SECTION I
GENERAL CONSIDERATIONS
1. Menopause is a transition phase from the reproductive to the nonreproductive phase in a woman's life. It is nature's protective phenomenon against reproductive morbidity and mortality in the aging population. It sets the stage for aging and accelerates the process of noncommunicable disorders
2. Menopause is diagnosed retrospectively by history. Markers for diagnosis of menopause are preferably restricted for use in special situations and for fertility issues. Levels of follicle-stimulating hormone (FSH) >10 IU/L are indicative of declining ovarian function. FSH levels >20 IU/L are diagnostic of ovarian insufficiency in the perimenopausal age group with vasomotor symptoms (VMS), even in the absence of cessation of menstruation.[1] FSH level >40 IU/L done at least 4 weeks apart is a reliable marker for menopause or impending menopause and associated with low estradiol levels. Anti-Mullerian hormone becomes undetectable, inhibin levels fall, and antral follicular count and ovarian volume decrease at menopause. Menstrual irregularity is the only objective marker to define and establish the menopause transition.[2]
TERMINOLOGY
3. Natural or spontaneous menopause: It is recognized to have occurred after 12 months of amenorrhea, for which there are no obvious pathological and physiological causes. It occurs due to the depletion of ovarian follicles, resulting in near-complete, but natural diminution of ovarian hormone secretion. There is no independent biological marker for menopause[3]
4. Premenopause: It is often used to refer the entire reproductive period, up to the final menstrual period (FMP).
5. Perimenopause: It is the period immediately before and up to 1 year after the FMP. It may last for 3–5 years. The characteristics are increased blood levels of FSH, anovulatory cycles, significantly reduced fertility and erratic menstrual periods, and onset of symptoms. This term is used interchangeably with menopause transition[4,5]
6. Menopause transition or perimenopause begins on an average 4 years before the FMP and is characterized by irregular menstrual cycles, endocrine changes, and symptoms such as hot flashes that may affect a woman's quality of life (QOL). It may be considered as a biological marker for chronic disease. The biology and symptomatology of menopause are blurred due to its relationship to the underlying aging process. Long-term effects on bone and heart have been related to estrogen deficiency. It is the term coined by the Stages of Reproductive Ageing Workshop (STRAW) group, and during this period, disturbed menstrual cycle and endocrine changes are observed. Anklesaria's staging of menopause has been adopted by the Indian Menopause Society (IMS) after modification[5,6,7]
7. Climacteric: Literally, it means the rungs of a ladder. It is interchangeable with perimenopause and menopause transition. When associated with symptoms, it is termed as the climacteric syndrome. This term is preferably not to be used in scientific papers[4]
8. Postmenopause: It is the span of time dating from the FMP, regardless of whether the menopause was spontaneous or iatrogenic[4]
9. Senescence: It is the period after the age of 60 years[3]
10. Premature menopause/premature ovarian insufficiency (POI): POI is replacing the term premature menopause. POI is described as amenorrhea due to loss of ovarian function before the age of 40 years. It is a state of female hypergonadotropic hypogonadism. It can manifest as primary amenorrhea with onset before menarche or secondary amenorrhea. Statistically, it is defined as spontaneous menopause occurring below two standard deviations (SDs) below the mean estimated age for the reference population for 5% level of significance. The estimated average age of menopause in India is reported to be 46 years; hence, we may consider POI as occurring below the age of 38 years. However, we do need to have population-based studies to derive at these cutoff values. The European Society of Human Reproduction and Embryology (ESHRE 2015) laid the following diagnostic criteria for POI-oligomenorrhea/amenorrhea for at least 4 months and an elevated FSH level >25 IU/l on two occasions >4 weeks apart.[8]
11. Induced menopause: It is cessation of menstruation that follows bilateral oophorectomy or iatrogenic ablation of ovarian function[4]
12. Temporary menopause: It is a term preferably not to be used since the definition of menopause is complete cessation of menstruation. Rarely, ovarian function is interrupted for a period of time and later resumes[4]
13. Early menopause: It is the time span between the spontaneous or iatrogenic menopause occurring between the age of 40 years and the accepted typical age of menopause for a given population. The consensus from various guidelines is to treat with hormone replacement therapy (HRT) till the age of menopause
14. Delayed menopause: It is not defined but may be important in terms of increased problems associated with the hyperestrogenism and is used in this guideline. It is two SDs above from the natural average age of menopause in a given population. We may consider it to be beyond 54 years. Population-based studies are needed to derive at these cutoff values
15. Postmenopausal bleeding (PMB): It is bleeding that occurs 12 months after the last normal period. However, it is recommended that any vaginal bleeding that occurs 6 months after the last period (presumed menopause) should be investigated
16. Staging system: The staging system of a physiological event is to improve comparability of strategies and facilitate clinical decision-making. In 1997, Ankelesaria in India published a simple clinical method of staging of menopause to understand and deal with the problems of the transition phase and beyond.[6,7] STRAW (2001) aimed to classify the woman's life in three phases: (1) reproductive, (2) menopause transition, and (3) postmenopause based on the menstrual cycle, endocrine parameters, and ovarian reserve markers. This was applicable only to healthy women. The 2012 STRAW + 10 provides a greater clarity for menstrual pattern and is applicable to most women, except for those with POI[9,10]
17. India, with a population of 1.2 billion people, is the second largest emerging economy and second most populated country in the world. According to the latest World Health Organization (WHO) data published in 2018, the life expectancy in India for a female is 70.3 years, expected to increase to 77 years by 2050. Noncommunicable diseases account for 60% of the total deaths in India. Currently, approximately 10% of India's population, i.e., more than 100 million, is aged over 50 years[11]
18. The estimated mean age of menopause is 46 years in India and is lower than that of the Caucasians.[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] From the available Indian data, it is hypothesized that an early age of menopause predisposes a woman to chronic health disorders a decade earlier than a Caucasian woman. It is reported that osteoporotic fractures occur 10–20 years earlier in Indians compared to Caucasians.[29,30] The first myocardial infarction (MI) attack occurs in 4.4% of Asian women at a younger age than in European women.[31] In India, type 2 diabetes mellitus (T2DM) occurs a decade earlier than the Caucasians.[32] Breast cancer is the most common cancer in Indian women, and the incidence peaks before the age of 50 years[33]
19. The overall prevalence of hypertension in India was 29.8%. Significant differences in hypertension prevalence were noted between rural and urban parts, i.e., 27.6% and 33.8%, respectively. Only 25% of rural and 38% of urban Indians are being treated for hypertension. The burden of cardiovascular disease (CVD) in India is projected to increase by 115% from 1990 to 2020[34] and cerebrovascular incidence by 104%.[35] The migrant population from the Indian subcontinent in the UK is known to be at a significantly higher risk of developing diabetes and CVD.[36] The mean bone mineral density (BMD) in India is about two SDs lower than in women in the Western population.[29] The prevalence of low bone mass is to the extent of 40% from the age of 40 years and increases to more than 62% by the age of 60 years and 80% by the age of 65 years.[37,38,39,40,41,42] The above facts indicate the need to have well-planned cost-effective systems in place to promote a healthy and an active aging population.
INDIVIDUALIZED PLAN FOR MENOPAUSE
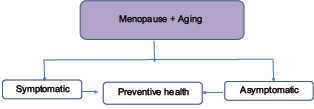
20. Each woman needs an individualized health plan management. It is most important to distinguish between a symptomatic and an asymptomatic menopausal woman.[43] Women may present at the menopausal clinic with menstrual problems, menopausal symptoms, or request for a general health checkup or as an opportunistic contact to be picked up by the health professional [Flowcharts 1 and 2].[44]
Flowchart 1.
The physician's role and approach
Flowchart 2.
Issues in symptomatic women
SECTION II
SYMPTOMS OF MENOPAUSE, ISSUES RELATED TO MENOPAUSE TRANSITION, AND AGING
Fertility
21. After the age of 30 years, if a woman does not conceive naturally within 6 months, the couple should have an infertility workup (Grade B)
22. In women with a single ovary, previous ovarian surgery, poor response to gonadotropins, previous exposure to chemotherapy or radiation, or unexplained infertility, they should undergo ovarian reserve testing even before the age of 30 years, and in all women, it is done beyond more than or equal to 30 years (Grade B)
23. In women aged >40 years who do not conceive within 1–2 cycles of controlled ovarian hyperstimulation, in vitro fertilization (IVF) should be considered (Grade B)
24. The only effective treatment for ovarian aging is oocyte donation. A woman with decreased ovarian reserve should be offered oocyte donation as an option, for pregnancy rates associated with this treatment are significantly higher than those associated with controlled ovarian hyperstimulation or IVF with a woman's own eggs (Grade B)
25. The risk of spontaneous pregnancy loss and chromosomal abnormalities increases with age, and the couple needs to be counseled on this aspect (Grade B)
26. Preconception counseling with an emphasis on optimal general health and screening for medical conditions, such as hypertension, diabetes, and pregnancy-related risks, should be addressed for women aged >40 years (Grade B).
Contraception
27. Pregnancies in the elderly women are associated with higher maternal and perinatal morbidity and mortality. There is an increased risk of fetal malformations. This can also lead to psychological and potential domestic and social consequences
28. The annual risk of deaths associated with using no method of contraception far exceeds that for use of any method among all age groups [Table 1][45]
Table 1.
Mortality rates
| Age | 35-39 years | 40-44 years |
|---|---|---|
| No method Contraception | 11.7/100,000 women | 20.6/100,000 |
| With oral contraceptive pills | 1/10,000 women | 1.9/100,000 |
29. Pattern of contraception use in the age group of 15–49 years is shown in Table 2[46]
Table 2.
Unmet need for family planning, and percentage of women currently married women and sexually active women 15-49 years who use any contraceptive method
| Criteria | Numbers in percentages |
|---|---|
| Total unmet need in % | 13% |
| Unmet need for spacing in % | 6% |
| Not using any method | 47% |
| Female sterilisation | 37% |
| Male sterilisation | 0.3% |
| Pill/Injectables | 4.1%/0.2% |
| IUD/PPIUD | 1.5% |
| Condom; male/female | 5.6%/0.1% |
| Rhythm/withdrawal | 3.5%/2.3% |
IUD - Intrauterine device; PPIUD - Postpartum Intrauterine device
30. Sterilization is a highly effective, safe, and single act; the case-fatality rate with tubectomy is 1–2/100,000 procedures. However, it is a permanent method. Vasectomy is even safer except for minor complications (Grade A)
31. Oral contraceptive pills (OCPs) are effective, easy to use, and reversible. Low-dose OCPs have noncontraceptive health benefits with an increased safety profile (Grade A)[47,48,49]
32. For women above the age of 35 years, careful personal and family history, and accurate measurement of blood pressure (BP), breast examination, screening for diabetes, and lipid profile should be performed (Grade A)[50]
33. Healthy women of normal weight, nonusers of tobacco, and doing well on a combination contraceptive pill can continue this method until the age of menopause and up to a year or 2 years later, after analyzing its risks and benefits (Grade B)
34. If OCPs are continued before major surgery, heparin prophylaxis may be considered (Grade B)
35. Depot medroxyprogesterone acetate (DMPA) is associated with bone loss, which returns to normal, after stopping DMPA. Yet, the caution needs to be exercised in women at a high risk of osteoporosis. Short- or long-term use of DMPA in healthy women should not be considered as an indication for dual X-ray energy absorptiometry or other tests that assess BMD (Grade C)
36. Change over from OCPs to hormone therapy (HT) is carried out at an arbitrary age of 45–50 years, if serum FSH: luteinzing hormone ratio of >1; low estradiol <20 pg/mL; FSH >30 IU/L done twice 6 weeks apart[51]
37. Progesterone-only contraceptive is an ideal method in women with a history of venous thromboembolism (VTE) and gallstones. Limitations are erratic and scanty periods. The levonorgestrel–intrauterine system, apart from being used as a contraception, is an effective HT for heavy menstrual bleeding and for treating bleeding disturbances associated with endometrial hyperplasia (EH) (Grade B)
38. Intrauterine contraceptive devices are effective but sometimes can cause menorrhagia and dysmenorrhea (Grade B)
39. Emergency contraception is an effective emergency method, but it is not as effective and consistent as the use of other contraceptive (Grade C).
Perimenopausal bleeding
40. It is suggested to incorporate the use of PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified) classification for abnormal uterine bleeding (AUB)[52]
41. The common causes are anovulatory bleeding, leiomyoma, endometrial polyp, EH, and endometrial cancer (EC)[53]
42. Endometrial tissue sampling may be performed in patients with AUB who are older than 40 years (Grade C). Office hysteroscopy is the preferred method for tissue sampling. Blind sampling procedures are justified if an abnormality is symmetrically “panuterine.” Directed biopsies are preferable, in cases of focal lesions[54]
43. Pipelle is a safe, accurate, noninvasive, and cost-effective outpatient procedure and may be offered to women at low risk of cancer.[55] It has a low sensitivity for detecting other intracavitary lesions, including polyps and submucosal fibroids, and small lesions may be missed. Hysteroscopy directed biopsy is the gold standard
44. A meta-analysis by van Hanegem et al., 2016 concluded that a positive test result of endometrial sampling is very accurate in diagnosing endometrial (pre) cancer or endometrial disease. However, endometrial sampling is not very accurate in ruling out endometrial (pre) cancer and endometrial disease, and therefore, further diagnostic workup for focal pathology is warranted, after a benign result of endometrial sampling in high-risk women. Endometrial sampling methods show good specificity (98%–100%), but the sensitivity is low (76%–90%)[56]
45. Transvaginal ultrasonography (TVS) is the primary screening test for AUB, and magnetic resonance imaging (MRI) should be considered when the diagnosis is inconclusive (Grade C)
46. Persistent bleeding with a previous benign pathology, such as proliferative endometrium, requires further testing to rule out focal endometrial pathology or a structural pathology, such as a polyp or leiomyoma (Grade B)
47. Management depends on the cause, the cost–benefit analysis of therapy, and the patient's choice (Grade C).
Postmenopausal bleeding
48. PMB is defined as uterine bleeding occurring after at least 1 year of amenorrhea. Its incidence is about 10%–15%[57]
49. Women with PMB have a 10%–15% chance of having EC. Conversely, 90% of EC in the postmenopausal period presents with PMB. Hence, immediate evaluation is required[58]
50. The common cause of PMB is due to atrophic changes in the vagina and the endometrium[59]
51. A detailed clinical and drug history is important as some over-the-counter drugs such as “ginseng” can cause PMB
52. A thorough clinical examination is carried out to rule out cervical, vulval, and vaginal cancer, atrophic vaginitis, and urinary and anal causes for bleeding
53. Women with PMB may be assessed initially with TVS and an endometrial biopsy (Grade A)
54. Endometrial thickness is measured as the maximum anteroposterior thickness of the endometrial echo on a long-axis transvaginal view of the uterus
55. Women with PMB with an endometrial thickness of ≤3–4 mm in the transvaginal scan do not require endometrial sampling unless they are at a high risk for EC or bleeding is episodic. In an asymptomatic early postmenopausal woman, an endometrial thickness of >11 should prompt an endometrial biopsy[60]
56. An endometrial thickness of more than 3–5 mm in TVS consider endometrial sampling. The sensitivity for detecting EC at 3 mm is 98%, at 4 mm is 95%, and at 5 mm is 90%. In women with homogeneous and normal morphology, those on MHT, and hypertensive medication, the acceptable combined thickness is 6 mm[61]
57. A focal increased echogenicity or a diffuse heterogeneity in the endometrium even in a thin endometrium warrants further investigations
58. Outpatient endometrial sampling devices such as Pipelle in low-risk woman and with global pathology may be used. Outpatient hysteroscopy is the preferred method for endometrial sampling
59. If the endometrial biopsy tissue is reported as insufficient for diagnosis, and endometrial thickness on TVS is <4 mm, follow-up is sufficient. Recurrent episode warrants further investigations
60. Saline infusion sonography and three-dimensional (3D) USG play a limited role in PMB evaluation.
Quality of life
61. The WHO defines QOL as an individual's perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards, and concerns.[62] The two terms in common usage are global QOL and health-related QOL (HRQOL). The WHO-several questionnaires are used to assess the HRQOL
62. QOL as it relates to menopausal women is usually referring to HRQOL, taking into account a woman's symptoms.[63] Commonly used are Menopause Rating Scale, Greene Climacteric Scale, Women's Health Questionnaire, and Utian QOL Scale
63. When evaluating drug therapies, besides safety and efficacy, it is important to know the effect of drug on QOL
64. Some studies show that menopausal hormone therapy (MHT) significantly improves overall measures of QOL in symptomatic women at menopause
65. Some studies show that low-dose MHT significantly improves overall measures of QOL. MHT had mixed effects on QOL among older women from the Heart and Estrogen or Progestin Replacement Study trial, whereas the Women's Health Initiative (WHI) Trial Investigators found that estrogen plus progestin did not have a clinically meaningful effect on HRQOL
66. An Indian study has shown an improvement in QOL in women receiving tibolone.[64]
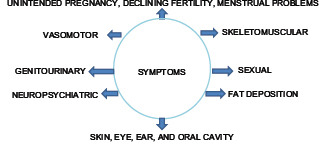
Symptoms of menopause
The symptom complex can be divided into VMS, somatic symptom, genitourinary syndrome of menopause (GSM), and neuropsychiatric symptom.
67. Menstrual cycle: Shortening of cycles that occurs in the late reproductive years is a clinical marker for the onset of menopause. Later, there is usually a lengthening of the intermenstrual interval, and some may have sudden amenorrhea.
