Abstract
Although diagnosed on the basis of deficits in social communication and interaction, autism spectrum disorder (ASD) is also characterized by superior performance on a variety of visuo-spatial tasks, including visual search. In neurotypical individuals, region-specific concentrations of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) are associated with individual differences in attention and perception. While it has been hypothesized that ASD may be associated with an excitatory-inhibitory imbalance, it remains unclear how this may contribute to accelerated visual search performance in individuals with ASD. To investigate this, 21 children with ASD and 20 typically developing (TD) children participated in a visual search task (VST) and a magnetic resonance spectroscopy (MRS) study to detect neurochemical concentrations, including GABA. Region-specific neurochemicals were examined in the right frontal eye fields (rFEF), right temporal parietal junction (rTPJ), and bilateral visual cortex (VIS). GABA concentrations did not differ between groups; however, in children with ASD, greater GABA concentration in the visual cortex was related to more efficient search. Additionally, lower VIS GABA levels were also associated with increased social impairment. Finally, we found reduced NAA, tCr, Glx, GABA/Glx in the rTPJ, suggestive of neuronal dysfunction in a critical network hub. Our results show that GABA concentrations in the visual cortex are related to efficient search in ASD, thus providing further evidence of enhanced discrimination in ASD.
Lay Abstract
Children with autism spectrum disorder (ASD) often perform better than their non-ASD peers on visual search tasks, however, it is unclear how they achieve this superior performance. Using magnetic resonance spectroscopy (MRS) to measure neurochemicals in the brain, we found the level of one, GABA, in the visual cortex was directly related to search abilities in children with ASD. These results suggest that faster search may relate to enhanced perceptual functioning in children with ASD.
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder diagnosed based on impairments in social communication and interaction as well as the presence of restricted and repetitive behaviors (APA, 2013). However, differences in non-social attentional functions – including both attentional strengths and weaknesses – have been identified as among the earliest features that distinguish infants likely to develop ASD (Elsabbagh et al., 2013; Gliga et al., 2015). One particular strength, performance on visual search tasks, which require participants to locate a target item embedded within an array of distractors, has been shown in infants at elevated risk for ASD (i.e., infants with an older sibling with ASD; Gliga et al., 2015), as well as toddlers, school-aged children, adolescents, and adults diagnosed with ASD (Kaldy, Giserman, Carter, & Blaser, 2016). Furthermore, faster, more efficient search abilities are associated with higher levels of ASD symptomatology (Gliga et al., 2015; Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009; Keehn & Joseph, 2016; Keehn, Shih, Brenner, Townsend, & Müller, 2013), suggesting that non-social attentional processes, and the differences in brain function that these behaviors reflect, may be of etiological significance in the development of ASD.
However, the precise mechanism(s) underlying enhanced visual search abilities in ASD remain unknown. That individuals with ASD evidence relatively greater search advantage compared to their typically developing (TD) peers with increasing target-distractor similarity has been used to support the hypothesis that enhanced discrimination contributes to faster search (O’Riordan & Plaisted, 2001), which is consistent with enhanced perceptual functioning in ASD (Mottron, Dawson, Soulières, Hubert, & Burack, 2006). Alternatively, faster search may be due to more focused attention rather than enhanced lower-level perceptual processing (Blaser, Eglington, Carter, & Kaldy, 2014; Kaldy et al., 2016). Lastly, evidence of increased perceptual capacity in ASD (Remington, Swettenham, Campbell, & Coleman, 2009; Remington, Swettenham, & Lavie, 2012) may facilitate enhanced peripheral selection, and, thus, faster target detection in ASD (Milne, Dunn, Freeth, & Rosas-Martinez, 2013). Thus, while evidence of superior visual search in ASD has been shown across the lifespan using a variety of experimental paradigms, there is no consensus about the attentional and/or perceptual processes that may contribute to accelerated search in ASD.
In neurotypical individuals, inter-individual differences in attention and perception are associated with region-specific concentrations of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) (e.g., Edden, Muthukumaraswamy, Freeman, & Singh, 2009; Sumner, Edden, Bompas, Evans, & Singh, 2010). For example, Sumner and colleagues (2010) showed that greater GABA levels in the frontal eye fields (rFEF) (but not the visual cortex) were associated with reduced distractor costs in a pro-saccade task. Others have also demonstrated that general failures of selective attention are related to cortical GABA levels (Sandberg et al., 2014). Furthermore, prior research has also shown that sensory perception thresholds are associated with region-specific GABA concentrations (e.g., orientation threshold-visual cortex: Edden et al., 2009; tactile perception-somatosensory cortex: Puts et al., 2017). Within the visual domain, occipital and parietal GABA levels uniquely influence perception of different visual features (orientation and size, respectively) (Song, Sandberg, Andersen, Blicher, & Rees, 2017). Together, these findings indicate that cortical GABA levels, which vary across distinct areas and between individuals, are associated with a variety of attentional and perceptual processes.
One model has hypothesized that ASD may result, in part, from atypically increased cortical excitation due to an imbalance of glutamatergic (excitatory) and GABAergic (inhibitory) signaling (Hussman, 2001; Rubenstein & Merzenich, 2003). Magnetic resonance spectroscopy (MRS) has been used successfully to measure neurochemicals in vivo, including N-acetyl aspartate (NAA), total Creatine (tCr), total Choline (tCho), myo-inositol (mI), with a particular focus on glutamate & glutamine (Glx) and GABA, where reliable GABA measurements requires edited MRS techniques. To date, these studies have provided evidence of equivalent GABA levels compared to TD individuals in the visual cortex (Gaetz et al., 2014; Robertson, Ratai, & Kanwisher, 2016), striatum (Harada et al., 2011; Horder et al., 2018), and medial prefrontal cortex (Horder et al., 2018), as well as lower GABA concentrations in the sensorimotor cortex (Puts et al., 2017; Sapey-Triomphe, Lamberton, Sonié, Mattout, & Schmitz, 2019), frontal lobe (Harada et al., 2011; Kubas et al., 2012), and auditory cortex (Gaetz et al., 2014; Port et al., 2017; Rojas, Singel, Steinmetz, Hepburn, & Brown, 2014). While there are several methodological differences between these studies, they suggest that discrete, region-specific differences in GABA may be present in individuals with ASD.
The objective of the present study was to use MRS to examine region-specific differences in GABA concentrations in three areas of interest, and to examine the relationship between GABA levels and visual search performance in ASD. These included two key nodes of dorsal and ventral attentional networks (Corbetta, Patel, & Shulman, 2008), the right frontal eye fields (rFEF) and the right temporal-parietal junction (rTPJ), which play an important role in visual search (Shulman, Astafiev, McAvoy, D’Avossa, & Corbetta, 2007; Shulman et al., 2003). Further, as discussed, greater GABA levels in the rFEF have been shown to be associated with increased filtering of distracting information (Sumner et al., 2010). Thus, if the ASD search advantage is related to differences in top-down, attentional filtering, faster performance will be associated with GABA levels in the rFEF. Alternatively, atypical activation and connectivity of the rTPJ in ASD has been shown during visual search (Keehn, Brenner, Palmer, Lincoln, & Müller, 2008; Keehn et al., 2013). If enhanced search is associated with differences in the re-orienting of attention to behaviorally relevant information (i.e., the target), then search metrics may be associated with rTPJ GABA concentrations. Finally, visual cortex (VIS) was chosen to determine whether superior search in ASD is associated with enhanced discrimination (O’Riordan & Plaisted, 2001), as occipital GABA levels are associated with orientation perceptual thresholds (Edden et al., 2009; Song et al., 2017). Finally, because others have hypothesized that an E/I imbalance may contribute to the development of ASD (Hussman, 2001; Rubenstein & Merzenich, 2003), and prior findings have shown an association between GABA and ASD symptom severity (Brix et al., 2015; Carvalho Pereira, Violante, Mouga, Oliveira, & Castelo-Branco, 2018) we examined the relationship between GABA levels and measures of ASD symptomatology.
