Abstract
Intravascular photoacoustic (IVPA) imaging technology enables the visualization of pathological characteristics (such as inflammation activities, lipid deposition) of the artery wall. Blood flushing is a necessary step in improving the imaging quality in in vivo IVPA imaging. But the limited imaging speed of the systems stretches their flushing time, which is an important obstacle of their clinical translations. In this paper, we report an improvement in IVPA/IVUS imaging speed to 100 frames per second. The high-speed imaging is demonstrated in rabbit in vivo, visualizing the nanoparticles accumulated on abdominal aorta wall at the wavelength of 1064 nm, in real time display. Blood flushing in vivo improves the IVPA signal-noise-ratio by around 3.5 dB. This study offers a stable, efficient and easy-to-use tool for instantaneous disease visualization and disease diagnosis in research and forwards IVPA/IVUS imaging technology towards clinical translations.
1. Introduction
Coronary artery diseases, in particular coronary atherosclerosis with subsequent thrombosis, has become one of the leading causes of morbidity and mortality worldwide. The pathological characteristics (lipid deposition, inflammation activities, etc) on artery wall have been documented for the evaluation and prognostication of disease [1,2]. Therefore, in vivo detection and visualization of the information in coronary arteries are instrumental for accurate diagnosis and treatment guidance. Although several noninvasive imaging modalities have been applied in artery information detection, intravascular imaging technologies are preferable in acquiring anatomical and functional information of plaque and artery wall. For example, intravascular ultrasound imaging (IVUS) offers the anatomical information of the entire artery wall with moderate imaging resolution [3]. The intravascular imaging modalities based on pure optics, such as intravascular optical coherence tomography (IVOCT) [4,5], near-infrared spectroscopy (NIRS) imaging [6] have been developed for imaging the fibrous cap and lipid of atherosclerotic plaque, respectively. Some researchers reported the near-infrared fluorescence (NIRF) imaging in contrast agents’ fluorescence detection with satisfactory imaging signal-noise-ratio (SNR), but the insufficient imaging depth and the lack of depth resolution of the optical modalities severely limit the visualization of the full plaque and artery wall [7,8].
Nowadays, intravascular photoacoustic (IVPA) systems based on compact and small-sized catheters have been developed in atherosclerotic plaque imaging. Photoacoustic imaging is a hybrid modality combining the advantages of high imaging contrast and large imaging depth, enabling accurate three-dimensional (3D) imaging in tissues [9,10]. It is a promising technology for visualizing pathological characteristics on artery wall by specific absorption of different tissues [11]. It has been reported in atherosclerotic lipid or inflammation identification by tuning the excitation wavelength to the absorption band of the targets, providing important functional information of artery disease [12–17]. Considering the scattering of the excitation laser by blood, flushing is generally a necessary step during the whole IVPA imaging process to displace the blood in the imaging area and subsequently improve the imaging SNR. But long-time flushing may have unfavorable impacts on study subjects, such as myocardial ischemia [18]. The application of high-speed imaging system can significantly reduce the amount of flushing, thereby minimizing these unintended effects. Thus, developing the high-speed IVPA imaging systems along with efficient blood flush is significant in their further applications. In 2017, Cheng’s group proposed an IVPA/IVUS imaging system capable of B-scan acquisition of approximately 25 frames/second (f/s). They applied the system for lipid imaging at the wavelength of 1700 nm and demonstrated for the first time that IVPA/IVUS imaging speed of 16 f/s was fast enough to avoid the distortion of motion artifacts generated from heartbeat pulsation. But their work was mainly based on phantom study and no flushing was applied [19]. In the same year, Van Soest’s group reported an IVPA system with the speed of 20 f/s and pullback speed of 0.5 mm/s, comparable to the speed of conventional commercial IVUS system. The system was successfully used for real-time visualization of the introduced lipid in swine coronaries in vivo at the wavelength of 1720 nm. During the experiment, they flushed blood with heavy water to improve the imaging contrast of lipid [20]. In 2019, they improved the system pullback speed to 1 mm/s and successfully applied it to the in vivo identification of the endogenous lipid naturally formed in the coronaries of swine atherosclerotic models at the wavelength of 1725 nm [21]. The above-mentioned researches pushed forward the in vivo application of IVPA systems, but higher requirements of imaging speed is always highly preferred in clinics. Taking clinical IVOCT (with blood flush operation) as an example, its imaging speed is generally as high as 100 f/s and the pullback speed is about 10 - 40 mm/s [4]. Thus, developing higher-speed IVPA/IVUS systems is still an important work in their clinical translations.
In this research, we develop an IVPA/IVUS system with imaging speed as high as 100 f/s and 5 mm/s pullback speed. The in vivo IVPA/IVUS imaging performance of the system is demonstrated in rabbit abdominal aorta marked by exogenous nanoparticles at the wavelength of 1064 nm. Blood flushing is applied and improves the image SNR by 3.5 dB. This study offers a blood flushing-integrated IVPA/IVUS imaging system with the imaging speed comparable to that of commercial IVOCT.
