WORDS: COVID-19, invasive mechanic ventilation, critically ill, APACHE II, PSI
Abstract
Background
Invasive mechanical ventilation (IMV) is a lifesaving strategy for critically ill patients with coronavirus disease 2019 (COVID-19). We aim to report the case series of critical patients receiving IMV in Wuhan and to discuss the timing of IMV in these patients.
Methods
Data of 657 patients admitted to emergency intensive care unit of Zhongnan Hospital and isolated isolation wards of Wuhan Union Hospital from January 1 to March 10, 2020, were retrospectively reviewed. All medical records of 40 COVID-19 patients who required IMV were collected at different time points, including baseline (at admission), before receiving IMV, and before death or hospital discharge.
Results
Among 40 COVID-19 patients with IMV, 31 died, and 9 survived and was discharged. The median age was 70 years (interquartile range [IQR], 62–76 years), and nonsurvivors were older than survivors. The median period from the noninvasive mechanic ventilation (NIV) or high-flow nasal cannula oxygen therapy (HFNC) to intubation was 7 hours (IQR, 2–42 hours) in IMV survivors and 54 hours (IQR, 28–143 hours) in IMV nonsurvivors. We observed that, when the time interval from NIV/HFNC to intubation was less than 50 hours (about 2 calendar days), together with Acute Physiology and Chronic Health Evaluation II (APACHE II) score of less than 10 or pneumonia severity index (PSI) score of less than 100, mortality can be reduced to 60% or less. Prolonged interval from NIV/HFNC to intubation and high levels of APACHE II and PSI before intubation were associated with higher mortality in critically ill patients. Multiple organ damage was common among these nonsurvivors in the course of treatment.
Conclusion
Early initial intubation after NIV/HFNC might have a beneficial effect in reducing mortality for critically ill patients meeting IMV indication. Considering APACHE II and PSI scores might help physicians in decision making about timing of intubation for curbing subsequent mortality.
Level of evidence
Therapeutic, level V.
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was introduced, causing the clustered cases of coronavirus disease 2019 (COVID-19) after the outbreak of pneumonia in Wuhan, one of the largest cities in the middle of China. Coronavirus disease 2019, characterized by a range of respiratory symptoms, including fever, cough, and moderate to severe pneumonia in most cases, was spread from Wuhan to other parts of China.1–4 Coronavirus disease 2019 has become a global pandemic with more than 400,000 people infected.5 Patients with mild or moderate pneumonia can rapidly progress to severe or critical conditions in some cases. As of March, 18, 2020, around 5% patients became critically ill and 4% died.6 The large number of critical patients presents challenges for treatment and finding novel therapies to curb mortality.
Mechanical ventilation (MV) is the main supportive therapy for critically ill patients with SARS-CoV-2 infection.2 A recent study has reported that a proportion of 6.1% COVID-19 patients required MV; among them, 2.3% is considered intubation invasive mechanical ventilation (IMV).7 In another study of 52 critically ill patients, 22 (42.3%) required IMV support, and 19 (86.4%) of them died.3 To reduce mortality, it is critical to explore the optimal time for intubation condition in these critically ill patients.
Here, we retrospectively reviewed 40 confirmed COVID-19 patients who required IMV during hospitalization. We aimed to describe and provide a preliminary investigation on clinical interventions and intubation condition to improve understanding and clinical practice.
PATIENTS AND METHODS
Patients
The retrospective multicenter study enrolled 40 laboratory-confirmed COVID-19 patients who were critically ill and required IMV for hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS). All patients were admitted to emergency intensive care unit in Zhongnan Hospital of Wuhan University (China) and isolated intensive care unit of Wuhan Union Hospital (China) from January 1 to March 10, 2020, and met the definition of SARS-CoV-2–induced pneumonia, according to World Health Organization interim guidance.8 The patients who were in hospital as of March 10, 2020, and coinfected with influenza, syncytial, coxsackie viruses, and adenovirus were excluded from this study. This study was approved by the Ethics Committee of Zhongnan Hospital, Wuhan University, and in accordance with the 1964 Declaration of Helsinki and its later amendments. The requirement for informed consent was waived because of the urgent need to collect data on this emerging pathogen.