68. VMS
In a multicenter hospital, urban-based study conducted by the IMS, the incidence of VMS was found to be 75%.[11] There is a wide variation in the prevalence of symptom reporting, ranging from 19% to 75% from various studies conducted in India.[20,21,65] The prevalence in the UK Asians was reported as 71%[66] and in the Australian Indians as 33%[67]
VMSs present as hot flushes, cold sweats, and night sweats. VMS may be reported in the menopause transition, reaches the maximum intensity during the first 2 years' postmenopause, and then declines over time. VMS generally lasts for 6 months to 2 years although some may experience for 10 years or longer. We need to exclude other causes of flushing before planning treatment
Grading of VMS is important to plan management, follow-up, and for research. Grades of hot flushes are classified as mild feeling of heat without sweating; moderate feeling of heat with sweating; and severe feeling of heat with sweating and palpitation that disrupt usual activity[68]
Lifestyle modifications may be recommended to reduce mild VMS (Grade B)
The most effective treatment for VMS is MHT (Grade A)
Low-dose OCPs if not contraindicated may be used at menopause transition phase for contraception and relief of symptoms (Grade A)
Nonhormonal prescription agents may relieve VMS but have their own side effects. These can be considered when MHT is contraindicated or not desired (Grade B)
Complementary and alternative treatments should be advised with caution as the data are still insufficient, especially in moderate-to-severe VMS (Grade B).
69. Somatic
Joint aches and pain are the commonly reported symptoms among women at midlife. While women who are obese or depressed are more likely to experience joint pain, there also appears to be an association with menopausal status, with peri- and post-menopausal women experiencing more joint pain than with premenopausal women
Body composition – Although women typically gain weight during midlife, it does not appear to be due to menopausal status or stage. In the early postmenopausal years, women typically gain abdominal fat mass and lose lean mass[69]
Skin changes – The collagen content of the skin and bones is reduced by estrogen deficiency. Decreased cutaneous collagen may lead to increased aging and wrinkling of the skin, frontal balding, and hirsutism[70]
Balance – Impaired balance in the postmenopausal women may be a central effect of estrogen deficiency additional to aging. Problems with balance may play a role in the incidence of forearm fractures in women.
70. GSM
The prevalence of urogenital symptoms in the postmenopause in the IMS study was 15%. It presents as vaginal dryness in 32%, pruritus vulvae in 10%–17%, and dyspareunia and urinary urgency in 10%. It is due to urogenital atrophy as a result of declining estrogen levels and may also present as recurrent urinary tract infections.[11,71] Although it effects the QOL, women in general do not complain about it; hence, suggestive questions need to be posed during history-taking
Physical signs of vulvovaginal atrophy are variable and include reduced vulval fat, reduced vaginal rugae, and blood flow, leading to a pale appearance; a change from moderately acidic range (pH 3.5–5.0) to a neutral range (pH 6.0–8.0) in the vaginal pH; there is a shift in the vaginal maturation index
Vaginal lubricants can be recommended for the subjective symptom improvement of dyspareunia (Grade B)
Vaginal moisturizers can be offered for vaginal dryness and dyspareunia (Grade B)
Local estrogen therapy (ET) is the first line of management, the only contraindication being active estrogen dependant tumors (Grade A)
Lifestyle modification, bladder drill, and pelvic floor exercises are recommended for urinary incontinence (Grade B).
71. Sexual problems
A woman's sexual response to her partner is significantly related to her baseline feelings for the partner, their relationship qualities, and partner's age and health
Sexual dysfunction is multifactorial and needs to be addressed accordingly[72]
Vaginal atrophy with aging may lead to dyspareunia causing sexual dysfunction, which is corrected by local ET
Acquired sexual desire disorder in some women responds to testosterone therapy. Formulations of testosterone for use in women are not available in India. Testosterone preparations meant for males should not be prescribed for women. Tibolone is a good option since it contains androgenic activity and can be used to treat libido problems.
Central nervous system
72. Dementia
In 2010, there are 3.7 million Indians with dementia, consisting of 2.1 million women and 1.5 million men, and the total societal costs is about 14,700 crore. While the numbers are expected to double by 2030, costs would increase three times. The prevalence of dementia is 0.6%–3.5% in rural India and 0.9%–4.8% in urban India[73]
The core mental functions are memory, communication and language, ability to focus and pay attention, reasoning and judgment, activities of daily living, and visual perception. Impairment of any two functions is suggestive of dementia
Many dementias are progressive; early diagnosis allows a person to get the maximum benefit from available treatments and provides an opportunity to plan for the future
Factors that increase the risk of dementia are family history, genetic factor apolipoprotein E, mild cognitive impairment, CVD risk factors, physical inactivity, diabetes, hypertension, dyslipidemia, smoking, obesity, autoimmune diseases, depression and stress, social engagement and diet, head trauma and traumatic brain injury, and age (Grade B)
An objective marker is the examination of cerebrospinal fluid for amyloid-beta or tau-protein and phosphorylated tau-protein concentration. They have a sensitivity between 94% and 100% (Grade A)
ET is not currently recommended for reducing risk of dementia developing in the postmenopausal women or retarding the progress of diagnosed Alzheimer's disease (Grade A)
For best preservation of memory and cognition, women should be advised about the importance of good overall health, good cardiac and vascular health, exercise, maintenance of active mind, avoidance of excessive alcohol consumption, and measures to reduce risk of diabetes and hypertension. At present, MHT is not indicated for neuroprotection
Introduction of accessible diagnostic and early-stage dementia care services, such as memory clinics, is recommended.
73. Depression
There is a significant increased risk of new-onset depression in women during the menopausal transition compared with their premenopausal years. In a within-woman, 8-year, longitudinal study to determine the risk factors for depressive disorders, a diagnosis of depression was 2.5 times more likely to occur in the menopausal transition compared with when a woman was premenopausal.
74. Cognitive changes
Evidence supports the relation of estrogen to cognitive function; women face problems with memory loss and difficulty in concentration at menopause transition. A definitive role of MHT for treating cognitive symptoms without VMS is lacking.
75. Sleep
In a study conducted in the UK Asians, sleep problems were noted in 32%. A large study of over 9000 older adults aged >65 years found that 42% of participants reported difficulty in initiating and maintaining sleep.[74] The estimate of the prevalence of sleep disorders in India, by the WHO extrapolated data from the US data, is 156,628,027 in 1,065,070,607 population
A detailed assessment of menopausal symptoms should always include questions about sleep pattern. Sleep questionnaires or sleep diaries can be useful to assess sleep in detail (Grade B)
Adverse lifestyle factors, social factors, and risk factors should be considered and treated accordingly (Grade C)
If insomnia is identified, medical or psychiatric causes of insomnia should be ruled out, and if present, it should be treated accordingly. If specific neurological or breathing disorders are suspected, further investigations and referrals to specialists should be initiated (Grade B)
Sleep hygiene measures and lifestyle modifications should be recommended as the first-line treatment. Psychological treatments, such as cognitive behavioral therapy, should also be considered (Grade B)
If insomnia is resistant to lifestyle modifications, then hypnotics, benzodiazepines, or melatonin agonists can be used in the short term, but there is no definite or convincing evidence to suggest its efficacy. These should only be prescribed by supervision or after liaison with psychiatrists or sleep experts (Grade C)
No recommendations can be made about the use of herbal remedies for insomnia as there is insufficient evidence. Mind–body therapies such as yoga and tai chi have some evidence but need further rigorous studies to prove its effectiveness (Grade D)
Skeletomuscular system
76. Osteoporosis: Refer Clinical Practice Guidelines on Postmenopausal Osteoporosis: An executive summary and recommendations: Indian Menopause Society.
77. Osteoarthritis
The prevalence of osteoarthritis in India as reported from community dwellers in a small study conducted in Delhi was 47.3%, and in others, it is reported to be between 22% and 39%[75,76,77]
Age, weight, female sex, quadriceps weakness, and overloading of the knee joint are the main contributors than menopause per se in the incidence of osteoarthritis. Those contributing factors should be addressed on a priority basis
Epidemiological studies of a potential role for estrogens in osteoarthritis showed two very different findings. First, estrogen deprivation at the menopause seems to be associated with increases in the frequency of knee, hip, and finger osteoarthritis and in the severity of hip osteoarthritis. Second, MHT for the menopause may decrease the incidence and progression of hip and knee osteoarthritis
The identification of the estrogen receptors (ERs) in normal and osteoarthritic cartilage and the effects of 17-beta-estradiol on cartilage in vivo in animals and in vitro confirm that the cartilage responds to estrogens. Finally, this response is dose dependent: physiological doses (as with MHT) are protective and higher dosages are deleterious
Once osteoarthritis sets in, there is no protection from MHT and osteoarthritis takes its own course. In such cases, osteoarthritis should be treated on its own merits
Age, weight, female sex, quadriceps weakness, and overloading of the knee joint are the main contributors than menopause per se in the incidence of osteoarthritis. Those contributing factors should be addressed on a priority basis
First two stages of osteoarthritis can be addressed by lifestyle modification, pharmacotherapy, and physical therapy (Grade A). A Cochrane review found that about 60% of the patients achieved at least 50% improvement in pain with topical nonsteroidal anti-inflammatory drugs, which was comparable to the effect obtained with oral formulations and slightly better than that observed with topical placebo.
Third and fourth stages need surgical intervention, for which total knee replacement is the gold standard (Grade B).
78. CVD.
Prevention and management
Lifestyle interventions (Grade A)
Encourage optimal BP <120/80 mmHg through lifestyle approaches (Grade A)
Pharmacotherapy if BP ≥140/90 mmHg to avoid end-organ damage, more so in diabetes (Grade A)
Use thiazide diuretics, unless there is an absolute contraindication
Optimal lipid targets (Grade A) – Low-density lipoprotein (LDL) <100 mg/dL, high-density lipoprotein (HDL) >50 mg/dL, triglycerides <150 mg/dL, non-HDL cholesterol <100 mg/dL (Grade A)
Women at high risk: Initiate statin if LDL >100 mg/dL (Grade A)
Women at intermediate risk: Initiate statin if LDL >130 mg/dL (Grade A)
Lifestyle approaches and pharmacotherapy to achieve near-normal glycosylated hemoglobin (HbA1C <7%) in women with diabetes (Grade A)
Aspirin in high-risk women (75–162 mg/day) (Grade A)
Routine use of aspirin in women <65 years of age is not recommended for MI prevention (Grade C)
MHT is not indicated solely for primary or secondary cardioprotection
A 2015 Cochrane review of randomized controlled trial (RCT) data found that MHT initiated fewer than 10 years after menopause onset lowered coronary heart disease (CHD) in the postmenopausal women (relative risk [RR], 0.52). It also found a reduction in all-cause mortality (RR, 0.70), no increased risk of stroke, but an increased risk of VTE (RR, 1.74), similar to the findings of an earlier meta-analysis of studies in women who initiated MHT within 10 years of menopause onset or in women aged younger than 60 years (Grade A)
Do not use antioxidant supplements for CVD prevention (Grade C)
Do not use folic acid, with or without B6 or B12 supplements for CVD prevention (Grade C).
79. Metabolic syndrome: Insulin resistance (IR)
The prevalence reported in India at perimenopause is 22.2%, rising to 32.2%–48% at postmenopause.[78,79] It is 1.5–2 times more common in women than in men
80. The metabolic syndrome is also known as IR syndrome and syndrome X, and an average of 40% of the Indian women are affected
81. Clinical conditions associated with IR include T2DM, CVD, polycystic ovary syndrome (PCOS), nonalcoholic fatty liver, obstructive sleep apnea, and certain cancers. It is also a prominent feature of the metabolic syndrome
82. Diagnosis of metabolic syndrome: National Cholesterol Education Program Adult Treatment Panel III (NCEP ATO III 2005 revision); abdominal obesity defined as >35 inche in females; serum triglycerides >150 mg/dL; BP >130/85 mm Hg; and fasting plasma glucose >110 mg/dL – any three out of the five criteria
83. Effect of MHT: A meta-analysis of pooled data from 107 trials concluded that MHT reduced IR, abdominal obesity, new-onset diabetes, lipids, BP, adhesion molecules, and procoagulant factors in women without diabetes and reduced fasting glucose and IR in women with diabetes. The effects were diminished by the addition of progestin (Grade A)
84. The basis of dietary recommendations is to reduce exposure to insulin both as a result of dietary stimulus and through decreased IR (Grade B). We should advocate exercise as it improves insulin sensitivity, aiming for a minimum of 30 min of moderate physical activity/exercise per day
85. Indications for the intervention by body mass index (BMI) category are shown in Table 3[79]
Table 3.
Body mass index in kg/m2 category and management
| Category and intervention | |||
|---|---|---|---|
| Category | WHO | Indian | Intervention |
| Under weight | <18.5 | <18 | Encourage balanced diet and exercise |
| Healthy | 18.5-24.9 | 18.0-22.9 | Encourage balanced diet and exercise |
| Overweight | 25.0-29.9 | 23.0-24.9 | Lifestyle (diet, exercise and behavior therapy) |
| Obese Grade I | >30.0-34.9 | >25 | Lifestyle, lifestyle plus drug therapy if comorbidities* exist |
| Obesity Grade II | 35.0-39.9 | Lifestyle plus drug therapy: If comorbidities* exist, bariatric surgery | |
| Obese Grade III | ≥40 | Lifestyle, drug therapy, and bariatric surgery | |
*Comorbidities: Hypertension, diabetes, and hyperlipidemia. WHO: World Health Organization
86. Abdominal obesity based on the waist circumference[79] is shown in Table 4
Table 4.
Obesity as defined by waist circumference
| WHO | Indian | Management |
|---|---|---|
| >80 cm | >72 cm | Action line 1 - Avoid weight gain or lose weight |
| >88 cm | >80 cm | Action line 2 - Supervised weight management |
WHO: World Health Organization
87. DM
India has 63 million people with diabetes and is second largest in numbers. The prevalence rate of diabetes in the last 30 years has increased from 2.3% in urban and 1.2% in rural areas (1971) to 15%–20% in urban and 10% in rural areas (2012)
The prevalence of diabetes in women in the age group of 15–49 years was 10.5% in the urban population and 7.5% in the rural, based on the data from the National Family Health Survey, 2015-2016 (NFHS-4)
The prevalence in hospital-based multicenter study by the IMS in the postmenopausal woman was 12%
In India, T2DM occurs a decade earlier than the Caucasians. More than 50% of the subjects are undiagnosed[80]
Screening: Opportunistic screening for all women above the age of 30 years, every 3 years for younger women with risk factors (Grade C), should be done. Diabetic women should be screened for hypertension, dyslipidemia, and microalbuminuria and undergo yearly eye check
Laboratory test for screening: The best test is a fasting plasma glucose or nonfasting HbA1c.[81] If fasting blood glucose is normal at <100 mg/dL or HbA1c <5.7%, retesting should be done at 3-year intervals. If the fasting blood glucose is 100–125 mg/dL or HbA1C is 5.7%–6.4%, repeat testing should be done in 1–3-year intervals. Prediabetes should be defined if the fasting blood glucose is 100–125 mg/dL or HbA1c is 5.7%–6.4%.
The goal in management is to maintain the HbA1c around <7% and control risk factors for CVD
It may be indicated to evaluate the endometrium by transvaginal scan before starting MHT.
88. Thyroid disease
The prevalence from hospital-based data in the postmenopausal women for hypothyroid in India is 3%–7%[82]
Hypothyroidism is much more common in older than younger individuals
Symptoms and signs include lethargy, constipation, dry skin, alopecia, memory impairment, and depression. The individual is often obese and may have elevated cholesterol
The prevalence of hypothyroidism is approximately 5% in otherwise healthy individuals. Thyroid-stimulating hormone (TSH) is a good screening test.
89. Anemia
Anemia is common in the elderly people in India. The overall prevalence of anemia was 68.7%.[83] The prevalence of iron deficiency anemia, Vitamin B12 deficiency, and folate deficiency is common and should be an integral part of the management of menopause.
90. Eye
Blindness was more likely with increasing age and decreasing socioeconomic status, in female subjects and in rural areas. The causes of blindness were easily treatable in 60.3% (cataract, 44%; refractive error, 16.3%).[84] Preventable corneal disease, glaucoma, complications of cataract surgery, and amblyopia caused another 19% of the blindness [Table 5][85]
Blindness due to primary angle-closure glaucoma is potentially avoidable if this condition is detected early and peripheral iridotomy or iridectomy is performed. This requires the detection of occludable angles, which lead to primary angle-closure glaucoma, using slit-lamp examination and gonioscopy. Blindness due to primary open-angle glaucoma is more difficult to prevent and medication in open-angle glaucoma could prevent the progression of the disease (Grade A)
There is increased risk of dry eye in both genders with age due to decreased tear production. The incidence is more in women than in men. Menopause also contributes to the ocular surface impairment due to hormonal imbalance
MHT after menopause, especially unopposed ET, has been implicated to cause the dry eye (Grade B).
Table 5.
Causes of blindness
| Disease | Percentage of contribution (%) |
|---|---|
| Cataract | 62.6 |
| Refractive error | 19.7 |
| Glaucoma | 5.8 |
| Corneal pathologies | 0.9 |
| Other causes | 11.00 |
91. Prevention of blindness
Improvement in the quality of cataract surgery and increase in the number of surgeries on persons blind in both eyes
Effective screening to detect the refractive error blindness and provision of spectacles
Initiation of long-term strategies to prevent corneal and glaucoma blindness
Effective control of diabetes and yearly eye checkup to prevent diabetic retinopathy.