Methods
Participants
Participants included 21 children with ASD and 20 age-, IQ-, sex-matched TD children (see Table 1). Clinical diagnoses were confirmed using the Autism Diagnostic Observation Schedule, Second Edition, (ADOS-2; Lord, Luyster, Gotham, & Guthrie, 2012), Social Communication Questionnaire (SCQ, Rutter, Bailey, & Lord, 2003), and expert clinical judgment according to DSM-5 criteria (RMK). Children with ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) were excluded. Participants in the TD group had no reported family history of ASD and were confirmed via parent report to be free of any other neurological or psychiatric conditions or clinically significant ASD symptoms as measured by the Social Responsiveness Scale (SRS-2; Constantino & Gruber, 2012) and the SCQ. Informed assent and consent were obtained from all participants and their caregivers in accordance with the Purdue University Institutional Review Board.
Table 1. Participant demographics.
Mean (SD); Range unless otherwise specified
| ASD | TD | p-value | ||
|---|---|---|---|---|
| N (females) | 21 (4) | 20 (4) | ||
| Age (Years) | 11.8 (1.2); 9.8 – 14.5 | 11.3 (1.7); 9.0 – 15.0 | 0.32 | |
| IQ | FSIQ | 104 (16); 76 – 132 | 112 (11); 98 – 133 | 0.07 |
| VIQ | 104 (18); 75 – 154 | 111 (11); 97 – 127 | 0.17 | |
| NVIQ | 103 (18); 59 – 132 | 110 (12); 94 – 134 | 0.13 | |
| SRS-2 (t-scores) | Total | 75.7 (10.9); 58 – 90 | 43.3 (4.7); 37 – 55 | <0.0001 |
| Social Communication and Interaction | 75.3 (10.8); 58 – 90 | 42.9 (4.3); 36 – 53 | <0.0001 | |
| Restricted and Repetitive Behavior | 74.8 (11.8); 50 – 90 | 45.6 (5.6); 41 – 59 | <0.0001 | |
| ADOS-2 | Social Affect | 10.7 (3.8); 5 – 17 | -- | |
| Restricted and Repetitive Behavior | 2.2 (1.4); 0 – 5 | -- | ||
| Total | 12.9 (4.4); 7 – 21 | -- | ||
| Comparison score | 7.3 (2.1); 4 – 10 | -- | ||
Visual Search Task
The experiment was presented using SR Research Experiment Builder 2.1 on a 19-inch LCD monitor. Participants were seated approximately 60cm from the display. Manual responses were registered using a Cedrus response pad (RB-740).
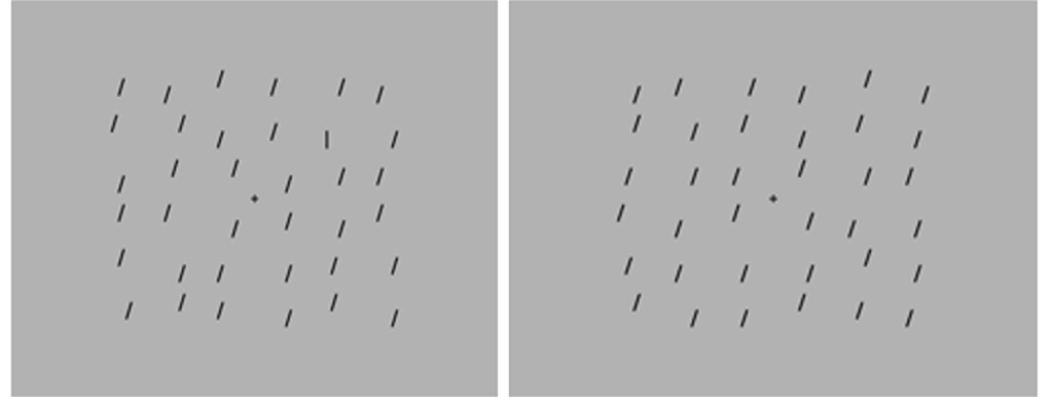
The procedure and stimuli were similar to previous studies, which have reported superior search in ASD (Hessels, Hooge, Snijders, & Kemner, 2014; Kemner, Van Ewijk, Van Engeland, & Hooge, 2008; O’Riordan & Plaisted, 2001). The target was a vertical line and the distractors were oblique lines (tilted 10°; Figure 1). At a viewing distance of 60cm, lines had a length of approximately 1.2° visual angle. Target and distractors were drawn in black on a gray background. Stimuli were randomly positioned on a 6 × 6 array of 1.1º x 1.1º squares. The size of the entire search array was 18.6° by 15.7°. Items were randomly positioned within each square and across the array to produce layout irregularity.
Figure 1. Example of visual search task (VST) for target present.
(left) and target absent (right) conditions (set size 36).
The VST was divided into four blocks, each consisting of 36 trials. Within each block, the target was present on 50% of trials, and target presence (present, absent) and set size (18, 24, 36) were varied in pseudorandom order.
A trial began with a fixation cross (“+”) presented alone for 1000ms. Next, with the fixation cross remaining on the screen, the stimulus array was presented until a response was made or until 7000ms had elapsed. Children responded with their dominant hand via a two-choice button box on which one button represented target present and the other target absent. The examiner first modeled the task and then participants completed a block of 12 practice trials. Participants were instructed to respond as quickly as possible without making errors. Reaction time (RT) was recorded and the median RT for correct trials at each set size was used to measure the slope and y-intercept of the RT x set size function for target present and absent conditions for each participant. Search efficiency is indexed by slope, and reflects the cost of each additional distractor, whereas the y-intercept of the RT x set size function is the RT that would theoretically be observed if search were eliminated from the task.
Social Responsiveness Scale, Second Edition (SRS-2).
The SRS-2 (Constantino & Gruber, 2012) is a 65-item caregiver-report questionnaire used to measure ASD symptomatology in children from 4 to 18 years of age, across the domains of Social Awareness, Social Cognition, Social Communication, Social Motivation, and Restricted Interests and Repetitive Behavior subscales. Social subscales (awareness, cognition, communication, and motivation) are combined to form the Social Communication and Interaction (SCI) scale. The SCI and Restricted and Repetitive Behavior (RRB) DSM-5 Compatible scales and the Total score t-scores were used for the correlational analyses.
Magnetic Resonance Imaging (MRI) and Spectroscopy (MRS)
MRI and MRS were performed on a 3T Prisma Siemens scanner with a 64-channel head coil separately from the VST (MRS and VST visits occurred no longer than 3 months apart; average duration between visits was 17 days). To minimize movement, participants’ heads were stabilized with foam padding and they were instructed to remain as still as possible and watch a video of their choice for the duration of the scan. A high-resolution T1-weighted image (MPRAGE, TE = 4.91, TR = 2000, TI = 977, FA = 9) was taken for MRS voxel placement and tissue segmentation. Gamma-aminobutyric acid (GABA) was acquired using MEGA-semi-LASER localization (TE = 68, TR = 2000, Averages =128, Acquisition Time = 8:56) (Andreychenko, Boer, Arteaga De Castro, Luijten, & Klomp, 2012; Klomp, Bitz, Heerschap, & Scheenen, 2009). Prior studies have shown that when performing spectral editing (Mescher, Merkle, Kirsch, Garwood, & Gruetter, 1998) (as is the case with MEGA-sLASER) some macromolecular signal contributes to the total GABA signal, therefore we refer to GABA as GABA+. To measure the neurochemicals Glx (Glutamate + Glutamine), N-acetylacetate (NAA), myo-Inositol (mI), choline (tCh), and creatine (tCr), 1H-MR spectroscopy was acquired using semi-LASER localization (TE = 35, TR = 2000, Averages = 64, Acquisition Time = 2:28) (Marjańska et al., 2013). Unsuppressed water acquisitions were acquired as reference scans both for phase and eddy-current corrections as well as for quantification (ratio of metabolite over water) for both semi-LASER and MEGA-sLASER. Parameters for the reference scans were the same as for water suppressed scans with the exception of only 8 averages acquired. Total acquisition time was approximately 45 minutes.