Definitions
We used the Berlin definition for ARDS, defined as the development of acute, bilateral pulmonary infiltrates (as determined by consensus of two trained physician reviewers) and hypoxemia (PaO2/FIO2, ≤300 mm Hg) not primarily due to heart failure or volume overload.9 Endotracheal intubation was considered in cases of a patient failed to maintain a PaO2/FIO2 level of >150 mm Hg and met conditions of respiratory rate of ≥30 breaths/min and SPO2 level of <90% despite optimal standard oxygen therapy or noninvasive ventilation (NIV).10 Once a patient fulfilled these diagnostic criteria, the final decision for intubation was made by the attending physician with consent from patients' family members. Sepsis was diagnosed according to the 2016 Third International Consensus Definition for Sepsis.4 Acute kidney injury was defined according to the KDIGO clinical practice guidelines.11 Acute cardiac injury (ACI) was diagnosed if serum levels of cardiac biomarkers (e.g., high-sensitive cardiac troponin I) were above the 99th percentile upper reference limit or if new abnormalities were shown in electrocardiography and echocardiography.4 Acute liver injury was considered as a patient presented with symptoms suggestive of liver disorder (nausea, vomiting abdominal pain, and/or jaundice) and had an increase of more than two times the upper limit of the normal range in alanine aminotransferase (ALT).12 Secondary infection was diagnosed when patients showed clinical symptoms or signs of pneumonia or bacteraemia and a positive culture of a new pathogen was obtained from lower respiratory tract specimens (qualified sputum, endotracheal aspirate, or bronchoalveolar lavage fluid) or blood samples after admission.4
According to the way of oxygen assistant, patients were classified as noninvasive ventilation (NIV) patients, high-flow nasal cannula oxygen therapy (HFNC) patients, IMV patients, and extracorporeal membrane oxygenation (ECMO) patients according to whether how the intubation was performed during hospitalization.
Data Collection
All medical record information including epidemiological, demographic, underlying comorbidities, physical examinations, laboratory data, and respiratory and physiologic parameters while receiving MV was obtained. Data were collected at different time points, including baseline (at admission), before receiving NIV/HFNC/IMV/ECMO, or hospital discharge/death. Subjects' severity of illness was evaluated at the same day of these time points by using Acute Physiology and Chronic Health Evaluation II and pneumonia severity index (PSI) score. Causes of death in the subjects were recorded.
Statistical Analysis
We divided the cohort into IMV survivor and IMV nonsurvivor group. We summarized continuous variables as medians with interquartile range (IQR) and categorical variables with n (%). Since our study is a case series study, statistical comparison is not necessary. The association of duration between NIMV and IMV, PSI, and Acute Physiology and Chronic Health Evaluation II with subsequent mortality was plotted with smoothing spline by Generalized Additive Models. All analyses were performed by using R software (version 3.6.1, The R Project for Statistical Computing; http://www.r-project.org) and EmpowerStats (X&Y Solutions, Inc., Boston, MA; http://www.empowerstats.com).
RESULTS
Clinical Features of COVID-19 Patients With IMV at Hospital Admission
All included 657 COVID-19–diagnosed patients presenting to emergency intensive care unit of Zhongnan Hospital of Wuhan University and isolation wards of Wuhan Union Hospital between January 1 to March 10, 2020, were retrospectively reviewed. Among them, 40 patients who required IMV during hospitalization were incorporated into this study. Baseline characteristics of the enrolled patients were presented in Table 1. Among these 40 patients, 31 (77.5%) died during the course of the treatment, defined as IMV nonsurvivors. Nine (22.5%) were hospital discharged, defined as IMV survivors. The median age of the 40 patients was 70 years (IQR, 62–76 years). The age of the IMV nonsurvivors (71 years; IQR, 66–78 years) was older than that of the IMV survivors (50 years; IQR, 47–62; p < 0.001) with slightly more female patients (56% vs. 19%). The three most frequent comorbidities were hypertension (42%), followed by coronary heart disease (25%) and diabetes (18%). The median period from the onset of symptom to intubation was 14 days (IQR, 10–15 days) in IMV survivors and 12 days (IQR, 8–17 days) in IMV nonsurvivors. Six IMV survivors (67%) and 19 IMV nonsurvivors (61%) showed an APACHE II score of between 8 and 15 at admission. The median PSI score was 78 (IQR, 75–95) in IMV survivors and 98 (IQR, 85–118) in IMV nonsurvivors. Higher levels of blood urea nitrogen (p = 0.033) and creatinine (p = 0.061) were observed in the IMV nonsurvivor group at baseline. Other laboratory findings at admission, including white blood cell, neutrophil, lymphocyte, prothrombin time, D-dimer, lactate dehydrogenase (LDH), ALT, C-reactive protein (CRP), creatine kinase MB (CK-MB), and high-sensitivity troponin I were comparable between IMV survivor and IMV nonsurvivor (Table 1). More than half of the patients exhibited decreased lymphocyte count and increased D-dimer, LDH, CRP, and CK-MB at the time of admission.