2. Methods and materials
2.1. IVPA/IVUS system design, real-time visualization and data processing
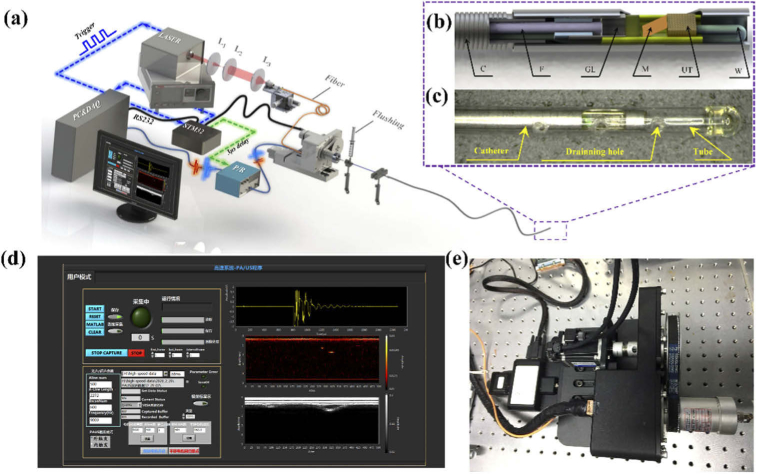
The schematic of the IVPA/IVUS system is shown in Fig. 1(a). A pulsed laser (Zylaser, China) with wavelength of 1064 nm and pulse width of 8 ns is used for optical excitation. The repetition rate of the laser source is tunable from 8 kHz to 100 kHz (9 kHz and 20 kHz are used in this research). The laser beam is coupled into an optical fiber with core size of 105 μm, which is connected to the fiber in IVPA/IVUS catheter through optical rotary joint (MJP-106-105-FC, Princetel, USA). The excitation light is guided by the fiber, then focused by a GRIN lens and finally reflected by a mirror out of the catheter to the artery wall [Fig. 1(b)]. The electrical signal is transmitted by an electrical slip ring (Jinpat, China) to the catheter [Fig. 1(e)]. The design of the catheter with outer diameter of 0.9 mm is same with that reported in Ref. [12]. The whole catheter is assembled inside a plastic tube for catheter protection from blood. A structure for saline flush is designed at the distal end of the catheter to flow the saline inside plastic tube. The proximal end of the plastic tube is enclosed by UV curable glue and several holes are drilled at the side of the tube for draining away the saline [Fig. 1(c)]. During the intervene in abdominal aorta, peristaltic pumps the saline flow inside plastic tube at the flow rate of 0.02 mL/s to prevent blood contaminating optical elements in catheter. During the imaging process, a syringe was used to flush the blood manually with the flow rate of about 0.3 mL/s.
Fig. 1.
(a) Schematic of high speed IVPA/IVUS imaging system; (b) Schematic and photo of the IVPA/IVUS imaging catheter; C: coil, F: fiber, GL: grin lens, M: mirror, UT: ultrasound transducer, W: electric wire; (c) The picture of the proximal end of a catheter with protection plastic tube; (d) The interface of real-time display on software of LabVIEW; (e) High-speed rotating and pullback machinery.
The ultrasound devices of the system have been reported in our previous studies [12]. The digitized photoacoustic and ultrasonic signals were processed by voltage conversion, digital filtering, envelop demodulation, and finally saved by a data acquisition card (ATS9325, Alazar, Canada). The real-time visualization of the imaging data was achieved and displayed in LabVIEW interface [Fig. 1(d), Visualization 1 (5.7MB, avi) ]. A time delay of 3 μs is set between photoacoustic and ultrasound signals for distinguishing the two signals to obtain the IVPA/IVUS images simultaneously. In this system, time sequence and the rotation and pullback scanning of the imaging catheter are controlled by one microcontroller STM32. The STM32 also communicates with the personal computer to set parameters for different imaging speeds.
2.2. Animal models in New Zealand rabbit abdominal aorta
All the procedures were performed according to a protocol approved by the Animal Study Committee of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. Healthy New Zealand rabbits (2-2.5 kg) were chosen as impaired endothelial models. The impaired artery endothelial were formed in the abdominal aorta wall after a balloon overstretch injury with a 3.5 F-sized balloon catheter [7,22]. After about 20 hours, we injected the CuS (Copper Sulfide) [23] nanoparticles into the rabbits by ear vein at the dose of 1.3 mg/kg. Then 4 hours post nanoparticles injection, we performed the in vivo imaging experiments or excised the abdominal aorta for ex vivo imaging experiments. During the ex vivo imaging experiments, the excised aorta segments were supported by a plastic tube and imaged by the IVPA/IVUS systems.
Aortic atherosclerotic plaque lesions were created in cholesterol-fed male New Zealand rabbits using balloon injury as previously reported [24,25]. The animals were fed with high cholesterol diet (2% cholesterol, 4% lard, 94% normal chow) for a total of 14 weeks, and balloon injury was performed on week 2. Upon successful anti-septics, anesthesia and heparinization, the animals were fixed on the operation table. A guidewire and a balloon catheter were inserted through a small incision on the left femoral artery and advanced distally to the aorta at about 12 cm in depth. The balloon was then inflated and gently retracted towards the femoral artery for 5 cm in length. This was repeated for three times at the same aortic segment. During the in vivo imaging experiment of the animal models, the nanoparticles with absorption wavelength at 1064 nm (CuS [23] or PBD-CD36 [16]) were directly injected into the atherosclerotic aorta wall in situ for further in vivo imaging.