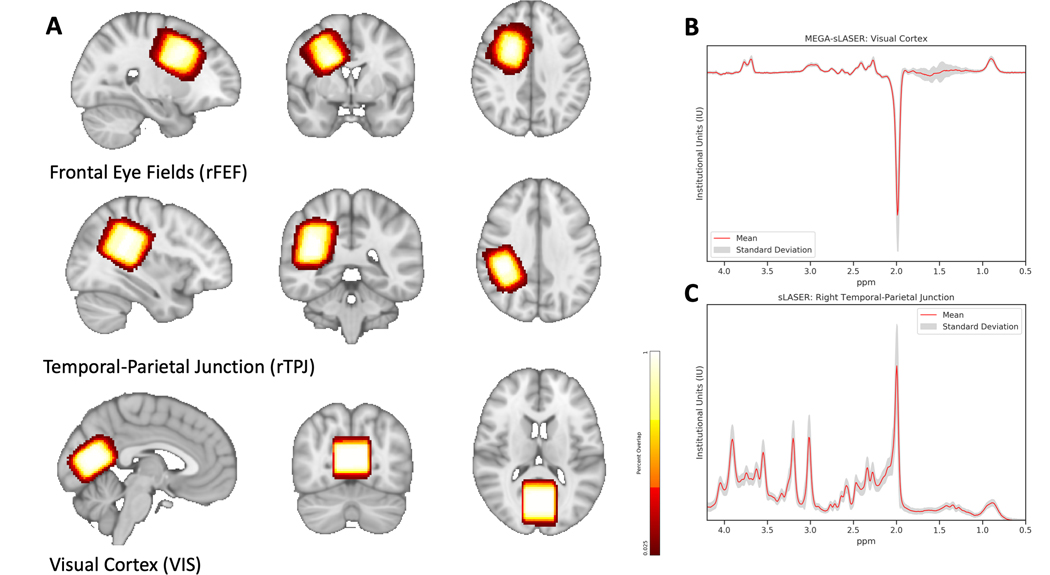
Volumes of interest (VOI) were placed in the right frontal eye fields (rFEF, 20 × 30, 25), right temporal-parietal junction (rTPJ, 30 × 20 × 30), and visual cortex (VIS, 30 × 30 × 20) using anatomical landmarks (Figure 2). The rFEF VOI was positioned at the junction of the precentral and superior frontal sulci and aligned with the cortical surface, while remaining anterior to the central sulcus (similar to Sumner et al., 2010). The rTPJ VOI was centered and aligned to the sylvian fissure and included superior temporal gyrus, supramarginal gyrus, angular gyrus, and posterior superior temporal sulcus, while remaining posterior to postcentral gyrus. Similar to Evans et al. (2010) and Muthukumaraswamy et al. (2009), the VIS VOI was centered on the midline, aligned with the cerebellar tentorium, and positioned anterior to the sagittal sinus (Evans, McGonigle, & Edden, 2010; Muthukumaraswamy, Edden, Jones, Swettenham, & Singh, 2009). Absolute quantification of GABA, Glx, NAA, tCr, tCho, and mI was performed on spectra from each VOI with LCModel V6.3–1B (Provencher, 1993). Basis sets used in LCModel were generated using density matrix simulation and using GABA coupling constants from Kaiser et al. (2007) (Kaiser, Young, & Matson, 2007). Results from LCModel for each neurochemical were in mM and only neurochemicals with Cramer-Rao lower bounds (CRLB) of < 20% across all participants were used for subsequent analyses. Tissue segmentation to obtain percentages of white matter, gray matter, and cerebrospinal fluid (CSF) was performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). All metabolites were CSF-corrected with the exception of GABA, which was tissue-corrected using the method as described in Harris et al. (2015) using an α = 0.5 (Harris, Puts, & Edden, 2015).
Figure 2. Volumes of Interest (VOI) for Magnetic Resonance Spectroscopy (MRS) Acquisition.
A) Representative locations and the % overlap for repeatability of voxel placement in volumes of interest (VOIs): right frontal eye fields (rFEF, 78.8% overlap), right temporal-parietal junction (rTPJ, 84% overlap), and visual cortex (VIS, 82.3% overlap). Shown in radiological orientation. B) Mean (standard deviation in grey) VIS difference spectrum acquired using MEGA-semi-LASER (MEGA-sLASER) with a TE of 68ms, TR of 2000ms, and 128 averages. C) Mean rTPJ 1H spectrum acquired using semi-LASER (sLASER) with an echo time (TE) of 35ms, repetition time (TR) of 2000ms, and 64 averages.
To determine repeatability of VOI placement, each participant’s VOI was aligned to a standard spatial template, MNI 152 (http://nist.mni.mcgill.ca). First, each participant’s T1-weighted image was registered to the standard volume with FSL’s FLIRT using 6 degrees of freedom. The resulting registered image then underwent non-linear transformation into standard space using FSL’s FNIRT. The resulting transformations from both FLIRT and FNIRT were then applied to the VOI mask using ApplyXFM and ApplyWarp, respectfully. The resulting masks were combined into one image which was used to assess the repeatability of VOI placement.
Data Analyses
Wilk-Shapiro tests were performed to assess normality for each variable. Most neurochemicals and VST measures were not normally distributed and were therefore transformed. MRS were log-transformed whereas, due to negative values, VST measures were transformed using y = log(x + min(x) + 1), where y is the transformed measure and x is the original measure.
For assessing VST reaction time (RT) and accuracy, a mixed-model repeated measures ANOVA with between-subjects factor group (ASD, TD) and within-subjects factors of set size (18, 24, and 36) and target presence (present, absent). Other VST metrics (i.e., slope & y-intercept) were assessed using similar repeated measures ANOVA with between-subjects factor group (ASD, TD) and within-subjects factor target presence (present, absent). Post-hoc analysis was performed using Tukey’s HSD.
To assess the effect of group (ASD, TD) on neurochemical (GABA+, Glx, NAA, mI, tCr, and tCh) concentration in each VOI (rFEF, rTPJ, and VIS), multivariate analysis of variance (MANOVA) were performed followed by post-hoc analysis using one-way ANOVA, correcting for multiple comparisons using FDR. Due to collinearity of neurochemicals within each VOI, principle component analysis (PCA) was used to transform data into principle components (PCs) for input into each MANOVA model. A number of PCs were used equal to 90% of total variance explained. Student t-tests were used to assess differences between groups in MRS quality assurance parameters.
Finally, to assess the relationships between neurochemicals and the VST metrics (RT, slope, and y-intercept), partial Pearson’s product-moment correlation tests were used. While groups were matched for age, behavioral and MRS measures vary across the ages tested, therefore we corrected for age. P-values were corrected for multiple comparisons using FDR. An alpha of 0.05 was used to determine statistical significance for both uncorrected and corrected values.
Analyses were performed in the R environment (R Core Team, 2019). Specific packages used included micompr (MANOVA, Fachada, Rodrigues, Lopes, Martins, & AC, 2016), emmeans (repeated measures ANOVA, Lenth, 2019), ppcor (partial correlations, Kim, 2015), and ggplot2 (figures, Wickham, 2016).
Results
Visual Search Task (VST)
Four children with ASD and one TD child were excluded from VST analyses due to accuracy rates below 60%. VST performance is summarized in Figure 3.
Figure 3. Visual Search Task Performance.
A) Accuracy is shown for target absent and target present conditions in the VST across set sizes of 18, 24, and 36. B) Reaction time (RT) for the target absent and target present conditions are shown
Accuracy.
There was a significant main effect of target presence, F(1, 37) = 65.459, p < 0.0001,p2 = 0.64), with greater accuracy for target present (M = 0.98; SD = 0.02 ) compared to target absent condition (M = 0.86; SD =0.10) (Figure 3A). There was no significant main effect for group, F(1, 37) = 2.64, p = 0.11) or set size, F(2, 74) = 0.54, p = 0.59, nor were there statistically significant interactions between group, target presence, or set size (all p > 0.05).
Reaction time.
