Abstract
Thrombospondins are encoded in vertebrates by a family of 5 THBS genes. THBS1 is infrequently mutated in most cancers, but its expression is positively regulated by several tumor suppressor genes and negatively regulated by activated oncogenes and promoter hypermethylation. Consequently, thrombospondin-1 expression is frequently lost during oncogenesis and is correlated with a poor prognosis for some cancers. Thrombospondin-1 is a secreted protein that acts in the tumor microenvironment to inhibit angiogenesis, regulate antitumor immunity, stimulate tumor cell migration, and regulate the activities of extracellular proteases and growth factors. Differential effects of thrombospondin-1 on the sensitivity of normal versus malignant cells to ischemic and genotoxic stress also regulate the responses to tumors to therapeutic radiation and chemotherapy.
Keywords: thrombospondin-1, matricellular, tumor angiogenesis, metastasis, resistance to genotoxic therapy
Identity
HGNC (Hugo): THBS1
Location: 15q14
Other names: THBS, TSP, THBS-1, TSP-1, TSP1
Local order: Telomeric to FLJ39531, centromeric to FSIPl (fibrous sheath interacting protein 1)
DNA/RNA
Description
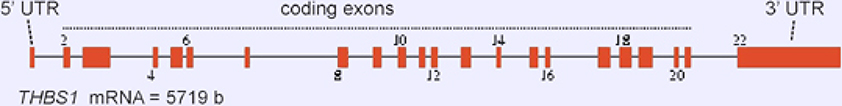
The THBS1 gene is 16,393 bases in size and is composed of 22 exons. Exons 2–21 encode the 5729 b mRNA (Figure 1).
Figure 1.
Exon/intron organization of the THBS1 gene.
Transcription
Egr-1 and Sp1 sites function in the transcription of THBS1 stimulated in most cell types by culture in the presence of serum (Shingu and Bornstein, 1994). Transcription is regulated by JUN (cjun or AP1) in cooperation with the repressor YinYang-I (YY1) and by TP53. USF2 and the aryl hydrocarbon receptor (AHR) mediate glucose-induced THBS1 transcription (Wang et al., 2004; Dabir et al., 2008). ID1 represses THBS1 transcription (Volpert et al., 2002).
The ATF1 transcription factor also down-regulates transcription of THBS1 through an ATF/cAMP- responsive element-binding protein binding site (Ghoneim et al., 2007). In contrast, MYC increases turnover of thrombospondin-1 mRNA (Janz et al., 2000). Transcription of THBS1 in some human cancers is suppressed through hypermethylation (Li et al., 1999; Yang et al., 2003). THBS1 expression is also regulated post-transcriptionally by micro-RNAs including MIR17HG (miR-17–92), MIR18A, MIR19A, MIR27B, MIR98, miR-194, MIR221, and MIRLET71 (let-7i-5p) (van Almen et al., 2011; Sundaram et al.,, 2011; Italiano et al., 2012; Yang et al., 2019; Miao et al., 2018; Farberov and Meidan 2018; Chen et al., 2017).
Pseudogene
None identified.
Protein
Description
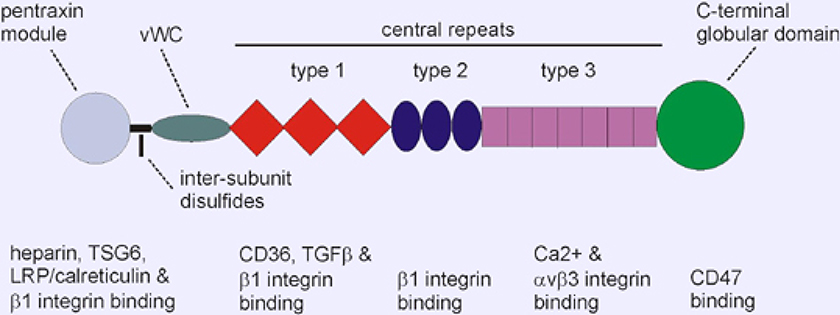
The THBS1 precursor contains 1170 amino acids; 129,412 Da. The mature secreted protein comprises residues 19–1170 after removal of the N-terminal signal peptide and assembles into a disulfide linked homotrimer (Figure 2). Secreted THBS1 is a glycoprotein with a molecular mass of 150–180 kDa that contains approximately 12 Asn-linked mono-, bi- tri-, and tetraantennary complex oligosaccharides and variable numbers of C-mannosylated Trp residues in the type 1 repeats, and O-fucosylation (Furukawa et al., 1989; Hofsteenge et al., 2001).
Figure 2.
Domain organization and localization of selected ligand binding sites in THBS1. THBS1 is a homotrimer linked via disulfide bonds.