TABLE 1.
Clinical Characteristics of COVID-19 Patients with IMV at Admission
| Total (n = 40) |
IMV Survivor (n = 9) |
IMV Nonsurvivor (n = 31) |
p | |
|---|---|---|---|---|
| Age, median (IQR), y | 70 (62–74) | 50 (47–62) | 70 (66–77) | <0.001 |
| Sex | 0.083 | |||
| Female | 11 (28%) | 5 (56%) | 6 (19%) | |
| Male | 29 (72%) | 4 (44%) | 25 (81%) | |
| Comorbidities | ||||
| Hypertension | 17 (42%) | 3 (33%) | 14 (45%) | 0.707 |
| Diabetes | 7 (18%) | 2 (22%) | 5 (16%) | 0.645 |
| Coronary heart disease | 10 (25%) | 1 (11%) | 9 (29%) | 0.404 |
| Chronic pulmonary disease | 1 (2%) | 0 (0%) | 1 (3%) | >0.99 |
| Chronic kidney disease | 2 (5%) | 0 (0%) | 2 (6%) | >0.99 |
| Cerebrovascular disease | 1 (2%) | 0 (0%) | 1 (3%) | >0.99 |
| Malignancy | 2 (5%) | 0 (0%) | 2 (6%) | >0.99 |
| Duration from symptoms to hospital admission, median (IQR), d | 8 (5–10) | 10 (8–10) | 7 (4–10) | 0.08 |
| Duration from symptoms to intubation, day, median (IQR) | 12 (9–17) | 14 (10–15) | 12 (8–17) | 0.649 |
| ECMO | 4 (10%) | 3 (33%) | 1 (3%) | 0.03 |
| APACHE II, median (IQR) | 8 (8–11) | 8 (8–10) | 9 (8–12) | 0.922 |
| ≤7 | 10 (25%) | 2 (22%) | 8 (26%) | |
| 8–15 | 25 (63%) | 6 (67%) | 19 (61%) | |
| ≥16 | 5 (13%) | 1 (11%) | 4 (13%) | |
| PSI score, median (IQR) | 92 (80–117) | 78 (75–95) | 98 (85–118) | 0.06 |
| ≤70 | 3 (8%) | 2 (22%) | 1 (3%) | |
| 71–90 | 16 (40%) | 4 (44%) | 12 (39%) | |
| 91–130 | 14 (35%) | 2 (22%) | 12 (39%) | |
| ≥131 | 7 (18%) | 1 (11%) | 6 (19%) | |
| Empiric systemic glucocorticoid therapy | 33 (82%) | 7 (78%) | 26 (84%) | 0.645 |
| Outcome/complication | ||||
| Sepsis | 27 (68%) | 3 (33%) | 24 (77%) | 0.038 |
| AKI | 24 (60%) | 0 (0%) | 24 (77%) | <0.001 |
| ACI | 17 (43%) | 1 (11%) | 16 (52%) | <0.001 |
| ALI | 20 (50%) | 1 (11%) | 19 (61%) | 0.02 |
| Second infection | 24 (60%) | 0 (0%) | 24 (77%) | <0.001 |
| Laboratory findings at admission, median (IQR) | ||||
| WBC count, ×109/L | 7.30 (4.61–11.42) | 5.62 (2.93–9.31) | 7.30 (5.11–11.42) | 0.421 |
| >9.5 | 12 (30%) | 2 (22%) | 10 (32%) | |
| Neutrophil count, ×109/L | 6.48 (3.24–9.75) | 4.52 (1.88–8.76) | 6.48 (3.88–9.75) | 0.296 |
| >6.3 | 20 (50%) | 4 (44%) | 16 (52%) | |
| Lymphocyte count, ×109/L | 0.58 (0.36–0.87) | 0.60 (0.40–0.81) | 0.58 (0.36–0.95) | 0.983 |
