ABSTRACT
CD47 is a ubiquitously expressed cell surface glycoprotein that functions as a signaling receptor for thrombospondin-1 and as the counter-receptor for signal regulatory protein-α (SIRPα). Engaging SIRPα on macrophages inhibits phagocytosis, and CD47 thereby serves as a physiological marker of self. However, elevated CD47 expression on some cancer cells also protects tumors from innate immune surveillance and limits adaptive antitumor immunity via inhibitory SIRPα signaling in antigen-presenting cells. CD47 also mediates inhibitory thrombospondin-1 signaling in vascular cells, T cells, and NK cells, and blocking inhibitory CD47 signaling on cytotoxic T cells directly increases tumor cell killing. Therefore, CD47 functions as an innate and adaptive immune checkpoint. These findings have led to the development of antibodies and other therapeutic approaches to block CD47 functions in the tumor microenvironment. Preclinical studies in mice demonstrated that blocking CD47 can limit the growth of hematologic malignancies and solid tumors and enhance the efficacy of conventional chemotherapy, radiation therapy, and some targeted cancer therapies. Humanized CD47 antibodies are showing promise in early clinical trials, but side effects related to enhanced phagocytic clearance of circulating blood cells remain a concern. Approaches to circumvent these include antibody preloading strategies and development of antibodies that recognize tumor-specific epitopes of CD47, SIRPα antibodies, and bivalent antibodies that restrict CD47 blockade to specific tumor cells. Preclinical and clinical development of antibodies and related biologics that inhibit CD47/SIRPα signaling are reviewed, including strategies to combine these agents with various conventional and targeted therapeutics to improve patient outcome for various cancers.
Keywords: humanized CD47 antibodies, bifunctional antibodies, immune checkpoint, immunotherapy, signal regulatory protein-α
Statement of Significance
Preclinical studies defining the function of CD47 in cancer cells and in modulating anti-tumor immunity have led to the development of humanized CD47 antibodies and related biologics. A growing number are entering clinical trials as single agents or used in combination with other therapeutics for treating various cancers.
INTRODUCTION
CD47 is a signaling receptor for thrombospondin-1 (TSP1) and the counter-receptor for signal regulatory protein-α (SIRPα) [1–3]. CD47 also associates laterally in the plasma membrane with a subset of integrins and regulates their function [1]. Integrin activation mediates CD47 functions in regulating cell adhesion and migration [1]. TSP1 interaction with CD47 regulates its intrinsic signaling functions in multiple cell types and controls nitric oxide/cGMP signaling in vascular cells [1]. The latter has physiological roles in regulating blood pressure, platelet hemostasis, tissue perfusion, and tissue responses to ischemic injuries and genotoxic stress. Conversely, CD47 serves as a ligand to induce inhibitory SIRPα signaling in macrophages [4], which has a physiological role in self-recognition [2]. Loss of inhibitory SIRPα signaling in macrophages results in more rapid turnover of circulating platelets and red blood cells (Fig. 1) [5, 6]. This accounts for the side effects of anemia and thrombocytopenia that are observed in animals and patients treated with CD47-targeted antibodies that block this interaction [7, 8].
Figure 1.
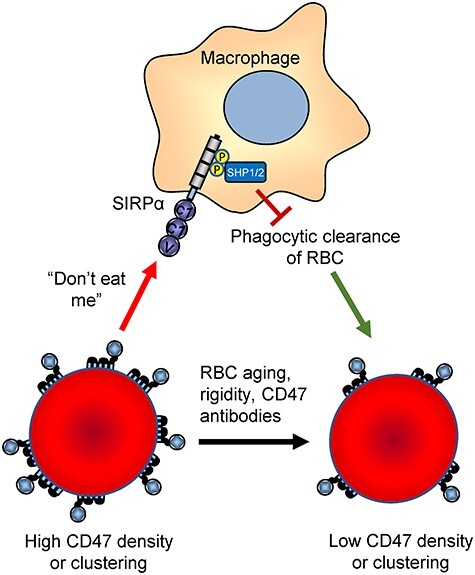
Antiphagocytic function of CD47 on red blood cells (RBCs). Young RBCs express ~25 000 copies of CD47, which inhibits their phagocytic clearance [5]. RBC aging [119], diseases that increase RBC rigidity [120], and exposure to function-blocking CD47 antibodies decrease the SIRPα-mediated “don’t eat me” signal and thereby increase erythrophagocytosis.
The increased CD47 expression on some cancer cells limits their phagocytic clearance by macrophages [2], despite increased expression of pro-phagocytic markers such as calreticulin on cancer cells (Fig. 2A,B) [9, 10] or suppression of the anti-phagocytic markers LILRB1 and CD24/Siglec-10 in cancer cells [11, 12]. Cell-intrinsic inhibitory CD47 signaling in T cells, macrophages, dendritic cells, and NK cells plays additional roles in immune regulation, cell survival, and death signaling (Fig. 2A) [1, 13–15]. Based on the hypothesis that the CD47/SIRPα interaction represents a major innate immune checkpoint in cancer [16], several antibodies and other antagonists of CD47 binding to SIRPα have entered Phase 1 and 2 clinical trials [3] (Table 1). This review focuses on the preclinical development of therapeutic antibodies targeting CD47 and SIRPα, ongoing clinical trials, and future challenges and opportunities for developing effective CD47-targeted cancer therapeutics.
Figure 2.
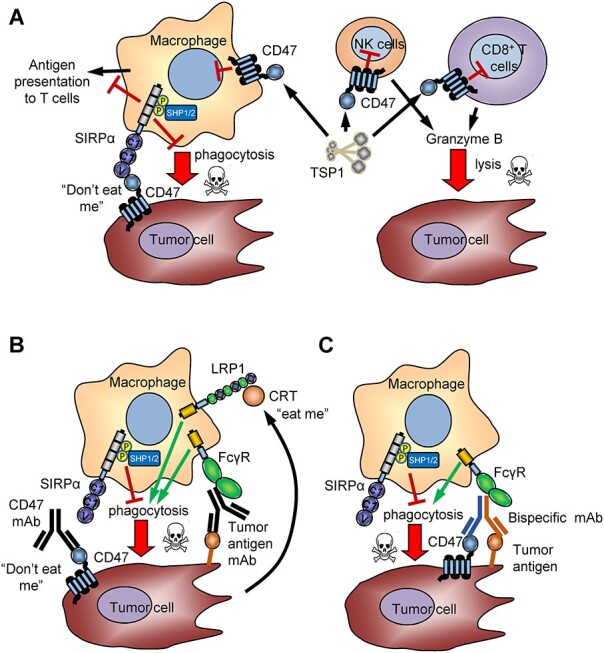
CD47 functions in the tumor microenvironment. A) CD47 on tumor cells induces inhibitory SIRPα signaling that prevents macrophage phagocytosis and antigen presentation. Thrombospondin-1 induces CD47 signaling in CD8 T cells and NK cells that inhibits lytic tumor cell killing [15, 113]. B) CD47 antibodies that block SIRPα binding relieve the inhibitory signal in macrophages and antigen-presenting cells, enabling pro-phagocytic signals from tumor-secreted calreticulin or tumor-specific antibodies to activate ADCP and ADCC. C) Bispecific CD47 antibodies enhance selective blocking of CD47 on tumor cells and induce ADCP and/orADCC.
Table 1.
