Abstract
In order to translate new treatments to the clinic, it is necessary to use animal models that closely recapitulate human disease. Lung cancer develops after extended exposure to carcinogens. It has one of the highest mutation rates of all cancer and is highly heterogenic. Topical treatment with N-nitrosotris-(2-chloroethyl)urea (NTCU) induces lung squamous cell carcinoma (SCC) with nonsynonymous mutation rates similar to those reported for human non-small cell lung cancer. However, NTCU induces lung cancer with variable efficacy and toxicity depending on the mouse strain. A detailed characterization of the NTCU model is needed. We have compared the effect of three different NTCU doses (20, 30 and 40 mM) in female and male of NIH Swiss, Black Swiss and FVB mice on tumor incidence, survival and toxicity. The main findings in this study are: (1) NIH Swiss mice present with a higher incidence of SCC and lower mortality compared with Black Swiss and FVB mice; (2) 30 mM NTCU dose induces SCC at the same rate and incidence as the 40 mM dose with lower mortality; (3) female mice present higher grade and incidence of pre-invasive lesions and SCC compared with males; (4) NTCU induced transformation is principally within the respiratory system; and (5) NTCU treatment does not impact the ability to elicit a specific adaptive immune response. This study provides a reference point for experimental designs to evaluate either preventive or therapeutic treatments for lung SCC, including immunotherapies, before initiating human clinical trials.
Keywords: Lung Squamous Cell carcinoma, N-nitrosotris-(2-chloroethyl)urea, lung cancer mouse model characterization
Introduction
Pre-clinical rodent models for cancer have not replicated human studies, due in part, to their inability to accurately recapitulate the complexity of human cancer. Lung cancer develops after an extended exposure to carcinogens. It has one of the highest mutation rates of all cancer and is highly heterogenic. Transgenic lung cancer models, such as the KrasG12D p53−/− for adenocarcinoma or the Lkb1 knock-out models for squamous cell carcinoma (SCC), do not represent the heterogeneity, mutational burden and genomic landscape found in human lung cancer (1). In addition, for the study of immunotherapy, they do not recapitulate the tumor microenvironment observed in humans (2). Syngeneic implanted tumors grow too fast in vivo to elicit adaptive immune responses, do not develop significant immune suppressive elements and have lower tumor infiltrating lymphocytes (TILs) than spontaneous tumors (3). Moreover, both the syngeneic implant tumors and the genetically engineered mouse (GEM) models lack the early pre-invasive events in lung cancer oncogenesis and therefore they are not ideal for chemoprevention studies (4).
Chemically induced tumors are often used to study lung cancer therapies. Tumors in these models present with a higher mutational load, significant heterogeneity, and slower tumor growth rates than GEM and tumor implant models. Yet chemically induced lung tumors have not been well characterized, especially regarding the kinetics of tumor growth and chemical treatment related toxicity. Topical treatment with N-nitrosotris-(2-chloroethyl)urea (NTCU) induces lung SCC in mouse with variable efficacy depending on the strain. (5,6). N-nitroso compounds, such as NTCU, are nicotine-derived compounds present in tobacco, which are transformed within the body to alkylating agents that attack DNA (7). Mice treated with NTCU develop bronchial epithelia dysplasia and metaplasia that progress to invasive SCC similarly to the development of human lung SCC. Exome sequencing of cell lines and tumors derived from the NTCU model have shown nonsynonymous mutation rates comparable to those reported for human non-small cell lung cancer (8,9). However, induction of lung SCC with NTCU severely reduces survival of treated animals, with mortality rates of 32–55% occurring before the development of tumors (10,11). There are no reported studies showing an NTCU dose titration to analyze the effect of mid-range doses on toxicity, survival and induction of lung SCC. This model has been extensively used for prevention studies and improved model characterization is necessary to evaluate the success of chemopreventive agents. Additionally, although sex effect on cancer susceptibility has been reported, it has not been studied if sex influences the development of SCC induced by NTCU in multiple strains of mice (12).
We questioned if lower doses of NTCU could still induce SCC while preventing chemical associated toxicity. We have compared the effect of three different doses of NTCU (20, 30 and 40 mM) in female and male NIH Swiss, Black Swiss and FVB mice. For each sex, NTCU dose and strain we have assessed: (1) mouse weight and survival, (2) incidence and grade of hyperplasia, dysplasia and SCC and (3) other respiratory tract and off-target lesions caused by NTCU. Finally, for the study of immune therapy it is critical to evaluate whether treatment with NTCU influences the ability to generate an immune response.
Materials and methods
Mouse lung cancer models.
FVB mice (FVB/NJ, Stock No. 001800) were purchased from the Jackson Laboratory. NIH Swiss (NIH(S)-550) and Black Swiss (NIHBL(S)-492) mice were purchased from Charles River Laboratory. Mice were housed in a specific pathogen-free facility (details in Supplementary Methods). At 5–9 weeks of age, animals were randomized to the different treatment groups. Six to 10 mice were included for untreated and acetone treated control groups and 12–16 mice were studied for each of the NTCU treated groups. Male and female mice were distributed at 50% in every group. NTCU (Toronto Research Chemicals Inc. (Toronto, Canada)) was dissolved in acetone. Forty-eight hours before treatment, the dorsal skin was shaved and acetone or NTCU at the appropriate concentration was applied every three days in 25 µl drops for 32 weeks. Animals were monitored twice a week for weight loss and health concerns such as labored respiration, dishevelled appearance, decreased activity and anorexia. All the procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Histologic evaluation of toxicity and lung neoplasia.
