Abstract
Oligodendrocytes are a subtype of glial cells found within the Central Nervous System (CNS), responsible for the formation and maintenance of specialized myelin membranes which wrap neuronal axons. The development of myelin requires tight coordination for the cell to deliver lipid and protein building blocks to specific myelin segments at the right time. Both internal and external cues control myelination, thus the reception of these signals also requires precise regulation. In late years, a growing body of evidence indicates that oligodendrocytes, like many other cell types, may use extracellular vesicles (EVs) as a medium for transferring information. The field of EV research has expanded rapidly over the past decade, with new contributions that suggest EVs might have direct involvement in communications with neurons and other glial cells to fine tune oligodendroglial function. This functional role of EVs might also be maladaptive, as it has likewise been implicated in the spreading of toxic molecules within the brain during disease. In this review we will discuss the field’s current understanding of extracellular vesicle biology within oligodendrocytes, and their contribution to physiologic and pathologic conditions.
Keywords: exosomes, microvesicles, extracellular vesicles, sphingolipids, myelin, oligodendrocytes, axon-glial communication, demyelination, remyelination
INTRODUCTION
Oligodendrocytes (OLs) are a subtype of glial cells found within the Central Nervous System (CNS), responsible for the formation and maintenance of specialized myelin membranes which wrap neuronal axons. Myelin sheath enables rapid saltatory conduction down an axon, functioning to allow synchronized signal propagation in a system with maximized spatial and temporal control. Mature myelinating oligodendrocytes may myelinate specific segments in multiple axons, each with unique demands for myelin composition.1 The development of these vast and elaborate membrane extensions requires precise coordination for the OL to deliver lipid and protein building blocks to the correct location at the right time. OLs incorporate both internal and external cues to adapt myelination patterns, and thus the reception of these signals also requires tight control.
Extracellular vesicles (EVs) have sparked interest in the neuroscience field because of their potential to facilitate intercellular communication, protect signaling molecules outside of the cell, and the potential to also harbor infectious agents or aberrant disease-causing molecules. Previous reviews have approached the importance of OL trafficking pathways with a particular focus on cell polarization2 and vesicle transport during myelin formation.3 In this review, we will provide a brief overview of vesicle-mediated signaling to and from OLs in both health and disease.
A BRIEF OVERVIEW OF OLIGODENDROCYTES AND MYELINATION
Myelin Composition and Ultrastructure
Myelin is chemically comprised of approximately 70% lipid and 30% protein. CNS and peripheral nervous system (PNS) myelin have compatible lipid composition, however the molar ratios of lipid species enriched in myelin are distinctly unique from that found in other biological membranes. The most abundant myelin lipids are cholesterol, galactosylceramides (galactocerebrosides) and glycerophospholipids, with primarily saturated long-chain fatty acids that enable tight packing of lipids in the myelin sheath.4 Myelin is a multilamellar structure characterized by electron microscopy to contain alternating electron dense and light layers, which are known as the major dense line and intraperiod line, respectively. The major dense line results from the apposition of cytoplasmic surfaces of an expanding myelinating OL process. Myelin Basic Protein (MBPs) are crucial for stabilizing the major dense line, by interacting with negatively charged lipids at the cytoplasmic surface of the lipid membrane.5,6 In contrast, the intraperiod line represents the apposition of the extracellular leaflets. Myelin proteolipid protein (PLP) and its splice variant DM20 are considered the primary integral proteins that enable tight compaction of normally repulsive extracellular membranes via hydrophilic interactions with other proteolipid molecules and galactocerebrosides.7,8 The specifics of how an OL process wraps around an axon to form concentric membrane layers within the CNS is still debated; meanwhile there is a greater consensus for myelin-wrapping by Schwann cells in the PNS. The myelin leading edge spirals and extends down the axon to form an internode, while the lateral edges become paranodal loops that participate in sodium channel clustering characteristic of an axonal Node of Ranvier. Removal of the cytoplasm and myelin membrane layer compaction initiates at the outermost layers and works its way inward toward the axon.9 Healthy myelin sheaths enable rapid saltatory conduction of neuronal signals by decreasing capacitance and increasing resistance; however, the layers of specialized membrane maintain some level of permeability through cytoplasmic channels to permit vesicular transport and trophic support of both the myelin and axon.10,11 For a more intensive insight into myelin dynamics, consider the review by Stadelmann et al. or the chapter by Rasband and Macklin.12,13
Oligodendrocyte origins, migration and maturation
Oligodendrocytes undergo many maturation states before reaching their destination and becoming a mature myelinating OL. Oligodendrocyte progenitor cells (OPCs) are proliferative and highly motile precursors derived from glia stem cells in two distinct regions, the ventral ventricular germinal zone (pMN) and the dorsal aspect of the spinal cord, during early embryonic development.14 OPCs proliferate in response to mitogens such as platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) and migrate from the germinal foci to form a tiling distribution through the CNS.15 Excess OPCs are removed by apoptosis or become quiescent, maintaining a progenitor pool capable of generating new OLs across the lifespan.16 As they mature, OLs become less motile and adopt a more complex radial morphology with ramified processes as they begin to produce a variety of sphingolipids and myelin specific proteins. Mature myelinating OLs are post-mitotic and non-motile with a distinct stellate morphology from fine processes in parallel or connected to myelinated internodes.17
Oligodendrocyte metabolism and susceptibility
