Abstract
Background
Controlled donation after circulatory death (cDCD) has emerged as one of the main strategies for increasing the organ donor pool. Because of the ischemic injury that follows the withdrawal of life-sustaining therapies, hearts from cDCD donors have not been considered for transplantation until recently. The ex-situ perfusion of hearts directly procured from cDCD donors has been used to allow the continuous perfusion of the organ and the assessment of myocardial viability prior to transplantation. Based on our experience with abdominal normothermic regional perfusion in cDCD, we designed a protocol to recover and validate hearts from cDCD donors using thoraco-abdominal normothermic regional perfusion without the utilization of an ex-situ device.
Case presentation
We describe the first case of a cDCD heart transplant performed with this approach in Spain. The donor was a 43-year-old asthmatic female diagnosed with severe hypoxic encephalopathy. She was considered a potential cDCD donor and a suitable candidate for multiorgan procurement including the heart via thoraco-abdominal normothermic regional perfusion. The heart recipient was a 60-year-old male diagnosed with amyloid cardiomyopathy. Cold ischemia time was 55 min. The surgery was uneventful.
Conclusions
This case report, the first of its kind in Spain, supports the feasibility of evaluating and successfully transplanting cDCD hearts without the need for ex-situ perfusion based on the use of thoraco-abdominal normothermic regional perfusion opening the way for multiorgan donation in cDCD.
Keywords: Controlled donation after circulatory death, Heart transplantation, Normothermic regional perfusion, Case report
Background
Controlled donation after circulatory death (cDCD) refers to the donation from individuals who have been declared dead following the decision for the withdrawal of life-sustaining therapies (WLST) that are no longer considered beneficial to patients with devastating brain injury or terminal lung, heart and neurodegenerative diseases [1]. cDCD is one of the most robust strategies for increasing the organ donor pool and is now carried out in a growing number of countries throughout the world [2]. cDCD was implemented in Spain approximately 10 years ago. Since then, this type of donation has increased exponentially to the point where, nowadays, it accounts for 32% of all deceased donation procedures in the country [3]. Amongst cDCD donors, the use of abdominal normothermic regional perfusion (A-NRP) based on the use of extracorporeal membrane oxygenation (ECMO) devices has become a common practice in several European countries mainly due to the favorable results obtained in liver grafts and the lower rate of delayed graft dysfunction observed in kidney recipients [4–8]. In Spain, A-NRP was used in nearly 50 % of all cDCD cases in 2019.
Initially, cDCD donors were not considered eligible heart donors. It was assumed that the hypoxia suffered by the heart following WLST and during cardiac arrest would deem the organ unsuitable for transplantation due to the irreversible myocardial injury, but different publications in children and adults have shown otherwise [9–11]. The use of either direct procurement followed by ex-situ Organ Care System (OCS™) or thoraco-abdominal normothermic regional perfusion (TA-NRP) with ECMO also followed in the majority of cases by OCS™, allows for the restoration of cardiac function and heart evaluation after the determination of death and prior to transplantation with successful post-transplant outcomes [10–14].
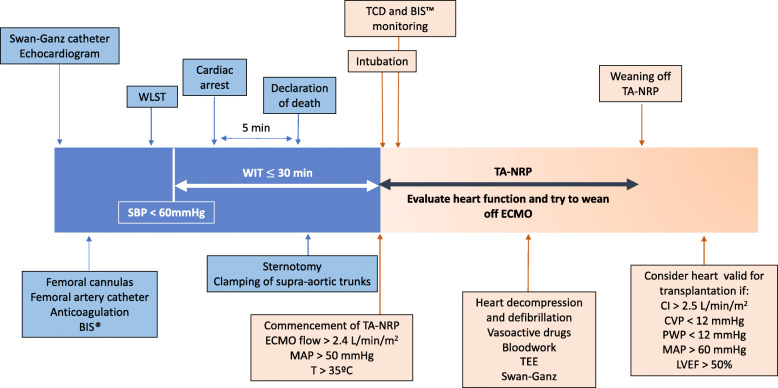
Based on these previous experiences and our own experience with the use of A-NRP in cDCD, the Donor Transplant Coordination Unit of Puerta de Hierro Majadahonda University Hospital (Madrid, Spain), together with the Departments of Cardiology, Cardiac Surgery, Thoracic Surgery and Intensive Care, designed a protocol for the procurement of cDCD donor hearts (Fig. 1), based on the use of TA-NRP with no ex-situ preservation devices. The protocol was approved by the Institutional Review Board, the Organización Nacional de Trasplantes [National Transplant Organization] and the Transplant Committee of the National Healthcare System at the end of 2019. The protocol was shared with all donor hospitals in the region, with the request to refer any potential cDCD donor who met the eligibility criteria for heart donation. We report the first case of a heart transplantation from a cDCD donor in Spain using this approach.