Cancers
92. A population-based study (Million Death Study Cancer Mortality in India: A Nationally Representative Survey, 2012) revealed that 1 in 22 men or women aged 30 years alive today in rural India is likely to die of cancer before 70 years of age based on the rates of actual deaths and in the absence of other disorders. In urban areas, the risks are 1 in 20 for men and 1 in 24 for women.[86,87]
Breast cancer
93. Breast cancer in India is now the most common cancer in most cities and second most common in the rural areas. In 2012, it is estimated that approximately 145,000 new patients were diagnosed with breast cancer in India, and nearly 70,000 women died of the disease. The data from Atlas Project suggest that breast cancer in urban areas of India is three times higher than in rural parts of the country[88,89,90]
94. More younger women are getting diagnosed with breast cancer. 25 years back, 69% of the patients were above 50 years of age. At present, almost 48% of patients are below 50 years age. Breast cancers in the young are hormone positive in 48%; the rest are negative and tend to be more aggressive. Indian women are more likely to develop breast cancer at earlier ages than their Western counterparts[91,92]
95. In the United States, 89 women out of 100 are likely to survive for 5 years after breast cancer. In India, a rough estimate is not even 60% and present late in Stage III[93,94]
96. Nonmodifiable risk factors for breast cancer are age, family history, benign breast disease, BRCA (breast cancer) 1 or 2 carriers, early menarche (<12 years), late age at menopause (after age 55 years), increased breast density, and a chest irradiation between ages of 25 and 55 years
97. Modifiable risk factors are age at first child, breastfeeding, parity, obesity, physical activity, and menopausal HT.
Screening in breast cancer
98. The debate about value of screening continues. There is no organized, systematic, government-funded screening program for breast cancer in India. The screening in developing countries can be regarded as “opportunistic screening.” There are no evidence-based guidelines for breast cancer screening in India at present
99. There are no validated breast screening tools in India. The 5-year NCI or IBIS breast cancer risk assessment is arrived at and classified as low, intermediate, or high. These tools are simple to implement in the population. The limitation is that they have not being validated in the Indian population. MHT needs to be avoided in the moderate and high risk category.
100. Methods
Breast cancer screening includes three methods of early detection (Grade C).
Breast self-examination (BSE) monthly starting in the 20s
Clinical breast examination (CBE) every 3 years starting in the 20s till 39 and annually thereafter
Mammography screening (annually) starting at the age of 40 years.
101. BSE
BSE is performed by the woman herself and involves examination of the breast, skin, and axillae – based on the palpations by her hands
The woman should examine the look and feel of her breasts as well as any signs, symptoms, or changes to the breasts
BSE is recommended so that women understand their breasts for detecting any suspicious changes over time
Initially, BSE should be performed very frequently and regularly so that a woman understands the physiological changes that occur during the different phases of menstrual cycle and then continue monthly around 7th or 8th day of cycle. They are encouraged to report any recent or persistent changes
Nodular and lumpy feel of the breasts and increased pain and tenderness, which is a physiological finding before menstruation, needs to be explained to the patient
Women can be taught to examine the breasts in any of the following ways in supine as well as standing positions.
102. CBE
CBE and increasing awareness of breast cancer are viable alternative in view of limited healthcare resources and advanced stage of disease distribution for Indian women aged 50 years
Early results of trial by the WHO in India (Journal of the National Cancer Institute [JNCI 2011]) and studies for cost-effectiveness of screening in Indian women support that CBE is an effective way and survival can be improved by up to 16% at half the cost[93]
For women between 50 and 70 years of age, annual CBE and selective use of mammography, once in 3 years, in high-risk groups, determined by the above-mentioned criteria have been found to be equally effective (JNCI 2011)
CBE is performed by a clinician or other health professional and involves a systematic examination of the breast skin and tissue
The health professional is looking for signs and symptoms or if any changes occur, including development of a lump or swelling, skin irritation or dimpling, nipple pain or retraction (turning inward), redness or scaliness of the nipple or breast skin, or a discharge other than breast milk
CBE should include all the four quadrants of the breast and the central nipple areola complex followed by examination of the axilla and supraclavicular fossae
Fibroadenoma, a benign condition, feels as a firm and freely mobile swelling, characteristically described as a “mouse in the breast,” whereas an irregular hard painless lump is a characteristic of malignancy
These findings are generalized and all lumps may not classically fit into these descriptions
Normal breasts may feel lumpy and tender before menstruation, especially if felt with the tips of the fingers; hence, the use of a flat hand is recommended.
103. Mammogram
In India, breast cancer incidence peaks before the age of 50 years, and a recent review of the evidence in younger women (aged 39–49 years), based on eight trials conducted between 2001 and 2008, suggests that mammography screening is also beneficial in this younger age group
An approximate 12%–15% reduction in breast cancer mortality is associated with mammography screening for women aged 40–69 years[94]
Imitations of mammography in developing countries are economic constraints and quality assurance. Cost-affectivity and false-positive rates are the other limitations in the use of mammography in India
The decision to perform mammography should be determined with shared decision-making about risks and benefits and by individual patient values
104. MRI
Currently, MRI screening in combination with mammography is targeted to high-risk patients, which includes:
BRCA 1 or 2 mutation carriers
Untested women who have a first-degree relative with a BRCA 1 or 2 mutation
Life time risk of breast cancer of 20%–25% or more
Received radiation treatment to the chest between ages of 10 and 30 years
Women with silicon implants.
105. Role of positron-emission tomography (PET) imaging
PET has currently a limited role in breast cancer, due to its low sensitivity, and is not recommended in most of the cases, especially in early disease. The most useful application of PET or computed tomography is monitoring the changes in 18-fludeoxyglucose uptake during chemotherapy to detect an early response to treatment.
106. Breast cancer prevention
The risk of breast cancer may be lowered to some extent by lifestyle changes, working on modifiable risk factors, and diligent use of MHT
The best way to protect one's self is through early detection
Prevention in high-risk population
107. Indications of risk reducing surgery, mastectomy, salpingo-oophorectomy, and chemoprevention can be discussed with experts. The decision is individualized.
Cancer cervix
108. The number of new cases of cervical cancer detected in India is 96,922 every year. Deaths due to cervical cancer in India are 60,078/year[95]
109. Opportunistic screening coverage varied from 6.9% in Kerala to 0.006% and 0.002% in the western state of Maharashtra and southern state of Tamil Nadu, respectively[96]
110. India contributes to over 25% of the disease burden and more than 26% of the deaths due to cervical cancer, worldwide. More than 75% of the cases presenting in the late stage of the disease render poor prospects for survival and cure. About 134,420 new case are being diagnosed every year[97]
111. Risk factors are human papilloma virus (HPV), sexual intercourse at an early age, multiple sexual partners, sexual partners who have had multiple partners, HIV-positive status, and smoking
112. Screening tests available
Visual inspection
Visual inspection with acetic acid (VIA)
Visual inspection with Lugol's iodine
Papanicolaou (PAP) smear both conventional and liquid-based cytology
HPV-DNA testing
Cervicography
Polar probe.
113. The first three are useful at community and low-resource setting
114. Cost-effectiveness studies on VIA screening also suggest that, once in a lifetime, screening at the age of 35 years involving one or two visits reduced the lifetime risk of cancer by approximately 25%–36%. The relative risk further decreased by 40% with two rounds of screenings (at 35 and 45 years of age). 98
Screening at different levels of care
115. Primary care (rural/urban)
Cytology-based screening has made little impact in developing countries due to relatively high false-negative rate and lack of organized screening program and referral pattern
Several studies have shown the benefit of a single-visit approach in the form of “see and treat,” which involves VIA followed by cryotherapy. This unique approach is based on the principle that the screening test should provide rapid and accurate results and the treatment modality should be appropriate, adequate, and effective. VIA and cryotherapy satisfied these criteria and yielded satisfying results. A randomized trial in South India done by Sankaranarayanan et al., in 2007, has shown 25% reduction in cervical cancer incidence and 35% reduction in mortality compared to control with VIA and cryotherapy.[98] This approach is useful in primary care level to make the screening program more cost-effective. This can be carried out both by physicians and by trained nurses and midwives[99,100,101,102,103,104,105]
Colposcopy: For diagnostic confirmation with guided biopsies in screen-positive women, post any primary screening method adopted. Because of hormonal changes, many postmenopausal women will have an unsatisfactory colposcopy. Estrogen treatment (estrogen cream application intravaginally each evening for 2 weeks and stopped 1 week before repeat cytology) will cause enough ectropion of the endocervical cells to result in a satisfactory examination
HPV testing also has been tried in a screen-and-treat approach. A few studies reported screening with HPV DNA testing followed by cryotherapy. However, it has two limitations: time and infrastructure required for the current HPV testing and a lack of consensus about appropriate follow-up for test positives and also treatment strategy. Hence, in some other studies, HPV DNA-positive women had VIA followed by cryotherapy if VIA was positive
Some studies suggest that cryotherapy is protective against the future development of cervical disease among women with current HPV infection. Because of this, and due to the low morbidity of cryotherapy, the occasional treatment of screen-positive women without confirmed cervical disease is acceptable.
116. Secondary and tertiary level
PAP smear and HPV-DNA testing are being used commonly at secondary and tertiary care level.
117. Screening techniques' recommendations from different organizations may be applicable at different settings both in rural and in urban [Table 6]
Table 6.
Screening at different levels of cancer cervix
| Level of care | Intervention |
|---|---|
| Primary | VIA + cryotherapy/HPV + cryotherapy |
| Secondary | PAP±HPV cotesting |
| Tertiary | PAP±HPV cotesting |
Pap: Papanicolaou, HPV: Human papilloma virus, VIA: Visual inspection with acetic acid
118. HPV co-testing is to be performed only if the woman crosses 30 years of age as most of the HPV infection clears by then with natural immunity. If both PAP and HPV are negative, the screening interval can be increased, which again becomes cost-effective
119. Colposcopy: For diagnostic confirmation with guided biopsies in screen-positive women, post any primary screening method adopted. Because of hormonal changes, many postmenopausal women will have an unsatisfactory colposcopy. Estrogen treatment (estrogen cream application intravaginally each evening for 2 weeks and stopped 1 week before repeat cytology) will cause enough ectropion of the endocervical cells to result in a satisfactory examination
120. Screening recommendations from different organizations are presented in Table 7
Table 7.
Screening recommendations from different organizations
| Age (years) | USPSTF | ACS/ASCCP |
|---|---|---|
| 30-65 | Co-testing with PAP + HPV every 5 years (preferred) PAP alone every 3 years (acceptable) (Grade A) | Same as USPSTF Recommendations |
| >65 | Screening not recommended in women who have had adequate prior screening and or not high risk for cervical cancer (Grade D) | Adequate prior negative screening no history of high-grade CIN in the last 20 years, no screening should not be resumed even a woman reports having a new sexual partner |
| Posthysterectomy, artificial menopause | No screening if hysterectomy with removal of the cervix is performed for benign lesions and no history of high-grade CIN (Grade D) | No screening if hysterectomy is performed with no history of high-grade CIN |
| HPV vaccinated | Screening as per the age | Screening as per age |
USPSTF: United States Preventive Services Task Force, ACS: American Cancer Society, ASSCP: American Society for Colposcopic and Cervical Pathology, CIN: Cervical intraepithelial neoplasia, PAP: Papinocolau, HPV: Human papilloma virus
121. Women with negative PAP and positive HPV testing can be either rescreened with co-testing in 1 year or with a test specific for type of HPV
122. All these screening methods may be sometimes inconclusive in the menopausal women whose transformation zone is inside the cervical canal or due to atrophic changes. Hence, choosing the appropriate test is important.
High-risk (oncogenic) HPV DNA testing could be adopted for appropriate triage management of postmenopausal women with unequivocal cytology results
HPV DNA testing is indicated in postcolposcopy management of women of any age with initial cytological result of atypical glandular cells (or atypical squamous cells cannot exclude-high-grade squamous intraepithelial lesion in initial workup does not identify a high-grade lesion)
If a low-cost and rapid HPV is available, this may be used as primary screening test every 5 years up to the age of 65 years. With HPV testing as the primary screening method, PAP or VIA testing can be used to triage to evaluate those with HPV-positive test results to plan for appropriate treatment options
Above recommendation holds true for women seeking opportunistic services in apex and secondary care levels in public and private sector health facilities where good-quality PAP cytology services and molecular testing for HPV-DNA are available
In the absence of organized cervix cancer screening for the vast women population in rural and urban areas, once in a lifetime, screening by co-testing by combined use of cervical cytology and high-risk HPV-DNA testing would be appropriate [Table 7].
Primary prevention
123. Women should be educated early on to think of cervical cancer as an extension of a sexually transmitted disease
124. Behavioral changes to reduce the risk of cervical cancer include limiting the number of sexual partners, delaying initial age of sexual intercourse, and avoiding sexually transmitted disease. The association of cigarette smoking with cervical cancer should also be emphasized
125. An HPV vaccine needs to be promoted, especially in the age group of 9 years to the age of first sexual debut. Data from a large placebo-controlled trial showed that the vaccine reduced the incidence of both HPV-16 infection and HPV-16-related cervical intraepithelial neoplasia.[106]
Endometrial cancer
126. Indian incidence of EC is 4.3/100,000 as per the Delhi population-based cancer registry. EC commonly occurs in the postmenopausal women[107]
127. Overall morbidity and mortality of EC are low because most patients present at an early stage because of abnormal bleeding or PMB
128. Risk factors
Factors that increase the risk of EC are those associated with increase in endogenous ET or HT with estrogens
A strong modifiable risk factor is increasing obesity; the relative risk of EC with obesity is 3 in women 9.5 -22 kg overweight and 10 in women more than 22 kg overweight
Women taking tamoxifen for more than 2 years have a 2.3-fold to 7.5-fold relative risk of EC[108]
Adenomatous hyperplasia and EH are the common precursors of endometrial carcinoma
Unopposed ET in women with an intact uterus increases the risk of EC 2–10-fold, and the risk increases with the duration of use
The lifetime risk of EC for women with hereditary nonpolyposis colorectal cancer (HNPCC) and for women who are at high risk for HNPCC is as high as 60%[109]
Cyclic or continuous progestin given along with estrogens reduces the risk of EC.
129. There is no evidence that screening by USG or endometrial biopsy reduces mortality from EC. Most cases of EC (85%) are diagnosed at low stage because of symptoms, and the survival rates are high[110]
130. There is no indication that screening for EC is warranted for women who have no identified risk factors 111
131. It is recommended that, at the time of menopause, women at average risk should be informed about the risks and symptoms of EC and strongly encouraged to report any unexpected bleeding or spotting
132. For those with increased risk and special situations, such as on MHT (tibolone sequential MHT), genetic risk, and tamoxifen therapy, they should have a follow-up and complete diagnostic evaluation as needed. Regular screening for high-risk group for EC has not been fully evaluated
133. Women diagnosed with EC should have the benefit of multidisciplinary team approach.
Endometrial hyperplasia
134. Definition: EH is a pathological condition that most often results from persistent, prolonged exposure of unopposed estrogenic (endogenous production or exogenous administration of estrogens) stimulation of the endometrium. It is known to be precursor of EC
135. There are two methods of classifying EH:
The 2014 WHO EH classification system has only two categories – hyperplasia without atypia (nonneoplastic) and atypical hyperplasia (endometrial intraepithelial neoplasm [EIN])[112]
The EIN classification system defines two classes of endometrial changes: benign and intraepithelial neoplasia[113]
136. EH is a histologic diagnosis made based upon the results of evaluation of an endometrial biopsy, endometrial curettage sample, or hysterectomy specimen
137. Clinical significance: EH is of clinical significance because it is often a precursor lesion to Type I endometrioid adenocarcinoma of the endometrium. Endometrial stripe thickness and age were the strongest predictors of concurrent endometrial cancer at time of hysterectomy for EIN. An endometrial stripe of ≥2 cm was associated with 4.0 times the odds of concurrent EC (95% confidence interval, 1.5–10.0), controlling for age
138. Management
All management strategies should also be accompanied by the removal of the extrinsic or intrinsic source of unopposed estrogen since excess exposure to estrogen is a main etiology of endometrial neoplasia
Conservative management: if the risk of an occult cancer or progression to cancer is low and the inciting factor that resulted in endometrial proliferation has been eliminated (e.g., patient with anovulation, now corrected, who had developed simple hyperplasia without atypia) and requires vigilant follow-up with two negative biopsies 6 months apart[114]
Benign EH: (1) Conservative with use of continuous progesterones and follow-up (2) Surgical—total hysterectomy (TH) when associated with high-risk factors, postmenopause
EIN: TH with bilateral salpingo-opherctomy (BSOP) for EIN provides definitive assessment of a possible concurrent carcinoma and effectively treats premalignant lesions in a postmenopausal woman. In premenopausal women, decision regarding BSOP needs to be individualized. Supracervical hysterectomy, morcellation, and endometrial ablation are unacceptable for the treatment of EIN.[115]
Cancer ovary
139. The general or lifetime risk of ovarian cancer is 1.4%
140. The most common sign of ovarian cancer is enlargement of the abdomen caused by the accumulation of fluid or a large ovarian mass
141. However, many women have bloating or weight gain in the abdominal area, making this sign nonspecific
142. In women aged over 40 years, digestive disturbances that persist and cannot be explained by any other cause indicate the need for a thorough evaluation for ovarian cancer, including a carefully performed pelvic examination and ultrasound (US)
143. Risk factors
A first-degree relative with ovarian cancer (mother, sister, or daughter)
Personal history of breast cancer <40 years or age
Personal history of breast cancer <50 years or age and one or more close relative with breast or ovary cancer at any age; two or more close relative with breast cancer <50 years of age or ovarian cancer at any age.
144. Screening
No screening guidelines are available for mass screening for ovarian cancer. Recommendation for screening is dependent on the risk status of women[116]
A heightened awareness of the symptoms of early ovarian cancers on the parts of the patients and practitioners may help reduce the delay in diagnosis and hopefully result in an improvement in the outcome of some progress
For general population, annual pelvic examination, PAP smear, and transvaginal sonography are recommended as a part of postmenopausal surveillance
145. Primary prevention: Limited data are available on the efficacy of prophylactic oophorectomy in decreasing the risk of ovarian cancer in mutation carriers. Still, it is recommended that prophylactic surgery be considered in BRCA mutation carriers who have completed childbearing.