As myelin formation requires high levels of protein production, there is significant metabolic susceptibility for OLs. ER-protein quality control (ERQC) checkpoints ensure that only accurately translated proteins exit the ER, otherwise protein products are transported to the proteasome for degradation. When there is accumulation of aberrant proteins, this triggers the unfolded protein response (UPR) which in turn increases ERQC signaling. Left unresolved, the UPR and ER-stress lead to apoptosis. OLs are susceptible to ERQC failure and such deficits have been identified in a multitude of myelin disorders.18 Similarly, mitochondria are involved in many pivotal functions of the cell; beyond energy production, mitochondria are involved in many signaling processes such as calcium homeostasis and production of molecular signals exemplified by pro-death proteins. As such, mitochondrial dysfunction has a significant impact on glial cell health and neurological disease development and progression.19 The review by Reddman et al. provides clarification on the intersection of autophagy, mitophagy, and lysosomal function in normal and aberrant nervous system physiology.20 The highly organized structure of myelin crafted by OLs underscores their vulnerability to disease and the heavy impact of OL loss on neuronal function.21 An increasing interest focuses on understanding the mechanisms of myelin-axon cross talk, particularly those involving transfer of information via membranous vesicles.22
A BRIEF OVERVIEW OF EXTRACELLULAR VESICLES
Standardized Terminology and Research of Extracellular Vesicles
Vesicle secretion is an evolutionarily conserved mechanism that can be utilized by all cell types. While originally thought to function only as waste disposal, the field is now captivated by the capacity of vesicles to deliver signals between cells. Extracellular vesicle (EV) is a generalized term used to describe highly heterogenous populations of secreted membrane vesicles, which vary in both biogenesis and signaling cargo, such as nucleic acids, proteins and lipids. The composition of EVs are influenced by cell type, differentiation stage, age, metabolic status and especially by disease state of the secreting cells.23,24 Much like myelin, current understanding of EV dynamics was established by biochemical analysis and electron microscopy, but there are still many unknowns surrounding the composition, biodynamics and physiological roles of EVs in health and disease. EVs can be subcategorized into 2 broad categories based on their size and biogenesis; exosomes are intraluminal vesicles between 50–200 nm released via fusion of a multivesicular body with the plasma membrane (PM) while microvesicles (ectosomes) are between 200–1000 nm and are derived from evaginations that lead to PM shedding (Figure 1).25–27 For a deeper review of vesicle biogenesis, release and targeting, see van Niel et al.28 International societies and databases have been established to consolidate the field behind likened terminology and to standardize sample collection, isolation, storage and analysis of extracellular vesicles (Table 1).29–34
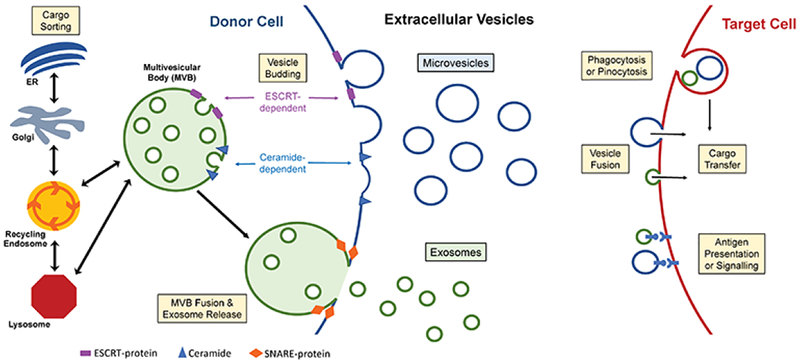
Figure 1. Extracellular Vesicle Release and Uptake.
Within the donor cell, cargo is sorted from various organelles and targeted for extracellular vesicle (EV) incorporation by cargo- and context-dependent mechanisms. EVs are formed by 2 major pathways: inward budding into an endosome to form a multivesicular body (MVB), or by direct outward budding of the plasma membrane (PM). Both methods employ multiple molecular components to alter membrane topology, such as ESCRT proteins and the lipid ceramide. Vesicles released from the MVB following SNARE protein facilitated fusion with the PM are termed exosomes, while the vesicles derived from PM fission are microvesicles. Heterogenous populations of EVs traverse interstitium and systemic circulation to reach their target, where they may bind to surface receptors, fuse with the cell membrane, or be internalized by the recipient cell to execute their function
Table 1.
Resources for Extracellular Vesicle Research
| Resource | Purpose | Website | Source & Support | Citation |
|---|---|---|---|---|
| Databases | ||||
| EV-TRACK | Platform to report and assess isolation methodology and characterization of EVs, as well as corresponding datasets. | http://evtrack.org/ | International Consortium of EV experts outlined on their website Last updated: Sept. 2018 | 29–31 |
| EVpedia | Database for profiles of proteins, lipids, RNAs and metabolites identified in various populations of EVs. Search and comparison tools of these datasets are also offered. | http://evpedia.info/ | Pohang University of Science and Technology Last updated: April 2018 | 32 |
| Vesiclepedia | Database of molecular readouts, including lipid, RNA and protein identified in different classes of EVs. | http://microvesicles.org/ | La Trobe Institute for Molecular Sciences Last updated: May 2019 | 33 |
| ExoCarta | Database for profiles specific to exosomes and their cargo. | http://exocarta.org/ | La Trobe Institute for Molecular Sciences Last updated: May 2019 | 34 |
| Professional Societies | ||||
| International Society for Extracellular Vesicles | A professional society run by scientists that provides education, event and publication opportunities for those conducting EV research. This society also supports many national societies in the EV field. | https://www.isev.org/ | Founded in 2012, with its headquarters located in the United States. The Executive Board is elected by the General Assembly at the Annual meeting. | |
Machinery Involved in Extracellular Vesicle Biogenesis
The process of vesicle formation may follow many different mechanisms which are influenced by the physiology and health status of the cell, however, the progression of events that result in EV release are generalizable. First, cargo are targeted to microdomains at the site of vesicle formation, whether that be an endosomal compartment in the case of exosomes, or the PM for microvesicle-mediated delivery. These cargo are the first regulators of vesicle formation,35 but generation and targeting of cargo to membrane microdomains is dependent on individual cellular contexts.36 The Rab family of small guanosine triphosphatases (GTPases), in conjunction with additional effector proteins, are the best studied regulators of vesicle trafficking.37 Cargo may originate from the Golgi apparatus, endoplasmic reticulum (ER) or through internalization at the PM before they are sorted for vesicle incorporation and secretion (Figure 1). Cycling through the endosomal sorting compartment is pivotal for proper incorporation of cargo into exosomes or microvesicles, and as such any impairment in endosome cycling, transport, or interconnected degradative organelles will largely impact on the vesicle biosynthesis and release.