Expression
THBS1 is expressed in many tissues during embryonic development but has limited expression in the healthy adult. THBS1 is the most abundant protein in alpha granules of platelets, but normal plasma levels are very low (typically 100–200 ng/ml). Expression in other cell types and tissues is induced by wounding, ischemia, ischemia reperfusion, during tissue remodeling, in atherosclerotic lesions, rheumatoid synovium, glomerulonephritis, in response to high glucose and fat, and in the stroma of many tumors. THBS1 expression increases with aging and in age-related conditions including type 2 diabetes and cardiovascular disease. THBS1 may also play a role in hematologic conditions such as sickle cell disease. Conversely, most but not all malignant cells in tumors exhibit loss of THBS1 expression during malignant progression (Isenberg et al., 2009). This loss is due to diminished positive regulation of the THBS1 gene by suppressor genes such as TP53 and NMEI and increased negative regulation by oncogenes including RAS and MYC. THBS1 expression is induced by TGF-beta, vitamin A, progesterone, and retinoids and suppressed by nickel, IDI, and HGF (hepatocyte growth factor).
Localisation
THBS1 is secreted by many cell types in response to injury or specific cytokines. THBS1 is present transiently in extracellular matrix but is rapidly internalized for degradation by fibroblasts and endothelial cells. THBS1 is abundant in megakaryocytes and platelets and is constitutively expressed at the dermal-epidermal boundary in skin and in subendothelial matrix of some blood vessels. However, THBS1 levels are generally low or undetectable in most healthy adult tissues.
Function
THBS1 binds to extracellular matrix ligands including fibrinogen, fibronectin, some collagens, latent and active TGFβ1 (transforming growth factor-beta-1), TNFAIP6 (TSG6), heparin, plasmin, CTSG (cathepsin G), ELANE (neutrophil elastase), some MMPs, tissue factor pathway inhibitor, and heparan sulfate proteoglycans (Resovi et al., 2014). THBS1 binds to cell surface receptors including CD36, CD47, some syndecans, LRP1 (LDL receptor-related protein-1) (via CALR (calreticulin)) and the integrins ITGA5/ITGB3 (alpha-5/beta-3), ITGA3/ITGB1 (alpha-3/beta-1), ITGA4/ITGB1 (alpha-4/beta-l), and ITGA6/ITGB1 (alpha-6/beta-1) (Calzada and Roberts, 2005). THBS1 is a slow tight inhibitor of several proteases including plasmin, cathepsin G, and neutrophil elastase. THBS1 directly binds and activates latent TGFβ1 (Murphy-Ullrich and Suto, 2018).
THBS1 in a context-dependent and cell-specific manner stimulates or inhibits cell adhesion, proliferation, motility, and survival. THBS1 is a potent inhibitor of angiogenesis, but N-terminal proteolytic and recombinant parts of THBS1 have clear pro-angiogenic activities mediated by beta-1 integrins. In the immune system, THBS1 is a potent inhibitor of T cell and dendritic cell activation and mediates clearance of apoptotic cells by phagocytes (Soto-Pantoja et al., 2015). In the CNS, THBS1 secreted by astrocytes promotes synaptogenesis (Risher and Eroglu, 2012).
Based on studies of Thbs1 null mice, platelet THBS1 is not essential for platelet aggregation, but Thbs1 null mice have impaired excisional but improved ischemic wound repair, increased retinal angiogenesis, and are hyper-responsive to several inflammatory stimuli (Soto-Pantoja et al., 2015).
Time stimulates pathologic production of reactive oxygen species (ROS) by targeting NOXl. Mitochondria from CD47 null mice produce less ROS. Inhibition of H2S signaling contributes to the inhibition of T cell activation by THBS1 mediated through the CD47 receptor (Miller et al., 2015).
THBS1 through interacting with CD47, plays a broader role in primary non-cancer and cancer tissue survival of genotoxic damage caused by ionizing radiation and chemotherapy (Soto-Pantoja et al., 2015; Feliz-Mosquea et al., 2018). Animals lacking either THBS1 or CD47 tolerated high-dose regional radiation with minimal soft-tissue injury or loss of bone marrow (Isenberg et al., 2008). Suppressing THBS1-CD47 signaling renders non-cancer cells and tissues resistant to radiation- and chemotherapy-mediated injury by promoting protective autophagy and enhancing anabolic metabolic repair pathways (Soto-Pantoja et al., 2012; Miller et al., 2015). Blocking the THBS1-CD47 axis also enhanced survival to lethal whole-body radiation (Soto-Pantoja et al., 2013). Conversely, interruption of THBS1-CD47 signaling increases radiation- and chemotherapy-mediated killing of cancers (Maxhimer et al., 2009; Feliz-Mosquea et al., 2018). This latter effect is mediated through activation of T and NK cell killing of tumors (Soto-Pantoja et al., 2014; Nath et al., 2019).
THBS1 is also a proximate inhibitor of stem cell self-renewal (Kaur et al., 2013). Acting via its cell surface receptor CD47, THBS1 limits the expression of important self-renewal transcription factors including POU5F1 (Oct3/4), SOX2, KLF4, and MYC in nonmalignant cells (Kaur et al., 2013). However, the ability of THBS1 to limit stem cell self-renewal is lost in cancer cells where MYC is amplified or dysregulated, and loss of CD47 expression or function consequently can suppress cancer stem cells (Kaur et al., 2013; Lee et al., 2014; Kaur and Roberts, 2016).