One animal from untreated and acetone groups and two animals, one female and one male, from the NTCU groups were sacrificed at 8, 16 and 24 weeks after initiation of treatment. The remaining mice in each group were sacrificed at 32 weeks. Mice were euthanized by overdose with isoflurane and cervical dislocation followed by complete necropsy. Lungs, skin from application site, stomach, kidney, liver and head (including brain, ear canal, and oral and nasal cavities) were collected for histological analysis. Lungs were removed, weighed and inflated with 10% neutral buffered formalin. All tissues were immersed fixed in 10% formalin. Samples were processed routinely, embedded in paraffin, stained with haematoxylin and eosin (H&E) and examined by a board-certified veterinary pathologist (PMT) for evidence of neoplasia, non-neoplastic lesions and toxicity. Two serial sections, 4–6 µm thick, were analyzed for each tissue and animal. Lung proliferative and neoplastic grading was carried out under bright field microscopy on H&E samples using criteria previously described (6,11,13,14). Images were captured using a Nikon Eclipse 80i with a Nikon Digital Sight DS-Fi1 camera and processed (global white balance and contrast) and plated with Adobe Photoshop 2015. Slides were scanned using Hamamatsu NanoZoomer S60 (Hamamatsu Photonics K.K.). Percentage of tumor area for each mouse was manually selected and quantified using QuPath (15).
Immunohistochemistry.
The following antibodies were used: anti-CD4 (eBioscience, clone 4SM95), anti-CD8 (eBioscience, clone 4SM15) and anti-PD-L1 (Cell Signalling, clone D5V3B). Full details about immunohistochemistry staining protocols are provided in the Supplementary methods. Nine NTCU- treated NIH Swiss mice (5 female, 4 male) presenting tumors were used for quantification. Two to three representative areas containing SCC were randomly selected and the number of CD4+ and CD8+ cells was quantified using QuPath (15). For PD-L1, three representative tumor areas with the highest number of PD-L1 positive cells were used for quantification. Percentage of positive cells was corrected for the total number of SCC cells in the counted area.
Evaluation of adaptive immune response.
FVB or NIH Swiss mice, female, five animals per group, were vaccinated four times every 10 days with an IGF1R vaccine containing three peptides (100 µg/each): IGF1R p1166–1182, IGF1R p1212–1226 and IGR1R p1302–1325 (16). Mice received a 5th booster vaccine 15 days before sacrifice. NTCU 40mM was applied twice a week for a total of four weeks, before or concomitant to vaccination. Spleens were processed according to our previously published methods and the immune response to vaccinated antigen was analyzed by IFN-γ ELISPOT assays as published (17). Antigens tested included the IGF1R peptide pool contained in the vaccine (10 µg/ml each peptide), HIVp17 (ERFALNPGLLETSEGCK, 10 µg/ml) and concanavalin A (ConA, 2.5 µg/ml). Data are reported as corrected spots per well (CSPW, mean number of spots for each experimental antigen minus the mean number of spots detected in no-antigen control wells).
Immune phenotype evaluation by flow cytometry.
Antibodies and reagents used for flow cytometry are listed in the Supplementary methods. Cells were acquired using FACSCanto flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Results are reported as the mean ± standard deviation (SD) of the total percentage of each cell population as indicated.
RNA sequencing.
Five NIH Swiss mice presenting lung SCC and five control healthy mice were used for LCM. Microdissection was performed in a LMD7000 microscope (Leica Microsystems GmbH, Wetzlar, Germany). Full details about the protocol are provided in the Supplementary methods. RNA was extracted using the RNeasy FFPE kit (Qiagen, Hilden, Germany) and libraries were prepared from using the SMARTer Stranded Total RNA-Seq Kit – Pico Input Mammalian (Clontech Laboratories, Inc., Mountain View, CA, USA). Sequencing was performed using an Illumina HiSeq 2500 generating 50 base pair paired end reads. Reads of low quality were filtered, and adapters trimmed using Cutadapt v1.9.1. Alignment to the reference genome (UCSC mm10) was performed using TopHat v2.0.1, and RNA counts were generated for each gene using the Python package HTSeq v0.6.1, employing the “intersection-strict” overlap mode. DESeq2 (18) was used to normalize and transform the data, examine the relationship between samples (by plotting the first two principal components) and to test for differential expression. Data derived from the RNA sequencing has been deposited in GEO (https://www.ncbi.nlm.nih.gov/geo/), accession number [pending]. Three human microarray datasets were analyzed using the web based GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). The most significantly differentially expressed genes were converted to their mouse counterpart using HCOP (https://www.genenames.org/tools/hcop/).
Statistical analysis.
Graphs and ANOVA comparisons were completed using GraphPad Prism v7 software. A one-way ANOVA with Tukey’s post-test was used for comparison of one variable between the different treatment groups and a two-way ANOVA with Bonferroni’s or Tukey’s post-test was used for grouped comparisons with more than one variable. Unless reported separately, there were no sexes differences noted by two-way ANOVA with Bonferroni’s correction test and therefore sexes were combined for analysis. Kaplan-Meier curves were generated to show overall survival. Log-rank (Mantel-Cox) Test was used for survival curves comparison. Chi-square, with a confidence interval of 95% was used to compare proportions in survival for each mouse strain treated with NTCU. Linear regression was used to evaluate the relation between lung weight and disease burden. Significance was considered at p<0.05 for statistical tests, except for the survival curves comparison. Survival curves corrected p-value was 0.0083.