Fig. 1.
Protocol for cardiac procurement with thoraco-abdominal NRP after cDCD. BIS™: Bispectral Index. WLST: withdrawal of life sustaining therapies. SBP: systolic blood pressure. WIT: warm ischemia time. TA-NRP: thoraco-abdominal normothermic regional perfusion. ECMO: extracorporeal membrane oxygenation. MAP: mean arterial pressure. TCD: transcranial Doppler. TEE: transesophageal echocardiogram. CI: cardiac index. CVP: central venous pressure. PWP: pulmonary wedge pressure. LVEF: left ventricular ejection fraction
Case presentation
A 43-year-old female, with no previous medical history except asthma, suffered a prolonged cardiac arrest secondary to respiratory distress due to a status asthmaticus. After the return of spontaneous circulation, following advanced cardio-pulmonary resuscitation, she was admitted to the Intensive Care Unit (ICU) where she developed persistent myoclonus. A magnetic resonance imaging scan of the brain and an electroencephalogram were performed in the following days, and she was diagnosed with severe hypoxic encephalopathy. After 16 days, her neurological situation had not improved, and the decision was made with the patient’s family for WLST. Once this decision had been made, the option of organ donation was presented to the family, who agreed to proceed after considering that organ donation was consistent with the patient’s principles and values. The hospital’s Donor Coordinator considered the potential cDCD donor as a suitable candidate for multiorgan procurement including the heart. Because heart recovery was contemplated, our hospital was contacted, and the potential donor was transferred to our center with authorization from the donor’s family after specific and detailed information about the cDCD procedure had been given to them.
Once the patient was admitted to our ICU, a Swan-Ganz catheter was inserted through the right jugular vein and a transthoracic echocardiogram was obtained as part of the heart evaluation. After the heart and the abdominal organs were evaluated and deemed suitable for transplantation, she was transferred to the operating room where two femoral cannulas, artery and vein, were inserted using a Seldinger’s technique. This technique was also used to place a catheter in the contralateral femoral artery that would be used during TA-NRP to measure arterial pressures. During these procedures, analgesia and sedation were adjusted according to the donor’s needs. Once the cannulas and the femoral artery catheter were in place, the donor was anticoagulated using unfractionated heparin as previously reported [15]. Brain activity was monitored using the Bispectral Index (BIS™) in order to guarantee adequate levels of sedation during the WLST [16].
Once the surgical field was prepared, the family was brought into the operating room. WLST and end-of-life care was conducted following the hospital protocol and performed by the intensive care physician in charge of the patient’s care. Cardiac arrest was diagnosed by the absence of a pulse wave in the femoral artery, and death was declared after a five-minute no-touch period.
Following the declaration of death, a sternotomy was performed, the pericardium was opened, the supra-aortic trunks were clamped and TA-NRP was started with an ECMO flow of 3 L/min/m2, aiming for a mean arterial pressure > 50 mmHg and a T > 35 °C. At that point, a norepinephrine infusion was started, reaching a maximum dose of 0.1 mcg/kg/min during organ procurement. Simultaneously, the donor was intubated, and mechanical ventilation was started using a FiO2 of 1, a PEEP of 5 and a tidal volume of 6–8 ml per kg of predicted body weight. We consider the warm ischemia time (WIT) as the time from significant hypoperfusion, defined by a systolic blood pressure < 60 mmHg, until TA-NRP is started. A WIT < 30 min for the heart and liver and < 60 for the kidneys is considered valid [15]. The WIT for our case was 16 min.
One minute after the start of the TA-NRP, a spontaneous effective heartbeat was observed with a normal sinus rhythm. Cardiac output was measured using both the Swan-Ganz catheter and a transesophageal echocardiogram, which also addressed cardiac contractility. The absence of cerebral blood flow was confirmed by transcranial Doppler performed both in the anterior and posterior territories. Brain activity was monitored using the BIS™ which showed values of 00 and a suppression rate of 100 throughout the TA-NRP procedure (Fig. 2). Blood samples to determine arterial blood gas, lactate, troponin I, hematocrit, hemoglobin and liver function parameters were collected every 30 min during TA-NRP (Table 1).