Homology
THBS1 is a member of the thrombospondin family that also contains THBS2, THBS3, THBS4, and COMP (cartilage oligomeric matrix protein) which arose from gene duplication of a single primordial thrombospondin in insects (Adams and Lawler, 2012). The central type 1 repeats are also known as thrombospondin-repeats (TSRs) and are shared with the larger thrombospondin/properdin repeat superfamily (Adams and Tucker, 2000; Apte 2009; de Lau et al., 2012). Orthologs of THBS1 are widely conserved in mammals and have also been identified in birds (Gallus gallus NP_001186382.1), amphibians (Xenopus tropicalis XP_002937245.1) and fish (Dania rerio XP_005160819.1).
Mutations
Germinal
Deep exon sequencing of THBS1 from 60,706 humans identified 4 putative loss of function mutations, which was significantly below the 37.6 expected loss of function mutations for a non-essential gene of this size (Lek et al., 2016). The resulting calculated probability that THBS1 is loss intolerant (pLI =1.0) exceeds the pLI > 0.9 cut-off, which predicts a strong selective pressure against inactivation of this gene. The basis for this apparent selective pressure against loss of THBS1 in humans remains unclear. Thbs1−/− mice are viable and fertile but exhibit defects in inflammatory responses and wound repair that may compromise their viability outside a protected laboratory environment (Lawler et al., 1998; Crawford et al., 1998; Lamy et al., 2007; Qu et al., 2018). Coding polymorphisms in THBS1 associated with altered disease risk in humans include a221Og (Asn700Ser), which is associated with premature familial myocardial infarction and small for gestational age infants. This mutation alters calcium binding to THBS1 and protein stability (Carlson et al., 2008; Hannah et al., 2004). The coding polymorphism g1678a (Thr523Ser) was identified as a genetic risk factor of cerebral thrombosis in a Chinese population (Liu et al., 2004). Several noncoding SNPs in THBS1 have been associated with cancer risk as detailed below.
Somatic
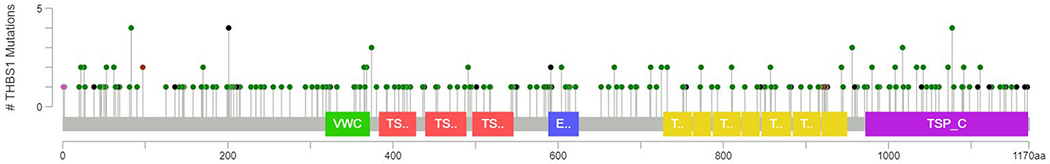
The frequency of somatic THBS1 mutation in human cancers is low. Somatic mutations have been identified at a frequency of 1.9% in The Cancer Genome Atlas (cbioprortal.org). A total of 233 mutations have been identified, most of which are missense or nonsense. However, the random distribution of these mutations indicates a lack of cancer-specific mutation hotspots (Figure 3). THBS1 is most frequently mutated in cutaneous melanomas (12%) followed by uterine cancers (7%), but rare or absent in other cancer types (Figure 4). The higher mutation rate of THBS1 in melanomas may simply reflect the high overall mutation burden of this malignancy.
Figure 3.
Identified mutations in thrombospondin-1 in human cancers include 187 missense (green), 41 truncating nonsense (black), 3 in frame (brown), and 2 other (purple). Data is from The Cancer Genome Atlas (TCGA) using cBioPortal tools to analyze data from 10,953 patients.
Figure 4.
Frequency of THBS1 mutations in TCGA PanCancer data classified by cancer type using cBioPortal tools (green = mutation, purple= fusion, blue= deletion, red = amplification, grey= multiple alterations).
Epigenetics
Most down-regulation of THBS1 in cancers is epigenetic, resulting from promoter hypermethylation (Yang et al., 2003), altered expression of regulatory noncoding RNAs (van Almen et al., 2011; Sundaram et al., 2011; Italiano et al., 2012; Yang et al., 2019; Miao et al., 2018; Farberov and Meidan 2018; Chen et al., 2017), or altered levels of oncogenic transcription factor. Epigenetic silencing of THBS1 is associated with a poor prognosis in several cancers (Guerrero et al., 2008; Isenberg et al., 2009).
Implicated in
Gastric carcinoma
Disease
THBS1 rs1478605 T>C
Carriers of the CC genotype exhibited a decreased risk of developing gastric cancer compared to the carriers of the CT and TT genotypes [adjusted OR, 0.56; 95% confidence interval (CI), 0.39–0.79; P=0.001] (Hong et al., 2015). The CC genotype of rsl478605 was negatively associated with gastric cancer lymph node metastasis (OR, 0.41; 95% CI, 0.23–0.71; P=0.001) and was associated with a reduced risk of lymph node metastasis in male patients (OR, 0.27; 95% CI, 0.14–0.52; P = 0.001).
THBS1 rs2292305 T>C, rs1478604 A>G
Significant association was found between the homozygous CC variant of THBS1 (rs2292305 T>C) and development of highly differentiated gastric carcinoma (Lin et al., 2012). The rs1478604 A>G variant was associated with invasion and lymph node metastasis in gastric cancer. Based on logistic regression and stratification analysis, rs1478604 A>G was more strongly associated with lymph node metastasis in highly differentiated gastric cancer.