Vulvar cancer
146. Epidemiology: Cancer of the vulva is a rare disease that accounts for approximately 5% of gynecological cancers. The median age of onset is approximately 65–70 years for invasive cancer and approximately 45–50 years for carcinoma in situ
147. Risk factors for vulvar cancer including HPV, previous genital warts, greater number of sexual partners, current smoking, abnormal PAP smear, diabetes, obesity, chronic vulvar pruritus, and poor personal hygiene have also been suggested as contributing to risk
148. Protected intercourse, monogamy, and adequate hygiene of the external genitalia protect against vulvar cancer
149. Prevention and detection: The prevention of vulvar cancer rests in the avoidance of risk factors and application of protective factors as summarized above. Annual examinations should be performed to check for vulvar cancer. High-risk patients should be examined every 6 months. White lesions and chronic ulcerative lesions should be biopsied for evaluation.
Stomach cancer
150. According to the GLOBOCAN 2018 data, in women aged 30–69 years, the fourth most common fatal cancer was stomach (14.1%). Stomach cancer rates were higher in rural than in urban areas of India due to increased prevalence of chronic Helicobacter pylori infection. Treating for H. pylori may prevent stomach cancer
151. Million Death Study Cancer Mortality in India: A Nationally Representative Survey 2012: This may include stomach and primary liver cancer. The prevalence of hepatitis B virus (HBV) in India was <1.9% in 72,000 pregnant women aged 15–49 years who were tested in 2002
152. Nearly, 37% of all female cancer deaths were from infection-related cervical, stomach, and liver cancers and 18.3% were from tobacco-related cancers. This underscores the importance of vaccination and control of infection. Vaccination against HBV would reduce future liver cancer deaths and cirrhosis. Use of tobacco in pan and beedi should be strongly discouraged.
Hyperandrogenism at menopause
153. Postmenopause is a state of relative androgen excess in relation to estrogen; the postmenopausal ovary remains hormonally active, secreting significant amounts of androgens and relatively fewer estrogens years after menopause
154. There is no consensus regarding specific clinical and hormonal indices and/or imaging modalities required for diagnostic certainty[117]
155. Hyperandrogenism in the postmenopausal women can be generally categorized as functional/nontumorous or tumorous
156. The causes of functional hyperandrogenism, such as PCOS and nonclassic congenital adrenal hyperplasia, are clinically manifested before menopause
157. Androgen-secreting neoplasm should be suspected in cases of clitoromegaly (clitoral size >1.5 cm × 2.5 cm) and other signs of virilization even if symptom progression is not rapid. Very high serum testosterone (>150–200 ng/dL) and/or DHEAS (>6000 ng/mL) levels are indicative of an androgen-secreting tumor of the ovarian and adrenal origin, respectively
158. The long-term sequelae of nontumorous hyperandrogenism in the postmenopausal women in respect to cardiovascular morbidity and mortality are not known.[117]
Polycystic ovary syndrome at menopause
159. Increased androgen secretion by the ovarian theca cells is a primary defect in PCOS and may persist as hyperandrogenism in a subset of the population
160. Androgen excess favors the development of abdominal adiposity, IR, and compensatory hyperinsulinism, and this further increases androgens, leading to a vicious circle that predisposes these women to metabolic dysfunction and cardiovascular risk[118]
161. Menopausal transition does not seem to be associated with worsening of cardiometabolic profile in PCOS patients. This may be related to the improvement in phenotypic features of PCOS with aging. The phenotype of PCOS plays an important role in determining the cardiometabolic risk in patients with the syndrome[119]
162. Population-based, longitudinal studies examining cardiometabolic morbidity and mortality in women with PCOS are lacking
163. Identifying clinical and biochemical characteristics of PCOS in the postmenopausal period may provide a reliable method for following women to help define the true cardiovascular morbidity and mortality
164. As a part of menopause workup, vigilance is needed in women with hyperandrogenism and PCOS or a history of PCOS as this forms an additional risk factor for cancers. Persistent thickened endometrium and/or risk factors including prolonged amenorrhea, abnormal vaginal bleeding, or excess weight need to be investigated[117]
165. Therapeutic strategies currently in use for PCOS, including lifestyle modification, oral contraceptives (OCs), antiandrogens, and insulin sensitizers, are used throughout the lifespan such that these women enter menopause with a healthy metabolic profile.
Endometriosis and menopause
166. Endometriosis in the postmenopausal women is a rare condition
167. Postmenopausal endometriosis may have a greater tendency to spread and involve extragonadal organs and structures that may develop into constrictive and/or obstructive lesions[120]
168. Surgical treatment is the first-line therapy to exclude malignancy case of postmenopausal endometriosis, but drug treatment may be an option in case of pain recurrence after surgery[121]
169. In a Cochrane review, authors concluded that MHT may increase the risk of endometriosis symptoms and disease recurrence after surgically induced menopause. The current data are, however, insufficient to justify not administering HRT for symptomatic women after surgically induced menopause[122]
170. The evidence is currently insufficient to support any conclusions about the optimal MHT regimen for women with endometriosis. Few data suggest a higher malignancy risk with estrogen-only MHT compared with combined MHT[121]
171. Postmenopausal women should be conscientiously evaluated before initiating MHT. Patients with endometriosis receiving MHT should be monitored regularly for pain recurrence.
Fibroid and menopause
Fibroids are benign tumors that probably arise from a somatic myometrial stem cell. They have ERs and progesterone receptors (PRs). After menopause, fibroids generally shrink, and some may become calcified due to the loss of estrogen
Malignant transformation into sarcoma is possible but exceedingly rare (around 1/2000)[123]
At menopause transition, depending on the patient's symptoms and age, fibroids can be managed expectantly, medically, radiologically, or surgically
MHT use in the menopausal women with asymptomatic fibroid demonstrates variable effects on the volume and size of uterine fibroids
Some combination estrogen and progestin therapy may increase in size of preexisting myomas and increase in frequency of new myoma formation, especially with higher doses of MPA (5 mg/day). Use the lowest possible dose of progestin with estrogen
The use of tibolone for menopausal symptoms is generally associated with fewer episodes of irregular spotting and no significant changes in volume or size of myoma compared with estrogen, progestin therapy
Raloxifene appears to be relatively safe for menopausal women at risk of osteoporosis or breast cancer, who present with asymptomatic fibroids
If on MHT, periodic follow-ups with an ultrasound every 3 months for appearance of symptoms and growth of myoma are advisable in the initial year.
SECTION III
ABNORMAL MENOPAUSE
Premature ovarian insufficiency
The NFHS of 2015-16 collected information from a sample of more than 90,000 married women aged between 15 and 49 years and covering 99% of the India's population living in 26 states; 3.7% of the women are already in menopause by the age of 30–34 years; and the incidence rises to 8% for the age bracket of 35–39 years. At the age of 48–49 years, 55.8% of the women are amenorrheic. It is probably an overestimate for the study that did not differentiate between natural, surgical, or secondary causes
Menopause occurring at an age less than 2 SD below the mean estimated age for the reference population is called as POI. It is a spectrum ranging from occult to overt POI
Conventionally, the diagnosis of overt POI is more likely if there is amenorrhea of 3–4 months and serum FSH levels >40 IU/mL (that is the menopause range) repeated a month apart accompanied by low estradiol levels. The ESHRE 2015 guidelines recommend these diagnostic criteria for POI: (1) the presence of menstrual disturbance such as oligomenorrhea or amenorrhea for at least 4 months and (2) an elevated FSH level >25 IU/L on two occasions at least 4 weeks apart (Grade C)
Appropriate counseling, lifestyle modification, and HT form the mainstay of treatment. HT should be started as early as possible in women with POI and continued till the age of natural menopause.[124,125] OCs may be used.[126] However, emerging evidence suggests that physiological dose of HT is superior to hormone contraceptives. Androgen replacement may be considered for women with persistent fatigue and loss of libido, in spite of estrogen replacement. Counsel women that HT is not a contraceptive, and erratic ovulation and pregnancy may occur in POI
There is no evidence that HT increases risk of breast cancer, CVD, or dementia, over and above that found in menstruating women with a normally timed menopause
Women with untreated premature menopause are at increased risk of developing osteoporosis, CVD, dementia, cognitive decline, and Parkinson's and all-cause mortality[127,128,129]
Women receiving chemotherapy or radiotherapy (pelvis) should be cautioned about iatrogenic premature menopause
Premenopausal hysterectomy alone may cause early menopause[130,131]
Iatrogenic menopause
The exact prevalence of surgical menopause is not known but varies from rural to urban areas and across states
A significant number of hysterectomies along with bilateral oophorectomies are performed at a young age. This trend of unwarranted hysterectomies and surgical castration for fear of cancer by the professional and the women should be discouraged
The physicians should have appropriate knowledge to recognize menopausal symptoms and whenever in doubt should get the tests done (FSH >40 IU/mL, E2 <20 pg/mL)
Women who need oophorectomy before menopause should be counseled about the risk of surgical menopause
Routine MHT is not recommended for surgical menopause in a postmenopausal woman as the primary prevention for chronic conditions
MHT should be considered in women aged <50 years who have undergone surgical menopause
Results of the WHI studies in older women do not apply to women with early menopause, and observational evidence suggests benefit with the use of HT taken up to the average age of menopause
Younger women may require higher doses for symptom relief or protection against bone loss
HT may have a beneficial effect on BP when compared with a combined OC
HT has bone protection effect. Combined OCs may protect bone depending on the estrogen status of the woman.[125,126]
SECTION IV
CLINICAL EVALUATION
General considerations
172. Clinical examination includes a holistic approach to health, rather than simply looking for the features of menopause in isolation; this leads to diagnose the latent and overt noncommunicable diseases NCD.[132,133,134,135] The age-related factors, lifestyle, and socioeconomic status need to be evaluated the woman and plan the management strategies
173. A detailed history, followed by general and physical examination, and assessment and categorization for risk of disease is done based on history and by using validated tools described below which are simple and can be easily incorporated in practice. These help in individualizing and plan long-term management for the midlife woman [Refer Appendix 1]
174. Menopause-related symptoms may be documented using the menopause rating scale [Refer Appendix 2]
175. Breast: A woman may be categorized of her risk for developing invasive breast cancer by Gail Model, the limitation being that it has not being validated in India (https://www.cancer.gov/bcrisktool) [Refer Appendix 3]
176. CVD: 10-year probability risk of MI/stroke – based on the WHO SEAR, WHO/ISH Risk Prediction Charts for India – uses the SEAR D. They are simple to use by the paramedics and the clinician (https://www.who.int'ncds'management'WHO_ISH_Risk_Prediction_) [Refer Appendix 4]
177. Skeletal health: Risk assessment for osteoporosis may be done by OSTA/SCORE/FRAX (http//www.shef.ac.uk/FRAX) [Refer Appendix 5]
178. Muscle health is assessed by the SARC-F, a 5-item questionnaire [Refer Appendix 6]
Results for risk assessment for diseases are documented in Table 8
Table 8.
Comprehensive chart for grading the health status of an individual based on tools for invasive breast cancer, CVD and Skeletomuscular health
| Tool | 5/10-Year risk score | Lifetime risk score | Status |
|---|---|---|---|
| Invasive breast Cancer Gail | |||
| WHO/ISH | |||
| OSTA/SCORE/FRAX | |||
| SARC-F |
WHO: World Health Organization, FRAX: Fracture Risk Assessment Tool
179. Examination can be broadly divided into three main categories: general physical including BMI and waist circumference, breast examination, and pelvic examination
180. Recommended laboratory tests include complete blood picture, urine test routine, fasting glucose level/Hb1Ac, lipid profile, serum TSH, stool for occult blood, PAP smear, vaginal pH, TVS, mammogram, or US
181. Investigations given in Table 9 should be chosen judiciously depending on the women's history and examination
Table 9.
Tests performed solely on indication
| Test | Indication |
|---|---|
| FSH | Premature ovarian insufficiency, women on contraceptive pills, women who had hysterectomy, doubt as to the cause of secondary amenorrhea or hot flushes, women on patches to rule out accumulation, fertility |
| Estradiol | Premature ovarian insufficiency, women on contraceptive pills, women who had hysterectomy, doubt as to the cause of secondary amenorrhea or hot flushes |
| Tests to assess increased risk of thrombosis | Where there is relevant past or family history, women with previous history of unexplained thromoembolic episodes antithrombin III, Tissue factor pathway inhibitor activity, protein C and protein S are to be estimated. Lupus anticoagulant, anticardiolipin antibodies should also be assessed |
| Endometrial biopsy | Postmenopausal bleeding, recent irregular bleeding, previous use of unopposed estrogen in the presence of uterus |
| Bone mass measurement | For specific indication (Refer Flowchart 2) |
| LFT | When relevant as with suspected liver disease or recent history of liver disease |
| Urodynamic study | To diagnose and differentiate on the severity and type of Incontinence before planning surgery |
| ECG, 2D Echo | CVD assessment |
| Vaginal pH, VMI | Vaginal atrophy |
| 25, OH Vitamin D | Rule out secondary causes of osteoporosis |
FSH: Follicle-stimulating hormone, LFT Liver function tests, ECG: Electrocardiogram, 2D Echo: Two-dimensional echocardiography, CVD: Cardiovascular disease, VMI: Vaginal saturation index
182. Dental and eye checkup should also be done.
SECTION V
MANAGEMENT OPTIONS
183. Classifying a woman into the following categories: It is helpful in planning management (adapted and modified from Morris Notelovitz)
The information obtained from the MRS scale and health status obtained from the risk screening results aids in classifying the women into two major groups.
Group 1-Women with no menopausal symptoms (VMS)
Group 2-Women with menopausal symptoms
In practise, charting out an individualised management plan becomes simple.
Group 1-Women with no VMS
Healthy, no VMS - institute preventive care
Healthy with risk factors for disease, no VMS —-institute preventive health care
Women with latent disease, no VMS- institute preventive health care, treat the disease
Women with comorbidities, no VMS-institute preventive health care, treat the disease
Group 2-Women with menopausal symptoms
Healthy with VMS - institute preventive health care, treat with MHT
Healthy with risk factors for disease, with VMS -institute preventive health care, risk-benefit analysis before therapeutic intervention
Women with latent diseases, with VMS —-institute preventive health care, risk benefit analysis before therapeutic intervention
Women with comorbidities, with VMS - institute preventive health care, treat the disease, risk-benefit analysis before therapeutic intervention. [Refer Appendix 7]
Counseling
184. Today, the art of medical counseling and translating the statistics in simple language is an important part of the consultation
185. The objectives of counseling include addressing women's questions and concerns, providing patient's education, and enhancing the patient's confidence in decision-making. If a therapy is chosen, the patient and clinician should agree on the goals, risks, and benefits, whether they are short term (menopause symptom relief), long term (primary or secondary prevention of diseases associated with aging), or both[136,137,138]
186. Classification of frequency of drug reactions according to the WHO and the Council for International Organisations of Medical Sciences is presented in Table 10.[139] This tool is effective in counseling the woman on the risks of MHT.
Table 10.
Council for International Organisations of Medical Sciences definitions are as follows which can be easily communicated to the lay person
| Term | n | Colloquial |
|---|---|---|
| Very common | 1/1 to 1/10 | A person in family |
| Common | 1/10 to 1/100 | A person in street |
| Uncommon | 1/100 to 1/1000 | A person in village |
| Rare | 1/1000 to 1/10000 | A person in a small town |
| Very rare | Less than 1/10000 | A person in a large town |
Dietary prescription
187. The National Institute of Nutrition plan for an adult sedentary woman is a good strategy for healthy living [Table 11].[140]
Table 11.
Nutrition plan for an adult sedentary women
| Food source | g/day |
|---|---|
| Cereals and millets | 270 |
| Pulses (vegetarian) | 60 |
| Nonvegetarian | 30 |
| Vegetables | 300 |
| Fruits | 100 |
| Dairy products | 0.3 |
| Fats and oils | 20 |
| Sugar | 20 |
| Salt | 5 |
| Water | 8-10 glasses |
Exercise prescription
188. Physical exercise helps maintain a healthy weight, improves bone density, coordination and balance, muscle strength and joint mobility, lipid profiles, genitourinary problems, relieves depression, and induces sleep
189. Social interactions, either in an exercise program or otherwise, help the postmenopausal women to improve mood, relieve depression, and relieve anxieties
190. Euphoria created with activity promotes QOL.
Immunization prescription
191. Hepatitis B vaccination is indicated for all unvaccinated adults at risk for HBV infection and all adults seeking protection from HBV infection including postexposure prophylaxis. Prevaccination screening in general population has not been found to be cost-effective in India (Grade B)[141]
192. The Expert Group of Association of Physicians of India recommends vaccination of the entire community at risk during an outbreak situation (Grade B)[141]
193. Two doses of varicella vaccine are strongly recommended in adults at increased risk for exposure of varicella (Grade B).[142]
Pharmacotherapy
Complementary and alternative therapies
194. Nonhormonal prescription agents may relieve VMS but have their own side effects. These can be considered when MHT is contraindicated or not desired (Grade A). Complementary and alternative treatments should be advised with caution as the data are still insufficient, especially in moderate-to-severe VMS.