Exosomes are primarily formed in the endosomal pathway through inward budding of the endosomal membrane to form intraluminal vesicles (ILVs), transforming that endosome into a newly matured multivesicular body (MVBs). These MVBs are segregated for either lysosomal degradation or release into the extracellular milieu. Endosomal sorting complex required for transport (ESCRT) proteins play a fundamental role in vesicle biogenesis by clustering ubiquitylated transmembrane cargo (ESCRT-0 and -I) before microdomain budding and fission (ESCRT-III).38 While defects in ESCRT proteins impact the capacity of cells to produce exosomes, there are also ESCRT-independent mechanisms of ILV formation (Figure 1).39 Local production of ceramide at the PM is mediated by the hydrolysis of sphingomyelin via neutral sphingomyelinase-2, contributes to microdomain formation for vesicle budding.40 Ceramide is a bioactive lipid that confers a negative (outward) curvature of membranes owing to its biophysical chemistry. Reducing ceramide production either genetically41 or chemically with the pharmacologic inhibitor GW486940,42 significantly decreases the capacity of the cell to release extracellular vesicles. Additionally, the formation of tetraspanin enriched membrane (TEM) domains serve as a platform for ESCRT-independent vesicle formation.43,44 The tetraspanin family of proteins, including CD63, CD81, CD82, and CD9 are all highly enriched in extracellular vesicles and thus serve as good biomarkers for exosome vesicle populations.
Microvesicles, or ectosomes, are instead formed by evaginations of the PM into the extracellular milieu. There are some commonalities between the machinery employed in exosome and microvesicle biogenesis, but we are only just starting to uncover the unique mechanistic pathways involved in the creation of microvesicles. Significant changes to the abundance and distribution of both protein and lipid species in the plasma membrane are initiated to create an asymmetry that promotes domain formation for microvesicle release. Phospholipids, namely phosphatidylserine, are shifted to the external leaflet of the phospholipid bilayer to enable outward membrane bending and contribute to actin cytoskeleton restructuring.45,46 The enzymes that direct these site changes include aminophospholipid translocases (such as flippase and floppase), scramblases, calpain, and lipid catabolism enzymes.47
Machinery Involved in Extracellular Vesicle Fission & Fusion
Microvesicles simply close off and are released from the PM, while exosomes follow a more regulated and complicated pathway to secretion. A fully formed MVB is transported along the cytoskeleton to fuse with the PM to release its ILVs as exosomes into the extracellular space. Fusion events are mediated by SNARE complexes (soluble N-ethylmaleimide-sensitive factor attachment protein receptor), which act by specific pairing of vesicle (R)- and target (Q)-SNAREs (Figure 1). While these proteins are highly conserved, each cell type has distinct pairings, requiring context-specific investigation to understand the role of SNARE proteins in vesicle trafficking. Feldman et. al. provides an extensive outline of SNARE localization and complex formation within Oli-neu cells, primary OLs and myelin isolates.48
EVs can travel short or long distances through the extracellular matrix (ECM) and into more systemic circulation, as EVs have been found in a variety of biological fluids.49 Targeting of the EVs is facilitated by specific surface protein interactions on the EV and recipient cell, such as EV integrins and cellular intercellular adhesion molecules (ICAMs). It has also been demonstrated that the ECM is involved in the process of pairing EVs with their cell targets, acting as a zipper through fibronectin and laminin proteins. Many additional target pairings have been identified, based in a variety of molecular, cellular and systemic specifics.28 Promoting selective or specific targeting of EVs is an area of intense investigation for the potential use of EVs in therapy.