Fig. 2.
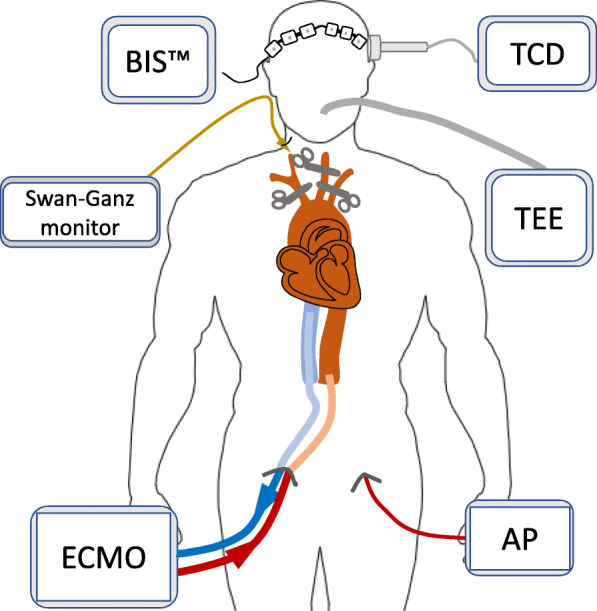
Donor monitoring during thoraco-abdominal NRP. BIS™: Bispectral Index. TCD: transcranial doppler. TEE: transesophageal echocardiogram. AP: femoral arterial pressure. ECMO: extracorporeal membrane oxygenator
Table 1.
Data collected before the withdrawal of life-sustaining therapies (baseline) and during thoraco-abdominal normothermic regional perfusion
| Baseline | TA-NRP | |||||
|---|---|---|---|---|---|---|
| 1′ | 15′ | 30′ | 60′ | 90′ | ||
| ECMO flow (L/min) | 0 | 3 | 0 | 1 | 1 | 1 |
| Cardiac index (L/min/m2)a | 3.9 | 3.7 | 3.2 | 3.7 | 3.7 | 3.7 |
| LVEF (%) | 74 | 54 | ||||
| SpO2 (%) | 97 | 100 | 89 | 100 | 100 | 100 |
| paO2 (mmHg) | 91.8 | 423 | 62 | 486 | 455 | 383 |
| paCO2 (mmHg) | 48.8 | 55.5 | 57.2 | 25.8 | 22.8 | 24.0 |
| pH | 7.37 | 7.09 | 7.16 | 7.44 | 7.49 | 7.46 |
| HCO3− (mEq/L) | 28.4 | 17.2 | 20.5 | 17.8 | 17.7 | 17.3 |
| Lactate | 0.7 | 8.4 | 4.8 | 4.2 | 3.8 | 3.6 |
| Hematocrit (%) | 34.5 | 38.4 | 29.3 | 29.7 | 29.9 | 26.6 |
| Hemoglobin (g/dL) | 11.3 | 12.6 | 9.5 | 9.7 | 9.8 | 8.7 |
| Troponin I (μg/dl) | 0.13 | 1.91 | 1.51 | 1.91 | 2.18 | 2.12 |
| ALT (IU/L) | 21 | 21 | 20 | 93 | 138 | |
| AST (IU/L) | 24 | 35 | 33 | 130 | 181 | |
| Bilirubin (mg/dL) | 0.5 | 0.4 | 0.3 | 0.5 | 0.4 | |
LVEF Left Ventricular Ejection Fraction measured by echocardiography, TA-NRP Thoraco-Abdominal Normothermic Regional Perfusion
aCalculated by Swan-Ganz monitoring
Once heart function was assessed, the ECMO flow was brought down until it reached 0 L/min/m2 15 min after its commencement. Because the donor had a history of severe asthma, SpO2 and PaO2 worsened after TA-NRP was stopped. For this reason, we decided not to wean the donor off ECMO completely, and a flow of 1 L/min/m2 was maintained during organ recovery. The duration of organ procurement was 120 min, and the heart, the liver and the kidneys were recovered and transplanted.