195. These are not as effective as MHT and include drugs used as antidepressants and anticonvulsants, i.e., the selective serotonin reuptake inhibitors/ serotonin-norepinephrine reuptake inhibitors. For women with insomnia as the predominant symptom, choose gabapentin, and for women with predominance of mood disorders, prefer fluoxetine and paroxetine.[143] In breast cancer patients, fluoxetine and paroxetine should not be used as they inhibit the effect of tamoxifen.[144] In women with dyslipidemia, fluoxetine and citalopram exert the most favorable effect on the lipid profie
196. Awareness should be created regarding the phytoestrogens and lycopene-rich foods in the Indian diet[145,146](Grade C)
197. It is recommended to validate the effects of locally used herbs in the Indian context, according to the modern medicine, and prescribe them rationally using clinical research tools and well-designed and documented RCTs. While prescribing or recommending herbs, it would be essential to fully inform the women that very little human data are available on the usefulness of these formulations and side effects of the herbs have not been studied. It is important to read labels to determine isoflavone content and to warn them that in India, there are no regulations to ensure the content or quality of such products (Grade C).[147]
Indian data are sparse on the use of MHT; hence, this section is based on the guidelines developed by various societies.[8,147,148,149,150,151]
Menopausal Hormone Therapy
Terminology
198. MHT covers therapies including estrogens, progestogens, combined therapies, androgens, and tibolone
Terminology used in MHT: ET-estrogen therapy: EPT-estrogenprogesterone therapy: AT-androgen therapy:SERMs-selective estrogen receptor modulators (raloxifene and bazedoxifene): Gonadomimetics- tibolone, which contains estrogen, progestogen, and androgen activity
199. Three indications for postmenopausal HT, which have constantly withstood the test of time, derived from the results of various clinical trials are the beneficial effects of estrogens on symptom relief, urogenital atrophy, and bone. Hormone thearpy is indicated in POI and early menopause
200. Contraindications for MHT: Active endometrial and gynecological hormone-dependent cancers, active breast cancer, high risk for breast cancer, severe active liver disease with impaired/abnormal liver function, venous thrombosis, established CVD and at severe increased risk of CVD, known or suspected pregnancy, and undiagnosed and abnormal vaginal bleeding.
Patient characteristics that may be favorable for estrogen/androgen combination
201. Surgical menopause with continued VMS despite estrogen replacement, decreased well-being despite estrogen replacement, and acquired sexual desire dysfunction.
Indications for hormone therapy
Vasomotor symptoms
202. The most effective treatment for VMS is MHT at menopause transition (Grade A). Progesterones or low-dose OCPs may be used at menopause transition phase for relief of symptoms.
Genitourinary syndrome of menopause
203. Vaginal ET is most effective in the treatment of urogenital atrophy. Low-dose vaginal preparations are as effective as systemic therapy. Some women on oral ET may require additional local therapy
204. Treatment should be started early to prevent irreversible atrophic changes and may need long-term treatment to maintain benefits. Regular sexual activity, including vaginal coitus, should be encouraged to maintain vaginal health (Grade A)
205. Recurrent attacks of atrophic vaginitis require the use of the smallest effective dose over a period of time. After control of acute symptoms, the dose of local estrogen can be tapered for long-term maintenance therapy. Treatment may be continued indefinitely although safety data from studies do not go beyond 1 year (Grade C)
206. Limited data are available on the use of vaginal ET in women with breast cancer and EC. Lifestyle management and nonhormonal treatments would be the first option, but, in resistant cases, joint consultation with the oncologist and counseling of the woman is done before prescribing vaginal estrogen. The preferred therapy may be low dose with low-potency agent like estriol
207. Recurrent urinary tract in this age after ruling out other causes may benefit from the local application of ET (Grade A)
208. Progesterone supplement for endometrial protection is not needed along with the use of vaginal estrogen (Grade C)
209. Endometrial surveillance is not necessary in low-risk asymptomatic woman. Unscheduled bleeding should be investigated by transvaginal US and endometrial biopsy (Grade A)
210. Low-dose vaginal estrogen formulations reported no incidence of increase in CHD, stroke, and VTE. There are no contraindications for use in nonestrogen-dependent cancers (Grade A).
Osteoporosis
211. All preparations including low-dose, nonoral routes of estrogen are effective in preserving bone mass
Estrogen–progesterone therapy (EPT) or ET may be used for the prevention and treatment of osteoporosis in the early postmenopause in symptomatic women unless there is a contraindication. ET/EPT prevents all osteoporotic fractures even in low-risk population; it increases lumbar spine BMD up to 7.6% and femoral neck BMD up to 4.5% over 3 years. It reduces the risk of spine, hip, and other osteoporotic fractures by 33%–40% (Grade A).
212. MHT should not be started solely for bone protection after 10 years of menopause. Extended use of MHT in women with reduced bone mass is an option after considering the risk–benefit analysis compared to the other available therapies for osteoporosis (Grade B)
213. POI: HT should be offered to women with POI and early menopause (and it can be recommended until the age of natural menopause) (Grade B)
214. Depression: Estrogen may be prescribed to enhance mood in women with depressive symptoms after ruling out other causes. The effect appears to be greater for perimenopausal symptomatic women than for postmenopausal women (Grade A).
Hormone therapy use in disease
215. In women with hypertriglyceridemia (>400 mg/dL), obesity, history of deep vein thrombosis, and tobacco users, nonoral route should be preferred (Grade B).
For women at moderate risk of CVD, observational data suggest transdermal rather than oral estrogen, with micronized progesterone or dydrogesterone for those with a uterus. Non-HTs are advised for symptomatic women who are at high risk (>10% 10-year risk) for CVD.
216. MHT has a favorable effect on glucose metabolism, both in women with and without T2DM, while it may delay the onset of T2DM
217. MHT in women with T2DM should be administered according to their risk of CVD. In women with T2DM and low CVD risk, oral estrogens may be preferred, while transdermal 17 β-estradiol is preferred for women with T2DM and coexistent CVD risk factors, such as obesity. Progestogen with neutral effects on glucose metabolism should be used, such as progesterone and dydrogesterone
218. Women who have low to moderate of breast cancer may be prescribed MHT according to their need after a detailed history, examination, and counseling. They should be provided information about breast cancer risk with MHT as per evidence
219. Women at moderate risk of breast cancer, a benifit risk analysis and the choice of MHT should be discussed
220. In women with high risk and breast cancer survivors, it is recommended to use non MHT
221. HT does not appear to influence the clinical pattern of benign breast disease in a postmenopausal woman (Grade C).
Preexisting cancers and menopausal hormone therapy (Grade C)
222. MHT is neutral – Low-grade, early-stage EC type I, cervical adenocarcinoma, epithelial ovarian cancer, germ cell tumors, hematological malignancies, local cutaneous malignant melanoma, colorectal cancer, hepatocellular cancer BRCA 1/2 mutation carriers without cancer, most types of ovarian cancer, squamous cell carcinomas of cervix, vaginal and vulvar squamous cell carcinoma, prolactinoma, kidney cancer, pancreatic cancer, and thyroid cancer
223. MHT is relatively contraindicated – Leiomyosarcoma, EC type II, advanced metastatic malignant melanoma, lung cancer, gastric cancer, and bladder cancer
224. MHT is contraindicated – Breast cancer, endometrial stromal sarcoma, uterine carcinosarcoma, ovarian cancer (estrogen-dependent granulosa cell, low-grade serous, and Sertoli–Leydig, endometroid types of ovarian tumors), adenocarcinoma of the cervix meningioma, glioma, and hormone receptor-positive gastric and bladder cancer
225. Low-dose, local vaginal ET may be an option for the treatment of vaginal dryness related to hormone deficiency after certain types of cancers, even if systemic MHT is not recommended.
Precautions
226. Progesterone in adequate dose and duration should be supplemented along with oral estrogens in women with uterus (Grade A)
227. Estrogen alone is given in hysterectomized women (Grade A)
228. Progesterone supplement for endometrial protection is not needed along with the use of vaginal estrogen (Grade C)
229. Investigations are needed, if bleeding starts at the start of progesterone therapy in cyclical regimens, or there is a change in the duration or intensity of blood flow which is not normal for that woman. Investigations are needed, if bleeding is heavy or continuous and continues after 6–12 months of use with continuous combined regimens, and in women with a high risk for uterine cancer
230. Pre-MHT workup and annual follow-up are essential when prescribing MHT. A full gynecological assessment is mandatory before starting MHT and at regular intervals thereafter. SBE is advised monthly. Mammogram or the breast US, where available, should be carried out 1–3 yearly if the initial mammogram is normal (Grade C)
231. The dose and duration of use of MHT should be individualized, and a risk–benefit assessment is carried out annually. Follow-up after 1 month, 3 months, 6 months and then annually (Grade C)
232. Regimens
Continuous-cyclic regimen, estrogen is used every day, with progesterone added cyclically for 10–14 days during each month. Progestogen is started on day 1 or day 15 each month. This regimen is suitable for women at perimenopause
Uterine bleeding occurs in about 80% of women when progestogen is withdrawn, although bleeding can begin 1–2 days earlier, depending on the type and dose of progestogen used
In the continuous-combined EPT regimen, fixed doses of estrogen and progesterone are administered every day. It is the treatment of choice at postmenopause
Approximately 40% incidence of irregular spotting or bleeding in the first 6 months
Estrogen alone is prescribed in hysterectomized woman. Progesterone is added along with estrogens in hysterectomized woman in cases of endometriosis, endometrial ablation, and supracervical hysterectomy.
233. Routes
Estrogen: Oral, transdermal, and vaginal
Progestogen: Oral, vaginal, and intrauterine levonorgestrel system.
234. Duration of use
235. POI: HT can be prescribed up to the natural age of menopause; further continuation of therapy is a shared decision between the woman and the physician according to the indication and the need (Grade C)
236. Natural menopause: Safety data on EPT with CEE + MPA use are 3–5 years, and with ET, safety data are 7 years of treatment with 20 years follow-up. Role of extended use of HT is a shared decision between the woman and the physician and may be considered in cases of recurrence of symptoms after stopping therapy, in case of management of osteoporosis when other therapies are contraindicated (Grade A)
237. Stopping MHT may be abrupt or the dose and duration may be tapered off gradually (Grade C).
Potency and nonoral routes
238. Minimum effective dose is the principle to be followed while prescribing MHT. The potency needed by the woman may change over time. After starting standard-dose therapy, dose can be lowered and maintained accordingly. Low-dose and ultralow-dose therapies are effective in relieving symptoms and maintaining bone mass
239. Transdermal estrogen has a neutral effect on triglycerides, CRP, and sex hormone-binding globulin and is preferable for use in women with hypertriglyceridemia, obesity, glucose intolerance, high risk of deep vein thrombosis, and tobacco users.
CONCLUSIONS ON THE RISKS AND BENEFITS OF SYSTEMIC AND LOCAL HORMONE THERAPY-BASED ON AVAILABLE EVIDENCE
240. MHT may be given to women below the age of 60 years or within 10 years of menopause, and the risks are rare.
Risks of systemic therapy
241. The risks of MHT may be explained to the woman in terms defined by CIOMS. Refer Table 12.
Table 12.
Based on the Women’s Health Initiative, number of excess events on hormone therapy versus placebo per 10,000 women per year of hormone therapy
| Disease | Estrogen | WHO/CIOMS definition of risk | Estrogen + progesterone | WHO/CIOMS definition of risk |
|---|---|---|---|---|
| Venous thromboembolism | 4 | Rare | 11 | Rare |
| Stroke | 1 | Rare | 4 | Rare |
| Breast cancer | 5 | Rare | ||
| Cardiovascular disease | 5 | Rare |
Use between the age group of 50-59 years. CIOMS: Council for International Organisations of Medical Sciences, WHO: World Health Organization
Coronary heart disease
242. Systemic therapy is not to be used for primary or secondary prevention of CHD. At any age, avoid MHT in women at high risk of CVD or in established CVD
243. ET may be cardioprotective, if started around the time of menopause “window of opportunity” in a healthy woman. It reduces the risk of diabetes and has positive effects on the lipid profile and metabolic syndrome
244. Less than 60 years old, recently menopausal and without evidence of CVD, at mild-to-moderate risk of CVD, the initiation of MHT does not cause early harm and may reduce morbidity and mortality from CHD. At moderate-risk women, if indicated, transdermal MHT is preferable
245. MHT beyond the age of 60 years should be decided as a part of the overall risk–benefit analysis.
Venous thromboembolism
246. The risk of VTE is increased by smoking, increasing age, obesity, and oral MHT. VTE risk is as high as 29/10,000 woman-years during pregnancy and 300–400/10,000 woman-years in the postpartum period
247. HT increases VTE risk by twofold (Grade A). Transdermal ET and the natural progesterones may not have an effect. Population screening for thrombophilia is not indicated before MHT use.
Stroke
248. Unlike CHD, the timing hypothesis does not appear to apply to the risk of stroke, most likely because the risk of stroke is probably due to thrombotic rather than atherosclerotic mechanisms. Hence, literature is suggestive of an increased risk of stroke with oral MHT, there is a dose-dependent relationship, and the event rate is extremely low, especially in younger women
249. Standard-dose oral MHT increased stroke risk by about one-third in generally healthy postmenopausal women (Grade B)
250. The risk increases with estrogen dose, age, and BMI and is greater during the first years of therapy. The absolute risk is 1.5 more cases/10,000 women per year at the age of 50–54 years, 2.2 at the age of 59 years, 2.8 at the age of 60–64 years
251. Low-dose ET, transdermal route may not increase the risk of stroke (Grade C).
MHT and breast cancer
252. MHT may not initiate breast cancer and may have promotional effect on hormone receptor breast cancer in susceptible women. Tumors in MHT used women are usually ER-positive and lobular type (Level C)
253. The increased risk for breast cancer attributable to MHT is less than 1 per 1000 women per year of use in the age group of 50–59 years. This increased risk is similar or lower than those associated with common lifestyle factors, such as reduced physical activity, obesity, and alcohol consumption
254. Losing weight and keeping it off were associated with lower breast cancer risk for women aged 50 years and over in The Nurses' Health Study (1980–2014; n = 73,196) and The Health Professionals Follow-Up Study (1986–2014; n = 38,366)
255. The effect of MHT on breast cancer risk may depend on the type of MHT, dose, duration of use, regimen, route of administration, prior exposure, and individual characteristics
256. Avoid MHT in women with severe risk for breast cancer and in breast cancer survivors.
Estrogen alone
257. In the WHI trial, prior randomized use of CEE alone compared with placebo, among women who had a previous hysterectomy, was significantly associated with lower breast cancer incidence and lower breast cancer mortality[151]
258. For women who are BRCA positive and have undergone risk reducing BSOP, observational data suggest that the use of systemic HT to the median age of menopause aimed to decrease health risks associated with premature loss of estrogen does not increase breast cancer risk. Decisions are on an individual basis.
Estrogen plus progesterone
259. In the WHI trial, prior randomized use of CEE plus MPA, compared with placebo, among women who had an intact uterus, was significantly associated with a higher breast cancer incidence but no significant difference in breast cancer mortality[151]
260. Emerging data from two independent studies report that progesterone (micronized progesterone/dydrogesterone) with estrogen may not increase the risk if given for less than 5 years (Level C)
261. The risk returns to approximately that of nonusers within 3 years of cessation (Level B).
Gallbladder
262. Risk of gallstones, cholecystitis, and cholecystectomy is increased with oral estrogen-alone and combination MHT
263. Observational studies report lower risk with transdermal MHT than with oral and with oral estradiol compared with CEE, but neither observation is confirmed in RCTs.
Benefits of systemic therapy
The absolute benefits of MHT are given in Table 13.
Table 13.
Based on WHI: number of less events on ET and EPT versus placebo per 10,000 women per year of HT use between the age group of 50 years and 59 years
| Disease | Number of less events with ET |
|---|---|
| Heart Disease | 12 |
| Breast Cancer | 8 |
| Disease | Number of less events with E / EPT |
| Overall Mortality | 10 |
| Fractures | 5 |
| Colorectal cancer | 6 |
Other possible benefits
MHT significantly reduces the diagnosis of new-onset T2DM
MHT may help reduce the abdominal adipose tissue and the weight gains that are often associated with the menopause transition and thus help in reducing the emergence of new onset of metabolic syndrome
Estrogens may have a protective effect on osteoarthritis (Grade B)
Estrogen benefits verbal memory over the short period when initiated soon after surgical menopause (Grade B)
MHT reduces the neovascular macular lesions (Grade C)
MHT use has shown to reduce colorectal cancers
MHT in the early menopausal period improves QOL by its effects on VSMs and urogenital symptoms and improvement on sleep and mood.
Benefits and risks of local therapy
Local therapy is indicated when the woman suffers only from urogenital atrophy
Vaginal ET is most effective in the treatment of urogenital atrophy. Low-dose vaginal preparations are as effective as systemic therapy. Some women on oral ET may require additional local therapy
Low-dose vaginal estrogen formulations reported no incidence of increase in CHD, stroke, and VTE. There are no contraindications for use in nonhormone-dependent cancers.
Androgens
264. Available data are of low quality and conflicting regarding the risk of breast cancer relating to use of androgens (Grade D)
265. Androgen replacement for use in women is not available in India.
Raloxifene
266. It decreases the risk of development of breast cancer. The indication for use is postmenopausal osteoporosis especially in women at high risk for breast cancer given in a dose of 60mg/day
267. Tissue selective estrogen complex: Newer formulations of combination therapy of estrogen and selective ER modulators are soon to be available.
Tibolone
268. Tibolone is a selective tissue estrogenic activity regulator. It is a synthetic steroid compound, which has estrogenic, progestogenic, and androgenic properties. It has an estrogenic effect on bone, inhibiting bone resorption by reducing osteoclastic activity
269. Tibolone is effective in treating VMS and improves urogenital atrophy (Grade A)
270. It improves mood and libido (Grade B)
271. It is prescribed in a single daily dose of 2.5 mg orally. A lower dose of 1.25 mg has been found to be equally effective for most indications, including osteoporosis. It should be prescribed 1 year after amenorrhea (Grade A).