Results
Animals treated with NTCU lose weight in a dose-dependent manner
Mice treated with 20 mM NTCU lose less weight compared with the 30 mM and 40 mM groups. For each analyzed mouse strain, weight loss in the 20 mM group was significantly lower than the 40 mM treatment group (20 mM vs. 40 mM, p<0.0001), and not significantly different from the acetone control group (Supplementary Table S1). Weight loss in the 30 mM group was dependent on strain. For the NIH Swiss mice, weight change in mice treated with 30 mM was not significantly different from the 40 mM NTCU group (Fig. 1A, Supplementary Table S1). For the Black Swiss, mice weight loss in the 30 mM group was significantly lower than the 40 mM treatment group (p=0.0189) (Fig. 1B). FVB mice show no differences in weight change between the 30 mM and the 40 mM groups (Fig. 1C). No significant differences were observed between the untreated control group and the acetone group in any mouse strain. Comparison of weight loss between females and males was not significantly different for any of the strains or NTCU doses assayed (Supplementary Table S2) and data from males and females were combined for analysis.
Figure 1. Dose of NTCU correlates with animal weight loss and mortality of treated animals.

NIH Swiss (A, D), Black Swiss (B, E) and FVB mice (C, F) untreated (black line) or treated with acetone (black dotted), 20 mM NTCU (green), 30 mM NTCU (blue) or 40 mM NTCU (red). Change in animal weight calculated as the difference between weight at a certain time point and the weight at day 0 of treatment. Weight graphs *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Healthy animals euthanized at intermediate time-points for analysis have not been included in the survival curve. Survival curves corrected p-value 0.0083. *p<0.0083, **p<0.0008, ***p<0.0001.
Survival of mice treated with NTCU is dose and strain dependent
NIH Swiss and Black Swiss mice treated with 20 mM and 30 mM NTCU showed significantly lower mortality compared to the standard 40 mM NTCU dose (NIH Swiss: 20 mM vs.40 mM p<0.0001; 30 mM vs.40 mM p=0.0004; Black Swiss: 20 mM vs.40 mM p<0.0001; 30 mM vs.40 mM p<0.0025) (Fig. 1D and 1E). For the FVB mice, survival rates of the three NTCU-treatment groups do not significantly differ from each other (20 mM vs. 40 mM, p=0.0288, 20 mM vs. 30 mM p=0.0326, 30 mM vs. 40 mM p=0.6364) (Fig. 1F).
The mean of survival days in the NIH Swiss mice for each treatment group was: 20 mM NTCU 219 ± 40 (95% CI: 191, 248), 30 mM NTCU 187 ± 37 (95% CI: 163, 210) and 40 mM NTCU 132 ± 36 (95% CI: 109, 155). The mean of survival for the Black Swiss mice was: 20 mM NTCU 144 ± 8 (95% CI: 137, 151), 30 mM NTCU 122 ± 16 (95% CI: 109, 134) and 40 mM NTCU 112 ± 6 (95% CI: 108, 116). The mean of survival days for the FVB strain was: 20 mM NTCU 179 ± 33 (95% CI: 149, 210), 30 mM NTCU 136 ± 72 (95% CI: 70, 203) and 40 mM NTCU 161 ± 22 (95% CI: 144, 178). Mean survival was not reached in the untreated and acetone groups and no differences in survival were found between them. In the FVB cohort, two female mice died in the control groups, one in the untreated group and one in the acetone group. Unfortunately, we were not able to perform necropsy in these mice and the cause of death remains unknown. We cannot exclude that some of the mice that died in NTCU groups were unrelated to the treatment or to the development of lung disease.
A significant interaction between mortality and strain was found. For any NTCU treatment group, Black Swiss and FVB were more likely to die than NIH Swiss mice. There is a 91.7% (n = 36) probability of dying in Black Swiss, 47.2% (n = 36) in FVB and 43.5% (n = 46) in NIH Swiss mice treated with NTCU (χ2 (2, n = 118) = 22.58, p<0.0001). Comparison of mean of survival between female and male mice was not significant for any of the strains and NTCU doses assayed (Supplementary Table S3). Data from male and female mice have been combined for analysis.
Development and grade of pre-invasive lesions, hyperplasia and dysplasia, depend on NTCU dose, sex and mouse strain
Hyperplasia and dysplasia were observed earlier in Black Swiss and NIH Swiss mice treated with NTCU compared to FVB. Hyperplasia was present at eight weeks after NTCU treatment for all the strains, with grade increasing at higher NTCU doses. Minimal-mild squamous and adenomatous atypical dysplasia was observed after eight weeks of treatment for the NIH Swiss and Black Swiss, and at 16 weeks for the FVB. High-grade hyperplasia, squamous metaplasia and high-grade dysplasia were found at 16 weeks in the Black Swiss mice, and at 24 weeks for the NIH Swiss and FVB mice.
Females presented with a higher degree (moderate-severe versus minimal-mild) squamous metaplasia and dysplasia compared to male. In the NIH Swiss strain, high-grade metaplasia was present in 80% of females vs. 17% of males in the 20 mM NTCU group, and 60% of females vs. 50% of males in the 30 mM group. No difference between females and males was observed in the 40 mM dose. For FVB mice, high-grade squamous metaplasia was found in 25% of females vs. 0% of males in the 30 mM group, and 60% of females vs. 33% of males in the 40 mM cohort.
NTCU-induced SCC develops at the same incidence and rate in the 30 mM and 40 mM NTCU doses and shows a gene expression profile comparable to human SCC
In NIH Swiss and FVB mice, SCC are present after 24 weeks of NTCU treatment in the 30 mM and 40 mM NTCU groups. NIH Swiss mice treated with 20 mM NTCU developed invasive SCC after 32 weeks of NTCU treatment, while SCC were not present in the FVB mice treated with the 20 mM dose. The percentage of mice treated with NTCU for 24–32 weeks presenting SCC in the 20 mM, 30 mM and 40 mM NTCU groups was: (1) NIH Swiss: 36%, 60% and 57%; and (2) FVB: 0%, 20% and 25%. Only two mice from the Black Swiss strain treated with 30 mM NTCU developed small SCC at 16 weeks, and no tumors were observed for the 20 mM and 40 mM doses at any time point. In addition to the higher percentage of mice found with tumors in the NIH Swiss mice, this strain presented with the largest tumors (>5 mm) as compared to FVB mice. Female NIH Swiss presented with the largest SCC (>5 mm) as compared with male. No SCC were found in the untreated or acetone treated control animals.