There were significant main effects of target presence, F(1, 37) = 70.92, p <0.0001,p2 = 0.66) and set size, F(2, 74) = 30.29, p < 0.0001, p2 = 0.45). Post-hoc analyses showed that RT was faster for present compared to absent (p < 0.0001) and increased at larger set sizes (p < 0.0001). Additionally, there was a significant interaction between target presence and set size, F(2, 74) = 35.07, p < 0.0001, p2 = 0.49, as there was no difference in RT in the present condition across set size (p = 1), but RT did increase across set sizes in the absent condition (p < 0.0001) (Figure 3B). However, there was no main effect of group, F(1, 37) = 0.52, p = 0.48, nor an interaction between group and target presence, F(1, 37) = 2.63, p = 0.11, or set size, F(2, 74) = 1.95, p = 0.17.
Slope and Y-intercept.
For slope of the RT x set size function there was a significant main effect of target presence, F(1, 37) = 33.791, p < 0.0001, p2 = 0.48, as search was more efficient in target present compared to target absent trials (i.e., lower slope in present compared to absent). However, there was no significant difference for ASD and TD groups, F(1,37) = 2.17, p = 0.15, nor was there an interaction between target presence and group, F(1,37) = 0.17, p = 0.68. Likewise, for y-intercept there was a significant main effect of target presence, F(1, 37) = 5.82, p = 0.02, p2 = 0.14, but no significant main effect of group, F(1, 37) = 0.01, p = 0.92, nor an interaction between group and target presence, F(1, 37) = 1.73, p = 0.20.
Magnetic Resonance Spectroscopy (MRS)
Quality Assurance.
Measures of quality assurance can be found in Table 2. Group differences between shims and spectral SNR were present. The TD group had larger shim values, as obtained from the Siemens preparation scans, than the ASD group in the rFEF (13.1 Hz versus 12.0 Hz, p = 0.02) and the rTPJ (14.2 Hz versus 12.8 Hz, p = 0.01). However, it should be noted that all of these shim values represent good shims and produced high quality spectra. SNR was also higher in the TD group for MEGA-sLASER spectra in the rTPJ (58.5 versus 46.2, p = 0.01). Semi-LASER spectra had higher SNR in the TD group for rTPJ spectra (78.7 versus 69.5, p = 0.01) and rFEF spectra (64.6 versus 56.2, p = 0.003). However, again, SNR is still considered high for all groups in this cohort. Linewidth (FWHM), obtained from LCModel analysis, was consistent across groups as was tissue segmentation in fractional grey matter (fGM), fractional white matter (fWM), and fractional cerebrospinal fluid (fCSF). Repeatability of VOI placement is shown in Figure 2. Combined with the consistent tissue fractions, the VOI placement was reasonably consistent across all participants with 78.8% overlap in the rFEF, 84% overlap in the rTPJ, and 82.3% overlap in the VIS.
Table 2. Spectroscopy Quality Assurance.
Mean (SD). Abbreviations: rTPJ = right temporal-parietal junction, rFEF = right frontal eye fields, VIS = visual cortex, fGM = fractional gray matter, fWM = fractional white matter, fCSF = fractional cerebrospinal fluid, MEGA = MEGA-semi-LASER, SVS = semi-LASER.
| Shim [Hz] | |||||||||
| rTPJ | rFEF | VIS | |||||||
| ASD | 12.8 (1.4) | 12.0 (0.7) | 12.8 (0.7) | ||||||
| TD | 14.2* (1.6) | 13.1* (1.8) | 13.2 (0.9) | ||||||
| Segmentation (GM, WM and CSF fractions) | |||||||||
| rTPJ | rFEF | VIS | |||||||
| fGM | fWM | fCSF | fGM | fWM | fCSF | fGM | fWM | fCSF | |
| ASD | 0.35 (0.14) | 0.62 (0.14) | 0.03 (0.04) | 0.28 (0.09) | 0.70 (0.1) | 0.02 (0.02) | 0.72 (0.04) | 0.22 (0.04) | 0.07 (0.03) |
| TD | 0.41 (0.07) | 0.58 (0.07) | 0.02 (0.01) | 0.29 (0.05) | 0.70 (0.05) | 0.01 (0.004) | 0.73 (0.04) | 0.21 (0.04) | 0.06 (0.02) |
| MRS QA | |||||||||
| FWHM [Hz] | |||||||||
| rTPJ | rFEF | VIS | |||||||
| MEGA | SVS | MEGA | SVS | MEGA | SVS | ||||
| ASD | 0.03 ( 0.009) | 0.03 (0.004) | 0.03 (0.003) | 0.03 (0.008) | 0.03 (0.006) | 0.03 (0.004) | |||
| TD | 0.03 (0.008) | 0.03 (0.008) | 0.03 (0.006) | 0.02 (0.004) | 0.03 (0.01) | 0.03 (0.005) | |||
| SNR | |||||||||
| rTPJ | rFEF | VIS | |||||||
| MEGA | SVS | MEGA | SVS | MEGA | SVS | ||||
| ASD | 46.2 (13.7) | 69.5 (14.9) | 43.9 (10.1) | 56.2 (9.1) | 53.3 (13.0) | 91.8 (10.3) | |||
| TD | 58.5 (12.5)* | 78.7* (9.91) | 50.2 (14.0) | 64.6** (5.9) | 55.4 (15.2) | 92.2 (7.0) | |||
p < 0.01,
p < 0.05
Neurochemistry.
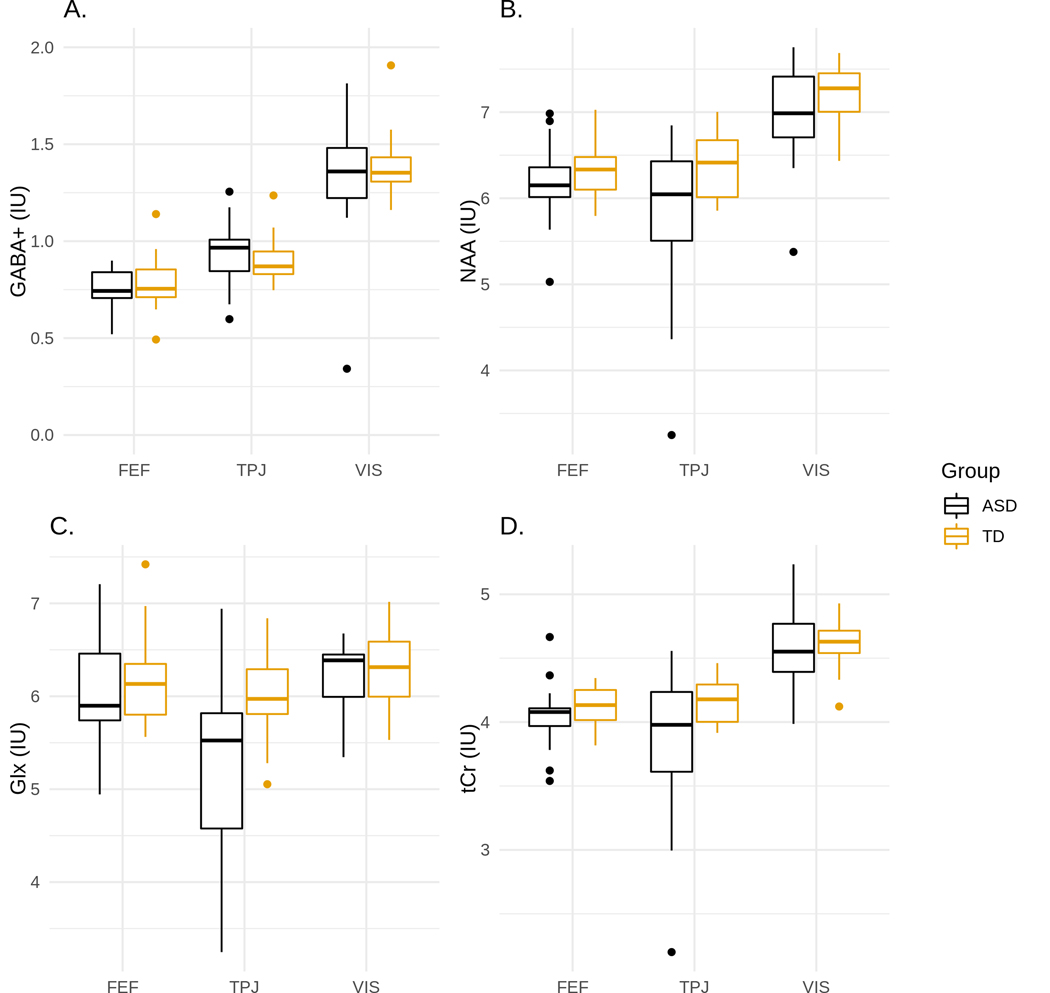
No significant differences in GABA+ were found between TD and ASD (Figure 4A). There were no significant differences in other neurochemicals for the rFEF or VIS VOI; however, groups did differ in the rTPJ VOI, Pillai’s Trace = 0.42, F(1, 33) = 3.38, p = 0.01. Post-hoc analysis showed significant differences between TD and ASD in NAA, F(1, 34) = 7.17, p = 0.02, adj. p = 0.03, P2 = 0.17 (Figure 4B), tCr, F(1, 34) = 7.70, p = 0.02, adj. p = 0.03, P2 = 0.18 (Figure 4C), and Glx, F(1, 34) = 16.3, p = 0.008, adj. p = 0.03, P2 = 0.32 (Figure 4D). Due to differences in Glx between ASD and TD, there was a significant difference in GABA/Glx, F(1,34) = 7.69, p = 0.009, adj. p = 0.03, P2 = 0.18. Internal neurochemical ratios were not used because of significant differences in NAA and tCr, instead absolute values for neurochemicals were used.