Use of oral contraceptives during the menopausal transition
272. A low-dose estrogen OC is an option, unless contraindicated for perimenopausal (40–50 years) women who seek relief of menopausal symptoms, also desire contraception, and may need control of bleeding when it is heavy. A continuous 3-monthly regimen may be beneficial to control VMS.
SECTION VI
ECONOMICS OF MENOPAUSE MANAGEMENT
273. Indian healthcare system is one of the most privatized systems where government spends much less and individual has to pay for health insurance[152]
274. Insurance may be described as a social device to reduce or eliminate the risk of life and property. Under the plan of insurance, many people associate themselves by sharing risk, attached to individual insurance plan that covers only healthcare costs and is called health insurance
275. It is indeed very important to enroll in any of the good health insurance schemes for a secure future. Healthcare insurance provides a cushion against medical emergencies. Most companies stop enrolment after 65–70 years of age. Menopause management has significant direct and indirect costs
276. Direct costs include physician's visits, specialist's visit, and traditional pharmacotherapy or alternative and complementary medicine modality
277. Indirect costs include laboratory testing, management of adverse events, loss of productivity at home and at work, and treatment of associated medical disorders
278. Various groups of molecules are available for prevention and treatment of osteopenia and osteoporosis. Cost of therapy varies according to the indication whether they are prescribed for prevention or treatment of osteopenia or osteoporosis
279. Menopause is a critical window of opportunity for preventing and reducing risk for CVD and maintaining skeletomuscular and urogenital health. The importance of lifestyle counseling for smoking cessation, diet modification, and physical activity in the menopausal women has to be emphasized. Adopting a healthy lifestyle is cost effective.
280. Establishing menopause clinics at primary and specialized centers would help in implementing the concept of critical window of opportunity at menopause.[153]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1: CHRONIC DISEASES WITH THE MODIFIABLE AND NON MODIFIABLE RISK FACTORS AND METHODS OF SCREENING
| Diseases | Risk factors | Refer appendix 3,4,5,6 | |
|---|---|---|---|
| Modifiable | Nonmodifiable | Method | |
| Osteoporosis | Low BMI (≤20) Malnutrition Nutritional deficiency Physical inactivity Prolonged immobilization Vitamin D deficiency Estrogen deficiency Smoking Excessive alcohol intake |
Female sex Advanced age Family history of osteoporosis history of osteoporotic fracture Diseases and disorders hypogonadal status, Endocrine disorders -Cushing’s insufficiency, Malabsorption syndrome, renal Insufficiency Hematological disorders Medications, steroids and anticonvulsants |
OSTA SCORE FRAX |
| CHD | Diabetes Hypertension Obesity Smoking Hyperlipidemia (high LDL, Low HDL, elevated TG) |
Age >55 years Primary ovarian insufficiency, Family history of CVD Gestational Hypertension |
WHO/ISH Risk prediction charts |
| Diabetes mellitus | Physical inactivity Obesity Polycystic ovary syndrome |
Family history Advancing age Personal history of gestational diabetes mellitus or impaired glucose tolerance | FBS/Hb1Ac/2 hours 75 gms oral Glucose tolerance test (GTT) |
| Thyroid dysfunction | Family history of thyroid disease Prior thyroid dysfunction Hyperthyroidism Autoimmune disorder |
Serum TSH | |
| AD | Physical inactivity Diabetes Hypertension Dyslipidemia Smoking Obesity Depression Stress and social engagement Diet |
Age >65 Female Family history: First degree Genetic factor: APOE-4 x MCI Autoimmune diseases Head trauma Traumatic brain injury |
Criteria for probable AD Dementia of insidious onset Progression of symptoms No disturbance of consciousness Absence of other systemic or brain diseases that produce cognitive and behavioral change |
| Deep vein thrombosis | Prolonged immobilization Oral contraceptive pills MHT, Norethisterone, norpregnane derivetives |
Personal or family history History of treatment with anticoagulants |
Activated partial thromboplastin time and prothrombin time, antithrombin, protein C and S, factor V Leiden, lupus anticoagulant and anticardiolipid antibodies (immunoglobulins G and M) |
CHD: Coronary heart disease, MCI: Minimal cognitive impairment, APOE-4: Apolipoprotein E-4, TG: Triglyceride, LDL: Low-density lipoprotein, AD: Alzheimer’s disease, HbA1C: Glycosylated hemoglobin AIC, FBS: Fasting blood sugar, BMI: Body mass index, WHO: World Health Organization, FRAX: Fracture Risk Assessment Tool, MHT: Menopause hormone therapy
APPENDIX 2: MENOPAUSE RATING SCALE
The menopause rating scale is a standardized menopause-specific instrument to accurately document the severity of symptoms. It consists of 11 main factors with scales.[154]
| Item | Description |
|---|---|
| 1 | Hot flushes, sweating (episode of sweating) |
| 2 | Heart discomfort (unusual awareness of heart beat, heart skipping, heart racing, and tightness) |
| 3 | Sleep problems (difficulty falling sleep, difficulty in sleeping through the night, and waking up too early) |
| 4 | Depressive mood (feeling “down,” sad, on the verge of tears, lack of derive, and mood swings) |
| 5 | Irritability (feeling nervous, inner tension, and feeling aggressive) |
| 6 | Anxiety (inner restlessness, feeling “panicky”) |
| 7 | Physical and mental exhaustion (general decrease in performance, impaired memory, decrease in concentration, forgetfulness, fatigue, headache, and dizziness) |
| 8 | Sexual problems (change in sexual desire, in sexual activity, and satisfaction) |
| 9 | Bladder problems (difficulty in urinating, increase need to urinate, and bladder incontinence) |
| 10 | Dryness of the vagina (sensation of dryness or burning in the vagina, difficulty with sexual intercourse) |
| 11 | Joint and muscular discomfort (joint pain, muscle pain, and backache) |
According to the World Health Organization (WHO) standards, the degree of severity is consistent with the following:
| Symptom | Degree | percentage |
|---|---|---|
| No problem | None, absent, negligible | 0%-4% |
| Mild problems | Slight, low | 5%-24% |
| Moderate problems | Medium, fair | 25%-49% |
| Severe problems | High, extreme | 50%-95% |
| Complete problems | Total | 95%-100% |
The degree of symptomatology, in terms of percentage at each visit, on the menopause pro forma for assessing the severity of symptom follow-up and effectiveness is documented.
APPENDIX 3: RISK ASSESSMENT FOR BREAST CANCER
The Breast Cancer Risk Assessment Tool, Gail Model allows health professionals to estimate a woman's risk of developing invasive breast cancer over the next 5 years and up to age 90 years (life time risk).[155]
The tool uses a woman's personal medical and reproductive history and the history of breast cancer among her first-degree relatives (mother, sisters, and daughters) to estimate absolute breast cancer risk – her chance or probability of developing invasive breast cancer in a defined age interval.
It needs to be validated in India. This simple tool may be used in the absence of country-specific validated tool (https://www.cancer.gov/bcrisktool) and classify the women into three groups.
| Method | Low | Moderate | High |
|---|---|---|---|
| Gail model | <1.67 | 1.6-5 | >5 |
Breast self-examination
Breast self-examination (BSE) is performed by the woman herself and involves examination of the breast, skin, and axillae based on palpations by her hands. BSE is recommended so that women understand their breasts for detecting any suspicious changes over time.
BSE should be done immediately after periods or any fixed day of the month if there is not menstruating. Nodular and lumpy feel of the breast and increased pain and tenderness, which is a physiological finding before menstruation, needs to be explained to the patient.
Women can be taught to examine the breasts in any of the following ways in supine as well as standing positions.
APPENDIX 4: WORLD HEALTH ORGANIZATION/ISH — CARDIOVASCULAR RISK PREDICTION CHART
The WHO/ISH Risk Prediction Charts predict 10-year risk of combined myocardial infarction and stroke risk, fatal and non-fatal. These have been developed from best available mortality and risk factor data of low- and middle-income country populations.[156]
At present, these charts are necessarily crude but are simple, safe, and useful tools for guiding the management and treatment decisions for individuals.
The charts can have an impact on prevention of heart attacks and stroke, particularly if they can be used by health workers in primary healthcare.
When are the charts useful for stratifying risk?
Charts are useful for stratifying risk for people with blood pressure <160/100 mmHg or blood cholesterol <8 mmol/L or uncomplicated diabetes.
An individual is classified as high, medium, or low risk over 10 years.
The following come under high-risk category, no risk assessment, they need to be treated
Persistent raised blood pressure ≥160/100 mmHg or blood cholesterol ≥8 mmol/L or established ischemic heart disease or diabetes with renal disease.
Note that cardiovascular disease risk may be higher than indicated by the charts in the presence of the following
Already on antihypertensive therapy, premature menopause, obesity (including central obesity); sedentary lifestyle; family history of premature coronary heart disease or stroke in first-degree relative (male <55 years, female <65 years); raised levels of C-reactive protein, microalbuminuria (increases the 5-year risk of diabetics by about 5%).
There are two sets of charts.
One set can be used in settings where blood cholesterol cannot be measured
Second set is for settings in which blood cholesterol can be measured.
How do you use the charts to assess cardiovascular risk?
First make sure that you select the appropriate chart
If blood cholesterol cannot be measured due to resource limitations, use the charts that do not have total cholesterol
-
Before applying the chart to estimate the 10-year cardiovascular risk of an individual, the following information is necessary.
- Presence or absence of diabetes
- Gender
- Smoker or nonsmoker
- Age
- Systolic blood pressure
- Total blood cholesterol (if in mg/dl divide by 38 to convert to mmol/L).
Once the above information is available proceed to estimate the 10-years cardiovascular risk as follows.
Step 1: Select the appropriate chart depending on the presence or absence of diabetes
Step 2: Female tables
Step 3: Select smoker or nonsmoker boxes
Step 4: Select age group box (if age is 50–59 years select 50, if 60–69 years select 60, etc.)
Step 5: Within this box find the nearest cell where the individuals systolic blood pressure (mmHg) and total blood cholesterol level (mmol/L) 4 cross.
The color of this cell determines the 10-year cardiovascular risk.
An individual is classified as high risk (maroon and red), medium risk (orange and yellow), or low risk (green) over 10 years.
| Low (green) | Moderate | High |
|---|---|---|
| >10% | 10%-30% | 30%->40% |
World Health Organization/ISH risk prediction charts for India
Ten-year risk prediction chart for cardiovascular disease event by gender, age, systolic blood pressure, smoking status, cholesterol, and presence or absence of diabetes mellitus is given below.[156]
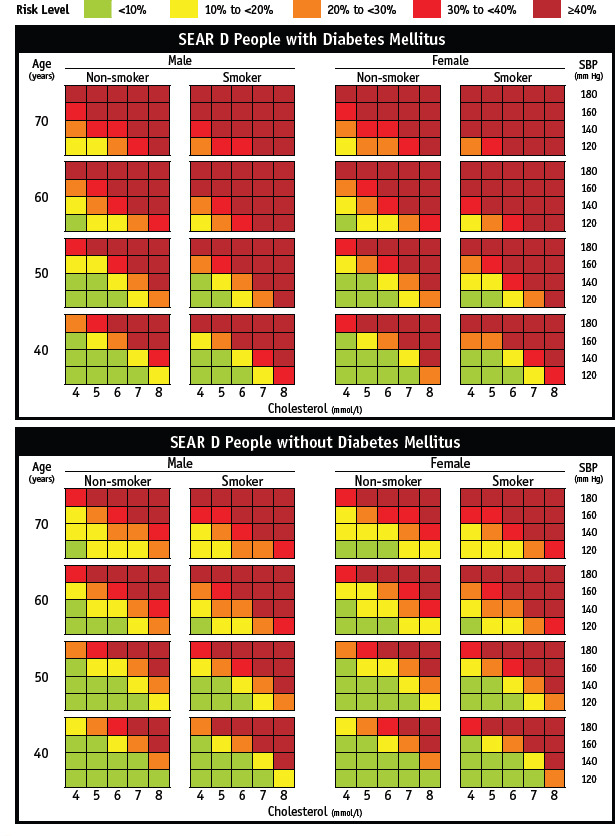
Ten-year risk prediction chart for cardiovascular disease event by gender, age, systolic blood pressure, smoking status, cholesterol, and presence or absence of diabetes mellitus is given below.
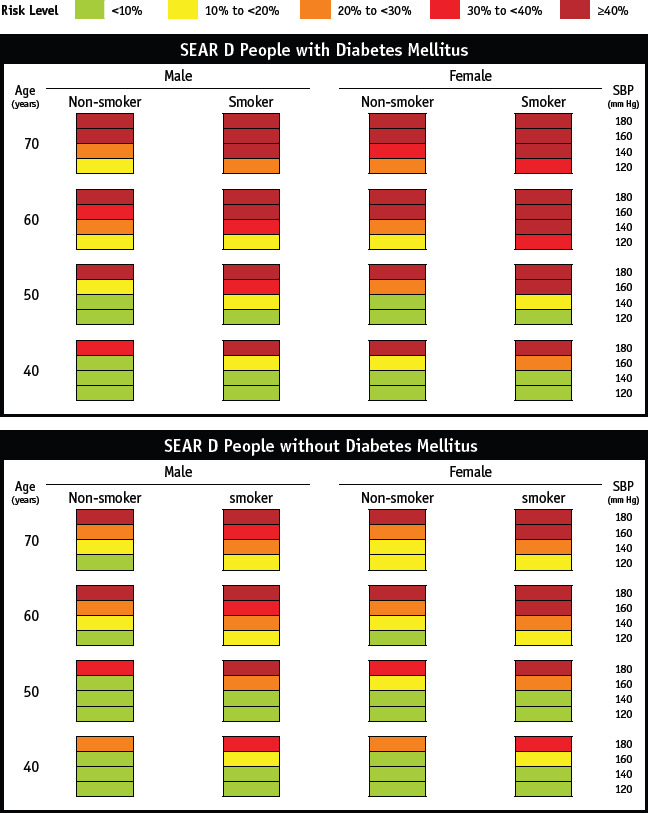
APPENDIX 5: OSTEOPOROSIS SCREEN
The WHO developed the OSTA score a risk assessment tool based simply on age and body weight to identify the women at risk for osteoporosis.
The OSTA is calculated using the following formula: (body weight [kg] − age [years]) × 0.2, with the decimal digits being disregarded or the chart below may be used.[157,158]
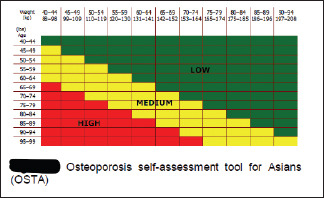
Women are stratified into three groups at risk for sustaining osteoporosis.
| Method | Low | Moderate | High |
|---|---|---|---|
| OSTA | >−1 | −1-−4 | <−4 |
Recommendation based on risk
High-risk patients: To measure bone mineral density (BMD), if possible, and consider drug treatment even if BMD if not available (about 61% of individuals in the high-risk group have osteoporosis)
Moderate-risk patients: To measure BMD and consider drug treatment if BMD is low (about 15% of individuals in the moderate-risk group have osteoporosis)
Low-risk patients: Not to measure BMD unless other risk factors are present (only about 3% of individuals in the low-risk group have osteoporosis).
WORLD HEALTH ORGANIZATION FRACTURE RISK ASSESSMENT TOOL
WHO Fracture Risk Assessment Tool (FRAX) is country specific and an online tool is available for India (http: www.shef.ac.uk/FRAX). FRAX is used to identify patients in the osteopenia group most likely to benefit from treatment. It predicts the 10-year absolute risk for a fracture in an individual and the cost-effective analysis determines the interventional threshold above which treatment is cost-effective. FRAX is country specific, and until more Indian data are available on prevalence of osteoporotic fractures and mortality rates, it may not serve the true purpose for the usage of FRAX in the Indian context.
10-year absolute risk for a fracture in an individual to decide for treatment is given below.
| Fracture | Percentage |
|---|---|
| Hip | >3% |
| Major fracture | >20% |
APPENDIX 6: SARCOPENIA SCREENING
European Working Group on Sarcopenia in Older People (EWGSOP) advises use of the SARC-F questionnaire or clinical suspicion to find sarcopenia-associated symptoms.[159]
Screen by history: A case-finding strategy starts when a patient reports symptoms or signs of sarcopenia, i.e., falling, feeling weak, slow walking speed, difficulty rising from a chair, or unintentional weight loss/muscle wasting
In such cases, testing for sarcopenia is recommended by using SARC-F questionnaire, SARC-F is a self-administered questionnaire, which has five components, including strength, assistance in walking, rise from a chair, climb stairs, and falls with a 3-level score range of 0–2 points for each item
SARC-F is an inexpensive and convenient method for sarcopenia risk screening
SARC-F has a low-to-moderate sensitivity and a very high specificity to predict low muscle strength.
| Component | Question | Scoring |
|---|---|---|
| Strength | How much difficulty do you have in lifting and carrying 10 lb? | None - 0 Some - 1 A lot or unable - 2 |
| Assistance in walking | How much difficulty do you have walking across a room? | None - 0 Some - 1 A lot, use aids, or unable - 2 |
| Rise from a chair | How much difficulty do you have transferring from a chair or bed? | None - 0 Some - 1 A lot or unable without help - 2 |
| Climb stairs | How much difficulty do you have climbing a flight of 10 stairs? | None - 0 Some - 1 A lot or unable - 2 |
| Falls | How many times have you fallen in the past year? | None - 0 1-3 falls - 1 ≥4 falls - 2 |
SARC-F score
The total score range is from 0 to 10, with scores of ≥4 points indicative of the risk of sarcopenia
Assess for evidence of sarcopenia: EWGSOP recommends use of grip strength or a chair stand measure with specific cutoff points for each test.