Oncogenesis
The mechanism by which this polymorphism regulates carcinogenesis remains to be determined.
Bladder cancer
Disease
THBS1 696 C/T polymorphism (rs2664139) Compared with the CT/TT genotypes, the CC genotype was associated with a significantly increased risk of bladder cancer (adjusted odds ratio [OR] 1.43, 95% CI 1.01–2.04) (Gu et al., 2014).
Oncogenesis
The mechanism by which this polymorphism regulates carcinogenesis remains to be determined.
Colorectal cancer
Cancer progression associated with loss of THBS1 expression in the absence of known gene mutations.
Prognosis
Mutation of THBS1 is rare in most cancers, but loss of THBS1 expression due to hypermethylation, transcriptional regulation by oncogenes or tumor suppressor genes, or altered mRNA stability is commonly reported (Isenberg et al., 2009). Decreased THBS1 expression has been correlated with malignant progression and decreased survival in several cancers (Isenberg et al., 2009). To date, the strongest data is for colorectal carcinomas. Multiple independent studies have shown significant association of reduced THBS1 expression with increased invasion, microvascular densities, and poor prognosis (Miyanaga et al., 2002; Maeda et al., 2001; Isenberg et al., 2009; Teraoku et al., 2016). Increased circulating levels of THBS1 were also a favourable prognostic marker in patients with colon cancer (HR 0.43, p = 0.007) (Marisi et al., 2018).
Oncogenesis
The specific role of THBS1 in colorectal oncogenesis has been studied in the APCMin/+ mouse model. Mice lacking Thbs1 on the ApcMin/+ background exhibited increased intestinal adenoma formation with increased vascularization compared to Thbs1+/+: ApcMin/+ mice, consistent with the known anti-angiogenic activity of THBS1 (Gutierrez et al., 2003). Lack of THBS1 also decreased colorectal carcinogenesis in mice exposed to the carcinogen azoxymethane in combination with oral administration of dextran sulfate to induce intestinal inflammation (Lopez-Dee, et al., 2015). Again, angiogenesis was increased in the lesions formed in the Thbs1−/− mice. However, the protective role of THBS1 expression to limit colorectal carcinogenesis was lost when ApcMin/+ mice were fed a high fat Western diet, and metabolomic analysis identified systemic alterations including in eicosanoid metabolism that may mediate this effect (Soto-Pantoja et al., 2016).
Various cancers
Disease
Cancer progression associated with loss of THBS1 expression in the absence of known gene mutations.
Prognosis
Studies have shown associations of decreased THBS1 with poor prognosis in various cancers including non-small cell lung carcinoma (Rouanne et al., 2016), pancreatic adenocarcinoma, gastric (Nakao, et al., 2011), invasive cervical carcinoma, and oral squamous cell carcinomas (Isenberg et al., 2009). Reports are mixed regarding THBS1 as a prognostic factor in breast cancers (Rice et al., 2002). Stromal THBS1 expression in breast cancer was inversely related to lymph node involvement (Ioachim et al., 2012). Evidence indicates that the failure of THBS1 to protect in breast cancer is due to an escape mechanism involving increased VEGFA expression (Fontana et al., 2005). Hypermethylation of THBS1 was associated with a poor prognosis in prostate cancers (Guerrero et al., 2008). However, THBS1 was positively correlated with invasion in hepatocellular carcinomas (Poon et al 2004). Evidence is mixed regarding the clinical significance of THBS1 expression in prostate cancer, and urothelial cancer (Miyata and Sakai, 2013).
Oncogenesis
Several transgenic mouse models support an indirect tumor suppressor activity of THBS1. Mice lacking THBS1 developed tumors earlier in a tp53 null background (Lawler et al., 2001). Loss of THBS1 expression was associated with local invasive behavior, tumor neovascularization, and metastasis. A study of DVB-induced skin carcinogenesis in wildtype versus Thbs1−/− hairless SKH1 mice found that the protective activity of the flavone apigenin was lost in the absence of THBS1 (Mirzoeva et al., 2018). The protective role of THBS1 to limit carcinogenesis in skin was associated with decreased levels of circulating inflammatory cytokines and infiltrating macrophages and neutrophils.
Conversely, transgenic mice overexpressing THBS1 in skin or mammary tissue were resistant to chemical or oncogene-driven carcinogenesis (Streit et al., 1999; Rodriguez-Manzaneque et al., 2001). In addition to inhibiting the angiogenic switch required for tumor growth and hematologic metastasis, over- expression of THBS1 in tumor cells was associated with increased M1 polarization of tumor-associated macrophages in xenograft tumors, and THBS1 treatment increased superoxide production and killing of tumor cells by macrophages in vitro (Martin-Manos et al., 2008).
Familial pulmonary artery hypertension
Disease
THBS1 missense mutant Asp362Asn
The THBS1 missense mutation (Asp362Asn) alters a residue in the first type 1 repeat of THBS1 (Maloney et al., 2012). The Asp362Asn THBS1 mutant had less than half of the ability of wild-type THBS1 to activate latent TGFβ1. Mutant 362Asn THBS1 also lost the ability to inhibit growth of pulmonary arterial smooth muscle cells and was over three-fold less effective at inhibiting endothelial cell growth.