Figure 4. Box plots of metabolite concentrations for ASD and TD children.
Distributions for neurochemicals in all three regions (rFEF, rTPJ, and VIS) are shown in A) GABA+, B) NAA, C) Glx, and D) tCr. Post-hoc analysis showed there were significant differences in NAA, Glx, and tCr between ASD and TD in the TPJ. * indicates statistical significance where p < 0.05.
Correlations between VST, MRS, and ASD Symptomatology
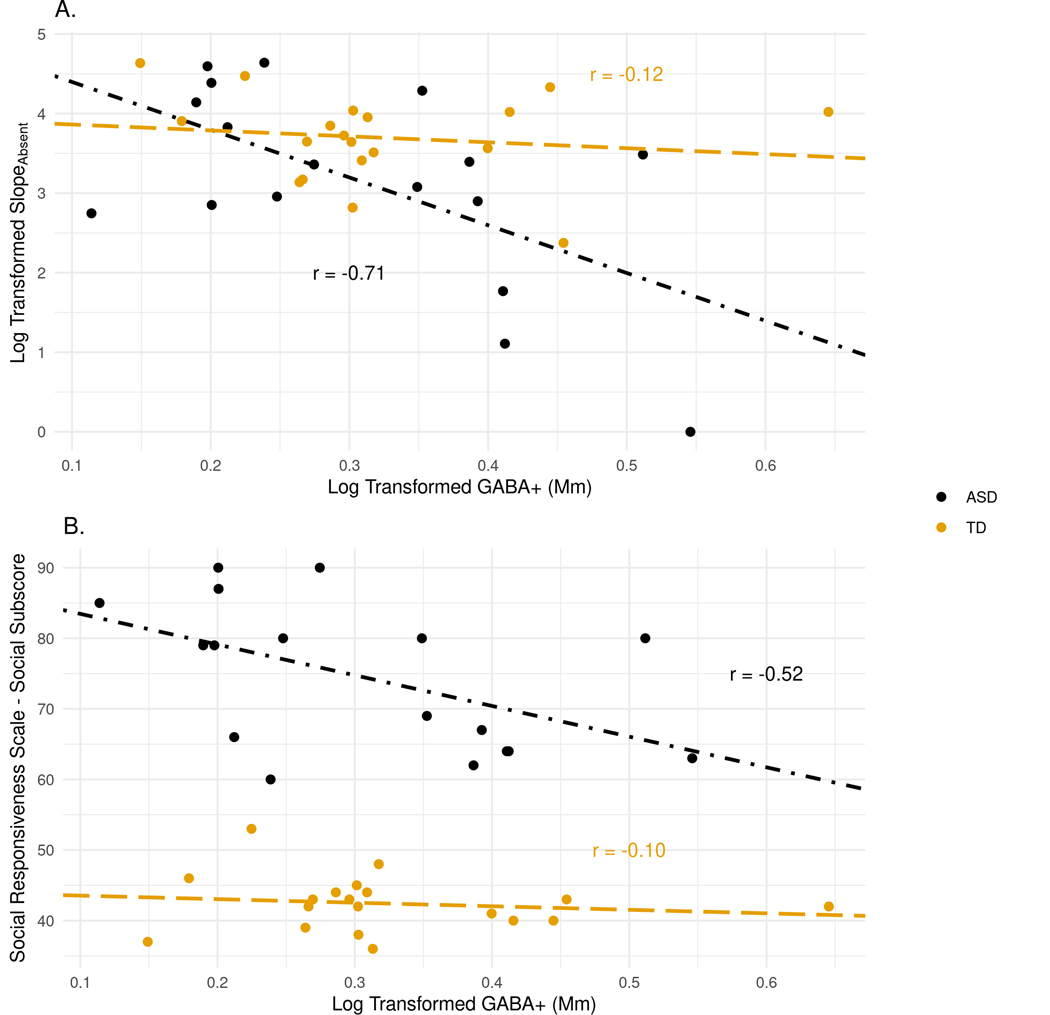
As illustrated in Figure 5A, there was a significant association between VIS GABA+ and slope in the absent condition, r(15) = −0.71, p = 0.0001, adj. p = 0.005, for the ASD group, but not for TD group(r = −0.12). These two correlations were significantly different from one another (Fisher’s z = −2.209, p = 0.03). Additionally, the SRS-2 Social Communication Index (SCI) t-score was significantly correlated with VIS GABA+, r(15) = −0.52, p = 0.02, adj. p = 0.26), as was the SRS Total t-score, r(15) = −0.50, p = 0.02, adj. p = 0.26, in ASD (but neither survived multiple comparison adjustment; Figure 5B). There was no significant relationship between ADOS-2 Comparison score and GABA+ levels. Additionally, despite differences between ASD and TD for NAA, tCR, and Glx in the rTPJ, there were no significant correlations between any rTPJ neurochemical and ASD symptoms or VST performance.
Figure 5. Correlations between GABA and VST performance and ASD symptomatology in the visual cortex (VIS).
A) Scatterplot of VIS VOI GABA+ values and RT x set size slope for the absent condition. B) Scatterplot of VIS VOI GABA+ values and Social Responsiveness Scale (SRS) Social Communication Index (SCI).
Discussion
The objective of the present report was to investigate whether region-specific GABA concentrations may underlie superior search in children with ASD. Across all three VOI, GABA+ levels did not differ between groups; however, for children with ASD (but not TD), GABA+ concentration in the visual cortex was associated with more efficient visual search abilities. In addition, reduced visual cortex GABA+ values were related to increased ASD symptomatology. Finally, significantly lower NAA, tCr, and Glx in the rTPJ provide further evidence of regional neurochemistry differences in ASD. Each will be discussed in turn.
No Evidence of Reduced GABA Concentrations
Contrary to our original hypothesis, we did not find any differences in GABA+ concentrations between ASD and TD. Other studies have investigated differences in GABA and showed no differences in the visual cortex (Gaetz et al., 2014; Robertson et al., 2016), striatum (Harada et al., 2011; Horder et al., 2018), and medial prefrontal cortex (Horder et al., 2018). However, other studies have reported lower GABA levels in other areas, such as the sensorimotor cortex (Puts et al., 2017; Sapey-Triomphe et al., 2019), frontal lobe (Harada et al., 2011; Kubas et al., 2012), and auditory cortex (Gaetz et al., 2014; Port et al., 2017; Rojas et al., 2014).