2.3. Ex vivo IVPA/IVUS imaging
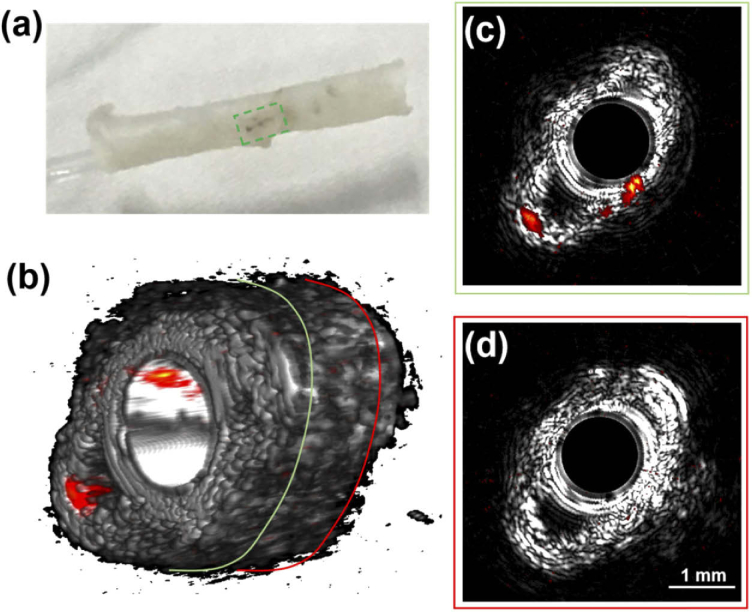
After balloon injury and CuS injection according to the procedure in section 2.2, we excised the abdominal aorta and performed ex vivo imaging of the excised aorta in water using the IVPA/IVUS system at the speed of 60 f/s. The excitation pulse energy was fixed at 30 μJ. An excised ex vivo abdominal aorta is shown in Fig. 2(a). CuS particles are visible in the excised aorta in the dashed green square.
Fig. 2.
IVPA/IVUS images of excised abdominal aorta ex vivo. (a) Photo of an ex vivo abdominal aorta, CuS particles are located in the dashed green square; (b) 3D volumetric fused IVPA/IVUS image; (c) and (d) are the IVPA/IVUS fused B-scan images at the location enclosed by the green and red circles in (b), respectively.
2.4. In vivo IVPA/IVUS imaging in rabbit abdominal aorta models
Four hours post CuS injection, the IVPA/IVUS imaging was performed in rabbit models in vivo to test the imaging ability of the system. The rabbits were anesthetized by pentobarbital sodium in saline solvent (1% concentration) with the dose of 1 mL/kg. After the anesthesia, the rabbit were fixed and a guiding catheter was introduced through iliac artery. Then the imaging catheter was guided through the guiding catheter into abdominal aorta. IVPA/IVUS pullback was performed with the B-scan step of around 50 μm to locate the target area and each pullback distance was fixed at 3 cm. In vivo IVPA/IVUS imaging of rabbit abdominal aorta is shown in Visualization 2 (3.8MB, mp4) .
In in vivo experiments, the imaging with the speed of 20, 60 and 100 f/s were performed with no blood flushing first to compare the imaging results at different imaging speed. In this process, the excitation laser intensity was fixed at 40 μJ (fluence: 0.1 J/cm2). 9 kHz laser pulse repetition rate was chosen for 20 and 60 f/s imaging with 450 A-lines and 150 A-lines per B-scan in IVPA/IVUS images; 20 kHz pulse repetition rate was chosen for 100 f/s imaging with 200 A-lines per B-scan in IVPA image and 100 A-lines per B-scan in IVUS image (limited by 10 kHz maximum repetition frequency of ultrasound pulser).
We also compared the in vivo imaging results with and without blood flush. During this process, we performed a block operation at the proximal end of the abdominal aorta. We flushed the aorta with saline for the imaging in all blood flush groups. Heparin sodium was included in the saline to prevent the blood from forming a thrombus. The flow rate was about 0.3 mL/s. In the comparison experiment, the abdominal aorta was also blocked but the imaging was performed with no blood flush. In this experiment, the excitation laser pulse energy was fixed at 40 μJ (fluence: 0.1 J/cm2) and one pullback distance was fixed at 3 cm.
2.5. Quantification of signal-noise-ratio (SNR)
The signal-noise-ratio (SNR) of the IVPA image was quantified according to the following procedures: choose the region of interest (ROI) where the photoacoustic signal locates; the ratio between the signals in ROI and the standard deviation (STD) of the background is the quantified SNR.
3. Results
3.1. Ex vivo IVPA/IVUS imaging of abdominal aorta from Switzerland rabbit models
The volumetric fused IVPA and IVUS images are visualized in Fig. 2(b), showing 3D distribution of CuS where the high photoacoustic signals locate. The SNR is about 24 dB with no averaging, demonstrating the capability of the system in capturing the photoacoustic signal from CuS nanoparticles accumulated on aorta wall. The IVPA/IVUS fused B-scan images are shown in Figs 2(c) and 2(d) at the selected locations of excised aorta. The aorta with CuS particles accumulated, showing in the green circle in Fig. 2(b), exhibits obvious photoacoustic signal at 5 o’clock and 7 o’clock in Fig. 2(c). While the aorta without CuS particles in the red circle in Fig. 2(b) shows no photoacoustic signal in Fig. 2(d). The signals in Fig. 2(c) demonstrate that the imaging depth of the system covers at least 0.6 mm to 1.5 mm.