Active and completed clinical trials using CD47 antibodies and related biologics
| Antibody | Molecule | Indications | Type of trial | Registry |
|---|---|---|---|---|
| Hu5F9-G4/Magrolimab (Forty Seven Inc/Gilead) | CD47 mAb | Solid tumors | Single agent | NCT02216409, NCT02678338 |
| Hu5F9-G4/Magrolimab | CD47 mAb | B cell lymphoma | +Rituximab | NCT02953509 |
| Hu5F9-G4/Magrolimab | CD47 mAb | Colorectal cancer | +Cetuximab | NCT02953782 |
| Hu5F9-G4/Magrolimab | CD47 mAb | Hematologic | +Azacitidine | NCT03248479 |
| Hu5F9-G4/Magrolimab | CD47 mAb | Non-Hodgkin’s Lymphoma | PRISM Study | NCT03527147 |
| CC-90002 (Celgene) | CD47 mAb | AML, MDS | Single agent | NCT02488811, NCT02641002 |
| CC-90002 | CD47 mAb | Solid & hematologic cancers | +Rituximab | NCT02367196 |
| IBI188 (Innovent Biologics) | CD47 mAb | Advanced malignancies | Single agent | NCT03763149 |
| IBI188 (Innovent Biologics) | CD47 mAb | Advanced malignancies | +Rituximab | NCT03717103, |
| TTI-621 (Trillium Therapeutics) | SIRPα-Ig fusion protein | Solid tumors and mycosis fungoides | Single agent +PD-1/PDL1 Inhibitor +PEG-IFN2a +T-Vec + radiation |
NCT02890368 |
| TTI-621 | SIRPα-Ig fusion protein | Solid & hematologic cancers | +Rituximab +Nivolumab |
NCT02663518 |
| TTI-622 (trillium) | SIRPα-IgG4 fusion protein | Lymphoma or myeloma | Single agent + Rituximab + PD-1 Inhibitor +Proteasome-inhibitor |
NCT03530683 |
| SRF231 (Surface Oncology) | CD47 mAb | Solid tumors, hematologic | Single agent | NCT03512340 |
| AO-176 (Arch Oncology) | CD47 mAb | Solid tumors | Single agent | NCT03834948 |
| ALX148 (Alexo Therapeutics) | SIRPα-Fc* fusion protein | Solid tumors and non-Hodgkin’s lymphoma | +Pembrolizumab +Trastuzumab +Rituximab +Trastuzumab, Ramucirumab, Paclitaxel |
NCT03013218 |
| ALX148 | SIRPα-Fc* fusion protein | Myelodysplastic SYNDROME | + Azacitidine | NCT04417517 |
| CC-95251 (Celgene) | SIRPα mAb | Solid tumors, hematologic cancers | +Rituximab +Cetuximab |
NCT03783403 |
| TG-1801 (TG Therapeutics) | CD47/CD19 bsAb | B-cell lymphoma | Single agent | NCT03804996 |
| BI765063/OSE-172 (Boehringer Ingelheim/OSE) | SIRPα mAb | Solid tumors | + PD-1 mAb | NCT03990233 |
| SGN CD47M (Seattle Genetics) | mAb-drug conjugate | Solid tumors | Single agent | NCT03957096 |
| IBI-322 (Innovent Biologics) | CD47/PD-L1 bsAb | Advanced tumors | Single agent | NCT04338659, NCT04328831 |
| HX-009 (Waterstone Hanxbio) | PD-1/CD47 bsAb | Solid tumors | Single agent | NCT04097769 |
| IMC-002 (ImmuneOncia) | CD47 mAb | Solid tumors and lymphoma | Single agent | NCT04306224 |
Preclinical studies using CD47 antibodies
Several antibodies have been identified that alter specific functions of CD47 by directly inhibiting its interactions with TSP1, SIRPα, integrins, or growth factor receptors (Table 2). Other CD47 antibodies exhibit agonist or antagonist activities that may result from altering clustering or inducing conformational changes that alter the intrinsic intracellular signaling functions of CD47. B6H12 is a function-blocking antibody that inhibits CD47 interactions with TSP1, SIRPα, some integrins, and EGF receptor [17–20]. B6H12 inhibits TSP1-dependent activation of αvβ3 integrin while increasing the activation of α4β1 integrin [21, 22]. Relevant to the role of CD47 in tumor immunology, B6H12 directly induces killing of tumor cells by NK cells [23], B cell migration [24], and regulatory T cell differentiation [25]. B6H12 blocks the inhibition of T and NK cell activation by TSP1 [13, 15]. B6H12 inhibits homotypic aggregation of monocytic cells [26], extravasation of neutrophils [27], production of mature IL-1β by macrophages [14], and the maturation and function of dendritic cells [28]. B6H12 also acts on other cell types in the tumor microenvironment, inhibiting nitric oxide signaling and angiogenic responses [29, 30], protecting nonmalignant cells from death and DNA damage caused by ionizing radiation or cytotoxic chemotherapy [31, 32], inhibiting uptake and functional responses in endothelial cells induced by extracellular vesicles released by breast cancer stem cells (CSC) [33], forcing differentiation of CSC [20], and inhibiting CD47-dependent platelet adhesion under flow [34]. Thus, although preclinical cancer studies generally interpret B6H12 inhibition as evidence for SIRPα-mediated functions of CD47 in vitro and in xenograft models, such inhibition may result from SIRPα-independent mechanisms including perturbing TSP1- or integrin-mediated activities ofCD47.
Table 2.
Properties of preclinical and clinical CD47 antibodies and related inhibitors of CD47 function
| Antibody Name | Source | Origin | Isotype | Specificity | CD47/SIRPα blockade | Agonist activity |
|---|---|---|---|---|---|---|
| MIAP301 | Research | rat | IgG2a | muCD47 | Yes | |
| A4 | Research | Alpaca | Nanobody | muCD47 | Yes | |
| A4-Fc | Research | Fusion | IgG2a | nuCD47 | Yes | |
| OX101 | Research | Mouse | IgG1 | ratCD47 | Yes | |
| B6H12 | Research | Mouse | IgG1 | huCD47 | Yes | Yes/No |
| 2D3 | Research | Mouse | IgG1 | huCD47 | No | |
| Vx1000R | Arch Oncology | Mouse | huCD47 | Yes | ||
| MAB4670 | Research | Mouse | IgG1 | huCD47 | Yes | |
| BRIC126 | Research | Mouse | IgG2b | huCD47 | Yes | Yes/No |
| CC2C6 | Research | Mouse | IgG1 | huCD47 | Yes | Yes |
| 1F7 | Research | Mouse | IgG1 | huCD47 | Yes | |
| Ad22 | Research | Mouse | IgG1 | huCD47 | Yes | |
| mAb400 | Research | Mouse | IgG2a | hu/muCD47 | Yes | |
| MIAP410 | Research | Mouse | IgG1 | hu/muCD47 | Yes/No | |
| MABL sc(Fv)2 | Research | Mouse | (scFv)2 | huCD47 | Yes | |
| MABL scFv | Research | Mouse | scFv | huCD47 | No | |
| Hu5F9-G4 | 47/Gilead | Humanized | IgG4 | huCD47 | Yes | |
| AO-176 | Arch Oncology | Humanized | IgG2 | huCD47 | Yes | Yes |
| AO-104 | Arch Oncology | Humanized | IgG4 | huCD47 | Yes | |
| SRF231 | Surface Oncology | Fully human | IgG4 | huCD47 | Yes | |
| SHR-1603 | Hengrui | Humanized | IgG4 | huCD47 | Yes | |
| CC90002 | Celgene | Humanized | IgG4 | huCD47 | Yes | |
| HuNb1 | Shanghai Novamab | Camel VHH-fusion | IgG4 | huCD47 | Yes | |
| C47B | Janssen | Fully human | IgG1 | huCD47 | Yes | |
| IBI188 | Innovent | Fully human | IgG4 | huCD47 | Yes | |
| ZF1 | Beijing Inst. of Biotechnology | Fully human | IgG1 | huCD47 | Yes | |
| AMMS4-G4 | Beijing Inst. of Biotechnology | Fully human | IgG4 | huCD47 | Yes | |
| h4C1 | CAS Key Laboratory | Humanized | huCD47 | Yes | ||
| CD47xPD-L1 | CAS Key Laboratory | bsAb from h4C1 | IgG4 | CD47xPD-L1 | Yes | |
| IBI-322 | Innovent | bsAb | CD47xPD-L1 | Yes | ||
| HX-009 | Waterstone Hanxbio | bsAb | CD47xPD1 | Yes | ||
| CD47xMSLN | Novimmune | bsAb | IgG1 | CD47xMSLN | Yes | |
| OV-TL3/CD3 | research | bsAb F(ab’)2 | No Fc | CD47xCD3 | ? | |
| CD47xEGFR | Xinxiang Medical University | SIRPαV-bsAb | IgG1 | CD47xEGFR | Yes | |
| HuNb1-rituximab | Shanghai Novamab | Bispecific scFv | No Fc | CD47xCD20 | Yes | |
| RTX-CD47 | University of Groningen | Tandem-scFv | No Fc | CD47xCD20 | Yes | |
| TG-1801 | TG Therapeutics | Fully human bsAb | IgG1 | CD47xCD19 | Yes | |
| CC-95251 | Celgene | Humanized | huSIRPα | Yes | ||
| OSE-172 | OSE Immunotherapeutics | huSIRPα | Yes | |||
| TTI-621 | Trillium | SIRPα fusion | IgG1 | huCD47 | Yes | |
| TTI-622 | Trillium | SIRPα fusion | IgG4 | huCD47 | Yes | |
| ALX148 | Alexo | SIRPα-Fc* fusion | Inactive | huCD47 | Yes |
Several CD47 antibodies directly induce death of cancer cells including 1F7, AD22, MABL, and CC2C6 (Table 2) [35–37]. These antibodies are selective CD47 agonists that in some cases do not induce death of nonmalignant cells and instead exhibit cytoprotective activities in the latter (Fig. 3). CD47 agonist antibodies are also being developed as potential cancer therapeutics [38]. Soluble AD22 and 1F7 antibodies induced apoptosis of Jurkat T lymphoma cells as well as CD3ε-stimulated PBMC [35]. Similar results were reported for CC2C6 [36]. AD22 recognizes the IgV domain of CD47 proximal to epitopes defined by B6H12 and 1F7, whereas 2D3 reacts with a distant epitope. Soluble B6H12 and 2D3 antibodies did not induce cell death, but B6H12 can induce death of some cancer cells when immobilized [35, 39]. 1F7 also induced death of several breast cancer cell lines [40]. 1F7-induced killing of breast cancer cells was not mediated by active caspases, Bcl-2 degradation, or mitochondrial cytochrome c release but involved inhibiting protein kinase A via Giα [40]. Similar induction of apoptosis was observed in B cell chronic lymphocytic leukemia cells exposed to immobilized CD47 antibody BRIC126 but not when the antibody was used in solution [39]. This was mediated by changes in the actin cytoskeleton that resulted in type III programmed cell death [41].