The mutation was found in two unrelated probands from 60 familial pulmonary arterial hypertension (PAH) kindreds but not in any healthy or chronic disease control cohorts. Several affected family members carried a mutation in BMPR2, which is known to be associated with PAH risk, and one family member with the THBS1 mutation but lacking the BMPR2 mutation was not diagnosed with PAR. Therefore, the THBS1 mutation alone may not be sufficient to cause PAH, and THBS1 was proposed to be a modifier gene for familial PAH. The frequency of other common THBS1 polymorphisms did not differ between PAH and control cohorts.
THBS1 intronic mutation (IVS8+2SS G/A)
THBS1 intronic mutation (IVS8+2SS G/A) was identified in a proband with familial pulmonary hypertension (Maloney et al., 2012). This mutation decreased and/or eliminated local binding of the transcription factors SP1 and MAZ in aortic smooth muscle cells. The mutation was confirmed to not alter splicing of THBS1 mRNA but is predicted to alter gene expression.
The mutation was found in multiple members of the single proband family with nine members diagnosed with PAH but absent in healthy and chronic disease control cohorts. Some of the affected family members were known to have BMPR2 mutations that are associated with PAH risk, and two family members with the THBS1 mutation but lacking the BMPR2 mutation were not diagnosed with PAH. Therefore, THBS1 was proposed to be a modifier gene. Because only one family was reported to date, the relative risk associated with this mutation remains to be determined.
Post-refractive surgery chronic ocular surface inflammation Disease
THBS1 SNPs (rs1478604 T>C, rs2228262
missense AAT> AGT, rs229230S missense ACA>GCA)
Increased risk for developing chronic inflammation in patients undergoing refractive eye surgery or receiving corneal allografts.
Prognosis
Patients with the minor alleles were more susceptible to developing chronic keratoconjunctivitis (rs1478604: odds ratio [OR], 2.5; 95% confidence interval [CI], 1.41–4.47; P = 2.5 × 10–3; rs2228262 and rs2292305: OR, 1.9; 95% CI, 1.05–3.Sl; P = 4.8 × 10–2. The rs1478604 A SNP was significantly associated with increased risk of corneal allograft rejection (odds ratio [OR], 1.58; 95% confidence interval [CI], 1.02–2.45; P = 0.04) (Contreras-Ruiz et al., 2014; Winton et al., 2014).
Familial premature myocardial infarction, Small for gestational age (SGA) infants
Disease
THBS1 variant A2210G (Ser700Asn)
The THBS1 S700N variant is a significant risk factor for familial premature myocardial infarction in both homozygous and heterozygous carriers of the variant allele (Topol et al., 2001; Zwicker et al., 2006; Stenina et al., 2004).
Paternal and neonatal THBS1 A2210G was also associated with small gestational age. Maternal THBS1 A2210G was associated with reduced maternal birth weight adjusted for gestational age at delivery (P = 0.03) (Andraweera et al., 2011).
Prognosis
The THBS1 S700N variant may be a general risk factor for vascular disorders throughout life.
Sickle cell disease
Disease
THBS1 SNPs (rs1478605 T > C and rs1478604 T>C)
The THBS1 SNPs rsl478604 (minor allele frequencies (MAF) 0.291) and rsl478605 (MAF 0.286) were negatively associated [OR 0.45 (95% CI 0.19, 1.08; p=0.069) and OR 0.33 (95% CI 0.12, 0.88; p=0.017, respectively)] with tricuspid regurgitant velocity (TRV) 2.5 in sickle cell disease patients (Jacob et al., 2017). Elevated TRV is a marker of pulmonary dysfunction. Of note, rs1478605 and rs1478604 are proximal to the THBS1 transcription start site and may alter THBS1 expression in patients with sickle cell disease.