3.2. In vivo IVPA/IVUS imaging of rabbit models with the speed of 20, 60 and 100 f/s with no flush
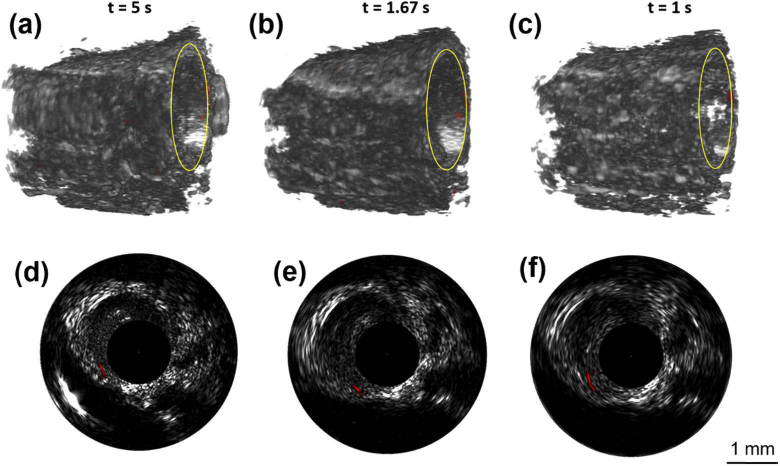
During 3 cm pullback distance, we used the shortest imaging time from 100 f/s group, with the imaging time of around 6 s; while the imaging time is 10 s and 30 s under the pullback speed of 60 and 20 f/s, respectively. The abdominal aorta pulsation was accurately captured by the imaging system and no motion artifacts were observed at the three imaging speed (Visualization 3 (3.6MB, mp4) , Visualization 4 (3.6MB, mp4) , Visualization 5 (3.7MB, mp4) ). The 3D images of 5 mm pullback containing the CuS target are shown in Figs. 3(a)–3(c). The imaging time at the three imaging speeds for the same pullback distance are 5 s, 1.67 s and 1 s, respectively. The B-scan images at the selected location enclosed by yellow circle in Figs. 3(a)–3(c) are shown in Figs. 3(d)–3(f), respectively. From the B-scan images at the same location where CuS nanoparticles exists, the SNR of the photoacoustic signal was about 17.7 dB and there is little difference in SNR between different imaging speeds. The entire IVPA/IVUS pullback data display with different imaging speed (3 cm pullback distance) is shown in Visualization 3 (3.6MB, mp4) , Visualization 4 (3.6MB, mp4) , and Visualization 5 (3.7MB, mp4) ) in supplementary materials.
Fig. 3.
IVPA/IVUS imaging in vivo with no flush. (a-c) 3D fused IVPA/IVUS images at the speed of 20, 60 and 100 f/s with the pullback distance of 5 mm, respectively; (d-f) IVPA/IVUS fused B-scan images at the selected locations enclosed by the yellow circle in (a)-(c), respectively.
3.3. Comparison of in vivo IVPA/IVUS imaging of rabbit models with and without blood flush
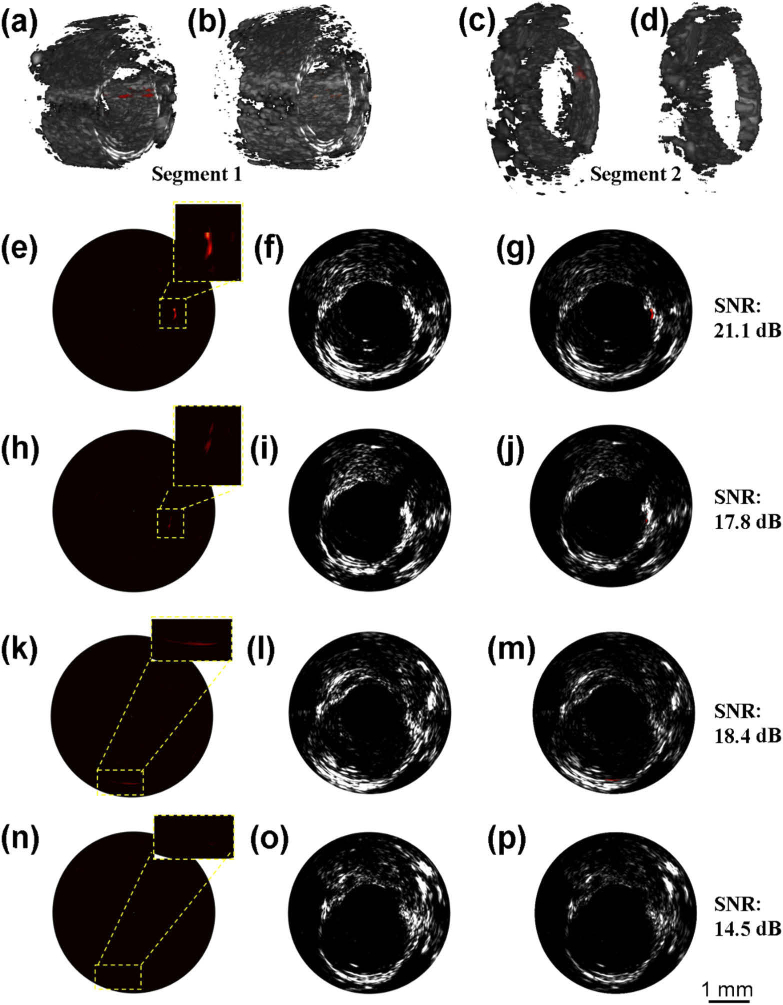
To test the flush influence on the imaging results, we performed and compared the IVPA/IVUS imaging with and without blood flush at the imaging speed of 60 f/s. The imaging results are shown in Fig. 4. 3D fused IVPA/IVUS images of two segments aorta containing CuS labeling with and without blood flush are shown in Figs. 4(a)–4(d). For the artery segment 1, we obtained higher photoacoustic signals with blood flush [Fig. 4(a)] than that obtained without blood flush [Fig. 4(b)]. The SNR of photoacoustic signal is improved from 17.8 dB [Fig. 4(h)] to 21.1 dB [Fig. 4(e)]. For the artery segment 2, we observed the photoacoustic signal with SNR of 18.4 dB in blood flush environment [Fig. 4(c)], while the signal is very faint (about 14.5 dB SNR) in the group of no blood flush [Fig. 4(d)]. The comparison results of the B-scans in this group is shown Figs. 4(k)–4(m) and Figs. 4(n)–4(p). The comparison experimental results demonstrate the SNR improvement by about 3.5 dB in the case of efficient blood flush in IVPA imaging. It is notable that the high imaging speed is especially important in the case of flushing, where we can minimize the flushing amount and further reduce the unfavorable impacts to experimental subjects.