| <1.1 | 34 (85%) | 8 (89%) | 26 (84%) | |
| PT, s | 13 (12–15) | 13 (12–16) | 14 (12–15) | 0.639 |
| >16 | 9 (23%) | 2 (22%) | 7 (23%) | |
| D-dimer, ng/L | 636 (254–2,000) | 500 (156–2,953) | 785 (260–2,000) | 0.56 |
| >500 | 21 (53%) | 4 (44%) | 17 (55%) | |
| LDH, U/L | 477 (329–637) | 440 (232–696) | 477 (337–595) | >0.99 |
| >245 | 33 (83%) | 5 (55%) | 28 (90%) | |
| ALT, U/L | 32 (21–48) | 24 (20–46) | 38 (21–48) | 0.674 |
| >40 | 12 (30%) | 3 (38%) | 9 (29%) | |
| BUN, mmol/L | 6.8 (4.7–10.3) | 4.9 (4.5–6.3) | 8.7 (5.0–11.5) | 0.033 |
| >8.2 | 16 (40%) | 0 (0%) | 16 (52%) | |
| Cr, μmol/L | 86 (68–104) | 77 (63–87) | 88 (75–125) | 0.061 |
| >90 | 17 (43%) | 2 (22%) | 15 (48%) | |
| CRP, mg/L | 143 (69–171) | 68 (17–151) | 149 (112–171) | 0.191 |
| >10 | 38 (95%) | 7 (78%) | 31 (100%) | |
| CK-MB, U/L | 12.3 (0.8–18.0) | 10.5 (7.7–17.0) | 14.0 (0.7–18.0) | >0.99 |
| >6.6 | 25 (63%) | 6 (67%) | 19 (61%) | |
| HsTNI, ng/mL | 21.5 (8.8–47.9) | 11.0 (10.5–33.5) | 21.6 (7.2–71.6) | 0.939 |
| >26.2 | 16 (40%) | 3 (33%) | 13 (42%) |
ALI, acute liver injury; AKI, acute kidney injury; WBC, white blood cell; PT, prothrombin time; BUN, blood urea nitrogen; Cr, creatinine; HsTNI, high-sensitivity troponin I.
Clinical Features of COVID-19 Patients Before IMV
All enrolled 40 COVID-19 patients required ventilation assistant like NIV or HFNC oxygen therapy at or following admission. As shown in Table 2, PaO2/FiO2 before intubation was comparable between IMV survivor and IMV nonsurvivor (73 vs. 81, p = 0.77). The median time from NIV or HFNC to intubation was 7 hours (IQR, 2–42 hours) for IMV survivors with lower APACHE II and PSI scores (APACHE II median, 10 [IQR, 9–14]; PSI score median, 98 [IQR, 85–112]) before IMV (Table 2, Fig. 1), while the median time from NIV or HFNC to intubation for IMV nonsurvivors showed a longer duration (54 hours; IQR 28–143 hours). Similar laboratory findings, such as white blood cell, neutrophil, lymphocyte, PT, D-dimer, LDH, ALT, CRP, CK-MB, and troponin I, were observed between these two groups except for blood urea nitrogen and troponin I, which have levels higher in IMV nonsurvivor group (Table 2). Compared with these indicators at the time of admission, lymphocyte count and D-dimer showed more worse changes at the time before IMV.
TABLE 2.