EVS AS MEDIATORS OF INTERCELLULAR COMMUNICATION IN THE BRAIN
There are many intrinsic characteristics of EVs which are intriguing in the context of nervous system health and disease. EVs are highly stable in the extracellular space, have the capacity to carry a wide array of molecules, are capable of crossing the blood-brain barrier,50 maintain donor cell markers to indicate their origin, and are often highjacked by invaders to evade immune detection and gain cell entry.51 In the normal developing CNS, EVs containing morphogens such as Wnt and Hedgehog may contribute to signaling gradients,52,53 while other EV cargo have been shown to participate in cell-fate determination.54
However, the cargo of EVs are not always benign or beneficial. Misfolded protein aggregates are now considered as contributors to pathology in most neurodegenerative diseases. Various groups have demonstrated that EVs contain and propagate prion,55 α-synuclein,56,57 and amyloid58 proteins, or similarly, the enzymes that lead to misfolding such as SOD1.59. Isolation of EVs has traditionally been limited to biological fluids such as serum or cerebrospinal fluid (CSF) of patients with neurological disorders, which have been found to contain EVs enriched in indicators of pathology.60,61 However, more recent studies have identified EVs enriched in indicators of pathology within the interstitium of parenchyma from animal models and human tissues of neurodegenerative diseases.59,62,63 This has opened up the possibility for EVs to serve as valuable biomarkers of disease, in diagnosis, prognosis and progression. Removal of these pathogenic EVs may also serve to decrease overall disease burden or alter its course, but whether depletion will have this effect in patients remains an open question. On the other hand, EVs have the potential to be manipulated as targeting mechanisms for drug delivery in experimental design and therapeutics of the CNS.64,65 EVs may be loaded with therapeutic molecules, such as pharmacologic agents or RNAs, to target delivery to specific cells of interest. The cargo may instead be tumor antigens, so that EVs could serve to activate the immune system against patient cancer cells. Both the negative and positive potential of EVs have significant implications for clinical application. This becomes especially pertinent to the study of neurological disorders because of the lack of reliable biomarkers and effective treatments for patients suffering from these conditions. A recent review by Basso and Bonetto provides a thorough summary of EV-mediated intercellular communication within the brain with a specific focus on neuron-glia interactions22 while Lai and Breakefield discuss the therapeutic potential of EVs within the CNS.66
VESICLE COMPARTMENTS OF OLIGODENDROCTYES
Oligodendroglial Endosome Cycling
Our understanding of the mechanisms that mediate the trafficking and recycling of myelin membrane components has advanced in recent years. Myelin is not a uniform structure, but rather a highly complex and dynamic membrane with many different domains comprised of components in a ratio specific to that location. Thus, each OL tightly regulates the temporal and spatial integration of components in myelin sheath formation and maintenance.
Most major myelin proteins are cycled through endosomes as they are trafficked to myelin membranes, but each follows a distinct pathway that employs various vesicular machinery. The myelin protein PLP (found at the intraperiod line) is recycled from myelin via clathrin-independent and ESCRT-independent endocytosis and is enriched in the late endosome/lysosome (LE/Lys) compartment.40,67,68 The proteins myelin-associated glycoprotein (MAG) and myelin-oligodendrocyte glycoprotein (MOG), which localize to periaxonal and outer tongue myelin, respectively, are both internalized by clathrin-dependent endocytosis. However, MAG is sorted to the LE/lys while MOG traffics to the recycling endosome.69 While these studies demonstrate the complex specificity of endosomal cycling for different myelin components, the mechanism to target these vesicles and the contextual relevance of specific myelinating conditions remain unanswered. Some preliminary evidence for major myelin proteins have indicated SNARE proteins as a mechanism utilized by OLs to specifically target myelin cargo. The PLP-containing vesicles in the LE/Lys compartment express the R-SNARE protein VAMP7 and are targeted to myelin membranes through interactions with target Q-SNARE proteins Syntaxin 3/SNAP23. Conversely, the recycling endosomes which contain PLP are enriched in VAMP3 and are directed to fuse with OL soma membranes via coupling with the Syntaxin 4/SNAP23 Q-SNARE complex.70 The coalescence of cargo is the first event to precipitate vesicle formation, and so it is curious that such different vesicle populations with distinct machinery are formed under the same pretext. This begs the question of what other molecular signaling pathways are involved in the fate determinations for these recycled myelin components and how are those incorporated into the vesicles that deliver the cargo to their intended destination.
The importance of lipid mediators to endosomal cycling have also been identified within OLs. Individual knockouts of the three components that comprise the enzyme complex that regulates phosphoinositides (phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) each lead to hypomyelination due to sequestration of MAG in the LE/Lys compartment.71 New molecular mechanisms involved in organizing and targeting myelin cargo are still being revealed, each with implications relevant to a specific set of myelin building blocks. With so many mechanisms at play, the field will need to take a systematic approach to fully understand the events involved in segregated trafficking to myelin membranes.
When endosomal cycling becomes dysregulated, there are catastrophic effects on oligodendroglial stability and myelin formation or maintenance. When the ESCRT-I protein TSG101 protein is knocked down within OLs, there was significant swelling of the endosomal compartment and lead to severe white matter vacuolation common to spongiform encephalopathy.72 The endosomal sorting protein SNX27 is required to sequester the G-protein coupled receptor GPCR17 within recycling endosomes and prevent its degradation in lysosomes. GPCR17 plays a vital role in OL maturation, so when SNX27 is reduced, GPCR17 is degraded and consequentially, increases OL differentiation without corresponding myelin protein expression.73 Decreased SNX27 gene expression has been reported in patients with Down Syndrome, and SNX27 knock out or knock down mice have similar deficits including cognitive impairment and hypomyelination.74 Conversely, mutations in the N-myc downstream regulated gene (NDRG1) cause aberrant sequestration of low density lipoprotein receptor (LDLR) in oligodendroglial endosomes, resulting in decreased uptake of LDL in Charcot-Marie-Tooth disease type 4D (CMT4D).75 NDGR1 silencing was also found to decrease OL differentiation through downregulation of the transcription factor Olig2. These factors likely impact on lipid processing in mature OLs and may explain the abnormal myelination seen in CMT4D. These examples demonstrate that regulation of endosomal compartments is pivotal to proper OL maturation and myelination, and that disruptions to these highly specialized systems which regulate trafficking can lead to devastating disease.