Figure 3.
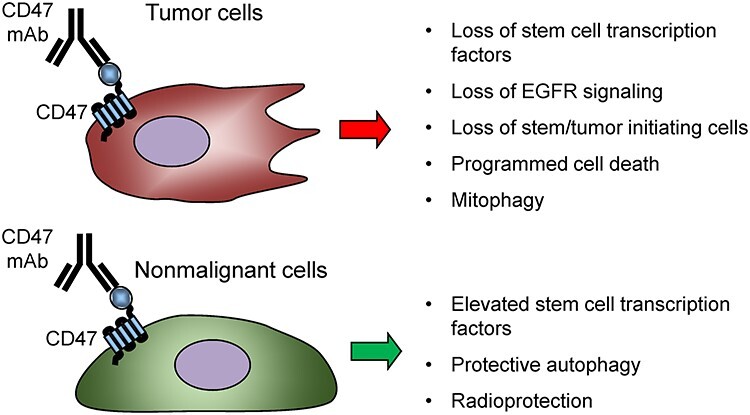
Direct effects of CD47 antibodies on tumor cells. Several CD47 antibodies induce signaling that causes cancer cell death (1F7, AD22, CC2C6, and AO-176) or suppress cancer stem cells (B6H12). In contrast, blocking CD47 in nonmalignant cells preserves stem cells and can be cytoprotective [31, 121].
CD47 antibodies that induce apoptosis only when immobilized may act by inducing clustering of CD47 in the membrane. This is consistent with the ability of dimeric bivalent CD47 scFv derived from MABL but not a MABL scFv monomer to induce apoptosis of leukemic cells in vitro and inhibit multiple myeloma (MM) growth in a mouse xenograft model [37, 42].
Another issue for evaluating CD47 antibodies is that binding may depend on or be limited by specific post-translational modifications of CD47. For example, the antibody CC2C6 binds selectively to CD47 with an N-terminal pyroglutamyl residue, which is formed by QPTCL-mediated enzymatic cyclization of the N-terminal Gln and is required for SIRPα binding [43]. CC2C6 resembles B6H12 in blocking binding to SIRPα [18], but CC2C6 differs from B6H12 in that only soluble CC2C6 antibody induces caspase-independent programmed cell death in acute lymphoblastic leukemia (ALL) cells [36]. CC2C6 also synergized with low dose cytotoxic chemotherapeutic agents to enhance apoptosis.
Preclinical CD47 antibodies that induce tumor cell phagocytosis by blocking SIRPα binding
B6H12
B6H12 was one for the first CD47 antibodies shown to inhibit its binding to SIRPα and thereby enhance macrophage phagocytosis [18]. This was extended to cancer cells with the observation that B6H12 enhanced antibody-dependent cellular phagocytosis (ADCP) of human acute myeloid leukemia (AML) cells in vitro by human and mouse macrophages [44]. This prophagocytic activity of B6H12 extends to many cancer cell types [2]. In mice, tolerance to human cell xenografts is conferred by the NOD mutation of murine SIRPα, which enables human CD47 to inhibit phagocytosis by engaging murine SIRPα with high affinity [45, 46]. Thus, B6H12 inhibited AML tumor growth in NOD/SCID/IL2Rγ− (NSG) mice by blocking this “don’t eat me” signal [44]. The antitumor activity of B6H12 in NSG mice extends to a number of other hematologic malignancy and solid tumor xenografts [2, 44, 47–59]. Conjugating B6H12 with a near-infrared photosensitizer enhanced its pro-phagocytic activity in vitro and efficacy in a bladder carcinoma xenograft photoimmunotherapy model [60]. B6H12 was also effective when combined with the small molecule TLR4 agonist pyrimido [5,4-b] indole [61]. Combination therapy resulted in enhanced macrophage-mediated ADCP of Daudi cells relative to pyrimido [5,4-b] indole or B6H12 monotherapy.
B6H12 was used in several preclinical studies to explore combining CD47 blockade with therapeutic antibodies that target specific cancers. B6H12 synergized with the CD20 antibody rituximab to promote phagocytosis in vitro and eradicated non-Hodgkin lymphoma in NSG mice [62]. The proposed mode of action of B6H12 (IgG1) as monotherapy and in this combination (Fig. 2A) involves increased ADCP but was independent of antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), or apoptosis induced by B6H12. However, SIRPα signaling enhances ADCC of both malignant and nonmalignant cells [63–65]. F(ab′)2 fragments of B6H12 potently and synergistically enhanced trastuzumab-mediated ADCC of breast cancer cells by neutrophils [65]. Combining B6H12 and the CD19/CD3-bispecific T cell engager antibody blinatumomab to treat Raji cell non-Hodgkin’s lymphoma (NHL) xenografts in NSG mice almost completely suppressed tumor growth if administered with human PBMCs [66]. Mono- and dual-therapies were significantly less effective in inhibiting tumor growth in the absence of PBMC injection or removal of macrophages via clodronate liposomes. These findings demonstrate roles for adaptive (CD8 T cell cytotoxicity) and innate (macrophage phagocytosis) immune responses in the anticancer activity of B6H12. Similar enhancement of NK cell-mediated ADCC by B6H12 was reported for ALL cells [67]. Treatment of CD16+ NK cells derived from cord blood with B6H12 increased their interferon-γ production. B6H12 similarly increased CD16+ NK cell-mediated ADCC of ALL cells.
Several studies reported beneficial direct effects of B6H12 to limit the maintenance of CSC. B6H12 inhibited CSC maintenance in aggressive breast cancer cell lines [20]. B6H12 treatment decreased mRNA expression of the EGF receptor and acutely inhibited EGF-induced phosphorylation of the receptor. B6H12 inhibited breast CSC proliferation, asymmetric division, and expression of the stem cell transcription factor KLF4. Changes in KLF4 and EGFR mRNA expression induced by B6H12 were mediated in part by induction of miR7. Similar suppression of CSC and tumor growth was reported when human hepatocellular carcinoma (HCC) cells were treated with B6H12 or CD47 expression was suppressed using a shRNA [68, 69]. B6H12 suppressed the tumorigenicity, self-renewal, and chemoresistance of HCC cells, mediated by suppression of cathepsin S. B6H12 also enhanced macrophage-independent chemosensitivity of HCC cells to doxorubicin and cisplatin in vitro [69]. Combining B6H12 and doxorubicin suppressed patient-derived HCC xenografts in a mouse model. Treating pancreatic carcinomas in mice with B6H12 also suppressed CSC abundance, but in this case, in vitro studies indicated that the antibody reduced viability of the CSC by inducing apoptosis [70].
Vx1000R (Vasculox Inc., currently Arch Oncology)
The mouse anti-human CD47 antibody Vx1000R induced phagocytosis and killing of human MM cells in vitro but did not affect survival of MM cells in the absence of macrophages [71]. Vx1000R antibody showed similar activity to suppress MM growth in xenograft models to previously reported CD47 antibodies [37, 56].
BRIC126
BRIC126 inhibits CD47 binding to SIRPα [18]. BRIC126 enhanced phagocytosis of ALL cells by human macrophages in vitro [72]. However, only modest activity was reported in a laryngeal squamous cell carcinoma model in mice, and B6H12 had significantly greater tumor inhibitory activity relative to BRIC126 [73].
mAb400
CD47 mAb400 recognizes both human and murine CD47 and was compared to B6H12 in a HCC xenograft model in NSG mice [74]. B6H12 and mAb400 significantly increased macrophage-mediated ADCP of human HCCs in vitro but not in a cytotoxic manner. Both antibodies significantly inhibited tumor growth in heterotopic and orthotopic HepG2 xenograft models and increased macrophage recruitment into the tumors. However, mAb400 was significantly more potent relative to B6H12 in the heterotopic HepG2 model [74].