Fig. 4.
IVPA/IVUS imaging results with blood flushing. (a), (b) 3D fused IVPA/IVUS images of artery segment 1 with and without blood flush, respectively; (c), (d) 3D fused IVPA/IVUS images of artery segment 2 with and without blood flush, respectively; (e) - (g) IVPA, IVUS and IVPA/IVUS fused B-scan images of (a); (h) - (j) IVPA, IVUS and IVPA/IVUS fused B-scan images of (b), the location is corresponding to that in (e)-(g); (k) - (m) IVPA, IVUS and IVPA/IVUS fused B-scan images of (c); (n) - (p) IVPA, IVUS and IVPA/IVUS fused B-scan images of (d), the location is corresponding to that in (k) - (m).
3.4. In vivo IVPA/IVUS imaging of rabbit atherosclerotic models
To demonstrate the capability of the system in atherosclerotic plaque imaging, we performed in vivo imaging using rabbit models with atherosclerotic plaques successfully built on abdominal aorta. The imaging results of CuS injection are shown in Fig. 5, showing the feasibility of the current IVPA/IVUS system in acquiring the anatomic information of the plaque from IVUS image (enclosed by the yellow line) and the functional information of the contrast agents from IVPA image (area indicated by CuS). The photoacoustic signal of PBD-CD36 can also be captured with the high speed system as shown in Fig. S1.
Fig. 5.
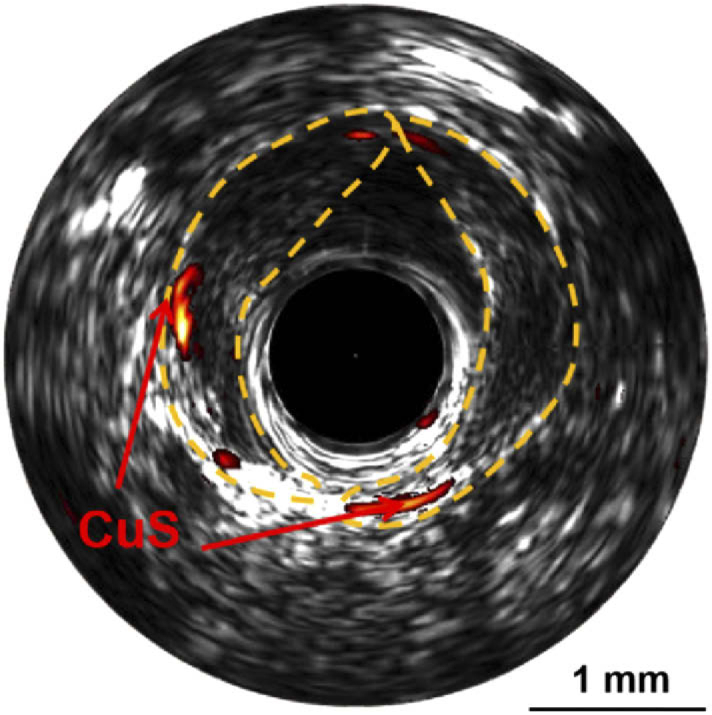
The fused IVPA/IVUS B-scan image of the abdominal aorta with atherosclerotic plaque built and CuS nanoparticles injected in situ. The area enclosed by yellow line is plaque and the signal indicated by CuS is photoacoustic signal of CuS.
4. Discussion
In this work, we improved an IVPA/IVUS system with the imaging speed as high as 100 f/s based on a miniature catheter of 0.9 mm. With a flushing structure integrated, the system is efficient enough for acquiring comprehensive imaging information of an animal model in the complex in vivo environment at the wavelength of 1064 nm. The high-speed IVPA system was developed to eliminate some obstacles of current IVPA technology translation to in vivo and even clinical applications. In these applications, the influence of blood scattering to excitation light was not negligible. Efficient blood flush in the imaging area was generally necessary to improve the image contrast and SNR. In this study, we performed blood flushing and evaluated its influence to IVPA imaging. The results demonstrated an obvious SNR improvement in IVPA images in flushing group (Fig. 4).