Clinical Characteristics of COVID-19 Patients Before IMV
| Total (n = 40) |
IMV Survivor (n = 9) |
IMV Nonsurvivor (n = 31) |
p | |
|---|---|---|---|---|
| Duration from NIV/HFSC to IMV, median (IQR), h | 46 (22–90) | 7 (2–42) | 54 (28–143) | 0.008 |
| APACHE II, median (IQR) | 14 (10–16) | 10 (9–14) | 14 (12–16) | 0.032 |
| ≤7 | 1 (3%) | 0 (0%) | 1 (3%) | |
| 8–15 | 27 (68%) | 8 (89%) | 19 (61%) | |
| ≥16 | 12 (30%) | 1 (11%) | 11 (35%) | |
| PSI score, median (IQR) | 112 (104–126) | 98 (85–112) | 116 (108–126) | 0.028 |
| ≤70 | 2 (5%) | 1 (11%) | 1 (3%) | |
| 71–90 | 2 (5%) | 2 (22%) | 0 | |
| 91–130 | 28 (70%) | 5 (56%) | 23 (74%) | |
| ≥131 | 8 (20%) | 1 (11%) | 7 (23%) | |
| PaO2/FiO2 before IMV, median (IQR), mm Hg | 79 (64–98) | 73 (65–98) | 81 (64–98) | 0.77 |
| Laboratory findings before IMV, median (IQR) | ||||
| WBC count, ×109/L | 9.57 (7.55–13.40) | 8.65 (8.11–10.44) | 10.19 (7.25–14.26) | 0.543 |
| >9.5 | 19 (48%) | 3 (33%) | 16 (52%) | |
| Neutrophil count, ×109/L | 8.78 (7.00–12.55) | 7.95 (7.03–9.75) | 9.04 (6.76–13.14) | 0.567 |
| >6.3 | 33 (84%) | 8 (100%) | 25 (81%) | |
| Lymphocyte count, ×109/L | 0.44 (0.30–0.76) | 0.33 (0.28–0.51) | 0.47 (0.30–0.76) | 0.252 |
| <1.1 | 34 (85%) | 8 (100%) | 26 (84%) | |
| PT, s | 14 (12–17) | 16 (14–17) | 14 (12–17) | 0.599 |
| >16 | 13 (33%) | 3 (33%) | 10 (32%) | |
| D-dimer, ng/L | 2,000 (1,123–3,296) | 786 (207–6,687) | 2,000 (1,402–3,270) | 0.26 |
| >500 | 32 (80%) | 5 (56%) | 27 (87%) | |
| LDH, U/L | 466 (404–651) | 397 (388–513) | 489 (435–651) | 0.25 |
| >245 | 39 (98%) | 9 (100%) | 30 (97%) | |
| ALT, U/L | 39 (20–67) | 27 (21–48) | 40 (18–77) | 0.465 |
| >40 | 17 (43%) | 3 (33%) | 14 (45%) | |
| BUN, mmol/L | 6.7 (4.8–10.9) | 4.9 (3.6–6.3) | 7.3 (5.1–11.9) | 0.02 |
| >8.2 | 13 (33%) | 0 (0%) | 13 (42%) | |
| Cr, μmol/L | 68 (60–98) | 63 (54–67) | 72 (62–107) | 0.096 |
| >90 | 9 (23%) | 0 (0%) | 9 (29%) | |
| CRP, mg/L | 143 (78–179) | 206 (115–237) | 132 (83–168) | 0.594 |
| >10 | 40 (100%) | 9 (100%) | 31 (100%) | |
| CK-MB, U/L | 3.0 (0.6–14.2) | 3.3 (0.3–7.2) | 3.0 (0.8–16.7) | 0.51 |
| >6.6 | 14 (35%) | 3 (33%) | 11 (35%) | |
| HsTNI, ng/mL | 33.6 (9.4–127.6) | 19.5 (7.8–75.6) | 48.3 (10.5–138.3) | 0.377 |
| >26.2 | 18 (45%) | 2 (22%) | 16 (52%) |
HFSC, high-flow nasal cannula oxygen therapy; WBC, white blood cell; PT, prothrombin time; BUN, blood urea nitrogen; Cr, creatinine; HsTNI, high-sensitivity troponin I.
Figure 1.
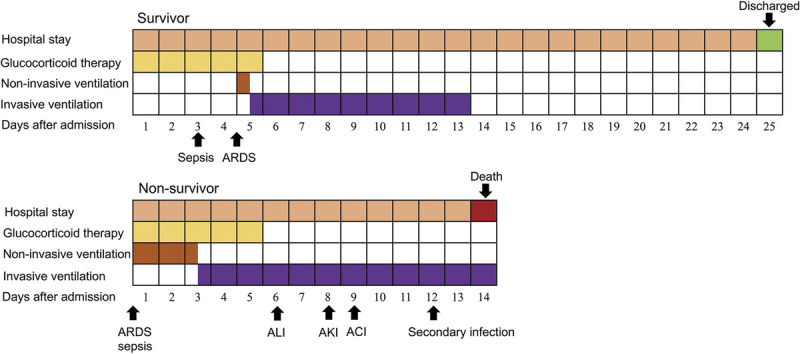
Clinical courses of ventilation and complications from admission in patients hospitalized with COVID-19. Figure shows median duration of hospital stay, ventilation, onset of complications, and outcomes. ALI, acute liver injury; AKI, acute kidney injury.