Total gene expression of normal lung and SCC from NIH Swiss mice was determined by RNA sequencing and compared with three independent public human datasets. Mouse expression data was consistent with genes up- and downregulated in the human data. The analysis of the top 150 genes upregulated in the human datasets showed they were also upregulated in the mouse SCC, while the top 150 genes downregulated in the human data were downregulated in mouse (Supplementary Fig. S1).
In addition to lung SCC, there was one example of adenosquamous carcinoma with a central adenomatous foci and peripheral squamous cell differentiation in the NIH Swiss 30 mM NTCU group as previously described (19). Spontaneous adenomas/adenocarcinomas without squamous differentiation were also observed at low frequency (3%) in all the groups of the FVB strain, as previously described, and have not been included in the analysis (11,13).
NTCU induces inflammation and damage of upper and lower respiratory tract epithelium with variable incidence depending on mouse strain
In addition to lung SCC, NTCU induced pre-neoplastic changes in the nasal and trachea epithelia and was associated with non-neoplastic lesions in the lung, such as peribronchiolar fibrosis, inflammation, foamy macrophage accumulations and pneumonia. Incidence of other respiratory tract and off-target lesions on NTCU-treated animals is summarized in Table 1. Neutrophilic exudative rhinitis and sinusitis was found in the three mouse strains examined, being more frequent in the Black Swiss mice (70%) compared to the NIH Swiss and FVB (28% and 9% respectively). In the Black Swiss strain, rhinitis and sinusitis was frequently accompanied by nasal epithelia erosions with hyperplasia and metaplasia. Trachea epithelia developed hyperplasia and dysplasia in the three mouse strains assayed with similar incidence (97% of NIH Swiss, 88% of Black Swiss and 82% of FVB). Non-neoplastic lesions were present in the lungs and included pneumonia and peribronchiolar fibrosis, typically accompanied by loss of respiratory epithelium and lymphoid aggregates (Supplementary Fig. S2). Pneumonic lesions varied from acute neutrophilic to histiocytic to organizing with fibrosis. The highest incidence of pneumonia occurred in the Black Swiss group, with 73% of the mice developing pneumonia. Black Swiss mice from the three NTCU doses prematurely died between 77 to 154 days of treatment, probably due to the development of NTCU related rhinitis, sinusitis and pneumonia. Lower incidence of pneumonia was present in NIH Swiss mice (7%) and FVB (9%). No differences were observed by sex.
Table 1. Incidence of respiratory tract and off-target lesions on NTCU-treated animals.
Percentage of animals with the indicated lesions is shown.
| NIH Swiss | Black Swiss | FVB | ||||
|---|---|---|---|---|---|---|
| Acetone | NTCU | Acetone | NTCU | Acetone | NTCU | |
| Respiratory Tract | ||||||
| Nasal cavity | ||||||
| Hyperplasia / Metaplasia | 0 | 0 | 0 | 13 | 0 | 0 |
| Rhinitis / Sinusitis | 0 | 28 | 0 | 70 | 0 | 9 |
| Trachea Hyperplasia / Dysplasia | 0 | 97 | 0 | 88 | 0 | 82 |
| Lung | ||||||
| Peribronchiolar Fibrosis | 0 | 37 | 0 | 47 | 0 | 18 |
| Lymphoid Aggregates | 0 | 17 | 0 | 33 | 0 | 12 |
| Pneumonia | 0 | 7 | 0 | 73 | 0 | 9 |
| Off target effects | ||||||
| Dermatitis at application site | 0 | 83 | 25 | 67 | 0 | 19 |
| Ear auricular dermatitis | 0 | 15 | 0 | 0 | 0 | 0 |
| Hyperplastic accessory sex glands | 0 | 0 | 0 | 17 | 0 | 58 |
The most common off-target effect of the NTCU was dermatitis at the application site for the three mouse strains studied. Ulcerative chronic proliferative dermatitis at the application site, frequently accompanied by dermal fibrosis, was found in 83% of NIH Swiss mice treated with NTCU and at lower frequency in Black Swiss (67%) and FVB (19%). NTCU-related severe ear auricular chondritis and dermatitis was present at low incidence (15%) exclusively in the NIH Swiss mice. There was no histologic evidence of neoplasia in the pinnae of three of the more severely affected. We also observed the development of perianal swelling in FVB and Black Swiss mice treated with NTCU, being more frequent in FVB mice (58%). Swelling occurred in both female and male with the same incidence. Histologic analysis of this region showed cystic hyperplastic sexual glands in the treated mice. One FVB mouse developed esophageal and gastric SCC. No signs of further NTCU-meditated toxicity or neoplasia was present in the other organs histologically examined.