But long time blood flushing during the whole imaging process and accompanying blood displacement in coronary arteries is potentially unfavorable for the operation subjects. Thus developing high-speed imaging systems with minimum flushing remains the focus of interests among the researchers and physicians. For example, blood flushing is one indispensable prerequisite of IVOCT in its clinical use and the imaging speed of current clinical IVOCT imaging system is set at 100 f/s. Considering the same requirement of blood flushing between IVPA and IVOCT systems, improving the IVPA imaging speed to that of IVOCT is an important step. In this study, we put forward the IVPA imaging system with the imaging speed as high as 100 f/s. The experimental results acquired the same image information along with the same pullback distance using the shortest imaging time at the speed of 100 f/s compared that at the speed of 60 f/s and 20 f/s (Fig. 3).
Based on our previous low-speed IVPA system, we developed the high-speed system and ensured its stable imaging by solving a series of issues. Firstly, during high-speed rotating, mechanical resonance severely influence the system stability; thus the mechanical structure was carefully redesigned to reduce the vibration of the system greatly and improved the imaging stability. Secondly, we customized the rotary joint with a high-speed electrical rotary joint and optical rotary joint, and designed a torque coil. The rotary joint is the core element to offer the imaging speed as high as 100 f/s and the torque coil ensured the stability of torque transmission during the high-speed imaging. In addition, LabVIEW interface was developed to control the imaging operation conveniently and display the IVPA and IVUS information during the imaging in real-time, which played an important role in observing the intravascular environment instantaneously. At last, the high speed rotation generated higher electrical noise, which degraded the SNR of the final image; we redesigned the electrical wire route and used shielding methods to weaken the noise and improve the image SNR.
Blood flushing is also a necessary step to ensure the imaging SNR. During blood flushing, we flowed the flushing media (saline) through the channel between the imaging catheter and the protection plastic tube; and drained away the media through several small holes on side wall of the plastic tube at the proximal end (Fig. 1). We flushed the blood in imaging area efficiently with little flushing speed (flow rate: 0.3 mL/s) in the case of artery block at proximal end. The design was different from that of current clinical IVOCT, which flowed the flushing media in the channel between protection plastic tube (about 0.9 mm diameter) and guiding catheter (guiding catheter is an important component to guide the imaging catheter into coronary artery and the catheter size is about 1.8 mm) [26]. The clinical blood flush requires larger flushing speed (flow rate: about 3 mL/s) and works well with no artery block. We tried this scheme in our system but failed to get satisfying results. It was mainly because our protection tube was larger (1.3 mm outer diameter), while the guiding catheter size was the same as that used in IVOCT. It means we didn’t have enough space between the guiding catheter and protection tube for the flushing media to achieve the flow rate as large as 3 mL/s. But the flushing scheme used in this study with little amount of flushing and artery block can still offer us an efficient blood displacement in short time.
The potential applications of the system in the future focus firstly on atherosclerotic inflammation identification, which involves the development of novel contrast agents. Although the simple CuS nanoparticles were successfully accumulated on impaired aorta wall and offered us a good target to test the imaging performance of the system in an in vivo environment in this study, direct labeling of nanoparticles in the inflammation cells of naturally formed atherosclerotic plaque by circulation was not achieved. This is mainly due to the limited inflammation activity of the atherosclerotic plaques and moderate labeling efficiency of the nanoparticles. We expect the system will be applied to identify and quantify the inflammation in naturally built atherosclerotic plaque by labeling the inflammatory cells with high-efficiency contrast agents in the near future. For this application, the development of the contrast agents is especially important. The contrast agents should have good photoacoustic signal, high-efficiency labeling to inflammatory cells with specificity, proper circulation time in blood, and enough quantity for the animal use. It is notable that the mature commercial laser source and little light scattering at 1064 nm make the contrast agents at 1064 nm absorption especially popular nowadays.
Our current system was only valid for intravascular photoacoustics at 1064 nm detecting exogenous contrast in an aortic injury rabbit model. But the high-speed IVPA/IVUS system is absolutely necessary to be applied in other experimental circumstances, which will generate other results. Take the application in human coronary artery for atherosclerotic lipid or inflammation identification as an example: there are several issues to address. The first issue is that our current 0.9 mm-sized catheter makes it very difficult to achieve efficient blood flushing with no artery block; it is necessary to further minimize the catheter size by optimizing the optical / ultrasonic elements and choosing smaller protection plastic tube, thus we can have enough space between the guiding catheter and protection tube for efficient blood flushing with no artery block. The second issue is the much more complex vasculature in human coronary arteries. Although the size of rabbit abdominal aorta is comparable with that of human coronary artery, the vasculature of rabbit abdominal aorta is much simpler than human coronary arteries, which makes the catheter easy to go through and locate in this study. But in clinical practice, the catheter length, flexibility and transmissibility should be improved to adapt to the human arteries environment. Another important factor limiting the expansion of the applications is the stable laser source with high repetition rate. For example, stable lasers with high repetition rate at 1210 nm or 1720 nm (commonly used lipid absorption spectrum) for lipid identification are highly desirable in this field nowadays. Considering the requirement of lipid quantification using photoacoustic spectrum method, developing the laser source with multi-wavelengths at the two absorption bands is important. Although some novel high-speed laser sources with multi-wavelengths have been developed [27], more proper laser sources are still in urgent need to push forward the expanded applications. Next step we will make the improvement for the system and test its imaging performance for lipid or inflammation in large animal models (such as swine), which is closer to human coronaries.