MIAP301
MIAP301 is specific for murine CD47 and blocks its binding to murine SIRPα [63]. This antibody has been useful to examine the efficacy of CD47 blockade in immunocompetent syngeneic mouse tumor models. Consistent with its documented blocking of SIRPα binding [75], MIAP301 enhanced phagocytosis of GL261 glioma cells and CD133+ glioma stem cells by mouse macrophages [76]. MIAP301 inhibited tumor growth and increased survival in an orthotopic glioma/glioma stem cell mouse model by upregulating both innate and adaptive immune responses. However, treating mice bearing MT1A2 mouse breast carcinoma tumors with MIAP301 yielded no significant inhibition of tumor growth relative to an IgG control [47]. This lack of activity is consistent with the observation that genetic deletion of SIRPα signaling by truncation of its cytoplasmic domain did not impair growth of a syngeneic melanoma [65]. Therefore, antibody blocking or genetic ablation of CD47/SIRPα signaling is not sufficient to enhance phagocytic clearance of all tumors in the absence of additional pro-phagocytic signals [75]. Such additional signals can include expression of cell damage markers, calreticulin, or engagement of activating Fc receptors (FcR). In contrast to MIAP301, MIAP410 (IgG1), an anti-mouse CD47 antibody previously reported to not inhibit SIRPα binding [75], inhibited growth of MT1A2 tumors [47], but a replication study failed to reproduce this result [77]. In another study, combination therapy using MIAP301 F(ab’)2 and anti-CD19 (ID3-IgG2a) increased mouse macrophage-mediated ADCP of B cells in vitro, but the combination did not differ from ID3-IgG2a monotherapy in vivo [78]. These reports suggest that CD47 neutralizing antibodies can sensitize tumors in an immune competent host by mechanisms other than SIRPα-mediated activation of ADCP (Fig. 2A). One critical difference between these syngeneic tumor models and the widely used NSG xenograft models is the absence of xenoantigens that can activate phagocytosis of human tumor cells by mouse macrophages.
As noted above for B6H12, MIAP301 interacts with multiple immune effector cells (Fig 2A). MIAP301 treatment of mice bearing B16 melanomas inhibited tumor growth, accompanied by increased tumor infiltration of NK cells that were positive for interferon-γ and granzyme B [15]. Treatment of isolated NK cells with MIAP301 in vitro produced a similar enhancement of interferon-γ expression. MIAP301 also alters CD47 functions by SIRPα-independent mechanisms including elevating blood pressure when administered intravenously in mice [79] and maintaining tissue perfusion and enhancing protective autophagy in ischemic tissues [80, 81]. Therefore, antitumor activities of MIAP301 may not be exclusively mediated by the SIRPα pathway.
MAB4670
MAB4670 (IgG1) blocks human CD47 binding to SIRPα. The antibody was used in combination with the CD38 antibody daratumumab to treat CD14+ bone marrow mononuclear cells and MM cells (CD138+) obtained from 29 patients with MM [82]. CD47 was significantly upregulated on CD138+ MM cells relative to CD138− MM cells. Treatment with daratumumab upregulated autologous ex-vivo killing of CD138+ MM cells by CD14+ and CD14+/CD16+ monocytes. Monocyte-mediated killing of MM cells was further upregulated by combination therapy with MAB4670.
A4 (CD47 nanobody)
CD47 nanobodies with picomolar affinity were developed as an approach to evade the CD47 antigen sink on RBC [83, 84]. The alpaca anti-mouse CD47 nanobody A4 inhibited SIRPα interaction but lacked effector function due to the absence of an antibody Fc-domain. A4 synergized with anti-PD-L1, but not anti-CTLA4, therapy in the syngeneic B16F10 melanoma model. However, A4 synergized with an anti-TRIP-1 (TA99, IgG2a) to promote ADCP of B16-F10 cells in vitro but not with either anti-CD200 (OX-90) or anti-EGFR (cetuximab) to promote ADCP of Tubo-EGFR cells. A4 synergized with anti–PD-L1 to increase ADCP of B16F10 cells treated with IFN-γ to induce PD-L1 expression. A4-secreting A12 CAR-T cells combined with melanoma-specific TA99 significantly decreased tumor growth rate and increased survival of immunocompetent hosts bearing syngeneic B16F10 tumors. This combination treatment did not affect the persistence of the CAR T cells. An IgG2A/A4 fusion protein (A4Fc) was constructed to enhance phagocytosis but induced severe anemia in mice that limited its utility [84]. A4Fc A12 CAR-T cells did not have cytotoxic effects, but decreased tumor volume and increased M1-macrophage infiltration in the mice bearing MC38 tumors [85].
CD47 antibody conjugates and nanoparticles
Azide-modified exosomes derived from M1 macrophages were conjugated with dibenzocyclooctyne-modified CD47 and SIRPα antibodies through pH-sensitive linkers. The modified exosomes enhanced phagocytosis in vitro and increased the survival of BALB/c mice bearing 4 T1 breast carcinoma tumors relative to animals treated with exosomes or CD47 antibody alone [86]. A CD47 antibody drug conjugate, SGN CD47M, has been developed by Seattle Genetics and is enrolling cancer patients for a phase 1 trial (NCT03957096), but no preclinical data have been published.
Chitosan/hyaluronic acid polyelectrolyte complex nanoparticles were loaded with CD47 antibody and administered to atherosclerotic ApoE−/− mice fed a high fat diet [87]. The particles significantly inhibited atherosclerotic plaque development. The particles were also used to coat foam cells before administration, which increased targeted inhibition of atherosclerotic plaques. Potential applications for cancer remain to be examined.
HUMANIZED THERAPEUTIC CD47 ANTIBODIES
Hu5F9/Magrolimab (Forty Seven, Inc./Gilead)
Enhanced macrophage phagocytosis and the antitumor efficacy of humanized Hu5F9/magrolimab (IgG4) were demonstrated using various tumor cell types in NSG xenograft models [88, 89]. Intravenous administration of magrolimab to cynomolgus monkeys resulted in dose-dependent anemia, but a protocol utilizing a 1–3 mg/kg priming dose, to occupy the RBC sink and induce compensatory erythropoiesis, followed by 30 mg/kg maintenance dosing achieved a sustained effective circulating concentration [88]. The same protocol was effective to decrease anemia in a Phase 1 human trial (Table 1), and receptor occupancy in a patient tumor was documented with a 30 mg/kg dose following priming [8]. A Phase 1b clinical trial combining magrolimab and rituximab enrolled 22 patients: 15 with diffuse large B cell lymphoma (DLBCL) and 7 with follicular lymphoma [7]. Patients had received a median of four prior therapies, and 95% had disease that was resistant to rituximab. Eleven patients responded to the drug, with eight complete responses, five in DLBCL and three in follicular lymphoma. Additionally, at a median follow-up of 6.2 months in the DLBCL group and 8.1 months in follicular lymphoma group, 91% of patients continued to respond. The most common side effects (chills, headache, and anemia) were observed in 41% of patients; one patient had grade 4 neutropenia and one grade 4 thrombocytopenia. Magrolimab combined with azacytidine increased tumor cell calreticulin expression and phagocytosis by macrophages in vitro and yielded increased survival in an AML xenograft model in NSG mice [89]. Recently presented Phase 1b results demonstrate tolerability of this combination and suggest efficacy [90]. Additional combination trials are in progress (Table 1).
SHR-1603
SHR-1603 is a humanized CD47 IgG4 antibody that includes active Fc domains to mediate CDC/ADCC functions [91]. This antibody blocked the interaction of CD47 with SIRPα and enhanced phagocytosis. SHR-1603 is being tested in a Phase 1 trial (Table 1).
CC-90002 (Celgene)
CC-90002 is an IgG4 high-affinity CD47 antibody that blocks its binding to SIRPα and enhanced macrophage-dependent killing of tumor cells in preclinical studies [92]. A Phase I Study of CC-90002 was completed in patients with relapsed and/or refractory AML and high-risk myelodysplastic syndromes (MDS). A Phase 1 trial using CC-90002 in combination with rituximab is ongoing for patients with CD20+ NHL (Table 1). CC-90002 antibody did not induce hemagglutination of red blood cells or hemolysis in preclinical studies. CC-90002 treatment combined with rituximab demonstrated tolerability and modest clinical activity in this early-phase study of heavily pretreated refractory/recurrent NHL subjects, with adverse events predominantly grade 1/2 cytopenias and dose-limiting thrombocytopenia [92].
AMMS4-G4
The fully human IgG4 CD47 antibody ZF1 blocked interaction between CD47 and human or mouse SIRPα slightly weaker than B6H12 but induced in vitro macrophage-mediated phagocytosis as robust as B6H12 [93]. ZF1 induced phagocytic killing of ALL or CML leukemic cells in mouse xenografts and increased survival comparable to B6H12 and significantly greater than control IgG. However, neither CD47 antibody completely eradicated the tumors. A fully human anti-CD47 antibody, AMMS4-G4, was derived from ZF1 using affinity maturation [94]. AMMS4-G4 had higher affinity for human CD47, blocked CD47/SIRPα interaction, and significantly increased macrophage recruitment, tumor phagocytosis, and survival in mice engrafted with human hematologic cancers. AMMS4-G4 inhibited tumor growth through increased macrophage recruitment in mice engrafted with SKOV3 ovarian cancer solid tumors. Combination therapy with the VEGF antagonist bevacizumab modestly enhanced this anti-tumor activity. Combination therapy with the EGFR antibody AC21 (IgG1) significantly inhibited tumor growth in mice bearing LOVO colon cancer xenografts. As observed for other CD47 antibodies, AMMS4-G4 infusion resulted in reversible anemia in cynomolgus monkeys, but no hemagglutination was observed.