Evaluation of tumor burden by lung weight is not specific for cancer due to the confounding pneumonia
Increase in lung weight was observed in NIH Swiss and Black Swiss mice treated with NTCU, but not in the FVB group. For the NIH Swiss mice, lung weight was significantly higher in mice treated with 30 mM NTCU for 24 weeks (control 0.30 ± 0.03 g, 30 mM NTCU 0.50 ± 0.09 g, p=0.0009), and in mice treated with 20 mM and 30 mM NTCU for 32 weeks (control group: 0.32 ± 0.04 g; 20 mM NTCU: 0.42 ± 0.07 g, p=0.0023; 30 mM NTCU: 0.46 ± 0.07 g, p=0.0001). In the Black Swiss cohort, lung weight was significantly higher in mice treated with 30 mM NTCU for 16 weeks (30 mM NTCU 0.48 ± 0.10 g vs. control 0.28 ± 0.05 g, p=0.043), and in mice treated with 20 mM NTCU for 24 weeks (20 mM NTCU 0.58 ± 0.07g vs. untreated and acetone control 0.32 ± 0.04g, p<0.0001). No significant differences in lung weight were observed in the FVB mice between NTCU treated and untreated groups. Tumor burden, calculated as the percentage of tumor area of the total lung area in histological sections, showed correlation with lung weight only for the NIH Swiss cohort (R2 = 0.5769, F(1,19) = 25.91, p<0.0001) (Fig. 2A). No correlation between lung weight and tumor burden was found for the FVB group (R2 = 0.0831, F(1,3) = 0.2720, p=0.6381). For the Black Swiss mice, there was not enough data to study correlation between lung weight and tumor burden, as only two mice in this group develop SCC. However, Black Swiss mice developed NTCU related pneumonia and lung weight was significantly higher for those mice with moderate to severe pneumonia compared with those mice with no inflammation (mean ± SD; no inflammation 0.283 ± 0.05 g, minimal 0.329 ± 0.04 g, mild 0.377 ± 0.05 g, moderate 0.439 ± 0.11 g, and severe 0.542 ± 0.09 g; no inflammation vs. moderate p=0.0064, no inflammation vs. severe p<0.0001). (Fig. 2B).
Figure 2. Lung weight correlates with tumor burden determined by histology and inflammation grade.
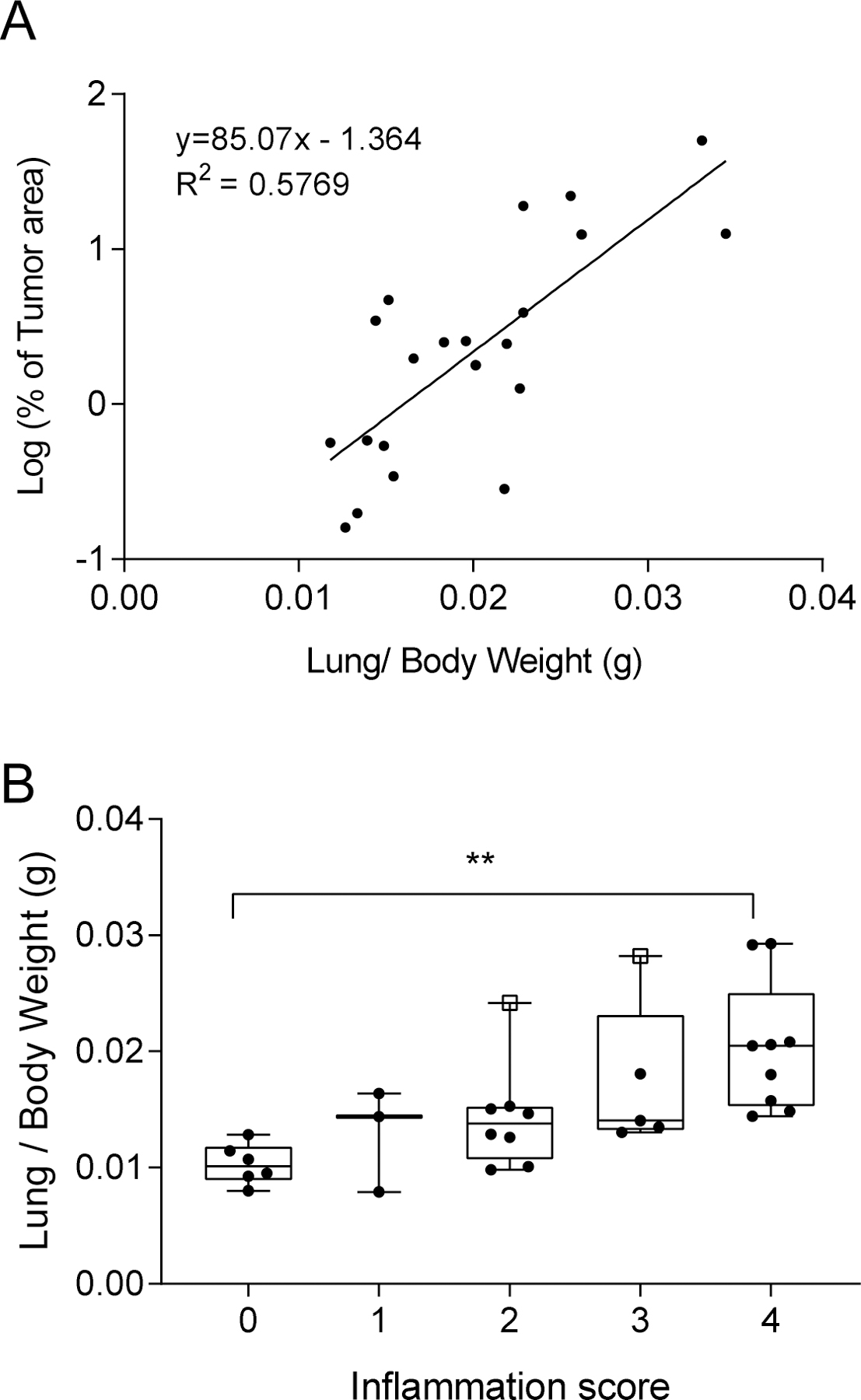
(A) Percentage of tumor area measured in H&E sections related to lung weight in NIH Swiss mice. (B) Lung weight of Black Swiss mice without pneumonia (Grade 0) and mice with pneumonia classified by inflammation grade. No inflammation (0), minimal (1), mild (2), moderate (3) and severe (4). The two Black Swiss mice presenting tumors are shown as open squares.