The endosomal compartment coordinates distribution of components within the OL, as well as those that have been internalized from the surrounding environment. OLs synthesize and recycle lipid components from myelin, but it has been shown that external sources of lipid may also contribute to myelin formation by OLs. A study that utilized astrocyte specific knock out of sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP), found hypomyelination of axonal segments with increased compensatory incorporation of diet-derived sterols into myelin membranes.76 This suggests that OLs receive lipid products from astrocytes and the environment, posing the question of how these lipids are taken up by OLs. Endocytosis of external cues is especially important to OLs, as it is strongly held that myelination is cued by neuronal activity. Different experimental approaches have been utilized to test whether the neuronal signals are relayed through points of contact with OLs or if there is a secreted factor that recruits oligodendroglial interaction and subsequent myelination.77–79 While there are a number of repulsive signals, no robust instructive signals have been identified.80 Neuronal release of glutamate has been shown to cause and influx of Ca2+ through AMPA and NMDA receptors on OLs, leading to the release of the OL extracellular vesicle pool.81 These vesicles are endocytosed by neurons, revealing a bidirectional route of communication between the 2 cell types. This endocytic machinery is also commonly used by viruses to infiltrate cells, surpassing the common infection pathway through viral receptors required for entry. One such example is the JC Polyomavirus (JCPyV), which is known to infect both OLs and astrocytes, despite these cells lacking the attachment receptor lactoseries tetrasaccharide c. This virus was recently shown to be packaged inside EVs, which when released from the origin cell, were able to transmit virus to naïve OLs and astrocytes while remaining protected from antisera against viral antigens.82 This has significant implications for clinical applications of the immunomodulatory drugs used in the autoimmune demyelinating disease Multiple Sclerosis (MS). Some of these therapies increase patient susceptibility to progressive multifocal leukoencephalopathy (PML) caused by JCPyV.83 Knowing this mechanism of viral infection provides useful information to establish more reliable metrics to predict risk of PML and can inform the development of therapeutics to decrease viral load for patients on immunomodulatory therapies. Introducing a foreign invader, such as JCPyV, into the complex and dynamic endosomal system of OLs poses a large challenge and existential threat to the cell, the myelin membranes it maintains, and the axons that they support.
Oligodendroglial Exocytosis
The release of EVs has become an important topic for consideration in intercellular communication, broadening the scale of system dynamics that were previously thought to be limited by proximity. EVs have the potential to relay signals between cells and may also serve as a window into the state of cellular health at any given time. In this way, EVs could be used as valuable biomarkers to understand nervous system cell reactivity in health and disease states.84 OLs have been shown to release high levels of exosome-like vesicles.40,85 A screen of small Rab-GTPase proteins identified Rab35 and its activating protein TBC1D10A-C as important regulators of OL exocytosis.86 EV release from primary OLs can be induced by calcium treatment and have been found to contain major myelin proteins CNP, PLP, MBP and MOG together with proteins involved in cell-stress relief when analyzed for protein and lipid content.85 This was taken to mean that these OL-derived EVs may provide trophic support to axons, but no testing of neuronal outcomes following EV treatment were performed in this study. The mechanism by which the EVs were formed was not determined and remains an important question; most often the EV target and functional impact is of primary interest. EVs isolated from rat primary OLs demonstrated an autoregulatory effect that lead to decreased cell surface expansion and myelination through Rho-ROCK-myosin interactions on the receiving OL.87 Interestingly, these OL-derived EVs counteracted the pro-myelinating effect of neuronal conditioned medium.
As mentioned above, neuronal activity is known to influence OL myelination of axons, but this would suggest that communication from other OLs may supersede the neuronal signals to myelinate. Microglia are a likely candidate to receive EVs due to their function as surveillance cells of the CNS. One study described a MHC-II negative subpopulation of microglia that preferentially took up EVs derived from Oli-neu cells via micropinocytosis, without subsequent immune activation.88 This finding adheres to the common notion that EVs have low immunogenicity, recognized as “self-antigens” when presented to antigen presenting cells. However, this may change when the same vesicular machinery is being utilized for viral uptake and secretion. A recent study characterized OPCs as antigen presenting cells that are targeted during demyelination,89 and it has been previously demonstrated that EVs from APCs express Major Histocompatibility Complex (MHC) molecules to stimulate immune activation.90 These ideas taken together evoke interesting implications for the potential of EVs to propagate antigen presentation in autoimmune demyelinating disorders.
There are many different disease states that affect lipid processing, and as such have a substantial impact on both myelin and vesicle production by OLs.38 In Krabbe Disease, decreased lysosomal galactosylceramidase activity leads to the toxic accumulation of psychosine (galactosylsphingosine) and global demyelination. Psychosine inserts itself into membranes, disrupting normal fluidity and architecture.91,92 These membrane changes were shown to cause myelin membrane vesiculation and an increase in OL vesicle release in both primary OLs and CG4 cells.93 In the lysosomal storage disorder Niemann-Pick type C1 (NPC), cholesterol accumulates in the LE/Lys compartment resulting in demyelination. In a similar fashion, this leads to increased extracellular vesicle release by Oli-neu cells and patient fibroblasts.94 Both of these disorders lead to significant changes in lipid composition in cellular membranes, with consequential increases in EV production. Whether the vesicle secretion is occurring because of changed membrane dynamics or as a purposeful mechanism to unburden the cell remains an open question. By either of these mechanisms, the effect of these lipid-rich EVs have on recipient cells is likely to be similarly taxing.