Although these mixed results may result from region-specific group differences, methodological variability across studies may also contribute to these inconsistent findings. We used a measure for GABA that included the co-edited macromolecule signal (GABA+) and corrected for GM, WM, and CSF composition of the VOI (Harris, Puts, Barker, & Edden, 2015). A majority of the studies listed above that found decreases in GABA used internal metabolite references, such as GABA/tCr (Gaetz et al., 2014; Kubas et al., 2012; Port et al., 2017; Rojas et al., 2014) or GABA/NAA (Harada et al., 2011). Additionally, we used an advanced method of voxel localization, semi-LASER, that minimizes the chemical shift displacement artifact (CSDA), which allows unwanted contributions of neurochemicals from outside the VOI to contribute to the signal. CSDA is a known complication in point-resolved spectroscopy, or PRESS, which most previous studies used, and therefore consensus papers have suggested to cease using this sequence in research (Wilson et al., 2019). Therefore, comparisons between prior and future studies that report GABA levels should be done mindful of these differences in methodology, especially in cases where internal references or PRESS were used. Nevertheless, our results provide further evidence that group differences in GABA+ concentrations are limited, and if present are likely to be region-specific.
Relationship between GABA and VST Performance
Our study provides further evidence of potential atypical GABA signaling in the visual cortex of individuals with ASD. Similar to reported deficits in binocular rivalry proportionate to lower GABA+ in children with ASD (Robertson et al., 2016) without differences in GABA+ between groups, findings from the present report show a strong negative relationship between visual cortex GABA+ and VST performance in children with ASD, but not in TD children (absent a difference in GABA+ in the visual cortex between ASD and TD). Children with ASD who had greater GABA+ concentrations demonstrated more efficient search performance.
In the present study target identification required orientation discrimination, which has shown to be enhanced or altered in ASD. For example, Dickinson et al (2016) showed that individuals with ASD observed differences in orientation earlier than neurotypical individuals, suggesting improved orientation discrimination. This was accompanied with increased gamma band response, further suggestive of increased neural inhibition in the visual cortex (Dickinson, Bruyns-Haylett, Smith, Jones, & Milne, 2016). Using contextual illusions, Song et al. (2017) identified a significant positive correlation between occipital GABA+/tCr and orientation magnitude, whereas they found a significant positive correlation between parietal GABA+/tCr and size magnitude. Together, these results suggest that GABA levels in different cortical regions have an influence on different visual features (Song et al., 2017). Therefore, it is possible that enhanced visual perception, in the form of improved orientation discrimination, might be the underlying cause of improved VST performance with higher GABA+, perhaps due to greater inhibition, in ASD.
In contrast to the findings from the present study, it should be noted, that others have reported region-specific reductions in GABA linked to poorer non-visual discrimination abilities. For example, Puts and colleagues (2017) showed decreased somatosensory GABA levels in ASD, which were associated with higher tactile discrimination thresholds (Puts et al., 2017). Similarly, reduced GABA in the auditory cortex and atypical gamma-band response to tones has been shown in ASD (Port et al., 2017). Thus, while our findings are consistent with previous psychophysics and electrophysiology findings related to visual perception, these may differ from other sensory modalities.
GABA and ASD Symptomatology
Correlational analyses also suggested that there was an association between GABA+ and ASD symptomology. Specifically, higher SRS-2 scores (indicative of greater ASD symptomatology) were associated with reduced concentrations of GABA+ in the visual cortex. This finding is consistent with previous findings linking reductions in GABA to increases in ASD symptoms (Brix et al., 2015; Carvalho Pereira et al., 2018). However, in light of the inverse relationship between VIS GABA+ and VST performance, this result appears to be conflicting, especially given that prior reports have shown that faster, more efficient search is related to ASD symptom severity (Joseph et al., 2009; Keehn et al., 2013). Due to these conflicts, as well as the result not surviving correction for multiple corrections, this finding should be interpreted with caution.
Differences in Neurochemical Concentrations in the rTPJ
While GABA+ levels did not differ between groups, results from the present study did show significant differences in other neurochemical concentrations (NAA, tCr, and Glx) between ASD and TD in the rTPJ. In children with ASD, NAA has been found to be lower in various regions of the cortex (Carvalho Pereira et al., 2018; Harada et al., 2011; Kleinhans, Schweinsburg, Cohen, Müller, & Courchesne, 2007; Kubas et al., 2012). NAA is a neuronal marker representing neurodysfunction or neuronal loss (de Graaf, 2018). Kubas et al. (2012) also found lower NAA/Cr and Glx/Cr in the frontal cortex, with no changes in Cr. The combined term of phosphocreatine and creatine, tCr, is a marker for energy metabolism where it functions as an energy buffer and shuttle. Decreases in tCr have been found in the occiptal (DeVito et al., 2007; Levitt et al., 2003), temporal (DeVito et al., 2007), frontal (Friedman et al., 2006), and parietal lobes (Friedman et al., 2003), as well as the thalamus (Friedman et al., 2003; Hardan et al., 2008). Glx findings appear to be regionally specific as well, with prior studies showing both elevated (Bejjani et al., 2012) and decreased Glx levels (Corrigan et al., 2013; DeVito et al., 2007). Combined, these results suggest dysfunction in the rTPJ that may be associated with decreased Glx, and thus resulting in an excitatory/inhibitory destabilization, perhaps in regions projecting from the rTPJ.
The rTPJ is a supramodal association area, which plays a role in both social and non-social cognitive abilities (Carter & Huettel, 2013) and follows a unique developmental trajectory (Sowell, 2004). Atypical activation, possibly due to neuronal dysfunction in the rTPJ, in individuals with ASD has been shown using a variety of paradigms, including social information processing paradigms, such as mentalizing tasks (e.g., Castelli, 2002; Lombardo et al., 2010), as well as non-social attention tasks (Gomot et al., 2006; Keehn, Nair, Lincoln, Townsend, & Müller, 2016). Functional connectivity studies have also demonstrated that this region is a critical node in multiple functional brain networks (Jakobs et al., 2012), and that children with ASD exhibit atypical functional differentiation and the absence of typical age-related changes in specialization of rTPJ-linked cortical networks (Shih et al., 2011). Collectively, these studies and our results suggest further exploration into differences between ASD and TD may be needed for further understanding the role of this area in the development of ASD.
Limitations
Children with ASD in the present study did not demonstrate faster, more efficient search. The ASD search advantage is greater for more difficult visual search paradigms. The present task may have been too easy to elicit significant differences in performance between ASD and TD. Nonetheless, we were able to identify differences between ASD and TD in terms of neurochemistry and their contribution to VST performance. Second, medication use was not an exclusionary criterion for the present study, and some children with ASD (n = 10) in the current study were prescribed psychotropic medication. Although these drugs did not specifically target the GABAergic or glutamatergic systems, our results should be interpreted with caution. Finally, MRS cannot determine the type of GABA measured, whether within a neurotransmitter or metabolite pool. Therefore, while GABA is the major inhibitory neurotransmitter in the CNS, we are unable to identify whether concentration differences are present in the synapse versus storage vesicles. Future research employing more advanced techniques, specifically diffusion-weighted MRS (Ligneul et al., 2019; Ronen, Ercan, & Webb, 2013), may be able to determine what type of GABA contributes to the measured GABA signal.
Conclusions
Together, our findings are in agreement with prior reports that show equivalent GABA levels in ASD and TD children (Gaetz et al., 2014; Harada et al., 2011; Horder et al., 2018; Robertson et al., 2016); however, correlational findings indicate that visual search superiority in individuals with ASD may be associated with GABA signaling in the visual cortex, and suggest that more efficient visual search is achieved via enhanced visual discrimination. Additionally, children with ASD showed decreased NAA, tCr, and Glx in the rTPJ compared to their TD peers. Evidence of neurochemical differences in the rTPJ along with prior findings of atypical activation and connectivity suggest this region, which plays a key role in sensory integration and social and non-social cognition, may be disrupted in ASD.