We further plotted the smoothing splines to present the association of time interval between NIV/HFNC and IMV, APACHE II score, and PSI score before IMV with mortality. The mortality tends to increase with the prolonged time interval between NIV/HFNC and IMV as well as increased APACHE II and PSI scores and reached a high plateau (Fig. 2). We observed that less than 50 hours (about 2 days) of NIV and IMV interval, APACHE II score of <10, and PSI score of <100 correspond to the mortality below 60%.
Figure 2.
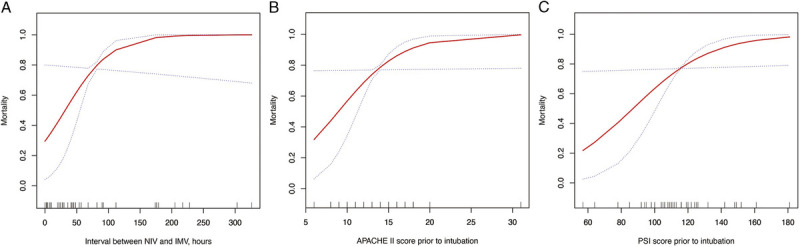
Smoothing splines present the association of (A) time interval from NIV/HFNC to IMV, (B) APACHE II score, and (C) PSI score before IMV with mortality. The red line indicates the estimated mortality, and the blue dot line indicates 95% confidence intervals.
A Case Report of Four COVID-19 Patients With ECMO
Among the four patients who received ECMO during hospitalization, three (75%) of them were relieved and discharged, while one was dead. Clinical characteristics of the four patients were presented in Supplementary Digital Content (Supplementary Table 1, http://links.lww.com/TA/B801). The age of the four ECMO patients was ranged from 45 to 72 years. Two of them had underlying disease. One survivor had diabetes and hypertension, and the one nonsurvivor had coronary heart disease. From the three ECMO survivors, the period from a cluster of symptoms emerging to hospital admission was 8 to 14 days. Two patients required intubation once hospitalized; one of them received ECMO therapy simultaneously with IMV. The third ECMO survivor required ECMO 7 days later of IMV support. All these three patients were discharged after 27 to 62 days of hospitalization and had elevated APACHE II and PSI scores before ECMO therapy (Fig. 3). For the ECMO nonsurvivor, the time from symptom initiation to NIV was 13 days. Acute liver injury was observed on day 10 after symptom onset. Sepsis was developed during the period of NIV accompanied by an elevated APACHE II and PSI scores of 12 and 149, respectively. Invasive mechanical ventilation and ECMO were performed after 4 days of NIV. Following this, this patient was attacked by secondary infections (Acinetobacter baumannii and fungal) and ACI. Finally, she died from cardiac arrest and multiple organ dysfunction syndrome.
Figure 3.
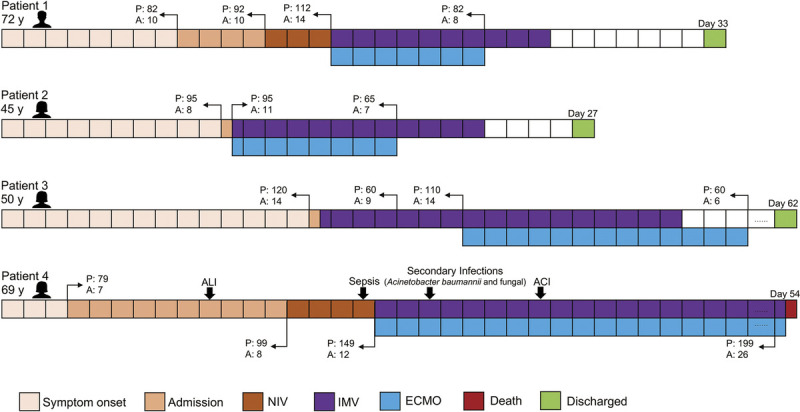
Clinical courses of four critical patients with COVID-19 receiving ECMO. ALI, acute liver injury; A, APACHE II; P, PSI.