Treatment with NTCU reduces spleen cellularity without affecting ability to mount a robust immune response
The total number of splenocytes was lower in mice treated with 40 mM NTCU for four weeks (Fig. 3A) compared with the untreated cohorts, although no change in viability was observed between the groups (Supplementary Table S4). Regardless of the decrease of cellularity in spleen, animals treated with NTCU demonstrated a robust and specific immune response similar to the vaccinated untreated group (Fig. 3B). Analysis of the different immune cell populations in splenocytes by flow cytometry showed a significant decrease in the regulatory myeloid-derived suppressor cell population (MDSC, CD11b+ Gr1+) in the NIH Swiss mice treated with NTCU simultaneously to vaccination (p=0.0024), but not for the FVB mice (Supplementary Table S4). In addition, in NIH Swiss mice, the percentage of CD8+ PD-1+ T cells is lower in mice treated with NTCU either subsequently (p=0.0248) or simultaneously (p<0.0001) to vaccination. Percentage of CD4+ PD-1+ T cells is also lower in NIH Swiss mice treated with NTCU concomitantly to vaccination (p=0.0406). No changes in PD-1 expression was observed in FVB mice. Percentage of CD3+, CD4+, CD8+ T cells and CD4+FoxP3+ T-regulatory cells (Treg) did not change in the different treatment groups.
Figure 3. Treatment with NTCU reduces spleen cellularity without affecting ability to mount a robust immune response.

NIH Swiss (left) and FVB (right) mice vaccinated with PBS (white), IGF-IR (black), IGF-IR followed by NTCU treatment (dark grey) or IGF-IR concomitant with NTCU treatment (light grey). (A) Total number of splenocytes. (B) IFN-γ ELISPOT conducted on splenocytes from vaccinated animals, five animals per group. Concanavalin A (Con A) was used as positive control and HIV peptide (HIVp17) was used as non-specific response control. CSPW: corrected spots per well. Mean and SEM is shown. * p<0.05, ** p<0.01, *** p<0.001.
The effect of long-term treatment with NTCU on the immune system was evaluated in mice untreated or treated with 20 mM or 30 mM NTCU for 24–32 weeks. NIH Swiss mice treated with 30 mM NTCU showed a moderate but significant higher percentage of CD4+ FoxP3+ Tregs cells compared to the untreated and acetone treated groups (p=0.01, Supplementary Table S5). There were no significant changes in the percentage of CD3+, CD4+, CD8+ T cells or MDSC cells (Supplementary Table S5). In addition, no differences in the percentage of PD-1 expressing CD4+ and CD8+ T cells were observed between the different treatment groups.
NIH Swiss mice treated with NTCU presented CD4+ and CD8+ T cells accumulated in lymphoid aggregates close to main bronchi and around blood vessels, and were also present in the lung tissue surrounding the tumor (Supplementary Fig. S3). However, percentages of CD4+ and CD8+ T cells within the tumor were low (<5%) and there were not significant differences between female and male mice (female vs. male % of CD4+ p=0.9731, % of CD8+ p=0.9913). PD-L1 expression was heterogeneous within the tumor and between mice, and there was not significant difference between female and male (p=0.0730). At an arbitrary cutoff of 1% for PD-L1 expression, 67% of the mice were PD-L1 positive.
Discussion
The main findings in this study are: (1) NIH Swiss mice present with a higher incidence of SCC and lower mortality and toxicity compared with Black Swiss and FVB mice; (2) 30 mM NTCU dose induces SCC at the same rate and incidence than the 40 mM dose with lower toxicity and mortality in treated mice; (3) female mice present higher grade and incidence of pre-invasive lesions and SCC compared with male; (4) NTCU induced transformation is principally within the respiratory system; and (5) NTCU treatment does not impact the ability to elicit a specific adaptive immune response in FVB and NIH Swiss strains.
Variation in mouse strain vulnerability to cancer has been previously reported in the literature (20–22). Wang et al. studied the mouse strain susceptibility to lung cancer after treatment with 40 mM NTCU and classified the strains as resistant, intermediate or susceptible to NTCU lung carcinogenesis (5). The study included C57BL/6N (resistant, no SCC), FVB (intermediate, SCC incidence 44%) and NIH Swiss mice (susceptible, SCC incidence 83%) among others. In addition to NIH Swiss and FVB mice, we have included Black Swiss strain, which has not been previously evaluated in the NTCU lung cancer model. Our study confirms the previously reported susceptibility for NIH Swiss and FVB (5,11). Confounding pneumonia together with the low incidence of SCC in Black Swiss mice dissuades the use of this strain for this model. NIH Swiss presented with a higher incidence of SCC and lower mortality as compared with FVB. Therefore, we proposed that NIH Swiss mice should be used for those studies where endpoint is prevention or treatment of SCC.
Treatment with 40 mM NTCU twice a week has been the standard to induce SCC in mice, yet this dose severely reduce survival of mice (10,11). Hudish et al showed that doses of 4 and 10 mM in FVB mice are better tolerated, although do not induce dysplasia nor SCC. Here we show that decreasing concentration of NTCU to 20 mM or 30 mM improves mouse health and reduces mortality associated with treatment, while still inducing high-grade dysplasia and SCC. Furthermore, treatment with 30 mM NTCU results in development of dysplasia and SCC at the same rate and incidence as the 40 mM dose. Therefore, we suggest standardizing the 30 mM NTCU dose for those studies evaluating treatments for SCC or prevention of progression from dysplasia to SCC. Treatment with 20 mM NTCU can also be useful for prevention studies, as the disease progress slowly at this dose, giving more time for the preventive agents to work.