In summary, we have built an IVPA/IVUS system with the imaging speed as high as 100 f/s and demonstrated a volumetric in vivo imaging of nanoparticles located on rabbit abdominal aorta wall at 1064 nm with a flexible miniature catheter of 0.9 mm. The imaging speed is suitable for clinical IVPA/IVUS imaging, especially in the case of blood flushing. The influence of blood flushing to imaging was evaluated and the SNR of IVPA images was significantly improved by 3.5 dB. Our study promoted the high-speed IVPA/IVUS system with blood flushing, which are quite important for further expanded applications of in vivo vulnerable plaque identification.
Funding
National Natural Science Foundation of China10.13039/501100001809 (61705250, 61975226, 81827807, 81971638); National Key Research and Development Program of China10.13039/501100012166 (2018YFC0116302); CAS Key Laboratory of Health Informatics (2011DP173015); Guangdong Provincial Key Laboratory of Biomedical Optical Imaging Technology (2020B121201010); Medical Science and Technology Foundation of Guangdong Province10.13039/501100009330 (A2019074); Science and Technology Planning Project of Guangdong Province10.13039/501100012245 (2016A020220007); Shenzhen Science and Technology Innovation Program10.13039/501100017610 (JCYJ20160608214524052, JCYJ20170818163841756); Shenzhen Science and Technology Innovation Committee10.13039/501100010877 grants (ZDSY20130401165820357); Hong Kong Research Impact Fund (R5029-19); Innovation and Technology Fund10.13039/501100010428 (ITF/082/18).
Disclosures
The authors declare no conflicts of interest.
See Supplement 1 (1.2MB, pdf) for supporting content.
References
- 1.Libby P., Ridker P. M., Maseri A., “Inflammation and atherosclerosis,” Circulation 105(9), 1135–1143 (2002). 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 2.Libby P., “Inflammation in Atherosclerosis,” Nature 420(6917), 868–874 (2002). 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 3.Garcìa-Garcìa H. M., Gogas B. D., Serruys P. W., Bruining N., “IVUS-based imaging modalities for tissue characterization: similarities and differences,” Int. J. Cardiovasc. Imaging 27(2), 215–224 (2011). 10.1007/s10554-010-9789-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ughi G. J., Adriaenssens T., Sinnaeve P., Desmet W., D’hooge J., “Automated tissue characterization of in vivo atherosclerotic plaques by intravascular optical coherence tomography images,” Biomed. Opt. Express 4(7), 1014–1030 (2013). 10.1364/BOE.4.001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahara S., Morooka T., Wang Z., Bezerra H. G., Rollins A. M., Simon D. I., Costa M. A., “Intravascular Optical Coherence Tomography Detection of Atherosclerosis and Inflammation in Murine Aorta,” Arterioscler., Thromb., Vasc. Biol. 32(5), 1150–1157 (2012). 10.1161/ATVBAHA.111.243626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaguszewski M., Klingenberg R., Landmesser U., “Intracoronary Near-Infrared Spectroscopy (NIRS) Imaging for Detection of Lipid Content of Coronary Plaques: Current Experience and Future Perspectives,” Curr. Cardiovasc. Imaging Rep. 6(5), 426–430 (2013). 10.1007/s12410-013-9224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozhko D., Osborn E. A., Rosenthal A., Verjans J. W., Hara T., Kellnberger S., Wissmeyer G., Ovsepian S. V., McCarthy J. R., Mauskapf A., Stein A. F., Jaffer F. A., Ntziachristos V., “Quantitative intravascular biological fluorescence-ultrasound imaging of coronary and peripheral arteries in vivo,” Eur. Heart J. - Card. Img. 18(11), 1253–1261 (2017). 10.1093/ehjci/jew222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verjans J. W., Osborn E. A., Ughi G. J., Calfon Press M. A., Hamidi E., Antoniadis A. P., Papafaklis M. I., Conrad M. F., Libby P., Stone P. H., Cambria R. P., Tearney G. J., Jaffer F. A., “Targeted Near-Infrared Fluorescence Imaging of Atherosclerosis,” J. Am. Coll. Cardiol. Img. 9(9), 1087–1095 (2016). 10.1016/j.jcmg.2016.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong Z., Yao J., Wang L. V., “Tutorial on photoacoustic tomography,” J. Biomed. Opt. 21(6), 061007 (2016). 10.1117/1.JBO.21.6.061007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J.-M., Favazza C., Chen R., Yao J., Cai X., Maslov K., Zhou Q., Shung K. K., Wang L. V., “Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo,” Nat. Med. 18(8), 1297–1302 (2012). 10.1038/nm.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L. V., Hu S., “Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs,” Science 335(6075), 1458–1462 (2012). 10.1126/science.1216210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Gong X., Liu C., Lin R., Hau W., Bai X., Song L., “High-speed intravascular spectroscopic photoacoustic imaging at 1000 A-lines per second with a 0.9-mm diameter catheter,” J. Biomed. Opt. 20(6), 065006 (2015). 10.1117/1.JBO.20.6.065006 [DOI] [PubMed] [Google Scholar]