DISCUSSION
In the current study, we described the clinical features and courses of 40 critically ill COVID-19 patients who required IMV during hospitalization. The clinical characteristics of these patients are very similar to those reported in previous studies.3,13 The most common symptoms were fever, dry cough, and difficulty breathing, followed by sputum, fatigue, myalgia, and expectoration (data not shown). The age of IMV nonsurvivors was older, and the mortality rate was highly influenced by their predisposition multiple underlying health conditions. We observed that, when the time interval from NIV/HFNC to intubation was less than 50 hours (about 2 calendar days), together with APACHE II score of less than 10 or PSI score of less than 100, mortality can be reduced to 60% or less, supporting that early tracheal intubation in reducing mortality is very crucial (Fig. 2).
The lungs are the organs most affected by SARS-CoV-2 because coronavirus enters into host cells via the angiotensin-converting enzyme 2, which is most abundant in the type II alveolar cells of the lungs.14 The pathological findings of a 50-year-old COVID-19 patient indicated bilateral diffuse alveolar damage with cellular fibromyxoid exudates.15 This pathological process produces exudates and hyaline membrane formation in the alveoli, which precipitates lung ventilatory dysfunction, indicative of ARDS.16 Notably, this is a gradual and progressive process. In the initial stage of hyaline membrane formation in the alveoli, the peripheral oxygen saturation level was maintained for 90% with high-flow oxygen or NIV due to the compensatory lung function. The oxygen-saturation phenomenon becomes deceptive during this period when the pathological changes may already occur. In further progression of hyaline membrane formation and diffuse alveolar damage, mild activity of patient is accompanied by large increases in oxygen consumption with a sudden decline in oxygen saturation level. During the patients' clinical and therapeutic course, we observed that the oxygen saturation level suddenly decreased from 90% to below 80%, accompanied by polypnea, cyanosis of lips, and dyspnea, even after a very mild activity (e.g., going to the toilet) in some cases. If oxygen saturation level remains below 90% with NIV, we usually performed intubation at this stage.
It is noteworthy that the cause of death is not only pulmonary failure. Among 31 IMV nonsurvivors in the present study, we found high rates of sepsis (77%), acute kidney injury (77%), ACI (52%), AHI (61%), and secondary opportunistic infection (77%), suggesting that multiple organ failure is common among these patients. This finding is consistent with a previous report that more than half of their nonsurvivors with SARS-CoV-2 infection developed sepsis and multiorgan dysfunction.13 According to the Chinese management guideline for COVID-19 (version 7.0),17 the IMV performance is primarily based on the respiratory function. We observed comparable PaO2/FiO2 before intubation between IMV survivor and nonsurvivor, indicating similar pulmonal inflammation and injure between these two groups. However, IMV nonsurvivor group showed higher PSI and APACHE II scores, which may be explained by more serious multiorgan damage in IMV nonsurvivor. Multiorgan damage is important to be sustainably monitored in the course of COVID-19 in critically ill patients. Two aspects should be included in the assessment for severe pneumonia: the severity of pneumonia itself and the assessment of organ damage.18 The PSI and APACHE II scores were assessed together in our study presenting as good evaluation indicators for these two aspects. We found a positive association of preintubation APACHE II and PSI scores with subsequent mortality (Fig. 2). Therefore, we suggested that APACHE II (presenting multiple organ state) and PSI (presenting pulmonary inflammatory state) should be considered in evaluating the timing of IMV in clinical practice.
In the present study, we retrospectively analyzed the clinical course of four IMV patients with ECMO therapy, and the cure rate was 75% (3 of 4 patients). As an extracorporeal life support therapy, ECMO has been increasingly used in circulatory or respiratory support for critically ill patients during the past decade.19,20 Extracorporeal membrane oxygenation maintains adequate gas exchange and acts as a lung protective ventilation strategy, which allows lung to be rest and recovered to rescue patients with ARDS.19–22 In Chinese management guideline for COVID-19 (version 7.0),17 ECMO has been considered as a treatment option in salvage setting for severe ARDS. According to the data in our present study, ECMO introducing is recommended before multiorgan dysfunction develops. However, because of the limited sample size in our study, further evidence is necessary and critical.