This is the first study evaluating NTCU toxicity in multiple organs. NTCU induces chronic inflammation and transformation of respiratory epithelium. Dysplasia was observed in the trachea epithelia for all the strains evaluated, as previously described for FVB (23). Extensive NTCU-induced pneumonia was present in Black Swiss mice. Other NTCU-related lesions included ear auricular chondritis, dermatitis and cystic changes in the accessory sex glands. One FVB mouse developed esophageal and gastric SCC, which is likely related to NTCU- exposure, as these are extremely rare spontaneous tumors in the FVB strain. No signs of further NTCU-meditated toxicity or neoplasia were present in the other organs histologically examined in any of the mouse strains.
Sex is an important factor in cancer incidence, prognosis and outcome (24–26). There are also significant sex differences in the prevalence and grade of respiratory diseases and lung cancer (27–29). Women are more susceptible to tobacco carcinogens and present increased risk for lung cancer development compared with men when adjusting for age and smoking history (30–32). In addition, females develop lung cancer at a younger age, even with less cigarettes consumed than males (25,33). However, in vivo studies exploring cancer therapies in both sexes are rare. There is only one study for the NTCU lung cancer model that included female and male FVB mice, and the authors reported that females have greater dysplasia per area of lung compared with males (11). We have shown that for every strain studied, females present higher-grade dysplasia, higher SCC incidence and larger SCC lesions compared to males. Female C57BL/6 mice have greater numbers of type 2 inflammation cells in the lungs (34), and sex hormones contribute to lung inflammation and modulate the PD-1/PD-L1 pathway (35,36). In order to account for this sex based heterogeneity in tumor development and treatment responses, both female and male animals should be included in the evaluation of lung cancer therapies.
The percentage of CD4+ and CD8+ T cells, as well as PD-L1 expression was heterogeneous within the tumor. Heterogeneity in TILs and PD-L1 expression has also been reported in human non-small cell lung cancer (37–39). Percentage of T cells in the mouse model was similar to the percentages observed in human non-small cell lung cancer (<5% TILs in >70% of patients) (38). At a cut-off of 1% for PD-L1 expression, 48% of human lung SCC are PD-L1+ (39), which is comparable to the 67% PD-L1+ SCC observed in the mouse model.
Finally, we found that NTCU treatment reduces the total number of splenocytes in a dose dependent manner. However, reduced cellularity does not affect the ability to mount a robust and specific T cell response. In addition, we did not find increase in the expression of checkpoint inhibitors (PD-1) in the splenocytes of mice treated with NTCU, although there was a modest increase of CD4+ T regulatory cells in NIH Swiss mice treated with higher doses of NTCU. Therefore, NTCU does not have immunosuppressive effects, and we believe that this chemically induced lung cancer model is appropriate for evaluation of immunotherapies.
The NTCU SCC mouse model has several properties that resemble human lung cancer: heterogeneity of tumors, increased mutation burden, slow progression and presence of pre-malignant lesions that evolve to invasive SCC similarly to the disease in lung cancer patients. Characterization of chemically induced tumor models is necessary to understand their advantages and limitations. This study provides a reference point for experimental designs to evaluate either preventive or therapeutic treatments for lung SCC, including immunotherapies, before initiating human clinical trials.
Supplementary Material
Acknowledgements
The authors wish to thank the staff of the University of Washington Comparative Pathology Program/Histology and Imaging Core Research Laboratory, especially Brian Johnson and Megan Larmore, for their contributions to slide production and histochemical staining. Thanks to Rebecca Hull-Meichle and Nishi Ivanov, from the University of Washington Diabetes Research Center, for the help with laser capture microdissection. We would also like to thank the staff at the NextGen Sequencing Core Facility at the Fred Hutchinson Cancer Research Center (FHCRC) for their support for the FFPE RNA sample preparation and RNA sequencing.
Financial support. This work was supported by the National Institutes of Health [HHSN261201200013I-HHSN26100009]. MLD is supported by the Helen B. Slonaker Endowed Professor for Cancer Research and the American Cancer Society Clinical Research Professor
Footnotes
Conflict of Interest Statement. L.R., E.A.G., P.M.T., A, E. T., E.A.H., L.R.C. and E.R. have no conflicts of interest. M.L.D. is a stockholder in Epithany and receives grant support from Celgene, EMD Serono, Epithany, Pfizer, Silverback Therapeutics, and Seattle Genetics.