- 13.Wang B., Yantsen E., Larson T., Karpiouk A. B., Sethuraman S., Su J. L., Sokolov K., Emelianov S. Y., “Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques,” Nano Lett. 9(6), 2212–2217 (2009). 10.1021/nl801852e [DOI] [PubMed] [Google Scholar]
- 14.Jansen K., van Soest G., van der Steen A. F. W., “Intravascular photoacoustic imaging: a new tool for vulnerable plaque identification,” Ultrasound Med. Biol. 40(6), 1037–1048 (2014). 10.1016/j.ultrasmedbio.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Lei P., Wen X., Zhang P., Yang S., “Tapered fiber-based intravascular photoacoustic endoscopy for high-resolution and deep-penetration imaging of lipid-rich plaque,” Opt. Express 27(9), 12832–12840 (2019). 10.1364/OE.27.012832 [DOI] [PubMed] [Google Scholar]
- 16.Xie Z., Yang Y., He Y., Shu C., Chen D., Zhang J., Chen J., Liu C., Sheng Z., Liu H., Liu J., Gong X., Song L., Dong S., “In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging,” Theranostics 10(10), 4694–4704 (2020). 10.7150/thno.41211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P., Ma T., Slipchenko M. N., Liang S., Hui J., Shung K. K., Roy S., Sturek M., Zhou Q., Chen Z., “High-speed intravascular photoacoustic imaging of lipid-laden atherosclerotic plaque enabled by a 2-kHz barium nitrite raman laser,” Sci. Rep. 4(1), 6889 (2015). 10.1038/srep06889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suter M. J., Kashiwagi M., Gallagher K. A., Nadkarni S. K., Asanani N., Tanaka A., Conditt G. B., Tellez A., Milewski K., Kaluza G. L., Granada J. F., Bouma B. E., Tearney G. J., “Optimizing flushing parameters in intracoronary optical coherence tomography: an in vivo swine study,” Int. J. Cardiovasc. Img. 31(6), 1097–1106 (2015). 10.1007/s10554-015-0668-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui J., Cao Y., Zhang Y., Kole A., Wang P., Yu G., Eakins G., Sturek M., Chen W., Cheng J.-X., “Real-time intravascular photoacoustic-ultrasound imaging of lipid-laden plaque in human coronary artery at 16 frames per second,” Sci. Rep. 7(1), 1–11 (2017). 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M., Springeling G., Lovrak M., Mastik F., Iskander-Rizk S., Wang T., Van Beusekom H. M., Van Der Steen A. F. W., Van Soest G., “Real-time volumetric lipid imaging in vivo by intravascular photoacoustics at 20 frames per second,” Biomed. Opt. Express 8(2), 943–953 (2017). 10.1364/BOE.8.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iskander-Rizk S., Wu M., Springeline G., van Beusekom H. M. M., Mastik F., Te Lintel Hekkert M., Beurskens R. H. S. H., Hoogendoorn A., Hartman E. M. J., van der Steen A. F. W., Wentzel J. J., van Soest G., “In vivo intravascular photoacoustic imaging of plaque lipid in coronary atherosclerosis,” Eurointervention : Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 15(5), 452–456 (2019). 10.4244/EIJ-D-19-00318 [DOI] [PubMed] [Google Scholar]
- 22.Moore S. J. E., “Endothelial injury and atherosclerosis,” Exp. Mol. Pathol. 31(1), 182–190 (1979). 10.1016/0014-4800(79)90019-4 [DOI] [PubMed] [Google Scholar]
- 23.Gao D., Sheng Z., Liu Y., Hu D., Zhang J., Zhang X., Zheng H., Yuan Z., “Protein-Modified CuS Nanotriangles: A Potential Multimodal Nanoplatform for In Vivo Tumor Photoacoustic/Magnetic Resonance Dual-Modal Imaging,” Adv. Healthcare Mater. 6(1), 1601094 (2017). 10.1002/adhm.201601094 [DOI] [PubMed] [Google Scholar]
- 24.Phinikaridou A., Hallock K. J., Qiao Y., Hamilton J. A., “A robust rabbit model of human atherosclerosis and atherothrombosis,” J. Lipid Res. 50(5), 787–797 (2009). 10.1194/jlr.M800460-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goertz D. E., Frijlink M. E., Tempel D., van Damme L. C. A., Krams R., Schaar J. A., ten Cate F. J., Serruys P. W., de Jong N., van der Steen A. F. W., “Contrast harmonic intravascular ultrasound - a feasibility study for vasa vasorum imaging,” Invest. Radiol. 41(1), 8–14 (2006). 10.1097/01.rli.0000188027.34400.f3 [DOI] [PubMed] [Google Scholar]
- 26.Roleder T., Jąkała J., Kałuża G. L., Partyka Ł, Proniewska K., Pociask E., Zasada W., Wojakowski W., Gąsior Z., Dudek D., “The basics of intravascular optical coherence tomography,” Postep. Kardiol. Inter. 2, 74–83 (2015). 10.5114/pwki.2015.52278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C., Shi J., Wang X., Wang B., Gong X., Song L., Wong K. K. Y., “High-energy all-fiber gain-switched thulium-doped fiber laser for volumetric photoacoustic imaging of lipids,” Photonics Res. 8(2), 160–164 (2020). 10.1364/PRJ.379882 [DOI] [Google Scholar]