Acknowledgements:
Funding for this study was provided by NIH/NIMH R21 MH114095 (B.K.), NIH/NIEHS F31 ES028081 (D.A.E.), NIH S10 OD012336 (U.D.), and the Purdue Institute for Integrative Neuroscience (#209257; B.K. and U.D.). Furthermore we acknowledge the receipt of the sLASER and MEGA-sLASER sequences by the Center for Magnetic Resonance Research, University of Minnesota, developed by Edward Auerbach and Malgorzata Marjanska.
Footnotes
Conflict of Interest:
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Andreychenko A, Boer VO, Arteaga De Castro CS, Luijten PR, & Klomp DWJ (2012). Efficient spectral editing at 7 T: GABA detection with MEGA-sLASER. Magnetic Resonance in Medicine, 68(4), 1018–1025. 10.1002/mrm.24131 [DOI] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington D.C. [Google Scholar]
- Bejjani A, O’Neill J, Kim JA, Frew AJ, Yee VW, Ly R, … Levitt JG (2012). Elevated Glutamatergic Compounds in Pregenual Anterior Cingulate in Pediatric Autism Spectrum Disorder Demonstrated by 1H MRS and 1H MRSI. PLoS ONE, 7(7), e38786. 10.1371/journal.pone.0038786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser E, Eglington L, Carter AS, & Kaldy Z. (2014). Pupillometry reveals a mechanism for the Autism Spectrum Disorder (ASD) advantage in visual tasks. Scientific Reports, 4, 1–5. 10.1038/srep04301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix MK, Ersland L, Hugdahl K, Grüner R, Posserud M-B, Hammar Å, … Beyer MK (2015). Brain MR spectroscopy in autism spectrum disorder—the GABA excitatory/inhibitory imbalance theory revisited. Frontiers in Human Neuroscience, 9(June), 1–12. 10.3389/fnhum.2015.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, & Huettel SA (2013). A nexus model of the temporal–parietal junction. Trends in Cognitive Sciences, 17(7), 328–336. 10.1016/j.tics.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Pereira A, Violante IR, Mouga S, Oliveira G, & Castelo-Branco M. (2018). Medial Frontal Lobe Neurochemistry in Autism Spectrum Disorder is Marked by Reduced N-Acetylaspartate and Unchanged Gamma-Aminobutyric Acid and Glutamate + Glutamine Levels. Journal of Autism and Developmental Disorders, 48(5), 1467–1482. 10.1007/s10803-017-3406-8 [DOI] [PubMed] [Google Scholar]
- Castelli F. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain, 125(8), 1839–1849. 10.1093/brain/awf189 [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber C. (2012). The Social Responsiveness Scale, Second Edition (SRS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan NM, Shaw DWW, Estes AM, Richards TL, Munson J, Friedman SD, … Dager SR (2013). Atypical developmental patterns of brain chemistry in children with autism spectrum disorder. JAMA Psychiatry, 70(9), 964–974. 10.1001/jamapsychiatry.2013.1388 [DOI] [PubMed] [Google Scholar]
- de Graaf RA (2018). In Vivo NMR Spectroscopy - Dynamic Aspects In Vivo NMR Spectroscopy, 129–210. 10.1002/9781119382461.ch3 [DOI] [Google Scholar]
- DeVito TJ, Drost DJ, Neufeld RWJ, Rajakumar N, Pavlosky W, Williamson P, & Nicolson R. (2007). Evidence for Cortical Dysfunction in Autism: A Proton Magnetic Resonance Spectroscopic Imaging Study. Biological Psychiatry, 61(4), 465–473. 10.1016/j.biopsych.2006.07.022 [DOI] [PubMed] [Google Scholar]
- Dickinson A, Bruyns-Haylett M, Smith R, Jones M, & Milne E. (2016). Superior orientation discrimination and increased peak gamma frequency in autism spectrum conditions. Journal of Abnormal Psychology, 125(3), 412–422. 10.1037/abn0000148 [DOI] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, & Singh KD (2009). Orientation Discrimination Performance Is Predicted by GABA Concentration and Gamma Oscillation Frequency in Human Primary Visual Cortex. Journal of Neuroscience, 29(50), 15721–15726. 10.1523/JNEUROSCI.4426-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, & Johnson MH (2013). Disengagement of Visual Attention in Infancy is Associated with Emerging Autism in Toddlerhood. Biological Psychiatry, 74(3), 189–194. 10.1016/j.biopsych.2012.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, McGonigle DJ, & Edden RAE (2010). Diurnal stability of γ-aminobutyric acid concentration in visual and sensorimotor cortex. Journal of Magnetic Resonance Imaging, 31(1), 204–209. 10.1002/jmri.21996 [DOI] [PubMed] [Google Scholar]
- Fachada N, Rodrigues J, Lopes V, Martins R, & AC R. (2016). micompr: An R Package for Multivariate Independent Comparison of Observations. The R Journal, 8(2), 405–420. [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, … Dager SR (2003). Regional brain chemical alterations in young children with autism spectrum disorder. Neurology, 60(1), 100–107. 10.1212/WNL.60.1.100 [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DWW, Artru AA, Dawson G, Petropoulos H, & Dager SR (2006). Gray and White Matter Brain Chemistry in Young Children With Autism. Archives of General Psychiatry, 63(7), 786 10.1001/archpsyc.63.7.786 [DOI] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, & Roberts TPLL (2014). GABA estimation in the brains of children on the autism spectrum: Measurement precision and regional cortical variation. NeuroImage, 86, 1–9. 10.1016/j.neuroimage.2013.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Bedford R, Charman T, Johnson MH, Baron-Cohen S, Bolton P, … Tucker L. (2015). Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms. Current Biology, 25(13), 1727–1730. 10.1016/j.cub.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, & Baron-Cohen S. (2006). Change detection in children with autism: An auditory event-related fMRI study. NeuroImage, 29(2), 475–484. 10.1016/j.neuroimage.2005.07.027 [DOI] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, & Matsuda T. (2011). Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 Tesla instrument. Journal of Autism and Developmental Disorders, 41(4), 447–454. 10.1007/s10803-010-1065-0 [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, … Stanley JA (2008). An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Research: Neuroimaging, 163(2), 97–105. 10.1016/j.pscychresns.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Barker PB, & Edden RAE (2015). Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magnetic Resonance in Medicine, 74(6), 1523–1529. 10.1002/mrm.25549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, & Edden RAE (2015). Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging, 42(5), 1431–1440. 10.1002/jmri.24903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessels RS, Hooge ITC, Snijders TM, & Kemner C. (2014). Is There a Limit to the Superiority of Individuals with ASD in Visual Search? Journal of Autism and Developmental Disorders, 44(2), 443–451. 10.1007/s10803-013-1886-8 [DOI] [PubMed] [Google Scholar]
- Horder J, Petrinovic MM, Mendez MA, Bruns A, Takumi T, Spooren W, … Murphy DG (2018). Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Translational Psychiatry, 8(1), 1–11. 10.1038/s41398-018-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman JP (2001). Suppressed GABAergic Inhibition as a Common Factor in Suspected Etiologies of Autism. Journal of Autism and Developmental Disorders, 31(2), 247–248. 10.1016/0002-9610(92)90118-B [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, … Eickhoff SB (2012). Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus–context integration. NeuroImage, 60(4), 2389–2398. 10.1016/j.neuroimage.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, & Horowitz TS (2009). Why is visual search superior in autism spectrum disorder? Developmental Science, 12(6), 1083–1096. 10.1111/j.1467-7687.2009.00855.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Young K, & Matson GB (2007). Elimination of spatial interference in PRESS-localized editing spectroscopy. Magnetic Resonance in Medicine, 58(4), 813–818. 10.1002/mrm.21407 [DOI] [PubMed] [Google Scholar]
- Kaldy Z, Giserman I, Carter AS, & Blaser E. (2016). The Mechanisms Underlying the ASD Advantage in Visual Search. Journal of Autism and Developmental Disorders, 46(5), 1513–1527. 10.1007/s10803-013-1957-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Brenner L, Palmer E, Lincoln AK, & Müller R-A (2008). Functional brain organization for visual search in ASD. Journal of the International Neuropsychological Society, 14(6), 990–1003. 10.1017/S1355617708081356 [DOI] [PubMed] [Google Scholar]