Myelin Damage and Vesiculation
When myelin membranes become damaged, the physicochemical properties of the charged membranes cause them to fold on themselves, which has been aptly named myelin vesiculation or vacuolation.95 Damage to myelin in Multiple Sclerosis has been described as having “intramyelinic blebs” which represent lamellar separation and edema. This can affect long stretches of internodal myelin, leaving some portions with an intact lamellar structure.96 Many neurodegenerative disorders exhibit myelin pathology and loss, with similar histopathologic patterns of myelin decompaction, vacuolation, and fragmentation with vesicles present on both periaxonal and surface myelin.97,98 These structural alterations eventually give way to myelin dissolution and debris recruiting phagocytic cells with significant consequences for the exposed axon. Specific proteins found in myelin debris have been shown to decrease OPC differentiation through the Fyn-RhoA-ROCK signaling pathway, thus preventing remyelination from occurring.99 It is interesting to consider that both OL-derived EVs and myelin debris decrease myelination through a common signaling pathway.
FUTURE DIRECTIONS
The field of EV research is growing rapidly, with constant advancement in our understanding of both physiologic and pathologic EV function owing to improved methodologies, data collection standards and analytics. The cellular crosstalk that is potentiated by EVs as well as the propensity for EVs to harbor toxic entities involved in neurodegenerative disease underscore the importance of this research. Many questions still remain regarding the mechanisms that direct cargo to sites of vesicle formation, as well as the targeting of those vesicles from donor to recipient cells. Not only are vesicle populations and their cargo heterogenous, but their function as effectors to mediate or potentiate disease has been clearly demonstrated in many disorders, including those of the central nervous system. Thorough investigation of EV contents and the pathway they follow from biogenesis on through secretion and target cell uptake is required to elucidate the relevance of EVs and how they may be manipulated to improve outcomes. Furthermore, common and context-specific EV biology will be clarified as more studies follow recommended standards for EV collection, isolation, characterization and application. The foundation for EV research has been laid using in vivo and in vitro model systems, which have more reliable experimental paradigms to understand EV biogenesis, targeting and impact. EVs are found in most biological fluids and have been isolated from both healthy and diseased patients; given their stability, EVs have a high potential to serve as valuable biomarkers to monitor neurodegenerative disease progression and treatment efficacy. While there have been a number of preliminary studies which isolated EVs from biological fluids of patients with CNS disorders, research on the human implications for EV biology require much more investigative effort. Scientific interest in the study of EVs has expanded rapidly over the last decade, burgeoning improved tools and methodologies to better understand their biologic relevance. Improved standardization and sensitivity will continue to impact on the field’s understanding of the role that EVs play in both human patients and models of disease.
SIGNIFICANCE.
The discovery that most cells secrete small extracellular vesicles (EVs) into the extracellular milieu has underlined the notion that EVs contribute to intercellular communication and plausibly, to the spreading of pathogenic molecules. Oligodendrocytes, the myelin forming cells in the central nervous system, are also capable of using this route for releasing a variety of signals and myelin related compounds. This mechanism may be relevant to spreading toxicity in some myelin conditions. In this essay, we discuss working ideas on how EVs play important physiological roles in oligodendrocytes and how EVs contribute to disease.
ACKNOWLEDGEMENTS
This work was supported in part by the generous contribution of the Legacy of Angels Foundation, National Institute of Neurological Disorders And Stroke of the National Institutes of Health (R01NS065808) and Dr Ralph and Marian Falk Medical Research Trust to E.R.B.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Chong SY et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A 109, 1299–1304, doi: 10.1073/pnas.1113540109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maier O, Hoekstra D & Baron W Polarity development in oligodendrocytes: sorting and trafficking of myelin components. Journal of molecular neuroscience : MN 35, 35–53, doi: 10.1007/s12031-007-9024-8 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Larocca JN & Rodriguez-Gabin AG Myelin biogenesis: vesicle transport in oligodendrocytes. Neurochem Res 27, 1313–1329 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Schmitt S, Castelvetri LC & Simons M Metabolism and functions of lipids in myelin. Biochim Biophys Acta 1851, 999–1005, doi: 10.1016/j.bbalip.2014.12.016 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Brady ST et al. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J Neurosci 19, 7278–7288 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y et al. Synergistic interactions of lipids and myelin basic protein. Proc Natl Acad Sci U S A 101, 13466–13471, doi: 10.1073/pnas.0405665101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weimbs T & Stoffel W Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP. Biochemistry 31, 12289–12296, doi: 10.1021/bi00164a002 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Dupree JL, Coetzee T, Blight A, Suzuki K & Popko B Myelin galactolipids are essential for proper node of Ranvier formation in the CNS. J Neurosci 18, 1642–1649 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snaidero N et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290, doi: 10.1016/j.cell.2013.11.044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravel M et al. Overexpression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Molecular and cellular neurosciences 7, 453–466, doi: 10.1006/mcne.1996.0033 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Snaidero N et al. Antagonistic Functions of MBP and CNP Establish Cytosolic Channels in CNS Myelin. Cell Rep 18, 314–323, doi: 10.1016/j.celrep.2016.12.053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadelmann C, Timmler S, Barrantes-Freer A & Simons M Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol Rev 99, 1381–1431, doi: 10.1152/physrev.00031.2018 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Rasband MN & Macklin WB in Basic Neurochemistry (eds Brady ST, Siegel GJ, Albers W, & Price DL) Ch. 10, 180–199 (Academic Press, 2012). [Google Scholar]