HuNb1
HuNb1 is a high affinity nanobody that is specific for human CD47 but exhibits low binding to human RBC CD47. HuNb1 was identified by phage display screening [95]. It exhibited safety in cynomolgus monkeys. A recombinant HuNb1-IgG4 induced phagocytosis in vitro and showed anti-tumor activity in a human ovarian carcinoma xenograft model.
SRF231
SRF231 is a fully human IgG4 CD47 antibody that inhibits binding to SIRPα but does not agglutinate RBC or induce their clearance by macrophages [96]. In addition to enhancing macrophage phagocytosis of tumor cells by blocking SIRPα, SRF231 activated macrophages by its binding to the activating FcγR CD32a. Compared to the antibody CC2C6, which directly increased tumor cell apoptosis when used in solution, SRF231 induced less direct cytotoxicity. However, SRF231 exhibited stronger proapoptotic activity than CC2C6 when immobilized on a scaffold, and this activity was inhibited by a pan-caspase inhibitor. Therefore, SRF231 has a context-dependent ability to directly induce tumor cell death. SRF231 treatment reduced tumor growth in multiple mouse xenograft models. Treatment was associated with increased tumor macrophage infiltration and induction of the macrophage cytokines monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α. SRF231 is currently in a Phase 1 clinical trial (Table 1).
Therapeutic SIRPα antibodies and decoys
Monoclonal antibodies specific for SIRPα that block its interaction with CD47 represent another strategy to enhance tumor ADCP while avoiding the RBC sink, thus permitting use of lower dosages than CD47 antibodies. Prophagocytic and antitumor efficacy has been demonstrated in several preclinical models [65, 97, 98]. Two such antibodies have entered phase 1 trials (Table 1), but preclinical data for these antibodies have not been published.
A decoy strategy has also been advanced using the CD47-binding domains of SIRPα fused to an immunoglobulin Fc region [99, 100]. These offer a potential advantage over the first generation of humanized CD47 antibodies in that they exhibit minimal binding to RBC. TTI-621 is a bivalent human SIRPα construct with a IgG1 Fc that enhances macrophage-mediated phagocytosis in vitro and growth of several xenograft tumor models in vivo [99, 101]. TTI-621 and TTI-622, with a IgG4 Fc, are currently in Phase 1 trials (Table 1). ALX148 contains the D1 domain of SIRPαf used to an inactive human IgG1 Fc [100]. ALX148 enhances phagocytosis of tumor cells in vitro, is active in several tumor models, and enhances the activities of other immunotherapy antibodies. It is currently in phase 1 clinical testing (Table 1).
Humanized CD47 antibodies with cell-autonomous anticancer activities
AO-176
AO-176 is a humanized mouse anti-human CD47 IgG2 antibody with κ-light chain that inhibits SIRPα binding [38]. AO-176 exhibited negligible interaction with RBCs, platelets, and endothelial cells and did not induce RBC agglutination. AO-176 also bound well to cynomolgus monkey and murine CD47. Intravenous administration of AO-176 in cynomolgus monkeys was well tolerated without any hematologic effects, with stable RBC counts and hemoglobin values even after repeated dosing. This contrasts with other CD47 blocking antibodies including the control humanized CD47 antibody AO-104, which exhibited high binding to RBCs and induced the typical transient anemia in monkeys. Compared to AO-104, AO-176 also demonstrated significantly lower binding affinity to naïve and activated T-cells and other non-malignant cells. In addition to preferential binding to cancer cells, AO-176 selectively induced death in Jurkat T-ALL and OV90 ovarian cancer cells involving late and early apoptosis. AO-176 promoted human macrophage phagocytosis of hematologic (Jurkat T-ALL, Raji B-cell lymphoma) and solid tumor cells (OV90, Detroit 562, and FaDu). AO-176 inhibited growth in Raji lymphoma xenografts. A 5 mg/kg dose of AO-176 remained detectable in circulation after 18 days, while AO-104 did not.
The mechanism by which AO-176 selectively binds CD47 on to tumor cells remains unclear but may involve cell-specific posttranslational modifications or masking of the AO-176 epitope by CD47 binding partners (eg. components of the Rh complex in the erythrocyte membrane) [1]. Conversely, AO-176 binding may depend on a specific lateral CD47-interacting protein in cancer cells, differences in the surface mobility of CD47 [102], differential densities, or differential partitioning of CD47 into lipid rafts between normal and tumor cells [103].
CD47 bispecific antibodies (bsAb)
Based on the homeostatic function of CD47 on RBC to limit clearance of aging RBCs and the ability of some CD47 antibodies to induce hemagglutination, anemia is a common reported side effect for humanized CD47 antibodies including Hu5F9 [8]. One approach to minimize these adverse effects is to construct bsAb wherein the second epitope is a tumor-specific surface antigen that directs the antibody to bind preferentially to tumor cells and in some cases activate ADCC or ADCP mediated via its Fc region, while simultaneously blocking the do not eat me signal (Fig. 2C). Such CD47 bsAb generally exhibit decreased anemia [104, 105].
CD3xCD47 (OV-TL3/CD3)
The first CD47 bsAb was reported in 1994 and contained variable regions recognizing CD47 and CD3 (Fig. 4A) [106]. The antibody OV-TL3/CD3 enhanced killing of human ovarian cancer cells by peripheral blood leukocytes (PBL) in vitro. Treatment of nude mice bearing ovarian carcinoma ascites tumors with bivalent OV-TL3/CD3 F (ab’)2 combined with PBL and IL-2 increased survival relative to antibody or PBL + IL-2 alone. This F(ab’)2 resembles the current bispecific T cell enhancers [107].
Figure 4.
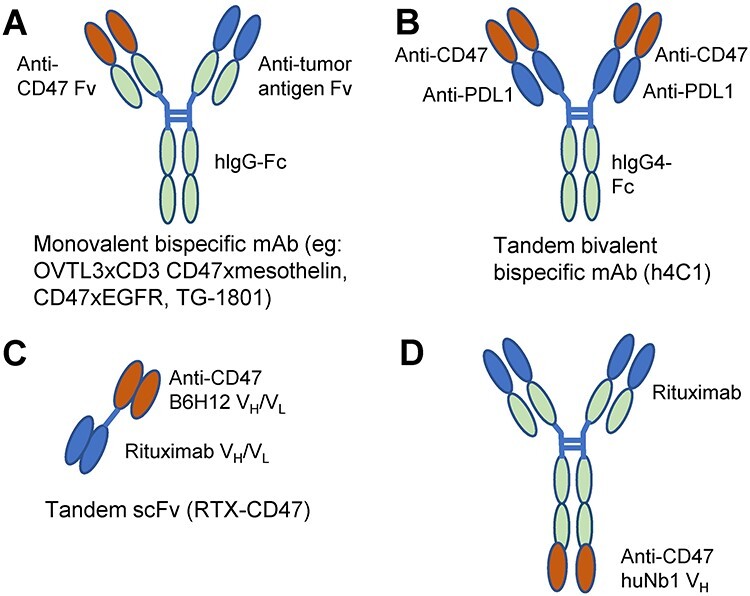
Structures of therapeutic bispecific CD47 antibodies. A) Conventional bispecific antibodies combine Fv regions from two antibodies and are monovalent for CD47 and a tumor-specific or immune cell antigen. B) A tandem bispecific that is bivalent for CD47 and PD-L1 [109]. C) A bispecific antibody containing scFv domains recognizing CD47 and CD20 [108]. D) A bispecific combining rituximab with the CD47 nanobody huNb1 [95].
CD20xCD47 (RTX-CD47, HuNb1-rituximab)
A bispecific tandem scFv antibody lacking an Fc region (RTX-CD47) was constructed from B6H12 and rituximab Fv regions (Fig. 4C) [108]. RTX-CD47 had enhanced avidity for CD20+/CD47+ tumor cells, but CD47/SIRPα blockade depended on CD20-restricted cooperativity. RTX-CD47 enhanced ADCP of primary malignant B cell lines by both macrophages and granulocytes in a CD20-restricted cooperative manner. Combination therapy with RTX further enhanced phagocytosis of some cell lines. Combining the CD20 antibody obinutuzumab with RTX-CD47 further improved ADCP without competing for the CD20 epitope for the RTX portion of RTX-CD47. The absence of a Fc-domain allowed this bispecific scFv to avoid triggering of FcR-mediated ADCC, ADCP, orCDC.
Another CD47xCD20 bsAb was constructed using the CD47 nanobody HuNb1 (IgG4) and the variable domain from rituximab (Fig. 4D), which exhibited increased efficacy in mouse xenograft B cell lymphoma models relative to parental HuNb1 [95].