References
- 1.McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, et al. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America 2016;113(42):E6409–E17 10.1073/pnas.1613601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busch SE, Hanke ML, Kargl J, Metz HE, MacPherson D, Houghton AM. Lung Cancer Subtypes Generate Unique Immune Responses. Journal of immunology 2016;197(11):4493–503 10.4049/jimmunol.1600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor-bearing neu transgenic mice predicts human tumor antigens. Cancer research 2006;66(19):9754–61 10.1158/0008-5472.CAN-06-1083. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Rouggly L, You M, Lubet R. Animal models of lung cancer characterization and use for chemoprevention research. Prog Mol Biol Transl Sci 2012;105:211–26 10.1016/B978-0-12-394596-9.00007-X. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang Z, Yan Y, Lemon WJ, LaRegina M, Morrison C, et al. A chemically induced model for squamous cell carcinoma of the lung in mice: histopathology and strain susceptibility. Cancer research 2004;64(5):1647–54. [DOI] [PubMed] [Google Scholar]
- 6.You MS, Rouggly LC, You M, Wang Y. Mouse models of lung squamous cell carcinomas. Cancer Metastasis Rev 2013;32(1–2):77–82 10.1007/s10555-012-9406-4. [DOI] [PubMed] [Google Scholar]
- 7.Goodsell DS. The molecular perspective: nicotine and nitrosamines. Oncologist 2004;9(3):353–4. [DOI] [PubMed] [Google Scholar]
- 8.Azpilikueta A, Agorreta J, Labiano S, Perez-Gracia JL, Sanchez-Paulete AR, Aznar MA, et al. Successful Immunotherapy against a Transplantable Mouse Squamous Lung Carcinoma with Anti-PD-1 and Anti-CD137 Monoclonal Antibodies. J Thorac Oncol 2016;11(4):524–36 10.1016/j.jtho.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Xiong D, Pan J, Yin Y, Jiang H, Szabo E, Lubet RA, et al. Novel mutational landscapes and expression signatures of lung squamous cell carcinoma. Oncotarget 2018;9(7):7424–41 10.18632/oncotarget.23716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosini V, Nanni C, Pettinato C, Fini M, D’Errico A, Trepidi S, et al. Assessment of a chemically induced model of lung squamous cell carcinoma in mice by 18F-FDG small-animal PET. Nucl Med Commun 2007;28(8):647–52 10.1097/MNM.0b013e32823f9ffa. [DOI] [PubMed] [Google Scholar]
- 11.Hudish TM, Opincariu LI, Mozer AB, Johnson MS, Cleaver TG, Malkoski SP, et al. N-nitroso-tris-chloroethylurea induces premalignant squamous dysplasia in mice. Cancer prevention research 2012;5(2):283–9 10.1158/1940-6207.CAPR-11-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet 2012;3:268 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer research 2004;64(7):2307–16. [DOI] [PubMed] [Google Scholar]
- 14.Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, et al. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol 2009;37(7 Suppl):5S–73S 10.1177/0192623309353423. [DOI] [PubMed] [Google Scholar]
- 15.Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep 2017;7(1):16878 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disis ML, Gad E, Herendeen DR, Lai VP, Park KH, Cecil DL, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer prevention research 2013;6(12):1273–82 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer research 2008;68(20):8400–9 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15(12):550 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehm S, Lijinsky W, Singh G, Katyal SL. Mouse bronchiolar cell carcinogenesis. Histologic characterization and expression of Clara cell antigen in lesions induced by N-nitrosobis-(2-chloroethyl) ureas. Am J Pathol 1991;139(2):413–22. [PMC free article] [PubMed] [Google Scholar]
- 20.Puccini J, Dorstyn L, Kumar S. Genetic background and tumour susceptibility in mouse models. Cell Death Differ 2013;20(7):964 10.1038/cdd.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly KM. The Effects of Genetic Background of Mouse Models of Cancer: Friend or Foe? Cold Spring Harb Protoc 2016;2016(3): 10.1101/pdb.top076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klopstock N, Katzenellenbogen M, Pappo O, Sklair-Levy M, Olam D, Mizrahi L, et al. HCV tumor promoting effect is dependent on host genetic background. PLoS One 2009;4(4):e5025 10.1371/journal.pone.0005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh M, Dwyer-Nield LD, Kwon JB, Barthel L, Janssen WJ, Merrick DT, et al. Tracheal dysplasia precedes bronchial dysplasia in mouse model of N-nitroso trischloroethylurea induced squamous cell lung cancer. PLoS One 2015;10(4):e0122823 10.1371/journal.pone.0122823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 2011;20(8):1629–37 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visbal AL, Williams BA, Nichols FC 3rd, Marks RS, Jett JR, Aubry MC, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg 2004;78(1):209–15; 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Hsu LH, Chu NM, Liu CC, Tsai SY, You DL, Ko JS, et al. Sex-associated differences in non-small cell lung cancer in the new era: is gender an independent prognostic factor? Lung Cancer 2009;66(2):262–7 10.1016/j.lungcan.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Carey MA, Card JW, Voltz JW, Arbes SJ Jr., Germolec DR, Korach KS, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 2007;18(8):308–13 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsslund H, Yang M, Mikko M, Karimi R, Nyren S, Engvall B, et al. Gender differences in the T-cell profiles of the airways in COPD patients associated with clinical phenotypes. Int J Chron Obstruct Pulmon Dis 2017;12:35–48 10.2147/COPD.S113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto JA, Vallejos CS, Raez LE, Mas LA, Ruiz R, Torres-Roman JS, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3(3):e000344 10.1136/esmoopen-2018-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88(3–4):183–92. [DOI] [PubMed] [Google Scholar]
- 31.Lubin JH, Blot WJ. Assessment of lung cancer risk factors by histologic category. J Natl Cancer Inst 1984;73(2):383–9. [DOI] [PubMed] [Google Scholar]
- 32.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol 1993;138(5):281–93. [DOI] [PubMed] [Google Scholar]
- 33.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer 1987;59(10):1825–30. [DOI] [PubMed] [Google Scholar]
- 34.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, et al. A Major Population of Functional KLRG1(−) ILC2s in Female Lungs Contributes to a Sex Bias in ILC2 Numbers. Immunohorizons 2018;2(2):74–86 10.4049/immunohorizons.1800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 2006;84(2):370–8 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 36.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1). Int Immunol 2007;19(3):337–43 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 37.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015;107(3) 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14(16):5220–7 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 39.Munari E, Zamboni G, Lunardi G, Marchionni L, Marconi M, Sommaggio M, et al. PD-L1 Expression Heterogeneity in Non-Small Cell Lung Cancer: Defining Criteria for Harmonization between Biopsy Specimens and Whole Sections. J Thorac Oncol 2018;13(8):1113–20 10.1016/j.jtho.2018.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