The heart recipient was a 60-year-old male diagnosed with amyloid cardiomyopathy. Due to the novelty of the case, the recipient had previously been informed of the peculiarities of the transplant which he agreed to by signing an specific informed consent were the different aspects of the procedure were detailed. Cold ischemia time was 55 min. The surgery was uneventful. He was easily weaned off cardiopulmonary bypass, and effective heartbeat was achieved after one defibrillation. He was then admitted to the ICU on low dose norepinephrine (< 0.2 mcg/kg/min) and low dose dobutamine (< 5 mcg/kg/min) and was extubated after 36 h. Follow-up echocardiograms showed a normal biventricular function. Five months after the transplant he has resumed a normal life.
Discussion and conclusion
Donor shortage worldwide has led to the development of different strategies to increase the organ donor pool. Amongst these strategies, cDCD has emerged as one of the cornerstones for this growth. It is estimated, according to different data, that the utilization of hearts from cDCD donors would have the potential of increasing the heart transplant activity between 15 to 50% depending on the countries [17, 18]. In Spain, based on a raw analysis that includes cDCD donors under 45 years of age, and excludes those with a suspicion of cardiac injury, it is estimated that the inclusion of these donors would account for a 5–10% increase in the number of heart donors, which would increase the number of hearts transplanted by 15–30 hearts per year.
In order to optimize heart function, one of the inclusion criteria of our protocol was that cDCD heart donors had to be under 45 years of age, a fact that narrowed the number of potential donors. For this reason, it was necessary to build a collaborative approach with other donor hospitals. Given the complexity of the recovery procedure in the case of a cDCD heart, together with the relatively small size of the Autonomous Community of Madrid (8.022 km2), we considered that the best approach would be to transfer the potential cDCD heart donor to our own hospital. The previous implementation of an intensive care program to facilitate organ donation in our country has made this a routine practice, whereas small donor hospitals cannot take on sophisticated cDCD procedures [19].
In Spain, ante-mortem interventions aimed at organ preservation that do not interfere with the dying process are not forbidden before WLST as long as the family consents [20]. Ante-mortem cannulation helps to reduce the duration of WIT. The possibility of restoring circulation to the brain – which would retroactively negate the diagnosis of death based on circulatory criteria - is one concern associated to the use of NRP. In the case of A-NRP, it is necessary to inflate an aortic occlusion balloon or to surgically clamp the abdominal aorta before A-NRP starts. In TA-NRP, the ECMO flow cannot start before the supra-aortic trunks have been clamped in order to avoid cerebral perfusion. This maneuver may prolong WIT but, in our case, the sternotomy-to-clamping time was only 4 min, resulting in a WIT of 16 min, which is below the 30-min limit that had been previously stipulated.
The absence of anterior and posterior cerebral perfusion during the procurement, one of the key points of this novel approach, was demonstrated by transcranial doppler, and cerebral electrical activity was monitored with BIS™ which always remained in the 00 mark and showed a suppression rate of 100 indicating an isoelectric electroencephalogram [13, 16, 21, 22].
At present, the majority of hearts recovered from cDCD donors have undergone a period of ex-situ perfusion in an OCS™ [10–12]. However, two recent reports have revealed the feasibility of evaluating and successfully transplanting cDCD hearts without the need for ex-situ perfusion based on the use of TA-NRP [13, 14]. In our case, due to our previous experience with A-NRP, we favored this approach [15]. We also consider that one of the advantages of in-situ heart evaluation is that it allows for a more realistic assessment of the heart through well validated techniques like Swan-Ganz or echocardiography. Also, because this was a case of a multiorgan donor, it was essential to guarantee adequate liver and kidney perfusion during their evaluation and procurement. For this reason, liver function tests were monitored every 30 min. Lactate levels were also measured as both markers of organ perfusion and liver function. Although no cut-off values of lactate were established, a decreasing trend was used as a surrogate for appropriate organ perfusion and adequate liver function during AT-NRP [8]. In all, the recovery procedure took around 120 min. In our case, the lungs were not considered due to the donor’s previous history of severe asthma.
Because both the procurement and the transplant were done in the same center, the cold ischemia time was only 55 min. In the future, it will be necessary to address if hearts procured by this technique will tolerate longer cold ischemia times in order to be implanted at a center different from where they have been retrieved. For the time being, our protocol only contemplates procurement and implantation at the same center in order to minimize the duration of cold ischemia, which may lead to better results in the recipient.