References
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol 2011. October 1;3(10):a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000. June;218(2):280–99 [DOI] [PubMed] [Google Scholar]
- Andraweera PH, Dekker GA, Thompson SD, North RA, McCowan LM, Roberts CT. A functional variant in the thrombospondin-1 gene and the risk of small for gestational age infants. J Thromb Haemost. 2011. November;9(11):2221–8 [DOI] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (raprolysin- type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009. November 13;284(46):31493–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada MJ, Roberts DD. Novel integrin antagonists derived from thrombospondins. Curr Pham, Des 2005;11(7):849–66 [DOI] [PubMed] [Google Scholar]
- Carlson CB, Liu Y, Keck JL, Mosher OF. Influences of the N700S thrombospondin-1 polymorphism on protein structure and stability. J Biol Chem 2008. July 18;283(29):20069–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu J, Chu X, Ju C. MicroRNA-98 interferes with thrombospondin 1 expression in peripheral B cells of patients with asthma. Biosci Rep 2017. August 30;37(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Ruiz L, Ryan OS, Sia RK, Bower KS, Dartt DA, Masli S. Polymorphism in THBS1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology 2014. Ju1;121(7):1389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin- 1 is a major activator of TGF-beta1 in vivo. Cell 1998. June 26;93(7):1159–70 [DOI] [PubMed] [Google Scholar]
- Dabir P, Marlnlc TE, Krukovets I, Stenina 0I. Aryl hydrocarbon receptor Is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res 2008. June 20;102(12):1558–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farberov S, Meidan R. Fibroblast growth factor-2 and transforming growth factor-beta1 oppositely regulate miR-221 that targets thrombospondin-1 in bovine luteal endothelial cells. Biol Reprod 2018. March 1;98(3):366–375 [DOI] [PubMed] [Google Scholar]
- Feliz-Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Melendez GC, Schwartz AL, Chen QR, Mathews Griner L, Guha R, Thomas CJ, Ferrer M, Merino MJ, Cook KL, Roberts DD, Soto-Pantoja DR. Combination of anthracyclines and anti-CD47 therapy inhibit Invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat 2018. November;172(1):69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Filleur S, Guglielmi J, Frappart L, Bruno-Bossie G, Boissier S, Cabon F, Clezardin P. Human breast tumors override the antiangiogenic effect of stromal thrombospondin-1 in vivo. Int J Cancer 2005. September 20;116(5):686–91 [DOI] [PubMed] [Google Scholar]
- Furukawa K, Roberts DD, Endo T, Kabala A. Structural study of the sugar chains of human platelet thrombospondin. Arch Biochem Biophys 1989. April;270(1):302–12 [DOI] [PubMed] [Google Scholar]
- Ghonelm C, Soula-Rothhut M, Blanchevoye C, Martiny L, Antonicelll F, Rothhut B. Activating transcription factor-1- mediated hepatocyte growth factor-induced down- regulation of thrombospondln-1 expression leads to thyroid cancer cell invasion. J Biol Chem 2007. May 25;282(21):15490–7 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gomez P, Bello MJ, Arjona D, Alonso ME, Lomas J, Amilioso C, de Campos JM, Sarasa JL, Gutierrez M, Rey JA. CpG Island methylation of tumor-related genes in three primary central nervous system lymphomas in immunocompetent patients. Cancer Genet Cytogenet 2003. April 1;142(1):21–4 [DOI] [PubMed] [Google Scholar]
- Gu J, Tao J, Yang X, Li P, Yang X, Qin C, Cao Q, Cai H, Zhang Z, Wang M, Gu M, Lu Q, Yin C. Effects ofTSP-1–696 CfT polymorphism on bladder cancer susceptibility and clinicopathologic features. Cancer Genet 2014. June;207(6):24 7–52 [DOI] [PubMed] [Google Scholar]
- Guerrero D, Guarch R, Ojer A, Casas JM, Ropero S, Mancha A, Pesce C, Lloveras B, Garcia-Bragado F, Puras A. Hypermethylation of the thrombospondln-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int 2008. September;102(6):747–55 [DOI] [PubMed] [Google Scholar]
- Hannah BL, Misenheimer TM, Pranghofer MM, Mosher OF. A polymorphism In thrombospondin-1 associated with familial premature coronary artery disease alters Ca2+ binding. J Biol Chem 2004. December 10;279(50):51915–22 [DOI] [PubMed] [Google Scholar]
- Hofsteenge J, Huwller KG, Macek B. Hess D, Lawler J, Mosher OF, Peler-Katallnic J. C-mannosylation and 0-fucosylation of the thrombospondin type 1 module. J Biol Chem 2001. March 2;276(9):6485–98 [DOI] [PubMed] [Google Scholar]
- Hong BB, Chen SQ, Qi YL, Zhu JW, Lin JY. Association of THBS1 rs1478605 T>C in 5’-untranslated regions with the development and progression of gastric cancer. Biomed Rep 2015. March;3(2):207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- loachim E, Damala K, Tsanou E, Briasoulis E, Papadlotls E, Mitselou A, Charhanti A, Doukas M, Lampri L, Arvanitis DL. Thrombospondin-1 expression in breast cancer: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. Histol Histopathol 2012. February;27(2):209–16 [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signaling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer 2009. March;9(3):182–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano A, Thomas R, Breen M, Zhang L, Crago AM, Singer S, Khanln R, Maki RG, Mihailovic A, Hafner M, Tuschl T, Antonescu CR. The miR 17–92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer 2012. June;51(6):569–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SA, Novelli EM, Isenberg JS, Garrett ME, Chu Y, Soldano K, Ataga Kl, Teien MJ, Ashley-Koch A, Gladwin MT, Zhang Y, Kato GJ. Thrombospondin-1 gene polymorphism is associated with estimated pulmonary artery pressure in patients with sickle cell anemia. Am J Hematol 2017. March;92(3):E31–E34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz A, Sevignani C, Kenyon K, Ngo CV, Thomas- Tikhonenko A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res 2000. June 1;28(11):2268–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Roberts DD. Divergent modulation of normal and neoplastic stem cells by thrombospondln-1 and CD47 signaling. Int J Biochem Cell Biol 2016. December;81(Pt A):184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondln-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 2013;3:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Miao WM, Duquette M, Bouck N, Bronson RT, Hynes RO. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am J Pathol 2001. November;159(5):1949–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondln-1 is regulated for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 1998. March 1;101(5):982–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng 10. Blockade of CD47-medlated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology 2014. July;60(1):179–91 [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minlkel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tuklainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Framer M, Gauthier L, Goldstein J, Gupta N, Howrlgan D, Kiezun A, Kurki Ml, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardisslno D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, Mccarroll S, McCarthy Ml, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomllehto J, Tsuang MT, Watkins HC, WIison JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016. August 18;536(7616):285–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ahuja N, Burger PC, Issa JP. Methylation and silencing of the Thrombospondin-1 promoter in human cancer. Oncogene 1999. May 27;18(21):3284–9 [DOI] [PubMed] [Google Scholar]
- Lin XD, Chen SQ, Qi YL, Zhu JW, Tang Y, Lin JV. Polymorphism of THBS1 rs1478604 A>G in 5-untranslated region is associated with lymph node metastasis of gastric cancer in a Southeast Chinese population. DNA Cell Biol 2012. April;31(4):511–9 [DOI] [PubMed] [Google Scholar]
- Linderholm B, Karlsson E, Klaar S, Lindahl T, Borg AL, Elmberger G, Bergh J. Thrombospondin-1 expression in relation to p53 status and VEGF expression in human breast cancers. Eur J Cancer 2004. November;40(16):2417–23 [DOI] [PubMed] [Google Scholar]
- Liu XN, Song L, Wang DW, Liao YH, Ma AQ, Zhu ZM, Zhao BR, Zhao JZ, Hui RT. [Correlation of thrombospondin-1 G1678A polymorphism to stroke: a study in Chinese population]. Zhonghua YI Xue Za Zhi 2004. December 2;84(23):1959–62 [PubMed] [Google Scholar]
- Lopez-Dee ZP, Chittur SV, Patel H, Chinikaylo A, Lippert B, Patel B, Lawler J, Gutierrez LS. Thrombospondin-1 in a Murine Model of Colorectal Carcinogenesis. PLoS One 2015. October 13;10(10):e0139918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Nishiguchi Y, Kang SM, Yashiro M, Onoda N, Sawada T, Ishikawa T, Hirakawa K. Expression of thrombospondin-1 inversely correlated with tumor vascularity and hematogenous metastasis in colon cancer. Oncol Rep 2001. Jul-Aug;8(4):763–6 [DOI] [PubMed] [Google Scholar]
- Maloney JP, Stearman RS, Bull TM, Calabrese OW, Tripp- Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, Loyd JE. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012. March 15;302(6):L541–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marisi G, Scarpi E, Passardi A, Nanni 0, Pagan F, Valglusti M, Casadei Gardini A, Neri LM, Frassineti GL, Amadori D, Ulivi P. IL-8 and thrombospondin-1 as prognostic markers in patients with metastatic colorectal cancer receiving bevacizumab. Cancer Manag Res 2018. November 14;10:5659–5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 2008. September 1;68(17):7090–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009. October 21;1(3):3ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Rahman MF, Jiang L, Min Y, Tan S, Xie H, Lee L, Wang M, Malmstrom RE, Lui WO, Li N. Thrombin-reduced miR-27b attenuates platelet angiogenic activities in vitro via enhancing platelet synthesis of anti-angiogenic thrombospondin-1. J Thromb Haemost 2018. April;16(4):791–801 [DOI] [PubMed] [Google Scholar]
- Miller TW, Kaur S, lvins-O’Keefe K, Roberts DD. Thrombospondln-1 is a CD47-dependent endogenous Inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol 2013. August 8;32(6):316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD. CD47 Receptor Globally Regulates Metabolic Pathways That Control Resistance to Ionizing Radiation. J Biol Chem 2015. October 9;290(41):24858–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoeva S, Tong X, Bridgeman BB, Plebanek MP, Volpert OV. Apigenin Inhibits UVB-induced Skin Carcinogenesis: The Role of Thrombospondln-1 as an Anti-Inflammatory Factor. Neoplasia 2018. September;20(9):930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanaga K, Kato Y, Nakamura T, Matsumura M, Amaya H, Horiuchi T, Chiba Y, Tanaka K. Expression and role of thrombospondin-1 in colorectal cancer. Anticancer Res 2002. Nov-Dec;22(6C):3941–8 [PubMed] [Google Scholar]
- Miyata Y, Sakai H. Thrombospondin-1 in urological cancer: pathological role, clinical significance, and therapeutic prospects. Int J Mol Sci 2013. June 7;14(6):12249–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Suto MJ. Thrombospondln-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol 2018. August;68–69:28–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, Utsunomiya T, Shimada M. Expression of thrombospondin- 1 and Ski are prognostic factors in advanced gastric cancer. Int J Clin Oncol 2011. April;16(2):145–52 [DOI] [PubMed] [Google Scholar]