CD47xPD-L1
The humanized IgG4 SIRPα-blocking CD47 mAb h4C1 increased phagocytosis of Raji cells by bone marrow-derived macrophages and improved survival and enhanced suppression of Raji cell xenografts in mice relative to a Hu5F9 positive control and IgG negative control. h4C1 binds to the FG loop of CD47, which interacts with SIRPα and several other known CD47/SIRPα-blocking mAb. Binding of h4C1 to CD47 was not affected by glycosylation modifications of CD47. The variable-domain from the PD-L1-blocking mAb #18 (CN Patent: 201810952740.3) was introduced in tandem onto h4C1 to generate a dual variable domain bsAb that binds bivalently to both CD47 and PD-L1 (Fig. 4B). CD47 binding affinity was reduced compared to h4C1, but PD-L1 binding remained comparable to monovalent PD-L1 mAb [109]. The construct retained blocking activity for both interactions but lacked the hemagglutinating activity of the parenth4C1.
IBI-322 is another bsAb that recognizes PD-L1 and CD47 and is in phase 1 trials for treating advanced tumors (Table 1). IBI-322 was also tested as a positron emission tomography imaging agent after conjugation with p-SCN-deferoxamine and loading with 89Zr [110].
CD47xEGFR
A CD47xEGFR bsAb was engineered from the EGFR antibody Pan and a SIRPα variant-Fc with a human IgG1 Fc [105]. The bsAb enhanced macrophage phagocytosis of human A431 epidermoid carcinoma cells in vitro and inhibited A431 xenograft tumor growth in mice. RBC counts assessed 20 days following injection in mice were higher than in mice injected with bivalent SIRPα variant-Fc, indicating reduced toxicity, although acute anemia at earlier times were not examined. Phagocytic activity and tumor inhibition were comparable to SIRPαV-Fc and Pan monotherapies, respectively.
CD47xMesothelin (Novimmune)
A bsAb recognizing CD47 and mesothelin increased ADCC and ADCP by promoting FcγR-IIIA signaling in NK cells and macrophages, respectively, relative to IgG1 control or either monospecific antibody [111]. Targeting a membrane-proximal epitope on mesothelin alone led to increased ADCC but not ADCP activity. However, the corresponding bsAb significantly increased ADCC and ADCP in an FcyR-IIIA-dependent manner and completely suppressed mesothelin-expressing HepG2 xenografts inmice.
CD47xCD19 (TG-1801/NI-1701, TG Therapeutics/Novimmune)
The CD47xCD19 bsAb TG-1801/NI-1701 induced potent ADCP against B cell malignancies in vitro and in vivo [104]. In contrast to either monospecific antibody, TG-1801/NI-1701 inhibited B cell receptor (BCR)-induced B cell proliferation in a non-cell death-dependent manner and inhibited BCR signaling, internalization, and cytokine production [112]. Simultaneously engaging CD47 was proposed to restrict mobility of CD19 to cluster and associate with the BCR. Combination therapy with the anti-CD20 rituximab further enhanced tumor growth inhibition in Raji cell xenograft mouse models. In vitro binding assays in whole blood as well as in vivo measurements in cynomolgus monkeys showed minimal-to-no binding of NI-1701 to RBCs, T cells, and platelets. Furthermore, no hematological or other adverse side effects were seen in cynomolgus monkeys at doses reaching 100 mg/kg. The high affinity of the anti-CD19 binding arm may specifically restrict CD47 blockade by NI-1701 to B-cells, and its IgG1 Fc may contribute to its ADCP potency. A clinical trial using TG-1801 for B cell lymphoma is in progress (Table 1).
CONCLUSIONS
The ubiquitous expression of CD47 on normal as well as tumor cells creates challenges for developing CD47 antibodies for cancer therapy. CD47 expressed on circulating blood cells and vascular endothelium serves as a sink for intravenously administered therapeutic CD47 antibodies and leads to the commonly observed side effects of transient anemia, hyperbilirubinemia, thrombocytopenia, and lymphopenia. Several strategies are emerging to circumvent these issues by achieving selective binding to CD47 on tumor cells, including identifying tumor-specific CD47 epitopes and developing bispecific antibody designs. A better understanding of the variations in posttranslational modifications of CD47 will facilitate this effort and may identify additional molecular targets that could selectively impair the function of CD47 on tumor cells. The finding that the glutaminyl cyclase QPCTL mediates a posttranslational modification of CD47 that is required for SIRPα binding suggests QPCTL is a relevant molecular target for future therapeutics [43].
Although antibodies targeting the CD47/SIRPα interaction may have significant efficacy as single agents for treating some cancers, data from immune-competent mouse and xenograft models indicate that efficacy for most cancers will require combination therapies. Such synergies have proven effective in preclinical models wherein CD47 therapeutics are combined with ionizing radiation or chemotherapy [31, 113–115] and immune checkpoint inhibitors targeting the PD-1/PD-L1 and CTLA4 pathways [116, 117]. Consequently, several new clinical trials are examining such therapeutic combinations (Table 1). In addition to minimizing binding to circulating blood cells, the emerging bispecific antibodies may provide specific targeting of CD47 blockade to tumor cells and effector functions to stimulate antitumor immunity.
The potential for CD47 therapeutics to achieve cancer-autonomous suppression of CSC or selectively induce programmed death of malignant cells is another promising path forward. Further investigation of how CD47 signaling controls the balance between protective autophagy and programmed cell death pathways in malignant versus nonmalignant cells is needed to realize this potential.
To date, CD47 therapeutics that have entered clinical testing are intended to block its engagement of SIRPα [2, 3]. However, preclinical studies have demonstrated that blocking TSP1 interactions also provides therapeutic benefits, especially when combined with radiation, chemotherapy, or adoptive immunotherapy [31, 32, 113, 114]. Because CD47 serves as an inhibitory signaling receptor on dendritic cells, T cells, and NK cells [15, 28, 113], therapeutics targeting the CD47/TSP1 axis could have broad immune-activating activities. This is supported by tumor models in immune competent mice that established the necessary role for CD8 T cell immunity and parallel increases in activation of adaptive immunity in Thbs1 and Cd47-null mice [83, 113, 118]. Based on the evidence that B6H12 inhibits both SIRPα and TSP1 binding, steric blocking of TSP1 binding by some antibodies intended to block SIRPα binding may be expected and merits further study for the antibodies currently in clinical trials. Further development of nanobody technologies may also enable more selective targeting of each CD47 function.
Contributor Information
Sukhbir Kaur, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Kyle V Cicalese, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Rajdeep Banerjee, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
David D Roberts, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
ABBREVIATIONS
ADCC, antibody-dependent cell-mediated cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; bsAb, bispecific antibody; CDC, complement-dependent cytotoxicity; CML, chronic myeloid leukemia; CSC, cancer stem cells; CTLA4, cytotoxic T-lymphocyte-associated protein 4; DLBCL, diffuse large B cell lymphoma; EGFR, epidermal growth factor receptor; HCC, hepatocellular carcinoma; MM, multiple myeloma; NK, natural killer; NSG, nonobese diabetic/severe combined immunodeficient/IL2Rγ−; PD-L1, programmed death ligand 1; QPTCL, glutaminyl-peptide cyclotransferase-like protein; RBC, red blood cells; scFv, single-chain variable fragment; SIRPα, signal regulator protein-α (CD172a); TSP1, thrombospondin-1.
Conflict of interest statement. None declared.
FUNDING
This work was supported by the Intramural Research Program of the NIH/NCI (ZIA SC009172).