- 14.Orentas DM & Miller RH The origin of spinal cord oligodendrocytes is dependent on local influences from the notochord. Dev Biol 177, 43–53, doi: 10.1006/dbio.1996.0143 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Bergles DE & Richardson WD Oligodendrocyte Development and Plasticity. Cold Spring Harb Perspect Biol 8, a020453, doi: 10.1101/cshperspect.a020453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barres BA & Raff MC Axonal control of oligodendrocyte development. The Journal of cell biology 147, 1123–1128, doi: 10.1083/jcb.147.6.1123 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumas L et al. Multicolor analysis of oligodendrocyte morphology, interactions, and development with Brainbow. Glia 63, 699–717, doi: 10.1002/glia.22779 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Volpi VG, Touvier T & D’Antonio M Endoplasmic Reticulum Protein Quality Control Failure in Myelin Disorders. Front Mol Neurosci 9, 162, doi: 10.3389/fnmol.2016.00162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose J et al. Mitochondrial dysfunction in glial cells: Implications for neuronal homeostasis and survival. Toxicology 391, 109–115, doi: 10.1016/j.tox.2017.06.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmann M, Darley-Usmar V & Zhang J The Role of Autophagy, Mitophagy and Lysosomal Functions in Modulating Bioenergetics and Survival in the Context of Redox and Proteotoxic Damage: Implications for Neurodegenerative Diseases. Aging Dis 7, 150–162, doi: 10.14336/AD.2015.0820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann N & Pham-Dinh D Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81, 871–927, doi: 10.1152/physrev.2001.81.2.871 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Basso M & Bonetto V Extracellular Vesicles and a Novel Form of Communication in the Brain. Front Neurosci 10, 127, doi: 10.3389/fnins.2016.00127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willms E et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6, 22519, doi: 10.1038/srep22519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo M, Raposo G & Thery C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30, 255–289, doi: 10.1146/annurev-cellbio-101512-122326 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Johnstone RM, Adam M, Hammond JR, Orr L & Turbide C Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262, 9412–9420 (1987). [PubMed] [Google Scholar]
- 26.Tricarico C, Clancy J & D’Souza-Schorey C Biology and biogenesis of shed microvesicles. Small GTPases 8, 220–232, doi: 10.1080/21541248.2016.1215283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trams EG, Lauter CJ, Salem N Jr. & Heine U Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645, 63–70, doi: 10.1016/0005-2736(81)90512-5 (1981). [DOI] [PubMed] [Google Scholar]
- 28.van Niel G, D’Angelo G & Raposo G Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol, doi: 10.1038/nrm.2017.125 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Consortium E-T et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14, 228–232, doi: 10.1038/nmeth.4185 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Lotvall J et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3, 26913, doi: 10.3402/jev.v3.26913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witwer KW et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2, doi: 10.3402/jev.v2i0.20360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DK et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles 2, doi: 10.3402/jev.v2i0.20384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalra H et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10, e1001450, doi: 10.1371/journal.pbio.1001450 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keerthikumar S et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 428, 688–692, doi: 10.1016/j.jmb.2015.09.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrowski M et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12, 19–30; sup pp 11–13, doi: 10.1038/ncb2000 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Kalra H, Drummen GP & Mathivanan S Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int J Mol Sci 17, 170, doi: 10.3390/ijms17020170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenmark H Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10, 513–525, doi: 10.1038/nrm2728 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Record M, Carayon K, Poirot M & Silvente-Poirot S Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841, 108–120, doi: 10.1016/j.bbalip.2013.10.004 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Colombo M et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126, 5553–5565, doi: 10.1242/jcs.128868 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Trajkovic K et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247, doi: 10.1126/science.1153124 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Aubin I et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nature genetics 37, 803–805, doi: 10.1038/ng1603 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Menck K et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles 6, 1378056, doi: 10.1080/20013078.2017.1378056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M & Sanchez-Madrid F Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19, 434–446, doi: 10.1016/j.tcb.2009.06.004 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Perez-Hernandez D et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 288, 11649–11661, doi: 10.1074/jbc.M112.445304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frey B & Gaipl US The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Semin Immunopathol 33, 497–516, doi: 10.1007/s00281-010-0228-6 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Li B, Antonyak MA, Zhang J & Cerione RA RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749, doi: 10.1038/onc.2011.636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segawa K, Suzuki J & Nagata S Flippases and scramblases in the plasma membrane. Cell Cycle 13, 2990–2991, doi: 10.4161/15384101.2014.962865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldmann A, Winterstein C, White R, Trotter J & Kramer-Albers EM Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J Neurosci Res 87, 1760–1772, doi: 10.1002/jnr.22020 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Thery C, Amigorena S, Raposo G & Clayton A Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3 22, doi: 10.1002/0471143030.cb0322s30 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto J, Stewart T, Banks WA & Zhang J The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr Pharm Des 23, 6206–6214, doi: 10.2174/1381612823666170913164738 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Raab-Traub N & Dittmer DP Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol 15, 559–572, doi: 10.1038/nrmicro.2017.60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gross JC, Chaudhary V, Bartscherer K & Boutros M Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14, 1036–1045, doi: 10.1038/ncb2574 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Vyas N et al. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep 4, 7357, doi: 10.1038/srep07357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratajczak J et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856, doi: 10.1038/sj.leu.2404132 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Vella LJ et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol 211, 582–590, doi: 10.1002/path.2145 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Danzer KM et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7, 42, doi: 10.1186/1750-1326-7-42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong SM et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet 23, 2816–2833, doi: 10.1093/hmg/ddu099 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Dinkins MB, Dasgupta S, Wang G, Zhu G & Bieberich E Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 35, 1792–1800, doi: 10.1016/j.neurobiolaging.2014.02.