- Keehn B, & Joseph RM (2016). Exploring What’s Missing: What Do Target Absent Trials Reveal About Autism Search Superiority? Journal of Autism and Developmental Disorders, 46(5), 1686–1698. 10.1007/s10803-016-2700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Nair A, Lincoln AJ, Townsend J, & Müller RA (2016). Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Developmental Cognitive Neuroscience, 17, 46–56. 10.1016/j.dcn.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Shih P, Brenner LA, Townsend J, & Müller R-A (2013). Functional connectivity for an “Island of sparing” in autism spectrum disorder: An fMRI study of visual search. Human Brain Mapping, 34(10), 2524–2537. 10.1002/hbm.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, Van Ewijk L, Van Engeland H, & Hooge I. (2008). Brief report: Eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. Journal of Autism and Developmental Disorders, 38(3), 553–558. 10.1007/s10803-007-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. (2015). ppcor: Partial and Semi-Partial (Part) Correlation. R package version 1.1. [Google Scholar]
- Kleinhans NM, Schweinsburg BC, Cohen DN, Müller R-A, & Courchesne E. (2007). N-acetyl aspartate in autism spectrum disorders: Regional effects and relationship to fMRI activation. Brain Research, 1162, 85–97. 10.1016/j.brainres.2007.04.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp DWJ, Bitz AK, Heerschap A, & Scheenen TWJ (2009). Proton spectroscopic imaging of the human prostate at 7 T. NMR in Biomedicine, 22(5), 495–501. 10.1002/nbm.1360 [DOI] [PubMed] [Google Scholar]
- Kubas B, Kułak W, Sobaniec W, Tarasow E, Łebkowska U, & Walecki J. (2012). Metabolite alterations in autistic children: A 1H MR spectroscopy study. Advances in Medical Sciences, 57(1), 152–156. 10.2478/v10039-012-0014-x [DOI] [PubMed] [Google Scholar]
- Lenth R. (2019). emmeans: Estimated Marginal Means, aka Least-Square Means. R package version 1.4.1. [Google Scholar]
- Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, … Alger JR (2003). Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biological Psychiatry, 54(12), 1355–1366. 10.1016/S0006-3223(03)00688-7 [DOI] [PubMed] [Google Scholar]
- Ligneul C, Palombo M, Hernández-Garzón E, Carrillo-de Sauvage M-A, Flament J, Hantraye P, … Valette J. (2019). Diffusion-weighted magnetic resonance spectroscopy enables cell-specific monitoring of astrocyte reactivity in vivo. NeuroImage, 191, 457–469. 10.1016/j.neuroimage.2019.02.046 [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, & Baron-Cohen S. (2010). Shared Neural Circuits for Mentalizing about the Self and Others. Journal of Cognitive Neuroscience, 22(7), 1623–1635. 10.1162/jocn.2009.21287 [DOI] [PubMed] [Google Scholar]
- Lord C, Luyster R, Gotham K, & Guthrie W. (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Moddule. Torrance, CA: Western Psychological Services. [Google Scholar]
- Marjańska M, Lehéricy S, Valabrègue R, Popa T, Worbe Y, Russo M, … Meunier S. (2013). Brain dynamic neurochemical changes in dystonic patients: A magnetic resonance spectroscopy study. Movement Disorders, 28(2), 201–209. 10.1002/mds.25279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, & Gruetter R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11(6), 266–272. [DOI] [PubMed] [Google Scholar]
- Milne E, Dunn SA, Freeth M, & Rosas-Martinez L. (2013). Visual search performance is predicted by the degree to which selective attention to features modulates the ERP between 350 and 600ms. Neuropsychologia, 51(6), 1109–1118. 10.1016/j.neuropsychologia.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, & Burack J. (2006). Enhanced Perceptual Functioning in Autism: An Update, and Eight Principles of Autistic Perception. Journal of Autism and Developmental Disorders, 36(1), 27–43. 10.1007/s10803-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, & Singh KD (2009). Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proceedings of the National Academy of Sciences, 106(20), 8356–8361. 10.1073/pnas.0900728106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan M, & Plaisted K. (2001). Enhanced discrimination in autism. The Quarterly Journal of Experimental Psychology Section A, 54(4), 961–979. 10.1080/713756000 [DOI] [PubMed] [Google Scholar]
- Port RG, Gaetz W, Bloy L, Wang DJ, Blaskey L, Kuschner ES, … Roberts TPL (2017). Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Research, 10(4), 593–607. 10.1002/aur.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine, 30(6), 672–679. 10.1002/mrm.1910300604 [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, & Edden RAE (2017). Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Research, 10(4), 608–619. 10.1002/aur.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Remington AM, Swettenham J, Campbell R, & Coleman M. (2009). Selective Attention and Perceptual Load in Autism Spectrum Disorder. Psychological Science, 20(11), 1388–1393. 10.1111/j.1467-9280.2009.02454.x [DOI] [PubMed] [Google Scholar]
- Remington AM, Swettenham JG, & Lavie N. (2012). Lightening the load: Perceptual load impairs visual detection in typical adults but not in autism. Journal of Abnormal Psychology, 121(2), 544–551. 10.1037/a0027670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Ratai EM, & Kanwisher N. (2016). Reduced GABAergic Action in the Autistic Brain. Current Biology, 26(1), 80–85. 10.1016/j.cub.2015.11.019 [DOI] [PubMed] [Google Scholar]
- Rojas DC, Singel D, Steinmetz S, Hepburn S, & Brown MS (2014). Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. NeuroImage, 86, 28–34. 10.1016/j.neuroimage.2013.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen I, Ercan E, & Webb A. (2013). Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Frontiers in Integrative Neuroscience, 7 10.3389/fnint.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, & Merzenich MM (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior, 2(5), 255–267. 10.1034/j.1601-183X.2003.00037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C. (2003). The social communication questionnaire: manual. Western Psychological Services. [Google Scholar]
- Sandberg K, Blicher JU, Dong MY, Rees G, Near J, & Kanai R. (2014). Occipital GABA correlates with cognitive failures in daily life. NeuroImage, 87, 55–60. 10.1016/j.neuroimage.2013.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapey-Triomphe LA, Lamberton F, Sonié S, Mattout J, & Schmitz C. (2019). Tactile hypersensitivity and GABA concentration in the sensorimotor cortex of adults with autism. Autism Research, 12(4), 562–575. 10.1002/aur.2073 [DOI] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, & Müller R-A (2011). Functional Differentiation of Posterior Superior Temporal Sulcus in Autism: A Functional Connectivity Magnetic Resonance Imaging Study. Biological Psychiatry, 70(3), 270–277. 10.1016/j.biopsych.2011.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, D’Avossa G, & Corbetta M. (2007). Right TPJ Deactivation during Visual Search: Functional Significance and Support for a Filter Hypothesis. Cerebral Cortex, 17(11), 2625–2633. 10.1093/cercor/bhl170 [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, D’Avossa G, & Corbetta M. (2003). Quantitative Analysis of Attention and Detection Signals During Visual Search. Journal of Neurophysiology, 90(5), 3384–3397. 10.1152/jn.00343.2003 [DOI] [PubMed] [Google Scholar]
- Song C, Sandberg K, Andersen LM, Blicher JU, & Rees G (2017). Human Occipital and Parietal GABA Selectively Influence Visual Perception of Orientation and Size. The Journal of Neuroscience, 37(37), 8929–8937. 10.1523/jneurosci.3945-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER (2004). Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. Journal of Neuroscience, 24(38), 8223–8231. 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Edden RAE, Bompas A, Evans CJ, & Singh KD (2010). More GABA, less distraction: a neurochemical predictor of motor decision speed. Nature Neuroscience, 13(7), 825–827. 10.1038/nn.2559 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, … Howe FA (2019). Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magnetic Resonance in Medicine, 82(2), 527–550. 10.1002/mrm.27742 [DOI] [PMC free article] [PubMed] [Google Scholar]