REFERENCES
- 1. Soto-Pantoja, DR, Kaur, S, Roberts, DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 2015; 50: 212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matlung, HL, Szilagyi, K, Barclay, NA, et al. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017; 276: 145–64. [DOI] [PubMed] [Google Scholar]
- 3. Jalil, AR, Andrechak, JC, Discher, DE. Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPalpha structure-function. Antib Ther 2020; 3: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okazawa, H, Motegi, S, Ohyama, N, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol 2005; 174: 2004–11. [DOI] [PubMed] [Google Scholar]
- 5. Oldenborg, PA, Zheleznyak, A, Fang, YF, et al. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051–4. [DOI] [PubMed] [Google Scholar]
- 6. Yamao, T, Noguchi, T, Takeuchi, O, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem 2002; 277: 39833–9. [DOI] [PubMed] [Google Scholar]
- 7. Advani, R, Flinn, I, Popplewell, L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 2018; 379: 1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sikic, BI, Lakhani, N, Patnaik, A, et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 2019; 37: 946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardai, SJ, McPhillips, KA, Frasch, SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123: 321–34. [DOI] [PubMed] [Google Scholar]
- 10. Chao, MP, Jaiswal, S, Weissman-Tsukamoto, R, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2010; 2: 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkal, AA, Weiskopf, K, Kao, KS, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol 2018; 19: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barkal, AA, Brewer, RE, Markovic, M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019; 572: 392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li, Z, He, L, Wilson, K, et al. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol 2001; 166: 2427–36. [DOI] [PubMed] [Google Scholar]
- 14. Stein, EV, Miller, TW, Ivins-O'Keefe, K, et al. Secreted Thrombospondin-1 regulates macrophage interleukin-1beta production and activation through CD47. Sci Rep 2016; 6: 19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nath, PR, Pal-Nath, D, Mandal, A, et al. Natural killer cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment. Cancer Immunol Res 2019; 7: 1547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon, SR, Maute, RL, Dulken, BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545: 495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Broom, OJ, Zhang, Y, Oldenborg, PA, et al. CD47 regulates collagen I-induced cyclooxygenase-2 expression and intestinal epithelial cell migration. PLoS ONE 2009; 4: e6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seiffert, M, Cant, C, Chen, Z, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999; 94: 3633–43. [PubMed] [Google Scholar]
- 19. Isenberg, JS, Annis, DS, Pendrak, ML, et al. Differential interactions of thrombospondin-1, −2, and −4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009; 284: 1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaur, S, Elkahloun, AG, Singh, SP, et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 2016; 7: 10133–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao, AG, Lindberg, FP, Dimitry, JM, et al. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol 1996; 135: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barazi, HO, Li, Z, Cashel, JA, et al. Regulation of integrin function by CD47 ligands. Differential effects on alpha vbeta 3 and alpha 4beta1 integrin-mediated adhesion. J Biol Chem 2002; 277: 42859–66. [DOI] [PubMed] [Google Scholar]
- 23. Kim, MJ, Lee, JC, Lee, JJ, et al. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol 2008; 29: 28–34. [DOI] [PubMed] [Google Scholar]
- 24. Yoshida, H, Tomiyama, Y, Ishikawa, J, et al. Integrin-associated protein/CD47 regulates motile activity in human B-cell lines through CDC42. Blood 2000; 96: 234–41. [PubMed] [Google Scholar]
- 25. Grimbert, P, Bouguermouh, S, Baba, N, et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol 2006; 177: 3534–41. [DOI] [PubMed] [Google Scholar]
- 26. Yamauchi, Y, Kuroki, M, Imakiire, T, et al. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol 2002; 21: 441–8. [DOI] [PubMed] [Google Scholar]
- 27. Cooper, D, Lindberg, FP, Gamble, JR, et al. Transendothelial migration of neutrophils involves integrin-associated protein (CD47). Proc Natl Acad Sci U S A 1995; 92: 3978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu, J, Lin, MF. Anti-CD47 monoclonal antibody (B6H12) impairs the maturation and function of human dendritic cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2005; 13: 192–7. [PubMed] [Google Scholar]
- 29. Lymn, JS, Patel, MK, Clunn, GF, et al. Thrombospondin-1 differentially induces chemotaxis and DNA synthesis of human venous smooth muscle cells at the receptor-binding level. J Cell Sci 2002; 115: 4353–60. [DOI] [PubMed] [Google Scholar]
- 30. Isenberg, JS, Ridnour, LA, Dimitry, J, et al. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 2006; 281: 26069–80. [DOI] [PubMed] [Google Scholar]
- 31. Maxhimer, JB, Soto-Pantoja, DR, Ridnour, LA, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009; 1: 3ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaur, S, Schwartz, AL, Jordan, DG, et al. Identification of Schlafen-11 as a target of CD47 Signaling that regulates sensitivity to ionizing radiation and topoisomerase inhibitors. Front Oncol 2019; 9: 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaur, S, Elkahloun, AG, Singh, SP, et al. A function-blocking CD47 antibody modulates extracellular vesicle-mediated intercellular signaling between breast carcinoma cells and endothelial cells. J Cell Commun Signal 2018; 12: 157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lagadec, P, Dejoux, O, Ticchioni, M, et al. Involvement of a CD47-dependent pathway in platelet adhesion on inflamed vascular endothelium under flow. Blood 2003; 101: 4836–43. [DOI] [PubMed] [Google Scholar]
- 35. Pettersen, RD, Hestdal, K, Olafsen, MK, et al. CD47 signals T cell death. J Immunol 1999; 162: 7031–40. [PubMed] [Google Scholar]
- 36. Leclair, P, Liu, CC, Monajemi, M, et al. CD47-ligation induced cell death in T-acute lymphoblastic leukemia. Cell Death Dis 2018; 9: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kikuchi, Y, Uno, S, Kinoshita, Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res 2005; 29: 445–50. [DOI] [PubMed] [Google Scholar]
- 38. Puro, RJ, Bouchlaka, MN, Hiebsch, RR, et al. Development of AO-176, a next-generation humanized anti-CD47 antibody with novel anticancer properties and negligible red blood cell binding. Mol Cancer Ther 2020; 19: 835–46. [DOI] [PubMed] [Google Scholar]
- 39. Mateo, V, Lagneaux, L, Bron, D, et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med 1999; 5: 1277–84. [DOI] [PubMed] [Google Scholar]
- 40. Manna, PP, Frazier, WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase a. Cancer Res 2004; 64: 1026–36. [DOI] [PubMed] [Google Scholar]
- 41. Barbier, S, Chatre, L, Bras, M, et al. Caspase-independent type III programmed cell death in chronic lymphocytic leukemia: the key role of the F-actin cytoskeleton. Haematologica 2009; 94: 507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kikuchi, Y, Uno, S, Yoshimura, Y, et al. A bivalent single-chain Fv fragment against CD47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun 2004; 315: 912–8. [DOI] [PubMed] [Google Scholar]
- 43. Logtenberg, MEW, Jansen, JHM, Raaben, M, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPalpha axis and a target for cancer immunotherapy. Nat Med 2019; 25: 612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Majeti, R, Chao, MP, Alizadeh, AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takenaka, K, Prasolava, TK, Wang, JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 2007; 8: 1313–23. [DOI] [PubMed] [Google Scholar]
- 46. Yamauchi, T, Takenaka, K, Urata, S, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 2013; 121: 1316–25. [DOI] [PubMed] [Google Scholar]
- 47. Willingham, SB, Volkmer, JP, Gentles, AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109: 6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chao, MP, Weissman, IL, Majeti, R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol 2012; 24: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murata, Y, Saito, Y, Kotani, T, et al. CD47-signal regulatory protein alpha signaling system and its application to cancer immunotherapy. Cancer Sci 2018; 109: 2349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Logtenberg, MEW, Scheeren, FA, Schumacher, TN. The CD47-SIRPalpha immune checkpoint. Immunity 2020; 52: 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu, L, Zhang, L, Yang, L, et al. Anti-CD47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front Immunol 2017; 8: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tseng, D, Volkmer, JP, Willingham, SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013; 110: 11103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaeteewoottacharn, K, Kariya, R, Pothipan, P, et al. Attenuation of CD47-SIRPalpha signal in Cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol 2019; 12: 217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edris, B, Weiskopf, K, Volkmer, AK, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 2012; 109: 6656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goto, H, Kojima, Y, Matsuda, K, et al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer 2014; 50: 1836–46. [DOI] [PubMed] [Google Scholar]
- 56. Kim, D, Wang, J, Willingham, SB, et al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012; 26: 2538–45. [DOI] [PubMed] [Google Scholar]
- 57. Zhu, H, Leiss, L, Yang, N, et al. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget 2017; 8: 12145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Michaels, AD, Newhook, TE, Adair, SJ, et al. CD47 blockade as an adjuvant immunotherapy for Resectable pancreatic cancer. Clin Cancer Res 2018; 24: 1415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshida, K, Tsujimoto, H, Matsumura, K, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric Cancer. cCancer Med 2015; 4: 1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiss, B, van denBerg, NS, Ertsey, R, et al. CD47-targeted near-infrared Photoimmunotherapy for human bladder cancer. Clin Cancer Res 2019; 25: 3561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hardie, J, Mas-Rosario, JA, Ha, S, et al. Macrophage activation by a substituted pyrimido [5,4-b] indole increases anti-cancer activity. Pharmacol Res 2019; 104452: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chao, MP, Alizadeh, AA, Tang, C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oldenborg, PA, Gresham, HD, Lindberg, FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001; 193: 855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Olsson, M, Oldenborg, PA. CD47 on experimentally senescent murine RBCs inhibits phagocytosis following Fcgamma receptor-mediated but not scavenger receptor-mediated recognition by macrophages. Blood 2008; 112: 4259–67. [DOI] [PubMed] [Google Scholar]