Overall, this is one of the few cases in the world, and the first in Spain, of a cDCD heart retrieved using only TA-NRP and successfully implanted, and it opens up the way for multiorgan donation in cDCD. Given the high cost of ex-situ machine perfusion, unaffordable in many settings, TA-NRP may become an option to make heart transplantation from cDCD donors economically feasible for some countries.
Acknowledgments
We appreciate the work of the nursing teams and central services of the hospital.
Abbreviations
- A-NRP
Abdominal normothermic regional perfusion
- AP
Arterial pressure
- BIS™
Bispectral index
- cDCD
Controlled donation after circulatory death
- CI
Cardiac index
- CVP
Central venous pressure
- ECMO
Extracorporeal membrane oxygenation
- ICU
Intensive Care Unit
- LVEF
Left ventricular ejection fraction
- MAP
Mean arterial pressure
- OCS™
Organ care system
- PWP
Pulmonary wedge pressure
- SAP
Systolic arterial pressure
- TA-NRP
Thoraco-abdominal normothermic regional perfusion
- TCD
Transcranial doppler
- TEE
Transesophageal echocardiogram
- WIT
Warm Ischemia time
- WLST
Withdrawal of life-sustaining therapies
Authors’ contributions
Marina Pérez Redondo, MD. ORCID: 0000–0002–2889-4312. Department of Intensive Care Medicine Department of Donor and Transplant Coordination. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in research design. Participated in the writing of the paper. Participated in the performance of the research. Sara Alcántara Carmona, MD, PhD. ORCID: 0000–0002–3941-5743. Department of Intensive Care Medicine. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in the writing of the paper. Participated in the performance of the research. Susana Villar García, MD. ORCID: 0000–0002–8591-0120. Department Cardiac Surgery. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in research design. Participated in the performance of the research. Alberto Forteza Gil, MD, PhD. ORCID: 0000–0001-6811-132X. Department of Cardiac Surgery. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in the performance of the research. Héctor Villanueva Fernández, MD. ORCID: 0000–0003–0847-0392. Department of Intensive Care Medicine. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in research design. Francisco José Hernández-Pérez, MD. ORCID: 0000–0001–6343-8112. Department of Cardiology. Advanced Heart Failure and Transplant Unit. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in research design. Participated in the performance of the research. José Luis Campo-Cañaveral de la Cruz, MD. ORCID: 0000–0003-3496-875X. Thoracic Surgery and Lung Transplantation Department. Hospital Universitario Puerta de Hierro-Majadahonda, Madrid. Participated in research design. Rocío Velasco Calvo, MD. ORCID 0000–0003–0338-9253. Department of Neurology. Hospital Universitario Puerta de Hierro de Majadahonda. Madrid. Spain. Participated in the performance of the research. Javier Segovia-Cubero, MD, PhD. ORCID: 0000–0002–4606-3416 Department of Cardiology. Advanced Heart Failure and Transplant Unit. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. CIBER cardiovascular, Instituto de Salud Carlos III, Madrid, Spain. Participated in the performance of the research. Beatriz Alonso Menárguez, MD. ORCID: 0000–0001–9221-2307. Division of Transplant and Cardiac Anesthesia, Department of Anesthesiology. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in the performance of the research. Francisco del Río Gallegos, MD, PhD. ORCID: 0000–0001–7572-4685. Regional Transplant Coordinator Community of Madrid. Madrid. Spain. Participated in research design. Elisabeth Coll, MD. PhD. ORCID 0000–0001–6876-7476. Organización Nacional de Trasplantes, Madrid, Spain. Participated in research design. Beatriz Domínguez-Gil González, MD, PhD. ORCID: 0000–0002–5695-8993. Organización Nacional de Trasplantes. Madrid. Spain. Participated in substantively revise the work. Juan José Rubio Muñoz, MD, PhD. ORCID: 0000-0001-7325-8473. Department of Intensive Care Medicine. Department of Donor and Transplant Coordination. Hospital Universitario Puerta de Hierro Majadahonda. Madrid. Spain. Participated in research design. Participated in the writing of the paper. Participated in the performance of the research. AND All of the authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study); and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Nominal grant from Consejería de Sanidad de la Comunidad de Madrid in favor of Fundación de Investigación Biomédica del Hospital Universitario Puerta de Hierro Majadahonda.
Availability of data and materials
Being a case report, data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent for publication was obtained.