- Nath PR, Pal-Nath D, Mandal A, Cam MC, Schwartz AL, Roberts DD. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment Cancer. Immunol Res 2019. September;7(9):1547–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli EM, Little-Ihrig L, Knupp HE, Rogers NM, Yao M, Baust JJ, Meijles D, St Croix CM, Ross MA, Pagano PJ, DeVallance ER, Miles G, Patoka KP, Isenberg JS, Gladwin MT. Vascular TSP1-CD47 signaling promotes sickle cell- associated arterial vasculopathy and pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 2019. June 1;316(6):L1150–L1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT, Chung KK, Cheung ST, Lau CP, Tong SW, Leung KL, Yu WC, Tuszynski GP, Fan ST. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin Cancer Res 2004. June 15;10(12 Pt 1):4150–7 [DOI] [PubMed] [Google Scholar]
- Qu Y, Olonisakin T, Bain W, Zupetic J, Brown R, Hulver M, Xiong 2, Tejera J, Shanks RM, Bomberger JM, Cooper VS, Zegans ME, Ryu H, Han J, Pilewski J, Ray A, Cheng Z, Ray P, Lee JS. Thrombospondln-1 protects against pathogen- Induced lung injury by limiting extracellular matrix proteolysis. JCI Insight 2018. February 8;3(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol 2014. July;37:83–91 [DOI] [PubMed] [Google Scholar]
- Rice AJ, Steward MA, Quinn CM. Thrombospondin 1 protein expression relates to good prognostic indices in ductal carcinoma in situ of the breast. J Clin Pathol 2002. December:55(12):921–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, lruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA 2001. October 23;98(22):12485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, Sharifl-Sanjanl M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol 2014. July;37:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouanne M, Adam J, Goubar A, Robin A, Ohana C, Louvet E, Cormier J, Mercier O, Dorfmuller P, Fattal S, de Montpreville VT, Lebret T, Dartevelle P, Fadel E, Besse B, Olaussen KA, Auclair C, Soria JC. Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-smalI cell lung cancer. BMC Cancer 2016. July 15;16:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingu T, Bornstein P. Overlapping Egr-1 and Sp1 sites function in the regulation of transcription of the mouse thrombospondin 1 gene. J Biol Chem 1994. December 23;269(51):32551–7 [PubMed] [Google Scholar]
- Soto-Pantoja DR, Sipes JM, Martin-Manso G, Westwood 8, Morris NL, Ghosh A, Emenaker NJ, Roberts DD. Dietary fat overcomes the protective activity of thrombospondln-1 signaling in the Apc(Min/+) model of colon cancer. Oncogenesis 2016. May 30;5(5):e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. Int J Biochem Cell Biol 2004. June;36(6):1013–30 [DOI] [PubMed] [Google Scholar]
- Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol 1999. August;155(2):441–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Scheller JM, Huang Q, Cleary MA, Volpert OV, Thomas-Tikhonenko A. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res 2011. December 15;71(24):7490–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoku H, Morine Y, Ikemoto T, Saito Y, Yamada S, Yoshikawa M, Takasu C, Higashijima J, lmura S, Shimada M. Role of thrombospondin-1 expression in colorectal liver metastasis and its molecular mechanism. J Hepatobillary Pancreat Sci 2016. September;23(9):565–73 [DOI] [PubMed] [Google Scholar]
- Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina 0, Daley GQ. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 2001. November 27;104(22):2641–4 [DOI] [PubMed] [Google Scholar]
- Volpert OV, Pili R, Sikder HA, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. ld1 regulates angiogenesis through transcriptional repression of thrombospondin-1 Cancer Cell 2002. December;2(6):473–83 [DOI] [PubMed] [Google Scholar]
- Wang 8, Guo W, Huang Y. Thrombospondins and synaptogenesis. Neural Regen Res 2012. August 5;7(22):1737–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Skorczewski J, Feng X, Mel L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem 2004. August 13;279(33):34311–22 [DOI] [PubMed] [Google Scholar]
- Winton HL, Bidwell JL, Armitage WJ. Thrombospondin-1 polymorphisms influence risk of corneal allograft rejection. Invest Ophthalmol Vis Sci 2014. April 7;55(4):2115–20 [DOI] [PubMed] [Google Scholar]
- Wolf FW, Eddy RL, Shows TB, Dixit VM. Structure and chromosomal localization of the human thrombospondin gene. Genomics 1990. April;6(4):685–91 [DOI] [PubMed] [Google Scholar]
- Yang HD, Kim HS, Kim SY, Na MJ, Yang G, Eun JW, Wang HJ, Cheong JV, Park WS, Nam SW. HDAC6 Suppresses Let-71–5p to Elicit TSP1/CD47-Medlated Anti-Tumorigenesis and Phagocytosis of Hepatocellular Carcinoma. Hepatology 2019. October;70(4):1262–1279 [DOI] [PubMed] [Google Scholar]
- Yang QW, Liu S, Tian Y, Salwen HR, Chlenskl A, Weinstein J, Cohn SL. Methylation-associated silencing of the thrombospondin-1 gene in human neuroblastoma. Cancer Res 2003. October 1;63(19):6299–310 [PubMed] [Google Scholar]
- Zwicker JI, Peyvandi F, Palla R, Lombardi R, Canciani MT, Cairo A, Ardissino D, Bernardinelli L, Bauer KA, Lawler J, Mannucci P. The thrombospondin-1 N700S polymorphism Is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood 2006. August 15;108(4):1280–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol 2012;13(3):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Haymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 2011. October;10(5):769–79 [DOI] [PMC free article] [PubMed] [Google Scholar]