- 65. Zhao, XW, vanBeek, EM, Schornagel, K, et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A 2011; 108: 18342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu, L, Wang, S, Li, J, et al. CD47/SIRPalpha blocking enhances CD19/CD3-bispecific T cell engager antibody-mediated lysis of B cell malignancies. Biochem Biophys Res Commun 2019; 509: 739–45. [DOI] [PubMed] [Google Scholar]
- 67. Valipour, B, Abedelahi, A, Naderali, E, et al. Cord blood stem cell derived CD16(+) NK cells eradicated acute lymphoblastic leukemia cells using with anti-CD47 antibody. Life Sci 2020; 242: 117223. [DOI] [PubMed] [Google Scholar]
- 68. Lee, TK, Cheung, VC, Lu, P, et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology 2014; 60: 179–91. [DOI] [PubMed] [Google Scholar]
- 69. Lo, J, Lau, EY, So, FT, et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int 2016; 36: 737–45. [DOI] [PubMed] [Google Scholar]
- 70. Cioffi, M, Trabulo, S, Hidalgo, M, et al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res 2015; 21: 2325–37. [DOI] [PubMed] [Google Scholar]
- 71. Sun, J, Muz, B, Alhallak, K, et al. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers (Basel) 2020; 12:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chao, MP, Alizadeh, AA, Tang, C, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011; 71: 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang, C, Gao, S, Zhang, H, et al. CD47 is a potential target for the treatment of laryngeal squamous cell carcinoma. Cell Physiol Biochem 2016; 40: 126–36. [DOI] [PubMed] [Google Scholar]
- 74. Xiao, Z, Chung, H, Banan, B, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett 2015; 360: 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soto-Pantoja, DR, Miller, TW, Frazier, WA, et al. Inhibitory signaling through signal regulatory protein-alpha is not sufficient to explain the antitumor activities of CD47 antibodies. Proc Natl Acad Sci U S A 2012; 109: E2842.author reply E2844-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li, F, Lv, B, Liu, Y, et al. Blocking the CD47-SIRPalpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 2018; 7: e1391973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Horrigan, SK, Project, R. Cancer B. replication study: the CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. elife 2017; 6: e18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gallagher, S, Turman, S, Lekstrom, K, et al. CD47 limits antibody dependent phagocytosis against non-malignant B cells. Mol Immunol 2017; 85: 57–65. [DOI] [PubMed] [Google Scholar]
- 79. Bauer, EM, Qin, Y, Miller, TW, et al. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 2010; 88: 471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Isenberg, JS, Romeo, MJ, Abu-Asab, M, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res 2007; 100: 712–20. [DOI] [PubMed] [Google Scholar]
- 81. El-Rashid, M, Ghimire, K, Sanganeria, B, et al. CD47 limits autophagy to promote acute kidney injury. FASEB J 2019; 33: 12735–49. [DOI] [PubMed] [Google Scholar]
- 82. Storti, P, Vescovini, R, Costa, F, et al. CD14(+) CD16(+) monocytes are involved in daratumumab-mediated myeloma cells killing and in anti-CD47 therapeutic strategy. Br J Haematol 2020. [DOI] [PubMed] [Google Scholar]
- 83. Sockolosky, JT, Dougan, M, Ingram, JR, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A 2016; 113: E2646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ingram, JR, Blomberg, OS, Sockolosky, JT, et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci U S A 2017; 114: 10184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie, YJ, Dougan, M, Ingram, JR, et al. Improved antitumor efficacy of chimeric antigen receptor T cells that secrete single-domain antibody fragments. Cancer Immunol Res 2020; 8: 518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nie, W, Wu, G, Zhang, J, et al. Responsive exosome Nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl 2020; 59: 2018–22. [DOI] [PubMed] [Google Scholar]
- 87. Yu, J, Ruan, Q, Nie, X, et al. Synthetic CD47 antibody-chitosan/hyaluronic acid polyelectrolyte complex mediates targeted inhibition of atherosclerotic plaques by exogenous foam-like cells via the NLRP3 pathway. J Biomater Appl 2020; 885328220905181. [DOI] [PubMed] [Google Scholar]
- 88. Liu, J, Wang, L, Zhao, F, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE 2015; 10: e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chao, MP, Takimoto, CH, Feng, DD, et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol 2019; 9: 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sallman, DA, Al Malki, M, Asch, AS, et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: phase Ib results. J Clin Oncol 2020; 38: 7507–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao, Y, Zhang, D, Yang, C, et al. Two validated liquid chromatography-mass spectrometry methods with different pretreatments for the quantification of an anti-CD47 monoclonal antibody in rat and cynomolgus monkey serum compared with an electrochemiluminescence method. J Pharm Biomed Anal 2019; 175: 112792. [DOI] [PubMed] [Google Scholar]
- 92. Abrisqueta, P, Sancho, J-M, Cordoba, R, et al. Anti-CD47 antibody, CC-90002, in combination with rituximab in subjects with relapsed and/or refractory non-Hodgkin lymphoma (R/R NHL). Blood 2019; 134: 4089–9. [Google Scholar]
- 93. Zeng, D, Sun, Q, Chen, A, et al. A fully human anti-CD47 blocking antibody with therapeutic potential for cancer. Oncotarget 2016; 7: 83040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yu, XY, Qiu, WY, Long, F, et al. A novel fully human anti-CD47 antibody as a potential therapy for human neoplasms with good safety. Biochimie 2018; 151: 54–66. [DOI] [PubMed] [Google Scholar]
- 95. Ma, L, Zhu, M, Gai, J, et al. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J Nanobiotechnology 2020; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Peluso, MO, Adam, A, Armet, CM, et al. The fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J Immunother Cancer 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yanagita, T, Murata, Y, Tanaka, D, et al. Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2017; 2: e89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murata, Y, Tanaka, D, Hazama, D, et al. Anti-human SIRPalpha antibody is a new tool for cancer immunotherapy. Cancer Sci 2018; 109: 1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Petrova, PS, Viller, NN, Wong, M, et al. TTI-621 (SIRPalphaFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res 2017; 23: 1068–79. [DOI] [PubMed] [Google Scholar]
- 100. Kauder, SE, Kuo, TC, Harrabi, O, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 2018; 13: e0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin, GHY, Chai, V, Lee, V, et al. TTI-621 (SIRPalphaFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS ONE 2017; 12: e0187262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dahl, KN, Westhoff, CM, Discher, DE. Fractional attachment of CD47 (IAP) to the erythrocyte cytoskeleton and visual colocalization with Rh protein complexes. Blood 2003; 101: 1194–9. [DOI] [PubMed] [Google Scholar]
- 103. McDonald, JF, Zheleznyak, A, Frazier, WA. Cholesterol-independent interactions with CD47 enhance alphavbeta 3 avidity. J Biol Chem 2004; 279: 17301–11. [DOI] [PubMed] [Google Scholar]
- 104. Buatois, V, Johnson, Z, Salgado-Pires, S, et al. Preclinical development of a Bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and Leukemia. Mol Cancer Ther 2018; 17: 1739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang, Y, Guo, R, Chen, Q, et al. A novel bispecific antibody fusion protein co-targeting EGFR and CD47 with enhanced therapeutic index. Biotechnol Lett 2018; 40: 789–95. [DOI] [PubMed] [Google Scholar]
- 106. van Ravenswaay Claasen, HH, Eggermont, AM, Nooyen, YA, et al. Immunotherapy in a human ovarian cancer xenograft model with two bispecific monoclonal antibodies: OV-TL 3/CD3 and OC/TR. Gynecol Oncol 1994; 52: 199–206. [DOI] [PubMed] [Google Scholar]
- 107. Kaiser, J. Forced into battle. Science 2020; 368: 930–3. [DOI] [PubMed] [Google Scholar]
- 108. van Bommel, PE, He, Y, Schepel, I, et al. CD20-selective inhibition of CD47-SIRPalpha "don't eat me" signaling with a bispecific antibody-derivative enhances the anticancer activity of daratumumab, alemtuzumab and obinutuzumab. Oncoimmunology 2018; 7: e1386361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shi, R, Chai, Y, Duan, X, et al. The identification of a CD47-blocking "hotspot" and design of a CD47/PD-L1 dual-specific antibody with limited hemagglutination. Signal Transduct Target Ther 2020; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang, Y, Pan, D, Huang, C, et al. Dose escalation PET imaging for safety and effective therapy dose optimization of a bispecific antibody. MAbs 2020; 12: 1748322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hatterer, E, Chauchet, X, Richard, F, et al. Targeting a membrane-proximal epitope on mesothelin increases the tumoricidal activity of a bispecific antibody blocking CD47 on mesothelin-positive tumors. MAbs 2020; 12: 1739408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hatterer, E, Barba, L, Noraz, N, et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. MAbs 2019; 11: 322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Soto-Pantoja, DR, Terabe, M, Ghosh, A, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res 2014; 74: 6771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Feliz-Mosquea, YR, Christensen, AA, Wilson, AS, et al. Combination of anthracyclines and anti-CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat 2018; 172: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gholamin, S, Youssef, OA, Rafat, M, et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun 2020; 26: 130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lian, S, Xie, X, Lu, Y, et al. Checkpoint CD47 function on tumor metastasis and immune therapy. Onco Targets Ther 2019; 12: 9105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schwartz, AL, Nath, PR, Allgauer, M, et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol Immunother 2019; 68: 1805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liu, X, Pu, Y, Cron, K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015; 21: 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Burger, P, Hilarius-Stokman, P, deKorte, D, et al. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012; 119: 5512–21. [DOI] [PubMed] [Google Scholar]
- 120. Sosale, NG, Rouhiparkouhi, T, Bradshaw, AM, et al. Cell rigidity and shape override CD47's "self"-signaling in phagocytosis by hyperactivating myosin-II. Blood 2015; 125: 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kaur, S, Soto-Pantoja, DR, Stein, EV, et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 2013; 3: 1673. [DOI] [PMC free article] [PubMed] [Google Scholar]