Our study has several limitations. First, the retrospective nature of the study and the missing data, such as PaO2/FiO2 data at admission, do not allow us to present more evidence to make our conclusion more reliable. Prospective controlled study is necessary to be incorporated. Second, the small case series with only four patients received ECMO-precluded reliable conclusions on the timing of use, cure rates, and complications of ECMO. Third, we cannot exclude the residual confounding and potential bias because of the observational nature of the study.
In conclusion, our study suggested that the decision on the timing of IMV might include APACHE II and PSI scores as auxiliary references (albeit the PaO2/FiO2 level is ≥150 or oxygen saturation level is ≥90% during the period). Although we know that initiation of intubation after NIV/HFNC as early as possible allows lung for resting and contributes to gain more time for further ARDS treatment, we are focusing more on the timing of IMV, and it needs more considerable attention.
AUTHORSHIP
Y.Z. and X.Z. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Q.Z., L.C., X.Z., and Y.Z. made substantial contributions to the study concept and design. Q.Z., J.S., W.Z., and L.L. collected the data. H.M. took responsibility for obtaining ethical approval and confirming data accuracy. L.C. was in charge of the statistical analysis. Q.Z., L.C., and J.S. were in charge of the article draft. X.Z. and Y.Z. contributed to critical revision of the report. All authors reviewed and approved the final version.
ACKNOWLEDGMENT
We thank all the doctors, nurses, disease control workers, and researchers who have fought bravely and ceaseless against the virus on the frontline during the SARS-CoV-2 epidemic.
DISCLOSURE
For all authors, no conflicts are declared. This study was supported by the National Natural Science Foundation of China (grant number 8170080939 to Q.Z.) and the Emergency Response Project of Hubei Science and Technology Department (2020FCA023 to Y.Z.). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
The Ethics Committees of Zhongnan Hospital of Wuhan University approved this study.
The data that support the findings of this study are available from the corresponding authors on reasonable request. We can provide participant data without names and identifiers but not the study protocol or statistical analysis plan. After publication of study findings, the data will be available for others to request. Once the data can be made public, the research team will provide an email address for communication. The corresponding authors have the right to decide whether to share the data based on the research objectives and plan provided.
Footnotes
Q.Z., J.S., and L.C. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Qian Zhang, Email: 32837843@qq.com.
Jun Shen, Email: 383329309@qq.com.
Liangkai Chen, Email: clk@hust.edu.cn.
Sumeng Li, Email: sumengli618@163.com.
Wenkai Zhang, Email: 43139290@qq.com.
Cheng Jiang, Email: chengjiang@whu.edu.cn.
Haoli Ma, Email: mahaoli@whu.edu.cn.
Lian Lin, Email: linlian@whu.edu.cn.
REFERENCES
- 1.Chen N Zhou M Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D Hu B Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X Yu Y Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc. 2007;102(478):527–537. [Google Scholar]
- 6.WHO Coronavirus disease 2019 (COVID-19). Situation Report—58. Published March 18, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 23, 2020.
- 7.Guan WJ Ni ZY Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance 25 January 2020.
- 9.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 10.General Office of the National Health Commission, Office of the State Administration of Traditional Chinese Medicine Diagnosis and treatment of COVID-19 (Version 6, in Chinese). Published February 18, 2020. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed March 23, 2020.
- 11.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. [DOI] [PubMed] [Google Scholar]
- 12.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11(2):272–276. [DOI] [PubMed] [Google Scholar]
- 13.Zhou F Yu T Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z Shi L Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health Commission of the People's Republic of China Chinese management guideline for COVID-19 (version 7.0, in Chinese). Published March 3, 2020. Availble at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Accessed March 23, 2020.
- 18.Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008;133(3):610–617. [DOI] [PubMed] [Google Scholar]
- 19.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914. [DOI] [PubMed] [Google Scholar]
- 20.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(2):163–172. [DOI] [PubMed] [Google Scholar]
- 21.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322(6):557–568. [DOI] [PubMed] [Google Scholar]
- 22.Peek GJ Mugford M Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. [DOI] [PubMed] [Google Scholar]