Competing interests
The authors of this manuscript have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rubio Muñoz JJ, Domínguez-Gil González B, Miñambres García E, et al. Papel de la perfusión normotérmica con oxigenación de membrana extracorpórea en la donación en asistolia controlada en España. Med Int. 2020;S0210-5691(20):30066–30068. doi: 10.1016/j.medin.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Global Observatory of Donation and Transplantation. http://www.transplant-observatory.org/data-charts-and-tables/chart. Last access June 2020.
- 3.Organización Nacional de Trasplantes. Actividad de donación y trasplante España 2019. http://www.ont.es/infesp/Memorias/ACTIVIDAD%20DE%20DONACIÓN%20Y%20TRASPLANTE%20ESPAÑA%202019.pdf. Last access June 2020.
- 4.Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70(4):658–665. doi: 10.1016/j.jhep.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Miñambres E, Suberviola B, Dominguez-Gil B, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant. 2017;17(8):2165–2172. doi: 10.1111/ajt.14214. [DOI] [PubMed] [Google Scholar]
- 6.Farney AC, Singh RP, Hines MH, et al. Experience in renal and extrarenal transplantation with donation after cardiac death donors with selective use of extracorporeal support. J Am Coll Surg. 2008;206(5):1028–1037. doi: 10.1016/j.jamcollsurg.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Lomero M, Gardiner D, Coll E, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33(1):76–88. doi: 10.1111/tri.13506. [DOI] [PubMed] [Google Scholar]
- 8.Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant. 2019;19(6):1745–1758. doi: 10.1111/ajt.15241. [DOI] [PubMed] [Google Scholar]
- 9.Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359(7):709–714. doi: 10.1056/NEJMoa0800660. [DOI] [PubMed] [Google Scholar]
- 10.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 11.Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36(12):1311–1318. doi: 10.1016/j.healun.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Messer S, Axell RG, Colah S, et al. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant. 2016;35(12):1443–1452. doi: 10.1016/j.healun.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Messer S, Page A, Colah S, et al. Human heart transplantation from donation after circulatory-determined death donors using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2018;37(7):865–869. doi: 10.1016/j.healun.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Tchana-Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38(6):593–598. doi: 10.1016/j.healun.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Pérez Redondo M, Alcántara Carmona S, Fernández Simón I, et al. Implementation of a mobile team to provide normothermic regional perfusion in controlled donation after circulatory death: Pilot study and first results. Clin Transpl. 2020:e13899. [DOI] [PubMed]
- 16.Rosow C, Manberg PJ. Bispectral index monitoring. Anesthesiol Clin North Am. 2001;19(4):947–966. doi: 10.1016/S0889-8537(01)80018-3. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald P, Dhital K. Heart transplantation from donation-after-circulatory-death (DCD) donors: Back to the future. Evolving trends in heart transplantation from DCD donors. J Heart Lung Transplant. 2019;38(6):599. doi: 10.1016/j.healun.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Messer S, Page A, Rushton S, et al. The potential of heart transplantation from donation after circulatory death donors within the United Kingdom. J Heart Lung Transplant. 2019;38(8):872–874. doi: 10.1016/j.healun.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Sociedad Española de Medicina Intensiva y Unidades Coronarias. Organización Nacional de Trasplantes. Cuidados intensivos orientados a la donación de órganos. Recomendaciones. Grupo de trabajo SEMICYUC-ONT. http://www.ont.es/mailings/CIOD_Recomendaciones%20SEMICYUC-ONT_Septiembre2017.pdf. Last access June 2020.
- 20.Matesanz R CE, Domínguez-Gil B, Perojo L. Donación en asistolia en España: situación actual y recomendaciones. http://www.ont.es/infesp/DocumentosDeConsenso/DONACIÓN%20EN%20ASISTOLIA%20EN%20ESPAÑA.%20SITUACIÓN%20ACTUAL%20Y%20RECOMENDACIONES.pdf. Last access June 2020.
- 21.Escudero D, Otero J, Muñiz G, et al. The bispectral index scale: its use in the detection of brain death. Transplant Proc. 2005;37(9):3661–3663. doi: 10.1016/j.transproceed.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro RWÁJS, Gomes B, Hondjeu A, et al. Assesment of cerebral perfusion and activity during normothermic regional perfusion in a porcine model of donation after circulatory death [abstract] J Heart Lung Transplant. 2020;39(4S):S352. doi: 10.1016/j.healun.2020.01.411. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Being a case report, data sharing not applicable to this article as no datasets were generated or analysed during the current study.