EWGSOP2 sarcopenia cutoff points for low strength by chair stand and grip strength is given below.
Grip strength <16 kg
Chair stand >15 s for five rises.
Methods
Grip strength
Handgrip strength is the most widely used method for the measurement of muscle strength.
Time of administration: 5 min.
Equipment: A well-calibrated handheld dynamometer.
Method
Six measures should be taken, three with each arm. Ideally, the patients should be encouraged to squeeze as hard and as tightly as possible during 3–5 s of the measure; the highest reading of the six measurements is reported as the final result.
Chair stand test
The chair stand test (also called chair rise test) can be used as a proxy for strength of leg muscles (quadriceps muscle group)
Time of administration: 1–2 min
Equipment: A chair with a straight back without arm rests and a stopwatch.
Method
The subject is first asked to stand from a sitting position without using their arms. If he/she is able to perform the task, he/she is then asked to stand up and sit down five times, as quickly as possible with arms folded across their chests. The time to complete five stands is recorded. The chair stand test measures the amount of time needed for a patient to rise five times from a seated position without using his or her arms.
APPENDIX 7
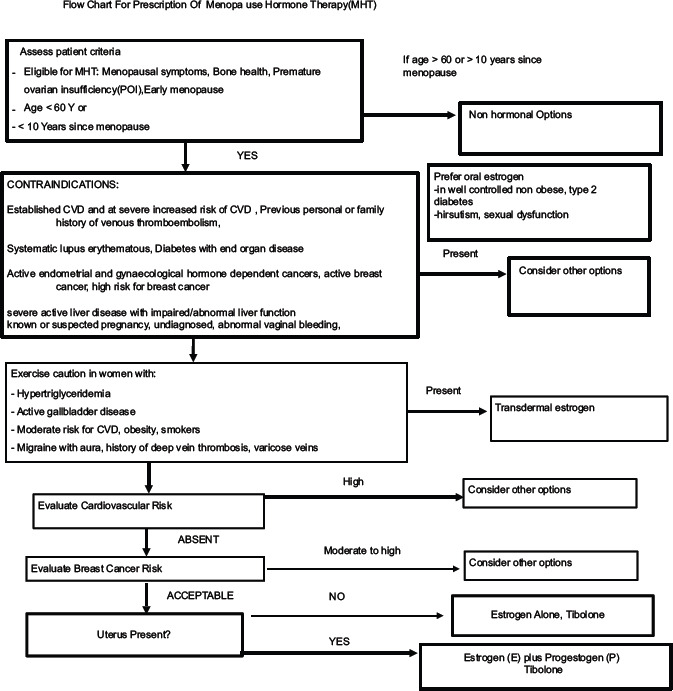
Systemic Regime Depending On The Stage Of Menopause
In a women with uterus
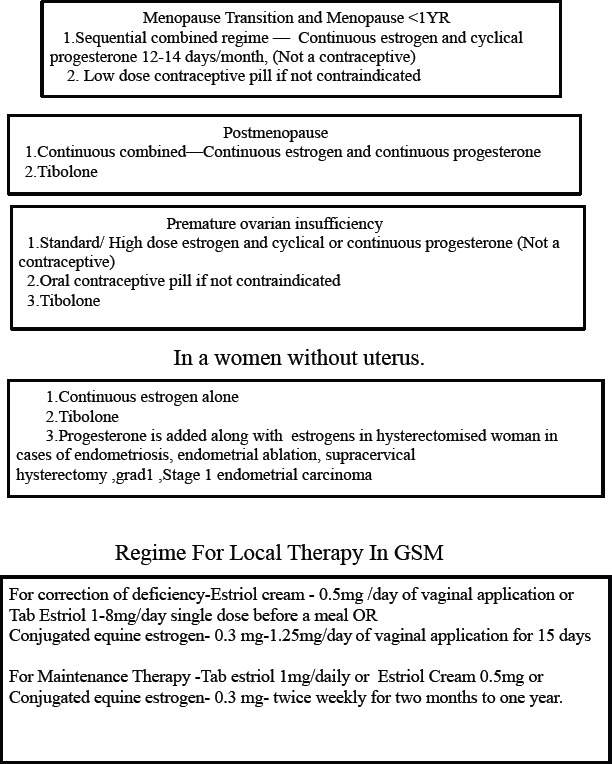
Terminology–Dosage and types of Systemic Estrogens used in MHT
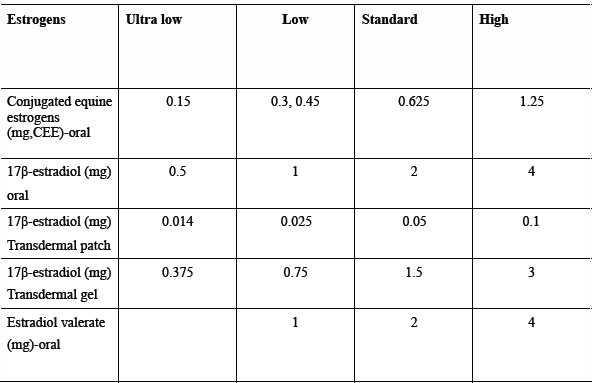
Types of progesterone used in MHT
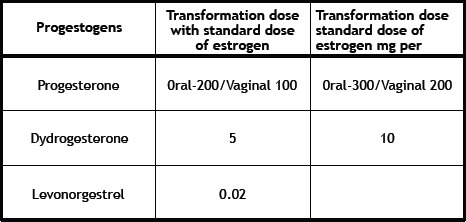
MHT Formulations Available In India
Systemic Therapy
Estrogen:
Oral-CEE 0.3 mg/0.625 mg,17 beta- estradiol 1 mg/2 mg Estradiol valerate 1 mg/2 mg
Transdermal estrogen gel - 0.1.25mg per 2.5 gm of gel
Progesterone
Dydrogesterone 10mg, Micronized Progesterone-100,200,300,400 mg, Levonorgesterol Intrauterine Device-52mg-release 20 mcg/day
Combination of Estrogen and Progesterone
Continuous sequential-17-beta estradiol 1 mg and 10 mg of dydrogesterone
Continuous combined-17 beta-estradiol and dydrogesterone 5 mg daily
Tibolone -2.5mg/day orally
Estrogen Therapy For Genitourinary Syndrome
Estriol cream -0.5mg/ 0.5gm of cream; Oral Estriol-1/2 mg
Conjugated equine estrogen-0.625mg/1gm of cream
REFERENCES
- 1.de Carvalho BR, Gomes Sobrinho DB, Damasceno Vieira AD, et al. Ovarian reserve assessment for infertility investigation. Obstet Gynecol. 2012;2012:576385. doi: 10.5402/2012/576385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritz MA, Speroff L. Menopause and the perimenopausal transition. In: Speroff L, Glass RH, Kase NG, editors. Clinical Gynecological Endocrinology and Infertility. 8th ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2011. p. 681. [Google Scholar]
- 3.Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–07. [PubMed] [Google Scholar]
- 4.Utian WH. The International Menopause Society menopause-related terminology definitions. Climacteric. 1999;2:284–6. doi: 10.3109/13697139909038088. [DOI] [PubMed] [Google Scholar]
- 5.Sherman S. Defining the menopausal transition. Am J Med. 2005;118:3S–7S. doi: 10.1016/j.amjmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Anklesaria BS, Soneji RM. Staging, symptoms and urological problems in the climacteric. In: Krishna UR, Shah D, editors. Menopause Obstetrics and Gynecology in Perspective. 1st edition. Chennai: Orient Longman Pvt Ltd; 1996. p. 13. [Google Scholar]
- 7.Anklesaria BS. The staging of menopause. In: Kumar P, Malhotra N, editors. Jeffcoate's Principles of Gynecology. 7th ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2008. pp. 862–4. [Google Scholar]
- 8.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 9.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of reproductive aging workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8:402–7. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97:843–51. doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Government of India: Ministry of Home Affairs 2011, Office of the Registrar General & Census Commissioner, India. 2011. [Last accessed on 2020 Jul 10]. Available from: http://censusindia.gov.in .
- 12.Sharma S, Tandon VR, Mahajan A. Menopausal symptoms in urban women. J K Sci J Med Educ Res. 2007;9:13–7. [Google Scholar]
- 13.Singh M. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric. 2012;15:581–6. doi: 10.3109/13697137.2011.643514. [DOI] [PubMed] [Google Scholar]
- 14.Singh L, Ahuja S. Trend of menopause among the women of Punjab. Anthropol Anz. 1980;38:297–300. [PubMed] [Google Scholar]
- 15.Sengupta S, Gogoi G. Menarche and menopause among the Kai- barta women of Dibrugarh, Assam. J Assam Sci Soc. 1993;35:113–9. [PubMed] [Google Scholar]
- 16.Kulkarni VS, Joshi M. Reproductive life of two endogamous groups of Maharashtra. Man India. 1979;59:71–90. [PubMed] [Google Scholar]
- 17.Kar RK, Mahanta S. Menarche and menopause among the women of Arunachal Pradesh. Indian J Phys Anthro Hum Genet. 1975;1:51–7. [Google Scholar]
- 18.Balgir RS. Age at menarche and menopause among the Sikligars of Punjab. J Indian Med Assoc. 1985;83:195–7. [PubMed] [Google Scholar]
- 19.Sharma N, Singh R. Age at menarche and menopause of Brahmins and Choudhary females of Kangra valley. Proceedings of International Symposium on Human Growth. Patiala India. 1980 [Google Scholar]
- 20.Gosh AK, Kumari S. Effect of menarcheal age on fertility. J Indian Anthropol Soc. 1973;8:165–72. [Google Scholar]
- 21.Singh A, Arora AK. Prole of menopausal women in rural North India. Climacteric. 2005;8:177–84. doi: 10.1080/13697130500117920. [DOI] [PubMed] [Google Scholar]
- 22.Shah R, Kalgutkar S, Savardekar L, Chitlange S, Iddya U, Baliah D. Menopausal symptoms in urban Indian women. Obstet Gynaecol Today. 2004;IX:667–70. [Google Scholar]
- 23.Bagga A. Age and symptomatology of menopause: A case study. Obstet Gynaecol Today. 2004;11:660–6. [Google Scholar]
- 24.Rakshit S. Reproductive life of Maharashtrian Brahmin women. Man India. 1962;56:65–70. [Google Scholar]
- 25.Sengupta S, Rjkhowa M. Menarche and menopause in Ahom women of Dibrogarh in Assam. J Hum Ecol. 1996;7:211–3. [Google Scholar]
- 26.Kaw D, Khanna B, Vasishtha K. Factors that influence the age at natural menopause. J Obstet Gynaecol India. 1994;44:273–7. [Google Scholar]
- 27.Mastana SS. Age at menopause among the Lobanas of North West India. J Hum Ecol. 1996;7:151–3. [Google Scholar]
- 28.Kriplani A, Banerjee K. An overview of age of onset of menopause in Northern India. Maturitas. 2005;52:199–204. doi: 10.1016/j.maturitas.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Shatrugna V, Kulkarni B, Kumar PA, Rani KU, Balakrishna N. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827–35. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A. Osteoporosis in India the nutritional hypothesis. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Lucknow, UP, India: Hindustani Book Depot; 1998. p. 115. [Google Scholar]
- 31.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer: GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide. 2012. [Last accessed on 2020 Jul 10]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx .
- 34.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 35.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 36.Smith GD, Morris JN, Shaw M. Acheson Report: The independent inquiry into inequalities in health. Is welcome, but its recommendations are too cautious and vague. BMJ. 1998;317:1465–6. doi: 10.1136/bmj.317.7171.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nangia S, Arya V, Gujral RB. Spinal bone mineral density in normal Indian females. Presented at 27th Annual Meeting of the Endocrine Society of India. Lucknow, Uttar Pradesh, India. 1997 [Google Scholar]
- 38.Singh M, Magon N, Singh T. Major and minor discordance in the diagnosis of postmenopausal osteoporosis among Indian women using hip and spine dual-energy X-ray absorptiometry. J Midlife Health. 2012;3:76–80. doi: 10.4103/0976-7800.104457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unni J, Garg R, Pawar R. Bone mineral density in women above 40 years. J Midlife Health. 2010;1:19–22. doi: 10.4103/0976-7800.66989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patni R. Normal BMD values for Indian females aged 20–80. J Mid-life Health. 2010;2:70–3. doi: 10.4103/0976-7800.76215. Aoki TT, Grecu EO, Srinivas PR, Prescott P, Benbarka M, Arcangeli MM. Prevalence of osteoporosis in women: Variation with skeletal site of measurement of bone mineral density. Endocr Pract 2000;6:127-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy PG, Mithal A, Rao DS. Bone mineral density in healthy Asian Indian women: Development of a reference database and implications for diagnosis of osteoporosis in Indian women living in the United States. J Bone Miner Res. 2002;17(Suppl 1):SA270. [Google Scholar]
- 42.Paul TV, Thomas N, Seshadri MS, Oommen R, Jose A, Mahendri NV. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: Relationship to calcium nutrition and Vitamin D status. Endocr Pract. 2008;14:665–71. doi: 10.4158/EP.14.6.665. [DOI] [PubMed] [Google Scholar]
- 43.Notelovitz M. Individualizing Hormone Therapy: Principles and Practice. [Last accessed on 2020 Jun 10]. Available from: https://www.medscape.org/viewarticle/412853 .
- 44.Lakshmi RM, Kusumalatha K, Shraddha S. Analysis of 200 perimenopausal women: A Prospective Study, 12th National Indian Menopause Society Meeting, Rajkot. 2007:25. [Google Scholar]
- 45.CDC. US medical eligibility criteria for contraception use. MMWR More Mortal Wkl Rep. 2010;59:1–88. [Google Scholar]
- 46.Kulkarni S. Fred Arnold-Family Planning; International Institute for Population Sciences (IIPS). National Family Health Survey (NFSH-2); 1998-99. 2000. [Last accessed on 2020 Jul 05]. pp. 85–7. Available from https://www.dhsprogram.com/pubs/pdf/FRIND2/FRIND2.pdf .
- 47.Haines CJ, Ludicke F. Contraception in the late perimenopause. Proceedings of the First Consensus Meeting on Menopause in the East Asian Region. Geneva. 1997. [Last accessed on 2020 Jul 05]. p. 55. Available from: http://www.gfmer.ch/Books/bookmp/52.htm .