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silverman JM et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294, 3744–3759, doi: 10.1074/jbc.RA118.004825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiasserini D et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics 106, 191–204, doi: 10.1016/j.jprot.2014.04.028 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Sproviero D et al. Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients. Front Neurosci 12, 487, doi: 10.3389/fnins.2018.00487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Gonzalez R, Gauthier SA, Kumar A & Levy E The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287, 43108–43115, doi: 10.1074/jbc.M112.404467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593, doi: 10.1038/nn.4132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarez-Erviti L et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29, 341–345, doi: 10.1038/nbt.1807 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Andre F et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. Journal of immunology (Baltimore, Md. : 1950) 172, 2126–2136, doi: 10.4049/jimmunol.172.4.2126 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Lai C & Breakefield X Role of Exosomes/Microvesicles in the Nervous System and Use in Emerging Therapies. Frontiers in Physiology 3, doi: 10.3389/fphys.2012.00228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trajkovic K et al. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. The Journal of cell biology 172, 937–948, doi: 10.1083/jcb.200509022 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons M et al. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. The Journal of cell biology 157, 327–336, doi: 10.1083/jcb.200110138 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winterstein C, Trotter J & Kramer-Albers EM Distinct endocytic recycling of myelin proteins promotes oligodendroglial membrane remodeling. J Cell Sci 121, 834–842, doi: 10.1242/jcs.022731 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Feldmann A et al. Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J Neurosci 31, 5659–5672, doi: 10.1523/JNEUROSCI.6638-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mironova YA et al. PI(3,5)P2 biosynthesis regulates oligodendrocyte differentiation by intrinsic and extrinsic mechanisms. eLife 5, doi: 10.7554/eLife.13023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker WP, Oehler A, Edinger AL, Wagner KU & Gunn TM Oligodendroglial deletion of ESCRT-I component TSG101 causes spongiform encephalopathy. Biol Cell 108, 324–337, doi: 10.1111/boc.201600014 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Meraviglia V et al. SNX27, a protein involved in down syndrome, regulates GPR17 trafficking and oligodendrocyte differentiation. Glia 64, 1437–1460, doi: 10.1002/glia.23015 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Powell D et al. Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiol Aging 35, 1562–1569, doi: 10.1016/j.neurobiolaging.2014.01.137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietiainen V et al. NDRG1 functions in LDL receptor trafficking by regulating endosomal recycling and degradation. J Cell Sci 126, 3961–3971, doi: 10.1242/jcs.128132 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Camargo N et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15, e1002605, doi: 10.1371/journal.pbio.1002605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9, 917–922, doi: 10.1038/nmeth.2105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wake H, Lee PR & Fields RD Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651, doi: 10.1126/science.1206998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demerens C et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 93, 9887–9892, doi: 10.1073/pnas.93.18.9887 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simons M & Lyons DA Axonal selection and myelin sheath generation in the central nervous system. Current opinion in cell biology 25, 512–519, doi: 10.1016/j.ceb.2013.04.007 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Fruhbeis C et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11, e1001604, doi: 10.1371/journal.pbio.1001604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris-Love J et al. JC Polyomavirus Uses Extracellular Vesicles To Infect Target Cells. MBio 10, doi: 10.1128/mBio.00379-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mills EA & Mao-Draayer Y Understanding Progressive Multifocal Leukoencephalopathy Risk in Multiple Sclerosis Patients Treated with Immunomodulatory Therapies: A Bird’s Eye View. Front Immunol 9, 138, doi: 10.3389/fimmu.2018.00138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delpech JC, Herron S, Botros MB & Ikezu T Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci 42, 361–372, doi: 10.1016/j.tins.2019.02.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kramer-Albers EM et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl 1, 1446–1461, doi: 10.1002/prca.200700522 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Hsu C et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. The Journal of cell biology 189, 223–232, doi: 10.1083/jcb.200911018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakhti M, Winter C & Simons M Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem 286, 787–796, doi: 10.1074/jbc.M110.190009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzner D et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124, 447–458, doi: 10.1242/jcs.074088 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Kirby L et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10, 3887, doi: 10.1038/s41467-019-11638-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raposo G et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183, 1161–1172, doi: 10.1084/jem.183.3.1161 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White AB et al. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci 29, 6068–6077, doi: 10.1523/JNEUROSCI.5597-08.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hawkins-Salsbury JA et al. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J Lipid Res 54, 3303–3311, doi: 10.1194/jlr.M039610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D’Auria L et al. Psychosine enhances the shedding of membrane microvesicles: Implications in demyelination in Krabbe’s disease. PLoS One 12, e0178103, doi: 10.1371/journal.pone.0178103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strauss K et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 285, 26279–26288, doi: 10.1074/jbc.M110.134775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaharabani R, Ram-On M, Talmon Y & Beck R Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc Natl Acad Sci U S A 115, 11156–11161, doi: 10.1073/pnas.1804275115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui QL et al. Sublethal oligodendrocyte injury: A reversible condition in multiple sclerosis? Ann Neurol 81, 811–824, doi: 10.1002/ana.24944 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Matute C, Domercq M, Perez-Samartin A & Ransom BR Protecting white matter from stroke injury. Stroke 44, 1204–1211, doi: 10.1161/STROKEAHA.112.658328 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP & Armstrong RC Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol 74, 218–232, doi: 10.1097/NEN.0000000000000165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baer AS et al. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132, 465–481, doi: 10.1093/brain/awn334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]