- 48.Rosenblatt KA, Thomas DB. Hormonal content of combined oral contraceptives in relation to the reduced risk of endometrial carcinoma. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Cancer. 1991;49:870–4. doi: 10.1002/ijc.2910490612. [DOI] [PubMed] [Google Scholar]
- 49.Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, et al. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. SHARE Study Group. Steroid Hormones and Reproductions. Am J Epidemiol. 2000;152:233–41. doi: 10.1093/aje/152.3.233. [DOI] [PubMed] [Google Scholar]
- 50.ACOG technical bulletin. Health maintenance for perimenopausal women. Number 210--August 1995. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1995;51:171–81. [PubMed] [Google Scholar]
- 51.Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101–4. doi: 10.1016/s0015-0282(16)58394-0. [DOI] [PubMed] [Google Scholar]
- 52.Munro MG, Critchley HO, Broder MS, Fraser IS FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein SR, Lumsden MA. Abnormal uterine bleeding in perimenopause. Climacteric. 2017;20:414–20. doi: 10.1080/13697137.2017.1358921. [DOI] [PubMed] [Google Scholar]
- 54.van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: A systematic review and meta-analysis. BJOG. 2007;114:664–75. doi: 10.1111/j.1471-0528.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 55.Gou J, Li ZY. Accuracy of endometrial biopsy by Pipelle: A systematic review and meta-analysis. Ann Oncol. 2019;30:IX84–5. [Google Scholar]
- 56.van Hanegem N, Breijer MC, Khan KS, Clark TJ, Burger MP, Mol BW, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: An evidence-based approach. Maturitas. 2011;68:155–64. doi: 10.1016/j.maturitas.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Astrup K, Olivarius Nde F. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83:203–7. doi: 10.1111/j.0001-6349.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 58.Smith PP, O'Connor S, Gupta J, Clark TJ. Recurrent postmenopausal bleeding: A prospective cohort study. J Minim Invasive Gynecol. 2014;21:799. doi: 10.1016/j.jmig.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Dowdy SC, Mariani A, Lurain JR. Uterine cancer. In: Berek JS, editor. Berek & Novak's Gynecology. 15th ed. Baltimore: Lippincott, Williams & Wilkins; 2011. pp. 1250–3. [Google Scholar]
- 60.Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick. When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding? Ultrasound Obstet Gynecol. 2004;24:558. doi: 10.1002/uog.1704. [DOI] [PubMed] [Google Scholar]
- 61.Timmermans A, Opmeer BC, Khalid KS, Bachmann LM, Epstein E, T Justin Clark TJ, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:160–167. doi: 10.1097/AOG.0b013e3181e3e7e8. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. Measurement of Quality of Life in Children. Geneva: Division of Mental Health. World Health Organization; 1993. [Google Scholar]
- 63.Kaur S, Walia I, Singh A. How menopause affects the lives of women in suburban Chandigarh, India. Climacteric. 2004;7:175–80. doi: 10.1080/13697130410001713779. [DOI] [PubMed] [Google Scholar]
- 64.Bhattacharya SM. Effects of tibolone on health-related quality of life in menopausal women. Int J Gynaecol Obstet. 2007;99:43–5. doi: 10.1016/j.ijgo.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Bipasa S. Diversity of menopausal symptoms in urban women of West Bengal, 11th National Indian Menopause Society Meeting, Chandigarh, Souvenir. 2003:203. [Google Scholar]
- 66.Gupta P, Sturdee DW, Hunter MS. Mid-age health in women from the Indian subcontinent (MAHWIS): General health and the experience of menopause in women. Climacteric. 2006;9:13–22. doi: 10.1080/13697130500515776. [DOI] [PubMed] [Google Scholar]
- 67.Ha ZI, Liu J, Eden J. A quantitative analysis of the menopause experience of Indian women living in Sydney. Aust N Z J Obstet Gynaecol. 2007;47:329–34. doi: 10.1111/j.1479-828X.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- 68.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 69.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D.P. Villaseca & as the Writing Group of the International Menopause Society for World Menopause Day 2012 (2012) Understanding weight gain at menopause. Climacteric. 2015;5:419–429. doi: 10.3109/13697137.2012.707385. DOI: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 70.Brincat Y, Baron M, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–23. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal M, Sinha R, Gupta N, Goel P. Faridabad, Souvenir: 17th National Indian Menopause Society; Study of Urogenital Complaints in Postmenopausal in Women over 45 yrs in Safdarjung Hospital. 2012:48. [Google Scholar]
- 72.Majumdar S, Shah JM, Aggarwal N, Sharma S. Olayi RAttitude towards sexuality in the perimenopausal and postmenopausal women in India 2014;121. Issue s2 Special Issue:Abstracts of the RCOG World Congress [Google Scholar]
- 73.Shaji KS, Jotheeswaran AT, Girish N, Bharath S, Dias A, Pattabiraman M, et al. Alzheimer's and Related Disorders Society of India. The Dementia India Report: Prevalence, Impact, Costs and Services for Dementia. 2010 [Google Scholar]
- 74.Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res. 2010;131:302–10. [PubMed] [Google Scholar]
- 75.Chopra A, Patil J, Billempelly V, Relwani J, Tandle HS WHO-ILAR COPCORD Study. WHO International League of Associations from Rheumatology Community Oriented Program from Control of Rheumatic Diseases. Prevalence of rheumatic diseases in a rural population in western India: A WHO-ILAR COPCORD Study. J Assoc Physicians India. 2001;49:240–6. [PubMed] [Google Scholar]
- 76.Mahajan A, Jasrotia DS, Manhas AS, Jamwal SS. Prevalence of Major Rheumatic Disorders in Jammu. JK Science. 2003;5:62–6. [Google Scholar]
- 77.Pal CP, Singh P, Chaturvedi S, Pruthi KK, Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016;50:518–22. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandey S, Srinivas M, Agashe S, Joshi J, Gavankar P, Prakasam CP, et al. Menopause and metabolic syndrome: A study of 498 urban women from Western India. J Midlife Health. 2010;1:63–9. doi: 10.4103/0976-7800.76214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 80.Public Health Foundation of India (PHFI), Evidence-Based Diabetes Management. New Delhi. Dr. Mohan's Education Academy, Chennai. 2010:31. [Google Scholar]
- 81.Ghazanfari Z, Haghdoost AA. A comparison of HbA1c and fasting blood sugar tests in general population. Int J Preve Med. 2010;1:187–94. [PMC free article] [PubMed] [Google Scholar]
- 82.Shringi MS, Vaidya RA, Joshi JV. Subclinical hypothyroidism in perimenopausal-A study of 648 women in Maitreyi's Healthcare Programme. Obstet Gynecol Today. 2004;9:671. [Google Scholar]
- 83.Abhishek P, Partha H. Prevalence of anemia among elderly persons residing in old age homes in National Capital Territory, Delhi, India. Indian J Public Health. 2019;63:288–92. doi: 10.4103/ijph.IJPH_412_18. [DOI] [PubMed] [Google Scholar]
- 84.Dandona L, Dandona R, Srinivas M, Giridhar P, Vilas K, Prasad MN, et al. Blindness in the Indian state of Andhra Pradesh. Invest Ophthalmol Vis Sci. 2001;42:908–16. [PubMed] [Google Scholar]
- 85.National Programme for Control of Blindness and Visual Impairment (NPCB&VI), Directorate General of Health Services Ministry of Health & Family Welfare, Government of India. 2017. [Last accessed on 2020 Jul 05]. Available from: https://dghs.gov.in/content/1354_3_NationalProgrammeforControlofBlindnessVisual.aspx .
- 86.Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: A nationally representative survey. Lancet. 2012;379:1807–16. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 87.Ferlay JS, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care (Basel) 2008;3:21–7. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal G, Ramakant P, Forgach ER, Rendon JC, Chaparro JM, Basurto CS, et al. Breast cancer care in developing countries. World J Surg. 2009;33:2069–76. doi: 10.1007/s00268-009-0150-z. [DOI] [PubMed] [Google Scholar]
- 90.National cancer registry programme. Three-year report of population based cancer registries 2012-2014. WWW page. 2016. [Last accessed 2018 July 19]. Available from: http://ncdirindia.org .
- 91.National Centre for Disease Informatics and Research, National Cancer Registry Programme, Indian Council of Medical Research (ICMR). Time Trends in Cancer Incidence Rates 1982-2010. 2013. [Last accessed on 2016 Aug 10]. Available from: www.icmr.nic.in/ncrp/trend%20report%201982_2010/ALL_PDF/Preliminary_Pages.pdf .
- 92.Sandhu GS, Erqou S, Patterson H, Mathew A. Prevalence of Triple-Negative Breast Cancer in India: Systematic Review and Meta-Analysis? J Glob Oncol. 2016;2:412–421. doi: 10.1200/JGO.2016.005397. doi:10.1200/JGO.2016.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008? Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. doi:10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 94.HighWire D, Klarenbach S, Sims JN, NLewin G, Singh, Harminder Thériault GT. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Canadian Medical Association Journal. 2018 doi: 10.1503/cmaj.180463. 2018-12-1000:00:00E1441-E145110.1503/cmaj.1804631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vallikad E. Cervical cancer: The Indian perspective. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S215–33. doi: 10.1016/S0020-7292(06)60037-4. [DOI] [PubMed] [Google Scholar]
- 96.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: A cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 97.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 98.Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, et al. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. 2003;106:404–8. doi: 10.1002/ijc.11245. [DOI] [PubMed] [Google Scholar]
- 99.World Health Survey. Health System Performance Assessment: World Health Survey. 2003. [Last accessed 2016 Aug 19]. Available from: http://www.who.int/healthinfo/survey/whs_hspa_book.pdf .
- 100.Sankaranarayanan R, Shastri SS, Basu P, Mahé C, Mandal R, Amin G, et al. Role of low-level magnification in visual inspection with acetic acid for the early detection of cervical neoplasia. Cancer Detect Prev. 2004;28:345–51. doi: 10.1016/j.cdp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89(Suppl 2):S4–12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 102.Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, et al. Pooled analysis of the accuracy of ve cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–60. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 103.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in ve developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 104.Legood R, Gray AM, Mahé C, Wolstenholme J, Jayant K, Nene BM, et al. Screening for cervical cancer in India: How much will it cost. A trial based analysis of the cost per case detected? Int J Cancer. 2005;117:981–7. doi: 10.1002/ijc.21220. [DOI] [PubMed] [Google Scholar]
- 105.Usha Rani P, Meeta M, Batool L, Anjaneyulu V, Murthy Ramana Reddy CH. Evaluation of cervical smears by the Betesda system for cervical screening in postmenopausal women. 9th National Indian Menopause Society Meeting, Kolkata, Souvenir. 2008 [Google Scholar]
- 106.WHO; Weekly Epidemiological Record 2017, 92, 241-268 No 19. [Last accessed on 2020 Jun 01]. Available from: http://www.who.int/wer .
- 107.Shridhar K, Dey S, Bhan CM, Bumb D, Govil J, Dhillon PK, et al. Cancer detection rates in a population-based, opportunistic screening model, new delhi, India. Asian Pac J Cancer Prev. 2015;16:1953–8. doi: 10.7314/apjcp.2015.16.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cawthorn S, Hamed H, Cuzick J, Forbes JF, Sestak I, Holli K. Long-term results of tamoxifen prophylaxis for breast cancer-96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 109.Watson P, Vasen HF, Mecklin JP, Järvinen H, Lynch HT. The risk of endometrial cancer in hereditary nonpolyposis colorectal cancer? Am J Med. 1994;96:516–520. doi: 10.1016/0002-9343(94)90091-4. doi:10.1016/0002-9343(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 110.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a metaanalysis. Obstet Gynecol. 1995;85:304–313. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 111.PDQ Screening and Prevention Editorial Board. Endometrial Cancer Screening (PDQ®): Health Professional Version. 2020 Feb 27. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. [Last accessed on 2019 Aug 12]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65786/ [Google Scholar]
- 112.Emons G, Beckmann MW, Schmidt D, Mallmann P. Uterus commission of the Gynecological Oncology Working Group (AGO) (2015). New WHO classification of endometrial hyperplasias. Geburtshilfe Frauenheilkunde. 2015;75:135–6. doi: 10.1055/s-0034-1396256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mutter GL. Endometrial intraepithelial neoplasia (EIN): Will it bring order to chaos. The Endometrial Collaborative Group. Gynecol Oncol. 2000;76:287. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 114.RCOG/BSGE Management of Endometrial Hyperplasia, Joint Guideline; Feb. 2016 [Google Scholar]
- 115.Vetter MH, Smith B, Benedict J, Hade EM, Bixel K, Copeland LJ, et al. Preoperative predictors of endometrial cancer at time of hysterectomy for endometrial intraepithelial neoplasia or complex atypical hyperplasia. Am J Obstet Gynecol. 2020;222:60.e1. doi: 10.1016/j.ajog.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calonge N. U.S. Preventive Services Task Force. Screening for ovarian cancer, recommendation. Ann Fam Med. 2004;2:260–2. doi: 10.1370/afm.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markopoulos MC, Kassi E. Hyperandrogenism after menopause: Review. Eur J Endocrinol 2015;172:R79-91. Hormone replacement therapy and the risk of endometrial cancer: A systematic review. Maturitas. 2016;91:25–35. doi: 10.1016/j.maturitas.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 118.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 119.Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol Endocrinol. 2020;36:12–23. doi: 10.1080/09513590.2019.1650337. [DOI] [PubMed] [Google Scholar]
- 120.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, Bie BD, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod. 2014;29:400–12. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 121.Gemmell LC, Webster KE, Kirtley S, Vincent K, Zondervan KT, Becker CM. The management of menopause in women with a history of endometriosis: A systematic review. Hum Reprod Update. 2017;23:481–500. doi: 10.1093/humupd/dmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause? Cochrane Database Syst Rev. 2009:CD005997. doi: 10.1002/14651858.CD005997.pub2. Published 2009 Jan 21. doi:10.1002/14651858.CD005997.pub2. [DOI] [PubMed] [Google Scholar]
- 123.Pritts EA, Vanness DJ, Berek JS, Parker W, Feinberg R, Feinberg J, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: A meta-analysis. Gynecol Surg. 2015;12:165–77. doi: 10.1007/s10397-015-0894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106:1588–99. doi: 10.1016/j.fertnstert.2016.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sarrel PM, Sullivan SD, Nelson LM. Hormone replacement therapy in young women with surgical primary ovarian insufficiency. Fertil Steril. 2016;106:1580–7. doi: 10.1016/j.fertnstert.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gazarra LBC, Bonacordi CL, Yela DA, Benetti-Pinto CL. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020 Jun; doi: 10.1097/GME.0000000000001592. doi:10.1097/gme.0000000000001592. PMID: 32576798. [DOI] [PubMed] [Google Scholar]
- 127.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences? Maturitas. 2010;65:161–6. doi: 10.1016/j.maturitas.2009.08.003. doi:10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Honigberg MC, Zekavat SM, Aragam K, Phoebe Finneran, Derek Klarin, Deepak L Bhatt, et al. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease [published online ahead of print, 2019 Nov 18] JAMA. 2019 doi: 10.1001/jama.2019.19191. 10.1001/jama.2019.19191. doi:10.1001/jama.2019.19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu D, Chung HF, Dobson AJ, Nirmala Pandeya, Eric J Brunner, Diana Kuh, et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies [published online ahead of print, 2020 Jun 20] Hum Reprod. 2020:deaa124. doi: 10.1093/humrep/deaa124. doi:10.1093/humrep/deaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril. 1987;47(1):94–100. doi: 10.1016/s0015-0282(16)49942-5. doi:10.1016/s0015-0282(16)49942-5. [DOI] [PubMed] [Google Scholar]
- 131.Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118:1271–9. doi: 10.1097/AOG.0b013e318236fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bipasa S. First Time Detection of Medical Disorders in Forty-Plus Women in Health Camps. 15th National Indian Menopause Society Meeting, Chennai. 2010:68. [Google Scholar]
- 133.Santhakumar K, Nivedita Bharati K, Jaishree Gajraj A. Routine Investigations in Women 40 Years and Above. 15th National Indian Menopause Society Meeting, Chennai, Souvenir. 2010:69. [Google Scholar]
- 134.Ahuja M. Age of menopause and determinants of menopause age: A PAN India survey by IMS. J Midlife Health. 2016;7:126–31. doi: 10.4103/0976-7800.191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hina K, Manju T, Mehandale SS, Wagh GN. Midlife management clinic for screening of metabolic disorders and osteoporsis in women aged above 35 yrs. 17th National Indian Menopause Society. Faridabad, Souvenir. 2012:45. [Google Scholar]
- 136.Nimala V. Counselling 12th women: Is it worth the time? National Indian Menopause Society Meeting. Rajkot, Souvenir. 2007:34. [Google Scholar]
- 137.Shitole AD. Counselling: A basic management of perimenopause and menopause period. 17th National Indian Menopause Society Meeting, Faridabad, Souvenir. 2012:35. [Google Scholar]
- 138.Kakkar V, Arshdeep K, Darshanjot K. Combined effect of drug therapy and counselling in the relief of menopause symptoms, 11th National Indian Menopause Society Meeting, Chandigarh, Souvenir. 2003:209. [Google Scholar]
- 139.Calman KC, Royston G. Personal paper: Risk language and dialects. Br Med J. 1997;315:939–42. doi: 10.1136/bmj.315.7113.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dietary Guidelines for Indians - A Manual. Hyderabad: National Institute of Nutrition, ICMR; 2010. [Google Scholar]
- 141.Expert Group of the Association of Physicians of India on Adult Immunization in India. Association of Physicians of India evidence-based clinical practice guidelines on adult immunization. J Assoc Phys India. 2009;57:345–56. [PubMed] [Google Scholar]
- 142.Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317–25. doi: 10.7326/0003-4819-145-5-200609050-00004. [DOI] [PubMed] [Google Scholar]
- 143.Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: A randomized clinical trial. JAMA Intern Med. 2014;174:1058–66. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haque R, Shi J, Schottinger JE, Syed A, Craig Cheetham T, Joanie Chung, et al. Tamoxifen and Antidepressant Drug Interaction in a Cohort of 16,887 Breast Cancer Survivors. J Natl Cancer Inst. 2015;108(3):djv337. doi: 10.1093/jnci/djv337. Published 2015. doi:10.1093/jnci/djv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laxmi S. Clinical effects of soya our on menopausal symptoms. 12th National Indian Menopause Society Meeting, Rajkot, Souvenir. 2007:24. [Google Scholar]
- 146.Meeta S. Risk analysis of cardiovascular disease and osteoporosis in Indian menopausal women and its relationship with blood lycopene levels. 17th National Indian Menopause Society, Faridabad, Souvenir. 2012:39. [Google Scholar]
- 147.Meeta M, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical Practice Guidelines on Menopause: Indian Menopause Society. 2020 doi: 10.4103/jmh.JMH_137_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.National Institute for Health and Care Excellence, Menopause, Clinical Guideline; June. 2015 [Google Scholar]
- 149.Baber RJ, Panay NA. Fenton the IMS Writing Group.2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016;2:109–50. doi: 10.3109/13697137.2015.1129166. [DOI] [PubMed] [Google Scholar]
- 150.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728–753. doi: 10.1097/GME.0000000000000921. doi:10.1097/GME.0000000000000921q. [DOI] [PubMed] [Google Scholar]
- 151.Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K. Association of Menopausal Hormone Therapy With Breast Cancer; Incidence and Mortality During Long-term Follow-up of the Women's Health Initiative Randomized Clinical Trials. JAMA. 2020;324:369–80. doi: 10.1001/jama.2020.9482. doi:10.1001/jama.2020.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chaudhuri P, Sanhati Healthcare in India: Features of One of the Most Privatised Systems in the World. 2009. [Last accessed on 2020 Jun 10]. Available from: http://www.sanhati.com/excerpted/1759/
- 153.Meeta M, Tandon V. Evidence based clinical practice guidelines on menopause and postmenopausal osteoporosis (2019–2020): A step towards implementation of menopausal medicine. J Mid-life Health. 2020;11:51–2. doi: 10.4103/jmh.JMH_136_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heinemann LA, Potthoff P, Schneider HP. International versions of the Menopause Rating Scale (MRS) Health Qual Life Outcomes. 2003;1:28. doi: 10.1186/1477-7525-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Breast Cancer Risk Assessment Tool, National Cancer Institute, US Dept of Health Services. [Last accessed on 2016 Feb 01]. Available from https://bcrisktool.cancer.gov/https://bcrisktool.cancer.gov/
- 156.Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, Lim S, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25(8):1578–82. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 157.Koh LK, Sedrine WB, Torralba TP, Kung A, Fujiwara S, Chan SP. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 158.Latt TS, Than Than Aye, Ko Ko, Thein Myint, Ni Ni Hlaing, Myint Thaung. Tet Tun Chit Myanmar Clinical Practice Guideline for Osteoporosis. Journal of the Asian Federation of Endocrine Societies. 2002;27:151–155. DOI: 10.15605/jafes.027.02.03. [Google Scholar]
- 159.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]


